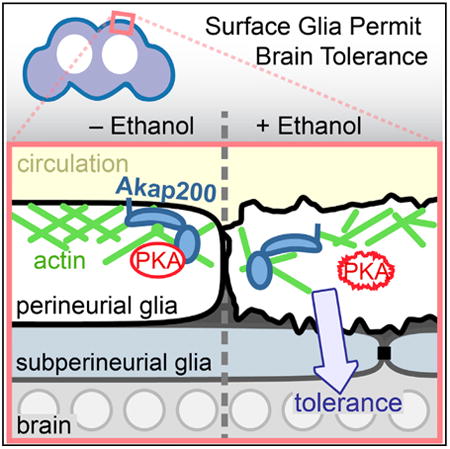
Summary
Ethanol is the most common drug of abuse. It exerts its behavioral effects by acting on widespread neural circuits; however, its impact on glial cells is less understood. We show that Drosophila perineurial glia are critical for ethanol tolerance, a simple form of behavioral plasticity. The perineurial glia form the continuous outer cellular layer of the blood-brain barrier and are the interface between the brain and the circulation. Ethanol tolerance development requires the A kinase anchoring protein Akap200 specifically in perineurial glia. Akap200 tightly coordinates protein kinase A, actin, and calcium signaling at the membrane to control tolerance. Furthermore, ethanol causes a structural remodeling of the actin cytoskeleton and perineurial membrane topology in an Akap200-dependent manner, without disrupting classical barrier functions. Our findings reveal an active molecular signaling process in the cells at the blood-brain interface that permits a form of behavioral plasticity induced by ethanol.
Graphical abstract
Parkhurst et. al show that glia at the interface of the Drosophila circulation and brain change shape in response to alcohol and permit the development of alcohol tolerance. This depends on the anchoring of signaling molecules to the plasma membrane in proximity to the actin cytoskeleton.
Introduction
Ethanol is the most widely used addictive drug, yet our partial understanding of how it affects brain function and behavior has hampered the development of treatments for alcohol use disorders. A simple neuroadaptive change induced by ethanol is the development of tolerance, which is the acquired resistance to its aversive and pleasurable effects, and which facilitates increased ethanol intake (Fadda and Rossetti, 1998). The fruit fly Drosophila is a promising model for identifying the molecular mechanisms of both simple and more complex behavioral responses to ethanol, including sensitivity, tolerance, preference, withdrawal, and reward (Ghezzi and Atkinson, 2011; Kaun et al., 2012).
Behavioral adaptations to ethanol exposure like tolerance are due to alterations in the synaptic transmission of neurons (Ghezzi and Atkinson, 2011; Hyman et al., 2006). The role of glia, the main non-neuronal cell type in the nervous system, is less well under-stood (Allen and Barres, 2009; Araque et al., 2014). Short-term exposure to moderate ethanol doses increases the levels of a glial-specific cytoskeletal protein (Blanco and Guerri, 2007; Bull et al., 2015; Goodlett et al., 1993). Binge and chronic drinking cause astrogliosis and changes in glial density (Bull et al., 2015; Evrard et al., 2003; Fattore et al., 2002; Goodlett et al., 1997; Miguel-Hidalgo, 2006). Changes in glial markers and glial proliferation following ethanol self-administration suggest that glia may also contribute to the long-lasting neurobehavioral effects of ethanol (Evrard et al., 2006; He et al., 2009; Nixon et al., 2008).
Glia are integral to the blood-brain barrier, which is affected by ethanol in as-yet-unclear ways (Haorah et al., 2005; Peng et al., 2013; Rubio-Araiz et al., 2017). The fly blood-brain barrier is composed of two closely apposed layers of glia and an overlying extracellular matrix, and it surrounds the CNS (Awasaki et al., 2008; Hindle and Bainton, 2014). The inner subperineurial glial layer forms the physical and much of the chemical barrier. The outer perineurial glia interfaces directly with the circulating hemolymph, and it transports sugar into the brain, provides some chemicalbarrier functions, and structures the overlying extracellular matrix(Seabrooke and O'Donnell, 2013; Stork et al., 2008; Volkenhoff et al., 2015). The subperineurial glia regulate ethanol sensitivity by adjusting the tightness of the physical barrier (Bainton et al., 2005).
Protein kinase A (PKA) is a key regulator of ethanol neurobehavioral action (Park et al., 2000; Ron and Barak, 2016). We sought to better understand how ethanol initiates behavioral plasticity by characterizing ethanol-regulated PKA interacting proteins. The Drosophila A kinase anchoring protein Akap200 is an intra-cellular scaffolding protein that spatially coordinates PKA, calmodulin (CaM), and actin, is regulated by protein kinase C (PKC), and that regulates the size of actin-rich ring canals in embryos (Jackson and Berg, 2002; Li et al., 1999; Rossi et al., 1999). We show here that Akap200 coordination of PKA, calcium, and actin at the membrane of the perineurial glia permits ethanol-induced behavioral plasticity.
Results
Akap200 Mutants Exhibit Decreased Behavioral Plasticity upon Repeated Ethanol Exposure
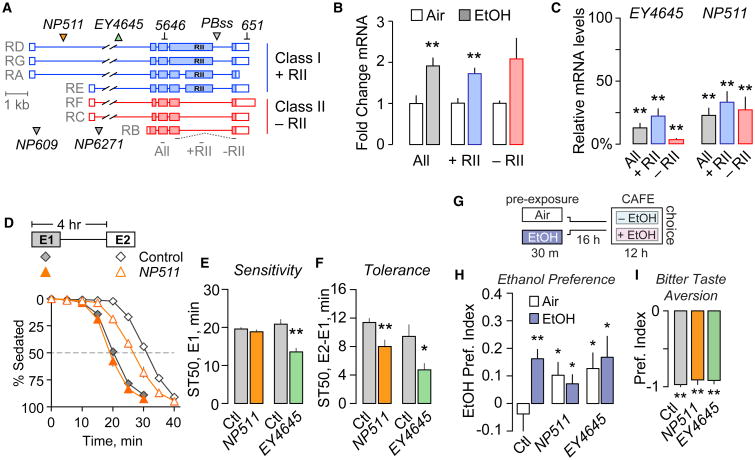
To understand whether Akap200 functions in ethanol behavioral responses, we first characterized its transcriptional response to ethanol. The Akap200 locus transcribes seven distinct transcripts that are translated into two major forms of Akap200 protein (Figure 1A). Class I transcripts encode Akap200L that contains a PKA regulatory subunit type II (RII) binding domain, and class II transcripts encode Akap200S that lacks the RII domain. Both protein isoforms are N-terminal myristoylated and contain a polybasic MARCKS-like domain that binds actin and Ca2+-bound CaM, and is PKC phosphorylated (Rossi et al., 1999). Ethanol increased Akap200 class I and II expression (Figure 1B). Two transposon insertions in the Akap200 locus, NP511 and EY4645, markedly decreased expression of both Akap200 transcript classes (Figure 1C). Two additional transposon insertions at different genomic locations also decreased Akap200 expression (Figure S1A). All Akap200 mutants were homozygous viable and sterile, with an incompletely penetrant wings-held-down posture defect. Sterility is an Akap200 loss-of-function phenotype due to its role in oogenesis (Jackson and Berg, 2002).
Figure 1. Akap200 Is Ethanol Responsive and Required for Ethanol Behaviors.
(A) Akap200 transcript classes that encode distinct proteins. Transposon insertions (triangles) and dsRNA targets are indicated. qPCR probe locations are shown below.
(B) Akap200 transcript levels by qPCR 1 hr after exposure to 30′ ethanol (just sedating) or air, normalized to air. One-way ANOVA/Dunn's, **p < 0.01. n = 7 biological replicates.
(C) Akap200 is reduced in flies homozygous for the indicated transposons. One-sample t test compared to 100, *p < 0.05, **p < 0.01. n = 5–7 biological replicates.
(D) Ethanol sedation sensitivity and tolerance time course. Two ethanol vapor exposures (E1, filled symbols, and E2, open symbols) separated by a rest period. ST50: time to 50% sedation.
(E and F) Sedation sensitivity (E) and tolerance (F) for flies homozygous for NP511 or EY4645. One-way ANOVA/Tukey's, n = 7–12 groups.
(G) Ethanol preference measured in the CAFE assay. Flies exposed to ethanol vapor (EtOH) or humidified air (Air), and after 16 hr placed in the CAFE assay to measure ethanol preference.
(H) Akap200 mutants developed precocious ethanol preference. One-sample t test compared to 0, *p < 0.05, **p < 0.01. n = 19–22 groups.
(I) Bitter taste aversion in flies choosing sucrose with and without quinine. A value of “−1” indicates aversion. One-sample t test compared to 0, **p < 0.01. n = 5–9 groups. “Ctl” is the genetic background. All bar graphs are mean with SEM.
See also Figure S1.
We tested NP511 and EY4645 flies for their behavioral response to ethanol using assays that measure sedation sensitivity (Figure 1D) and locomotor stimulation (Figure S1F). To induce and measure rapid tolerance to ethanol sedation, flies were exposed twice to ethanol vapor, with a 4-hr interval between the start of each exposure, allowing for complete ethanol metabolism between exposures. Flies are less sensitive to the sedating effects of ethanol upon the second exposure, and this tolerance is measured as the difference in time to 50% sedation (ST50) between exposures (Figure 1D). EY4645 flies showed increased ethanol sensitivity (Figure 1E), and both EY4645 and NP511 showed decreased ethanol tolerance (Figure 1F). Flies heterozygous for either mutation were unaffected for ethanol behavioral responses (Figures S1D and S1E), indicating that both Akap200 mutations are recessive. Placing EY4645 over the deficiency Df(2L)BSC201 resulted in fully penetrant lethality. The two additional alleles, NP609 and NP6271, also exhibited decreased ethanol tolerance (Figures S1B and S1C).
Ethanol stimulates locomotor activity that is sensitized during tolerance development (Figure S1F) (Kong et al., 2010). The difference in distance traveled between exposures is a measure of sensitization. Whereas NP511 and EY4645 differently affected ethanol-induced hyperactivity (Figure S1G), both decreased sensitization (Figure S1H). Ethanol absorption was unaffected in the Akap200 mutants (Figure S1I).
Ethanol preference is a distinct measure of drug-induced plasticity (Devineni and Heberlein, 2009; Devineni et al., 2011; Ja et al., 2007). Drug naive flies equally prefer food and food-plus-ethanol, whereas pre-exposure to an inebriating dose of ethanol vapor induces preference for food-plus-ethanol (Figures 1G and 1H) (Peru Y Coló n de Portugal et al., 2014). Interestingly, Akap200 mutant flies preferred food-plus-ethanol even without the priming pre-exposure (Figure 1H). This precocious ethanol preference was not due to an inability to detect aversive tastants like ethanol: Akap200 mutant flies given a choice between highly sweet but bitter and less sweet foods chose the less sweet option (Figure 1I). These data show that Akap200 affects ethanol sensitivity and promotes behavioral plasticity (tolerance, sensitization, and preference) upon repeated or chronic ethanol exposure.
Akap200 Expression Is Glial and Neuronal
Akap200 is expressed almost exclusively in the nervous system during embryonic development (Bonin and Mann, 2004; Freeman et al., 2003). We used the GFP protein trap Akap200PBss (Figure 1A) to assess Akap200 distribution in the adult nervous system. In Akap200PBss, GFP-encoding sequences splice to the Akap200 open reading frame upstream of the final coding exon, and downstream of all characterized functional domains. We confirmed Akap200L- and Aka200S-GFP fusion proteins in Akap200PBss fly heads by western analysis (Figure 2A). Akap200-GFP was non-nuclear and was widely distributed in the brain (Figure 2B). Particularly strong expression surrounding neuronal nuclei in the cortex suggested expression in the cortex glia. Diffuse Akap200 was evident throughout the synaptic neuropil, suggesting a broad neuronal distribution. We used the Gal4/UAS binary system to express two Akap200 dsRNAs, 651 and 5646, that target distinct regions of Akap200 that are common to all isoforms (Figure 1A). When expressed ubiquitously (using Act5C-Gal4), both dsRNAs decreased Akap200 expression to barely detectable levels (Figure 2E). We confirmed both RNAis affected ethanol behaviors (Figures S2F and S2G) and used the behaviorally stronger 651 for subsequent experiments. Akap200 RNAi specifically in all neurons reduced Akap200-GFP throughout the synaptic neuropil, making cortex glia and ensheathing glia Akap200-GFP expression more prominent (Figures 2C and 2C′). Akap200 RNAi specifically in all glia also reduced Akap200-GFP expression, especially in the cortex (Figures 2D and 2D′). Thus, Akap200 is present in both neurons and glia in the adult brain, and Akap200 expression is widespread.
Figure 2. Akap200 Is Neuronal and Glial.
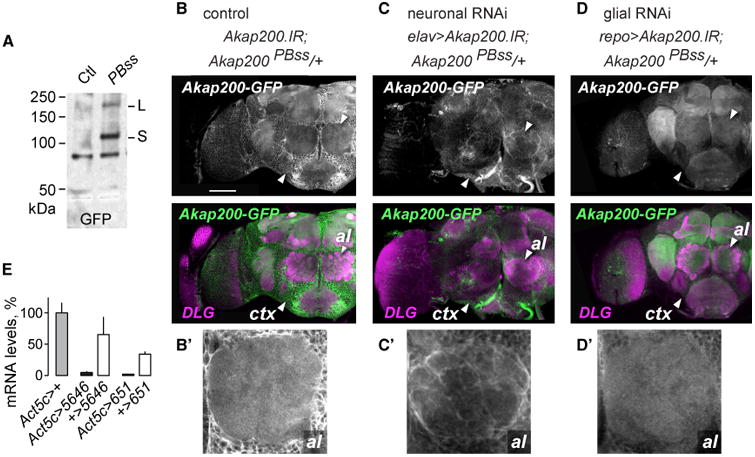
(A) Western blot of Akap200-GFP protein trap and control extracts probed with GFP antibodies.
(B) Akap200-GFP is expressed broadly in the adult brain, labeled with GFP (green) and DLG (magenta) antibodies. ctx: cortex region containing glia and neuron cell bodies; al: antennal lobe. Genotype: w,UAS-Akap200.IR/+;Akap200PBss/+. Scale bar: 50 μm.
(B′) Akap200-GFP in the antennal lobe.
(C) Akap200 RNAi in all neurons reduced synaptic neuropil Akap200-GFP.
(C′) Akap200-positive ensheathing glia. Genotype: w,elav(c155)-Gal4,UAS-Akap200.IR/+;Akap200PBss/+.
(D) Akap200 RNAi in all glia reduced Akap200-GFP expression.
(D′) Loss of glial staining in the antennal lobe. Genotype: w,UAS-Akap200.IR/+;Akap200PBss/+;repo-Gal4/+.
(E) Akap200 qPCR for all transcripts in fly heads when Akap200 RNAi is expressed ubiquitously with Act5c-Gal4, normalized to Act5c > +. All bar graphs are mean with SEM.
Akap200 Coordinates PKA in the Outer Layer of the Blood-Brain Barrier to Promote Ethanol Tolerance
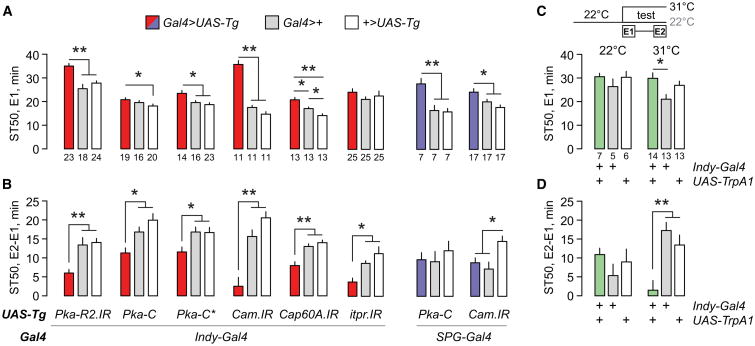
We next asked where Akap200 was required for ethanol behavioral responses. Akap200 RNAi in all neurons (elav-Gal4) did not alter ethanol sedation sensitivity or tolerance, whereas Akap200 RNAi in all glia (repo-Gal4) reduced ethanol tolerance (Figures 3A and 3B). Diverse glia types perform specific functions (Figure 3C). We used a panel of Gal4 transgenes that express only in specific glial classes to reduce Akap200 expression (Figure 3B, lower table). Akap200 RNAi specifically in perineurial glia (using Indy-Gal4), but not in any other type of glia, decreased ethanol sedation sensitivity and sedation tolerance (Figures 3A and 3B). Indy-Gal4 is expressed exclusively the perineurial glia in the adult nervous system (Figures S2A–S2E); however, it may be expressed in other tissues in the animal (DeSalvo et al., 2011). To confirm that Akap200 functions in perineurial glia, we introduced repo-Gal80 that expresses the GAL4 inhibitor GAL80 in all glia and found that it blocked the behavioral effects of Akap200 RNAi driven by Indy-Gal4 (Figures 3A and 3B). Locomotor sensitization was also decreased when Akap200 expression was reduced in all glia and specifically in perineurial glia (Figures S2F and S2G). Thus, Akap200 promotes ethanol behavioral responses through its actions in the perineurial glia that form the outermost cellular layer of the Drosophila blood-brain barrier. Akap200-GFP is expressed in perineurial glia (Figure 3D). Perineurial-specific Akap200 RNAi decreased Akap200-GFP expression only in these cells, and the perineurial layer appeared to be intact (Figure 3D).
Figure 3. Akap200 Coordinates PKA at the Membrane in Perineurial Glia for Ethanol Responses.
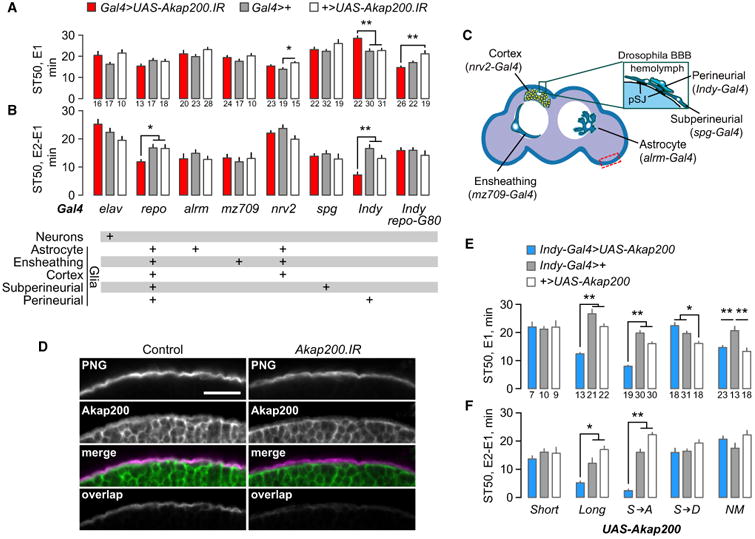
(A and B) Ethanol sedation sensitivity (A) and tolerance (B) for Akap200 RNAi driven with the Gal4 strains and in the patterns indicated below the graphs. repo-G80 expresses the GAL4 inhibitor GAL80 specifically in all glial cells. One-way ANOVA/Tukey's, *p < 0.05, **p < 0.01; n indicated below each bar.
(C) Location of the glia types, adapted with permission (Ou et al., 2014).
(D) Akap200 is in the perineurial glia (PNG, Indy > CD2mCherry, anti-dsRed). “Overlap” show pixels that are common with Akap200-GFP. Control: w;Akap200PBss/UAS-CD2mCherry;Indy-Gal4/+. Akap200.IR: w,UAS-Akap200.IR;Akap200PBss/UAS-CD2mCherry;Indy-Gal4/+. Area imaged is dashed rectangle in (C). Scale bar: 25 μm.
(E and F) Ethanol sensitivity (E) and tolerance (F) when either wild-type or mutated Akap200 transgenes were expressed in perineurial glia of wild-type flies. Akap200 sequence changes were S → A: PKC non-phosphorylatable; S → D: PKC pseudo-phosphorylated; NM: myristoylation blocked (details in Experimental Procedures). One way ANOVA/Tukey's, the number of groups tested is indicated below each bar for all panels in figure. *p < 0.05, **p < 0.01.
All bar graphs are mean with SEM. See also Figure S2.
To ask how Akap200 dictates ethanol tolerance, we overexpressed Akap200 specifically in the perineurial glia. Akap200L overexpression acted like a dominant negative, increasing ethanol sensitivity and decreasing ethanol tolerance, similar to EY4645 (Figures 3E and 3F). Decreasing endogenous Akap200 by one-half did not alter the Akap200L overexpression phenotype (Figure S2H). Importantly, overexpression of Akap200S had no effect. Akap200L contains the Pka-RII binding domain, suggesting that coordination of PKA by Akap200 is key for tolerance development. We tested this using transgenes harboring mutations of the known molecular functions of Akap200L (Rossi et al., 1999). PKC phosphorylation sites in the N-terminal positively charged domain were made either nonphosphorylatable (S → A) or psuedophosphorylated (S → D), and the myristoylation consensus sequence was mutated (NM). Both psuedophosphorylation and blocking myristoylation eliminated the effects of overexpression on ethanol sensitivity and tolerance (Figures 3E and 3F). These two manipulations are predicted to delocalize overexpressed Akap200L from the membrane (Rossi et al., 1999). Taken together, these results suggest that ethanol tolerance is promoted in the perineurial glia through reversible tethering of PKA to the plasma membrane by Akap200.
PKA and Calcium Acutely Regulate Ethanol Tolerance in the Perineurial Glia
Our findings suggest that Akap200 positions PKA and other proteins at the membrane where they can respond to cellular signals in a localized fashion. We therefore asked whether altering PKA and intracellular calcium in perineurial glia affects tolerance. RNAi-mediated reduction of the PKA RII regulatory subunit PKA-R2 decreased ethanol sedation sensitivity and tolerance (Figures 4A and 4B). Overexpression of the PKA catalytic subunit (UAS-Pka-C), either the wild-type or a constitutively active form (UAS-Pka-C*), had a similar effect. These PKA manipulations are predicted to increase and delocalize PKA activity. Furthermore, reducing CaM, which competes with actin for Akap200 binding, in the perineurial glia led to marked ethanol sedation resistance and a near absence of ethanol tolerance (Figures 4A and 4B) (Rossi et al., 1999). Consistent with a role for internal stores, as in other glia, reduced expression of the sarco/endoplasmic reticulum calcium-ATPase channel Cap60A or the inositol 1,4,5-trisphosphate receptor Itpr resulted in decreased ethanol tolerance (Figures 4A and 4B). Thus, PKA and calcium signaling may be coordinated by Akap200 in the perineurial glia to promote ethanol sensitivity and tolerance. These same manipulations done specifically in subperineurial glia decreased ethanol sensitivity but had no effect on ethanol tolerance (Figures 3A, 4A, and 4B). Thus, blood-brain barrier promotion of ethanol tolerance through Akap200, PKA, and calcium is specific to the perineurial layer, whereas ethanol sensitivity is controlled by both layers.
Figure 4. Akap200 Interacting Proteins and Calcium Homeostasis Are Required in Perineurial Glia for Ethanol Responses.
(A and B) Ethanol sedation sensitivity (A) and tolerance (B) for flies expressing the indicated transgenes (UAS-Tg) in perineurial (Indy-Gal4) or subperineurial (SPG-Gal4) glia. Pka-R2.IR: PKA regulatory subunit RII RNAi; Pka-C: wild-type PKA catalytic subunit; Pka-C*: constitutively active PKA catalytic subunit; Cam.IR: CaM RNAi; Cap60A.IR: SERCA RNAi; itpr.IR: InsP3R Itp-r83A RNAi.
(C and D) Ethanol sedation sensitivity (C) and tolerance (D) for flies expressing the TrpA1 cation channel in perineurial glia. Flies were raised at 22°C(TrpA1 off), and held at 22°C (control) or shifted to 31°C (TrpA1 on, experimental) just prior to ethanol vapor exposure. One-way ANOVA/Tukey's or Kruskal-Wallis/Dunn's, *p < 0.05, **p < 0.01. n is indicated below each graph.
All bar graphics are mean with SEM.
Finally, we asked whether altering the properties of the perineurial glia acutely during ethanol exposure affected behavior. We depolarized the perineurial glia, increasing calcium influx, using the heat-activated TrpA1 cation channel at the onset of ethanol exposure. Acute TrpA1 activation did not alter ethanol sensitivity, but it strongly decreased ethanol tolerance (Figures 4C and 4D). Therefore, the perineurial glia are actively involved in the development of ethanol tolerance.
Ethanol Alters Perineurial Glial Morphology in an Akap200-Dependent Manner
The perineurial layer of the blood-brain barrier is composed of elongated cells that tile the surface of the adult brain (Awasaki et al., 2008; Kremer et al., 2017; Stork et al., 2008). Labeling the perineurial glia with plasma membrane bound GFP revealed their tiled organization on the front surface of the brain (Figure 5A, Air; Figure S3). Akap200 RNAi in the perineurial glia did not affect perineurial morphology, indicating that their development and overall structure are unaffected by loss of Akap200 (Figure 5B, Air). Ethanol exposure resulted in a marked change in perineurial glia membrane topology (Figure 5A, Ethanol), appearing more disorganized and masking the tiled appearance. We used a stochastic multicolor labeling technique to assess the morphology of individual cells (Nern et al., 2015). Whereas the spatial arrangement of perineurial cells was unaffected by ethanol, the plasma membranes were less uniform (Figures S3A and S3B). Because Akap200 binds actin through its MARCKS-like domain, which is critical for cell morphology, we expressed the Lifeact-GFP actin-binding protein in perineurial glia, which was expressed in a subset of perineurial cells. The actin cytoskeleton in untreated perineurial cells formed a reticulated structure (Figure 5C). Ethanol exposure decreased reticulation, and at the extreme resulted in the accumulation of discrete actin blobs (Figures 5C and 5D).
Figure 5. Ethanol Alters Perineurial Morphology and Actin Organization.
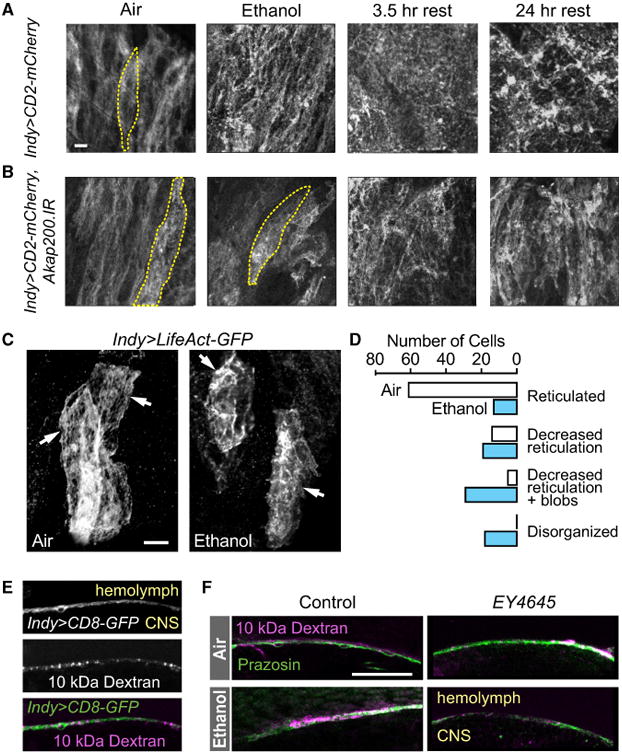
(A) Front surface of brain expressing membrane-bound mCherry in the perineurial cells, revealing their columnar distribution in ethanol naïve flies (Air). One cell is outlined. Membrane topology became disordered following exposure to a sedating dose of ethanol (Ethanol, rest). Scale bar: 10 μm.
(B) Ethanol effects were delayed or reduced when Akap200 expression was reduced in the perineurial glia.
(C) Reticular organization of perineurial actin (arrows) in individual cells becomes disorganized following ethanol exposure, as revealed with LifeAct-GFP. Scale bar: 10 μm.
(D) Quantification of perineurial actin organization with ethanol treatment. p < 0.0001, Mann-Whitney test for treatment, n = 83 cells in 10 brains per treatment, from three independent experiments.
(E) High-molecular-weight dye (10-kDa Texas Red dextran) injected into the hemolymph accumulated at the perineurial glia layer (Indy > CD8:GFP),and did not penetrate into the brain (CNS).
(F) Barrier functions were unaffected by ethanol exposure or lack of Akap200. 10-kDa Texas Red dextran (physical, septate junctions, magenta) and BODIPY-prazosin (chemical, green) co-injected into the hemolymph were excluded from the CNS.3-μm frontal sections midway through optic lobe surface. Scale bar: 25 μm.
See also Figure S3.
Surprisingly, the altered appearance of the perineurial glia persisted at least 24 hr after recovery from the ethanol exposure (Figure 5A, 24-hr rest). In flies with Akap200 expression reduced in the perineurial glia, ethanol exposure affected perineurial glia membrane topology less severely or in a delayed manner (Figure 5B). These findings suggest that ethanol induces morphological changes in the perineurial glia through an Akap200-dependent mechanism, possibly by facilitating changes in the actin cytoskeleton.
Ethanol may promote tolerance by structurally or functionally incapacitating the perineurial glia. We tested this by killing the perineurial layer specifically in adults. Flies of the genotype tub-Gal80ts, Indy-Gal4>UAS-rpr,UAS-hid express pro-apoptotic Rpr and Hid in the perineurial glia at 29°C but not at 18°C. Flies raised at 18°C were viable and outwardly normal. When shifted to 29°C as adults the experimental flies died within 3 days (28/30 dead), whereas temperature controls maintained at 18°C (0/20) and genetic controls lacking UAS-rpr and UAS-hid shifted to 29° C (2/34) did not die. Moreover, wild-type flies showed no decreased viability for at least a week after exposure to ethanol (not shown). Therefore, the adult perineurial glia are essential for viability, consistent with their role in transporting circulating sugars into the brain, and they retain their vital functions following ethanol exposure (Volkenhoff et al., 2015).
The perineurial glia make extensive contact with the subperineurial glia that form most of the physical and chemical barrier (Hindle and Bainton, 2014). We asked whether ethanol or loss of Akap200 changes barrier permeability, potentially altering the molecular composition of the brain extracellular fluid. Dye-coupled high-molecular-weight dextrans injected into the hemolymph are excluded from the brain by subperineurial septate junctions (Figure 5E). Similarly, prazosin is excluded from the brain by subperineurial transporter proteins: BODIPY-prazosin accumulated at the barrier along with dextran (Figure 5F) (Mayer et al., 2009). Neither loss of Akap200 nor ethanol exposure increased penetration of either molecule into the brain (Figure 5F). Tests with mutants known to disrupt the physical (moodyc17) and chemical (mdr65Pex8) barrier confirmed our ability to detect barrier defects (not shown) (Bainton et al., 2005; Mayer et al., 2009). Therefore, the classical partitioning functions of the blood-brain barrier appear to be intact following ethanol treatment and with loss of Akap200, indicating that Akap200 serves a different role in the perineurial glia.
Discussion
Akap200 scaffolding of signaling in the perineurial glial layer at the interface of the circulation and the brain is critical for the development of ethanol tolerance. Prior work shows that tolerance and other rapidly induced changes in behavior are due to ethanol's effects on neuronal excitability and synaptic plasticity (Ghezzi and Atkinson, 2011; Lovinger and Roberto, 2013). We propose that ethanol generates tolerance, and perhaps other forms of plasticity, in part by Akap200-tethered PKA signaling and actin organization. Akap200, membrane polarization, PKA activity, and calcium homeostasis all contribute to perineurial competence for promoting tolerance. Moreover, classic partitioning functions of the barrier are unaffected by ethanol or loss of Akap200. These findings assign a function to the perineurial glia in adults in the regulation of behavior, and they provide an in vivo model for ethanol regulation of the actin cytoskeleton. A perineurial-CNS communication pathway appears to exist that is affected by ethanol.
Our findings uncover a perineurial Akap200 scaffold that gates ethanol behavioral plasticity. Specifically, tight spatial control of PKA activity by Akap200 appears to be key. Overexpression of PKA-binding Akap200L, but not non-PKA-binding Akap200S interferes with tolerance, indicating that Akap200-PKA association is critical. Blocking Akap200L myristoylation or pseudophosphorylation of Akap200L PKC sites, both predicted to prevent Akap200L MARCKS-like membrane localization, abolishes tolerance interference. Consistent with this, removal of Akap200L PKC phosphorylation sites, predicted to lock Akap200L to the membrane and to actin, dramatically reduces tolerance. These data indicate that PKA kept in proximity of the membrane by Akap200 promotes tolerance, likely to keep PKA close to sources of cAMP production and actin. Moreover, loss of Akap200 causes a reduced or delayed change in perineurial membrane topology, which may be due to decreases in local PKA concentration at membrane sites of signaling.
PKA signaling in perineurial cells must also be tempered for tolerance development: Pka-RII downregulation that delocalizes and activates PKA, and constitutive PKA catalytic activity both decrease ethanol tolerance. Acute ethanol exposure generally activates PKA signaling in mammals to promote ethanol sensitivity and regulate ethanol consumption (Lai et al., 2007; Pandey et al., 2003; Ron and Messing, 2013; Thiele et al., 2000; Wand et al., 2001). In flies, precise PKA localization regulates ethanol sensitivity: flies lacking Pka-RII or expressing a dominant-negative form in neurons show decreased ethanol sensitivity (Park et al., 2000; Rodan et al., 2002). Furthermore, genetic manipulation of adenylyl cyclase or phosphodiesterase activity in flies shows that neuronal PKA signaling promotes ethanol tolerance and preference (Ruppert et al., 2017; Xu et al., 2012).
We demonstrate, in vivo, that the actin cytoskeleton is reorganized with acute ethanol exposure. Ethanol regulation of the actin cytoskeleton in the nervous system is implicated genetically in acute ethanol sensitivity and the acquisition of ethanol preference in flies, and in ethanol reward and ethanol seeking in mice (Laguesse et al., 2017; Ojelade et al., 2015a, 2015b; Rothenfluh and Cowan, 2013). Ethanol affects the cytoskeleton of cultured glia as well: astrocytes lose actin stress fibers and mislocalize the focal adhesion protein paxillin in response to brief ethanol exposure (Allansson et al., 2001; Guasch et al., 2003). Our findings, together with prior biochemical studies, suggest that Akap200 brings signaling pathways into tight proximity with filamentous actin at the membrane, allowing ethanol to control actin organization (Rossi et al., 1999). Accordingly, the delayed effects of ethanol on perineurial morphology with Akap200 knockdown may be accounted for by decreased local concentration of signaling molecules at actin substrates. A candidate mechanism involves the PKA-regulated Rho-family GTPases that are critical for both actin dynamics and ethanol behavioral responses (Ojelade et al., 2015a; Ridley, 2006). Whether actin reorganization in perineurial glia is a key step or collateral to tolerance development needs to be determined.
CaM and endoplasmic reticulum stores of calcium in the perineurial glia are important for promoting ethanol sensitivity and tolerance. Furthermore, TrpA1 activation causes a calcium influx and a specific disruption of ethanol tolerance. This suggests that calcium homeostasis or calcium-dependent signaling contributes to Akap200 regulation of behavioral plasticity. Mammalian Akap12, functionally related to Akap200L, is upregulated by ethanol and it shifts from the plasma membrane to the cytoplasm on calcium influx (Lee et al., 2003; Pignataro et al., 2013; Schott and Grove, 2013).
How do the perineurial cells regulate ethanol behaviors? Akap200, coordinating signaling pathways, may impact a currently known perineurial function like sugar transport from the hemolymph and its metabolism, organic anion flux, or structuring of the overlying extracellular matrix (Petley-Ragan et al., 2016; Seabrooke and O'Donnell, 2013; Volkenhoff et al., 2015). For example, ethanol might change energy availability in the nervous system to favor nervous system plasticity. Alternatively, precedence exists for the barrier releasing neuropeptidergic signals to the nervous system in response to humoral signals (Alvarez et al., 2013; Spéder and Brand, 2014). As the main interface between the circulation and the brain, the Drosophila perineurial glia and cells serving equivalent functions in mammals are well-positioned to provide communications between the body and the brain.
Experimental Procedures
Flies were raised on standard food containing agar (1.2% w/v), cornmeal (6.75% w/v), molasses (9% v/v), and yeast (1.7% w/v) at 25°C and 70% humidity, unless otherwise indicated. Drosophila strains (stock number) were from Bloomington Drosophila Stock Center: Akap200EY4645 (15759), UAS-Akap200.IR (35651), repo-Gal4 (7415), nrv2-Gal4 (6800), Act5C-Gal4 (4414), UAS-Itp-r83A.IR (25937), UAS-Ca-p60A.IR (25928), UAS-Pka-R2.IR (27680), UAS-Lifeact-GFP (58718), UAS-MCFO (64085); Drosophila Genetic Resource Center: Akap200NP511 (103626), Akap200NP609 (103674), Akap200NP6271 (105177); Vienna Drosophila Resource Center: UAS-Akap.IR (v5646), UAS-CaM.IR (v28242); Richard Mann: Akap200PBss; Roland Bainton: Indy-Gal4, SPG-Gal4; Marc Freeman: alrm-Gal4, mz709-Gal4; Paul Garrity: UAS-TrpA1; Bing Ye: UAS-CD2mCherry; Ulrike Heberlein: UAS-Pka-C and UAS-Pka-C*. UAS-Akap200 transgenes in the pUAST vector were created by Zhuhao Wu in the laboratory of Alex Kolodkin for P-element transgenesis. The amino acid changes were, in Akap200L isoform PA: UAS-Akap200.NM (non-myris-toylated mutation): G-2-A; UAS-Akap200.DN (non-phosphorylated mutation): S-132/135/137-A; UAS-Akap200.CA (pseudo-phosphorylated mutation): S-132/135/137-D. Ubiquitous expression with Act5c-Gal4 of UAS-Akap200 transgenes, except UAS-Akap200S, caused lethality or severe motor impairment, and UAS-Akap200S suppressed UAS-Akap200L lethality, indicating that the transgenes expressed Akap200 proteins.
All strains were outcrossed for at least five generations to the Berlin genetic background. Ethanol vapor and humidified air were produced as previously described (Wolf et al., 2002). The experimenter was blinded to genotype for all behavioral tests. Groups of 20 male flies (for n = 1) were acclimated to a stream of humidified air for 5 min in the booz-o-mat and then exposed to a continuous stream of ethanol vapor. The flies were filmed for locomotion or counted for loss of the righting reflex for sedation (Wolf et al., 2002). Sedation sensitivity was the ST50 per group. Sedation tolerance was ST50 exposure 2 minus 1. We developed a miniaturized booz-o-mat that mounted atop a Peltier thermal controller (IC20; Torrey Pines Scientific) for TrpA1 behavior. Control and experimental groups were tested side-by-side and across multiple days to account for variation in behavior. see also Supplemental Experimental Procedures.
Statistical analysis was with GraphPad Prism v6.0. Typically, one-way ANOVA was used followed with Tukey's multiple-comparison test. If the data were not distributed normally (Brown-Forsythe test), we used the Kruskal-Wallis test followed with Dunn's multiple-comparisons test. t tests were two-tailed. Sample sizes were chosen based on prior experience with each experimental paradigm. All graphs show the mean and SEM.
Supplementary Material
Highlights.
Perineurial glia at blood-brain interface physically respond to ethanol
Akap200 anchoring is required in perineurial glia for ethanol behavioral plasticity
Akap200 localizes PKA to reorganize actin in response to ethanol
In vivo model for spatial and temporal regulation of PKA signaling by ethanol
Acknowledgments
We thank Eric C. Kong for technical assistance, Zhuhao Xu and Alex Kolodkin for the generous gift of the UAS-Akap200 transgenic flies, and Nestor Oviedo for help with dye injections. This work was supported by NIH Grants R01AA018799 and R21AA025560 (F.W.W.).
Footnotes
Supplemental information: Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.01.049.
Author Contributions: S.J.P, P.A., A.L., and F.W.W. designed the experiments. S.J.P, P.A., J.S.N., A.L., M.M., and F.W.W. conducted the experiments. S.J.P, P.A., A.L., and F.W.W. analyzed the results. F.W.W. prepared the figures and wrote the manuscript.
Declaration of Interests: The authors declare no conflicts of interest.
References
- Allansson L, Khatibi S, Olsson T, Hansson E. Acute ethanol exposure induces [Ca2+]i transients, cell swelling and transformation of actin cytoskeleton in astroglial primary cultures. J Neurochem. 2001;76:472–479. doi: 10.1046/j.1471-4159.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Barres BA. Neuroscience: glia—more than just brain glue. Nature. 2009;457:675–677. doi: 10.1038/457675a. [DOI] [PubMed] [Google Scholar]
- Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia. 2013;61:1939–1958. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SHR, Robitaille R, Volterra A. Gliotransmitters travel in time and space. Neuron. 2014;81:728–739. doi: 10.1016/j.neuron.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton RJ, Tsai LTY, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123:145–156. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Guerri C. Ethanol intake enhances inflammatory mediators in brain: role of glial cells and TLR4/IL-1RI receptors. Front Biosci. 2007;12:2616–2630. doi: 10.2741/2259. [DOI] [PubMed] [Google Scholar]
- Bonin CP, Mann RS. A piggyBac transposon gene trap for the analysis of gene expression and function in Drosophila. Genetics. 2004;167:1801–1811. doi: 10.1534/genetics.104.027557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull C, Syed WA, Minter SC, Bowers MS. Differential response of glial fibrillary acidic protein-positive astrocytes in the rat prefrontal cortex following ethanol self-administration. Alcohol Clin Exp Res. 2015;39:650–658. doi: 10.1111/acer.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo MK, Mayer N, Mayer F, Bainton RJ. Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila. Glia. 2011;59:1322–1340. doi: 10.1002/glia.21147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, Heberlein U. Preferential ethanol consumption in Drosophila models features of addiction. Curr Biol. 2009;19:2126–2132. doi: 10.1016/j.cub.2009.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, McClure KD, Guarnieri DJ, Corl AB, Wolf FW, Eddison M, Heberlein U. The genetic relationships between ethanol preference, acute ethanol sensitivity, and ethanol tolerance in Drosophila melanogaster. Fly (Austin) 2011;5:191–199. doi: 10.4161/fly.5.3.16987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evrard SG, Vega MD, Ramos AJ, Tagliaferro P, Brusco A. Altered neuron-glia interactions in a low, chronic prenatal ethanol exposure. Brain Res Dev Brain Res. 2003;147:119–133. doi: 10.1016/j.devbrainres.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR, Brusco A. A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that are not fully recovered even after a long abstinence: an immunohistochemical study. Exp Neurol. 2006;200:438–459. doi: 10.1016/j.expneurol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Fattore L, Puddu MC, Picciau S, Cappai A, Fratta W, Serra GP, Spiga S. Astroglial in vivo response to cocaine in mouse dentate gyrus: a quantitative and qualitative analysis by confocal microscopy. Neuroscience. 2002;110:1–6. doi: 10.1016/s0306-4522(01)00598-x. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Delrow J, Kim J, Johnson E, Doe CQ. Unwrapping glial biology: Gcm target genes regulating glial development, diversification, and function. Neuron. 2003;38:567–580. doi: 10.1016/s0896-6273(03)00289-7. [DOI] [PubMed] [Google Scholar]
- Ghezzi A, Atkinson NS. Homeostatic cont of neural activity: a Drosophila model for drug tolerance and dependence. In: Atkinson N, editor. International Review of Neurobiology. Academic Press; 2011. pp. 23–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Leo JT, O'Callaghan JP, Mahoney JC, West JR. Transient cortical astrogliosis induced by alcohol exposure during the neonatal brain growth spurt in rats. Brain Res Dev Brain Res. 1993;72:85–97. doi: 10.1016/0165-3806(93)90162-4. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Peterson SD, Lundahl KR, Pearlman AD. Binge-like alcohol exposure of neonatal rats via intragastric intubation induces both Purkinje cell loss and cortical astrogliosis. Alcohol Clin Exp Res. 1997;21:1010–1017. [PubMed] [Google Scholar]
- Guasch RM, Tomas M, Miñambres R, Valles S, Renau-Piqueras J, Guerri C. RhoA and lysophosphatidic acid are involved in the actin cytoskeleton reorganization of astrocytes exposed to ethanol. J Neurosci Res. 2003;72:487–502. doi: 10.1002/jnr.10594. [DOI] [PubMed] [Google Scholar]
- Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, Miller DW, Persidsky Y. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin Exp Res. 2005;29:999–1009. doi: 10.1097/01.alc.0000166944.79914.0a. [DOI] [PubMed] [Google Scholar]
- He J, Overstreet DH, Crews FT. Abstinence from moderate alcohol self-administration alters progenitor cell proliferation and differentiation in multiple brain regions of male and female P rats. Alcohol Clin Exp Res. 2009;33:129–138. doi: 10.1111/j.1530-0277.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- Hindle SJ, Bainton RJ. Barrier mechanisms in the Drosophila blood-brain barrier. Front Neurosci. 2014;8:414. doi: 10.3389/fnins.2014.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SM, Berg CA. An A-kinase anchoring protein is required for protein kinase A regulatory subunit localization and morphology of actin structures during oogenesis in Drosophila. Development. 2002;129:4423–4433. doi: 10.1242/dev.129.19.4423. [DOI] [PubMed] [Google Scholar]
- Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Hum Genet. 2012;131:959–975. doi: 10.1007/s00439-012-1146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong EC, Allouche L, Chapot PA, Vranizan K, Moore MS, Heberlein U, Wolf FW. Ethanol-regulated genes that contribute to ethanol sensitivity and rapid tolerance in Drosophila. Alcohol Clin Exp Res. 2010;34:302–316. doi: 10.1111/j.1530-0277.2009.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer MC, Jung C, Batelli S, Rubin GM, Gaul U. The glia of the adult Drosophila nervous system. Glia. 2017;65:606–638. doi: 10.1002/glia.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguesse S, Morisot N, Shin JH, Liu F, Adrover MF, Sakhai SA, Lopez MF, Phamluong K, Griffin WC, 3rd, Becker HC, et al. Prosa-pip1-dependent synaptic adaptations in the nucleus accumbens drive alcohol intake, seeking. and reward Neuron. 2017;96:145–159.e8. doi: 10.1016/j.neuron.2017.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CC, Kuo TI, Lin HH. The role of protein kinase A in acute ethanol-induced neurobehavioral actions in rats. Anesth Analg. 2007;105:89–96. doi: 10.1213/01.ane.0000263030.13249.36. [DOI] [PubMed] [Google Scholar]
- Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- Li Z, Rossi EA, Hoheisel JD, Kalderon D, Rubin CS. Generation of a novel A kinase anchor protein and a myristoylated alanine-rich C kinase substrate-like analog from a single gene. J Biol Chem. 1999;274:27191–27200. doi: 10.1074/jbc.274.38.27191. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Roberto M. Synaptic effects induced by alcohol. Curr Top Behav Neurosci. 2013;13:31–86. doi: 10.1007/7854_2011_143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci. 2009;29:3538–3550. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol Alcohol. 2006;41:379–385. doi: 10.1093/alcalc/agl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern A, Pfeiffer BD, Rubin GM. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci USA. 2015;112:E2967–E2976. doi: 10.1073/pnas.1506763112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, Crews FT. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiol Dis. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojelade SA, Acevedo SF, Kalahasti G, Rodan AR, Rothenfluh A. RhoGAP18B Isoforms Act on distinct rho-family GTPases and regulate behavioral responses to alcohol via cofilin. PLoS One. 2015a;10:e0137465. doi: 10.1371/journal.pone.0137465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojelade SA, Jia T, Rodan AR, Chenyang T, Kadrmas JL, Cattrell A, Ruggeri B, Charoen P, Lemaitre H, Banaschewski T, et al. IMAGEN Consortium. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc Natl Acad Sci USA. 2015b;112:E4085–E4093. doi: 10.1073/pnas.1417222112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J, He Y, Xiao X, Yu TM, Chen C, Gao Z, Ho MS. Glial cells in neuronal development: recent advances and insights from Drosophila melanogaster. Neurosci Bull. 2014;30:584–594. doi: 10.1007/s12264-014-1448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin Exp Res. 2003;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Park SK, Sedore SA, Cronmiller C, Hirsh J. Type II cAMP-dependent protein kinase-deficient Drosophila are viable but show developmental, circadian, and drug response phenotypes. J Biol Chem. 2000;275:20588–20596. doi: 10.1074/jbc.M002460200. [DOI] [PubMed] [Google Scholar]
- Peng C, Li WA, Fu P, Chakraborty T, Hussain M, Guthikonda M, Rafols JA, Ding Y. At low doses ethanol maintains blood-brain barrier (BBB) integrity after hypoxia and reoxygenation: a brain slice study. Neurol Res. 2013;35:790–797. doi: 10.1179/1743132813Y.0000000198. [DOI] [PubMed] [Google Scholar]
- Peru Y Coló n de Portugal RL, Ojelade SA, Penninti PS, Dove RJ, Nye MJ, Acevedo SF, Lopez A, Rodan AR, Rothenfluh A. Long-lasting, experience-dependent alcohol preference in Drosophila. Addict Biol. 2014;19:392–401. doi: 10.1111/adb.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petley-Ragan LM, Ardiel EL, Rankin CH, Auld VJ. Accumulation of laminin monomers in Drosophila glia leads to glial endoplasmic reticulum stress and disrupted larval locomotion. J Neurosci. 2016;36:1151–1164. doi: 10.1523/JNEUROSCI.1797-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Varodayan FP, Tannenholz LE, Protiva P, Harrison NL. Brief alcohol exposure alters transcription in astrocytes via the heat shock pathway. Brain Behav. 2013;3:114–133. doi: 10.1002/brb3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16:522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Rodan AR, Kiger JA, Jr, Heberlein U. Functional dissection of neuroanatomical loci regulating ethanol sensitivity in Drosophila. J Neurosci. 2002;22:9490–9501. doi: 10.1523/JNEUROSCI.22-21-09490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Barak S. Molecular mechanisms underlying alcohol-drinking behaviours. Nat Rev Neurosci. 2016;17:576–591. doi: 10.1038/nrn.2016.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Messing RO. Signaling pathways mediating alcohol effects. Curr Top Behav Neurosci. 2013;13:87–126. doi: 10.1007/7854_2011_161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EA, Li Z, Feng H, Rubin CS. Characterization of the targeting, binding, and phosphorylation site domains of an A kinase anchor protein and a myristoylated alanine-rich C kinase substrate-like analog that are encoded by a single gene. J Biol Chem. 1999;274:27201–27210. doi: 10.1074/jbc.274.38.27201. [DOI] [PubMed] [Google Scholar]
- Rothenfluh A, Cowan CW. Emerging roles of actin cytoskeleton regulating enzymes in drug addiction: actin or reactin'? Curr Opin Neurobiol. 2013;23:507–512. doi: 10.1016/j.conb.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Araiz A, Porcu F, Pérez-Hernández M, García-Gutiérrez MS, Aracil-Fernández MA, Gutierrez-López MD, Guerri C, Manzanares J, O'Shea E, Colado MI. Disruption of blood-brain barrier integrity in postmortem alcoholic brain: preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict Biol. 2017;22:1103–1116. doi: 10.1111/adb.12376. [DOI] [PubMed] [Google Scholar]
- Ruppert M, Franz M, Saratsis A, Velo Escarcena L, Hendrich O, Gooi LM, Schwenkert I, Klebes A, Scholz H. Hangover links nuclear RNA signaling to cAMP regulation via the phosphodiesterase 4d ortholog dunce. Cell Rep. 2017;18:533–544. doi: 10.1016/j.celrep.2016.12.048. [DOI] [PubMed] [Google Scholar]
- Schott MB, Grove B. Receptor-mediated Ca2+ and PKC signaling triggers the loss of cortical PKA compartmentalization through the redistribution of gravin. Cell Signal. 2013;25:2125–2135. doi: 10.1016/j.cellsig.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrooke S, O'Donnell MJ. Oatp58Dc contributes to blood-brain barrier function by excluding organic anions from the Drosophila brain. Am J Physiol Cell Physiol. 2013;305:C558–C567. doi: 10.1152/ajpcell.00408.2012. [DOI] [PubMed] [Google Scholar]
- Spéder P, Brand AH. Gap junction proteins in the blood-brain barrier control nutrient-dependent reactivation of Drosophila neural stem cells. Dev Cell. 2014;30:309–321. doi: 10.1016/j.devcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klämbt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci. 2000;20:RC75. doi: 10.1523/JNEUROSCI.20-10-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkenhoff A, Weiler A, Letzel M, Stehling M, Klämbt C, Schirmeier S. Glial glycolysis is essential for neuronal survival in Drosophila. Cell Metab. 2015;22:437–447. doi: 10.1016/j.cmet.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Wand G, Levine M, Zweifel L, Schwindinger W, Abel T. The cAMP-protein kinase A signal transduction pathway modulates ethanol consumption and sedative effects of ethanol. J Neurosci. 2001;21:5297–5303. doi: 10.1523/JNEUROSCI.21-14-05297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf FW, Rodan AR, Tsai LTY, Heberlein U. High-resolution analysis of ethanol-induced locomotor stimulation in Drosophila. J Neurosci. 2002;22:11035–11044. doi: 10.1523/JNEUROSCI.22-24-11035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Chan T, Shah V, Zhang S, Pletcher SD, Roman G. The propensity for consuming ethanol in Drosophila requires rutabaga adenylyl cyclase expression within mushroom body neurons. Genes Brain Behav. 2012;11:727–739. doi: 10.1111/j.1601-183X.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.