Abstract
Hepatocyte growth factor (HGF) is a glycoprotein produced by mesenchymal cells and operates as a key molecule for tissue generation and renewal. During corneal injury, HGF is primarily secreted by stromal fibroblasts and promotes epithelial wound healing in a paracrine manner. While this mesenchymal-epithelial interaction is well characterized in various organs and the cornea, the role of HGF in corneal stromal and endothelial wound healing is understudied. In addition, HGF has been shown to play an anti-fibrotic role by inhibiting myofibroblast generation and subsequent production of a disorganized extracellular matrix and tissue fibrosis. Therefore, HGF represents a potential therapeutic tool in numerous organs in which myofibroblasts are responsible for tissue scarring. Corneal fibrosis can be a devastating sequella of injury and can result in corneal opacification and retrocorneal membrane formation leading to severe vision loss. In this article, we concisely review the available literature regarding the role of HGF in corneal wound healing. We highlight the influence of HGF on cellular behaviors in each corneal layer. Additionally, we suggest the possibility that HGF may represent a therapeutic tool for interrupting dysregulated corneal repair processes to improve patient outcomes.
Keywords: HGF, wound healing, myofibroblast, fibrosis, TGF-β
1. Introduction
The cornea is a protective barrier and the primary refractive element of the visual system. The cornea generally consists of four transparent and avascular layers: epithelium, stroma, Descemet's membrane and endothelium. Additionally, in some species (e.g. humans, lizards, birds), the cornea can possess a Bowman's layer, a thickened acellular collagenous zone that lies between the epithelium and stroma. The cornea is continuously subjected to physical, chemical and biological insults from the external environment that can result in wounding. Corneal injury by trauma, infection, or surgery initiates multiple complex cellular processes including cell migration, proliferation, re-stratification, as well as deposition of extracellular matrix (ECM) and tissue remodeling which are coordinated to restore a healthy and functional cornea. These wound healing processes are regulated by numerous soluble cytoactive factors, the intrinsic chemistry of ECM elements as well as by biophysical attributes of the microenvironment of corneal cells. Soluble cytoactive factors include growth factors, cytokines, proteases, and neuropeptides. These factors work through autocrine and paracrine mechanisms and are derived from epithelial cells, stromal fibroblasts, corneal nerves, lacrimal glands, and cells of the immune system (Ljubimov and Saghizadeh, 2015).
Hepatocyte growth factor (HGF) is one of the growth factors which mediate tissue regeneration in numerous organs (Nakamura and Mizuno, 2010). The liver is a potently regenerative organ, which can renew even after removal of two-thirds of its volume. The factors controlling this process have been heavily studied (Nakamura and Mizuno, 2010; Nakamura et al., 2011). In 1984, HGF was identified in rat platelets as a potent mitogenic factor for hepatocytes in vitro (Nakamura et al., 1984; Russell et al., 1984). A few years later, scatter factor, originally identified as a protein which modulates cell motility of renal tubular cells (Stoker et al., 1987; Weidner et al., 1991), was shown to be structurally identical to HGF. Tumor cytotoxic factor, a fibroblast-derived factor that induces cell death for some kinds of cancer cells, was also shown to be indistinguishable from HGF (Shima et al., 1991). In aggregate, these various functions demonstrate the diverse biologic roles that HGF can assume depending on the target tissue of interest.
Hepatocyte growth factor is primarily secreted by mesenchymal cells and stimulates morphogenesis, migration, proliferation and survival of epithelial cells that express its specific receptor, c-Met (Montesano et al., 1991; Sonnenberg et al., 1993; Matsumoto and Nakamura, 1996; Birchmeier and Gherardi, 1998). Hepatocyte growth factor is known as a key mediator for organ generation and maturation at defined stages of development (Schmidt et al., 1995; Uehara et al., 1995; Bladt et al., 1995). In addition to organ development, proliferative activities of epithelial cells are critical for wound healing (Yoshida et al., 2003; Chmielowiec et al., 2007). Though there are numerous reports regarding the importance of HGF in wound healing processes in an array of organs (Conway et al., 2006; Nakamura and Mizuno, 2010), its role in corneal biology and repair has been understudied. Here, we concisely summarize the available literature regarding the role of HGF in corneal homeostasis and wound healing and discuss its potential as a therapeutic tool in the management of corneal fibrosis.
2. Structure of HGF
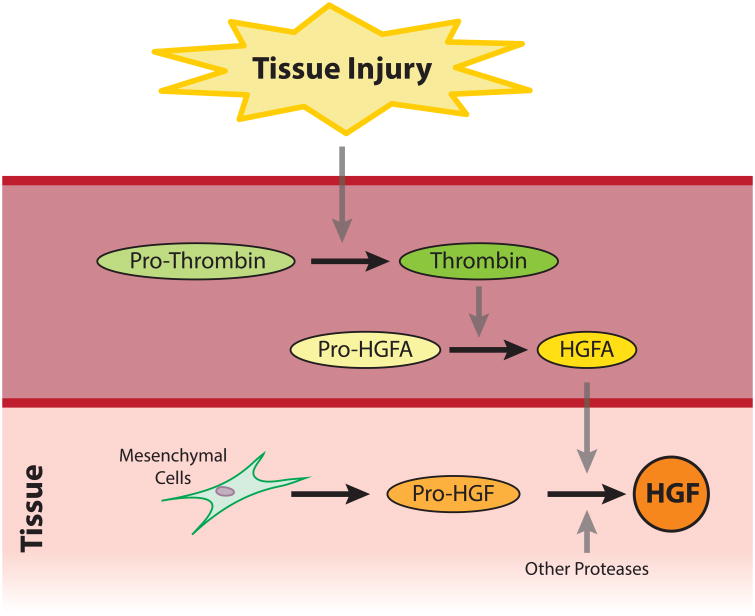
The primary structure of HGF was determined in 1989 (Nakamura et al., 1989; Miyazawa et al., 1989; Tashiro et al., 1990), and multiple splice variants encoding different isoforms have been subsequently reported (Schultz et al., 2009). Hepatocyte growth factor is synthesized in an inactive pre-pro form, consisting of 728 amino acids, and secreted by mesenchymal cells such as fibroblasts and macrophages. Inactive HGF becomes activated through two-cleavage processes. First, the signal peptide comprised of the first 31 amino acids is degraded, generating the pro-HGF. The single-chain pro-HGF is subsequently cleaved between Arg 494 and Val 495 by serine proteases. Numerous serum and cell-membrane proteases are involved in this cleaving process, including HGF activator (HGFA), urokinase- and tissue-type plasminogen activator (u-PA and t-PA), plasma kallikrein, coagulation factors XII and XI, matriptase and hepsin (Miyazawa et al., 1993; Tamagnone and Comoglio, 1997; Lee et al., 2000; Herter et al., 2005). Matriptase and hepsin are transmembrane proteases involved in pericellular activation of HGF, whereas the other proteases are resident in serum. In vascularized tissues, HGFA is the primary HGF protease and is also regulated by proteolytic cleavage in response to injury (Conway et al., 2006; Miyazawa, 2010; Kataoka and Kawaguchi, 2010; Rodgers et al., 2017). Inactive pro-HGFA is produced by hepatocytes in the liver and circulates in serum (Shimomura et al., 1993; Okajima et al., 1997). Upon tissue injury, activated thrombin, which plays to prevent further hemorrhage in blood coagulation system, concomitantly activates HGFA (Fig. 1). Therefore, HGFA represents the link between tissue injury and activation of HGF (Miyazawa, 2010). The activation process in avascular tissues such as the cornea is less well understood and remains understudied. Amongst these proteases, u-PA and t-PA are known to be present in the cornea and t-PA is found in tears (Geanon et al., 1987; Stevens et al., 1992; Watanabe et al., 2003; Warejcka et al., 2011). These proteases represent the most likely candidates to activate HGF through enzymatic processing (Mars et al., 1993). While the cleaving activities of u-PA and t-PA are weak in vitro in comparison to other proteases such as HGFA, matriptase, and hepsin, their activity may be amplified by the in vivo microenvironment following wounding (Naldini et al., 1995; Grierson et al., 2000). Additional studies are required to better define the activation process of HGF in the cornea in health and disease.
Fig. 1. The Activating Process of HGF upon the Tissue Injury.
Tissue injury activates the blood coagulation system leading to conversion of pro-thrombin to thrombin to form blood clots and prevent further hemorrhage. Concomitantly, thrombin activates HGFA by processing an enzymatically inactive pro-HGFA produced by hepatocytes in the liver into an active HGFA that possesses HGF-processing enzymatic activity. Therefore, HGFA represents the link between tissue injury and activation of HGF. This diagram was modified after Conway et al., 2006.
Mature HGF is a heterodimeric molecule consisting of a 69 kDa α-chain and a 34 kDa β-chain linked by a disulfide bond. The C-terminus of the α-chain is followed directly by the N-terminus of β-chain. The α-chain has a high affinity for c-Met, but the activation of the HGF/c-Met signaling is dependent on the subsequent binding of the β-chain (Hartmann et al., 1992; Matsumoto et al., 1998; Gherardi et al., 2006; Merchant et al., 2013). The binding of HGF to c-Met induces phosphorylation of tyrosine residues of intracellular tyrosine kinase domain of c-Met, which results in biological activities including mitogenic, motogenic and morphogenic activities via downstream signaling pathways (Birchmeier and Gherardi, 1998; Nakamura et al., 2011).
3. Corneal epithelial wound healing and HGF
The anterior corneal epithelium is a stratified, squamous, non-keratinized epithelium. Surface cells make tight junctional complexes between their neighbors, which create the first barrier against the external environment. Like other epithelial barriers in the human body, the corneal epithelium is a self-renewing tissue with a distinct stem cell niche residing in the limbal basal region to provide an unlimited supply of proliferating cells for epithelial regeneration (Schermer et al., 1986; Cotsarelis et al., 1989; Li et al., 2007a; Xie et al., 2011). Proper healing of the corneal epithelium is important for maintenance of transparency and thus for preserving vision. The corneal epithelium is subjected continuously to physical, chemical, and biological insults that can result in frank defects and loss of its barrier function (Lu et al., 2001). Corneal epithelial cells respond rapidly to injury, proliferating and migrating to cover the defect and to re-establish its barrier function. This process requires the coordinated interaction of numerous growth factors and cytokines, including transforming growth factor (TGF-β), platelet derived growth factor (PDGF), fibroblast growth factor (FGF), insulin-like growth factor (IGF-1) and epidermal growth factor (EGF), secreted by epithelial cells (Pancholi et al., 1998; Andresen and Ehlers, 1998; Yu et al., 2010). Furthermore, HGF and keratinocyte growth factor (KGF) are secreted by fibroblasts following epithelial injury, and contribute to re-epithelialization via their individual receptors expressed in epithelial cells (Wilson et al., 1994; Wilson et al., 1999a). While HGF receptor c-Met is highly expressed in central corneal cells, the KGF receptor is more abundant in limbal cells (Li and Tseng, 1995; Li et al., 1996).
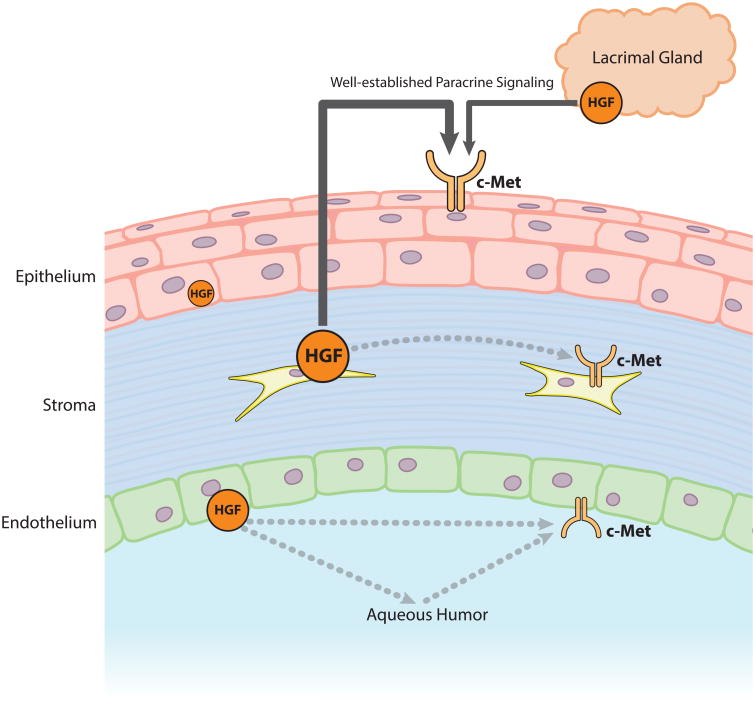
Hepatocyte growth factor and c-Met are expressed in the corneal epithelium, stromal cells, endothelium, as well as in the lacrimal grand, although the amount of HGF in the epithelium appears to be extremely low (Wilson et al., 1993; Wilson et al., 1994; Li et al., 1996; Wilson et al., 1999b). Human tears also contain about 500 pg/ml of HGF, which is derived from corneal stromal cells and the lacrimal grand (Li et al., 1996; Tervo et al., 1997). The sources of HGF in the cornea and its surroundings are depicted in Fig. 2.
Fig. 2. The Sources of HGF in the Cornea.
Hepatocyte growth factor and c-Met are expressed in the corneal epithelium, stromal cells, endothelium, as well as in the lacrimal grand. The size of the HGF and c-Met icons and width of arrows represent relative contributions. In addition to its classical paracrine mechanism, this expression pattern shows that HGF has the potential to act in an autocrine manner.
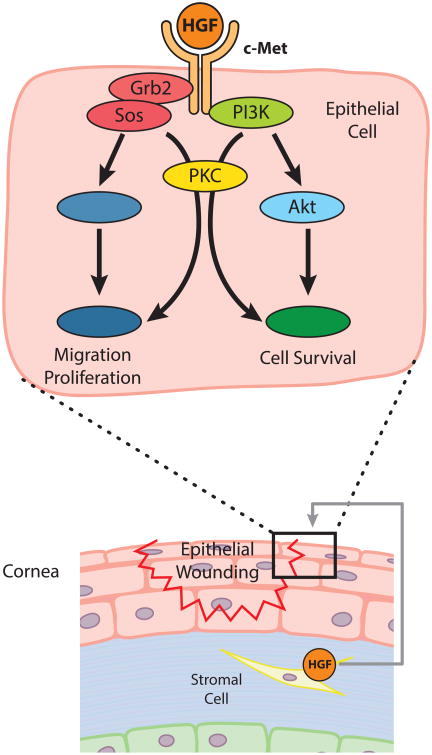
In corneal epithelial healing, HGF acts as a paracrine growth factor mediating mesenchymal-epithelial interactions. The binding of HGF to c-Met activates mitogen activated protein kinase (MAPK) pathways in human corneal epithelial cells via the receptor-Grb2/Sos complex to the Ras pathway or through protein kinase C (PKC) (Liang et al., 1998). Phosphatidylinositol-3 kinase (PI3K) and p70 S6 kinase (S6K), which are regulated by PKC or Akt (also known as protein kinase B), are also critical for epithelial cell survival (Chandrasekher et al., 2001; Kakazu et al., 2004). The scheme of HGF signaling in corneal epithelial cells during wound healing is depicted in Fig. 3. Additionally, HGF is also known to induce cell motility through transactivation of the EGF receptor (Spix et al., 2007).
Fig. 3. The Role of HGF in Epithelial-Mesenchymal Crosstalk in Corneal Epithelial Wound Healing.
Upon epithelial wounding, HGF mRNA is highly induced in stromal fibroblasts, while the expression of c-Met is upregulated in epithelial cells. The binding of HGF to c-Met activates the MAPK pathway via Grb2/Sos complex to the Ras or through PKC to promote epithelial wound healing. PI3K-S6K pathway mediated by PKC or Akt is another route influenced by HGF that promotes epithelial cell survival.
Hepatocyte growth factor also facilitates corneal epithelial cell migration (Daniels et al., 2003; McBain et al., 2003), proliferation (Wilson et al., 1993; Yanai et al., 2006), and inhibits apoptosis (Kakazu et al., 2004). These activities suggest that HGF is capable of enhancing epithelial wound healing (Chandrasekher et al., 2001). However, in one study employing an ex vivo bovine corneal model, the retardation of re-epithelialization by HGF was reported (Carrington and Boulton, 2005). Thus, in vivo studies are required to further elucidate the effect of HGF on corneal epithelial wound healing.
4. Corneal stromal wound healing and HGF
The stroma of the cornea is a highly organized ECM comprised of a network of a heterodimeric complex of Type I and Type V collagen fibers, containing water, inorganic salts, proteoglycans, and glycoproteins (Birk et al., 1986; Birk, 2001). Keratocytes are the primary cells of the corneal stroma and serve to maintain the extracellular environment by synthesizing collagen molecules and glycosaminoglycans, and remodeling the stroma with matrix metalloproteinases (MMPs) that are crucial to stromal homeostasis and ECM renewal (DelMonte and Kim, 2011).
Corneal stromal wounding typically results in direct damage to both stromal and epithelial elements. Wounding triggers a release of inflammatory cytokines from epithelial cells, mainly interleukin-1 (IL-1) which induces apoptosis of anterior keratocytes expressing the IL-1 receptor (Wilson et al., 1996; Wilson et al., 2001; Wilson et al., 2007). Upon stromal injury, keratocytes differentiate into spindle-shaped fibroblasts which acquire a migratory phenotype through the increased expression of actin, to generate traction forces enabling them to proliferate and migrate towards the region of injury, repopulating the region that had been depleted of keratocytes through apoptosis (Moller-Pedersen et al., 1998; Zieske et al., 2001; Hinz et al., 2001b). As described above, corneal wounding leads to epithelial cells secretion of growth factors and cytokines, including TGF-β, PDGF, FGF-2, IGF-1 and EGF, which have all been implicated in this differentiation (Funderburgh et al., 2001; Maltseva et al., 2001; Jester and Ho-Chang, 2003; Musselmann et al., 2005; He and Bazan, 2008). In the process of transdifferentiation to activated fibroblasts, there is downregulation of keratocyte proteins, such as corneal crystallins and keratan sulfate proteoglycans, and the simultaneous initiation of increased proteinase activity (mostly MMPs) necessary to remodel the wounded ECM (Fini, 1999; Jester et al., 1999; Carlson et al., 2003; West-Mays and Dwivedi, 2006).
Upon arrival at the corneal wound bed, fibroblasts differentiate into myofibroblasts that elaborate ECM and generate contractile forces engaged in corneal wound closure (Ishizaki et al., 1993; Petroll et al., 1993; Jester et al.,1995; Kurosaka et al., 1998). Myofibroblasts are characterized by the expression of α-smooth muscle actin (α-SMA) whose expression directly correlates with corneal wound contraction (Jester et al., 1995). Keratocyte-fibroblast-myofibroblast (KFM) transformation is triggered by TGF-β1 and PDGF (Jester et al., 1999; Carrington et al., 2006; Kaur et al., 2009; Singh et al., 2014).
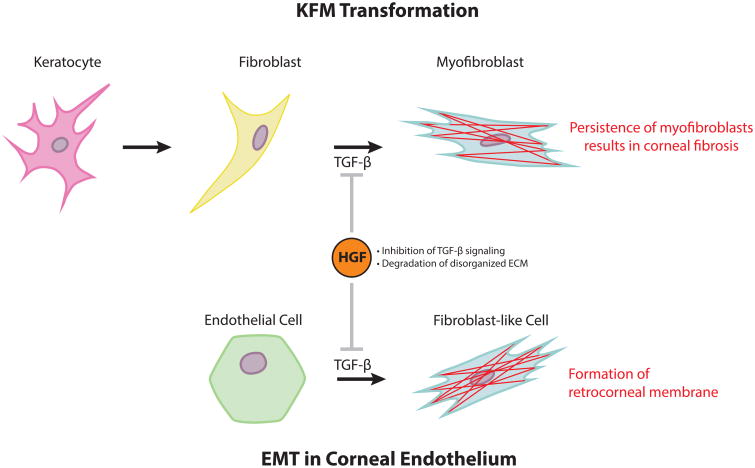
Upon proper healing, the corneal stroma is remodeled so that its arrays of collagen lamellae are orderly to ensure transparency. However, multiple reports document long-term corneal opacity from excessive numbers and/or prolonged persistence of myofibroblasts after healing (Wilson et al., 2001; Ljubimov and Saghizadeh, 2015). Myofibroblasts are themselves opaque and produce a disorganized ECM, leading to the development of corneal stromal opacity and fibrosis. If the epithelial basement membrane was ablated upon initial wounding, fibrosis is often more severe than if the basement membrane was left intact (Fini and Stramer, 2005; West-Mays and Dwivedi, 2006; Torricelli et al., 2016). Hepatocyte growth factor is a well-known antifibrotic molecule that counteracts TGF-β1 to reduce fibrosis in various organs (Dai and Liu, 2004; Mizuno and Nakamura, 2004; Okayama et al., 2012). Specifically, HGF activates Smad7 which prevents Smad2 phosphorylation resulting in inhibition of the TGF-β signaling pathway (Shukla et al., 2009; Yong et al., 2016). Additionally, HGF promotes apoptosis of myofibroblasts by inducing MMPs to degrade the ECM in general including fibronectin, specifically, which is an essential anchor for myofibroblasts (Pepper et al., 1992; Mizuno et al., 2005). A recent study documented that the administration of HGF can restore corneal transparency after wounding in a murine model (Mittal et al., 2016). Therefore, exogenous HGF represents a potential therapeutic tool in promoting improved corneal stromal wound healing and patient outcomes (Fig. 4).
Fig. 4. HGF represents a potential therapeutic tool to minimize corneal fibrosis.
In the cornea, TGF-β induces a myofibroblast-phenotype following KFM transformation in the stroma and EMT in the endothelium, which can result in corneal fibrosis and retrocorneal membrane formation, respectively. Hepatocyte growth factor could play an antifibrotic role to counteract TGF-β promotion of myofibroblast generation by activating Smad7, an inhibitory Smad. Additionally, HGF promotes apoptosis of myofibroblasts by inducing MMPs which degrade the ECM including fibronectin, which is an essential anchor for myofibroblasts.
In recent years, our lab has focused its investigation on the impact of the biophysical attributes of the microenvironment of corneal cells on wound healing, and shown that biophysical cues represent potent modulators of KFM transformation (Myrna et al., 2009; Pot et al., 2010; Myrna et al., 2012; Dreier et al., 2013). Thus, HGF may be capable of affecting corneal stiffness by inhibiting the myofibroblast phenotype or degrading ECM through the induction of MMPs and u-PA (Ueki et al., 1999; Ono et al., 2004; Mizuno et al., 2005; Kim et al., 2005). The effect of HGF on the biophysical attributes of the cornea and KFM transformation represents a promising avenue to explore with this molecule to increase our understanding of compounds that may reverse unwanted fibrotic scars.
In addition to its classical paracrine mechanism, HGF may act on corneal fibroblasts in an autocrine manner. The c-Met receptor is expressed in not only corneal epithelium but also in corneal stromal and endothelial cells (Wilson et al., 1993) (Fig. 2). While HGF does not induce proliferation of corneal fibroblasts, an autocrine HGF/c-Met loop is known to operate in other cell types (Sheehan et al., 2000; Warn et al., 2001; Xie et al., 2001; Kawase et al., 2006). Thus, it remains poorly understood how endogenous HGF may exert its effects in corneal stromal wound repair.
5. Corneal endothelial wound healing and HGF
The intact human corneal endothelium is a layer of simple cuboidal cells, which appears as a honeycomb-like mosaic when viewed from the posterior aspect. Corneal endothelial cells are essential to maintain corneal transparency through preservation of stromal deturgescence. Damage to the corneal endothelium can be inflicted both directly by trauma, corneal endotheliitis and surgical removal of dysfunctional endothelium or indirectly by cataract surgery. Corneal endothelial cells exhibit certain peculiarities in their healing processes. Specifically, in vivo, with few exceptions, corneal endothelial cells have a very low regenerative capacity, and typically fill any areas devoid of cells by migration and increased cell spreading (Joyce et al., 1990; Ichijima et al., 1993; Mimura et al., 2013). Recently, corneal endothelial cells have been found to be capable of proliferative capacity under certain conditions in vitro (Nayak and Binder, 1984; Blake et al., 1997; Senoo et al., 2000; Li et al., 2007b; Okumura et al., 2009), and inhibition of Rho kinase has been reported to be able to stimulate the proliferation of corneal endothelial cells in vivo (Koizumi et al., 2014; Okumura et al., 2016). Similar to other corneal cells, corneal endothelial cells express mRNAs for HGF and c-Met, and the addition of HGF to culture medium stimulates endothelial cell proliferation (Wilson et al., 1993). One recent study supports the possibility that HGF acts on c-Met of corneal endothelial cells and promote their growth in an autocrine manner (Kimoto et al., 2012), as described above. Also, HGF is found in the aqueous humor, and its concentration is correlated with corneal endothelial cell density (Araki-Sasaki et al., 1997; Grierson et al., 2000), suggesting that corneal endothelial cells may contribute to aqueous HGF. Therefore, HGF is thought to be capable of maintaining corneal endothelial cells not only in vitro but also in vivo.
In cases of severe corneal endothelial injury such as alkaline burns and syphilitic interstitial keratitis, corneal endothelial cells can undergo epithelial-mesenchymal transformation (EMT) (Ishizaki et al., 1993; Saika et al., 1993; Kawaguchi et al., 2001). Additionally, cultured corneal endothelial cells can result in a phenotypic switch that changes their morphology from polygonal to spindle-shaped in vitro (Peh et al., 2013; Okumura et al., 2013; Roy et al., 2015). In a model of freeze injury, EMT occurs along the migrating front, whereby cells lose the tight junction protein ZO-1 and begin expressing α-SMA (Petroll et al., 1997). These findings suggest that corneal endothelial cells, like KFM transformation in corneal stroma or EMT in epithelial cells, require a transient acquisition of a fibroblast-like morphology and actin stress fibers for migration to close the wound gap (Hinz et al., 2001a). A potent inducer of EMT is TGF-β which leads to abnormal ECM accumulation and production of a fibrous retrocorneal membrane on the posterior surface of the Descemet's membrane (Sumioka et al., 2008; Miyamoto et al., 2010). The overexpression of Smad7, an inhibitor of TGF-β signaling, can suppress corneal endothelial fibrosis without compromising endothelial wound healing (Sumioka et al., 2008). Therefore, exogenous HGF holds promise as a therapeutic agent to prevent fibrogenic EMT and the formation of retrocorneal membranes via Smad7 activation (Shukla et al., 2009; Yong et al., 2016) (Fig. 4).
6. Conclusion
In this review, we have highlighted the roles of HGF in the normal cornea as well as during corneal wound healing. Hepatocyte growth factor is mainly secreted by fibroblasts, and accelerates proliferative activities of epithelial and endothelial cells. Besides operating as a key molecule in corneal wound healing state, the ability of HGF to modulate the transdifferentiation of cells implicated in the development of fibrosis motivates its investigation as a potential therapeutic tool to minimize corneal fibrosis and improve wound healing outcomes. While corneal cells in each layer respectively have nuances to their engagement in wound healing processes, corneal stromal and endothelial cells share the involvement of the myofibroblast phenotype to close a wound gap. Since TGF-β is one of the strongest profibrotic factors inducing differentiation to myofibroblasts, the inhibition of TGF-β activation by HGF presents a promising tool to ameliorate fibrosis.
Highlights.
HGF directs mesenchymal-epithelial interaction in corneal wound healing.
The persistence of the myofibroblast phenotype results in corneal fibrosis.
HGF can improve corneal fibrosis by inhibiting the myofibroblast phenotype.
HGF is also involved in maintaining corneal endothelial cells in vivo.
Corneal cells need the myofibroblast phenotype to close a wound gap.
Acknowledgments
This study was funded by grants from the National Institute of Health K08 EY021142, R01 EY019970, R01 EY016134, and P30 EY12576.
Abbreviations
- ECM
extracellular matrix
- HGF
hepatocyte growth factor
- HGFA
hepatocyte growth factor activator
- u-PA
urokinase-type plasminogen activator
- t-PA
tissue-type plasminogen activator
- TGF-β
transforming growth factor
- PDGF
platelet derived growth factor
- FGF
fibroblast growth factor
- IGF-1
insulin-like growth factor-1
- EGF
epidermal growth factor
- KGF
keratinocyte growth factor
- MAPK
Ras-mitogen activated protein kinase
- PKC
protein kinase C
- PI3K
phosphatidylinositol-3 kinase
- S6K
p70 S6 kinase
- MMP
matrix metalloproteinase
- IL-1
interleukin-1
- α-SMA
α-smooth muscle actin
- KFM
keratocyte-fibroblast-myofibroblast
- EMT
epithelial-mesenchymal transformation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res. 1998;17(1):79–87. doi: 10.1076/ceyr.17.1.79.5261. [DOI] [PubMed] [Google Scholar]
- Araki-Sasaki K, Danjo S, Kawaguchi S, Hosohata J, Tano Y. Human hepatocyte growth factor (HGF) in the aqueous humor. Jpn J Ophthalmol. 1997;41(6):409–413. doi: 10.1016/s0021-5155(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8(10):404–410. doi: 10.1016/s0962-8924(98)01359-2. [DOI] [PubMed] [Google Scholar]
- Birk DE. Type V collagen: heterotypic type I/V collagen interactions in the regulation of fibril assembly. Micron. 2001;32(3):223–237. doi: 10.1016/s0968-4328(00)00043-3. [DOI] [PubMed] [Google Scholar]
- Birk DE, Fitch JM, Linsenmayer TF. Organization of collagen types I and V in the embryonic chicken cornea. Invest Ophthalmol Vis Sci. 1986;27(10):1470–1477. [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376(6543):768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Blake DA, Yu H, Young DL, Caldwell DR. Matrix stimulates the proliferation of human corneal endothelial cells in culture. Invest Ophthalmol Vis Sci. 1997;38(6):1119–1129. [PubMed] [Google Scholar]
- Carlson EC, Wang IJ, Liu CY, Brannan P, Kao CW, Kao WW. Altered KSPG expression by keratocytes following corneal injury. Mol Vis. 2003;9:615–623. [PubMed] [Google Scholar]
- Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006;47(5):1886–1894. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- Carrington LM, Boulton M. Hepatocyte growth factor and keratinocyte growth factor regulation of epithelial and stromal corneal wound healing. J Cataract Refract Surg. 2005;31(2):412–423. doi: 10.1016/j.jcrs.2004.04.072. [DOI] [PubMed] [Google Scholar]
- Chandrasekher G, Kakazu AH, Bazan HE. HGF- and KGF-induced activation of PI-3K/p70 s6 kinase pathway in corneal epithelial cells: its relevance in wound healing. Exp Eye Res. 2001;73(2):191–202. doi: 10.1006/exer.2001.1026. [DOI] [PubMed] [Google Scholar]
- Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W. c-Met is essential for wound healing in the skin. J Cell Biol. 2007;177(1):151–162. doi: 10.1083/jcb.200701086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway K, Price P, Harding KG, Jiang WG. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006;14(1):2–10. doi: 10.1111/j.1743-6109.2005.00081.x. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57(2):201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15(6):1402–1412. doi: 10.1097/01.asn.0000130568.53923.fd. [DOI] [PubMed] [Google Scholar]
- Daniels JT, Limb GA, Saarialho-Kere U, Murphy G, Khaw PT. Human corneal epithelial cells require MMP-1 for HGF-mediated migration on collagen I. Invest Ophthalmol Vis Sci. 2003;44(3):1048–1055. doi: 10.1167/iovs.02-0442. [DOI] [PubMed] [Google Scholar]
- DelMonte DW, Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37(3):588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- Dreier B, Thomasy SM, Mendonsa R, Raghunathan VK, Russell P, Murphy CJ. Substratum compliance modulates corneal fibroblast to myofibroblast transformation. Invest Ophthalmol Vis Sci. 2013;54(8):5901–5907. doi: 10.1167/iovs.12-11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999;18(4):529–551. doi: 10.1016/s1350-9462(98)00033-0. [DOI] [PubMed] [Google Scholar]
- Fini ME, Stramer BM. How the cornea heals: cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005;24(8 Suppl):S2–s11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta – induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001;276(47):44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geanon JD, Tripathi BJ, Tripathi RC, Barlow GH. Tissue plasminogen activator in avascular tissues of the eye: a quantitative study of its activity in the cornea, lens, and aqueous and vitreous humors of dog, calf, and monkey. Exp Eye Res. 1987;44(1):55–63. doi: 10.1016/s0014-4835(87)80025-8. [DOI] [PubMed] [Google Scholar]
- Gherardi E, Sandin S, Petoukhov MV, Finch J, Youles ME, Ofverstedt LG, Miguel RN, Blundell TL, Vande Woude GF, Skoglund U, Svergun DI. Structural basis of hepatocyte growth factor/scatter factor and MET signalling. Proc Natl Acad Sci U S A. 2006;103(11):4046–4051. doi: 10.1073/pnas.0509040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson I, Heathcote L, Hiscott P, Hogg P, Briggs M, Hagan S. Hepatocyte growth factor/scatter factor in the eye. Prog Retin Eye Res. 2000;19(6):779–802. doi: 10.1016/s1350-9462(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Hartmann G, Naldini L, Weidner KM, Sachs M, Vigna E, Comoglio PM, Birchmeier W. A functional domain in the heavy chain of scatter factor/hepatocyte growth factor binds the c-Met receptor and induces cell dissociation but not mitogenesis. Proc Natl Acad Sci U S A. 1992;89(23):11574–11578. doi: 10.1073/pnas.89.23.11574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Bazan HE. Epidermal growth factor synergism with TGF-beta1 via PI-3 kinase activity in corneal keratocyte differentiation. Invest Ophthalmol Vis Sci. 2008;49(7):2936–2945. doi: 10.1167/iovs.07-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herter S, Piper DE, Aaron W, Gabriele T, Cutler G, Cao P, Bhatt AS, Choe Y, Craik CS, Walker N, Meininger D, Hoey T, Austin RJ. Hepatocyte growth factor is a preferred in vitro substrate for human hepsin, a membrane-anchored serine protease implicated in prostate and ovarian cancers. Biochem J. 2005;390(Pt 1):125–136. doi: 10.1042/BJ20041955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001a;12(9):2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001b;159(3):1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichijima H, Petroll WM, Barry PA, Andrews PM, Dai M, Cavanagh HD, Jester JV. Actin filament organization during endothelial wound healing in the rabbit cornea: comparison between transcorneal freeze and mechanical scrape injuries. Invest Ophthalmol Vis Sci. 1993;34(9):2803–2812. [PubMed] [Google Scholar]
- Ishizaki M, Zhu G, Haseba T, Shafer SS, Kao WW. Expression of collagen I, smooth muscle alpha-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993;34(12):3320–3328. [PubMed] [Google Scholar]
- Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77(5):581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha-smooth muscle (alpha-SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995;36(5):809–819. [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res. 1999;18(3):311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Joyce NC, Meklir B, Neufeld AH. In vitro pharmacologic separation of corneal endothelial migration and spreading responses. Invest Ophthalmol Vis Sci. 1990;31(9):1816–1826. [PubMed] [Google Scholar]
- Kakazu A, Chandrasekher G, Bazan HE. HGF protects corneal epithelial cells from apoptosis by the PI-3K/Akt-1/Bad- but not the ERK1/2-mediated signaling pathway. Invest Ophthalmol Vis Sci. 2004;45(10):3485–3492. doi: 10.1167/iovs.04-0372. [DOI] [PubMed] [Google Scholar]
- Kataoka H, Kawaguchi M. Hepatocyte growth factor activator (HGFA): pathophysiological functions in vivo. Febs j. 2010;277(10):2230–2237. doi: 10.1111/j.1742-4658.2010.07640.x. [DOI] [PubMed] [Google Scholar]
- Kaur H, Chaurasia SS, de Medeiros FW, Agrawal V, Salomao MQ, Singh N, Ambati BK, Wilson SE. Corneal stroma PDGF blockade and myofibroblast development. Exp Eye Res. 2009;88(5):960–965. doi: 10.1016/j.exer.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi R, Saika S, Wakayama M, Ooshima A, Ohnishi Y, Yabe H. Extracellular matrix components in a case of retrocorneal membrane associated with syphilitic interstitial keratitis. Cornea. 2001;20(1):100–103. doi: 10.1097/00003226-200101000-00019. [DOI] [PubMed] [Google Scholar]
- Kawase T, Okuda K, Yoshie H. A Hepatocyte Growth Factor (HGF)/receptor autocrine loop regulates constitutive self-renewal of human periodontal ligament cells but reduces sensitivity to exogenous HGF. J Periodontol. 2006;77(10):1723–1730. doi: 10.1902/jop.2006.060031. [DOI] [PubMed] [Google Scholar]
- Kim WH, Matsumoto K, Bessho K, Nakamura T. Growth inhibition and apoptosis in liver myofibroblasts promoted by hepatocyte growth factor leads to resolution from liver cirrhosis. Am J Pathol. 2005;166(4):1017–1028. doi: 10.1016/S0002-9440(10)62323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto M, Shima N, Yamaguchi M, Amano S, Yamagami S. Role of hepatocyte growth factor in promoting the growth of human corneal endothelial cells stimulated by L-ascorbic acid 2-phosphate. Invest Ophthalmol Vis Sci. 2012;53(12):7583–7589. doi: 10.1167/iovs.12-10146. [DOI] [PubMed] [Google Scholar]
- Koizumi N, Okumura N, Ueno M, Kinoshita S. New therapeutic modality for corneal endothelial disease using Rho-associated kinase inhibitor eye drops. Cornea. 2014;33(11):S25–31. doi: 10.1097/ICO.0000000000000240. [DOI] [PubMed] [Google Scholar]
- Kurosaka H, Kurosaka D, Kato K, Mashima Y, Tanaka Y. Transforming growth factor-beta 1 promotes contraction of collagen gel by bovine corneal fibroblasts through differentiation of myofibroblasts. Invest Ophthalmol Vis Sci. 1998;39(5):699–704. [PubMed] [Google Scholar]
- Lee SL, Dickson RB, Lin CY. Activation of hepatocyte growth factor and urokinase/plasminogen activator by matriptase, an epithelial membrane serine protease. J Biol Chem. 2000;275(47):36720–36725. doi: 10.1074/jbc.M007802200. [DOI] [PubMed] [Google Scholar]
- Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163(1):61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- Li Q, Weng J, Mohan RR, Bennett GL, Schwall R, Wang ZF, Tabor K, Kim J, Hargrave S, Cuevas KH, Wilson SE. Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Invest Ophthalmol Vis Sci. 1996;37(5):727–739. [PubMed] [Google Scholar]
- Li W, Hayashida Y, Chen YT, Tseng SC. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007a;17(1):26–36. doi: 10.1038/sj.cr.7310137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Sabater AL, Chen YT, Hayashida Y, Chen SY, He H, Tseng SC. A novel method of isolation, preservation, and expansion of human corneal endothelial cells. Invest Ophthalmol Vis Sci. 2007b;48(2):614–620. doi: 10.1167/iovs.06-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Q, Mohan RR, Chen L, Wilson SE. Signaling by HGF and KGF in corneal epithelial cells: Ras/MAP kinase and Jak-STAT pathways. Invest Ophthalmol Vis Sci. 1998;39(8):1329–1338. [PubMed] [Google Scholar]
- Ljubimov AV, Saghizadeh M. Progress in corneal wound healing. Prog Retin Eye Res. 2015;49:17–45. doi: 10.1016/j.preteyeres.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Reinach PS, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood) 2001;226(7):653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42(11):2490–2495. [PubMed] [Google Scholar]
- Mars WM, Zarnegar R, Michalopoulos GK. Activation of hepatocyte growth factor by the plasminogen activators uPA and tPA. Am J Pathol. 1993;143(3):949–958. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K, Kataoka H, Date K, Nakamura T. Cooperative interaction between alpha- and beta-chains of hepatocyte growth factor on c-Met receptor confers ligand-induced receptor tyrosine phosphorylation and multiple biological responses. J Biol Chem. 1998;273(36):22913–22920. doi: 10.1074/jbc.273.36.22913. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119(4):591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- McBain VA, Forrester JV, McCaig CD. HGF, MAPK, and a small physiological electric field interact during corneal epithelial cell migration. Invest Ophthalmol Vis Sci. 2003;44(2):540–547. doi: 10.1167/iovs.02-0570. [DOI] [PubMed] [Google Scholar]
- Merchant M, Ma X, Maun HR, Zheng Z, Peng J, Romero M, Huang A, Yang NY, Nishimura M, Greve J, Santell L, Zhang YW, Su Y, Kaufman DW, Billeci KL, Mai E, Moffat B, Lim A, Duenas ET, Phillips HS, Xiang H, Young JC, Vande Woude GF, Dennis MS, Reilly DE, Schwall RH, Starovasnik MA, Lazarus RA, Yansura DG. Monovalent antibody design and mechanism of action of onartuzumab, a MET antagonist with anti-tumor activity as a therapeutic agent. Proc Natl Acad Sci U S A. 2013;110(32):E2987–2996. doi: 10.1073/pnas.1302725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Yamagami S, Amano S. Corneal endothelial regeneration and tissue engineering. Prog Retin Eye Res. 2013;35:1–17. doi: 10.1016/j.preteyeres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Mittal SK, Omoto M, Amouzegar A, Sahu A, Rezazadeh A, Katikireddy KR, Shah DI, Sahu SK, Chauhan SK. Restoration of Corneal Transparency by Mesenchymal Stem Cells. Stem Cell Reports. 2016;7(4):583–590. doi: 10.1016/j.stemcr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto T, Sumioka T, Saika S. Endothelial mesenchymal transition: a therapeutic target in retrocorneal membrane. Cornea. 2010;29(1):S52–56. doi: 10.1097/ICO.0b013e3181efe36a. [DOI] [PubMed] [Google Scholar]
- Miyazawa K. Hepatocyte growth factor activator (HGFA): a serine protease that links tissue injury to activation of hepatocyte growth factor. Febs j. 2010;277(10):2208–2214. doi: 10.1111/j.1742-4658.2010.07637.x. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Shimomura T, Kitamura A, Kondo J, Morimoto Y, Kitamura N. Molecular cloning and sequence analysis of the cDNA for a human serine protease reponsible for activation of hepatocyte growth factor. Structural similarity of the protease precursor to blood coagulation factor XII. J Biol Chem. 1993;268(14):10024–10028. [PubMed] [Google Scholar]
- Miyazawa K, Tsubouchi H, Naka D, Takahashi K, Okigaki M, Arakaki N, Nakayama H, Hirono S, Sakiyama O, Takahashi K, et al. Molecular cloning and sequence analysis of cDNA for human hepatocyte growth factor. Biochem Biophys Res Commun. 1989;163(2):967–973. doi: 10.1016/0006-291x(89)92316-4. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Matsumoto K, Li MY, Nakamura T. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. Faseb j. 2005;19(6):580–582. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- Mizuno S, Nakamura T. Suppressions of chronic glomerular injuries and TGF-beta 1 production by HGF in attenuation of murine diabetic nephropathy. Am J Physiol Renal Physiol. 2004;286(1):F134–143. doi: 10.1152/ajprenal.00199.2003. [DOI] [PubMed] [Google Scholar]
- Moller-Pedersen T, Li HF, Petroll WM, Cavanagh HD, Jester JV. Confocal microscopic characterization of wound repair after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 1998;39(3):487–501. [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67(5):901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Alexandrou B, Kane B, Hassell JR. Maintenance of the keratocyte phenotype during cell proliferation stimulated by insulin. J Biol Chem. 2005;280(38):32634–32639. doi: 10.1074/jbc.M504724200. [DOI] [PubMed] [Google Scholar]
- Myrna KE, Mendonsa R, Russell P, Pot SA, Liliensiek SJ, Jester JV, Nealey PF, Brown D, Murphy CJ. Substratum topography modulates corneal fibroblast to myofibroblast transformation. Invest Ophthalmol Vis Sci. 2012;53(2):811–816. doi: 10.1167/iovs.11-7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrna KE, Pot SA, Murphy CJ. Meet the corneal myofibroblast: the role of myofibroblast transformation in corneal wound healing and pathology. Vet Ophthalmol. 2009;12(1):25–27. doi: 10.1111/j.1463-5224.2009.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Mizuno S. The discovery of hepatocyte growth factor (HGF) and its significance for cell biology, life sciences and clinical medicine. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(6):588–610. doi: 10.2183/pjab.86.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122(3):1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Sakai K, Nakamura T, Matsumoto K. Hepatocyte growth factor twenty years on: Much more than a growth factor. J Gastroenterol Hepatol. 2011;26(1):188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Bardelli A, Follenzi A, Galimi F, Comoglio PM. Biological activation of pro-HGF (hepatocyte growth factor) by urokinase is controlled by a stoichiometric reaction. J Biol Chem. 1995;270(2):603–611. doi: 10.1074/jbc.270.2.603. [DOI] [PubMed] [Google Scholar]
- Nayak SK, Binder PS. The growth of endothelium from human corneal rims in tissue culture. Invest Ophthalmol Vis Sci. 1984;25(10):1213–1216. [PubMed] [Google Scholar]
- Okajima A, Miyazawa K, Naitoh Y, Inoue K, Kitamura N. Induction of hepatocyte growth factor activator messenger RNA in the liver following tissue injury and acute inflammation. Hepatology. 1997;25(1):97–102. doi: 10.1053/jhep.1997.v25.pm0008985272. [DOI] [PubMed] [Google Scholar]
- Okayama K, Azuma J, Dosaka N, Iekushi K, Sanada F, Kusunoki H, Iwabayashi M, Rakugi H, Taniyama Y, Morishita R. Hepatocyte growth factor reduces cardiac fibrosis by inhibiting endothelial-mesenchymal transition. Hypertension. 2012;59(5):958–965. doi: 10.1161/HYPERTENSIONAHA.111.183905. [DOI] [PubMed] [Google Scholar]
- Okumura N, Kay EP, Nakahara M, Hamuro J, Kinoshita S, Koizumi N. Inhibition of TGF-beta signaling enables human corneal endothelial cell expansion in vitro for use in regenerative medicine. PLoS One. 2013;8(2):e58000. doi: 10.1371/journal.pone.0058000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura N, Sakamoto Y, Fujii K, Kitano J, Nakano S, Tsujimoto Y, Nakamura S, Ueno M, Hagiya M, Hamuro J, Matsuyama A, Suzuki S, Shiina T, Kinoshita S, Koizumi N. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci Rep. 2016;6:26113. doi: 10.1038/srep26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura N, Ueno M, Koizumi N, Sakamoto Y, Hirata K, Hamuro J, Kinoshita S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50(8):3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- Ono M, Sawa Y, Mizuno S, Fukushima N, Ichikawa H, Bessho K, Nakamura T, Matsuda H. Hepatocyte growth factor suppresses vascular medial hyperplasia and matrix accumulation in advanced pulmonary hypertension of rats. Circulation. 2004;110(18):2896–2902. doi: 10.1161/01.CIR.0000146342.30470.30. [DOI] [PubMed] [Google Scholar]
- Pancholi S, Tullo A, Khaliq A, Foreman D, Boulton M. The effects of growth factors and conditioned media on the proliferation of human corneal epithelial cells and keratocytes. Graefes Arch Clin Exp Ophthalmol. 1998;236(1):1–8. doi: 10.1007/s004170050034. [DOI] [PubMed] [Google Scholar]
- Peh GS, Toh KP, Ang HP, Seah XY, George BL, Mehta JS. Optimization of human corneal endothelial cell culture: density dependency of successful cultures in vitro. BMC Res Notes. 2013;6:176. doi: 10.1186/1756-0500-6-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper MS, Matsumoto K, Nakamura T, Orci L, Montesano R. Hepatocyte growth factor increases urokinase-type plasminogen activator (u-PA) and u-PA receptor expression in Madin-Darby canine kidney epithelial cells. J Biol Chem. 1992;267(28):20493–20496. [PubMed] [Google Scholar]
- Petroll WM, Barry-Lane PA, Cavanagh HD, Jester JV. ZO-1 reorganization and myofibroblast transformation of corneal endothelial cells after freeze injury in the cat. Exp Eye Res. 1997;64(2):257–267. doi: 10.1006/exer.1996.0211. [DOI] [PubMed] [Google Scholar]
- Petroll WM, Cavanagh HD, Barry P, Andrews P, Jester JV. Quantitative analysis of stress fiber orientation during corneal wound contraction. J Cell Sci. 1993;104(Pt 2):353–363. doi: 10.1242/jcs.104.2.353. [DOI] [PubMed] [Google Scholar]
- Pot SA, Liliensiek SJ, Myrna KE, Bentley E, Jester JV, Nealey PF, Murphy CJ. Nanoscale topography-induced modulation of fundamental cell behaviors of rabbit corneal keratocytes, fibroblasts, and myofibroblasts. Invest Ophthalmol Vis Sci. 2010;51(3):1373–1381. doi: 10.1167/iovs.09-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Schroeder MD, Ma C, Rando TA. HGFA Is an Injury-Regulated Systemic Factor that Induces the Transition of Stem Cells into GAlert. Cell Rep. 2017;19(3):479–486. doi: 10.1016/j.celrep.2017.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy O, Leclerc VB, Bourget JM, Theriault M, Proulx S. Understanding the process of corneal endothelial morphological change in vitro. Invest Ophthalmol Vis Sci. 2015;56(2):1228–1237. doi: 10.1167/iovs.14-16166. [DOI] [PubMed] [Google Scholar]
- Russell WE, McGowan JA, Bucher NL. Partial characterization of a hepatocyte growth factor from rat platelets. J Cell Physiol. 1984;119(2):183–192. doi: 10.1002/jcp.1041190207. [DOI] [PubMed] [Google Scholar]
- Saika S, Kobata S, Hashizume N, Okada Y, Yamanaka O. Epithelial basement membrane in alkali-burned corneas in rats. Immunohistochemical study. Cornea. 1993;12(5):383–390. doi: 10.1097/00003226-199309000-00003. [DOI] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373(6516):699–702. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Schultz JM, Khan SN, Ahmed ZM, Riazuddin S, Waryah AM, Chhatre D, Starost MF, Ploplis B, Buckley S, Velasquez D, Kabra M, Lee K, Hassan MJ, Ali G, Ansar M, Ghosh M, Wilcox ER, Ahmad W, Merlino G, Leal SM, Riazuddin S, Friedman TB, Morell RJ. Noncoding mutations of HGF are associated with nonsyndromic hearing loss, DFNB39. Am J Hum Genet. 2009;85(1):25–39. doi: 10.1016/j.ajhg.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senoo T, Obara Y, Joyce NC. EDTA: a promoter of proliferation in human corneal endothelium. Invest Ophthalmol Vis Sci. 2000;41(10):2930–2935. [PubMed] [Google Scholar]
- Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve. 2000;23(2):239–245. doi: 10.1002/(sici)1097-4598(200002)23:2<239::aid-mus15>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Shima N, Nagao M, Ogaki F, Tsuda E, Murakami A, Higashio K. Tumor cytotoxic factor/hepatocyte growth factor from human fibroblasts: cloning of its cDNA, purification and characterization of recombinant protein. Biochem Biophys Res Commun. 1991;180(2):1151–1158. doi: 10.1016/s0006-291x(05)81187-8. [DOI] [PubMed] [Google Scholar]
- Shimomura T, Kondo J, Ochiai M, Naka D, Miyazawa K, Morimoto Y, Kitamura N. Activation of the zymogen of hepatocyte growth factor activator by thrombin. J Biol Chem. 1993;268(30):22927–22932. [PubMed] [Google Scholar]
- Shukla MN, Rose JL, Ray R, Lathrop KL, Ray A, Ray P. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am J Respir Cell Mol Biol. 2009;40(6):643–653. doi: 10.1165/rcmb.2008-0217OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Barbosa FL, Torricelli AA, Santhiago MR, Wilson SE. Transforming growth factor beta and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp Eye Res. 2014;120:152–160. doi: 10.1016/j.exer.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg E, Meyer D, Weidner KM, Birchmeier C. Scatter factor/hepatocyte growth factor and its receptor, the c-met tyrosine kinase, can mediate a signal exchange between mesenchyme and epithelia during mouse development. J Cell Biol. 1993;123(1):223–235. doi: 10.1083/jcb.123.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spix JK, Chay EY, Block ER, Klarlund JK. Hepatocyte growth factor induces epithelial cell motility through transactivation of the epidermal growth factor receptor. Exp Cell Res. 2007;313(15):3319–3325. doi: 10.1016/j.yexcr.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JD, Marshall JM, Benjamin L, Cederholm-Williams SA, Bron AJ. Plasminogen activator in human tears. Eye (Lond) 1992;6(Pt 6):653–658. doi: 10.1038/eye.1992.140. [DOI] [PubMed] [Google Scholar]
- Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327(6119):239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Sumioka T, Ikeda K, Okada Y, Yamanaka O, Kitano A, Saika S. Inhibitory effect of blocking TGF-beta/Smad signal on injury-induced fibrosis of corneal endothelium. Mol Vis. 2008;14:2272–2281. [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM. Control of invasive growth by hepatocyte growth factor (HGF) and related scatter factors. Cytokine & Growth Factor Reviews. 1997;8(2):129–142. doi: 10.1016/s1359-6101(97)00007-5. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Hagiya M, Nishizawa T, Seki T, Shimonishi M, Shimizu S, Nakamura T. Deduced primary structure of rat hepatocyte growth factor and expression of the mRNA in rat tissues. Proc Natl Acad Sci U S A. 1990;87(8):3200–3204. doi: 10.1073/pnas.87.8.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo T, Vesaluoma M, Bennett GL, Schwall R, Helena M, Liang Q, Wilson SE. Tear hepatocyte growth factor (HGF) availability increases markedly after excimer laser surface ablation. Exp Eye Res. 1997;64(4):501–504. doi: 10.1006/exer.1996.0226. [DOI] [PubMed] [Google Scholar]
- Torricelli AA, Santhanam A, Wu J, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res. 2016;142:110–118. doi: 10.1016/j.exer.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, Kitamura N. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373(6516):702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Ueki T, Kaneda Y, Tsutsui H, Nakanishi K, Sawa Y, Morishita R, Matsumoto K, Nakamura T, Takahashi H, Okamoto E, Fujimoto J. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nat Med. 1999;5(2):226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- Warejcka DJ, Narayan M, Twining SS. Maspin increases extracellular plasminogen activator activity associated with corneal fibroblasts and myofibroblasts. Exp Eye Res. 2011;93(5):618–627. doi: 10.1016/j.exer.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warn R, Harvey P, Warn A, Foley-Comer A, Heldin P, Versnel M, Arakaki N, Daikuhara Y, Laurent GJ, Herrick SE, Mutsaers SE. HGF/SF induces mesothelial cell migration and proliferation by autocrine and paracrine pathways. Exp Cell Res. 2001;267(2):258–266. doi: 10.1006/excr.2001.5240. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Yano W, Kondo S, Hattori Y, Yamada N, Yanai R, Nishida T. Up-regulation of urokinase-type plasminogen activator in corneal epithelial cells induced by wounding. Invest Ophthalmol Vis Sci. 2003;44(8):3332–3338. doi: 10.1167/iovs.02-1280. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Arakaki N, Hartmann G, Vandekerckhove J, Weingart S, Rieder H, Fonatsch C, Tsubouchi H, Hishida T, Daikuhara Y, et al. Evidence for the identity of human scatter factor and human hepatocyte growth factor. Proc Natl Acad Sci U S A. 1991;88(16):7001–7005. doi: 10.1073/pnas.88.16.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol. 2006;38(10):1625–1631. doi: 10.1016/j.biocel.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Chaurasia SS, Medeiros FW. Apoptosis in the initiation, modulation and termination of the corneal wound healing response. Exp Eye Res. 2007;85(3):305–311. doi: 10.1016/j.exer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. 1999a;68(4):377–397. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Li Q, McDowall AW, Vital M, Chwang EL. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62(4):325–327. doi: 10.1006/exer.1996.0038. [DOI] [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Zieske JD, Jester JV, Schultz GS. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994;59(6):665–678. doi: 10.1006/exer.1994.1152. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Liang Q, Kim WJ. Lacrimal gland HGF, KGF, and EGF mRNA levels increase after corneal epithelial wounding. Invest Ophthalmol Vis Sci. 1999b;40(10):2185–2190. [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrosio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20(5):625–637. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Walker JW, Chwang EL, He YG. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Invest Ophthalmol Vis Sci. 1993;34(8):2544–2561. [PubMed] [Google Scholar]
- Xie HT, Chen SY, Li GG, Tseng SC. Limbal epithelial stem/progenitor cells attract stromal niche cells by SDF-1/CXCR4 signaling to prevent differentiation. Stem Cells. 2011;29(11):1874–1885. doi: 10.1002/stem.743. [DOI] [PubMed] [Google Scholar]
- Xie Q, Liu KD, Hu MY, Zhou K. SF/HGF-c-Met autocrine and paracrine promote metastasis of hepatocellular carcinoma. World J Gastroenterol. 2001;7(6):816–820. doi: 10.3748/wjg.v7.i6.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai R, Yamada N, Inui M, Nishida T. Correlation of proliferative and anti-apoptotic effects of HGF, insulin, IGF-1, IGF-2, and EGF in SV40-transformed human corneal epithelial cells. Exp Eye Res. 2006;83(1):76–83. doi: 10.1016/j.exer.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Yong KW, Li Y, Liu F, Bin G, Lu TJ, Wan Abas WA, Wan Safwani WK, Pingguan-Murphy B, Ma Y, Xu F, Huang G. Paracrine Effects of Adipose-Derived Stem Cells on Matrix Stiffness-Induced Cardiac Myofibroblast Differentiation via Angiotensin II Type 1 Receptor and Smad7. Sci Rep. 2016;6:33067. doi: 10.1038/srep33067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Yamaguchi Y, Itami S, Yoshikawa K, Tabata Y, Matsumoto K, Nakamura T. Neutralization of hepatocyte growth factor leads to retarded cutaneous wound healing associated with decreased neovascularization and granulation tissue formation. J Invest Dermatol. 2003;120(2):335–343. doi: 10.1046/j.1523-1747.2003.12039.x. [DOI] [PubMed] [Google Scholar]
- Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010;81(2-3):229–235. doi: 10.1016/j.brainresbull.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD, Guimaraes SR, Hutcheon AE. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72(1):33–39. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]