Abstract
Objective
To estimate the minimal clinically important difference (MCID) for 6-minute walk distance (6MWD) in patients with fibromyalgia (FM).
Design
Data from a recently completed trial that included 187 patients who completed the 6-minute walk test, Fibromyalgia Impact Questionnaire (FIQ), and Short-Form 36 (SF36) at 12 and 36 weeks were used to examine longitudinal changes in 6MWD. An anchor-based approach that used linear regression analyses was used to determine the MCID for 6MWD, using the total FIQ score (FIQ-Total) and SF36-physical function domain (SF36-PF) as clinical anchors.
Results
The mean (SD) change in 6MWD from baseline to week 36 was 34.4 (65.2) m (p<0.001). The anchor-based MCID’s for the 6MWD were 156 m and 167 m for the FIQ and SF36-PF, respectively. These MCID’s correspond with clinically meaningful improvements in FIQ (14% reduction) and SF36-PF (10 point increase).
Conclusion
The MCID for 6MWD in patients with FM was 156 to 167 m. These findings provide the first evidence of the change in 6MWD that is perceived by patients to be clinically meaningful. Further research using other MCID calculation methods is needed to refine estimates of the MCID for 6MWD in patients with FM.
Keywords: Fibromyalgia, 6-minute walk distance, Exercise test, Minimal clinically important difference
INTRODUCTION
Fibromyalgia (FM) is a complex rheumatologic syndrome characterized by chronic widespread pain and other non-specific symptoms, including fatigue, morning stiffness, cognitive difficulties, sleep disturbance, and exercise intolerance1. As the disorder worsens, many patients spiral downward as these symptoms typically exacerbate an already sedentary lifestyle that leads to progressive declines in functional performance2,3 and exercise capacity4. For many individuals, the ability to complete occupational tasks and/or daily living activities is significantly reduced, resulting in a diminished quality of life1.
The diagnosis of FM remains a challenge, particularly in primary care settings. Because of its complex nature and unknown etiology, an approach that uses a variety of tools is necessary in the clinical examination and evaluation of patients. In recent years, the inclusion of physical fitness testing has been recommended as a complementary tool for the diagnosis and monitoring of FM5–8. While most patients with FM can participate in symptom-limited maximal exercise tests to assess exercise capacity, many are deconditioned4,9, report increased symptoms during (and after) moderate-vigorous exercise10, or have a heightened perception of effort, particularly at higher exercise intensities10,11. For these reasons, submaximal exercise testing is generally preferred, as many patients with FM may not be intrinsically motivated to exercise to volitional fatigue.
The 6-minute walk test (6MWT) has gained widespread acceptance in the clinical community as a simple, practical, and inexpensive option for measuring functional exercise capacity in disease-based populations known to experience exercise intolerance. It requires no specialized equipment or advanced training, is more reflective of activities of daily living (ADLs), and is better tolerated by patients12. In FM research, the distance walked in six minutes (6MWD) has shown to be a clinically relevant measure that is reproducible, sensitive to change, and significantly related to the Fibromyalgia Impact Questionnaire (FIQ), Short-Form Health Survey 36 (SF36), and peak exercise capacity6,7,13–17. However, despite its widespread use as a clinical outcome measure in clinical trials of FM, the minimum amount of change in 6MWD necessary for patients to perceive an improvement in functional status remains unknown.
The concept of the minimal clinically important difference (MCID) was developed to help clinicians interpret changes in health status. The MCID of a specific instrument represents the smallest level of change that patients perceive as beneficial, and that might lead a clinician to consider changing a patient’s medical management18. Although no universally accepted standards exist for determining the MCID, most methods fall into two global categories19,20. Distribution-based methods rely on the statistical properties of the study results in a population to estimate the effect size, standardized response mean, or standard error of the measurement. One advantage of distribution-based approaches is the ability to account for change beyond some level of random variation. Additionally, this approach provides a common metric that is simple to calculate, easily interpretable, and has equivalent meaning across various measures and populations. However, distribution-based methods do not take into account the patient’s perspective of clinically important change. Thus, while MCID scores produced from distribution-based methods may produce statistical significance, they may lack clinical meaning. Conversely, anchor-based methods map change scores of a particular outcome measure to changes in clinical measures that are patient-specific. Global ratings of change scores are commonly used as anchors to define the MCID when attempting to identify change within a patient19,21. These changes can be calculated at a single point in time (cross-sectional), or across multiple time points (longitudinal). The latter approach is generally preferred as it is more directly linked to change. The primary advantage of the anchor-based approach is that it provides confidence that the identified changes in health status are clinically meaningful to patients. Importantly, for anchors to be useful, they must be valid and reliable, easily interpretable, and closely related to the outcome measure20.
Establishment of the MCID for 6MWD would aid the clinician’s ability to interpret improvement in measures of functional status after the implementation of a particular treatment. In addition, the MCID has implications for the design of clinical trials, in terms of sample size calculation and the selection of primary and secondary endpoints. Therefore, the aim of this study was to determine the MCID for the 6MWD in a large, well-defined sample of patients diagnosed with FM. This study used data from the Research to Encourage Exercise for Fibromyalgia (REEF) study, a randomized clinical trial that evaluated the efficacy of motivational interviewing on improving physical activity adherence in adults with FM22. In this trial, the 6MWT was used as a secondary outcome measure of functional exercise capacity. To estimate the MCID for 6MWD, an anchor-based approach was used, as this method aims to assess clinical significance by incorporating the perspective of the patient19,23.
MATERIALS AND METHODS
Study Design
This was a secondary analysis of data from a recently completed randomized, attention-controlled clinical trial of the efficacy of motivational interviewing (MI) to increase physical activity participation in patients with FM22. In the REEF study, eligible patients were randomized to either the MI intervention group or an education-based attention control (AC) group. Each patient received an individualized exercise prescription and two supervised exercise sessions from a qualified fitness instructor who was blinded to treatment assignment. After completing both exercise sessions, patients received either six exercise-based (MI group) or six FM-related health education (AC group) telephone calls over the subsequent 12 weeks. Outcome assessments were conducted at baseline, immediate post-intervention (week 12), and 6-month follow-up (week 36). All participants gave written informed consent after being clearly advised about the study protocol, which was approved by the Indiana University Institutional Review Board and conformed to the principles outlined in the Declaration of Helsinki.
Study Participants
The complete details of participant recruitment and eligibility criteria have been described in detail previously24. In brief, patients referred from specialty (rheumatology, neurology, pain management) or primary care clinics with an initial diagnosis of FM were invited to participate. All eligible patients met the following entry criteria: (a) male or female between 18–65 years old; (b) 1990 American College of Rheumatology classification criteria for FM25; and (c) Brief Pain Inventory (BPI) pain intensity core ≥ 426. Of the 216 patients enrolled in the primary study, 187 (86.5%) had complete baseline and follow-up data for all clinical and exercise capacity outcome measures and were included in this report. There were no significant differences in any baseline variable for patients excluded from the final analyses.
Measurement of Six-Minute Walk Distance (6MWD)
The 6MWT was performed indoors on 30-meter flat surface in accordance with guidelines recommended by the American Thoracic Society27. To improve reliability, each participant completed two trials of the 6MWT at study entry (baseline), immediate post-intervention (week 12), and at the 6-month follow-up (week 36)13,15. A 15-minute rest period separated each trial and the average of the two trials was recorded (meters) as the total distance walked. Participants were not made aware of how long they had walked for any trial.
Fibromyalgia Impact Questionnaire (FIQ)
The FIQ is a reliable, validated, self-administered questionnaire designed to assess several dimensions of health status that are most affected by FM28,29. The FIQ contains 10 subscales (score range 0–10), which are summed to yield the total FIQ score (FIQ-Total). The FIQ-physical impairment (FIQ-PI) subscale assesses the patient’s ability to perform different types of physical activity. Higher scores on each of the subscales and the FIQ-Total indicate a greater severity of symptoms and/or a higher negative impact of FM on the individual. A 14% change in the FIQ-Total score signifies a clinically meaningful change in FM status30.
Short-Form 36 (SF36)
The SF36 is a reliable and validated self-report questionnaire that assesses health-related quality of life. It contains 36 items grouped into eight dimensions: health, including physical function, body pain, role physical, role emotional, general health, vitality, social functioning, and mental heath31,32. These domains are combined to provide a total SF36 score, where a higher score indicates a better health outcome. For this study, the Physical Function subscale (SF36-PF) was used, which asks individuals if their health limits physical activity, basic mobility, and the ability to perform daily living activities. A 10-point increase in the SF36-PF domain score has been identified as a clinically relevant change in patients with chronic illnesses33.
Statistical Analyses
Descriptive statistics were calculated at baseline for all subjects with non-missing data. Change scores for each study variable were calculated by subtracting the baseline score from the follow-up score. A positive change score for SF36-PF indicates improvement in physical function. Conversely, negative change scores for FIQ-Total and FIQ-PI indicate an improvement in functional status.
To estimate the MCID for 6MWD, the FIQ-Total and SF36-PF were chosen as anchors since both instruments reflect global ratings of health, are responsive to change, and have established MCID’s30,33. External responsiveness was assessed using Pearson’s correlation to determine if the change in 6MWD was significantly associated with patient-reported clinical outcomes at each follow-up period, as well as with the change in these outcomes from baseline to follow-up. Because the usefulness of an anchor is dependent on how closely it relates to the target outcome19, correlation analyses (p<0.05) were used to assess the utility of these anchors. Using change in the anchors as the independent variables and change in 6MWD as the dependent variable, linear regression analyses, adjusted for treatment group assignment (MI vs. attention control), were performed to determine what change in 6MWD (from baseline to week 36) was equivalent to a clinically meaningful change in SF36-PF and FIQ-Total30,33. All analytic assumptions were verified and met. Analyses were performed using SAS v9.3 (SAS Institute, Cary, NC).
RESULTS
Of the 216 patients enrolled in the REEF trial, we included 187 patients who had no missing data for the 6MWT and all clinical outcome measures at baseline and all follow-up visits. The majority of participants were middle-aged females (95.2%) and white (87.2%) with moderate-to-severe physical impairment [(FIQ-PI=5.4 (1.6)]. The mean (SD) age at study entry was 46 (11) years; most had some education beyond high school (80.2%), and slightly more than half were employed at least part-time (54.6%). Descriptive characteristics for all participants are presented in Table 1.
Table 1.
Patient Characteristics (n=187)
| Demographics | |
|---|---|
| Age, years | 45.76 (11.01) |
| Gender, % female | 178 (95.2) |
| Race, % white | 163 (87.2) |
| Body Mass Index (kg/m2) | 31.30 (7.22) |
| Education, % > high school | 150 (80.2) |
| Marital status, % married | 114 (61.0) |
| Employment; % employed | 102 (54.6) |
| Duration of FM diagnosis (years) | 8.94 (6.52) |
| Medications, % prescribed | |
| Nontricyclic antidepressants | 101 (54.0) |
| Anticonvulsants | 56 (30.0) |
| Opioid analgesics | 59 (31.6) |
Values are means (standard deviation) for continuous variables and frequency (percent) for categorical variables.
Significant improvements were observed from baseline to week 12 in the average distance walked on the 6MWT (Δ=25.3±64.9) and clinical outcomes FIQ-Total (Δ=−12.3±16.2), FIQ-PI (Δ=−1.5±2.1), and SF36-PF (Δ=−10.6±16.7) (all p<0.001). Similar mean changes and p-values were observed in these outcomes from baseline to week 36 (Table 2).
Table 2.
Longitudinal changes in 6MWD and self-report clinical outcomes
| Measurement | Baseline | Wk 12 | Wk 36 | Mean change Baseline – Wk 36 |
p value |
|---|---|---|---|---|---|
| 6MWD (m) | 482.9 (80.5) | 510.8 (84.4) | 517.7 (80.9) | 34.4 (65.2) | <0.001 |
| FIQ-Total (0–100)† | 66.4 (12.6) | 54.4 (18.4) | 53.0 (20.6) | −13.8 (18.7) | <0.001 |
| FIQ-PI (0–10)† | 5.4 (1.6) | 3.9 (2.0) | 3.8 (2.4) | −1.6 (2.3) | <0.001 |
| SF36-PF (0–100)‡ | 41.5 (19.2) | 51.4 (21.5) | 52.2 (22.8) | 11.4 (20.3) | <0.001 |
Values are the means (standard deviation) unless otherwise indicated. Mean change is from baseline to week 36.
Higher score indicates a worse state of health.
Lower score indicates a worse state of health.
Abbreviations: 6MWD = six-minute walk distance; Mean change = change from baseline to week 36; FIQ = Fibromyalgia Impact Questionnaire (PI, physical impairment); SF36-PF = Short Form 36 (Physical Function).
Table 3 shows the correlations between changes in 6MWD and changes in clinical outcomes, assessed from baseline to follow-up. Significant associations were observed between change in 6MWD from baseline to week 12 and changes in FIQ-PI (ρ=−0.18) and SF36-PF (ρ=0.20). From baseline to week 36, significant associations were observed between change in 6MWD and changes in all clinical outcomes: FIQ-Total (ρ=−0.20), FIQ-PI (ρ=−0.25), and SF36-PF (ρ=0.20).
Table 3.
Correlations between changes in 6MWD and changes in self-report clinical outcomes
| Change (Δ) 6MWD (Baseline to Wk 12) | Pearson’s ρ (95% CI) | p-value |
|---|---|---|
| Δ FIQ-Total | −0.108 (−0.248, 0.036) | 0.142 |
| Δ FIQ-PI | −0.184 (−0.319, −0.040) | 0.012 |
| Δ SF36-PF | 0.201 (0.059, 0.335) | 0.006 |
| FIQ Ratio | −0.089 (−0.229, 0.056) | 0.229 |
| Δ 6MWD (Baseline to Wk 36) | ||
| Δ FIQ-Total | −0.201 (−0.336, −0.056) | 0.007 |
| Δ FIQ-PI | −0.247 (−0.378, −0.105) | 0.001 |
| Δ SF36-PF | 0.200 (0.056, 0.335) | 0.007 |
| FIQ Ratio | −0.203 (−0.338, −0.059) | 0.006 |
Abbreviations: 6MWD = six-minute walk distance; FIQ = Fibromyalgia Impact Questionnaire (PI, physical impairment); SF36-PF = Short Form 36 (Physical Function); FIQ Ratio = percentage change in FIQ-Total from baseline to follow-up.
As significant correlations were found between the change in 6MWD and changes in FIQ-Total and SF36-PF at week 36, it was reasonable to use these measures as anchors for estimating the MCID for 6MWD. Regression analysis results indicate that to see a 14% decrease in FIQ-Total, the 6MWD would have to increase by 156 meters (FIQ-Total = 0.82 – 0.0009(Δ6MWD); 95% CI for slope: −0.0015, −0.0003) (Figure 1). For a 10-point increase in SF36-PF, the 6MWD would have to change by 167 meters (ΔSF36-PF = 10.03 + 0.06(Δ6MWD); 95% CI for slope: 0.02, 0.10) (Figure 2).
Figure 1.
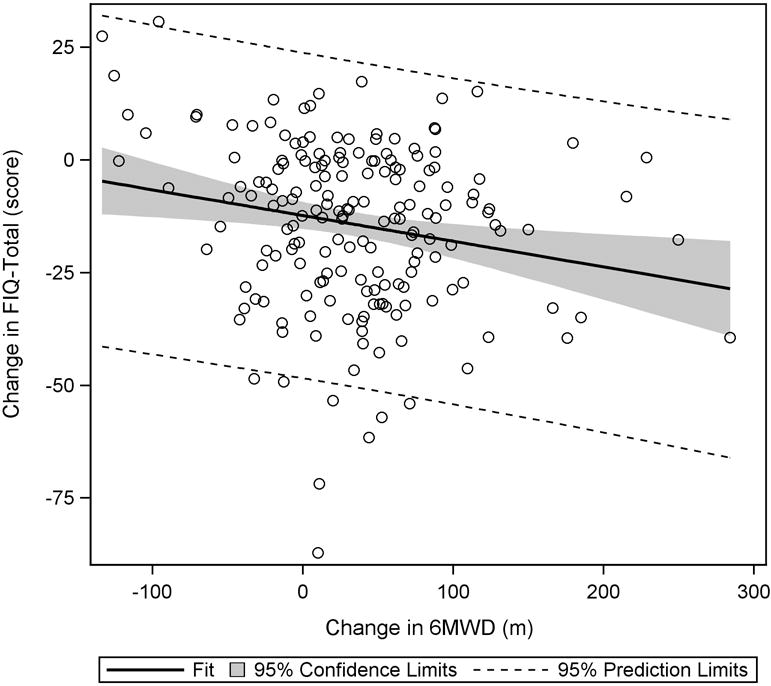
Plot of change in FIQ-Total to change in 6MWD. From baseline to week 36, a significant negative trend was observed, indicating that as 6MWD increased, FIQ-Total scores decreased.
Figure 2.
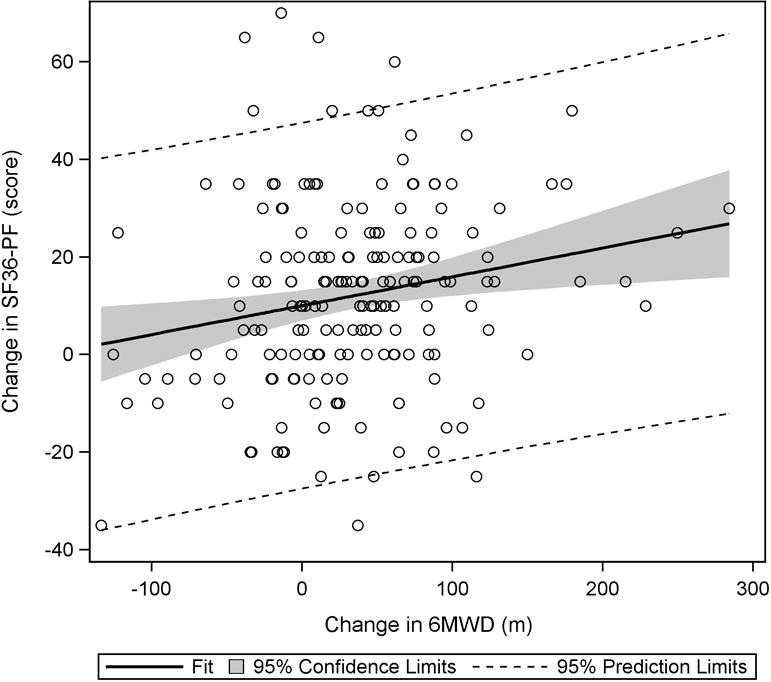
Plot of change in SF36-PF vs. change in 6MWD. From baseline to week 36, a significant positive trend was observed, indicating that as 6MWD increased, scores on the SF36-PF subscale also increased.
DISCUSSION
This is the first study to investigate the change in 6MWD that is necessary to be considered clinically meaningful in patients with FM. Using an anchor-based, longitudinal model approach with well-established clinical outcomes as anchors, and 6MWD as the target outcome measure, the MCID for 6MWD was determined to be 156 meters for the FIQ-Total and 167 meters for the SF36-PF. Significant correlations observed between 6MWD and self-report measures FIQ-Total and SF36-PF provide further support that the changes in walking distance were clinically meaningful.
Self-report questionnaires are commonly used in intervention-based clinical trials for FM to evaluate the efficacy of treatment on global ratings of pain, physical function, and quality of life. Unfortunately, recall bias may hinder the clinician’s ability to interpret meaningful change in health status from these questionnaires, as many patients with FM report subjective cognitive deficits in attention, concentration, and memory34–36. Linking an objective measure of function, such as the 6MWT, may aid interpretation of changes in health status identified in subjective measures. The 6MWT is frequently used in clinical trials for FM and has increasingly been recommended as a complementary tool in the clinical diagnosis, evaluation, and management of patients with FM5–8. In this regard, the ability to identify a stable, universally accepted MCID for 6MWD in FM is an appealing concept for researchers and clinicians.
Estimates of the MCID for 6MWD in FM are notably absent in the published literature; however, several studies have provided scores in other clinical populations37–43. A study on patients referred to cardiac rehabilitation with acute coronary syndrome found that the MCID for 6MWD was 25 m. This estimate was similar to that recently reported in patients with heart failure (30 m)40. In patients with lung disease, several studies have reported MCID estimates ranging from 10 to 80 m37,39,41–43. Compared to these studies, the MCID thresholds for 6MWD calculated in this study are notably higher, and possibly unattainable, considering the majority of patients with FM participate in low levels of physical activity and have below average exercise capacities4. In this regard, the 6MWT may have limited utility in clinical trials that aim to use change in walking distance as an outcome measure to interpret clinically meaningful change in patient health status. However, clinicians should be aware that MCID scores are not universal characteristics that can be transferred or compared across patient populations44. Rather, the MCID is context-specific, heavily influenced by several factors, including patient baseline and demographic characteristics, disease process and severity, the construct measured, and the instrument used21,44,45. Furthermore, the wide range of available calculation methods likely contributes to the large variability in MCID scores46. For example, Terwee et al.46 demonstrated that within the same patient group, the use of multiple MCID calculation methods on a single outcome measure produced a broad range of MCID scores. Thus, as the MCID is an evolving and complicated concept, clinicians should be knowledgeable of the various factors affecting MCID scores, as well as the strengths and limitations of current methodologies prior to use in the clinical setting.
This study does have several limitations that should be taken into consideration. First, to improve stability and reliability of walking distance scores, participants performed two trials of the 6MWT13,15. Given that fatigue is common in FM, the addition of a second trial of the 6MWT may have negatively influenced the mean walking distance performance in some patients. However, the first and the second trials were very highly correlated (r=0.92, p<0.001) and regression analysis showed that the slope (i.e., relationship between the first and second trials) was not significantly different from one. These findings suggest that fatigue did not interfere with the repeatability (or reproducibility) of the test, and that two trials of the 6MWT are unnecessary. Second, the REEF study included mostly female patients reporting a higher degree of pain severity. Thus, the results from this study may not be generalizable to male patients with FM, or those reporting less severe pain. In addition, the utility of the 6MWT for detecting clinically meaningful change may be limited in patients with higher exercise capacities due to an observed ceiling effect and resultant inability to substantially improve walking performance. However, this study sample included patients with clinical characteristics similar to other psychoeducational-based clinical trials for FM47; thus, it’s likely these findings have broad applicability. Third, the magnitude of the correlations between changes in 6MWD and changes FIQ-Total and SF36-PF were small. Some investigators have suggested an arbitrary minimum correlation of 0.3 or higher48,49; however, no consensus exists regarding the minimum threshold strength of the association. Given the multifaceted nature of FM and that significant associations were observed between change in 6MWD and changes in FIQ-Total and SF36-PF, the use of these measures as anchors was considered appropriate for estimating the MCID. Finally, this study was derived from a randomized controlled trial of exercise adherence that was not primarily designed to estimate the MCID for 6MWD. Thus, it’s unclear if these findings would be similar within the framework of other medical or exercise-based interventions. A prospective study, powered based on the MCID estimates presented here, would be an appropriate next step to validate the findings in this study. Despite these limitations, the strengths of this study include its longitudinal design, large sample size and longer study duration, and documented improvement in 6MWD from baseline to follow-up.
CONCLUSION
The 6MWT is a simple test of functional exercise capacity that is widely used in clinical trials for FM to assess the degree of dysfunction and response to medical and exercise interventions. To our knowledge, this is the first study to provide initial evidence of the change in 6MWD that is perceived by patients to be clinically meaningful. These findings have important research and clinical implications. For researchers, the MCID may assist in the design of future clinical trials that use change in 6MWD as an important outcome measure. Clinically, the MCID for 6MWD could be used to establish therapeutic thresholds that are objective, measurable, and patient-centered to evaluate the efficacy of various medical or exercise-based interventions. While these findings have the potential to aid clinical researchers in health outcomes research, further investigation using other anchors and calculation methods is needed to refine estimates of the MCID for 6MWD in this patient population.
Acknowledgments
Funding Support: National Institute of Arthritis & Musculoskeletal and Skin Diseases (1RO1AR054324-01A1)
References
- 1.Lachaine J, Beauchemin C, Landry PA. Clinical and economic characteristics of patients with fibromyalgia syndrome. Clin J Pain. 2010;26(4):284–290. doi: 10.1097/AJP.0b013e3181cf599f. [DOI] [PubMed] [Google Scholar]
- 2.Maquet D, Croisier JL, Renard C, Crielaard JM. Muscle performance in patients with fibromyalgia. Joint Bone Spine. 2002;69(3):293–299. doi: 10.1016/s1297-319x(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 3.Panton LB, Kingsley JD, Toole T, et al. A comparison of physical functional performance and strength in women with fibromyalgia, age- and weight-matched controls, and older women who are healthy. Phys Ther. 2006;86(11):1479–1488. doi: 10.2522/ptj.20050320. [DOI] [PubMed] [Google Scholar]
- 4.Valim V, Oliveira LM, Suda AL, et al. Peak oxygen uptake and ventilatory anaerobic threshold in fibromyalgia. J Rheumatol. 2002;29(2):353–357. [PubMed] [Google Scholar]
- 5.Aparicio VA, Segura-Jimenez V, Alvarez-Gallardo IC, et al. Fitness Testing in the Fibromyalgia Diagnosis: The al-Andalus Project. Med Sci Sports Exerc. 2015;47(3):451–459. doi: 10.1249/MSS.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 6.Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2007;(4):CD003786. doi: 10.1002/14651858.CD003786.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Mannerkorpi K, Svantesson U, Broberg C. Relationships between performance-based tests and patients’ ratings of activity limitations, self-efficacy, and pain in fibromyalgia. Arch Phys Med Rehabil. 2006;87(2):259–264. doi: 10.1016/j.apmr.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Latorre-Roman P, Santos-Campos M, Heredia-Jimenez J, Delgado-Fernandez M, Soto-Hermoso V. Analysis of the performance of women with fibromyalgia in the six-minute walk test and its relation with health and quality of life. J Sports Med Phys Fitness. 2014;54(4):511–517. [PubMed] [Google Scholar]
- 9.Norregaard J, Bulow PM, Lykkegaard JJ, Mehlsen J, Danneskiold-Samsooe B. Muscle strength, working capacity and effort in patients with fibromyalgia. Scand J Rehabil Med. 1997;29(2):97–102. [PubMed] [Google Scholar]
- 10.Cook DB, Stegner AJ, Nagelkirk PR, Meyer JD, Togo F, Natelson BH. Responses to exercise differ for chronic fatigue syndrome patients with fibromyalgia. Med Sci Sports Exerc. 2012;44(6):1186–1193. doi: 10.1249/MSS.0b013e3182417b9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielens H, Boisset V, Masquelier E. Fitness and perceived exertion in patients with fibromyalgia syndrome. Clin J Pain. 2000;16(3):209–213. doi: 10.1097/00002508-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119(1):256–270. doi: 10.1378/chest.119.1.256. [DOI] [PubMed] [Google Scholar]
- 13.King S, Wessel J, Bhambhani Y, Maikala R, Sholter D, Maksymowych W. Validity and reliability of the 6 minute walk in persons with fibromyalgia. J Rheumatol. 1999;26(10):2233–2237. [PubMed] [Google Scholar]
- 14.Pankoff BA, Overend TJ, Lucy SD, White KP. Reliability of the six-minute walk test in people with fibromyalgia. Arthritis Care Res. 2000;13(5):291–295. doi: 10.1002/1529-0131(200010)13:5<291::aid-anr8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 15.Pankoff B, Overend T, Lucy D, White K. Validity and responsiveness of the 6 minute walk test for people with fibromyalgia. J Rheumatol. 2000;27(11):2666–2670. [PubMed] [Google Scholar]
- 16.Ratter J, Radlinger L, Lucas C. Several submaximal exercise tests are reliable, valid and acceptable in people with chronic pain, fibromyalgia or chronic fatigue: a systematic review. J Physiother. 2014;60(3):144–150. doi: 10.1016/j.jphys.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Carbonell-Baeza A, Ruiz JR, Aparicio VA, Ortega FB, Delgado-Fernandez M. The 6-minute walk test in female fibromyalgia patients: relationship with tenderness, symptomatology, quality of life, and coping strategies. Pain Manag Nurs. 2013;14(4):193–199. doi: 10.1016/j.pmn.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controll Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 19.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 20.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Clinical Significance Consensus Meeting G. Methods to explain the clinical significance of health status measures. Mayo Clin Proc. 2002;77(4):371–383. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 21.Copay AG, Subach BR, Glassman SD, Polly DW, Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Ang DC, Kaleth AS, Bigatti S, et al. Research to encourage exercise for fibromyalgia (REEF): use of motivational interviewing, outcomes from a randomized-controlled trial. Clin J Pain. 2013;29(4):296–304. doi: 10.1097/AJP.0b013e318254ac76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turner D, Schunemann HJ, Griffith LE, et al. The minimal detectable change cannot reliably replace the minimal important difference. J Clin Epidemiol. 2010;63(1):28–36. doi: 10.1016/j.jclinepi.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Ang DC, Kaleth AS, Bigatti S, et al. Research to Encourage Exercise for Fibromyalgia (REEF): use of motivational interviewing design and method. Contemp Clin Trials. 2011;32(1):59–68. doi: 10.1016/j.cct.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfe F, Smythe HA, Yunus MB, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 26.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 27.American Thoracic Society. ATS Statement: Guidelines for the Six-Minute Walk Test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 28.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S154–162. [PubMed] [Google Scholar]
- 29.Burckhardt CS, Clark SR, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18(5):728–733. [PubMed] [Google Scholar]
- 30.Bennett RM, Bushmakin AG, Cappelleri JC, Zlateva G, Sadosky AB. Minimal clinically important difference in the fibromyalgia impact questionnaire. J Rheumatol. 2009;36(6):1304–1311. doi: 10.3899/jrheum.081090. [DOI] [PubMed] [Google Scholar]
- 31.Lyons RA, Perry HM, Littlepage BN. Evidence for the validity of the Short-form 36 Questionnaire (SF-36) in an elderly population. Age Ageing. 1994;23(3):182–184. doi: 10.1093/ageing/23.3.182. [DOI] [PubMed] [Google Scholar]
- 32.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res. 2005;40(2):577–591. doi: 10.1111/j.1475-6773.2005.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glass JM. Cognitive dysfunction in fibromyalgia and chronic fatigue syndrome: new trends and future directions. Curr Rheumatol Rep. 2006;8(6):425–429. doi: 10.1007/s11926-006-0036-0. [DOI] [PubMed] [Google Scholar]
- 35.Leavitt F, Katz RS. Distraction as a key determinant of impaired memory in patients with fibromyalgia. J Rheumatol. 2006;33(1):127–132. [PubMed] [Google Scholar]
- 36.Sephton SE, Studts JL, Hoover K, et al. Biological and psychological factors associated with memory function in fibromyalgia syndrome. Health Psychol. 2003;22(6):592–597. doi: 10.1037/0278-6133.22.6.592. [DOI] [PubMed] [Google Scholar]
- 37.du Bois RM, Weycker D, Albera C, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183(9):1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 38.Gremeaux V, Troisgros O, Benaim S, et al. Determining the minimal clinically important difference for the six-minute walk test and the 200-meter fast-walk test during cardiac rehabilitation program in coronary artery disease patients after acute coronary syndrome. Arch Phys Med Rehabil. 2011;92(4):611–619. doi: 10.1016/j.apmr.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221–225. doi: 10.1016/j.apmr.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 40.Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six-minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther. 2013;24(3):21–29. [PMC free article] [PubMed] [Google Scholar]
- 41.Nathan SD, du Bois RM, Albera C, et al. Validation of test performance characteristics and minimal clinically important difference of the 6-minute walk test in patients with idiopathic pulmonary fibrosis. Respir Med. 2015;109(7):914–922. doi: 10.1016/j.rmed.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD. 2005;2(1):125–129. doi: 10.1081/copd-200050527. [DOI] [PubMed] [Google Scholar]
- 43.Swigris JJ, Wamboldt FS, Behr J, et al. The 6 minute walk in idiopathic pulmonary fibrosis: longitudinal changes and minimum important difference. Thorax. 2010;65(2):173–177. doi: 10.1136/thx.2009.113498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright A, Hannon J, Hegedus EJ, Kavchak AE. Clinimetrics corner: a closer look at the minimal clinically important difference (MCID) J Man Manip Ther. 2012;20(3):160–166. doi: 10.1179/2042618612Y.0000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskelet Disord. 2006;7:82. doi: 10.1186/1471-2474-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terwee CB, Roorda LD, Dekker J, et al. Mind the MIC: large variation among populations and methods. J Clin Epidemiol. 2010;63(5):524–534. doi: 10.1016/j.jclinepi.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151(2):280–295. doi: 10.1016/j.pain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 48.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Schunemann HJ, Griffith L, Jaeschke R, Goldstein R, Stubbing D, Guyatt GH. Evaluation of the minimal important difference for the feeling thermometer and the St. George’s Respiratory Questionnaire in patients with chronic airflow obstruction. J Clin Epidemiol. 2003;56(12):1170–1176. doi: 10.1016/s0895-4356(03)00115-x. [DOI] [PubMed] [Google Scholar]


