Abstract
Mycobacteria produce carbohydrates of exceptional structures that are covalently modified by unique substituents, whose functional characterization could expand our understanding of how mycobacteria adapt to their environment.
Prokaryotes produce a variety of cell envelope glycans that play important roles in their physiology, their adaptation to the environment and their interactions with the host. Consistent with the role of these glycans in formation and preservation of the integrity of the inner and outer membranes, the core structure of the lipid A (endotoxin) moiety of lipopolysaccharides (LPS) in Gram-negative bacteria and that of the carbohydrate-based anionic polymers found in a wide range of Gram-positive bacteria (wall teichoic acids and lipoteichoic acids) are relatively well conserved1–3. These core structural elements, however, may be modified by the addition or removal of various sugars, amino acids, phosphates, or acyl groups to allow for bacterial adaptation and survival under various stress conditions. Although not required for growth per se, and variable from species to species, these discrete tailoring events affect the biosynthesis, export, physical properties, and biological activities of (lipo)polysaccharides and, as such, significantly impact the cell-envelope integrity and surface properties of the bacteria, the way that they interact with environment and host, and their resistance to antibiotics and antimicrobial peptides1–3.
The Mycobacterium genus includes paramount human pathogens including Mycobacterium tuberculosis, the etiological agent of tuberculosis and the most deadly infectious agent in the world; Mycobacterium leprae (leprosy); Mycobacterium ulcerans (Buruli ulcer); and a number of other nontuberculous Mycobacterium pathogens. Reflective of the unique composition and structure of their cell envelope (Fig. 1), mycobacteria lack the canonical LPS and (lipo)teichoic acids found in other prokaryotes. They do, however, produce a distinct array of (lipo)polysaccharides and (glyco)lipids with unique structures that play essential roles in their physiology and pathogenesis4,5, including three dominant mycobacterial cell envelope polysaccharides—arabinogalactan, (AG) lipomannan (LM), and lipoarabinomannan (LAM)—that are the focus of this Commentary (Fig. 2).
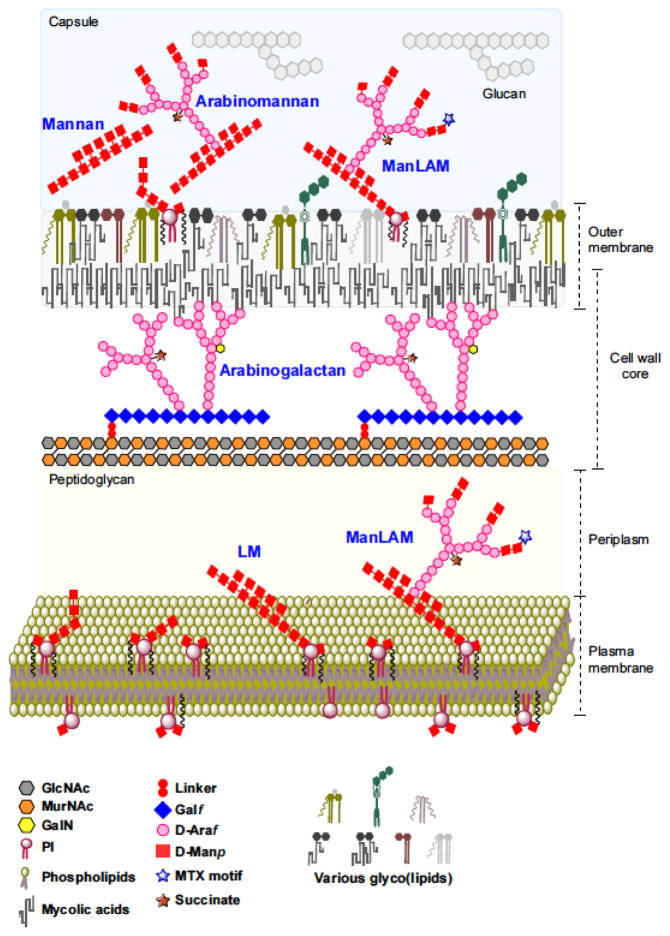
Fig. 1. A current perspective of the cell envelope of M. tuberculosis.
Schematic representation of the cell envelope of M. tuberculosis. The mycobacterial cell envelope is made of the following: a plasma membrane that is typical of prokaryotic membranes except for the presence of Mycobacterium-specific (lipo)proteins, (glyco)lipids and lipoglycans (LM and LAM); a cell wall core comprised of PG in covalent attachment with AG, which is in turn esterified at its nonreducing ends to α-alkyl, β-hydroxy long-chain (C60–C90) fatty acids known as the mycolic acids; an outer membrane (also referred to as ‘mycomembrane’) consisting of an inner leaflet primarily made of AG-bound mycolic acids and an outer leaflet containing a variety of noncovalently bound (glyco)lipids, (lipo)proteins, LM and LAM; and a loosely attached capsular-like structure made of proteins, lipids and polysaccharides (including AM). The overall schematic and individual structures are not drawn to scale. Proteins are not shown for the sake of clarity. PI, phosphatidyl-myo-inositol; GlcNAc, N-acetylglucosamine; MurNAc; N-acetylmuramic acid.
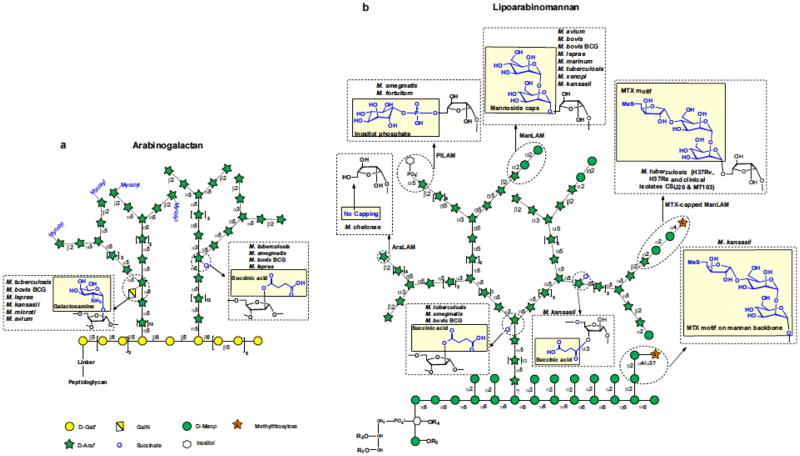
Fig. 2. Details of the chemical modifications affecting arabinogalactan (AG), lipomannan (LM), and lipoarabinomannan (LAM) in mycobacteria.
(a) AG contains, on average, 79 glycosyl residues that are distributed between a galactan domain made of 23 galactofuranosyl residues, two arabinan domains each containing about 26 Araf residues, and a specific linker disaccharide made of a rhamnosyl residue attached to a N-acetylglucosaminosyl-1-phosphate residue which serves in the covalent attachment of AG to PG. (b) LAM and its biosynthetic precursor, LM, are anchored in the inner membrane and outer membrane via their phosphatidyl-myo-inositol anchor (see Fig. 1). LM and LAM share a common linear α-1,6-linked mannan backbone made up of 20–25 mannopyranose (Manp) residues elaborated by single α-1,2-linked Manp units. The major LAM glycoforms contain about 110 glycosyl residues (approximately 60 Araf and 50 Manp units) and consist of a single D-arabinan chain structurally very similar to that found in AG attached to the α-1,6-D-mannan backbone. R1, R2, R3 and R4 are acyl chains (palmitic acid, stearic acid, oleic acid, and tuberculostearic acid). LAM capped at its nonreducing arabinan termini with one to three α-1,2-Manp-linked residues is known as ManLAM; LAM capped with phospho-inositol is known as PILAM. Noncapped LAM is known as AraLAM. The various chemical modifications found in the AG, LM, and LAM of mycobacteria are shown in yellow boxes.
Owing to their importance to the structural integrity of the cell, AG, LM, and LAM have been extensively studied from the perspective of drug discovery. This fundamental, yet restrictive, view of their role in mycobacterial physiology, however, has too often ignored the fact that discrete covalent substituents may confer upon these glycans additional fine-tuning functions that contribute to the ability of mycobacteria to adapt to their environment. Increasing evidence now points to chemical modifications affecting resistance to host defense mechanisms and host–pathogen interactions, thus supporting the notion that mycobacteria, in their own way, have evolved strategies to exploit tailoring substituents of polysaccharides to promote their survival in different environments. New details about the nature and biosynthetic origin of these chemical modifications are providing opportunities to explore their biological significance. This Commentary describes some of the rather unique chemical modifications found in AG, LM, and LAM, as well as what is known of their biological significance. It further highlights the recent developments in chemical biology and cell imaging that could complement traditional biochemistry, genetic and immunology approaches to help functionally characterize these modifications.
Arabinogalactan
Two covalently linked heteropolysaccharides, peptidoglycan (PG) and AG, make up the bulk of the mycobacterial cell wall core (Fig. 1). They provide a rigid structure outside the plasma membrane that is essential to the structural integrity of the cells and serves as a scaffold for the rest of the cell envelope.
The arabinan domain of AG is made of stretches of α-1,5-linked arabinofuranosyl (Araf) residues with precisely positioned α-3,5-branch sites (Fig. 2a). The C2 position of a portion of the internal α-3,5-branched Araf residues may be modified with galactosamine (α-D-GalN) or succinyl substituents4 (Fig. 2a). Thus far, the GalN substituent has only been found in M. tuberculosis and a number of other slow-growing pathogenic mycobacteria, but the conservation of genes responsible for the biosynthesis of this motif in some rapidly growing species such as Mycobacterium abscessus suggests that GalN may modify the AG of a greater diversity of Mycobacterium species than previously appreciated. Succinyl substituents have been reported in both slow- and fast-growing species4. GalN was estimated to occur at the level of about one residue per AG molecule and succinyl substituents at the level of one to three motifs per AG molecule.
The biosynthetic origin of the GalN substituent of AG in M. tuberculosis has been reported6 and, much like that of the glycosyl substituents of LM and LAM (see below), found to be reminiscent of the pathways leading to the modification of lipid A with GalN or aminoarabinose residues in Francisella and Escherichia coli6. The enzyme responsible for the addition of succinyl substituents has not yet been identified.
Emerging evidence suggests that the GalN substituent of AG plays a role in mycobacterial physiology and pathogenesis. A M. tuberculosis GalN-deficient transposon mutant was found to be attenuated for virulence in mouse spleen7. Studies with GalN-deficient M. tuberculosis knockout mutants further revealed that the presence of this substituent abrogates the complete maturation and activation of human dendritic cells (DCs), increases the bacilli surface affinity for a soluble DC-SIGN probe (dendritic cell-specific intercellular molecule-3-grabbing-non-integrin), stimulates increased IL-10 secretion, and decreases Toll-like-receptor-2-mediated NF-κB activation8. Because purified AG from either wild-type or GalN-deficient mutants did not alter human DC maturation, it was postulated that the presence of a protonated GalN residue on AG, through its interactions with anionic components of the outer membrane such as the lipid anchor of glycerophospho(glyco)lipids, may alter the topology of the cell surface, thereby modulating interactions with host-cell receptors and resulting in the downregulation of the initial immune response8. Though the decreased binding of DC-SIGN to the cell surface of GalN mutants points to changes in the amount and/or accessibility of its ligands, the underlying mechanism through which a minute substituent buried in the cell envelope may exert such dramatic changes at the cell surface remains to be determined. The biological significance of the succinyl substituents has not yet been defined. It has been proposed that the negatively charged succinyl residues interact with the protonated GalN, leading to a more tightened and rigid AG structure4. The observation that mycolylated arabinan chains are devoid of succinyl substituents has further led to the suggestion that succinylation may negatively control mycolylation4 (Fig. 2a).
Lipomannan and lipoarabinomannan
Two abundant lipoglycans, LM and LAM, populate the inner and outer membranes of all mycobacteria. ‘Capsular’ polysaccharides known as D-mannan and D-arabino-D-mannan (AM), which have mannan and arabinomannan domains structurally identical to those of LM and LAM, are further found at the cell surface of M. tuberculosis and in a number of other slow- and fast-growing Mycobacterium species5 (Fig. 1).
The arabinan domain of LAM and AM is very similar to that of AG (Fig. 2b) and correspondingly harbors a succinate group substituting the C2 position of a portion of the α-3,5-Araf interior residues. Succinates occur at the level of 1–4 per entire LAM molecule or 2–3 motifs per AM molecule. Additional reports of succinyl groups in mycobacterial lipoglycans include those in Mycobacterium kansasii, wherein succinates were found to substitute the C3 position of linear α-1,5-Araf residues rather than the C2 position of the branching Araf residue of LAM (Fig. 2b), and Mycobacterium smegmatis, in which succinates were proposed to acylate the mannan backbone of LM (although their attachment site was not defined). The presence of lactate groups associated with the arabinomannan domain of LAM was also reported in M. tuberculosis, M. smegmatis and M. leprae, but neither the existence of a covalent linkage nor their attachment site was defined, raising the possibility that the short-chain acids arose from an unknown contaminant. The nonreducing arabinan termini of LAM and AM display species-specific structural microheterogeneity that is key to the biological activity of these molecules. In M. tuberculosis and a number of other pathogenic mycobacteria, these termini are capped with one to three α-1,2-linked Manp residues, giving rise to mannosylated LAM (known as ManLAM)4 (Fig. 2b). The mannoside caps of ManLAM in M. tuberculosis may be further substituted with an α-1,4-linked methylthio-D-xylose (MTX) residue9 (Fig. 2b). This motif occurs at the level of one molecule per entire molecule of ManLAM. It is an unusual chemical modification in that it includes one of the few reports of a xylo-configured sugar outside the plant kingdom and the first report of a thiosugar in a bacterial polysaccharide. In M. kansasii, MTX appears to substitute the mannan backbone rather than the mannoside caps of ManLAM9 (Fig. 2b).
The biosynthetic pathways leading to the synthesis of the mannoside and MTX caps of ManLAM in M. tuberculosis have been elucidated4,10. The succinyl substituents are likely to be added by the same, as yet unknown, enzyme that modifies AG.
The mannoside caps of ManLAM bind to the macrophage mannose receptor and to DC-SIGN present on DCs, thus playing an important role in host-cell recognition and subsequent survival of the bacilli in the phagosomal environment11–13. Conservation of the MTX motif of ManLAM in all M. tuberculosis isolates analyzed to date and its exposure on the cell surface suggest that it may similarly play a beneficial role in the bacterium during infection. Accordingly, in vitro studies have indicated that the presence of MTX prevents a subset of mannose-binding receptors that are expressed at the surface of innate immune cells from binding to the mannoside caps of ManLAM13 and that disaccharide mimetics of this motif downregulate cytokine production by activated human macrophages9. MTX was also proposed to account for the anti-oxidative properties of the entire molecule9,14. How these phenotypes translate in the context of intact bacilli given the low abundance of the MTX substituent in the cell envelope and the likely existence of surface constituents with partially overlapping functions awaits infection studies with knockout mutants specifically lacking the MTX motif. In line with the proposed role of MTX in resistance to oxidative stress and virulence, gene-expression profiling experiments (http://www.tbdb.org) have indicated that genes involved in the biosynthesis of this motif are induced more than two-fold in M. tuberculosis grown on host fatty acids, inside macrophages, and under hypoxic conditions. The presence of the five core genes of the MTX pathway in a number of mycobacteria, including species such as M. smegmatis that are devoid of mannoside caps on LAM and other Actinomycetes in which no MTX substituents have yet been reported10, suggests that this motif may be more widespread among prokaryotes and may modify a greater diversity of acceptors than previously appreciated.
Other variations in the structures of LM and LAM, including the degree of acylation of their phosphatidyl-myo-inositol anchor, extent of the α-1,2-Manp branching of their mannan backbone, and length of the mannan and arabinan chains (Fig. 2b), have been shown to impact the interaction of these lipoglycans with host-cell receptors as well as their immunomodulatory properties4,12,15.
Understanding chemical modifications of mycobacterial polysaccharides
It is clear from the above examples that mycobacteria have evolved to modify their cell-envelope glycans with a distinct array of strategically placed substituents whose acceptor molecules and specific placement on the acceptor are likely to be tailored to the Mycobacterium spp. under consideration, their particular cell envelope compositions and unique lifestyles. Our understanding of the biological significance of these modifications, however, is still rudimentary, and many other fundamental questions remain open. In this context, new chemical tools and approaches tailored to the study of Mycobacterium-specific glycans will be valuable complements to traditional biochemistry-, genetics-, and ‘omics’-based methodologies.
Did we miss something?
Not least among open questions is whether any other important chemical modifications of glycans might have been missed given the remaining unknowns in their overall structure4, the low abundance and potential transient nature of some of these substituents, and the relatively harsh procedures of acid and base treatments typically used to purify glycans from the mycobacterial cell envelope. Does LAM harbor any lactate substituents? If not added to ManLAM, on what acceptor substrate(s) is the MTX residue produced by M. smegmatis10 transferred? Critical to the functional characterization of the succinyl, MTX, and GalN substituents will be a thorough investigation of their promiscuity in the cell; i.e., do other molecules aside from LM, LAM, and AG serve as acceptors for these motifs?
The milligram quantities of pure glycans required for accurate detection and structural determination of these rare substituents still represents a major obstacle for progression in the field. Possible approaches to address these limitations that would not require purification of the acceptor molecules of interest may include high-resolution–magic angle spinning (HR–MAS) NMR to characterize the glycoconjugate content of whole live mycobacterial cells16, as well as the targeted enzymatic digestion of glycans present at the surface of the bacilli followed by characterization of released fragments by mass spectrometry and/or NMR (Fig. 3a).
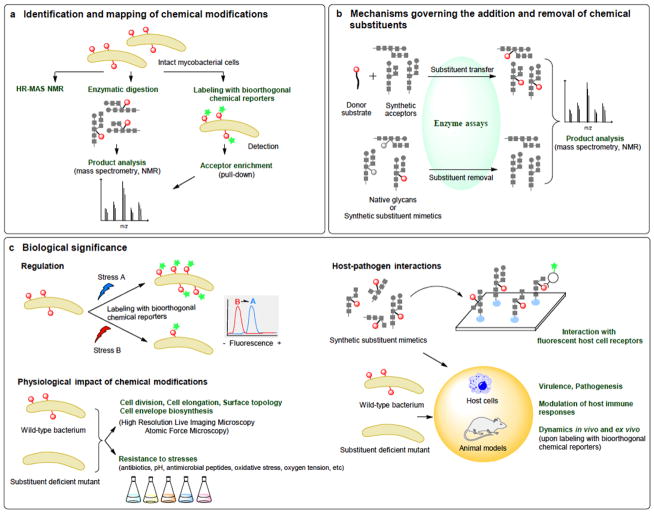
Fig. 3. A workflow for studies of chemical modifications of mycobacterial polysaccharides.
It is anticipated that chemical biology (bioorthogonal chemical reporters; synthetic glycan mimetics; synthetic acceptor and donor substrates) will play a key role in advancing the field as evidenced by the unmet needs for new technologies for (a) identifying new chemical modifications (in red) and their acceptor molecules; (b) studying the enzymatic mechanisms and timing of their potentially transient addition onto different acceptors and (c) defining their regulation and biological functions under various environmental conditions and in the host.
Another approach is be to broaden the utility of metabolic and chemoenzymatic labeling with bioorthogonal chemical reporters to study Mycobacterium glycans17–19. Specifically, refinement of unnatural azide-containing substituents that can efficiently be incorporated into glycans in place of MTX, GalN, and other motifs of interest and subsequently be conjugated to fluorescent dyes will enable their sensitive and specific detection in live bacilli or in crude cell lysates, and allow the identification of new acceptor molecules, whether they are polysaccharides, glycolipids or proteins (Fig. 3a). Conjugation to biotin tags for pull-down from complex cell lysates or partially purified extracts followed by enzymatic digestion of the acceptor molecule(s) may further facilitate the structural characterization of enriched motifs by mass spectrometry (Fig. 3a).
At what stage of biosynthesis are the modifications introduced and are they transient?
Nothing is known of the regulation and potential reversibility of the chemical modifications of AG, LM, and LAM. Yet, the timely introduction of these motifs in glycan structures, and their reversibility, is likely to be key to their function. Aside from allowing mycobacteria to remodel their cell envelope in situ in response to changing environmental conditions, transient modifications may serve as critical biosynthetic signals. For instance, the elongation and branching of AG, LM, and LAM appear to be tightly regulated4. Should the GalN and succinyl substituents be found to be introduced during the polymerization of the arabinan domain, this would suggest that they may act as molecular signatures regulating elongation and branching, as described with other bacterial polysaccharide substituents20. Precedent exists in Corynebacterineae, in which a transient chemical modification of glycolipids serves as a signal for export21. Detailed studies on the addition and potential removal of these motifs await the design of functional transfer assays using synthetic neoglycolipid acceptors that mimic the details of the structures of AG, LM, and LAM, as was done to study the mannose capping of LAM22 (Fig. 3b). These acceptors will be equally important in defining the mechanisms through which orthologous glycosyltransferases and succinyltransferases from different Mycobacterium species may transfer MTX and succinyl substituents to different positions of LM and LAM (Fig. 2b) and for understanding the functional significance of this regioselectivity.
Defining biological significance
The effects of chemical modifications on mycobacteria, including their adaptation to various stresses and to the host environment, could cover a broad range, and may be directly or indirectly mediated by changes impacting many different aspects of their physiology, including envelope composition and permeability, cell surface topology, and susceptibility to antimicrobials and oxidative stress, among others. As stated above, important to the understanding of the function of these motifs will be the determination of whether they modify more than one acceptor in the cell, whether they occur constitutively as is true of some LPS modifications2 or if they are regulated, and whether they are reversible, as well as the nature of the environmental cues triggering their addition or removal.
Thankfully, many of the chemical modifications described in this Commentary do not negatively impact the fitness of mycobacteria in vitro, and the growing number of isogenic mutants impaired in these modifications that have been reported in the last ten years are finally providing opportunities to explore their biological significance in vitro and in vivo. Complementing traditional gene-expression profiling approaches, the use of bioorthogonal chemical reporters specific to mycobacterial glycan substituents are expected to facilitate the study of their distribution and dynamics under various stress conditions, inside host cells and in vivo (Fig. 3c). The ability to detect these motifs in their native environments combined with advances in imaging probes and high-resolution live-imaging microscopy further provides opportunities to investigate spatiotemporal aspects of their biosynthesis and the impact of genetically abolishing their production on cell envelope biosynthesis, cell elongation, and cell division23 (Fig. 3c). Likewise, the application of state-of-the-art atomic-force-microscopy-based imaging provides unprecedented means of exploring the impact of glycan substituents on nanoscale features of the mycobacterial cell surface24 (Fig. 3c). With regard to host–pathogen interactions, the ability to determine whether glycan substituents interact with immune receptors will greatly benefit from the design of dedicated synthetic glycan arrays and their probing with fluorescently labeled host glycan-binding proteins, as was done recently to gain insight into the impact of the MTX motif on the interactions of ManLAM with mannose-binding receptors13 (Fig. 3c).
Outlook
The roles that the modifications affecting the cell-envelope glycans of mycobacteria are likely to play in the physiology of these microorganisms, in their adaptation to the environment, and in their interactions with the host emphasize the importance of the continued efforts to understand their biosynthetic origin, regulation, and biological significance. The time is now right to take on this challenge by refining the increasing diversity and power of the chemical biology and cell imaging tools at our disposal to leverage recent advances in our understanding of the biosynthetic origin and functions of these substituents. Progress in the field will require multidisciplinary approaches and is expected to be heavily reliant on synthetic and chemical biology.
As our understanding of the biological functions and biosynthetic origin of these chemical modifications progresses, new translational opportunities continue to emerge. Important steps in this direction were made with the development of mannodendrimers mimicking the α-1,2-linked oligomannoside appendages found in ManLAM as innovative synthetic immunomodulators for the treatment of lung inflammatory diseases25. Other exciting prospects include the identification of novel and Mycobacterium-specific therapeutic targets within the substituents biosynthetic machinery, whose inhibition may affect the fitness of mycobacterial pathogens during infection, and the possibility of specifically targeting mycobacterial bacilli expressing tagged surface glycans (upon labeling with azide-containing sugars) with covalent therapeutics19. Lastly, the restricted distribution of some of these modifications to pathogenic mycobacteria, in particular M. tuberculosis (for example, the MTX motif of ManLAM), and their antigenicity provide impetus for their study as potential biomarkers of infection. Here again, the possibility to metabolically label bacilli with azido substituents, which can be followed by conjugation to fluorescent probes and detection by microscopy or flow cytometry, could provide a more sensitive alternative to antibody- or lectin-based detection.
Acknowledgments
We thank P.J. Brennan for critical reading of the manuscript. Studies on the chemical modifications of mycobacterial carbohydrates in the authors’ laboratory were supported by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institutes of Health (NIH) grants AI064798 and AI116525. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Swoboda JG, Campbell J, Meredith TC, Walker S. ChemBioChem. 2010;11:35–45. doi: 10.1002/cbic.200900557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Needham BD, Trent MS. Nat Rev Microbiol. 2013;11:467–481. doi: 10.1038/nrmicro3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneewind O, Missiakas D. J Bacteriol. 2014;196:1133–1142. doi: 10.1128/JB.01155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angala SK, Belardinelli JM, Huc-Claustre E, Wheat WH, Jackson M. Crit Rev Biochem Mol Biol. 2014;49:361–399. doi: 10.3109/10409238.2014.925420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daffé M, Crick DC, Jackson M. Microbiol Spectrum. 2014;2:MGM2–0021–2013. doi: 10.1128/microbiolspec.MGM2-0021-2013. [DOI] [PubMed] [Google Scholar]
- 6.Skovierová H, et al. J Biol Chem. 2010;285:41348–41355. doi: 10.1074/jbc.M110.188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sassetti CM, Rubin EJ. Proc Natl Acad Sci USA. 2003;100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheat WH, et al. Tuberculosis (Edinb) 2015;95:476–489. doi: 10.1016/j.tube.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbull WB, Stalford SA. Org Biomol Chem. 2012;10:5698–5706. doi: 10.1039/c2ob25630d. [DOI] [PubMed] [Google Scholar]
- 10.Angala SK, et al. ACS Chem Biol. 2017;12:682–691. doi: 10.1021/acschembio.6b01071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torrelles JB, Schlesinger LS. Tuberculosis (Edinb) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergne I, Gilleron M, Nigou J. Front Cell Infect Microbiol. 2015;4:187. doi: 10.3389/fcimb.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng RB, et al. ACS Chem Biol. 2017;12:2990–3002. doi: 10.1021/acschembio.7b00797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J, Fan XD, Hunter SW, Brennan PJ, Bloom BR. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoop EJ, et al. Cell Microbiol. 2013;15:2093–2108. doi: 10.1111/cmi.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee RE, Li W, Chatterjee D, Lee RE. Glycobiology. 2005;15:139–151. doi: 10.1093/glycob/cwh150. [DOI] [PubMed] [Google Scholar]
- 17.Swarts BM, et al. J Am Chem Soc. 2012;134:16123–16126. doi: 10.1021/ja3062419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez Aguilar A, et al. ACS Chem Biol. 2017;12:611–621. doi: 10.1021/acschembio.6b01089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clark EL, et al. ACS Chem Biol. 2016;11:3365–3373. doi: 10.1021/acschembio.6b00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cuthbertson L, Kos V, Whitfield C. Microbiol Mol Biol Rev. 2010;74:341–362. doi: 10.1128/MMBR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belardinelli JM, et al. ACS Infect Dis. 2016;2:702–713. doi: 10.1021/acsinfecdis.6b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Appelmelk BJ, et al. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi JM, et al. Proc Natl Acad Sci USA. 2016;113:5400–5405. doi: 10.1073/pnas.1525165113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskandarian HA, et al. Nat Microbiol. 2017;2:17094. doi: 10.1038/nmicrobiol.2017.94. [DOI] [PubMed] [Google Scholar]
- 25.Blattes E, et al. Proc Natl Acad Sci USA. 2013;110:8795–8800. doi: 10.1073/pnas.1221708110. [DOI] [PMC free article] [PubMed] [Google Scholar]