Abstract
Background
Sequelae of venous thromboembolism (VTE) in children include recurrence, development of post thrombotic syndrome (PTS) when venous return from a limb is affected and chronic thromboembolic pulmonary hypertension (CTEPH) after pulmonary embolism. Identification of laboratory-based risk factors may be useful for individualized risk assessment for VTE sequelae. Coagulation activation and inflammation may contribute to their pathophysiology. We performed a systematic review to investigate the association between biomarkers of coagulation activation, inflammation and fibrinolysis and adverse VTE outcomes in children and young adults.
Methods
A systematic search of electronic databases, PubMed (NIH), EMBASE (Ovid), Web of Science (Thompson Reuters), and SCOPUS (Elsevier) for studies published through November 2016 was conducted using “VTE” including MeSH terms for “coagulation activation,” “inflammation” and “fibrinolysis,” with no limit on publication date. A study was eligible for inclusion when it evaluated patients (< 21 years) with VTE and biomarkers of coagulation activation, inflammation, and fibrinolysis and assessed for their association with development of adverse thrombotic outcomes. A modified Newcastle Ottawa Scale was applied to examine the quality of included studies.
Results
Our search strategy yielded 200 references. A total of 3 cohort studies representing 220 patients with VTE were included. Two authors independently assessed all references for inclusion. Three studies (2 prospective cohort and one mixed cohort study) were identified that reported on biomarkers of coagulation activation, inflammation and fibrinolysis, checked at least once after VTE diagnosis and assessed association with primary outcomes of recurrent VTE, PTS and CTEPH. Studies varied with regards to definition of outcomes, the type of biomarkers measured and time point of measurement. We were unable to meta-analyze results due to marked clinical heterogeneity and <3 studies available for each biomarker. Descriptively, a significant association was found for elevated plasma levels of FVIII and D-dimer for a compound outcome of PTS, recurrence and progression in one study, and positive lupus anticoagulant at DVT diagnosis and subsequent PTS by another study. No studies were found for CTEPH.
Conclusions
Elevated D-dimer, FVIII and lupus anticoagulant show promise for predicting recurrent VTE and PTS in children and young adults. Further research is needed to elucidate whether these markers might be useful to predict development of adverse outcomes after VTE in children.
Keywords: Pediatric thrombosis, long term outcomes, poor outcomes, venous thromboembolism
1. Introduction
Adverse outcomes after pediatric venous thromboembolism (VTE) include recurrent VTE, post thrombotic syndrome (PTS) [1] and chronic thromboembolic pulmonary hypertension (CTEPH) [2]. These complications develop after deep venous thrombosis (DVT) and pulmonary embolism (PE) despite appropriate anticoagulation [3, 4]. The incidence of VTE in children has increased in the recent years by 70% and affects 1 in 200 hospitalized children [5]. In parallel with this increase, sequelae of thrombosis are expected to rise in the ensuing years. There are no validated screening methods that allow risk stratification for poor outcomes of VTE in an individual after completion of anticoagulation. Management is, therefore, challenging and occurs once complications have already developed. The negative impact of VTE sequelae on children and young adults, anticipated to live 5 or more decades after the acute event, is likely to be significant, unless prevented. To that end, it is desirable to discover suitable biomarkers early in the course after VTE to identify patients at risk, who may benefit from targeted interventions aimed at preventing VTE complications.
A close relationship exists between inflammation and thrombosis [6, 7]. Acute DVT causes a systemic inflammatory response characterized by elevated plasma levels of interleukin-6, interleukin-8, and C-reactive protein [7]. The temporal accumulation of leukocytes in the forming (PMNs) and resolving (monocytes/macrophages) thrombus is part of a dynamic intravascular wound healing process that results in either early lysis of the thrombus or its stabilization [8–10]. The transition over time to a monocyte predominant thrombus environment results in plasmin and matrix metalloproteinase (MMP-2) mediated thrombus breakdown - also driven by the proinflammatory cytokine milieu. Inflammation, thus, influences not only thrombogenesis but also thrombus resolution and vein wall remodeling. Indeed, studies of patients with DVT treated with anticoagulation have shown that in most cases, return of normal physiologic function of the vein is rare [11]. Even in patients who achieve clot lysis, permanent damage to vein frequently occurs, conceivably via thrombus-induced activation of inflammation [7, 12]. It is plausible that pathological interactions among several factors at the site of the DVT affected vein may portend a poor prognosis. These may include the thrombus itself, inflammatory mediators secreted by blood cells and the venous endothelium, the process of fibrin degradation and vein recanalization. Coagulation activation, inflammation and impaired fibrinolysis may thus be the antecedents that are present in patients who subsequently develop adverse VTE sequelae.
We conducted a systematic review aimed at answering the question: Can markers of coagulation activation, inflammation and fibrinolysis predict the development of poor outcomes in pediatric VTE?
2. Methods
a. Eligibility criteria
We included randomized studies (RS) and non-randomized studies (NRS) in patients <21 years with venous thromboembolism (VTE) who had biomarkers of interest, excluding inherited thrombophilia (defined, for the purpose of this review as Factor V Leiden mutation, prothrombin gene mutation, protein (P) C, PS and antithrombin deficiencies) checked at least once after diagnosis of VTE, and assessed for association with development of poor outcomes of thrombosis. The primary outcomes selected for this review included recurrent VTE, PTS and CTEPH.
b. Information sources and search strategy
We searched PubMed (NIH), EMBASE (Ovid), Web of Science (Thompson Reuters), and SCOPUS (Elsevier) through November 2016. The searches combined terms to identify studies using “VTE” including MeSH terms for “coagulation activation,” “inflammation” and “fibrinolysis,” with no limit on publication date. The search strategy developed for PubMed (Appendix A) was adapted for use in the other databases.
d. Study selection and data extraction
Two authors (AZ and JJ) screened all studies that appeared relevant on the basis of “Title” and “Abstract” for full article review. Both authors (AZ and JJ) independently selected studies based on the full article for inclusion criteria into the review. Agreement was measured using simple agreement. Discordance was resolved by discussion and consensus between the two authors.
e. Assessment of risk of bias and quality in included studies
Two authors (AZ and JJ) independently assessed methodological quality of each included study. Discordance was resolved by discussion and consensus between the two authors. We implemented the Newcastle-Ottawa Scale (NOS) [13], developed to assess the quality of nonrandomized studies for this review (Appendix C). The NOS utilizes a ‘star system’ to judge a study based on: the selection of study groups (maximum of 4 stars); the comparability of the groups (maximum of 2 stars); and the ascertainment of either the exposure or outcome of interest (maximum of 3 stars) for case-control or cohort studies respectively.
For the studies recommended for inclusion in the secondary screen, data were extracted using a data extraction form. After study selection, both authors (AZ and JJ) independently extracted data. Collected data included: 1) basic study characteristics; 2) inclusion and exclusion criteria; 3) concomitant medical diagnoses that could influence the measured biomarkers; 4) biomarkers measured, method of measurement, time point at which measured and the outcome with which association was presented; 5) statistical analyses and measure of association; 6) type of outcome (s), definition of outcome used and if defined a priori, and whether any validated methods or scales used to measure outcome. Extracted data were compared and disagreements were resolved by consensus.
We divided time points of biomarker measurement in four subgroups: acute phase (< 1 month post diagnosis), sub-acute phase (≥ 1 to < 3 months post diagnosis), early chronic (≥3 to < 12 months post diagnosis) and late chronic (≥ 12 months post diagnosis) based on distinctive patterns of hypercoagulability and/or hypofibrinolysis post-VTE [14, 15]. We studied each biomarker with respect to these time points for each of the primary outcomes. We planned for meta-analysis only if there were three or more studies analyzing the same biomarker for its association with any of the primary outcomes.
For all outcomes, we planned to stratify biomarkers based on: a) age (28 days- 23.99 months, 2–6.99, 7–11.99, 12–17.99 and 18–20.99 years old), with additional analyses for neonate, adolescents and all others (taking in account the bimodal peak of VTE); b) sex; c) BMI (obese/overweight vs. normal weight); d) provoked vs. unprovoked VTE; e) DVT triggering event; f) positive family history (FMH) (we defined FMH of VTE as positive when there was a report of any first and/or second degree family member with VTE, myocardial infarction and/or stroke at age <40 years, recurrent spontaneous abortions and/or laboratory diagnosis of inherited or acquired thrombophilia) and; e) antiphospholipid antibody positivity at diagnosis.
Additionally, three subgroup analyses were planned for each individual primary outcome. For studies evaluating PTS, whether biomarkers were assessed in subjects stratified according to a) Initial complete vs. partial veno-occlusiveness; and b) proximal vs. distal DVT. For studies on recurrent VTE, whether biomarkers were assessed in subjects stratified according to race; African American (AA) race vs. non-AA race and for studies on CTEPH, whether biomarkers were assessed in subjects stratified according to a) location (initial central embolic burden vs. segmental or sub segmental emboli) on imaging and (ii) persistent embolic burden vs. recanalization on follow-up imaging. For all outcomes, we planned to also assess whether biomarkers measurements were done with on or off anticoagulant therapy.
3. Results
Descriptive Analyses
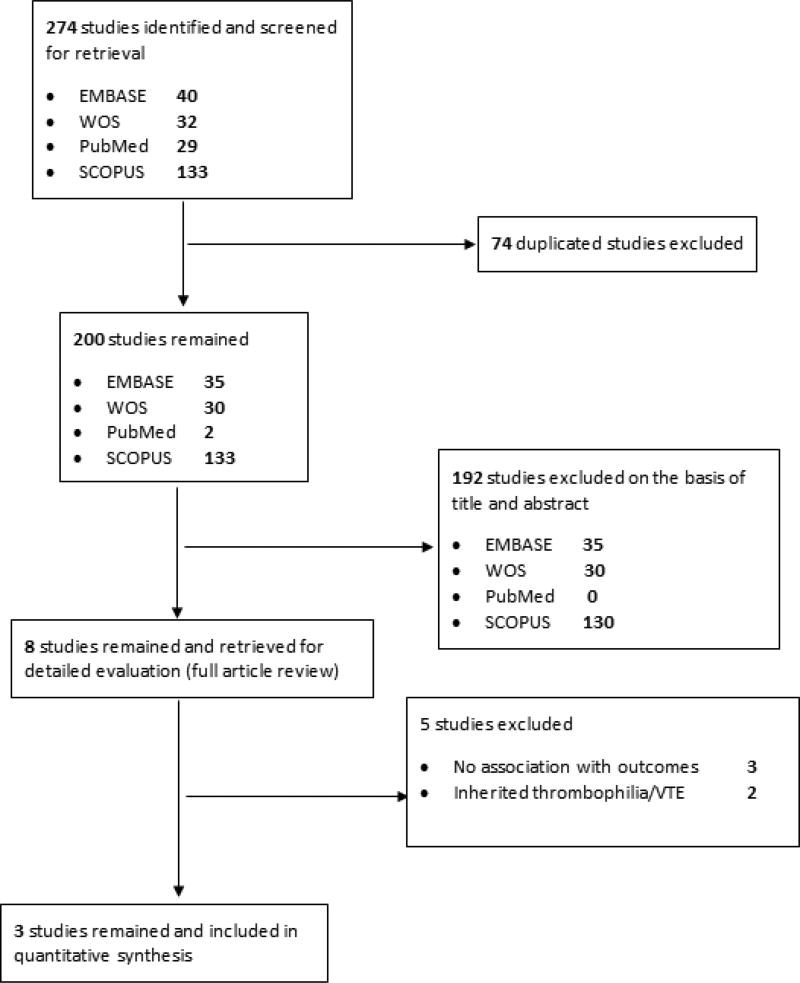
Figure 1 depicts the flow diagram for the process of the study selection. Five electronic databases were searched. The search provided 274 citations. After adjusting for duplicates, a total of 200 remained. Of these, we excluded 192 studies that did not meet the above inclusion criteria after title and abstract screening. The full text of the remaining citations was examined in more detail. After full text assessment we excluded an additional 5 papers. These studies measured global assays of plasmin generation and fibrinolysis at various time points after VTE [14], antiphospholipid antibodies in a pediatric antiphospholipid syndrome (APS) with recurrent VTE [16] and FVIII and D-dimer in subjects with recurrent VTE [17] but analyses on the association between biomarkers and outcomes were not reported. Two studies assessed inherited thrombophilia with recurrence [18, 19]. Only 3 studies met the inclusion criteria.
Figure 1.
Prisma flow diagram for study selection
Table 1 summarizes the included studies. Studies varied with regards to inclusion and exclusion criteria, outcomes studied and definition and timing of assessment.
Table 1.
Characteristics of included studies
| Author Year (ref.) |
Study design |
Inclusion criteria |
Exclusion criteria | n | Outcomes Assessed |
Prospective assessment of outcomes |
Criteria for recurrence diagnosis/ Time of assessment |
% Recurrence |
Criteria for PTS diagnosis/ Time of assessment |
% PTS | Biomarkers assessed |
Prospective assessment of biomarkers |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Goldenberg 2004 (16) | Prospective Cohort study | Acute VTE; birth -21 yrs. of age | Congenital deficiency of protein C, S, AT; long-term AC or prophylaxis at VTE; absence of D-dimer and FVIII at diagnosis | 82 | Recurrence PTS | Yes | Occurrence of a thrombus in a previously unaffected venous system/Within 2 yrs. of the initial VTE | 6 | Presence of pain with aerobic exercise, with activities of daily living, or at rest or by at least one of the following: visible or measurable edema, collateral formation, venous stasis dermatitis, or ulceration/Within 3–60 mo. | 33 | FVIII D-dimer | Yes |
| Kreuz 2006 (17) | Prospective Cohort study | First symptomatic VTE, age <18 | Age >18; nonwhite, incomplete clinical or lab work-up; lost to follow-up; arterial thrombosis; liver or renal disease; inflammatory disorders; malignancies; treatments known to increase FVIII levels | 103 | Recurrence ‘Transient’ PTS | Yes | A new intraluminal filling defect compared with previous defects on venography/Not specified | 7.8 | Diagnosed on objective signs of: an increase in calf and ankle circumference by 2–4 cm, dark pigmentation of the skin, venous telangiectasia, varicose veins, or open ulcer/Not specified | 8.5 | FVIII | No (cross sectional) |
| Lyle 2012 (18) | Mixed Cohort Prospective Study | Acute limb DVT, Age <21 years | Initial treatment with thrombolysis or thrombectomy; lack of PTS evaluation 12 or more months following DVT diagnosis | 35 | PTS | Yes | NA | NA | A score of at least 1 in both the following categories: functional limitations of activities of daily living or chronic lower extremity pain at rest or with physical activity and presence of physical examination abnormalities such as edema, superficial collateral veins, venous stasis dermatitis, and venous stasis ulcers/12 mo. post DVT | 23 | FVIII D-dimer LA | No (cross sectional) |
VTE, venous thromboembolism; AT, antithrombin deficiency; AC, anticoagulation; PTS, post thrombotic syndrome, DVT, deep venous thrombosis, NA, not applicable, LA, lupus anticoagulant
The limited number of studies identified suggested that conventional analytical approaches for systematic review were inapplicable and unnecessary (Appendix B). In the following, we compiled the findings of the three studies on the association of biomarkers with different outcomes:
a) Post thrombotic syndrome
All three studies assessed the association of biomarkers with PTS (Table 2). The prevalence of PTS was 33% (median follow-up of 12 months) [20], 8.5% (median follow-up of 48 months) [21] and 23% (median follow-up of 33 months) respectively [22]. The study by Goldenberg and colleagues [20] assessed PTS in combination with other poor outcomes of thrombosis (recurrence and lack of thrombus resolution) whereas PTS was the primary outcome defined a priori for the other two studies [21, 22]. This study found a statistically significant association between FVIII levels > 150 IU per deciliter, D-dimer above 500 ng per milliliter or both at diagnosis with the composite end point. At follow-up, in the sub-acute and early chronic phase, FVIII and D-dimer were checked in tandem and the same cut-offs were found to be statistically significant.
Table 2.
The association between biomarkers and PTS
| Author/year (ref.) |
Biomarker (s) | Measurement Method |
Relationship to AC | Association with poor outcomes |
Comments |
|---|---|---|---|---|---|
|
| |||||
|
Acute phase (up to one month post event)
| |||||
| Lyle 2012 (18) | FVIII | One-stage clotting assay | On AC | FVIII 226 IU per dL in 4/8 (50%) in PTS+ vs 208 in 15/27 (55%) in PTS -; p=0.55 | Adjustments via multivariate analysis were deemed not necessary for FVIII as only one variable (lupus anticoagulant) in this study met the criterion for which P < 0.1 in univariate logistic regression model a priori |
| Univariate OR 1.00 (0.99–1.02), p=0.78 | |||||
|
| |||||
| D-dimer | Quantitative or semiquantitative immunoturbidemtric assay or fluorescence immunoassay | On AC | D-dimer >500 ng per milliliter in 6/6 (100%) in PTS+ vs. 14/20 (70%) in PTS-; p=0.78 | Adjustments via multivariate analysis were deemed not necessary for D-dimer as only one variable (lupus anticoagulant) in this study met the criterion for which P < 0.1 in univariate logistic regression model a priori | |
| Univariate OR 2.14 (0.2–22.5);p=0.53 | |||||
|
| |||||
| Lupus anticoagulant | By dRVVT | On AC | Positive dRVVT in 6/7 (86%) in PTS+ vs. 5/19 (26%) in PTS-; p=0.02 | Adjustments via multivariate analysis were deemed not necessary as lupus anticoagulant was the only variable in this study which met the criterion of P < 0.1 in univariate logistic regression model determined a priori | |
| Univariate OR 16.8, 95% CI 1.60–176.2) | |||||
|
| |||||
|
Late chronic phase (>6 - <12 months post event)
| |||||
| Kreuz 2006 (17) | FVIII: Ag | ELISA | Off AC | OR 1.0; CI 0.99–1.01 | Association with ‘transient PTS’ diagnosed within 12 months of event |
|
| |||||
| FVIII: C | One-stage clotting assay | Off AC | OR 1.0; CI 0.97 to 1.02 | ||
AC, anticoagulation; dRVVT, dilute Russell viper venom test; ELISA, Enzyme linked immunosorbent assay
Kreuz et al assessed FVIII antigen and activity (early chronic phase) [21] and Lyle et al, lupus anticoagulant (acute phase) [22] in association with PTS. Elevated FVIII antigen or activity tested during early chronic phase after DVT, did not show any association with PTS [21], however, the presence of a lupus anticoagulant by dilute Russell Viper venom time testing (dRVVT) within two weeks of DVT diagnosis was associated with a 17 fold increased odds of developing clinically significant PTS [22]. Additional antiphospholipid antibodies were not evaluated in this study, as they were not uniformly tested at the two centers where the study was carried out. In the same study, elevated FVIII or D-dimer was not predictive of PTS.
The PTS definitions were not uniform in these studies. PTS diagnosis criteria included the presence of either physical abnormalities or functional limitations [20], only objective signs of venous insufficiency to diagnose ‘transient’ PTS [21] or ‘functionally significant PTS’ incorporating both subjective and objective signs and symptoms of PTS [22].
Timing of PTS assessment also varied between the three studies. Kreuz et al coined the term ‘transient PTS’ as PTS that developed within 12 months of the index event while Lyle and authors evaluated PTS at ≥ 1 year and used the latest measurement of PTS assessment for analysis[21, 22]. None of the studies diagnosed PTS after two consecutive, consistent exams, as is the current International Society of Thrombosis and Haemostasis (ISTH) recommendation[23, 24]. Only the study by Lyle and colleagues used a validated, Manco-Johnson Instrument PTS scale[1, 25]. PTS assessors were adequately trained for assessment of PTS at only one of the centers in this study but the assessments were not blinded.
b) Recurrent venous thromboembolism
Of the studies included, recurrent VTE was chosen as the primary end point in two studies [20, 21] but only one assessed biomarkers with the risk of recurrence [20]. The number of subjects with recurrence (8/103) were deemed to be too small in the study by Kreuz and authors for further statistical analysis despite having biomarker data available[21]. Both studies used different definition of recurrent VTE and the time point of measurement of biomarker assessed in the setting of recurrence (Table 1).
The cumulative incidence of recurrent VTE was 6% with a median follow-up of 12 months (3 months -5 years) [20] and 7.8% with a follow-up of 48 months (24 to 120 months) [21]. FVIII and D-dimer were measured in the acute phase (< 1 month post-DVT), and measurements were repeated at follow-up at 3–6 months post-DVT. The study defined recurrent thromboembolism a priori (Table 1) but did not use the ISTH recommended definition as it was completed before 2011 [26]. The biomarkers in this study were assessed in association with recurrent VTE as part of a composite end point of other poor thrombotic outcomes (PTS and thrombus progression) within two year. This study found a statistically significant association between FVIII levels > 150 IU per deciliter, D-dimer above 500 ng per milliliter or both at diagnosis and at follow-up with the development of the composite poor outcome including recurrence [20] (Table 3).
Table 3.
The association between proposed biomarkers and poor outcomes
| Author/year (ref.) |
Biomarker (s) | Measurement Method |
Relationship to anticoagulation (AC) |
Association between FVIII and recurrence |
Comments |
|---|---|---|---|---|---|
|
| |||||
|
Acute phase (< one month post event)
| |||||
| Goldenberg 2004 (16) | FVIII | One-stage clotting assay | On AC | FVIII >150 IU per dL in 28/55 (51%) in those with poor outcome vs. 3/27 (11%) of good outcome group; p=0.061 | OR of 8.9 (95% CI 2.2–36.3; P=0.002) described for FVIII and D-dimer in combination. |
|
|
|||||
| D-dimer | Latex-agglutination assay | On AC | D-dimer > 500 ng per milliliter in 67% good outcome group vs. 38% good outcome group; p=0.03 | FVIII and D-dimer were assessed together. Similarly, outcomes were not individually assessed, rather ‘poor outcome group’ constituted those with persistent thrombosis, recurrent VTE and PTS | |
|
| |||||
|
Sub-acute and early chronic phase (1–6 months post event)
| |||||
| Goldenberg 2004 (16) | FVIII | One-stage clotting assay | Not specified | FVIII > 150 IU per deciliter in 16/32(50%) those with poor outcome vs. 17/43 (39%) in those with good outcome group; p=0.01 | OR of 4.1 (95% CI 1.4–11.6; p=0.008) described for FVIII and D-dimer in combination. |
|
|
|||||
| D-dimer | Latex-agglutination assay | Not specified | D-dimer > 500 ng per milliliter in 56 % good outcome group vs. 24% good outcome group; p=0.03 | FVIII and D-dimer were assessed together. Similarly, outcomes were not individually assessed, rather ‘poor outcome group’ constituted those with persistent thrombosis, recurrent VTE and PTS | |
AC, anticoagulation
c) Chronic thromboembolic pulmonary hypertension
No studies were found that investigated any biomarkers for association with CTEPH.
4. Discussion
Defining predictive risk factors for poor outcomes of thrombosis is paramount in order to permit identification of children with VTE at high risk of sequelae, and to target appropriate prophylactic measures, such as thrombolysis, prolonged anticoagulation, and compression therapy. To date, data that allow stratification of pediatric patients after VTE according to their risk of complications is limited. We systematically reviewed literature on the association between biomarkers of coagulation activation and inflammation with VTE sequelae. Three studies were included, with very small numbers of patients who developed VTE complications.
Due to marked heterogeneity and a small number of studies, we felt a meta-analysis would not give a meaningful combined estimate. Descriptively, a statistically significant association for PTS was found with the presence of lupus anticoagulant in the acute phase after DVT, and for a compound outcome of lack of thrombus resolution, PTS and recurrent VTE with elevated D-dimer and FVIII in both the acute and sub-acute phase after DVT.
Studies differed in many aspects, including population studied (e.g. inclusion of patients with ischemic arterial stroke, VTE affecting different territories (i.e. organs) and with underlying inflammatory disorders) which might influence number of subjects available for assessment of poor outcomes; exclusion criteria (e.g. exclusion of patients on long-term prophylactic anticoagulant therapy or non-white subjects – populations that may be at higher risk of adverse outcomes, such as PTS after DVT or recurrent VTE respectively); biomarkers tested (FVIII and D-dimer vs. FVIII activity and FVIII antigen vs. lupus anticoagulant); definition of outcomes used (two studies were published before the ISTH recommended outcome definitions [26]; timing of outcomes assessment; and the method of analysis (FVIII activity above the cut-off value of 150 IU per deciliter vs. FVIII activity > 90th percentile).
The most commonly measured biomarkers were FVIII and D-dimer. The timing of biomarker measurement after VTE varied among studies. FVIII measurement during VTE onset reflects or may have a component of acute phase reaction, however, measurements during the sub-acute or chronic phases might be more useful. Similarly, elevated D-dimers in subacute-chronic phase is indicative of ongoing activation of coagulation and fibrinolytic pathways. As each phase carries its own importance toward the risk of poor outcomes, we grouped biomarkers from the included studies according to time of measurement.
All included studies analyzed FVIII for its association with PTS. Of these, a nearly 9- and a 4- fold risk of adverse thrombotic outcomes (composite of PTS and recurrence) was found with elevated FVIII at diagnosis and following 3–6 months of anticoagulant therapy; however, these data have yet to be validated individually for either recurrence or PTS in a large pediatric population. Elevated FVIII measured within the first two weeks of DVT presentation [22] and at 6–12 months after the acute thrombotic event [21] did not demonstrate an increased odd of PTS. Given the small number of subjects with PTS in the latter two studies, 5 and 8, respectively, an association cannot be definitively ruled out.
Elevated FVIII is frequently encountered due to acute phase response at the time of acute thrombosis due to high prevalence of acute infections and other proinflammatory states such as surgery and trauma. However, after adjustment for the presence or absence of a chronic inflammatory condition in [20] (present in only 10 percent of children in this cohort), elevated FVIII, D-dimer, or both remained independently predictive of poor outcomes of thrombosis, suggesting association between elevated FVIII and D-dimer was not mediated by overt infection or inflammation. Although neither FVIII nor D-dimer level was elevated in approximately half of the cohort at three to six months in this study, we cannot rule out reverse causality in the remaining, whereby increased FVIII or D-dimer in the poor outcome group may have been caused by PTS or lack of thrombus resolution.
D-dimer is a plasma-based biomarker of coagulation activation and fibrinolysis. It is used in many clinical risk prediction models for recurrent VTE in adults, both at the time of VTE diagnosis and after cessation of anticoagulant therapy, together with clinical predictors to differentiate patients at high or low risk of recurrence. Similarly, an elevated D-dimer suggests an increased risk of PTS. D-dimer, like FVIII, is also nonspecifically elevated in the presence of infection, trauma and systemic inflammation. In children, D-dimer predicted a compound poor outcome of PTS, recurrence and thrombosis progression when checked in the acute and sub-acute phases after DVT [20] but no association with PTS was found when D-dimer was checked within the first two weeks after acute DVT [22]. Further studies are needed to validate these data individually for PTS and recurrent VTE. D-dimer, ideally, should be expressed as a continuous variable, but a cut-off that separates patients on the basis of their risk of outcomes such as recurrence or PTS may be more clinically useful. Another important consideration is whether D-dimer should be measured on or off anticoagulation. Patients on anticoagulants generally have lower D-dimer values, which while being useful for the risk of recurrence, limits its usefulness as an early predictor of PTS. D-dimer values also vary by age and by the assay employed [27], which highlights the potential need for establishing an age and assay specific D-dimer in children with VTE. Overall, additional research is needed to ascertain the optimal manner in which D-dimer might be used as a predictor of poor VTE outcomes.
The antiphospholipid antibody syndrome is an acquired autoimmune disorder characterized by a persistent presence of antiphosphoipid antibodies, thrombosis and in some cases, vasculopathy. One study in our review assessed the association between lupus anticoagulant positivity during acute VTE and PTS development [22]. The authors determined a priori that antiphospholipid antibody would be highly collinear with dilute Russell viper venom test as a specific test for the lupus anticoagulant. A positive dRVVT within two weeks of DVT diagnosis was associated with a 17-fold risk of developing PTS. The lack of association of both the presence of an inflammatory or infectious trigger at presentation with PTS, and dRVVT positivity with the presence of an infectious/inflammatory disorder at diagnosis in this study suggests lupus anticoagulant may exert its effect independent of acute infection or inflammation. While promising as an early biomarker for future development of PTS, the small sample size and wide confidence intervals around the odds of clinically significant PTS for a positive dRVVT suggest the need for further studies to evaluate this association. In antiphospholipid antibody syndrome, vascular cellular infiltrates and fibrosis of the intima and media develop in various vascular territories[28]. It is plausible that a parallel process in veins affected by DVT also occurs. This coupled with impaired thrombus resolution [22], also caused by lupus anticoagulant, may lead to or exacerbate vascular stenosis. Future research is needed to investigate pathways that support these hypotheses. Similarly, the association of persistent antiphospholipid antibodies, in the subacute and chronic phases and the development and severity of PTS needs to be fully explored.
We did not find any study evaluating biomarkers for association with CTEPH. As the pathophysiology underlying the development of thrombofibrotic pulmonary artery occlusion involves inflammation, proliferation and angiogenesis [29], it can be hypothesized that coagulation activation and impaired fibrinolysis may also play a role in the development of this outcome. Elevated FVIII levels have been found in adult patients in association with CTEPH compared to healthy controls and those with nonthromboembolic pulmonary arterial hypertension [30, 31] whereas the association with antiphospholipid antibodies has been controversial [31, 32]. Venous thrombi have high fibrin and red cell content, and their interaction during VTE suggests that thrombi undergo substantial consolidation during their maturation. Several studies in adults have suggested that abnormal fibrin(ogen) concentration; structure and stability contribute to VTE [33]. On study found a high incidence of fibrinogen mutations in patients with CTEPH, suggesting that abnormal fibrinogen structure/and or stability contribute to persistent pulmonary emboli and consequently the development of CTEPH [34]. There is a dearth of such data in the pediatric population. While PTS is the most frequent outcome after DVT, CTEPH is another important under recognized outcome after PE with significant morbidity. Owing to a lack of data for children, ISTH has recently published recommendations for assessment and monitoring of CTEPH in children [2].
The comprehensive search strategy, with two reviewers involved in screening, study selection, and data extraction to perform a wide-ranging search of the available biomarker literature is the main strength of the present review. Finally, we attempted to analyze data within specific subgroups to decrease heterogeneity of results and highlight various phases after VTE when their measurement may be prognostically important. The small number of studies included and the heterogeneity with regards to the biomarkers tested and the timing of testing and the outcomes limit our review. Publication bias could not be assessed. Despite these limitations, this review highlights important findings and has highlighted knowledge gaps in the field. Ultimately, the goal is to identify very early in the course of VTE (acute or sub-acute phases) patients at highest risk of complications amenable to an individualized approach aimed at preventing poor outcomes of thrombosis.
This systematic review also emphasizes the challenges faced when interpreting and applying the currently available biomarker data – or the lack thereof - when determining the risk of complications for a pediatric patient with VTE. To address this issue, the ‘The TOP (Thrombosis Outcomes in Pediatrics) Study’ (www.clinicaltrials.gov; NCT030568923), initiated by the authors, will enroll and systematically follow unselected consecutive pediatric VTE patients over a 4-year period. We will measure conventional and global biomarkers of coagulation, with a standardized comprehensive program of clinical, radiological, functional and laboratory testing coupled to blinded assessments of adverse VTE outcomes. TOP will possess adequate power to provide answers to relevant questions regarding the temporal pattern of biomarkers of coagulation activation, inflammation and fibrinolysis and association with adverse outcomes post VTE. It will provide evidence for future guideline recommendations regarding selection of pediatric patients for long-term follow-up after VTE, the biomarkers that this follow-up should include, and the findings that should be interpreted as indicative of patients at risk of developing recurrent VTE, PTS and post-PE impairment.
5. Conclusions
Given the small number of studies, it is not currently possible to reach a firm conclusion and determine the type of biomarker, the timing of measurement or the method of measurement of a biomarker after an acute thrombotic event that is most predictive for adverse VTE outcomes in the pediatric population. Elevated D-dimer, FVIII and lupus anticoagulant show promise for predicting recurrent VTE and PTS and provide an impetus for future research. CTEPH remains under studied in pediatrics.
Supplementary Material
Highlights.
A systematic review highlighting biomarkers of pediatric VTE outcomes is presented.
D-dimer, FVIII and lupus anticoagulant show promise in predicting recurrence and PTS.
CTEPH remains understudied in children and young adults.
Further research is needed to study pathophysiology of adverse VTE outcomes.
Acknowledgments
AZ is supported by NIH NHLBI Career Development Award (1K23HL132054-01). We are grateful to Dr. Madhvi Rajpurkar for her critical review of the manuscript and thoughtful suggestions. AZ drafted and all other authors edited the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors declare no competing financial interests.
References
- 1.Goldenberg NA, Donadini MP, Kahn SR, Crowther M, Kenet G, Nowak-Gottl U, et al. Post-thrombotic syndrome in children: a systematic review of frequency of occurrence, validity of outcome measures, and prognostic factors. Haematologica. 2010;95:1952–9. doi: 10.3324/haematol.2010.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajpurkar M, Sharathkumar A, Williams S, Lau K, Ling SC, Chan AK, et al. Recommendations for the assessment of non-extremity venous thromboembolism outcomes: communication from the SSC of the ISTH. Journal of thrombosis and haemostasis : JTH. 2015;13:477–80. doi: 10.1111/jth.12809. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg NA, Brandao L, Journeycake J, Kahn S, Monagle P, Revel-vilk S, et al. Definition of post-thrombotic syndrome following lower extremity deep venous thrombosis and standardization of outcome measurement in pediatric clinical investigations. Journal of thrombosis and haemostasis : JTH. 2012;10:477–80. doi: 10.1111/j.1538-7836.2011.04594.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg NA. Long-term outcomes of venous thrombosis in children. Current opinion in hematology. 2005;12:370–6. doi: 10.1097/01.moh.0000160754.55131.14. [DOI] [PubMed] [Google Scholar]
- 5.Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children's hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124:1001–8. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 6.Esmon CT. Crosstalk between inflammation and thrombosis. Maturitas. 2008;61:122–31. doi: 10.1016/j.maturitas.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Roumen-Klappe EM, den Heijer M, van Uum SH, van der Ven-Jongekrijg J, van der Graaf F, Wollersheim H. Inflammatory response in the acute phase of deep vein thrombosis. Journal of vascular surgery. 2002;35:701–6. doi: 10.1067/mva.2002.121746. [DOI] [PubMed] [Google Scholar]
- 8.Wakefield TW, Greenfield LJ, Rolfe MW, DeLucia A, 3rd, Strieter RM, Abrams GD, et al. Inflammatory and procoagulant mediator interactions in an experimental baboon model of venous thrombosis. Thrombosis and haemostasis. 1993;69:164–72. [PubMed] [Google Scholar]
- 9.Wakefield TW, Myers DD, Henke PK. Mechanisms of venous thrombosis and resolution. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:387–91. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- 10.Saha P, Humphries J, Modarai B, Mattock K, Waltham M, Evans CE, et al. Leukocytes and the natural history of deep vein thrombosis: current concepts and future directions. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:506–12. doi: 10.1161/ATVBAHA.110.213405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22–30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 12.van Aken BE, Reitsma PH, Rosendaal FR. Interleukin 8 and venous thrombosis: evidence for a role of inflammation in thrombosis. British journal of haematology. 2002;116:173–7. doi: 10.1046/j.1365-2141.2002.03245.x. [DOI] [PubMed] [Google Scholar]
- 13.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 14.Bombardier C, Villalobos-Menuey E, Ruegg K, Hathaway WE, Manco-Johnson MJ, Goldenberg NA. Monitoring hypercoagulability and hypofibrinolysis following acute venous Thromboembolism in children: application of the CloFAL assay in a prospective inception cohort study. Thrombosis research. 2012;130:343–9. doi: 10.1016/j.thromres.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Meltzer ME, Bol L, Rosendaal FR, Lisman T, Cannegieter SC. Hypofibrinolysis as a risk factor for recurrent venous thrombosis; results of the LETS follow-up study. Journal of thrombosis and haemostasis : JTH. 2010;8:605–7. doi: 10.1111/j.1538-7836.2009.03715.x. [DOI] [PubMed] [Google Scholar]
- 16.Berkun Y, Padeh S, Barash J, Uziel Y, Harel L, Mukamel M, et al. Antiphospholipid syndrome and recurrent thrombosis in children. Arthritis Rheum. 2006;55:850–5. doi: 10.1002/art.22360. [DOI] [PubMed] [Google Scholar]
- 17.Gokce M, Altan I, Unal S, Kuskonmaz B, Aytac S, Cetin M, et al. Recurrent pediatric thrombosis: the effect of underlying and/or coexisting factors. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2012;23:434–9. doi: 10.1097/MBC.0b013e3283548e39. [DOI] [PubMed] [Google Scholar]
- 18.Nowak-Gottl U, Junker R, Kreuz W, von Eckardstein A, Kosch A, Nohe N, et al. Risk of recurrent venous thrombosis in children with combined prothrombotic risk factors. Blood. 2001;97:858–62. doi: 10.1182/blood.v97.4.858. [DOI] [PubMed] [Google Scholar]
- 19.Young G, Albisetti M, Bonduel M, Brandao L, Chan A, Friedrichs F, et al. Impact of inherited thrombophilia on venous thromboembolism in children: a systematic review and meta-analysis of observational studies. Circulation. 2008;118:1373–82. doi: 10.1161/CIRCULATIONAHA.108.789008. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg NA, Knapp-Clevenger R, Manco-Johnson MJ. Elevated plasma factor VIII and D-dimer levels as predictors of poor outcomes of thrombosis in children. The New England journal of medicine. 2004;351:1081–8. doi: 10.1056/NEJMoa040161. [DOI] [PubMed] [Google Scholar]
- 21.Kreuz W, Stoll M, Junker R, Heinecke A, Schobess R, Kurnik K, et al. Familial elevated factor VIII in children with symptomatic venous thrombosis and post-thrombotic syndrome: results of a multicenter study. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:1901–6. doi: 10.1161/01.ATV.0000227510.36653.ed. [DOI] [PubMed] [Google Scholar]
- 22.Lyle CA, Gibson E, Lovejoy AE, Goldenberg NA. Acute prognostic factors for post-thrombotic syndrome in children with limb DVT: a bi-institutional cohort study. Thrombosis research. 2013;131:37–41. doi: 10.1016/j.thromres.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Revel-Vilk S, Brandao LR, Journeycake J, Goldenberg NA, Monagle P, Sharathkumar A, et al. Standardization of post-thrombotic syndrome definition and outcome assessment following upper venous system thrombosis in pediatric practice. Journal of thrombosis and haemostasis : JTH. 2012;10:2182–5. doi: 10.1111/j.1538-7836.2012.04885.x. [DOI] [PubMed] [Google Scholar]
- 24.Goldenberg NA, Brandao L, Journeycake J, Kahn S, Monagle P, Revel-vilk S, et al. Definition of post-thrombotic syndrome following lower extremity deep venous thrombosis and standardization of outcome measurement in pediatric clinical investigations. Journal of thrombosis and haemostasis : JTH. 2012;10:477–80. doi: 10.1111/j.1538-7836.2011.04594.x. [DOI] [PubMed] [Google Scholar]
- 25.Goldenberg NA, Pounder E, Knapp-Clevenger R, Manco-Johnson MJ. Validation of upper extremity post-thrombotic syndrome outcome measurement in children. The Journal of pediatrics. 2010;157:852–5. doi: 10.1016/j.jpeds.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell LG, Goldenberg NA, Male C, Kenet G, Monagle P, Nowak-Gottl U, et al. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. Journal of thrombosis and haemostasis : JTH. 2011;9:1856–8. doi: 10.1111/j.1538-7836.2011.04433.x. [DOI] [PubMed] [Google Scholar]
- 27.Arslan FD, Serdar M, Merve Ari E, Onur Oztan M, Hikmet Kozcu S, Tarhan H, et al. Determination of Age-Dependent Reference Ranges for Coagulation Tests Performed Using Destiny Plus. Iran J Pediatr. 2016;26:e6177. doi: 10.5812/ijp.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. The New England journal of medicine. 2013;368:1033–44. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 29.Bochenek ML, Rosinus NS, Lankeit M, Hobohm L, Bremmer F, Schutz E, et al. From thrombosis to fibrosis in chronic thromboembolic pulmonary hypertension. Thrombosis and haemostasis. 2017 doi: 10.1160/TH16-10-0790. [DOI] [PubMed] [Google Scholar]
- 30.Bonderman D, Turecek PL, Jakowitsch J, Weltermann A, Adlbrecht C, Schneider B, et al. High prevalence of elevated clotting factor VIII in chronic thromboembolic pulmonary hypertension. Thrombosis and haemostasis. 2003;90:372–6. doi: 10.1160/TH03-02-0067. [DOI] [PubMed] [Google Scholar]
- 31.Wong CL, Szydlo R, Gibbs S, Laffan M. Hereditary and acquired thrombotic risk factors for chronic thromboembolic pulmonary hypertension. Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis. 2010;21:201–6. doi: 10.1097/MBC.0b013e328331e664. [DOI] [PubMed] [Google Scholar]
- 32.Wolf M, Boyer-Neumann C, Parent F, Eschwege V, Jaillet H, Meyer D, et al. Thrombotic risk factors in pulmonary hypertension. Eur Respir J. 2000;15:395–9. doi: 10.1034/j.1399-3003.2000.15b28.x. [DOI] [PubMed] [Google Scholar]
- 33.Undas A, Zawilska K, Ciesla-Dul M, Lehmann-Kopydlowska A, Skubiszak A, Ciepluch K, et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood. 2009;114:4272–8. doi: 10.1182/blood-2009-05-222380. [DOI] [PubMed] [Google Scholar]
- 34.Morris TA, Marsh JJ, Chiles PG, Magana MM, Liang NC, Soler X, et al. High prevalence of dysfibrinogenemia among patients with chronic thromboembolic pulmonary hypertension. Blood. 2009;114:1929–36. doi: 10.1182/blood-2009-03-208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.