Abstract
Background
In a pilot study, neutrophil CD64 surface expression was significantly elevated in newly diagnosed, pediatric-onset Crohn’s disease. We aimed to test the CD64 biomarkers (neutrophil CD64 surface expression and soluble CD64) as determinates for mucosal inflammation in a larger pediatric Crohn’s cohort with the hypotheses that the CD64 biomarkers would reliably detect intestinal inflammation and correlate with endoscopic severity scores.
Methods
We enrolled patients referred for colonoscopy for either suspected inflammatory bowel disease or with established Crohn’s. Neutrophil CD64 index was determined by flow cytometry using a commercial kit (Leuko64, Trillium) and soluble CD64 by ELISA (LifeSpan).
Results
A total of 209 patients (72 controls, 76 new inflammatory bowel disease patients, and 61 established Crohn’s) were enrolled. Both neutrophil CD64 index and soluble CD64 were significantly elevated in new Crohn’s compared with controls. The area under the curve (AUC) for neutrophil CD64 index ≥1 was 0.85 (95% confidence interval, 0.77–0.92), 75% sensitive and 89% specific for new Crohn’s. Comparatively, soluble CD64 ≥39 ng/mL was 92% sensitive and 85% specific (AUC, 0.93) for new Crohn’s. Neutrophil CD64 index, soluble CD64, and fecal calprotectin discriminated endoscopic inactive from moderate and severe activity while soluble CD64 differentiated endoscopic mild from moderate and severe activity. Neutrophil CD64 index (r = 0.46, P < 0.001) and fecal calprotectin (r = 0.55, P < 0.001) correlated well with the Simple Endoscopic Score–Crohn’s disease. Spearman correlation between the CD64 index and calprotectin was 0.39 (P < 0.001).
Conclusions
In a large Crohn’s disease cohort, we found that neutrophil CD64 index and soluble CD64 were significantly elevated during active gastrointestinal inflammation.
Keywords: CD64 biomarker, Crohn’s disease, pediatrics
Crohn’s disease (CD) is a chronic, progressive disease that over time may result in structural bowel damage.1 Novel treatment strategies that result in complete mucosal healing sparked a shift from managing symptoms to preventing disease progression.2 Notwithstanding, preventing bowel injury entails accurately assessing gut inflammation. Current standards to assess disease activity include logging gastrointestinal symptoms, the physical exam, assessing biologic activity with laboratory biomarkers, abdominal cross-sectional imaging, and endoscopy. While the diagnosis of inflammatory bowel disease (IBD) unequivocally requires endoscopy, clinicians rely on biomarkers to guide clinical management in children with established IBD receiving treatment. Minimally invasive CD-specific biomarkers that correlate with endoscopic severity are needed not only to gauge risk for potential IBD but to assess treatment response in established CD as mucosal healing has been shown to be one of the best predictors of favorable long-term outcomes.3, 4
Repeat colonoscopy to monitor treatment response has a significant role in IBD as it provides visual inspection of the gastrointestinal mucosa, histological assessment, and evaluation for IBD sequela. High-cost, undesirable colonic preparation, minor anesthesia and procedural risks,5 and highly sensitive stool biomarkers have led many clinicians, especially pediatric gastroenterologists, to rely on biomarkers to inform treatment decisions.6 Traditional blood biomarkers, such as c-reactive protein (CRP), erythrocyte sedimentation rate (ESR), hemoglobin, and albumin, are utilized as screening tests for patients with gastrointestinal symptoms and may wrongfully result in further diagnostics as abnormalities are nonspecific to the gastrointestinal tract. Fecal biomarkers, lactoferrin, and calprotectin are gut specific as both are released into the fecal stream following neutrophil degradation.7 In addition to the 2 fecal biomarkers of neutrophil activity, there is additional evidence by histology and radiolabeled granulocytes8 that active inflammation in CD is characterized by the migration of peripheral blood neutrophils to the intestinal lamina propria and crypts. In our pilot studies, we found that the ileal and rectal mRNA expression of Fcγ receptor IA (FcγRIA) was up to 3-fold higher in new-diagnosis CD patients compared with controls.9 This initial finding led to a pilot investigation where significant elevations in peripheral blood neutrophil FcγRI (CD64) were found in new-diagnosis CD patients and shown to be a significant risk factor for clinical relapse in treated, asymptomatic CD patients.9
The neutrophil CD64 index (an objective measurement of CD64 surface expression) was found to be a reliable blood biomarker of intestinal inflammation in pediatric CD with our initial pilot investigation.9 In this study, our primary aim was to evaluate the difference in neutrophil CD64 index between the 4 levels (inactive, mild, moderate, and severe) of endoscopic activity. As a secondary aim, we explored the difference in CD64 expression between patients with newly diagnosed CD and the non-IBD controls. The primary hypothesis is that neutrophil CD64 index is an alternative and reliable diagnostic test for active CD that could be utilized as a reliable biomarker for endoscopic severity. We further explored the performance of 2 additional CD64 biomarkers, soluble CD64 (sCD64) and neutrophil CD64 activity ratio (NCAR), in differentiating active and inactive intestinal inflammation.
MATERIALS AND METHODS
Participants
We enrolled pediatric and young adult patients at Cincinnati Children’s Hospital Medical Center between July 1, 2013, and January 31, 2017, referred for a colonoscopy for either suspected IBD or established CD. The results of the colonoscopy established 3 cohorts; (1) patients with new-onset IBD, (2) patients without IBD (controls) who were further divided into healthy controls (HCs) or non-IBD controls (NCs), and (3) patients with established Crohn’s. The diagnosis of CD, UC, or IBD-unclassified (IBD-U) was confirmed by the study team following a review of the subjects’ endoscopic, histologic, and radiographic findings. Similarly, we reviewed the charts of all controls (HC and NC) to confirm the diagnosis. We defined HC as study participants who had a normal esophagogastroduodenoscopy, ileocolonoscopy, and normal histology on both exams. NC consisted of study participants who had abnormal macroscopic or microscopic findings that were not consistent with IBD (such as esophagitis, gastritis, polyps, etc.). All study participants were between age 4 and 22 years. We did not include subjects with a known intestinal infection (including Clostridium difficile) in the previous 2 weeks prior to colonoscopy. Blood samples were obtained from each subject at the time of endoscopy.
Neutrophil CD64 Index
Blood collected immediately prior to colonoscopy was processed within 24 hours for the measurement of granulocyte CD64 expression by quantitative flow cytometry on a FACSCalibur (BD Biosciences, San Jose, California) using the Leuko64 assay kit (Trillium Diagnostics, Brewer, Maine). The kit includes fluorescent beads and antibodies to CD64 and CD163. The lymphocyte, monocyte, and granulocyte populations are defined by their forward and side scatter characteristics with CD163 staining to further define the monocyte population. The neutrophil CD64 index is the result of the ratio of the mean fluorescent intensity (MFI) of the granulocytes to that of the calibration beads.
Soluble CD64/Neutrophil CD64 Activation Ratio
Human FCGRI/CD64 ELISA was performed according to the manufacturer’s manual (LifeSpan BioSciences, Inc., Seattle, Washington) to determine sCD64. We also evaluated the NCAR in a subset of subjects. NCAR was measured by first defining the lymphocyte and granulocyte population by their forward and side scatter characteristics during flow cytometry with the FACSCalibur. Cell surface CD64 expression was detected using the antibodies in the aforementioned Leuko64 kit. The CD64 MFI of granulocytes was divided by the CD64 MFI of the lymphocytes to produce the NCAR.
Endoscopic Scores
The Simple Endoscopic Score–Crohn’s Disease (SES-CD) was chosen for endoscopic scoring given its excellent inter-rater variability and ease of scoring. Endoscopic scores were tabulated by a pediatric gastroenterologist (P.M.) blinded to the blood and fecal testing results, while viewing a streaming video of the procedure (retrospectively). For a subset of subjects, the gastroenterologist performing the colonoscopy also provided an SES-CD score following the procedure. The primary SES-CD scores for this study were provided by the central reader (P.M.) with the sole aim of the secondary readers (multiple providers) to establish a baseline inter-rater assessment. The SES-CD score is based on the assessment of 5 segments of the bowel (distal ileum, right colon, transverse colon, left colon, and rectum) with grading (0–3) of 4 parameters. The 4 parameters include (1) size of ulcers, (2) extent of ulcerated surface, (3) extent of affected surface, and (4) presence/type of luminal narrowing.10 We defined endoscopic healing as a total SES-CD score ≤2, while 3–6 was classified as mild, 7–15 as moderate, and ≥16 as severe endoscopic activity.11, 12
Laboratory Testing
Additional laboratory testing (blood or stool) was performed at the discretion of the treating clinician with the results included in our study if the tests were collected up to 35 days before the endoscopy (fecal calprotectin was not collected during the clean out or on the day of the endoscopy). Of note, the fecal calprotectin collected on the majority of the patients had an upper limit of detection of 2500 μg/g. However, with 7% of the calprotectin tests having an upper limit of detection of 1250 μg/g, all calprotectin results >1250 μg/g were input as 1251 μg/g. Fecal calprotectin <50 μg/g was used for upper cutoff of normal,13 and <250 μg/g was selected as an a priori cutoff for mucosal inflammation in patients with established CD14. The lower limit of detection for CRP was 0.29 mg/dL (input as 0.28 mg/dL).
Statistical Analysis
Statistical analyses were performed using GraphPad PRISM Version 7 and R (Core Team, 2012). Continuous variables are represented as means with standard deviations or medians with minimum to maximum range based on data distribution. For our primary aim, the neutrophil CD64 index was compared among 4 SES-CD groups (endoscopic healing 0–2, mild 3–6, moderate 7–15, severe ≥16) by Kruskal Wallis with Dunn’s post-test to test for differences between groups as the data were not normally distributed. Our a priori sample size calculation included 19 subjects in the 4 SES-CD groups to demonstrate 80% power (α = 0.05). We also tested for the area under the curve (AUC) of each biomarker to differentiate endoscopic active (SEC-CD ≥ 3) from inactive by performing receiver operator characteristic (ROC) curve analysis. The Youden index was utilized to define the ideal cut-point for endoscopic activity. Analyses included the comparison of the CD64 biomarkers between controls (HC and NC) and new-diagnosis CD patients by the Mann-Whitney U test. Our previous pilot investigation established a neutrophil CD64 index ≥1.0 as the ideal cutoff to distinguish Crohn’s disease from controls,9 with this cut-point investigated in this larger, distinct pediatric cohort. We produced additional ROC curves by combing the CD64 biomarkers with albumin and fecal calprotectin, respectively. DeLong’s test was used to test for statistical difference between the AUC for each biomarker. The Fisher exact test was used for categorical data. With SES-CD scores as continuous data, we performed inter-rater analysis with the intraclass correlation coefficient. We measured association between the biomarkers and SES-CD by the Spearman’s rank correlation coefficient. Sensitivity analysis was performed for the CD64 biomarkers by limiting the analyses to the subgroups of patients with available (1) CRP and (2) fecal calprotectin data. Finally, additional group comparisons were performed based on our a priori selection of potential confounders including age of diagnosis, sex, and concurrent corticosteroid exposures. Significance was set at 0.05.
Ethical Considerations
All subjects and/or their parents or guardians provided informed consent for this study before collection of any study data. The study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center.
RESULTS
Study Population
The diagnostic performance of the CD64 biomarkers to differentiate IBD from non-IBD was tested in patients referred for colonoscopy with a clinical suspicion for IBD. Of the 148 subjects with suspected IBD (Table 1), 60 were diagnosed with CD, 16 with UC/IBD-U, and 72 subjects were found not to have IBD (controls). The final diagnoses of the non-IBD patients are listed in Table 2. The primary aim of this investigation was to evaluate the diagnostic performance of the CD64 biomarkers to determine the degrees of endoscopic severity. Of the 121 CD who had endoscopic scores (Table 3), 60 were newly diagnosed CD subjects and 61 had established CD.
Table 1.
Disease Characteristics and Biomarker Results for Subjects With Suspected Inflammatory Bowel Disease
| Controls (n = 72) | UC/IBD-U (n = 16) | Crohn’s (n = 60) | |
|---|---|---|---|
| Median age at endoscopy (range), y | 15(6–20) | 15(4–18) | 14(4–21) |
| Female, % | 44 (61%) | 10 (63%) | 20 (33%)a |
| White race, % | 63 (88%) | 16 (100%) | 52 (87%) |
| Crohn’s location | |||
| Ileal only (L1) | 9 | ||
| Colon only (L2) | 12 | ||
| Ileocolonic (L3) | 39 | ||
| Proximal to ligament of Treitz (L4a) | 24 | ||
| Crohn’s behavior | |||
| Inflammatory (B1) | 48 | ||
| Stricturing (B2) | 6 | ||
| Penetrating (B3) | 6 | ||
| Stricturing/penetrating (B2B3) | 0 | ||
| Perianal | 7 | ||
| SES-CD, median (range) | NA | NA | 13(3–31) |
| Biomarker at endoscopy | |||
| Neutrophil CD64 index, median (range) | 0.65 (0.3–1.6) | 1.1 (0.4–2.6)b | 1.7 (0.4–4.4)c |
| Soluble CD64, median (range), ng/mL | 29 (12–73) | 43 (34–131)d | 73 (27–174)c |
| Neutrophil CD64 activity index, median (range) | 4 (2.1–14.6) | 6 (3.9–7.5)e | 7.6 (2.2–30.4)c |
| CRP, median (range), mg/dL | 0.28 (0.28–4.5) | 0.28 (0.28–5.7) | 2 (0.28–14)c |
| Fecal calprotectin, median (range), μg/g | 76 (15–1066) | 1173 (65–1251)b | 863 (55–1251)c |
Lower limit CRP detection for clinical laboratory is <0.29 mg/dL, input 0.28 mg/dL. Biomarkers were compared for (a) controls vs Crohn’s and (b) controls vs UC/IBD-U.
a P < 0.01 Crohn’s vs controls.
b P < 0.001 UC vs controls.
c P < 0.001 Crohn’s vs controls.
d P < 0.01 UC vs controls.
e P < 0.05 UC vs controls.
SES-CD = Simple Endoscopic Score–Crohn’s disease.
Table 2.
Demographics and Final Diagnosis of Controls
| Healthy Controls (n = 42) | Non-IBD Controls (n = 30) | |
|---|---|---|
| Mean age, (SD), y | 14 (3.5) | 14(3) |
| Female, % | 25 (60%) | 19 (63%) |
| White race, % | 39 (93%) | 24 (80%) |
| Neutrophil CD64 index, median (range) | 0.66 (0.42–1.6) | 0.65 (0.34–1.4) |
| Soluble CD64, median (range), ng/mL | 30 (12–73) | 23 (13–47) |
| Fecal calprotectin, median (range), μg/g | 54 (15–670) | 121 (15–1066) |
| Final diagnosis, % | ||
| Irritable bowel syndrome | 19 (45%) | 5 (17%) |
| Constipation | 7 (17%) | 2 (7%) |
| Functional abdominal pain | 10 (24%) | |
| Dyspepsia | 6 (14%) | |
| Eosinophilic esophagitis or EC | 3 (10%) | |
| Nonspecific esophagitis or gastritis | 7 (23%) | |
| Polyp(s) | 4 (13%) | |
| H. pylori | 1 (3%) | |
| Candida esophagitis | 1 (3%) | |
| Other (arthritis, self-limiting colitis) | 7 (23%) |
EC = eosinophilic colitis.
Table 3.
Disease Characteristics and Biomarker Results for Subjects With Endoscopic Inactive and Endoscopic Active Crohn’s Disease
| Endoscopic Inactive (n = 19) | Endoscopic Active (n = 102)a | |
|---|---|---|
| Mean age at endoscopy (SD), y | 16 (4) | 14 (4) |
| Mean age at diagnosis (SD), y | 12 (5) | 12 (4) |
| Median duration with Crohn’s (range), y | 4.2 (0.5–14) | 0 (0–16)b |
| Female, % | 5 (26%) | 35 (34%) |
| White race, % | 18 (95%) | 92 (90%) |
| Crohn’s location | ||
| Ileal only (L1) | 2 | 11 |
| Colon only (L2) | 6 | 22 |
| Ileocolonic (L3) | 11 | 69 |
| Crohn’s behavior | ||
| Inflammatory (B1) | 14 | 77 |
| Stricturing (B2) | 4 | 14 |
| Penetrating (B3) | 1 | 10 |
| Both (B2B3) | 0 | 1 |
| Perianal | 3 | 15 |
| Prior surgery, % | 3 (16%) | 5 (5%) |
| Medications, % | ||
| Prednisone | 1 (6%) | 11 (26%) |
| 6-mercaptopurine or methotrexate | 7 (39%) | 19 (45%) |
| Anti-TNF without IMM | 9 (50%) | 18 (43%) |
| Anti-TNF and IMM | 2 (11%) | 3 (7%) |
| Vedolizumab or ustekinumab | 0 | 3 (7%) |
| 5-aminosalicylate only | 2 (11%) | 3 (7%) |
| None | 1 | 60 |
| SES-CD, median (range) | 0 (0–2) | 12 (3–34)b |
| Neutrophil CD64 index, median (range) | 0.61 (0.3–1.2) | 1.3 (0.4–6)b |
| Soluble CD64, median (range), ng/mL | 38 (20–62) | 60 (15–179)b |
| Neutrophil CD64 activity ratio, median (range) | 3.6 (2.3–5.3) | 6.4 (2.2–32)b |
| C-reactive protein, median (range), mg/dL | 0.28 (0.28–0.49) | 1.6 (0.28–14)b |
| Fecal calprotectin, median (range), μg/g | 37 (15–1132) | 712 (55–1251)b |
aEndoscopic active includes 60 new-onset Crohn’s and 42 established Crohn’s. Medication percentage (%) based on those subjects receiving therapy at time of endoscopy.
b P < 0.001.
IMM = immunomodulator; SES-CD = Simple Endoscopic Score-Crohn’s disease.
Neutrophil CD64 Is Comparable With Fecal Calprotectin for New Diagnosis Crohn’s Disease
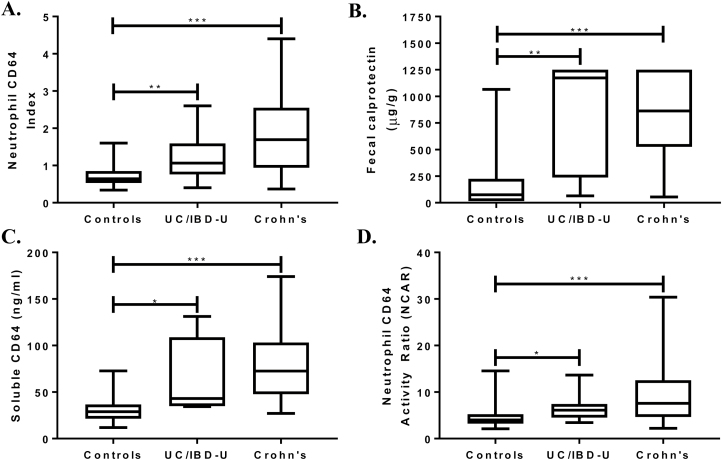
The majority of the 60 newly diagnosed CD subjects had an inflammatory behavior (B1, 80%) with ileocolonic distribution (L3, 65%). The median neutrophil CD64 index for new CD was 1.7 (range, 0.4–4.4) compared with 0.65 (range, 0.3–1.6) for the controls (P < 0.001) (Fig. 1A). At a neutrophil CD64 index cutoff ≥1, the test was 75% sensitive and 89% specific, with a positive predictive value (PPV) of 85% and a negative predictive value (NPV) of 81% (AUC, 0.85; 95% confidence interval [CI], 0.77–0.92; P < 0.001) for CD. We had fecal calprotectin results in 47% of the controls and 75% of new CD. The median for fecal calprotectin was also significantly higher in CD then controls (Fig. 1B). We found that at a fecal calprotectin cutoff ≥50 μg/g, the sensitivity was 100%, 47% specific, with a PPV of 71%, and an NPV of 100% (AUC, 0.91; 95% CI, 0.85–0.98; P < 0.001). In this cohort of controls and new CD patients, we found a significant correlation between the neutrophil CD64 index and fecal calprotectin (Spearman r = 0.39; P < 0.001).
FIGURE 1.
Neutrophil CD64 index, fecal calprotectin, soluble CD64, and neutrophil CD64 activity index were measured prior to endoscopy. The result of each biomarker was tested for each diagnosis by the Kruskal-Wallis test, with Dunn’s test used for pairwise comparisons. *P < 0.05; **P < 0.01; ***P < 0.001.
Additional Blood Biomarkers for New Crohn’s
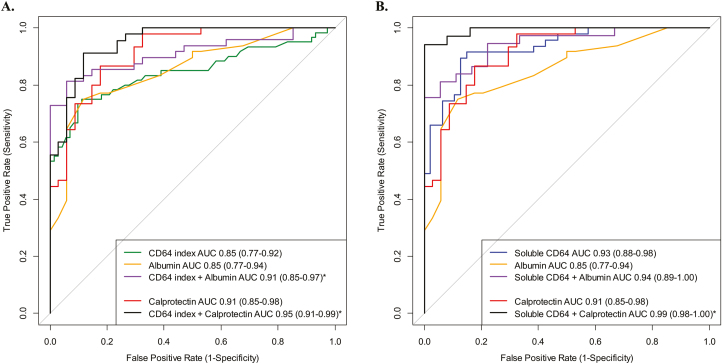
Two additional CD64 biomarkers were tested in the suspected IBD cohort. We found that both sCD64 (Fig. 1C) and NCAR (Fig. 1D) were significantly elevated in new CD compared with controls. Table 4 further details the testing characteristics of all blood biomarkers investigated. We found that the combination of the 2 blood biomarkers, neutrophil CD64 index + albumin, demonstrated a superior AUC than the AUC of CD64 index alone (P = 0.03) (Fig. 2A). The AUC for sCD64 alone was similar to the combination of sCD64 + albumin (P = 0.25) (Fig. 2B). The specificity of fecal calprotectin ≥50 μg/g to detect CD from controls was significantly improved with the addition of the neutrophil CD64 index from 47% to 88% specific (AUC, 0.95; 95% CI, 0.91–0.99; 91% sensitive). Although limited to 59 subjects (34 CD, 25 controls), the combination of sCD64 and fecal calprotectin was 94% sensitive, 100% specific, and demonstrated an AUC of 0.99 (95% CI, 0.98–1.0). We found no statistical difference in the median CD64 expression when controlling for age of diagnosis (<10 vs >10 years old) or sex. As the location of inflammation and Crohn’s phenotype of new CD cases were largely ileocolonic (L3) and inflammatory (B1), we were limited in performing any secondary analyses of the CD64 or fecal biomarkers by disease location or behavior.
Table 4.
Testing Characteristics of Blood and Stool Biomarkers to Distinguish New Diagnosis Crohn’s Disease From Controls
| Cutoff | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC (95% CI) | |
|---|---|---|---|---|---|---|
| Neutrophil CD64 index | ≥1.0 | 75 | 89 | 85 | 81 | 0.85 (0.77–0.92) |
| Soluble CD64 | ≥39 ng/mL | 92 | 85 | 86 | 91 | 0.93 (0.88–0.98) |
| Neutrophil CD64 activity ratio | ≥6.8 | 62 | 93 | 87 | 76 | 0.80 (0.71–0.88) |
| Fecal calprotectin | ≥50 μg/g | 100 | 47 | 71 | 100 | 0.91 (0.85–0.98) |
| C-reactive protein | ≥0.82 mg/dL | 73 | 82 | 86 | 67 | 0.81 (0.70–0.91) |
| Erythrocyte sedimentation rate | ≥14 mm/h | 93 | 73 | 83 | 88 | 0.87 (0.78–0.96) |
| Albumin | ≥3.4 g/dL | 75 | 88 | 90 | 71 | 0.85 (0.77–0.94) |
AUC = area under the curve; CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
FIGURE 2.
Patients with suspected IBD were referred for colonoscopy, with blood and stool biomarkers obtained prior to the endoscopy. The performance (AUC) of each biomarker to distinguish Crohn’s from controls was calculated. We also tested for statistical significance between the AUC for each biomarker curve. (A) The AUC for neutrophil CD64 index + albumin combination (81% sensitive, 94% specific) was significantly better than neutrophil CD64 index alone (P = 0.03) while the combination of CD64 index + calprotectin (91% sensitive, 88% specific) was also superior to CD64 index alone (P = 0.02). (B) The AUC of sCD64 + albumin was similar to soluble CD64 alone while the combination of soluble CD64 + calprotectin (94% sensitive, 100% specific) was significantly higher than soluble CD64 alone (P = 0.02). *P < 0.05.
Sensitivity Analysis for Serum Biomarkers
There were 27 controls and 41 new CD subjects who had a CRP measured prior to endoscopy and served as our subcohort for a sensitivity analysis of the blood biomarkers. Within this subcohort with CRP measured, the AUC for neutrophil CD64 index ≥1 was 0.86 (95% CI, 0.77–0.95; 76% sensitive, 93% specific) compared with a CRP ≥0.82 mg/dL and an AUC of 0.81 (95% CI, 0.70–0.91) with a sensitivity of 73% and specificity of 82% (AUC comparison with CD64 index, P = 0.31). Additionally, for the subcohort, both sCD64 ≥39 ng/mL (AUC, 0.95; 95% CI, 0.9–1.0; 91% sensitive, 93% specific) and fecal calprotectin ≥50 μg/g (AUC, 0.94, 95% CI; 0.86–1.0; 100% sensitive, 59% specific) remained superior in differentiating CD from controls than all other biomarkers. Finally, of the 16 newly diagnosed CD subjects with a neutrophil CD64 index <1 (false negatives), we found that the sCD64 was elevated (≥39 ng/mL) in 10 of the 14 (71%) subjects with sCD64 measured, while fecal calprotectin was elevated (≥50 μg/g) in all 12 subjects with calprotectin measured.
CD64 Expression Differentiates Endoscopic Active From Inactive
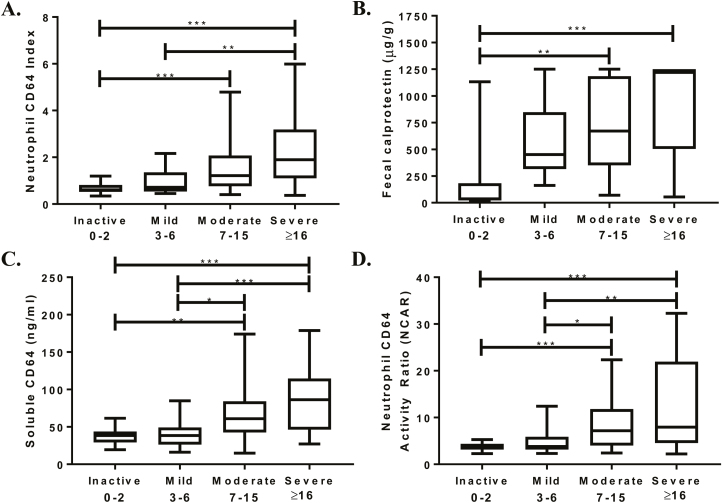
Table 3 details the differences in demographics, CD phenotype, and biomarker results of the 121 CD subjects who had a colonoscopy with endoscopic scoring performed. This cohort includes the previously discussed 60 new diagnosis CD subjects and an additional 61 subjects with established CD. The median SES-CD for both endoscopic active and inactive was 11 (range, 0–34), with 19 subjects endoscopically inactive (SES-CD ≤2). For the established CD subjects, there was no difference in the rate of endoscopic activity and anti-TNF use (66% SES-CD active on anti-TNF vs 69% active without anti-TNF; P > 0.99). We found the median CD64 index for endoscopic inactive was 0.61 (range, 0.3–1.2) and a fecal calprotectin of 37 μg/g (range, 15–1132) whereas the endoscopic active group had a median CD64 index of 1.3 (range, 0.4–6; P < 0.001) and a calprotectin of 712 μg/g (range, 55–1251; P < 0.001). We also found significant differences in the severity of the endoscopic scores and the intensity of the neutrophil CD64 index and fecal calprotectin (Fig. 3A, B). The ideal diagnostic cutoff values for each biomarker (calculated by ROC analyses for all biomarkers, the Youden index) are shown in Table 5. Neutrophil CD64 index (r = 0.46; P < 0.001) and fecal calprotectin (r = 0.55; P < 0.001) correlated well with SES-CD. Corticosteroids were previously shown to downregulate neutrophil CD64 expression in IBD patients.15 After excluding the 12 subjects receiving corticosteroids, our ROC curve analysis found minimal improvement (from 65% to 68%) in the sensitivity of a neutrophil CD64 index ≥0.9. The intraclass correlation coefficient was 0.80 (95% CI, 0.64–0.89; P < 0.001) on the 35 colonoscopies scored by the central reader and the multiple independent pediatric gastroenterologists.
FIGURE 3.
The CD64 biomarkers and fecal calprotectin were collected prior to endoscopy, with endoscopic severity determined by the Simple Endoscopic Score–Crohn’s Disease. (A) Neutrophil CD64 index, (B) fecal calprotectin, (C) soluble CD64, and (D) neutrophil CD64 activity ratio were evaluated across 4 levels of endoscopic severity by Kruskal-Wallis test with Dunn’s post-test for pairwise comparisons. *P < 0.05; **P < 0.01; ***P < 0.001.
Table 5.
Testing Characteristics of Blood and Stool Biomarkers to Distinguish Endoscopic Active Crohn’s From Endoscopic Inactive Crohn’s
| Cutoff | Sensitivity, % | Specificity, % | PPV, % | NPV, % | AUC (95% CI) | |
|---|---|---|---|---|---|---|
| Neutrophil CD64 index | ≥0.9 | 65 | 95 | 98 | 33 | 0.81 (0.73–0.89) |
| Soluble CD64 | ≥51 ng/mL | 59 | 94 | 98 | 32 | 0.78 (0.68–0.88) |
| Neutrophil CD64 activity ratio | ≥5.4 | 57 | 100 | 100 | 32 | 0.79 (0.70–0.88) |
| Fecal calprotectin | ≥250 μg/g | 84 | 88 | 98 | 44 | 0.90 (0.74–1.00) |
| C-reactive protein | ≥0.5 mg/dL | 73 | 100 | 100 | 32 | 0.86 (0.78–0.96) |
| Erythrocyte sedimentation rate | ≥16 mm/h | 74 | 100 | 100 | 36 | 0.91 (0.83–0.98) |
| Albumin | ≥3.8 g/dL | 77 | 85 | 97 | 38 | 0.86 (0.77–0.94) |
| Fecal calprotectin | ≥50 μg/g | 100 | 75 | 97 | 100 | 0.90 (0.74–1.00) |
AUC = area under the curve; CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
Additional CD64 Biomarkers for Active Crohn’s
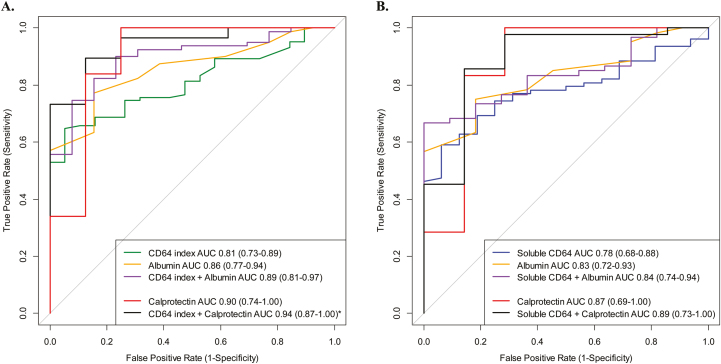
The testing characteristics for the biomarkers were similar or marginally lower in the CD cohort with endoscopic scoring (Table 5) performed compared with the biomarker results seen in the newly diagnosed cohort. NCAR (r = 0.43), sCD64 (r = 0.47), and CRP (r = 0.44) correlated significantly (P < 0.001) with the SES-CD. The neutrophil CD64 index correlated with both the NCAR (r = 0.80; P < 0.001) and the sCD64 (r = 0.61; P < 0.001). Similar to neutrophil CD64 index and fecal calprotectin, the intensity of sCD64 and NCAR significantly increased across the 4 SES-CD grades (Kruskal-Wallis P < 0.001) (Fig. 3C, D). We did not find the addition of albumin to either the neutrophil CD64 index or sCD64 to significantly increase the testing characteristics compared with either biomarker alone (Fig. 4A, B).
FIGURE 4.
Endoscopic active was defined by an SES-CD ≥3, with blood and stool biomarkers obtained prior to the endoscopy. The performance (AUC) of each biomarker to distinguish active Crohn’s from inactive Crohn’s was calculated. We also tested for statistical significance between the AUC for each biomarker curve. (A) The AUC for neutrophil CD64 index + albumin combination was not different than neutrophil CD64 index alone (P = 0.14) while combination CD64 index + calprotectin (89% sensitive, 88% specific) was superior to the neutrophil CD64 index alone (P = 0.02). (B) The AUC for soluble CD64 + albumin was not different than soluble CD64 alone (P = 0.42), while the combination of soluble CD64 + calprotectin (86% sensitive, 86% specific) was also not superior to soluble CD64 alone (P = 0.25).
Sensitivity Analysis for Crohn’s Biomarkers
Similar to the prior sensitivity analysis, we further evaluated the biomarkers based on the 71 (59%) subjects with CRP available and found 63/71 had an SES-CD ≥3. The neutrophil CD64 index (<0.9) and CRP (<0.5 mg/dL) were normal in 30% and 27%, respectively, in the endoscopically active patients while fecal calprotectin was <250 μg/g in 17% of the 41 active subjects who had both calprotectin and CRP measured (P = 0.17, calprotectin compared with CD64 index). The Spearman correlation (r) between neutrophil CD64 index and CRP was 0.49 (P < 0.001) while 7/17 of the endoscopically active subjects with a normal CRP were found to have a neutrophil CD64 index ≥0.9. For this subcohort of patients with CRP measured, the sensitivity and specificity for a neutrophil CD64 index ≥0.9 were 70% and 100%, respectively (AUC, 0.82; 95% CI, 0.71–92) which was comparable with the AUC for CRP.
We performed an additional sensitivity analysis to directly compare calprotectin and neutrophil CD64 index in CD subjects who had endoscopic scoring. In this cohort, there were 8 subjects with an SES-CD 0–2 (inactive) and 56 with an SES-CD ≥3 (active). The sensitivity of neutrophil CD64 index ≥0.9 for active inflammation improved from 67% (in the original cohort; n = 121) to 71% (n = 64; AUC, 0.82; 95% CI, 0.71–0.92; P = 0.004). The neutrophil CD64 index sensitivity (71%) was similar to the sensitivity (84%) of fecal calprotectin (cutoff ≥250 μg/g; Fisher exact P = 0.17) while the specificities of both biomarkers were 100% and 88%, respectively.
DISCUSSION
In this study, we have demonstrated that the peripheral blood CD64 biomarkers are significantly elevated during CD-induced intestinal inflammation. While we could not directly compare all biomarkers given the heterogeneity of biomarkers collected during our study, we found that sCD64 testing produced a similar ROC curve to fecal calprotectin, with a sensitivity of 92% and specificity of 85%, in distinguishing CD from controls in patients referred for colonoscopy for suspected IBD. Likewise, the neutrophil CD64 index and sCD64 differentiated the degree of endoscopic severity in CD patients with similar accuracy as fecal calprotectin.
Neutrophil CD64 was measured for gastrointestinal inflammation and was initially considered a candidate biomarker as neutrophil surface expression of CD64 is minimal during health. Tillinger et al. found a significant increase in the quantitative expression of neutrophil CD64 in clinically active IBD compared with healthy subjects,16 and our results further corroborate neutrophil CD64 surface expression (neutrophil CD64 index) as a biomarker of endoscopically verified gastrointestinal inflammation in pediatric-onset CD.
The diagnostic utility of sCD64 in peripheral blood, however, has yet to be explored in adult or pediatric IBD. Plasma levels of sCD64 were found to be significantly elevated in untreated adults with rheumatoid arthritis compared with controls.17 The authors also reported a significant reduction of sCD64 in rheumatoid arthritis patients responding to treatment.17 In our study, the sCD64 produced similar testing characteristics to the blood and fecal biomarkers for patients with suspected IBD but was more modest in accurately detecting endoscopic inflammation in established CD patients receiving treatment. The observed difference in the testing characteristics of all the CD64 biomarkers could be attributable to the higher inflammatory burden in new CD (SES-CD median, 13; range, 3–31) compared with the established CD patients with endoscopically active inflammation (SES-CD median, 10; range, 3–34; P < 0.01) or a reflection of the biomarker’s limitations in detecting subtle, low-grade inflammation. Both the CD64 biomarkers and fecal calprotectin poorly distinguished between mild (SES-CD score of 3–6) and inactive endoscopic severity.
The high sensitivity of fecal calprotectin has led to the widespread use of the test as a screening diagnostic for IBD in patients with gastrointestinal complaints.18, 19 However, the specificity of fecal calprotectin ≥50 μg/g has been shown to be as low as 44% in children, which is significantly lower than studies in adults.13, 20 In this study, we found that the neutrophil CD64 index >1 had a specificity of 89%, compared with a fecal calprotectin specificity of only 47% at a cutoff ≥50 μg/g. We noted that the combination of both biomarkers dramatically improved the sensitivity (91%) and specificity (88%) to diagnose CD. As a screening test, we found sCD64 to be a reliable biomarker for gastrointestinal inflammation with an AUC of 0.93, a sensitivity of 92%, and a specificity of 85% at a cutoff ≥39 ng/mL, with an NPV of 91%. With an aim to test the performance of the biomarkers in a “real-world” scenario, our control subjects were not necessarily healthy controls as we enrolled patients with a high pretest probability of gastrointestinal inflammation. However, as this is the population that is referred for gastroenterology opinion in clinical practice, we intentionally included all 148 consecutively enrolled patients, similar to the cohort studied by Henderson et al., who reported a specificity of 47% with a fecal calprotectin >50 μg/g.13 In our study, there was a modest increase in the specificity (from 47% to 56%) of fecal calprotectin when the cutoff was increased to ≥100 μg/g (data not shown), and there has been a similar trend noted in earlier calprotectin studies.13, 18
Previous studies have noted a reduced sensitivity of fecal calprotectin in CD patients with isolated or limited small bowel inflammation, with the sensitivity ranging from 59% to 89% at a fecal calprotectin >50 μg/g.21, 22 Although isolated small bowel CD represents a small percentage (2.9%–13%)23, 24 of newly diagnosed pediatric CD, unrecognized or poorly treated small bowel Crohn’s can lead to significant complications. Additional research is needed to determine the accuracy of CD64 testing in patients with isolated small bowel Crohn’s as we had a limited number (9/60) of L1 subjects.
With the primary emphasis on targeting mucosal healing to prevent intestinal damage, clinicians are now relying on repeat endoscopy to monitor treatment efficacy. The timing of repeat colonoscopy and location of disease are important factors when considering the treat-to-target management, which further highlights the need for reliable surrogate biomarkers for longitudinal assessment. For clinicians managing pediatric CD, the dilemma of repeat endoscopy is often a negotiation between child and parent and between clinician and parent as cost, school absence, the small risk of complications, and undesirable colonic preparation are all strongly considered. Historically, pediatric clinicians have relied on surrogate biomarkers (ESR, CRP, and fecal calprotectin) to track treatment efficacy, selecting repeat colonoscopy for concerning gastrointestinal symptoms, continued poor growth, anemia, or worsening inflammatory markers. If monitoring for endoscopic healing was the primary indication for a repeat colonoscopy, the high specificity and PPV with the CD64 biomarkers would strongly suggest that mucosal healing is unlikely with a CD64 elevation.
Although debate exists about the method to assess endoscopic healing in pediatric CD and the endoscopic severity score that correlates with mucosal healing, we chose the cutoff of ≤2 for SES-CD inactivity based on more recent data supporting this threshold.25 Although we reported on the testing characteristics of all biomarkers studied, our sample size calculation was only powered to detect differences across endoscopic severity using the neutrophil CD64 index. All the CD64 biomarkers differentiated SES-CD severe and mild, severe and inactive, while sCD64 and NCAR distinguished mild from moderate activity (no difference was noted between inactive and mild for any blood or stool biomarker). Although we did not detect differences for fecal calprotectin across all the endoscopic subscores, other investigations have found that fecal calprotectin does effectively distinguish between mild and moderate or moderate and severe using the SES-CD cutoffs we utilized in this study, as well as other proposed endoscopic scales.11, 26
Irrespective of the endoscopic cutoff utilized, there was a significant difference in the neutrophil CD64 surface expression and sCD64 between endoscopic active and endoscopic inactive, further suggesting a strong association between CD64 and CD inflammatory mechanisms. We have reported up to a 3-fold increase in ileal FcγRIA (CD64) mRNA in newly diagnosed CD subjects.9 Additionally, intestinal FcγRIA mRNA expression was found to be upregulated in infliximab nonresponders with a proposed mechanism of FcγRI capture of the infliximab/TNFα immune complex inducing transcription of proinflammatory cytokines (IL-6, IL-8) directly leading to anti-TNF failure in CD64high patients.27 In a previous investigation of 36 clinically quiescent CD patients on maintenance infliximab, we found that an elevated neutrophil CD64 index ≥1 was a significant risk factor (hazard ratio, 4.4; 95% CI, 1.6–12.4; P < 0.005)28 for treatment failure, suggesting a role for longitudinal CD64 assessments similar to serial monitoring with fecal calprotectin for early detection of CD recurrence following intestinal resection.29
Our data demonstrate that the CD64 biomarkers are effective in distinguishing CD from non-CD in children and young adults who are considered for colonoscopy. The sCD64 AUC was comparable with fecal calprotectin. The basis for the more modest diagnostic accuracy of the CD64 biomarkers in established CD patients receiving therapy is unknown at this time. Systemic corticosteroids have been shown to rapidly downregulate neutrophil CD64 expression15; however, we did not find that the sensitivity of the neutrophil CD64 index improved when controlling for steroid exposures. Alternatively, elevations in neutrophil CD64 expression may be limited to a CD phenotype driven largely by IFNγ.30, 31 Lastly, although CD64 upregulation in response to an inflammatory trigger (like IFNγ) predominates, there is the possibility of increased neutrophil CD64 receptor avidity rather than absolute CD64 upregulation. CD64 and the other activating Fcγ receptors have been found to increase inside-out signaling (avidity) where ligand binding capacity to Fcγ receptors increases with no change in CD64 cell surface expression.32
Specific limitations to our observational study include limited collection of the other blood and fecal biomarkers for direct diagnostic comparisons as well as the clinician’s diagnostic evaluation. The missing laboratory data could result in bias if the missing data were not completely random. The primary goal of the study for which the sample size was based was the difference in neutrophil CD64 index between the 4 groups of endoscopic severity (inactive, mild, moderate, and severe). However, we performed additional analyses that were considered secondary and were subject to the hazards of multiple testing. We chose to combine the CD64 biomarkers with albumin to perform the ROC curve analysis as this was the most common biomarker obtained (74%) in this and our previous studies. Additionally, the analysis for endoscopic severity across the 4 SES-CD rankings combined the 60 newly diagnosed CDs with 61 known CDs, raising the potential for increasing the proportion of patients with more severe endoscopic severity. As practice guidelines change and more patients in clinical remission undergo colonoscopy to evaluate treatment response, future studies will evaluate neutrophil CD64 cutoffs for endoscopic remission in a larger cohort of only established CD patients.
In conclusion, we have found that both neutrophil CD64 surface expression and sCD64 reliably distinguished CD from non-IBD in patients referred for colonoscopy for suspected IBD. We also found that the CD64 blood biomarkers are significantly increased in endoscopically active CD patients. The next steps are to investigate whether longitudinal CD64 measurements can provide individual assessments of treatment response and predict clinical relapse or disease complications. In a recent nationwide analysis of health care utilization in IBD using insurance claims data, blood biomarkers were more commonly used to monitor IBD activity than the fecal biomarkers.33 With the increasing need for more reliable blood biomarkers to monitor CD treatment targets, we are encouraged that elevations in the CD64 biomarkers are strongly indicative of gastrointestinal inflammation and could be utilized to prioritize referral for future colonoscopy in both established and suspected IBD patients.
The authors have no financial, professional, or personal arrangement(s) with a company whose product figures prominently in the submitted manuscript or with a company making a competing product.
Supported by National Institutes of Health (K23 DK105229 and K12 HD028827 to PM, K23 DK094832 to MJR), the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition Foundation/Crohn’s and Colitis Foundation Young Investigator Development Award (to P.M.), and a National Institutes of Health Public Health Service Grant (P30 DK078392 to the Digestive Disease Research Flow Core Center in Cincinnati, OH).
REFERENCES
- 1. Abraham C, Cho JH. Inflammatory bowel disease. n Engl j Med. 2009;361:2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pariente B, Cosnes J, Danese S et al. . Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colombel JF, Reinisch W, Mantzaris GJ et al. . Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn’s disease - a sonic post hoc analysis. Aliment Pharmacol Ther. 2015;41:734–746. [DOI] [PubMed] [Google Scholar]
- 4. Schnitzler F, Fidder H, Ferrante M et al. . Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295–1301. [DOI] [PubMed] [Google Scholar]
- 5. Thakkar K, El-Serag HB, Mattek N, Gilger M. Complications of pediatric colonoscopy: a five-year multicenter experience. Clin Gastroenterol Hepatol. 2008;6:515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149:1275–1285.e2. [DOI] [PubMed] [Google Scholar]
- 7. Abraham BP, Kane S. Fecal markers: calprotectin and lactoferrin. Gastroenterol Clin North Am. 2012;41:483–495. [DOI] [PubMed] [Google Scholar]
- 8. Becker W, Fischbach W, Weppler M et al. . Radiolabelled granulocytes in inflammatory bowel disease: diagnostic possibilities and clinical indications. Nucl Med Commun. 1988;9:693–701. [DOI] [PubMed] [Google Scholar]
- 9. Minar P, Haberman Y, Jurickova I et al. . Utility of neutrophil fcγ receptor i (cd64) index as a biomarker for mucosal inflammation in pediatric Crohn’s disease. Inflamm Bowel Dis. 2014;20:1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daperno M, D’Haens G, Van Assche G et al. . Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the ses-cd. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 11. Schoepfer AM, Beglinger C, Straumann A et al. . Fecal calprotectin correlates more closely with the simple endoscopic score for Crohn’s disease (ses-cd) than crp, blood leukocytes, and the cdai. Am j Gastroenterol. 2010;105:162–169. [DOI] [PubMed] [Google Scholar]
- 12. Moskovitz DN, Daperno M, Van Assche GA et al. . Defining and validating cut-offs for the simple endoscopic score for Crohn’s disease. Gastroenterology. 2007;132:A173–A173. [Google Scholar]
- 13. Henderson P, Casey A, Lawrence SJ et al. . The diagnostic accuracy of fecal calprotectin during the investigation of suspected pediatric inflammatory bowel disease. Am j Gastroenterol. 2012;107:941–949. [DOI] [PubMed] [Google Scholar]
- 14. Roblin X, Duru G, Williet N et al. . Development and internal validation of a model using fecal calprotectin in combination with infliximab trough levels to predict clinical relapse in Crohn’s disease. Inflamm Bowel Dis. 2017;23:126–132. [DOI] [PubMed] [Google Scholar]
- 15. Tillinger W, Gasche C, Reinisch W et al. . Influence of topically and systemically active steroids on circulating leukocytes in Crohn’s disease. Am j Gastroenterol. 1998;93:1848–1853. [DOI] [PubMed] [Google Scholar]
- 16. Tillinger W, Jilch R, Jilma B et al. . Expression of the high-affinity IgG receptor fcri (cd64) in patients with inflammatory bowel disease: a new biomarker for gastroenterologic diagnostics. Am j Gastroenterol. 2009;104:102–109. [DOI] [PubMed] [Google Scholar]
- 17. Matt P, Lindqvist U, Kleinau S. Elevated membrane and soluble cd64: a novel marker reflecting altered fcγr function and disease in early rheumatoid arthritis that can be regulated by anti-rheumatic treatment. Plos One. 2015;10:e0137474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diamanti A, Panetta F, Basso MS et al. . Diagnostic work-up of inflammatory bowel disease in children: the role of calprotectin assay. Inflamm Bowel Dis. 2010;16:1926–1930. [DOI] [PubMed] [Google Scholar]
- 19. Schoepfer AM, Trummler M, Seeholzer P et al. . Discriminating ibd from ibs: comparison of the test performance of fecal markers, blood leukocytes, crp, and ibd antibodies. Inflamm Bowel Dis. 2008;14:32–39. [DOI] [PubMed] [Google Scholar]
- 20. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kopylov U, Yung DE, Engel T et al. . Fecal calprotectin for the prediction of small-bowel Crohn’s disease by capsule endoscopy: a systematic review and meta-analysis. Eur j Gastroenterol Hepatol. 2016;28:1137–1144. [DOI] [PubMed] [Google Scholar]
- 22. Sipponen T, Haapamäki J, Savilahti E et al. . Fecal calprotectin and s100a12 have low utility in prediction of small bowel Crohn’s disease detected by wireless capsule endoscopy. Scand j Gastroenterol. 2012;47:778–784. [DOI] [PubMed] [Google Scholar]
- 23. Müller KE, Lakatos PL, Arató A et al. ; Hungarian IBD Registry Group (HUPIR) Incidence, paris classification, and follow-up in a nationwide incident cohort of pediatric patients with inflammatory bowel disease. j Pediatr Gastroenterol Nutr. 2013;57:576–582. [DOI] [PubMed] [Google Scholar]
- 24. Eidelwein AP, Thompson R, Fiorino K et al. . Disease presentation and clinical course in black and white children with inflammatory bowel disease. j Pediatr Gastroenterol Nutr. 2007;44:555–560. [DOI] [PubMed] [Google Scholar]
- 25. af Björkesten CG, Nieminen U, Turunen U et al. . Surrogate markers and clinical indices, alone or combined, as indicators for endoscopic remission in anti-tnf-treated luminal Crohn’s disease. Scand j Gastroenterol. 2012;47:528–537. [DOI] [PubMed] [Google Scholar]
- 26. Sipponen T, Savilahti E, Kolho KL et al. . Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis. 2008;14:40–46. [DOI] [PubMed] [Google Scholar]
- 27. Wojtal KA, Rogler G, Scharl M et al. . Fc gamma receptor cd64 modulates the inhibitory activity of infliximab. Plos One. 2012;7:e43361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Minar P, Jackson K, Tsai YT et al. . a low neutrophil cd64 index is associated with sustained remission during infliximab maintenance therapy. Inflamm Bowel Dis. 2016;22:2641–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wright EK, Kamm MA, De Cruz P et al. . Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148:938–947.e1. [DOI] [PubMed] [Google Scholar]
- 30. Haberman Y, Tickle TL, Dexheimer PJ et al. . Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. j Clin Invest. 2014;124:3617–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:374–388.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brandsma AM, Jacobino SR, Meyer S et al. . Fc receptor inside-out signaling and possible impact on antibody therapy. Immunol Rev. 2015;268:74–87. [DOI] [PubMed] [Google Scholar]
- 33. van Deen WK, van Oijen MG, Myers KD et al. . a nationwide 2010-2012 analysis of u.s. Health care utilization in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1747–1753. [DOI] [PubMed] [Google Scholar]