Abstract
The high throughput screening of chemicals for interaction with intracellular targets is gaining prominence in the toxicity evaluation of environmental chemicals. We describe ligand-mediated receptor assembly as an early event in receptor signaling and its application to the screening of chemicals for interaction with targeted receptors. We utilized bioluminescence resonance energy transfer (BRET) to detect and quantify assembly of the methyl farnesoate receptor (MfR) in response to various high-production volume and other chemicals. The hormone methyl farnesoate binds to the MfR to regulate various aspects of reproduction and development in crustaceans. The MfR protein subunits Met and SRC, cloned from Daphnia pulex, were fused to the fluorophore, mAmetrine and the photon generator, Rluc2, respectively. Ligand-mediated receptor assembly was measured by photon transfer from the photon donor to the fluorophore resulting in fluorescence emission. Overall, the BRET assay had comparable or greater sensitivity as compared to a traditional reporter gene assay. Further, chemicals that screened positive in the BRET assay also stimulated phenotypic outcomes in daphnids that result from MfR signaling. We concluded the BRET assay is an accurate, sensitive, and cost/time efficient alternative to traditional screening assays.
Introduction
Endocrine signaling pathways are crucial to survival and reproduction, however these pathways are often susceptible to disruption by environmental chemicals resulting in perturbations in normal physiology 1-4. Environmental exposure to endocrine disrupting chemicals has been associated with reproductive dysfunction 5, perturbations in reproductive development 6, 7, and population demise 8. As a result, significant effort has gone into the development of screening and testing methods for detecting endocrine-disrupting properties of chemicals, and hazards associated with the use of these chemicals9-13. Screening assays used to detect endocrine-disrupting activity of chemicals often consist of hormone-receptor binding assays or reporter gene transcription assays 9-11. Chemicals that screen positive in one or more of these assays then become a candidate for more definitive testing to assess whole organism consequences of this activity and the exposure concentrations at which effects occur 12. However, receptor-binding assays are relatively uninformative, because they provide no information on the consequence of binding (e.g., receptor activation, inhibition, or no consequence). Reporter gene transcription assays are more informative, however these assays rely upon reporter gene transcription and translation which increases the time required to provide a measurable endpoint.
Most hormone receptors consist of homo- or hetero-dimers 14. The first step in activation of many of these receptors is ligand-stimulated dimerization 14-16. This endpoint can serve as an initiating event in the adverse outcome pathway 17 for many endocrine signaling pathways. This study addresses the potential for protein dimerization to serve as an endpoint for the detection of chemical-induced receptor activation using the methyl farnesoate receptor as a model.
Methyl farnesoate has long been recognized as a hormone involved in reproduction and development in crustaceans 18-20. Methyl farnesoate stimulates male sex determination, in branchiopod crustaceans 18, 19 and male sex differentiation in some decapod crustaceans 21. Recently, we and others identified the protein receptor complex that mediates the actions of methyl farnesoate, the methyl farnesoate receptor (MfR) 22, 23. Further, we demonstrated methyl farnesoate stimulates the association of the protein methoprene tolerant (Met) with its partner, steroid receptor coactivator (SRC) 24. These assembled proteins comprise the active MfR 22-24.
Bioluminescence resonance energy transfer (BRET) technology has gained prominence as a means of measuring protein-protein interactions in cells and in real time25, 26. The method involves the construction of fusion proteins whereby one protein of interest is fused to a luciferase protein. The other protein of interest is fused to a fluorophore. When the proteins associate, photons generated by the luciferase can excite the fluorophore resulting in fluorescence emission. BRET has been extensively used in the study of G-protein coupled receptors 26-28, but more recently has been used in the study of homo- and hetero-dimerization of hormone nuclear receptors 15, 16, 29.
Herein, we describe the construction and optimization of a novel approach to screen chemicals for hormone receptor activation using the MfR cloned from daphnids (Daphnia pulex). We propose that this methodology could be applied to all receptor complexes whose subunit dimerization is agonist driven. Further, we screened several compounds for comparison of specificity and sensitivity to the more traditional luciferase reporter gene transcription assay. Finally, compounds that screened positive in the BRET assay were evaluated in vivo to determine whether results from the cell-based assay accurately predicted phenotypic outcomes in the whole organism.
Material and Methods
Methyl farnesoate (Echelon Biosciences Inc., Salt Lake City, Utah), and all other chemicals (Sigma-Aldrich Corp., St. Louis, MO) screened in BRET and luciferase reporter gene assays, were dissolved in DMSO for delivery to assay solutions. Final DMSO concentrations were 0.001% v/v in the BRET assays and 0.0005% v/v in the reporter gene assays. Hydroprene and diofenolan were dissolved in ethanol for in vivo experiments, where the final concentration of ethanol was 0.0003% v/v.
Fusion Protein Construction
Four fusion proteins were constructed to identify those constructs that provided the optimum BRET signal. The daphnid met open reading frame, described previously 22, was fused to the fluorophore mAmetrine open reading frame (mAme) (excitation: 510 nm, emission: 535nm) (Addgene, Cambridge MA) at either the 5′ or 3′end of met. The daphnid SRC open reading frame, described previously 24, was fused to the Renilla luciferase 2 open reading frame (RLuc2) (substrate: coelenterazine 400A, emission: 410 nm) (Dr. Sanjiv Gambhir, Stanford University, School of Medicine, Stanford, CA) at either the 5′ or 3′ end of SRC.
The met gene was amplified using primers harboring NotHFI (forward containing linker sequence, ATAGCGGAAGTGGTAGCGGAAGTGGT) and ApaI (reverse) restriction enzyme sites, and sub-cloned into the pMT-B vector (ThermoFischer Scientific). mAme was amplified from the pBad cloning vector using primers with KpnI (forward) and NotHFI (reverse) sites, and subcloned at the 5′-terminus of the pMT-met, to create pMT-mAme-linker-met (mAme-Met). A similar procedure was used to construct the pMT-met-linker-mAme (Met-mAme), with some exceptions. Primers harboring KpnI (forward) and NotHFI (reverse) sites were used to amplify met, while primers with NotHFI (forward containing linker sequence) and BstBI (reverse) sites amplified mAme.
SRC was amplified from the TOPO cloning vector using primers harboring BstB1 (forward) and AgeI (reverse) restriction enzyme sites, and was sub-cloned into the pMT-B vector. Rluc2 was amplified from the pcDNA storage vector using primers harboring XhoI (forward) and BstBI (reverse) sites. The reverse primer also contained a nucleotide “linker” sequence (AGCGGAAGTGGTAGCGGAAGTGGC) to lengthen the distance between the two proteins and decrease probability of incorrect folding. The Rluc2-linker sequence was sub-cloned at the 5′-terminus of the pMT-SRC plasmid, to create pMT- Rluc2-linker-SRC (Rluc2-SRC). A similar procedure was used to construct the pMT-SRC-linker-Rluc2 (SRC-Rluc2), with some exceptions. Primers harboring Xho1 (forward) and BstB1 (reverse) sites was used to amplify SRC, while primers with BstB1 (forward containing linker sequence) and Age1 (reverse) sites were used to amplify Rluc2. All four fusion proteins, Rluc2-SRC, SRC-Rluc2, mAme-Met, and Met-mAme, were sequenced (Eton Bioscience, San Diego, CA) to ensure the fluorescent/luminescent proteins were in frame with the respective MfR subunit protein. All fusion constructs were successfully sub-cloned without amino acid substitutions.
BRET Assay
BRET assays were performed as we described previously 24. Assays were performed in Drosophila Schneider (S2) cells (Invitrogen). Cells were grown in Schneider's medium, containing 10% heat inactivated fetal bovine serum (Gibco, Carlsbad, CA, USA), 50 mg/ml penicillin G and 50 mg/ml streptomycin sulfate (Fisher Scientific, Pittsburgh, PA), and incubated at 23°C under ambient air atmosphere. Cells were seeded at a density of 3 × 106 in 35 mm dishes in 6-well plates and transfected 24 hours after plating.
The relevant plasmids were transiently transfected into cells by calcium phosphate DNA precipitation. Total DNA concentration remained constant across all experiments at 2.8 ng/μL, while the photon donor: protein acceptor ratio was held at an optimized 1: 6 ratio (produced greatest energy transfer). Transcription was induced by exposing cells to 500 μM CuSO4 for 24 hours. Cells were treated with test chemical or vehicle control for 1 hour in 1× phosphate-buffered saline medium. The Rluc2 substrate, coelenterazine 400A (Biotium, Inc.), was then added to each well (5.0 μM), and light emission was measured immediately at 410 ± 40 nm (emission produced by Rluc2) and 535 ± 15 nm (emission produced by mAme) using a FluoroStar fluorimeter (BMG Labtech). The ratio of light emitted at 535 nm/410 nm (corrected for basal level donor emission of Rluc2 15, 25) is termed the BRET ratio. The BRET ratio provided a quantitative measure of the degree of MfR protein association. All treatments were replicated 3 times.
Luciferase Reporter Gene Assay
Luciferase-based transcription reporter gene assays were conducted for comparison to BRET with respect to specificity and sensitivity. S2 cells were transiently transfected with daphnid met full open reading frame fused to the Gal4 DNA binding domain22, daphnid SRC, Renilla Luciferase (pRL-CMV, internal transfection control, Promega) and the firefly luciferase reporter gene vector (pGL5-Luc, Promega) that contained five upstream GAL4 binding sites. Following transfection, transcription of Met-Gal4 and SRC was induced with CuSO4 (100 μM for 24 hours). Cells then were treated with test chemical in Ex-cellTM 420 insect serum-free medium with L-glutamine (SAFC Biosciences, Sigma, St. Louis, MO). Cells were harvested after 24 hours incubation. Emissions from the firefly luciferase and Renilla luciferases were measured using the Dual-Glo® luciferase system (Promega). Firefly luciferase emission was normalized to Renilla luciferase emission, and each chemical treatment group was normalized to DMSO control treated cells. All treatments were replicated 3times.
Male Sex Determination
D. magna were cultured under parthenogenetic rearing conditions where all offspring produced are female29,30. Hydroprene and diofenolan were used in in vivo exposure assays to determine their potency in stimulating male sex determination. Gravid adult female daphnids of the same age were selected from cultures, and placed individually in 50 mL beakers containing 40 mL daphnid media. The daphnids were exposed to serial dilutions of the evaluated chemicals, where each treatment consisted of ten individual daphnids. Solutions were renewed daily. Animals were fed 0.20 mg (dry wt) fish food and 7 × 106 algae cells (Pseudokirchneriella subcapitata), prepared as described elsewhere 4, daily and daphnids were assessed for survival, brood release. Survival of maternal organisms was ≥90% in all treatments. The percentage of males in the second brood was used to determine chemical potency. Experiments were terminated after the second brood release. Methods used are described in greater detail elsewhere 30,31.
Statistical Analysis
Comparisons of two means were evaluated for significance (p<0.05) using Student's t test. Equal variance between multiple treatment groups was confirmed with Brown-Forsythe's test. One-way analysis of variance (ANOVA), followed by Tukey's multiple comparison procedure, was used to evaluate significant differences between the control and multiple treatments. All statistics were performed using Origin software (OriginLab Corp., Northhampton, MA).
Results
BRET Assay
Fusion proteins were constructed (Fig. 1) in different configurations and used in BRET assays to determine which configuration provided the strongest BRET ratio. Significant increases in BRET ratio upon the addition of methyl farnesoate occurred with all fusion protein configurations (Fig. 2). However, the greatest increase in BRET ratio between the control and hormone treatment occurred in cells expressing both subunit proteins fused at the N-terminus with the fluorophore or luciferase protein: mAme-Met and Rluc2-SRC (Fig. 2). All subsequent BRET assays were performed with these two protein constructs.
Figure 1. The detection of MfR agonists using BRET.
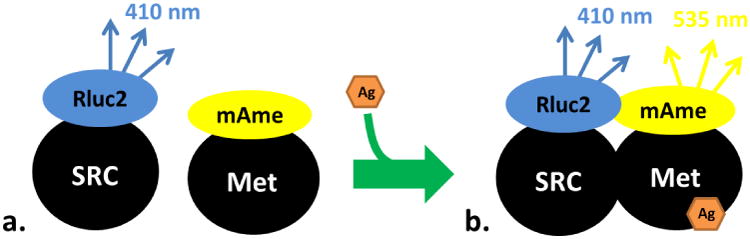
A) SRC is fused to Rluc2, which metabolizes coelenterazine 400A to emit light at 410 nm. B) In the presence of an agonist (Ag), dimerization with Met is stimulated and the light emitted at 410 nm excites the proximal yellow fluorescent protein, mAmetrine (mAme), that is fused to Met to emit a secondary light at 535 nm.
Figure 2. BRET signaling using MfR subunits with different bioluminescent/fluorescent protein configurations.
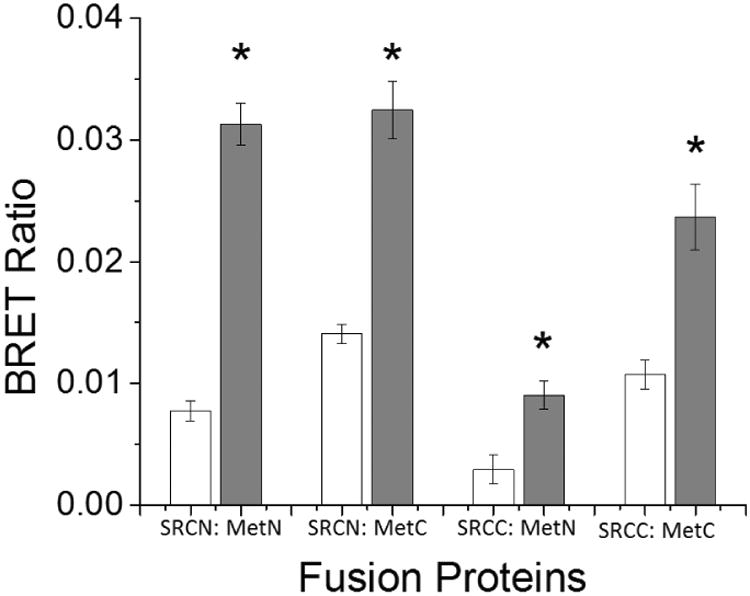
Cells were transfected with four different sets of fusion proteins, where Met was fused with a mAmetrine and SRC was fused with Renilla luciferase 2 at either the N- or C- terminus. White bars indicate cells treated with vehicle (DMSO) control and grey bars indicate cells treated with 100 μM methyl farnesoate. Data are presented as mean with standard deviation (n = 3) and an asterisk denotes a significant increase in Met: SRC binding compared to control (p<0.05).
Twenty-nine environmental chemicals (Table S1) were screened at 100 μM for their ability to stimulate MfR subunit association. Chemicals were selected based upon propensity for environmental exposure or structural similarity to methyl farnesoate. All compounds appeared to be non-toxic at 100 μM except for TBBPA which caused a significant (p<0.05, ANOVA) reduction in Rluc2 luciferase. The positive control, methyl farnesoate, and six of the test compounds significantly (p<0.05) stimulated MfR subunit association compared to controls (Fig. 3). These compounds, methoprene, kinoprene, pyriproxyfen, fenoxycarb, diofenoaln, and hydroprene, were all insect growth regulating (IGR) insecticides.
Figure 3. Chemical screen for MfR activation using BRET assay.
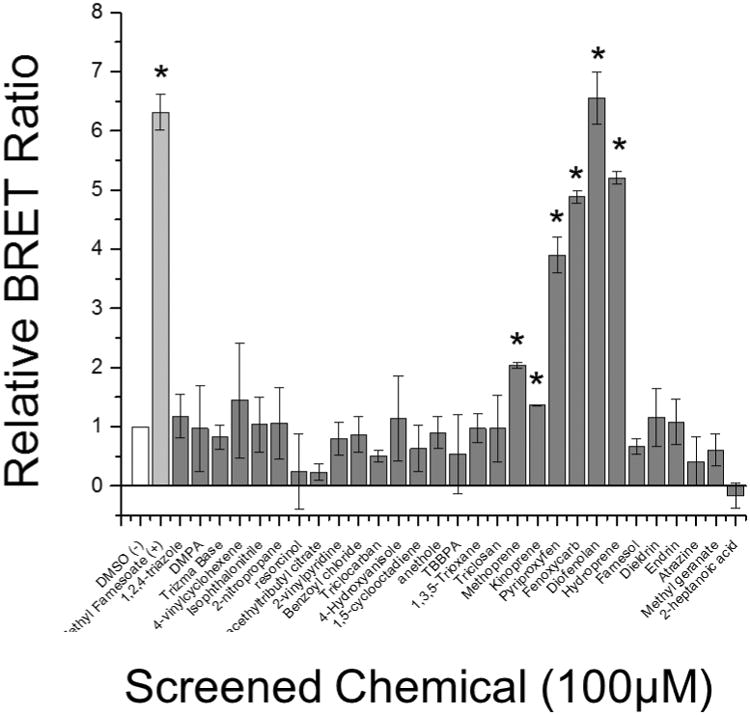
White bar indicates DMSO vehicle control, light grey bar indicates the positive positive control (methyl farnesoate), and dark grey bars indicate screened chemicals. Data are presented as means with standard deviations (n = 3) and an asterisk denotes a significant increase in Met-SRC dimerization compared to DMSO control (p<0.05).
The compounds that screened positive were assayed over a range of chemical concentrations to determine relative potency in stimulating MfR association. Chemical potency or assay sensitivity were judged by the lowest observed effect concentration (LOEC). The positive control, methyl farnesoate, significantly activated subunit association with an LOEC of 3.0 μM (Fig. 4A). Methoprene (LOEC = 10 μM) (Fig. 4B) and kinoprene (LOEC = 100 μM) (Fig. 4C) were both less potent at stimulating subunit assembly compared to methyl farnesoate. The remaining compounds, pyriproxyfen (LOEC = 1.0 μM) (Fig. 4D), hydroprene (LOEC = 0.3 μM) (Fig. 4E), diofenolan (LOEC = ≤0.0030 μM) (Fig. 4F), and fenoxycarb (LOEC = 0.0030 μM) (Fig. 4G) were all more potent than the hormone at stimulating MfR subunit association.
Figure 4. MfR BRET assay: concentration-response analysis for MfR activators identified in the initial BRET screen.
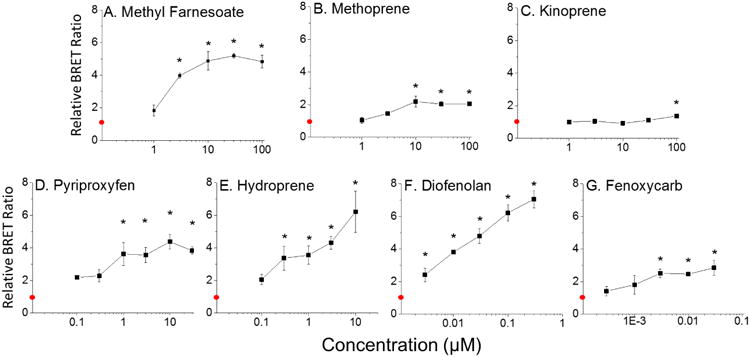
Cells containing the MfR BRET fusion proteins were treated with increasing concentrations of compounds that screened positive in the BRET assay: (A) Methyl Farnesoate, (B) Methoprene, (C) Kinoprene, (D) Pyriproxyfen, (E) Hydroprene, (F) Diofenolan, (G) Fenoxycarb. The red circle on the y-axis represents the control group where cells were treated with the DMSO vehicle control. Data are presented as mean with standard deviation (n = 3) and an asterisk denotes significant increase in Met: SRC dimerization compared to control (p<0.01, ANOVA, Tukey's Multiple Comparison test).
MfR Reporter Gene Activation
The same suite of twenty-nine environmental chemicals (Table S1) also were screened for their ability to stimulate MfR-initiated gene transcription (Fig. 5). Only the positive control and five of the six compounds that were active in the BRET assay significantly stimulated transcription of the reporter gene at 100 μM. Kinoprene, the weak agonist in the BRET assay (Fig. 3) failed to activate transcription in the reporter gene assay (Fig. 5).
Figure 5. Chemical screen for MfR activation using luciferase reporter gene assay.
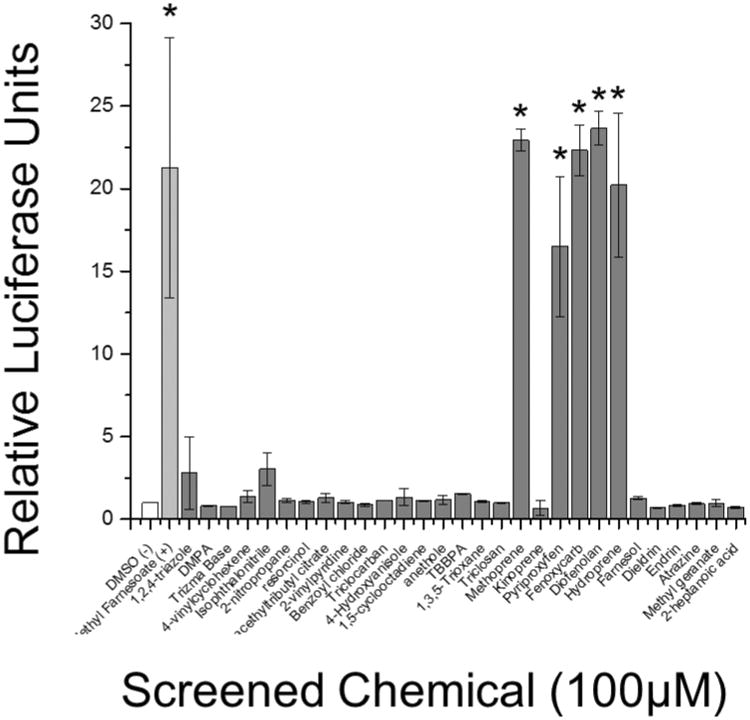
White bar indicates DMSO vehicle control, light grey bar indicates the positive control (methyl farnesoate), and dark grey bars indicate screened chemicals. Data are presented as means with standard deviations (n = 3) and an asterisk denotes significant increase in Met-SRC dimerization compared to DMSO control (p<0.05).
Concentration-response analyses were performed with respect to reporter gene transcriptional activation using methyl farnesoate and the same suite of compounds that were active in the BRET assay. Methyl farnesoate significantly stimulated transcription with an LOEC of 30 μM (Fig. 6A). Methoprene was comparable to the hormone in potency using the reporter gene assay (LOEC = 30 μM, Fig. 6B). Kinoprene, again did not activate transcription in the reporter gene assay (Fig. 6C). Pyriproxyfen (LOEC = 1.0 μM, Fig. 6D), hydroprene (LOEC = 0.30 μM, Fig. 6E), diofenolan (LOEC = 0.30 μM, Fig. 6F), and fenoxycarb (LOEC = 0.010 μM, Fig. 6G) were all more potent than methyl farnesoate in activating gene transcription.
Figure 6. Reporter gene assay: concentration response analysis for MfR activators identified in the initial BRET screen.
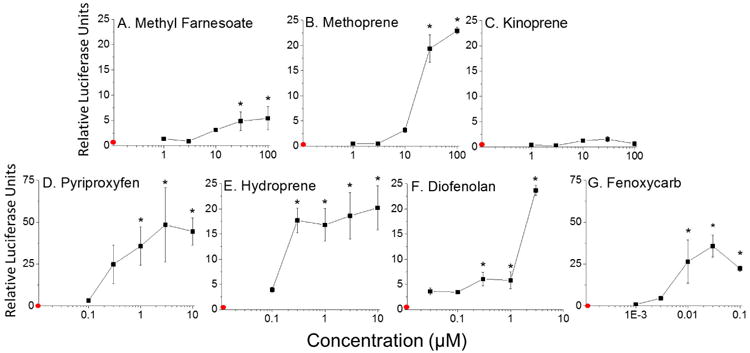
Cells expressing MfR subunits and a luciferase reporter gene were treated with increasing concentration compounds that screened positive in the BRET assay:(A) Methyl Farnesoate, (B) Methoprene, (C) Kinoprene, (D) Pyriproxyfen, (E) Hydroprene, (F) Diofenolan, (G) Fenoxycarb. The red circle on the y-axis represents the control group where cells were treated with the DMSO vehicle control. Data are presented as means with standard deviations (n = 3) and an asterisk denotes significant increase in transcription of the luciferase reporter gene compared to control (p<0.05, ANOVA, Tukey's Multiple Comparison test).
Male Sex Determination
Lastly, we evaluated the ability of the BRET MfR subunit association assay to predict male sex determination in D. magna. Two of the most potent chemicals, hydroprene and diofenolan, that generated a BRET signal also stimulated the production of male offspring at low exposure concentrations. Daphnids exposed to 0.001 nM diofenolan (Fig. 7A), and 0.03 nM hydroprene (Fig. 7B) produced all male offspring. All other compounds that screened positive in the BRET assay have been previously shown to stimulate male sex determination in vivo 31, 32, although kinoprene did not stimulate male sex determination in our previous in vivo assay31.
Figure 7. Male offspring production in response to diofenolan and hydroprene exposure.
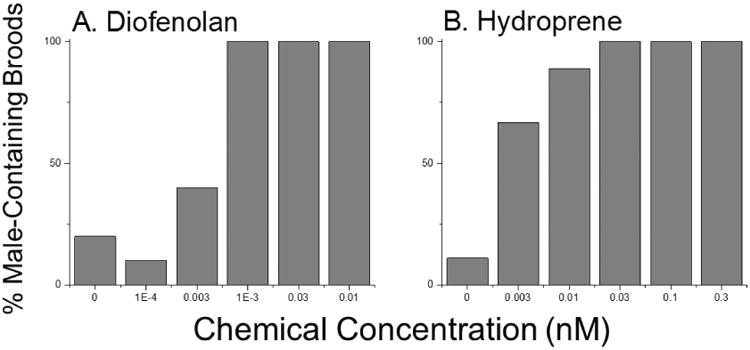
Adult female daphnids were exposed to diofenolan (A) and hydroprene (B). Data are presented as the percentage of females that produced a male-containing brood at each treatment concentration (n = 9-10).
Discussion
Screening assays that detect the potential for a chemical to elicit toxicity typically utilize an early event in a relevant toxicity pathway33, e.g. ligand-mediated gene transcription. In fact, transcriptional activation assays quantified by reporter genes are extensively used to detect nuclear receptor-chemical interactions in toxicity screening formats34-36. We have demonstrated the use of an endpoint upstream of gene transcription that is well-suited for the evaluation of chemical interactions with many different nuclear receptors.
Nuclear receptors typically function as homo- or hetero-dimers, with dimerization initiated by the binding of an activating ligand37. The BRET assay, as described herein, can be exploited to detect this initial receptor assembly response to agonist binding in living cells and in real-time with no requirement for transcription, translation, and accumulation of a reporter gene product.
We used the BRET assay to screen a battery of 29 chemicals for their ability to activate the methyl farnesoate receptor (MfR). Several of these compounds are designed for environmental use (e.g., pesticides) or designated as high production volume compounds (compounds produced or imported into the United States in quantities equal to or greater than one million pounds per year)38, 39 and thus have potential environmental exposure. Others were selected because they are known to stimulate male sex determination in daphnids or are structurally related to such compounds. In comparison to the reporter gene assay, the BRET assay was as or more efficient in detecting MfR agonists with no false negatives detected. There was one positive result from the BRET assay that was not detected using the reporter gene assay, kinoprene. Kinoprene is a member of the insect growth regulating class of insecticides, akin to all other assayed members of this chemical class which yielded positive weresults in both the BRET and reporter gene assays. This positive result likely represented greater sensitivity of the BRET assay. Furthermore, kinoprene yielded positive results in an in vivo assay for activity towards the MfR40. Based upon this limited dataset, the BRET assay appears to have comparable or greater sensitivity than the reporter gene assay (Table S2).
The MfR mediates the action of the hormone methyl farnesoate in crustaceans22 and the MfR signaling pathway is involved in larval development, metamorphosis, reproductive maturation, and sex determination of offspring21. The methyl farnesoate hormone is the unepoxidated form of an insect hormone ortholog, juvenile hormone III41. Results of the present study suggest that the MfR exhibits a high degree of ligand specificity, as the only compounds that stimulated dimerization, reporter gene activation, and in vivo responses were insect growth-regulating insecticides. These compounds are considered to elicit insecticidal activity by mimicking the action of juvenile hormone42. Further investigation is warranted to establish the extent of this apparent specificity.
All compounds that screened positive in the BRET assay have been shown to stimulate male sex determination in daphnids22, 31, 32, 43. We had not previously evaluated hydroprene and diofenolan in vivo, while, others had reported high potency of these compounds with respect to stimulating male sex determination40, 44. Therefore, we evaluated these compounds in the present study. Consistent with previous results, both compounds stimulate male sex determination at low nM concentrations.
A negative aspect of the BRET assay as compared to reporter gene assays is the lower signal intensity. Maximum BRET ratios detected in the present study were less than 10× those measured in controls. While, maximum reporter gene responses approached 50× control values. Despite the lower signal intensity, BRET results were sufficient to derive statistically discernible activation along with concentration-response relationships. Major positive attributes to the BRET assay are its rapidity, its conduciveness to real-time measurements in intact cells, and reduced cost due to reduced time commitment.
The BRET assay has demonstrated utility with other nuclear receptors such as the estrogen receptor dimer15, the peroxisome proliferator-activated receptor:retinoid × receptor heterodimer45, and the thyroid hormone receptor:retinoid × receptor heterodimer16. The assay typically has been used to assess receptor interaction with its endogenous ligand. Clearly, the methodology can be applied to the screening of environmental chemicals for activity with nuclear receptors. With proper validation46 such assays could significantly increase the capacity of screening programs47 to evaluate receptors and chemicals for interaction.
Supplementary Material
Acknowledgments
This research was supported by US EPA grant RD-835165 and NIEHS grant T32 ES007046.
Footnotes
Supporting Information: Table of the thirty compounds used in screening experiments, along with corresponding IUPAC name, commercial use and/or activity and the screening rationale.
Notes: The authors declare no competing financial interest.
References
- 1.LaLone CA, Villeneuve DL, Olmstead AW, Medlock EK, Kahl MD, Jensen KM, et al. Effects of a glucocorticoid receptor agonist, dexamethasone, on fathead minnow reproduction, growth, and development. Environ Toxicol Chem. 2012;31(3):611–622. doi: 10.1002/etc.1729. [DOI] [PubMed] [Google Scholar]
- 2.Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, et al. Effects of the androgenic growth promoter 17-β-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ Toxicol Chem. 2003;22(6):1350–1360. [PubMed] [Google Scholar]
- 3.Ye T, Kang M, Huang Q, Fang C, Chen Y, Shen H, Dong S. Exposure to DEHP and MEHP from hatching to adulthood causes reproductive dysfunction and endocrine disruption in marine medaka (Oryzias melastigma) Aquat Toxicol. 2014;146:115–126. doi: 10.1016/j.aquatox.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Olmstead AW, LeBlanc GA. The Environmental-Endocrine Basis of Gynandromorphism (Intersex) in a Crustacean. Int J Biol Sci. 2007;3(2):77–84. doi: 10.7150/ijbs.3.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olmstead AW, LeBlanc GA. Temporal and quantitative changes in sexual reproductive cycling of the cladoceran Daphnia magna by a juvenile hormone analog. J Exp Zool. 2001;290(2):148–155. doi: 10.1002/jez.1044. [DOI] [PubMed] [Google Scholar]
- 6.Gooding MP, Wilson VS, Folmar LC, Marcovich DT, LeBlanc GA. The biocide tributyltin reduces the accumulation of testosterone as fatty acid esters in the mud snail (Ilyanassa obsoleta) Environ Health Perspect. 2003;111(4):426–430. doi: 10.1289/ehp.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J, Wang C, Peng H, Zheng G, Zhang S, Hu J. p,p′-DDE induces gonadal intersex in Japanese Medaka (Oryzias latipes) at environmentally relevant concentrations: comparison with o,p′-DDT. Environ Sci Technol. 2016;50(1):462–469. doi: 10.1021/acs.est.5b05042. [DOI] [PubMed] [Google Scholar]
- 8.Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proc Nat Acad Sci USA. 2007;104(21):8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Judson RS, Magpantay FM, Chickarmane V, Haskell C, Tania N, Taylor J, et al. Integrated model of chemical perturbations of a biological pathway using 18 in vitro high-throughput screening assays for the estrogen receptor. Toxicol Sci. 2015;148(1):137–154. doi: 10.1093/toxsci/kfv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira-Fernandes A, Demaegdt H, Vandermeiren K, Hectors TLM, Jorens PG, Blust R, Vanparys C. Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS ONE. 2013;8(10):e77481. doi: 10.1371/journal.pone.0077481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerlach T, Knaust J, Kaiser C, Körner M, Hettwer K, Uhlig S, Simon K, Baronian K, Kunze G. Development and assessment of a novel Arxula adeninivorans androgen screen (A-YAS) assay and its application in analysis of cattle urine. Sci Total Environ. 2014;490:1073–1081. doi: 10.1016/j.scitotenv.2014.05.100. [DOI] [PubMed] [Google Scholar]
- 12.USEPA Endocrine Disruptor Screening Program (EDSP) Overview. https://www.epa.gov/endocrine-disruption/endocrine-disruptor-screening-program-edsp-overview.
- 13.Degitz SJ, Holcombe GW, Flynn KM, Kosian PA, Korte JJ, Tietge JE. Progress towards development of an amphibian-based thyroid screening assay using Xenopus laevis. Organismal and thyroidal responses to the model compounds 6-propylthiouracil, methimazole, and thyroxine. Toxicol Sci. 2005;87(2):353–364. doi: 10.1093/toxsci/kfi246. [DOI] [PubMed] [Google Scholar]
- 14.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83(6):841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 15.Powell E, Xu W. Intermolecular interactions identify ligand-selective activity of estrogen receptor α/β dimers. Proc Nat Acad Sci USA. 2008;105(48):19012–19017. doi: 10.1073/pnas.0807274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mulero M, Perroy J, Federici C, Cabello G, Ollendorff V. Analysis of RXR/THR and RXR/PPARG2 heterodimerization by bioluminescence resonance energy transfer (BRET) PLoS ONE. 2014;8(12):e84569. doi: 10.1371/journal.pone.0084569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem. 2010;29(3):730–741. doi: 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- 18.Tatarazako N, Oda S, Watanabe H, Morita M, Iguchi T. Juvenile hormone agonists affect the occurrence of male. Daphnia Chemosphere. 2003;53(8):827–833. doi: 10.1016/S0045-6535(03)00761-6. [DOI] [PubMed] [Google Scholar]
- 19.Olmstead AW, LeBlanc GA. Juvenoid hormone methyl farnesoate is a sex determinant in the crustacean Daphnia magna. J Exp Zool. 2002;293(7):736–739. doi: 10.1002/jez.10162. [DOI] [PubMed] [Google Scholar]
- 20.Laufer H, Biggers W. Unifying concepts learned from methyl farnesoate for invertebrate reproduction and post-embryonic development. Am Zool. 2001;(41):442–457. [Google Scholar]
- 21.LeBlanc GA. Crustacean endocrine toxicology: a review. Ecotoxicology. 2007;16(1):61–81. doi: 10.1007/s10646-006-0115-z. [DOI] [PubMed] [Google Scholar]
- 22.LeBlanc GA, Wang YH, Holmes CN, Kwon G, Medlock EK. A transgenerational endocrine signaling pathway in crustacea. PLoS ONE. 2013;8(4):e61715. doi: 10.1371/journal.pone.0061715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyakawa H, Toyota K, Hirakawa I, Ogino Y, Miyagawa S, Oda S, Tatarazako N, Miura T, Colbourne JK, Iguchi T. A mutation in the receptor methoprene-tolerant alters juvenile hormone response in insects and crustaceans. Nat Commun. 2013;4:1856. doi: 10.1038/ncomms2868. [DOI] [PubMed] [Google Scholar]
- 24.Medlock Kakaley EK, Wang HY, Le Blanc GA. Agonist-mediated assembly of the crustacean methyl farnesoate receptor. Scientific Reports. 2017;7:45071. doi: 10.1038/srep45071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfleger KDG, Eidne KA. Illuminating insights into protein-protein interactions using bioluminescence resonance energy transfer (BRET) Nat Meth. 2006;3(3):165–174. doi: 10.1038/nmeth841. [DOI] [PubMed] [Google Scholar]
- 26.Pfleger KDG, Seeber RM, Eidne KA. Bioluminescence resonance energy transfer (BRET) for the real-time detection of protein-protein interactions. Nat Protocols. 2006;1(1):337–345. doi: 10.1038/nprot.2006.52. [DOI] [PubMed] [Google Scholar]
- 27.Breton B, Sauvageau É, Zhou J, Bonin H, Le Gouill C, Bouvier M. Multiplexing of Multicolor Bioluminescence Resonance Energy Transfer. Biophys J. 2010;99(12):4037–4046. doi: 10.1016/j.bpj.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramsay D, Kellet E, McVey M, Rees S, Milligan G. Homo- and hetero-oligomeric interactions between G-protein-coupled receptors in living cells monitored by two variants of bioluminescence resonance energy transfer (BRET) Biochem J. 2002;365(2):429–440. doi: 10.1042/BJ20020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YH, Wang G, LeBlanc GA. Cloning and characterization of the retinoid × receptor from a primitive crustacean. Daphnia magna Gen Comp Endocrinol. 2007;150(2):309–318. doi: 10.1016/j.ygcen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Olmstead AW, LeBlanc GA. Insecticidal juvenile hormone analogs stimulate the production of male offspring in the crustacean. Daphnia magna. 2003;111(7):919–924. doi: 10.1289/ehp.5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HY, Olmstead AW, Li H, LeBlanc GA. The screening of chemicals for juvenoid-related endocrine activity using the water flea. Daphnia magna Aquat Toxicol. 2005;74(3):193–204. doi: 10.1016/j.aquatox.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T. Production of male neonates in four cladoceran species exposed to a juvenile hormone analog, fenoxycarb. Chemosphere. 2005;60(1):74–78. doi: 10.1016/j.chemosphere.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 33.NRC. Toxicity testing in the 21st century: a vision and strategy. The National Adademies Press; Washington, DC, USA: 2007. [Google Scholar]
- 34.Legler J, Zeinstra LM, Schuitemaker F, Lanser PH, Bogerd J, Brouwer A, Vethaak AD, de Voogt P, Murk AJ, van der Burg B. Comparison of in vivo and in vitro reporter gene assays for short-term screening of estrogenic activity. Environ Sci Technol. 2002;36(20):4410–4415. doi: 10.1021/es010323a. [DOI] [PubMed] [Google Scholar]
- 35.Wilson VS, Bobseine K, Lambright CR, Gray JLE. A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol Sci. 2002;66(1):69–81. doi: 10.1093/toxsci/66.1.69. [DOI] [PubMed] [Google Scholar]
- 36.Du G, Shen O, Sun H, Fei J, Lu C, Song L, Xia Y, Wang S, Wang X. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol Sci. 2010;116(1):58–66. doi: 10.1093/toxsci/kfq120. [DOI] [PubMed] [Google Scholar]
- 37.Robinson-Rechavi M, Garcia HE, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116(4):585–586. doi: 10.1242/jcs.00247. [DOI] [PubMed] [Google Scholar]
- 38.Green S, Goldberg AM, Zurlo J. The TestSmart–HPV Program—Development of an integrated approach for testing high production volume chemicals. Regul Toxicol Pharmacol. 2001;33(2):105–109. doi: 10.1006/rtph.2000.1435. [DOI] [PubMed] [Google Scholar]
- 39.USEPA High Production Volume Information System (HPVIS) [accessed May 2016]; https://ofmpub.epa.gov/oppthpv/hpv_hc_characterization.get_report_by_cas?doctype=2.
- 40.Oda S, Tatarazako N, Watanabe H, Morita M, Iguchi T. Production of male neonates in Daphnia magna (Cladocera, Crustacea) exposed to juvenile hormones and their analogs. Chemosphere. 2005;61(8):1168–1174. doi: 10.1016/j.chemosphere.2005.02.075. [DOI] [PubMed] [Google Scholar]
- 41.Laufer H, Sagi A, Ahl JSB, Homola E. Methyl farnesoate appears to be a crustacean reproductive hormone. Invertebr Reprod Dev. 1992;22(1-3):17–19. [Google Scholar]
- 42.Dhadialla TS, Carlson GR, Le DP. New insecticides with ecdysteroidal and juvenile hormone activity. Annu Rev Entomol. 1998;43(1):545–569. doi: 10.1146/annurev.ento.43.1.545. [DOI] [PubMed] [Google Scholar]
- 43.Abe R, Watanabe H, Yamamuro M, Iguchi T, Tatarazako N. Establishment of a short-term, in vivo screening method for detecting chemicals with juvenile hormone activity using adult Daphnia magna. J Appl Toxicol. 2015;35(1):75–82. doi: 10.1002/jat.2989. [DOI] [PubMed] [Google Scholar]
- 44.Abe R, Toyota K, Miyakawa H, Watanabe H, Oka T, Miyagawa S, et al. Diofenolan induces male offspring production through binding to the juvenile hormone receptor in Daphnia magna. Aquat Toxicol. 2015;159:44–51. doi: 10.1016/j.aquatox.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Wang YH, Kwon G, An L, Holmes CN, Haeba M, Le Blanc GA. Differential interactions of the flame retardant triphenyl phosphate within the PPAR signaling network. MOJ Toxicol. 2016;2(3):00039. [Google Scholar]
- 46.Judson R, Kavlock R, Martin M, Reif D, Houck K, Knudsen T, et al. Perspectives on validation of high-throughput assays supporting 21(st) century toxicity testing. ALTEX. 2013;30(1):51–56. doi: 10.14573/altex.2013.1.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol Sci. 2007;95(1):5–12. doi: 10.1093/toxsci/kfl103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


