Abstract
Emerging evidence shows that microbe interactions with the host immune system impact diverse aspects of cancer development and treatment. As a result, exciting new opportunities exist for engineering diets and microbe cocktails to lower cancer risks with fewer adverse clinical effects than traditional strategies. Microbe-based therapies may ultimately be used to reinforce host immune balance and extinguish cancer for generations to come.
Keywords: Bacteria, Neutrophil, Lymphocyte, Homeostasis, Health, Hygiene, Thymus
1. Introduction
Recent studies reveal that gut bacteria and immune cells exist within a whole host interactive network that dictates good health and disease [1–6]. An emerging paradigm links gut bacteria with whole body health, specifically involving microbial-immune networks influencing risk for many diseases including cancer [5–8]. In a whole body context, microbe-immune interactions constitute part of a vast gut-immune-brain signaling axis [9] that continuously modulates host hypothalamic-pituitary-adrenal hormones and inflammatory tone [3,6,10–12] in an optimal balance for sustained good health. In this way, gut microbes directly and indirectly influence immune system proficiency and cancer outcomes. Drilling deeper into the relationships between bacteria and the host immune system promises to reveal novel targets with vast potential for management of otherwise intractable diseases, such as cancer.
2. Microbes, immune homeostasis and good health
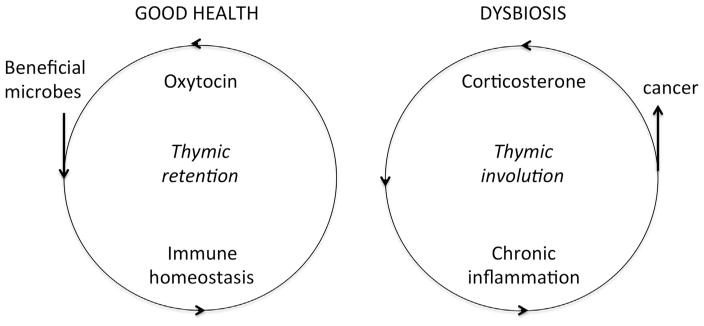
A properly functioning immune system is integral for counteracting pathogen invasion and infection, and also healing of tissue injuries [13]. Having such an important role in mammalian health and survival, the immune system does not work alone in homeostasis. Besides foreign antigen recognition, killing of pathogens, and tissue remodeling, the immune system imparts signals at the whole organism level to involve other systems in stabilizing the host animal during life-threatening conditions [13–17]. The increase of the body temperature (fever), loss of appetite, dulling of fur or skin, and changes in behavior and metabolic profile during infection are tangible outcomes of this mammalian signaling process [14,16,17]. In the present paradigm, the immune system interacts with the brain and the endocrine system to coordinate a holistic response to danger [14–17] (Fig. 1).
Fig. 1. Gut microbiota interact with whole body physiology to promote or undermine good health.
Bacteria in the gastrointestinal (GI) tract influence a gut-immune-endocrine system axis. Microbial symbiosis promotes an overall homeostatic balance by oxytocin and thymus gland dominant immune functions, with balanced effector and suppressive immunity culminating in good health. By contrast, gut dysbiosis, anxiety and stress contribute to corticosterone-biased undermining of thymus gland functions, promoting a smoldering pro-inflammatory systemic tone with high levels of neutrophils and increased risk of developing cancer.
In response to external challenges, the powerful and multidirectional signaling machinery of the immune system is capable of directly damaging host biological structures, explaining its important contributions in the pathogenesis of many different diseases [13]. These are not restricted to classical immune-associated pathologies caused by insufficient or excessive or abnormally prolonged inflammation [13,18]. They also include several metabolic and neuropsychological disorders, cardiovascular diseases and types of cancer, which previously were not considered to link with immune system imbalances [19–21].
Our understanding for the role of clinically detectable immune system malfunctions in the pathogenesis of many important diseases has been considerably increased over the last two decades [13,18–21]. This knowledge, however, inevitably leads to the next important questions connecting immune homeostasis with the preservation of good health and healthful longevity. Are there quantitatively and qualitatively different subclinical systemic inflammatory tones in healthy-appearing individuals that correlate with the risk of developing cancer or other diseases with advancing age [22–24]?
Previously unrecognized physiological axes link the gut microbiota and distant tissues including brain, skin and mucosal epithelia, and endocrine glands, with the immune system serving as the signaling “hub” [5,20,22–29]. A growing body of evidence suggests that the interaction of gut microbiota with hypothalamic-pituitary-adrenal axis signaling may influence the psychological condition of the host [9,10,27,30]. Gut bacteria with beneficial effects on host mood, behavior and sense of well-being (“psychobiotics”) may have potential as therapeutic modalities against neuropsychiatric disorders, including anxiety, stress and depression [9,17,31]. In the context of neoplastic disease, this may be particularly important given the long-suspected contributions of stress-induced neuroendocrine signaling in carcino-genesis and tumor growth [32–34].
3. Cancer as a failure of immune homeostasis
Earlier studies using enteropathogenic bacteria such as Helicobacter hepaticus have revealed that outcomes in cancer heavily rely upon not only host genotype but also immune system competency [7,35–37]. Studies using immune–deficient mouse models lacking functional T and B lymphocytes were among the first to unveil microbe-induced innate immune carcinogenic events otherwise suppressed by a competent host immune system [35,38]. In particular, among immune cells, neutrophils have emerged as a key cell in cancer development and growth [37,39–42].
Clinical studies in cancer patients statistically correlate high blood neutrophil numbers and especially a high neutrophil:lymphocyte ratio with poor clinical outcomes including shorter survival time and higher risk of metastases [43,44]. Likewise, neutrophils in the tumor micro-environment (tumor-associated neutrophils, TAN’s) have also been linked with poor neoplastic disease outcomes in the majority of studies, although with few exceptions [43–45]. Since the full appreciation of the strong link between inflammation and cancer, many studies in animal models of cancer focused on neutrophils and have revealed some of the complicated mechanisms of neutrophil contributions in the various stages of neoplastic disease [41,43–45].
The majority of data suggest a critical role of neutrophils mostly in promoting tumor growth and metastasis. Cytokines, chemokines, growth factors and serine proteases of neutrophils shape a microenvironment that favors important events in tumor growth, such as motility, migration, invasion and expansion of neoplastic cells and angiogenesis. Recent evidence suggests that neutrophils exert direct signals on neoplastic cells to promote their proliferation and escape from senescence [41,43–46]. The effects of neutrophils on tumor growth are also important for metastases, particularly by facilitating tumor cell intravasation. For this phenomenon, however, neutrophils seem to play additional roles. Indeed, neutrophils facilitate tumor cell intravasation, adhesion in the endothelium, and extravasation. They also localize in sites where metastases occur and shape the local tissue environment, thus setting the stage for the survival and thriving of metastatic foci [41,43–46].
One way neutrophils contribute to cancer is that the inflammatory environment of the tumor and tumor cells themselves release cytokines, such as IL-23, IL-17 and IL1-β that up-regulate granulocyte-colony stimulating factor (G-CSF). As a result the bone marrow receives signals for urgent neutrophil production and release. Under these circumstances mature neutrophils, as well as neutrophil precursor cells, pass in the circulation in high numbers. The immature granulocytes recognized by their ring-shaped or band nuclei [Fig. 2], associate with tumors and have been recently described as important tumor growth promoters [41,47,48].
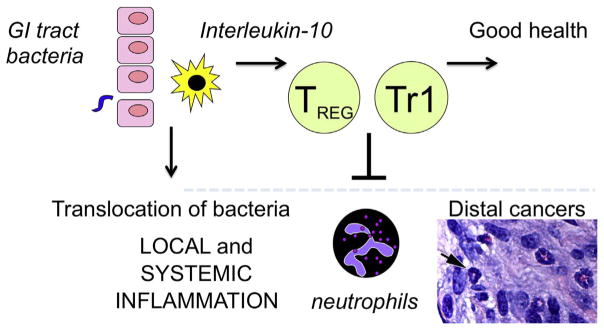
Fig. 2. Bacteria from the environment interface with mammalian biology via vast mucosal surfaces of the GI tract.
Gut epithelial cells and antigen-presenting cells comprise the initial interactions with GI tract bacteria. Innate and adaptive immune cells amplify signaling to eliminate pathogens and stimulate tissue repairs. Subsequently, Interleukin-10 and regulatory T (Treg) cell subsets enforce local homeostasis to minimize collateral tissue damage. Rapid return to homeostasis stabilizes epithelial barriers to reduce translocation of bacteria [sepsis] and restore lower systemic inflammatory tone. Infiltrating neutrophil precursors are important mediators of cancer. Shown are neutrophil precursor cells (arrow) in the stroma of mouse mammary cancer. Bar=25 μm.
Another aspect of neutrophils and granulocyte precursor cells known as myeloid-derived suppressor cells (MDSC’s) in cancer lies in their immunosuppressive activities. In the tumor microenvironment these cells are believed to suppress anti-tumoral effector T-cell action [41,43–47]. However, recent data suggest that the role of neutrophils is not one-dimensional in that regard. In a manner similar to what is known for T-lymphocytes and macrophages, which are polarized towards either phenotype 1 or 2 (Th1 vs Th2, M1 vs M2), tumor-associated neutrophils can also be separated in two types [41,45,46,49]. In the presence of Tgf-β, neutrophils polarize towards the N1 phenotype, which suppresses the desirable CD8+ T-cell responses and therefore is tumor-promoting. On the other hand, in the absence of Tgf-β the N2 neutrophils emerge in the tumor micro-environment and mount and anti-tumor cell response [41,45,46,49]. The strict categorization of neutrophils in either N1 or N2 may not reflect the complex reality and full spectrum of neutrophil functional diversities in the neoplastic tissue [41]. Nonetheless, this preliminary categorization reflects the plasticity of the tumor-associated neutrophils population and opens a window of therapeutic opportunities for their exogenous polarization towards desired phenotypes.
While roles of neutrophils in cancer growth and progression are now well established, their contributions to tumor initiation are less studied but quite intriguing. Reactive oxygen species (ROS) produced by neutrophils can induce genotoxic damage, thus contributing to the accumulating mutations in the initial steps of carcinogenesis [41,43,45]. Neutrophils, Tgf-β and oxidative stress/ROS may be interrelated in pathways supporting carcinogenesis and tumor growth [49,50]. Reactive nitrogen species (RNS) and proteases may also be important players in tumor initiation. Apart from direct damage on the genetic material of somatic cells, biologically active compounds of neutrophils that are being released to destroy invading pathogens may also damage epithelial cells. The destruction of the epithelial barrier can lead to bacterial translocation and chronic inflammation that promotes cancer [41].
4. Gut microbes and immune homeostasis
How does the host animal counteract pathogen invasion and infection, while at the same time healing life-threatening tissue injuries to restore homeostasis? The majority of studies suggest that local and systemic effects of gut bacteria rely on balanced interactions of host innate immunity with lymphocytes at the periphery [2,4,51–56]. Among lymphocytes, a growing body of research has identified CD4+ T lymphocytes subsets as pivotal in homeostasis of gut-associated lymphoid tissue, with lymphocytes continuously being generated, activated and balanced under the influence of bacterial antigen-driven signals [12,51–53,57–60]. This paradigm has been established and confirmed in vivo using adoptive cell transplant studies in mouse models. For example, cell transfers using highly purified CD4+ regulatory T lymphocyte populations showed that H. hepaticus-triggered Interleukin-10-dependent regulatory T (Treg) cells and Tr1 cells alone are sufficient to impart immune homeostasis within the bowel of mice [60,61]. Along these same lines, targeted orogastric infection with H. hepaticus was also found to stimulate transplantable cancer-protective CD4+ T cells inhibiting carcinogenesis in recipient animals by similar immune-mediated mechanisms [7,36,39,62]. In affiliated research, Rao et al (2007) proposed that microbe-driven immune-mediated events throughout the body evolved through two different mechanisms:
Modulation of systemic inflammatory events, and
translocation of bacteria from epithelial surfaces to organs throughout the body.
More specifically, those study authors postulated that CD4+ regulatory T (Treg) cells exert continuous control over gut microbe-triggered innate immune events including carcinogenic activities of neutrophils and precusor MDSC [6,39,63] (Fig. 2). Additional evidence suggests that microbe exposures earlier in life stimulate cancer-protective immune cells that subsequently lower risk of chronic inflammatory dysregulation and associated-diseases including cancer later in life [36,40,63].
The observation that prior microbe exposures improve potency of immune cells in anti-cancer protection [8,36] led us to conclude that bacteria-immune interactions have vast potential to prevent and treat cancer and bestow good health.
Indeed, both direct and indirect effects of microbes upon CD4+ T cell programming have been demonstrated in human subjects and animal models [2,4,5,64,65]. Although the peripheral immune tissues are a major contributor in this immune-programming process, in youth the centralized thymus gland also serves to routinely balance immune system activities via antigen-specific programming of T lymphocytes. As a result, premature thymic involution has been convincingly linked with a wide spectrum of immune disorders resulting in failure to properly distinguish self from non-self [66,67]. The relevancy of the thymus gland in sustaining good health has been convincingly demonstrated in mouse models lacking a functional thymus gland and thus without T lymphocytes [i.e., athymic nude mice], that are subsequently highly susceptible to infections and cancer [68,69]. A well-developed thymus gland helps assure robust yet balanced host responses to environmental challenges. When taken together, these data indicate that microbial programming of adaptive immunity is important in maintaining host animal homeostasis.
A more direct link between the microbiome and pathogenic neutrophils is now also emerging. The microbiota and its products such as short chain fatty acids (SCFA) have been shown to contribute to physiological myelopoiesis in the bone marrow [70–72]. Mice descending from mothers that are treated with certain antibiotics during pregnancy have low levels of bone marrow granulocyte precursors and circulating neutrophils [73]. The microbiota affect neutrophil homeostasis through enterocyte CXCL5-mediated signaling and IL-17 [74,75]. The aging process of neutrophils is also controlled by the microbiota through Toll-like receptor and myeloid differentiation factor 88 signaling [76]. These aged neutrophils have enhanced pro-inflammatory capabilities [41,43,76]. Therefore, a microbe-driven increase of neutrophil lifespan may affect inflammatory processes and cancer progression occurring throughout the body [41,43,76].
Along these same lines, dietary enrichment of the gut microbiota with a probiotic bacterium L. reuteri consistently decreases the numbers of circulating neutrophils [12,77,78]. This phenomenon may relate with immune-endocrine interactions actions involving Treg and hormone oxytocin, which both increase after L. reuteri consumption [10,11,77,79–81] and have the capacity to down-regulate neutrophilic responses [12,22,82–86].
While microbe-immune interplay is pivotal to health outcomes in many model systems, at the same time there is growing evidence that microbes can directly alter host risk for cancer [30]. Candidate bacteria such as E. coli have been identified in humans with breast cancer [1,87]; however, precisely how the carcinogenic gut microbes may translocate to mammary tissue remains to be determined. In contrast, the mammary cancer-protective microbe L. reuteri used in mouse model studies was originally isolated from human breast milk [81]. Nonetheless, immune-mediated tissue repair restores homeostasis and inhibits tumorigenesis. Taken together, this raises the likelihood that different types of host-microbe interactions: 1) modulation of systemic inflammatory events and 2) altered translocation of gut bacteria to other tissues in the body, are involved in systemic homeostatsis and regulation of carcinogenesis.
5. Early life microbe exposures and the ‘Hygiene Hypothesis’
The ‘hygiene hypothesis’ theory asserts that inhabitants of developed countries have immune systems with improved regulatory capacity when exposed to diverse microbiota early in life [20,88]. Conversely, immune system function is impaired with exposures to refined diets, antibiotics, Caesarian births and artificial milk that reduce beneficial microbe exposures [20,22,65]. To examine the potential of this ‘too clean’ concept further, Lakritz et al. [81] used a human source microbe, L. reuteri, which was once widespread in human populations and now occurs in less than 4% of people surveyed [89,90]. This group showed in mouse models that L reuteri served to inhibit mammary cancer later in life in murine models [81]. Additionally, introduction of these same bacteria during early life was found to convey multigenerational effects inhibiting a scurfy-like syndrome [premature thymic involution], massive systemic accumulations of neutrophils, and cancers of lymph nodes, lungs and liver [63]. Further studies using L. reuteri revealed this breast milk-derived microbe was sufficient to induce CD4+ lymphocytes, in particular CD4+ regulatory T (Treg) cells with transplantable health benefits to naïve animals [11,79,80,91].
It is becoming clear that perinatal bacteria exposures bestow immune system competency for a lifetime [20,22,63]. This effect may be peripheral but also include the central organ of lymphocyte differentiation i.e. the thymus gland. Indeed, recent findings by Varian et al. indicate that the dietary L. reuteri supplementation stimulates the thymus gland via up-regulation of the transcription factor FoxN1 in epithelial cells [12], an effect that may not require live microbiota for thymogenic effects [78]. Intestinal bacteria have also been shown to modulate the expression of another transcription factor, the autoimmune regulator (AIRE) pivotal in Treg programming, within thymic epithelial cells of mice [92]. Thus, emerging data shows that, in fact, immune tolerance may rely heavily on thymus-derived Treg [61,64,93,94]. To what extent L. reuteri-induced oxytocin [10,78] overlaps with central and peripheral host immune programming remains to be determined. However, oxytocin itself is connected with immune system elementary functions, since it is an integral component of both thymus gland and bone marrow [95,96]. The hormone has been shown to have a role in thymic maturation and selection of T-lymphocytes and in the generation and kinetics of bone marrow hematopoietic progenitor cells [95–98]. The effects of oxytocin in inflammatory processes parallel those of L. reuteri, with a balance of beneficial pro- and anti-inflammatory activities [95]. Oxytocin administration has been shown to balance neutrophil counts, oxidative stress and several proinflammatory cytokines. Importantly, it also imparts a wide array of more efficient host T-cell responses (5–7)[95,99–101].
Taken together, these data shape the current consensus that efficient immune tolerance and sustained good health is achieved by concerted actions of both thymic and peripherally-induced Treg.
6. Microbial metabolites also modulate host CD4+ T cells
Recent evidence suggests that CD4+ T cell subsets in the gut are responding to bacteria-derived signals [51–53,57–59], including fermentation products. Indeed, butyrate and propionic acid, which are byproducts of bacterial fermentation of starch and fiber in the bowel, can directly stimulate the expansion of local Treg and consequently ameliorate colitis in mice [102–104]. The effect of organic acids appears to be transmitted in Treg via the G coupled protein receptor 43 [103], depends upon the intronic enhancer CNS1 [104] and is accomplished via histone deacetylase (HDAC) inhibition [102–104]. The downstream gene target of this pathway has consistently been found to be Foxp3, the master nuclear transcription factor and signature molecule of suppressive TREG [102–104]. Interestingly, HDAC, such as HDAC3 affect not only peripheral induction of Treg but thymus-derived Treg as well [105]. Evidence, however, also exists that organic acids, such as n-butyrate suppresses CD4+ T effector cells directly, without Treg mediation [106]. In other studies, CD4+ Treg cells appear to mediate the bulk of the reported beneficial health effects of probiotic bacteria [6,23,31,107]. Further research is needed to determine the extent to which the benefits of organic acids in mammalian health [108] rely upon CD4+ T cell-mediated effects on intestinal and systemic health.
Microbe exposures may impart life-long immune homeostasis to animal hosts. Immune homeostasis is an otherwise intractable, complex, multi-factorial, and highly integrated process difficult to target via one immune cell molecule or type [109]. This inherent property of microbes makes them a tractable target for public and personalized health goals.
7. Microbes, immunity, and anti-cancer therapies
It has been known for some time that individual patients respond very differently to cancer treatments. There is a growing body of evidence that gut microbes play a role in these differences. Data from several different laboratories show that gut microbiota modulate patient responses to cancer immunotherapy [110,111]. Specifically, in those studies, microbe-associated immunomodulatory effects involved stimulating a Th-1 response in lymph nodes draining the tumor site. Interestingly, successful immunotherapy favored certain symbiotic microbe populations contributing to therapeutic efficacy. Recognizing the potential potency of microorganisms to boost immunotherapy raises hopes that engineering of gut bacteria for select immune outcomes will deliver a constructive host physiology. Earlier studies using L. reuteri indicate that even a single species of microbe can potently transform the host animal immune system, restore immune homeostasis, and convey sustained good health. In this way, gut microbes modulate whole host immune and hormonal factors impacting the fate of distant preneoplastic lesions toward malignancy or regression. This raises the possibility that the tumor microenvironment interacts with broader systemic microbial-immune networks. These accumulated findings suggest novel therapeutic opportunities for holobiont engineering in emerging tumor microenvironments [109].
8. Dietary microbes re-program host immunity, restore homeostasis, and combat cancer
There is an inherent appeal in the simplicity that symbiotic microbes may lessen inflammatory tone without compromising host defenses. In support of this, temporal dissection of epithelial wound healing showed that L. reuteri supplements served to balance activities of IFN-γ, neutrophils and Treg for constructive wound resolution [10]. While high levels of IFN-γ are beneficial for the early phase of epithelial proliferation and wound repair [112–114], excessive inflammation and chronic inflammation are ultimately detrimental to the host. In that setting, oral supplementation with a single probiotic organism was sufficient to rapidly restore homeostasis and at the same time minimize collateral tissue damage [6]. Recently, sterile lysates of bacteria were found to be sufficient for homeostatic effects [77,78]. Rapid recovery and restoration of homeostasis is pivotal in preventing chronic smoldering inflammation that otherwise contributes to individual risk of developing cancer. An exciting possibility exists that beneficial microbes may ultimately convey multi-generational anti-cancer effects, as well [63].
9. Conclusions
Recognizing the potential potency of microorganisms in whole body health raises hopes that engineering gut bacteria will deliver a constructive whole host balance for sustained cancer prevention and remission. Taken together, these data usher in a new era of microbe engineering seeking freedom from cancer, as well as other systemic disorders of the cardiovascular system and mental health.
Acknowledgments
This work was supported by National Institutes of Health grants P30-ES002109 (pilot project award to S.E.E), RO1CA108854 (to S.E.E), and U01 CA164337 (to S.E.E).
Footnotes
Conflict of interest
The authors have declared that no competing interests exist.
References
- 1.Erdman SE. Gut microbiota: microbes offer engineering strategies to combat cancer. Nat Rev Gastroenterol Hepatol. 2016;13:125–126. doi: 10.1038/nrgastro.2016.14. [DOI] [PubMed] [Google Scholar]
- 2.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troy EB, Kasper DL. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci (Landmark Ed) 2010;15:25–34. doi: 10.2741/3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinen T, Rudensky AY. The effects of commensal microbiota on immune cell subsets and inflammatory responses. Immunol Rev. 2012;245:45–55. doi: 10.1111/j.1600-065X.2011.01083.x. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg R, Powrie F. Microbiota, disease and back to health: a metastable journey (137rv137) Sci Transl Med. 2012;4:137rv137. doi: 10.1126/scitranslmed.3004184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erdman SE, Poutahidis T. Gut bacteria and cancer. Biochim Biophys Acta. 2015;1856:86–90. doi: 10.1016/j.bbcan.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2010;126:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;67:847–850. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- 9.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Microbial symbionts accelerate wound healing via the neuropeptide hormone oxytocin. PLoS One. 2013;8:e78898. doi: 10.1371/journal.pone.0078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varian BJ, Poutahidis T, Levkovich T, Ibrahim YM, Lakritz JR, Chatzigiagkos A, Scherer-Hoock A, Alm EJ, Erdman SE. Beneficial bacteria stimulate youthful thyroid gland activity. J Obes Weight Loss Ther. 2014;4:1–8. [Google Scholar]
- 12.Varian BJ, Goureshetti S, Poutahidis T, Lakritz JR, Levkovich T, Kwok C, Teliousis K, Ibrahim YM, Mirabal S, Erdman SE. Beneficial bacteria inhibit cachexia. Oncotarget. 2016;7:11803–11816. doi: 10.18632/oncotarget.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberl G. Immunity by equilibrium. Nat Rev Immunol. 2016;16:524–532. doi: 10.1038/nri.2016.75. [DOI] [PubMed] [Google Scholar]
- 14.Pittman QJ. A neuro-endocrine-immune symphony. J Neuroendocrinol. 2011;23:1296–1297. doi: 10.1111/j.1365-2826.2011.02176.x. [DOI] [PubMed] [Google Scholar]
- 15.Brestoff JR, Artis D. Immune regulation of metabolic homeostasis in health and disease. Cell. 2015;161:146–160. doi: 10.1016/j.cell.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol. 2013;216:84–98. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanisms of depression. Nat Neurosci. 2015;18:1386–1393. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuPage M, Bluestone JA. Harnessing the plasticity of CD4(+) T cells to treat immune-mediated disease. Nat Rev Immunol. 2016;16:149–163. doi: 10.1038/nri.2015.18. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzatti AJ, Retzlaff BM. Unmet needs in the management of atherosclerotic cardiovascular disease: is there a role for emerging anti-inflammatory interventions? Int J Cardiol. 2016;221:581–586. doi: 10.1016/j.ijcard.2016.07.061. [DOI] [PubMed] [Google Scholar]
- 20.Rook GA, Dalgleish A. Infection, immunoregulation, and cancer. Immunol Rev. 2011;240:141–159. doi: 10.1111/j.1600-065X.2010.00987.x. [DOI] [PubMed] [Google Scholar]
- 21.Kipnis J. Multifaceted interactions between adaptive immunity and the central nervous system. Science. 2016;353:766–771. doi: 10.1126/science.aag2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 2010;127:768–779. doi: 10.1002/ijc.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erdman SE, Poutahidis T. The microbiome modulates the tumor macroenvironment. Oncoimmunology. 2014;(3) doi: 10.4161/onci.28271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 26.Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci USA. 2013;110:18360–18367. doi: 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 28.Erdman SE, Poutahidis T. Probiotic ‘glow of health’: it’s more than skin deep. Benef Microbes. 2014;5:109–119. doi: 10.3920/BM2013.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120–123. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherwin E, Rea K, Dinan TG, Cryan JF. A gut (microbiome) feeling about the brain. Curr Opin Gastroenterol. 2016;32:96–102. doi: 10.1097/MOG.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 32.Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol. 2003;157:415–423. doi: 10.1093/aje/kwg002. [DOI] [PubMed] [Google Scholar]
- 33.Costanzo ES, Sood AK, Lutgendorf SK. Biobehavioral influences on cancer progression. Immunol Allergy Clin N Am. 2011;31:109–132. doi: 10.1016/j.iac.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Powell ND, Tarr AJ, Sheridan JF. Psychosocial stress and inflammation in cancer. Brain Behav Immun. 2013;30(Suppl):S41–47. doi: 10.1016/j.bbi.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 37.Lakritz JR, Poutahidis T, Mirabal S, Varian BJ, Levkovich T, Ibrahim YM, Ward JM, Teng EC, Fisher B, Parry N, Lesage S, Alberg N, Gourishetti S, Fox JG, Ge Z, Erdman SE. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 2015;6:9387–9396. doi: 10.18632/oncotarget.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 39.Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, Ge Z, Watanabe K, Davidson A, Horwitz BH, Fox JG, Erdman SE. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;28:2614–2623. doi: 10.1093/carcin/bgm180. [DOI] [PubMed] [Google Scholar]
- 40.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EJ, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2009;126:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coffelt SB, de Visser WM, KE Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:16. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 42.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, Tannenbaum SR, Fox JG. Nitric oxide and TNF-{alpha} trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumitru CA, Lang S, Brandau S. Modulation of neutrophil granulocytes in the tumor microenvironment: mechanisms and consequences for tumor progression. Semin Cancer Biol. 2013;23:141–148. doi: 10.1016/j.semcancer.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Singel KL, Segal BH. Neutrophils in the tumor microenvironment: trying to heal the wound that cannot heal. Immunol Rev. 2016;273:329–343. doi: 10.1111/imr.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–2416. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 46.Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. 2016;273:312–328. doi: 10.1111/imr.12444. [DOI] [PubMed] [Google Scholar]
- 47.Yang XD, Ai W, Asfaha S, Bhagat G, Friedman RA, Jin G, Park H, Shykind B, Diacovo TG, Falus A, Wang TC. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b(+)Ly6G(+) immature myeloid cells. Nat Med. 2011;17:87–95. doi: 10.1038/nm.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poutahidis T, Cappelle K, Levkovich T, Lee CW, Doulberis M, Ge Z, Fox JG, Horwitz BH, Erdman SE. Pathogenic intestinal bacteria enhance prostate cancer development via systemic activation of immune cells in mice. PLoS One. 2013;(8):e73933. doi: 10.1371/journal.pone.0073933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “n1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krstic J, Trivanovic D, Mojsilovic S, Santibanez JF. Transforming growth factor-beta and oxidative stress interplay: implicationsimplications in tumori-genesis and cancer progression. Oxid Med Cell Longev. 2015;2015:654594. doi: 10.1155/2015/654594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, Kim S, Fritz JV, Wilmes P, Ueha S, Matsushima K, Ohno H, Olle B, Sakaguchi S, Taniguchi T, Morita H, Hattori M, Honda K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 54.Ohnmacht C, Park JH, Cording S, Wing JB, Atarashi K, Obata Y, Gaboriau-Routhiau V, Marques R, Dulauroy S, Fedoseeva M, Busslinger M, Cerf-Bensussan N, Boneca IG, Voehringer D, Hase K, Honda K, Sakaguchi S, Eberl G. MUCOSAL IMMUNOLOGY. The microbiota regulates type 2 immunity through RORgammat(+) T cells. Science. 2015;349:989–993. doi: 10.1126/science.aac4263. [DOI] [PubMed] [Google Scholar]
- 55.Lindner C, Thomsen I, Wahl B, Ugur M, Sethi MK, Friedrichsen M, Smoczek A, Ott S, Baumann U, Suerbaum S, Schreiber S, Bleich A, Gaboriau-Routhiau V, Cerf-Bensussan N, Hazanov H, Mehr R, Boysen P, Rosenstiel P, Pabst O. Diversification of memory B cells drives the continuous adaptation of secretory antibodies to gut microbiota. Nat Immunol. 2015;16:880–888. doi: 10.1038/ni.3213. [DOI] [PubMed] [Google Scholar]
- 56.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, Rochet V, Pisi A, De Paepe M, Brandi G, Eberl G, Snel J, Kelly D, Cerf-Bensussan N. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31:677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 58.Chow J, Mazmanian SK. A pathobiont of the microbiota balances host colonization and intestinal inflammation. Cell Host Microbe. 2010;7:265–276. doi: 10.1016/j.chom.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 60.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–515. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Powrie F, Maloy KJ. Immunology. Regulating the regulators. Science. 2003;299:1030–1031. doi: 10.1126/science.1082031. [DOI] [PubMed] [Google Scholar]
- 62.Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–6050. [PubMed] [Google Scholar]
- 63.Poutahidis T, Varian BJ, Levkovich T, Lakritz JR, Mirabal S, Kwok C, Ibrahim YM, Kearney SM, Chatzigiagkos A, Alm EJ, Erdman SE. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015;75:1197–1204. doi: 10.1158/0008-5472.CAN-14-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, Nelson RW, Fife BT, Orr HT, Anderson MS, Hogquist KA, Jenkins MK. Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat Immunol. 2016;17:187–195. doi: 10.1038/ni.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat Immunol. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- 67.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176:6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 68.van den Akker TW, Tio-Gillen AP, Solleveld HA, Benner R, Radl J. The influence of T cells on homogeneous immunoglobulins in sera of athymic nude mice during aging. Scand J Immunol. 1988;28:359–365. doi: 10.1111/j.1365-3083.1988.tb01461.x. [DOI] [PubMed] [Google Scholar]
- 69.Palamaro L, Romano R, Fusco A, Giardino G, Gallo V, Pignata C. FOXN1 in organ development and human diseases. Int Rev Immunol. 2014;33:83–93. doi: 10.3109/08830185.2013.870171. [DOI] [PubMed] [Google Scholar]
- 70.Khosravi A, Yanez A, Price JG, Chow A, Merad M, Goodridge HS, Mazmanian SK. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15:374–381. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Balmer ML, Schurch CM, Saito Y, Geuking MB, Li H, Cuenca M, Kovtonyuk LV, McCoy KD, Hapfelmeier S, Ochsenbein AF, Manz MG, Slack E, Macpherson AJ. Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol. 2014;193:5273–5283. doi: 10.4049/jimmunol.1400762. [DOI] [PubMed] [Google Scholar]
- 72.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 73.Gomez de Aguero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. The maternal microbiota drives early postnatal innate immune development. Science. 2016;351:1296–1302. doi: 10.1126/science.aad2571. [DOI] [PubMed] [Google Scholar]
- 74.Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, O’Leary CE, Oliver PM, Kolls JK, Weiser JN, Worthen GS. The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med. 2014;20:524–530. doi: 10.1038/nm.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mei J, Liu Y, Dai N, Hoffmann C, Hudock KM, Zhang P, Guttentag SH, Kolls JK, Oliver PM, Bushman FD, Worthen GS. Cxcr2 and Cxcl5 regulate the IL-17/G-CSF axis and neutrophil homeostasis in mice. J Clin Investig. 2012;122:974–986. doi: 10.1172/JCI60588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith JJ, Burk RD, Kunisaki Y, Jang JE, Scheiermann C, Merad M, Frenette PS. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Varian BJ, Levkovich T, Poutahidis T, Ibrahim YM, Perrotta A, Alm EJ, Erdman SE. Beneficial dog bacteria up-regulate oxytocin and lower risk of obesity. J Probiotics Health. 2016;4:1–9. [Google Scholar]
- 78.Varian BJ, Poutahidis T, DiBenedictis BT, Levkovich T, Ibrahim Y, Didyk E, Shikhman L, Cheung HK, Hardas A, Ricciardi CE, Kolandaivelu K, Veenema AH, Alm EJ, Erdman SE. Microbial lysate upregulates host oxytocin. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.11.002. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, Chatzigiagkos A, Hafler DA, Alm EJ, Erdman SE. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One. 2013;8:e68596. doi: 10.1371/journal.pone.0068596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Levkovich T, Poutahidis T, Smillie C, Varian BJ, Ibrahim YM, Lakritz JR, Alm EJ, Erdman SE. Probiotic bacteria induce a ‘glow of health’. PLoS One. 2013;8:e53867. doi: 10.1371/journal.pone.0053867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, Mirabal S, Alm EJ, Erdman SE. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135:529–540. doi: 10.1002/ijc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al-Amran F, Shahkolahi M. Oxytocin ameliorates the immediate myocardial injury in rat heart transplant through downregulation of neutrophil-dependent myocardial apoptosis. Transpl Proc. 2013;45:2506–2512. doi: 10.1016/j.transproceed.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 83.Biyikli NK, Tugtepe H, Sener G, Velioglu-Ogunc A, Cetinel S, Midillioglu S, Gedik N, Yegen BC. Oxytocin alleviates oxidative renal injury in pyelonephritic rats via a neutrophil-dependent mechanism. Peptides. 2006;27:2249–2257. doi: 10.1016/j.peptides.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 84.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism. Peptides. 2005;26:483–491. doi: 10.1016/j.peptides.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 85.Iseri SO, Sener G, Saglam B, Gedik N, Ercan F, Yegen BC. Oxytocin protects against sepsis-induced multiple organ damage: role of neutrophils. J Surg Res. 2005;126:73–81. doi: 10.1016/j.jss.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 86.Petersson M, Wiberg U, Lundeberg T, Uvnas-Moberg K. Oxytocin decreases carrageenan induced inflammation in rats. Peptides. 2001;22:1479–1484. doi: 10.1016/s0196-9781(01)00469-7. [DOI] [PubMed] [Google Scholar]
- 87.Cheema AK, Maier I, Dowdy T, Wang Y, Singh R, Ruegger PM, Borneman J, Fornace AJ, Jr, Schiestl RH. Chemopreventive metabolites are correlated with a change in intestinal microbiota measured in A-T mice and decreased carcino-genesis. PLoS One. 2016;11:e0151190. doi: 10.1371/journal.pone.0151190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Molin G, Jeppsson B, Johansson ML, Ahrne S, Nobaek S, Stahl M, Bengmark S. Numerical taxonomy of Lactobacillus spp. associated with healthy and diseased mucosa of the human intestines. J Appl Bacteriol. 1993;74:314–323. doi: 10.1111/j.1365-2672.1993.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 90.Walter J, Britton RA, Roos S. Host-microbial symbiosis in the vertebrate gastrointestinal tract and the Lactobacillus reuteri paradigm. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4645–4652. doi: 10.1073/pnas.1000099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014;9:e84877. doi: 10.1371/journal.pone.0084877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakajima A, Negishi N, Tsurui H, Kadowaki-Ohtsuji N, Maeda K, Nanno M, Yamaguchi Y, Shimizu N, Yagita H, Okumura K, Habu S. Commensal bacteria regulate thymic Aire expression. PLoS One. 2014;9:e105904. doi: 10.1371/journal.pone.0105904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang P, Yang HP, Tian S, Wang L, Wang SC, Zhang F, Wang YF. Oxytocin-secreting system: a major part of the neuroendocrine center regulating immunologic activity. J Neuroimmunol. 2015;289:152–161. doi: 10.1016/j.jneuroim.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 96.Geenen V, Martens H, Brilot F, Renard C, Franchimont D, Kecha O. Thymic neuroendocrine self-antigens. Role in T-cell development and central T-cell self-tolerance. Ann NY Acad Sci. 2000;917:710–723. doi: 10.1111/j.1749-6632.2000.tb05435.x. [DOI] [PubMed] [Google Scholar]
- 97.Hansenne I, Rasier G, Pequeux C, Brilot F, Renard C, Breton C, Greimers R, Legros JJ, Geenen V, Martens HJ. Ontogenesis and functional aspects of oxytocin and vasopressin gene expression in the thymus network. J Neuroimmunol. 2005;158:67–75. doi: 10.1016/j.jneuroim.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 98.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 99.Hamasaki MY, Barbeiro HV, Barbeiro DF, Cunha DM, Koike MK, Machado MC, Pinheiro da Silva F. Neuropeptides in the brain defense against distant organ damage. J Neuroimmunol. 2016;290:33–35. doi: 10.1016/j.jneuroim.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 100.Yuan L, Liu S, Bai X, Gao Y, Liu G, Wang X, Liu D, Li T, Hao A, Wang Z. Oxytocin inhibits lipopolysaccharide-induced inflammation in microglial cells and attenuates microglial activation in lipopolysaccharide-treated mice. J Neuroinflamm. 2016;13:77. doi: 10.1186/s12974-016-0541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsuura T, Kawasaki M, Hashimoto H, Yoshimura M, Motojima Y, Saito R, Ueno H, Maruyama T, Sabanai K, Mori T, Ohnishi H, Sakai A, Ueta Y. Effects of central administration of oxytocin-saporin cytotoxin on chronic inflammation and feeding/drinking behaviors in adjuvant arthritic rats. Neurosci Lett. 2016;621:104–110. doi: 10.1016/j.neulet.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 102.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 103.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang L, Liu Y, Han R, Beier UH, Bhatti TR, Akimova T, Greene MI, Hiebert SW, Hancock WW. FOXP3+ regulatory T cell development and function require histone/protein deacetylase. J Clin Investig. 2015;125(3):1111–1123. doi: 10.1172/JCI77088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fontenelle B, Gilbert KM. n-Butyrate anergized effector CD4+ T cells independent of regulatory T cell generation or activity. Scand J Immunol. 2012;76:457–463. doi: 10.1111/j.1365-3083.2012.02740.x. [DOI] [PubMed] [Google Scholar]
- 107.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Guilloteau P, Martin L, Eeckhaut V, Ducatelle R, Zabielski R, Van Immerseel F. From the gut to the peripheral tissues: the multiple effects of butyrate. Nutr Res Rev. 2010;23:366–384. doi: 10.1017/S0954422410000247. [DOI] [PubMed] [Google Scholar]
- 109.Poutahidis T, Erdman SE. Commensal bacteria modulate the tumor micro-environment. Cancer Lett. 2015 doi: 10.1016/j.canlet.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, Poirier-Colame V, Roux A, Becharef S, Formenti S, Golden E, Cording S, Eberl G, Schlitzer A, Ginhoux F, Mani S, Yamazaki T, Jacquelot N, Enot DP, Berard M, Nigou J, Opolon P, Eggermont A, Woerther PL, Chachaty E, Chaput N, Robert C, Mateus C, Kroemer G, Raoult D, Boneca IG, Carbonnel F, Chamaillard M, Zitvogel L. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, Chang EB, Gajewski TF. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Northoff H, Berg A, Weinstock C. Similarities and differences of the immune response to exercise and trauma: the IFN-gamma concept. Can J Physiol Pharmacol. 1998;76:497–504. doi: 10.1139/cjpp-76-5-497. [DOI] [PubMed] [Google Scholar]
- 113.Tanno H, Kawakami K, Ritsu M, Kanno E, Suzuki A, Kamimatsuno R, Takagi N, Miyasaka T, Ishii K, Imai Y, Maruyama R, Tachi M. Contribution of invariant natural killer T cells to skin wound healing. Am J Pathol. 2015;185:3248–3257. doi: 10.1016/j.ajpath.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 114.Thelemann C, Eren RO, Coutaz M, Brasseit J, Bouzourene H, Rosa M, Duval A, Lavanchy C, Mack V, Mueller C, Reith W, Acha-Orbea H. Interferon-gamma induces expression of MHC class II on intestinal epithelial cells and protects mice from colitis. PLoS One. 2014;9:e86844. doi: 10.1371/journal.pone.0086844. [DOI] [PMC free article] [PubMed] [Google Scholar]