Abstract
Objective
Given the known side effects of opioids and its potential effect on cognition, we seek to evaluate the benefits of motivational interviewing (MI) to promote physical activity on two subsets of participants with fibromyalgia (FM): non-users and users of opioids.
Method
This is a secondary data analysis of a 36-week randomized controlled trial to assess the efficacy of MI to promote physical activity among participants with FM. Participants were randomized to 1 of 2 treatment arms: 6 phone-based MI sessions (n=107) or 6 sessions of FM self-management instructions (attention control/AC, n=109). The primary outcomes were changes in physical function (SF-36), pain severity (Brief Pain Inventory), global FM symptom severity (Fibromyalgia Impact Questionnaire), and the amount of light to moderate physical activity from baseline to each follow-up visit. At study entry, subjects were categorized as opioid non-users vs. users. Repeated measures analysis of variance (ANOVA) was used to assess treatment effects adjusting for potential confounders.
Results
Of the 216 participants, 145 (67%) were non-users and 71 (33%) were opioid users. Among non-users, MI was associated with improved physical function; reduced pain severity and global FM severity; and increased light to moderate physical activity at 6-month follow-up. Among opioid users, there were no significant differences in any outcome measures between the MI and AC groups.
Conclusion
Exercise-based MI was associated with sustained clinical benefits 6 months after completion of therapy, but only for those who were not taking opioids.
Key Indexing Terms: opioid risk, treatment moderator, motivational interviewing, fibromyalgia
Introduction
Five million patients in the United States (1) have fibromyalgia (FM) with estimated annual costs of $18,671 per patient (2). Exercise, both aerobic and resistance training, improves global well-being, physical function, and pain in FM patients (3) and is considered a cornerstone of FM management. However, despite the well-known benefits of exercise, FM patients are less active than healthy controls, and only 31% of FM patients reported walking regularly (4). For example, in a report of a 12-week supervised exercise program, adherence to aerobic exercise (participation in at least 120 minutes of aerobic exercise per week) was only about 40% (5).
Motivational interviewing (MI) is a collaborative conversational approach for strengthening a person's motivation and commitment to change. MI is associated with better adherence to medications (6), higher rates of successful smoking cessation (7), and increased physical activity (8). Given this evidence, we previously hypothesized that MI would promote and help maintain adherence to an exercise prescription. In our study, MI was associated with increased physical activity and improved global FM symptom severity, but only during the acute treatment phase. Six months after completion of therapy (i.e., primary endpoint), benefits with respect to self-reported physical activity and clinical outcomes were not sustained (9). The absence of long term benefits of MI is likely multifactorial, one of which is the presence of treatment moderators (e.g., use of opioids) that may diminish the effects of MI.
With more liberal prescribing pattern in the 1990's and wider availability of opioids, chronic opioid usage has increased significantly in the past 2 decades with associated increases in deaths from overdoses, misuse, and hospitalization for substance abuse treatment (10). Despite a consensus against the long-term use of opioids for FM from the American College of Neurology (11) and the European League Against Rheumatism (12), 11-69% of patients with FM are reported to take them(13). In patients with FM, the use of opioids is associated with worse clinical outcomes, namely pain severity, functional status, insomnia, depression, and disability (14). Moreover, opioids are associated with deficits in cognitive function, especially in domains of attention, inhibitory control, strategic planning, decision making and learning and memory - domains that are essential to behavioral changes (15). Given the potential complications of opioids in cognitive function (15) and FM-relevant clinical outcomes (14), we hypothesized that MI would be beneficial among non-users of opioids, but ineffective among opioid users. The objective of this report was to evaluate the benefits of MI over attention control (AC) on 2 subsets of participants with FM: non-users and opioid users.
Materials and Methods
Study Design
This is a secondary analysis of a 36-week randomized controlled trial (RCT) to assess the efficacy of MI to promote physical activity among patients with FM (9). Participants were randomized to one of 2 groups, an MI group or an attention control (AC) group. The MI group received 6 telephone-delivered exercise-based MI sessions over 12 weeks. The AC group received 6 telephone contacts over 12 weeks, to control for time and therapist attention. Outcome assessments were conducted at baseline, immediately post-treatment (week 12), and at 3-month (week 24), and 6-month (week 36) follow up. The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Study procedures, including written informed consent, were approved by the Indiana University-Purdue University Indianapolis Institutional Review Board (Study Number: 0708-62). The study enrollment started in December 2007 and the follow up was completed in March 2010.
Study Population
All participants met the following entry criteria: (1) 1990 American College of Rheumatology classification criteria for FM (16); (2) average Brief Pain Inventory (BPI) pain severity score ≥4 (17); (3) FM Impact Questionnaire-Physical Impact score ≥2 (18); (4) on stable doses of medication for FM ≥ 4 weeks; (5) between 18 and 65 years old. To avoid the floor effect we included only FM patients with moderate to severe symptoms. We excluded individuals with (1) known cardiovascular disease; (2) moderate-severe chronic lung disease; (3) uncontrolled hypertension; (4) orthopedic or musculoskeletal conditions that would prohibit moderate-intensity exercise; (5) active suicidal ideation; (6) planned elective surgery during the study period; (7) ongoing unresolved disability claims; (8) inflammatory rheumatic conditions (e.g., rheumatoid arthritis); (9) current use of heart rate lowering medications (e.g., β-blocker); (10) pregnancy; (11) psychosis; or (12) currently participating in a structured exercise program 3 times or more per week. The diagnosis was corroborated by the corresponding author of the study (DCA).
Opioid usage
Opioid users were defined as patients who were taking any opioids (e.g., oxycodone, hydrocodone, propoxyphene, morphine, fentanyl and codeine) for at least for 2 weeks at the time of enrollment. The information on dose was not collected. All participants were taking opioid regularly (e.g., hydrocodone twice daily); none were taking it PRN. We did not consider medication changes that occurred after enrollment.
Randomization
Participants were randomized to one of the 2 treatment arms stratified by depression status, gender, and referral source (specialty vs. primary care). Allocation to treatment arm was carried out by a computer-generated randomization list with permuted block size of 2.
Supervised Exercise Training
All participants received an aerobic exercise prescription and 2 individualized supervised exercise sessions from a qualified fitness instructor who was blinded to treatment assignment. The written exercise prescription included the initial exercise intensity (40-50% of the heart rate reserve/HRR), duration (10-12 min/session), and frequency (2 days/week). Participants were instructed to gradually increase their total volume of exercise to a maximum of 55 to 65% of HRR, 28 to 30 min/session, and 3-4 day/week over the ensuing 36 weeks. Details of the exercise prescription have been previously described (19).
After the 2 supervised exercise sessions, MI participants received the phone-delivered exercise-based MI and the AC group received the phone-delivered education on FM-relevant topics. Each participant interacted with the same interventionist (MI-trained health practitioner or health educator) throughout the study.
Interventions
Exercise-based MI
MI participants received 6 telephone calls over 12 weeks, delivered by trained interventionists who used a standard MI handbook (20). The first 2 MI session focused on enhancing patient motivation to exercise by: (1) eliciting self-motivational statements related to problem recognition and concern about the status quo; (2) intent to participate in graded aerobic exercise; and (3) optimism that exercise-related change is possible. Calls 3 and 4 were devoted to strategies that strengthen commitment to exercise by helping the participant develop a plan for change and reviewing the positive consequences of graded aerobic exercise. The last 2 calls focused on follow-through strategies to prevent relapse of inactivity. As previously reported (9), adherence to the principles and spirit of MI was generally well maintained in the MI group, as assessed by the Motivational Interviewing Treatment Integrity (MITI) (21).
Attention Control
Participants in the AC group received didactic health information delivered over the telephone on the following topics: (1) an overview of FM; (2) pain; (3) fatigue; (4) sleep; (5) stress; and (6) living well with FM.
Measures
All assessments were conducted in one day.
Body Mass Index (BMI)
BMI was calculated by dividing the weight in kilograms by height squared in meters.
Community Health Activities Model Program for Seniors (CHAMPS)
CHAMPS is a 15-minute survey that asks about the frequency and duration of physical activity in a typical week of the past month. The CHAMPS questionnaire provides a list of various activities ranging from light to vigorous intensity. Research supports the validity, reliability, and sensitivity to changes of CHAMPS among older adults (22). The questionnaire provides measures of estimated hours per week spent performing light, moderate and vigorous physical activity (22). We concentrated on light to moderate physical activity because our exercise prescription was focused on light to moderate physical activity, and vigorous exercise is poorly tolerated by FM patients (23).
Fibromyalgia Impact Questionnaire (FIQ)
The FIQ is a disease-specific well validated measure assessing a number of functioning domains related to FM (18). The FIQ includes the FIQ-physical impairment, 6 visual analog scales for measuring FM-related symptoms (e.g., pain and fatigue), and 2 single-item questions assessing work status and overall well-being. A higher FIQ total score (range, 0 to 100) indicates greater severity of global FM symptoms.
SF-36
The SF-36 is a multipurpose, short form health survey with 36 questions on various aspects of health. For the current study, we calculated the Physical Functioning (PF) scale to assess physical function. The 10-item PF scale measures patient's perception of their limitations in the performance of various types of physical activities. Scale scores range from 0 to 100, with higher scores indicating better functioning. The psychometric properties of the PF scale are well established in patients with chronic pain (24), with demonstrated responsiveness to both medical and nonmedical interventions in individuals with FM (25, 26).
Patient Health Questionnaire-8
The Patient Health Questionnaire 8-item (PHQ8) Depression Scale is a self-administered scale which assesses core symptoms of major depressive disorder. Scores can range from 0 to 24; higher scores indicate more severe depressive symptoms. (27).
BPI
The BPI is a measure of pain with proven reliability and validity across different pain conditions. (28), including fibromyalgia (29). In this report, BPI pain severity is the average of 4 items asking about current pain, worse, least, and average pain in the past week.
Statistical Analyses
Repeated measures analyses of variance were used to assess treatment effects within each category. Mixed Linear Models were used for this analysis in order to control for covariates and to use the appropriate covariance structure. All analyses were adjusted for the baseline value of the outcome and body mass index (BMI). BMI was the only baseline characteristic that was significantly different between MI and AC. All analytic assumptions for the statistical models were verified and all analyses were performed using SAS v9.4 (SAS Institute, Cary, NC).
Results
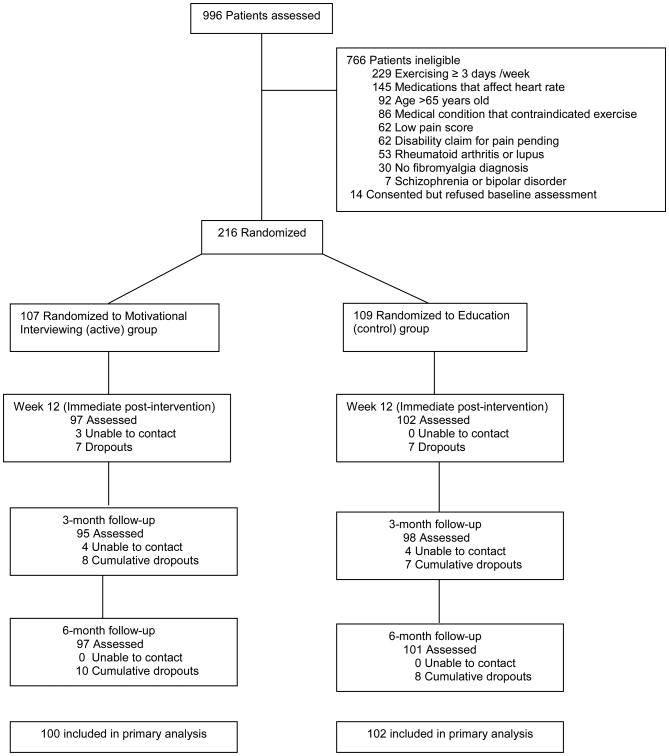
Figure 1 shows the flow of participants in the trial. As seen in Table 1, baseline characteristics were similar across treatment groups except for BMI. MI participants were slightly heavier than AC participants (p=0.07). At study entry, 71 (33%) were taking opioids, while 145 (67%) were not. Of the 71 participants taking opioids, 32 (45%) participants were taking hydrocodone, 13 (18.3%) were taking propoxyphene, 7 (9.8%) were taking oxycodone (short and/or long acting), 1 (1.4%) was taking morphine, and 18 (25.3%) were taking 2 or more classes of opioids. None of the participants were on codeine or tapentadol. Compared to non-users, opioid users had fewer years of education (≥ high school graduate 67.6% vs 82.8%, p=0.012); higher mean depression scores on the PHQ-8 questionnaire (14.08 ± 5.14 vs 11.79 ± 4.65; standard deviations [SD]; p=0.001); worse physical function on the SF-36 questionnaire (36.62 ± 18.47 vs 42.96 ± 18.94; SD; p=0.022); and worse total scores on the FIQ (70.99 ± 12.30 vs 65.12 ± 12.53; SD; p=0.001). There were no statistically significant differences between opioid non-users and users with regards to BPI pain severity [non-users: 5.86 (1.25) vs. users: 6.15 (1.30), p=0.115] and the amount of light to moderate physical activity [non-users: 6.58 (6.78) vs. users: 6.66 hours/week (5.98), p=0.927].
Figure 1. Flow of Participants in the Parent Trial.
Table 1. Baseline Characteristics.
| Motivational interviewing (n=107) | Attention control (n=109) | |
|---|---|---|
| Demographics | ||
| Age in years | 46.0 (11.4) | 45.7 (11.0) |
| Gender, % female | 96 | 95 |
| Ethnicity, % non-Hispanic | 99.1 | 98.2 |
| Race, % white | 90.7 | 86.2 |
| Education, % > high school | 76.6 | 78.9 |
| Marital status, % married | 57.9 | 64.2 |
| Employment, % employed | 57.9 | 49.5 |
| Clinical Variables | ||
| Body mass index (kg/m2) | 32.3 (7.6) | 30.5 (6.6) |
| Duration of fibromyalgia diagnosis (years) | 8.9 (6.4) | 9.1 (7.6) |
| PHQ-8 depression (range 0-24)ˆ | 12.4 (4.8) | 12.7 (5.1) |
| BPI pain severity (range 0-10)ˆ | 5.9 (1.2) | 6.0 (1.4) |
| SF-36 physical function | 41.36 (17.7) | 40.28 (20.2) |
| FIQ total (range 0-100)ˆ | 67.5 (12.0) | 66.6 (13.5) |
| Medications, % prescribed | ||
| Non-tricyclic antidepressants | 66.4 | 57.8 |
| Anticonvulsants | 31.8 | 27.5 |
| Opioid analgesics | 32.7 | 33.0 |
| Physical Activity (self-report) | ||
| CHAMPS | ||
| LMPA (hrs/wk) | 6.5 (5.6) | 6.7 (7.4) |
Values are the means (standard deviation) unless otherwise indicated.
Higher score indicates a worse state of health (except for SF-36 physical function).
Abbreviations: PHQ-8 = Patient Health Questionnaire-8; BPI = Brief Pain Inventory; FIQ = Fibromyalgia Impact Questionnaire; CHAMPS = Community Health Activities Model Program for Seniors; LMPA= light-moderate physical activity. n = number of patients
Main Outcomes
Table 2 shows the comparison between MI and AC groups stratified by opioid usage. Among opioid non-users, MI (vs. AC) was associated with significantly greater improvement in SF-36 physical function, greater reduction in BPI pain, global FM severity, and larger increase in the amount of light to moderate physical activity. On the other hand, among opioid users, there were no significant treatment group differences in SF-36 physical function, BPI pain, global FM severity, and the amount of light to moderate physical activity.
Table 2.
| Change from Baseline to Week 36 | MI | AC | p-value |
|---|---|---|---|
| Improvement in SF36 physical function | |||
| Opioid users | 10.25 (1.94) | 7.00 (1.87) | 0.234 |
| Opioid non-users | 14.47 (1.23) | 9.66 (1.22) | 0.006* |
| Reduction in BPI pain severity | |||
| Opioid users | -0.80 (0.17) | -0.76 (0.16) | 0.930 |
| Opioid non-users | -1.49 (0.12) | -0.98 (0.12) | 0.004* |
| Improvement in global FM severity (FIQ total) | |||
| Opioid users | -9.60 (1.71) | -9.49 (1.66) | 0.963 |
| Opioid non-users | -15.47 (1.21) | -11.85 (1.20) | 0.036* |
| Increase in light-moderate physical activity# | |||
| Opioid users | 3.64 (0.84) | 3.26 (0.82) | 0.746 |
| Opioid non-users | 4.68 (0.52) | 3.01 (0.51) | 0.023* |
Values are means (standard errors) adjusted for baseline outcome and body mass index
P<0.05; Analyses included subjects (N=198, 92% of 216) with 6-month follow-up data.
Number of hours per week
MI=motivational interviewing, AC=Attention control
Discussion
In this secondary analysis of a randomized controlled trial using MI to promote physical activity in FM, MI improved physical function, reduced pain and global FM severity, and increased the volume of light to moderate physical activity among opioid non-users, but not among opioid users.
MI was developed as a cognitive-behavioral strategy using collaborative conversation approach for strengthening a person's motivation and commitment to behavior change (30). MI has demonstrated effectiveness in non-pharmacological management of obesity (31), self-management of chronic pain (32), and medication adherence (33). A recent meta-analysis found moderate-level evidence that MI had a small effect in increasing physical activity levels in adults with obesity, cardiovascular condition or multiple sclerosis (8). Except for our previously completed randomized controlled trial (9), no study to date has employed MI to promote physical activity in individuals with chronic pain.
Unlike other medical patient populations, patients with chronic pain are fearful that exercise might exacerbate their existing pain or result in a new injury or pain site, which may interfere with their planned physical exercise program (34). Fear of pain is a potent inhibitor that keeps patients with chronic pain from keeping regular exercise regimens. Most patients with FM need more than advice to exercise; they need assistance overcoming the barriers to exercise. Our MI program was designed to elicit self-motivational statements, help participants develop a plan for change, and provide strategies and plans to overcome barriers and maintain the intended behavioral changes.
In our primary outcome paper (9), 6 months after completion of treatment, MI was not superior to AC in any of the physical activity and clinical measures. We postulate that the use of opioid may have attenuated the beneficial effects of MI to promote physical activity. One possible explanation is that opioids may impair cognition (35). Opioids decrease activation in regions involved in cognitive control and attention such as dorsolateral prefrontal cortex, anterior cingulate cortex, and inferior parietal lobes, resulting in deficits in executive function (36). Individuals who were dependent on opioids had volumetric loss in the amygdala, which is responsible for emotions, survival instincts, and memory (37); they also showed decreased functional connectivity for the anterior insula, nucleus accumbens and amygdala subdivisions (38). Opioid receptors, including the mu (MOR), delta (DOR) and kappa (KOR), interact with both endogenous and exogenous opioids (39). In animal studies, administration of a selective MOR agonist and endogenous MOR agonists impair working memory (40). In a study of 93 opioid dependent patients and 30 normal controls, the opioid dependents had poorer neuropsychological performance, including verbal fluency, attention and memory. After one week of detoxification, the opioid dependent group performed equally well compared to the normal controls (41). These studies suggest that opioid usage could impair cognition that may hinder processing and retention of information delivered during an MI session.
A second possible explanation is that the common side effects (i.e., fatigue and sleep disorder) of opioid might have (indirectly) resulted in lower physical activity (42). The association between opioid and sleep disordered breathing is well established (43). Among chronic opioid users who underwent overnight polysomnography, 36% had obstructive sleep apnea, 24% had central sleep apnea, 21% had combined obstructive and central sleep apnea, and only 15% did not have sleep apnea (44). Compared to well matched healthy controls, chronic opioid group had a significantly higher apnea-hypopnea index (AHI) (43.5/hour vs 30.2/hour, p<0.05), indicating more sleep disturbance (45). Opioids also reduce rapid eye movement (REM) and non-REM phases of sleep (46). Among our study participants, opioid-induced daytime sleepiness and fatigue may have rendered exercise-based MI seemingly less effective.
Finally, the use of opioid could be a surrogate marker of patients that are more complex to treat and less likely to respond to any treatment. In our study, compared to non-users, participants who used opioids had greater functional impairment (as measured by SF-36 physical function) and FM-related symptom burden (as measured by FIQ). The association of the use of opioids with greater psychological distress and worse health status is well reported in the literature (47, 48).
The current study has some limitations. Given that the current report was a post-hoc analysis of a previously completed RCT, our study findings could have resulted from type 1 error (i.e., false positive findings among non-users of opioids) and/or type 2 error (i.e., false negative among opioid users). As such, appropriate caution about the conclusions has to be made. However, our study findings were consistent across all 4 outcome measures in both groups of participants. Furthermore, there are plausible neurological, biological, and neuropsychological mechanisms (44) that support the concept that opioids may reduce the efficacy of MI. Future larger study is needed to confirm our study findings. Second, we defined opioid-users and non-users based on self-report (verified by the medication container) at the time of the enrollment. We did not consider drug changes during the study. Participants who self-reported use of opioids at study entry might have discontinued the medication during the study, and vice versa. However, cross-contamination between non-users and users would only weaken (rather than magnify) the differential treatment effects that we observed. Third, tramadol was not considered as an opioid in this study. However, the proportions of subjects taking tramadol were not significantly different between opiate non-users and users [30 (20.7%) vs. 13 (18.3%), p=0.7)]; and thus, should not biased the study results. Nevertheless, our study results did not change when the 30 non-users who were on tramadol were reclassified as opiate users (data not shown). Fourth, although we defined opioid users as participants who have been on opioids >2 weeks, we did not distinguish chronic vs. acute use of opioids. However, a previous study reported that a significant majority of patients with FM who were prescribed opioids were still taking them one year later (49). Thus, our participants were likely chronic users of opioids. Nonetheless, some opioid users might have been taking them for only a short time period (e.g., 2 - 12 weeks). In addition, we only measured patients reported outcomes, not performance based outcomes while it is known that there is disagreement between the two outcomes (50). Finally, it would have been interesting to see if the total daily dose of opioids influences the study outcome. Unfortunately, we did not collect such information to assess a possible dose-response relationship.
Our study is the first to show that exercise-based MI was associated with sustained benefits in increasing physical activity and improving clinical outcomes in FM patients who were non-opioid users. In today's world of cost-conscious heath care delivery, it is important to precisely predict treatment effect and tailor treatment to maximize effects. Clinicians may consider offering exercise-based MI among patients who are not taking opioids to enhance FM-relevant treatment outcomes. Furthermore, our study raises an important testable hypothesis that opioids may attenuate the benefits of psychoeducational treatment intervention (i.e., MI) in patients with FM. Using brain neuroimaging and neurophysiologic tools, future investigations should seek to understand the relationships among physical activity, opioids, and the neural substrate of motivation.
Acknowledgments
Source of support: This study was funded by the National Institute of Arthritis & Musculoskeletal and Skin Diseases (1RO1AR054324-01A1).
Footnotes
There is no conflicts of interested indicated by the authors.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skaer TL. Fibromyalgia: disease synopsis, medication cost effectiveness and economic burden. Pharmacoeconomics. 2014;32:457–66. doi: 10.1007/s40273-014-0137-y. [DOI] [PubMed] [Google Scholar]
- 3.Bidonde J, Busch AJ, Bath B, Milosavljevic S. Exercise for adults with fibromyalgia: an umbrella systematic review with synthesis of best evidence. Curr Rheumatol Rev. 2014;10:45–79. doi: 10.2174/1573403x10666140914155304. [DOI] [PubMed] [Google Scholar]
- 4.Segura-Jimenez V, Alvarez-Gallardo IC, Estevez-Lopez F, Soriano-Maldonado A, Delgado-Fernandez M, Ortega FB, et al. Differences in sedentary time and physical activity between female patients with fibromyalgia and healthy controls: the al-Andalus project. Arthritis Rheumatol. 2015;67:3047–57. doi: 10.1002/art.39252. [DOI] [PubMed] [Google Scholar]
- 5.Dobkin PL, Da Costa D, Abrahamowicz M, Dritsa M, Du Berger R, Fitzcharles MA, et al. Adherence during an individualized home based 12-week exercise program in women with fibromyalgia. J Rheumatol. 2006;33:333–41. [PubMed] [Google Scholar]
- 6.Palacio A, Garay D, Langer B, Taylor J, Wood BA, Tamariz L. Motivational Interviewing Improves Medication Adherence: a Systematic Review and Meta-analysis. J Gen Intern Med. 2016:929–40. doi: 10.1007/s11606-016-3685-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindson-Hawley N, Thompson TP, Begh R. Motivational interviewing for smoking cessation. Cochrane Database Syst Rev. 2015:CD006936. doi: 10.1002/14651858.CD006936.pub3. [DOI] [PubMed] [Google Scholar]
- 8.O'Halloran PD, Blackstock F, Shields N, Holland A, Iles R, Kingsley M, et al. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin Rehabil. 2014;28:1159–71. doi: 10.1177/0269215514536210. [DOI] [PubMed] [Google Scholar]
- 9.Ang DC, Kaleth AS, Bigatti S, Mazzuca SA, Jensen MP, Hilligoss J, et al. Research to encourage exercise for fibromyalgia (REEF): use of motivational interviewing, outcomes from a randomized-controlled trial. Clin J Pain. 2013;29:296–304. doi: 10.1097/AJP.0b013e318254ac76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webster LR. Chronic Pain and the Opioid Conundrum. Anesthesiol Clin. 2016;34:341–55. doi: 10.1016/j.anclin.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Franklin GM. Opioids for chronic noncancer pain: a position paper of the American Academy of Neurology. Neurology. 2014;83:1277–84. doi: 10.1212/WNL.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 12.Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluss E, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209724. [DOI] [PubMed] [Google Scholar]
- 13.Goldenberg DL, Clauw DJ, Palmer RE, Clair AG. Opioid Use in Fibromyalgia: A Cautionary Tale. Mayo Clin Proc. 2016;91:640–8. doi: 10.1016/j.mayocp.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Fitzcharles MA, Faregh N, Ste-Marie PA, Shir Y. Opioid use in fibromyalgia is associated with negative health related measures in a prospective cohort study. Pain Res Treat. 2013;2013:1–7. doi: 10.1155/2013/898493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ersche KD, Sahakian BJ. The neuropsychology of amphetamine and opiate dependence: implications for treatment. Neuropsychol Rev. 2007;17:317–36. doi: 10.1007/s11065-007-9033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 17.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–18. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23:S154–62. [PubMed] [Google Scholar]
- 19.Ang DC, Kaleth AS, Bigatti S, Mazzuca S, Saha C, Hilligoss J, et al. Research to Encourage Exercise for Fibromyalgia (REEF): use of motivational interviewing design and method. Contemp Clin Trials. 2011;32:59–68. doi: 10.1016/j.cct.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ang D, Kesavalu R, Lydon JR, Lane KA, Bigatti S. Exercise-based motivational interviewing for female patients with fibromyalgia: a case series. Clin Rheumatol. 2007;26:1843–9. doi: 10.1007/s10067-007-0587-0. [DOI] [PubMed] [Google Scholar]
- 21.Moyers TB, Martin T, Manuel JK, Hendrickson SM, Miller WR. Assessing competence in the use of motivational interviewing. J Subst Abuse Treat. 2005;28:19–26. doi: 10.1016/j.jsat.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–41. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 23.van Santen M, Bolwijn P, Landewe R, Verstappen F, Bakker C, Hidding A, et al. High or low intensity aerobic fitness training in fibromyalgia: does it matter? J Rheumatol. 2002;29:582–7. [PubMed] [Google Scholar]
- 24.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 25.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003;114:537–45. doi: 10.1016/s0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 26.Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Corbin AE, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:1264–73. doi: 10.1002/art.20983. [DOI] [PubMed] [Google Scholar]
- 27.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Schaefer C, Mann R, Masters ET, Cappelleri JC, Daniel SR, Zlateva G, et al. The Comparative Burden of Chronic Widespread Pain and Fibromyalgia in the United States. Pain Pract. 2016;16:565–79. doi: 10.1111/papr.12302. [DOI] [PubMed] [Google Scholar]
- 30.Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behavior. New York: Guilford Press; 1991. [Google Scholar]
- 31.Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. 2011;12:709–23. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- 32.Tse MM, Vong SK, Tang SK. Motivational interviewing and exercise programme for community-dwelling older persons with chronic pain: a randomised controlled study. J Clin Nurs. 2013;22:1843–56. doi: 10.1111/j.1365-2702.2012.04317.x. [DOI] [PubMed] [Google Scholar]
- 33.Moral RR, Torres LA, Ortega LP, Larumbe MC, Villalobos AR, Garcia JA, et al. Effectiveness of motivational interviewing to improve therapeutic adherence in patients over 65 years old with chronic diseases: A cluster randomized clinical trial in primary care. Patient Educ Couns. 2015;98:977–83. doi: 10.1016/j.pec.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Ramsay C, Moreland J, Ho M, Joyce S, Walker S, Pullar T. An observer-blinded comparison of supervised and unsupervised aerobic exercise regimens in fibromyalgia. Rheumatology (Oxford) 2000;39:501–5. doi: 10.1093/rheumatology/39.5.501. [DOI] [PubMed] [Google Scholar]
- 35.Fishbein DH, Krupitsky E, Flannery BA, Langevin DJ, Bobashev G, Verbitskaya E, et al. Neurocognitive characterizations of Russian heroin addicts without a significant history of other drug use. Drug Alcohol Depend. 2007;90:25–38. doi: 10.1016/j.drugalcdep.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev. 2013;37:2597–607. doi: 10.1016/j.neubiorev.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22:561–7. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- 38.Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, et al. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: implications for addiction. Curr Opin Neurobiol. 2013;23:473–9. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh J, Ukai M, Kameyama T. Dynorphin A-(1-13) potently improves the impairment of spontaneous alternation performance induced by the mu-selective opioid receptor agonist DAMGO in mice. J Pharmacol Exp Ther. 1994;269:15–21. [PubMed] [Google Scholar]
- 41.Guerra D, Sole A, Cami J, Tobena A. Neuropsychological performance in opiate addicts after rapid detoxification. Drug Alcohol Depend. 1987;20:261–70. doi: 10.1016/0376-8716(87)90036-6. [DOI] [PubMed] [Google Scholar]
- 42.Painter JT, Crofford LJ. Chronic opioid use in fibromyalgia syndrome: a clinical review. J Clin Rheumatol. 2013;19:72–7. doi: 10.1097/RHU.0b013e3182863447. [DOI] [PubMed] [Google Scholar]
- 43.Van Ryswyk E, Antic N. Opioids and Sleep Disordered Breathing. Chest. 2016 doi: 10.1016/j.chest.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 44.Mogri M, Desai H, Webster L, Grant BJ, Mador MJ. Hypoxemia in patients on chronic opiate therapy with and without sleep apnea. Sleep Breath. 2009;13:49–57. doi: 10.1007/s11325-008-0208-4. [DOI] [PubMed] [Google Scholar]
- 45.Walker JM, Farney RJ, Rhondeau SM, Boyle KM, Valentine K, Cloward TV, et al. Chronic opioid use is a risk factor for the development of central sleep apnea and ataxic breathing. J Clin Sleep Med. 2007;3:455–61. [PMC free article] [PubMed] [Google Scholar]
- 46.Cheatle MD, Webster LR. Opioid Therapy and Sleep Disorders: Risks and Mitigation Strategies. Pain Med. 2015;16(Suppl 1):S22–6. doi: 10.1111/pme.12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krebs EE, Kroenke K, Wu J, Bair MJ, Kozak MA, Yu Z. Opioid Use as a Predictor of Health Care Use and Pain Outcomes: Analysis of Clinical Trial Data. Pain Med. 2016 doi: 10.1093/pm/pnw002. [DOI] [PubMed] [Google Scholar]
- 48.Bair MJ, Bohnert AS. Overdoses in Patients on Opioids: Risks Associated with Mental Health Conditions and Their Treatment. J Gen Intern Med. 2015;30:1051–3. doi: 10.1007/s11606-015-3332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halpern R, Shah SN, Cappelleri JC, Masters ET, Clair A. Evaluating Guideline-recommended Pain Medication Use Among Patients with Newly Diagnosed Fibromyalgia. Pain Pract. 2015 doi: 10.1111/papr.12364. [DOI] [PubMed] [Google Scholar]
- 50.Segura-Jimenez V, Munguia-Izquierdo D, Camiletti-Moiron D, Alvarez-Gallardo IC, Ortega FB, Ruiz JR, et al. Comparison of the International Physical Activity Questionnaire (IPAQ) with a multi-sensor armband accelerometer in women with fibromyalgia: the al-Andalus project. Clin Exp Rheumatol. 2013;31:S94–101. [PubMed] [Google Scholar]