Abstract
Estimating blood demand to determine collection goals challenges many low-income countries. We sampled Tanzanian hospitals to estimate national blood demand. A representative sample based on probability proportional to size sampling of 42 of 273 (15%) Tanzanian transfusing hospitals was selected. Blood bank registers, patient medical records, and blood component disposition records were reviewed prospectively from June to September 2013 to determine the number of components requested and the number and proportion issued, not issued due to nonavailability, and not issued for other reasons. Data were estimated for an annual national estimate. Of an estimated 278 371 components requested in 2013, 6648 (2.4%) were not issued due to nonavailability, 34 591 (12.4%) were not issued for other reasons, and 244 535 (87.8%) were issued. Of these 278 371 components, 86 753 (31.2%) were requested by adult medical, 74 499 (26.8%) by pediatric medical, and 57 312 (20.6%) by obstetric units. In these 3 units, the proportion of units not issued due to nonavailability was 1.8%. Private (4.1%) and large (6%) hospitals had the largest proportion of units not issued because of nonavailability. Of 244 535 issued components, 91 690 (37.5%) were collected, tested, and issued from blood banks that are not part of the Tanzania National Blood Transfusion Services (TNBTS). Nearly 98% of blood component demand was met. However, a large portion of the blood supply for the hospitals came from non-TNBTS blood banks. TNBTS could increase availability of safe blood through assuring the quality of donor selection and donation testing at non-TNBTS blood banks.
Keywords: Blood management, Transfusion practices (adult), Transfusion practices (neonatal, pediatrics)
Improving blood transfusion safety is a priority for Tanzania, the World Health Organization (WHO), the US President’s Emergency Plan for AIDS Relief (PEPFAR), and the United States Centers for Disease Control and Prevention (CDC) [1–5]. To reduce the risk of HIV and other transfusion-transmitted infections (TTIs; eg, hepatitis B, hepatitis C, syphilis) [1–4], WHO recommends that national blood transfusion services collect blood from voluntary nonremunerated donors at low risk for HIV or other TTIs, provide quality screening of donated units for blood-borne pathogens, and maintain adequate blood availability to reduce emergency use of higher-risk blood donations. In 2004, as part of its comprehensive HIV prevention strategy, PEPFAR included blood safety activities based on WHO recommendations. Since then, PEPFAR’s support to strengthen the blood transfusion services in Tanzania and 33 other countries has increased voluntary nonremunerated donor collections in most countries and reduced TTIs among collected unit [3–6]. PEPFAR policy has evolved into a Partnership Framework which was signed in 2009 to transition the portion of blood safety program financed by PEPFAR to Tanzania. Past funding of the blood safety program included all areas of blood safety, whereas the current approved cooperative agreement includes only technical assistance.
With PEPFAR’s contribution to 95% of the Tanzania National Blood Transfusion Services (TNBTS) operational budget, Tanzania has reduced the proportion of units contaminated with HIV and other blood-borne pathogens and increased blood collections [6–9]. HIV prevalence among whole blood (WB) donations within the TNBTS network has dropped from 4.8% in 2005 to 1.5% in 2014. Collections increased from 12 597 donors in 2005 to 171 300 donors in 2014. Despite this increase, TNBTS collected 3.8 WB units per 1000 population in 2014, substantially less than 10 WB units per 1000 per year recommended by WHO for developing countries [10]. As a result, there is concern that safe blood availability is inadequate to meet the transfusion demands in Tanzania and may increase the potential for accepting unsafe donors and using poorly tested blood products.
In an evaluation of about a third of the country’s 273 hospitals that perform transfusions, the TNBTS estimated that 80% of blood issued was provided by the TNTBS [9]. To fulfill demand that would otherwise go unmet, the remaining 20% gap was collected, tested, and issued by non-TNBTS blood banks. Data regarding donor screening and laboratory testing of these non-TNBTS units are unavailable, thus raising concern that non-TNBTS donors may not be rigorously screened and their donations not adequately tested for TTIs [11–14].
As disease epidemiology and transfusion-ordering practices of health care providers change and as the ability to make components increases [15–17], a new approach to more frequently estimate blood demand in low-income countries is needed. Few studies have suggested methods to project blood demand in low-income countries [18–22]. Estimates based on data obtained from a limited population or sample size may not be generalizable to the extended population. Other studies collected data retrospectively and were limited by incomplete medical records and inadequate blood ordering forms [13,22–24]. The purpose of this study is to describe the clinical indications for transfusion in Tanzania by assessing the current ordering and utilization of blood in 42 public and private transfusion hospitals.
To determine the proportion of transfusion orders that are unmet nationally, we conducted a prospective review of blood bank registers and the medical records for all patients for whom transfusions were ordered in a nationally representative sample of hospitals that perform transfusions. Only a portion of the data collected is reported in this manuscript. Data on the estimate of patient needs including the diagnoses and ordering practices driving blood demand for the treatment of anemia in Tanzania are published separately.
Materials and Methods
Study Design
A total of 42 of 273 hospitals that transfuse blood were selected using a stratified probability proportional to size sampling design. Hospitals were stratified by size based on the number of hospital beds. Facilities were further stratified based on their location within Tanzania’s 7 health zones (Eastern, Lake, Northern, Southern, Southern Highland, Western, and Zanzibar) and by type (government, private, faith based). To ensure a nationally representative sample, 2 private, 3 government, and 2 faith-based hospitals were sampled in each zone. If a zone had fewer hospitals than required to meet the sample, then all transfusing hospitals within the zone were included.
All transfusion requests for WB, packed red blood cells (PRBCs), platelets, or fresh frozen plasma (FFP) submitted from June 17 through September 27, 2013, were identified. Data from the blood bank register and the medical record of the corresponding patient were abstracted. Each transfusion request was treated as an independent event. The survey period was specified to account for supply and demand variations and included low–malaria transmission months (August, September), high–malaria transmission months (June, July), and extended school holidays when blood supply is often low due to reduced blood collections [9,24–26].
Outcome Measures
The primary outcome measures for this analysis were the number of requested blood components, number of available components issued to patients (met demand), the number of components that were available but not issued (stock), and number of components not available for issue (unmet demand) due to blood being out of stock or no available replacement donor; outcomes were stratified by age and sex as well as hospital location, size, type, and medical service unit or ward. Reasons components were available but not issued included canceled requests because the patient died, surgery canceled or postponed, or intraoperative hemoglobin remained stable. Where it could not be determined at the blood bank if blood was issued, the patient’s medical record was reviewed to determine if and the number of blood units transfused against the number of units requested to determine the units issued. The assumption was that in these small numbers of cases, the number of blood units transfused was equal to the number of blood units issued.
The following data were collected for each patient that had a transfusion request: age (child defined as person <18 years old; adult defined as person ≥18 years old), sex, clinical diagnosis, medical service unit, hospital type, location (rural, urban), hospital size, source of issued blood components (TNBTS vs non-TNBTS), pretransfusion tests, and whether blood components were transfused or not. The physician or nurse was contacted to determine the specific condition for which the patient was being transfused.
Sample Size and Statistical Power
The sample size was determined using the percentage of blood units received by hospitals from the NBTS network for transfusion (ie, 100[#units from NBTS/#units all sources]), available resources, logistics, and the statistical precision of the estimates. The estimated number of blood unit requests per month per zonal area needed was used along with a design effect of 1.5 to account for the complex survey design [10]. The 95% confidence intervals (CIs) were calculated based upon an estimated NBTS blood supply of 40% for the precision levels of the point estimate for the zonal areas. TNBTS had reported that 80% of the blood supply came from TNBTS sources in 2006. After discussion with TNBTS staff, we assumed the TNBTS supply to be much lower at 40% for 2013 due to reduced resources and funding for blood collection activities. This is a conservative assumption for the sample size calculation, and precision level is guaranteed if the percentage is greater than 40. The expected precision level <0.07 (half CI width) was deemed reasonable and served as the justification for the study sample size.
Data Collection
After a study team of 57 data collectors and 22 data entry clerks were trained on procedures, medical record review, and data entry; a 2-day pilot study was conducted at 4 sites. Following training, study teams were assigned to 42 study hospitals in 7 zones to collect transfusion data from June 17 to September 27, 2013. Blood transfusion requests were identified through daily review of blood bank registers, which contained a patient blood request identification number. Information in the blood requests was entered in a blood request tracking sheet. For each new blood request, a unique study identification number was assigned. Data collectors reviewed the patients’ medical record daily for 7 days following the transfusion order unless the patient was discharged, was transferred, or died before 7 days. Data collectors distinguished between new and repeat requests to fill previously submitted ones using the patient blood request and study identification numbers. Study supervisors visited each study hospital at least once every 7–10 days to review 5%–10% of the questionnaires for accuracy and completeness.
Data Entry and Handling
The completed questionnaires were entered into a CSPro database (US Census Bureau) and uploaded to a secured file transfer system. Double data entry including edit checks for valid values, range checks, and inconsistency checking of any mismatched data points was performed. Through review of data, errors were corrected while at the study site by the study supervisors. Zonal and hospital-specific query reports of blood transfusion requests were generated bimonthly.
Statistical Analysis
The 3.5-month data from the 42-hospital sample were extrapolated to generate annual national estimates. The survey sample design used data weighting together with strata and cluster information to obtain blood demand projections and calculate standard errors for the estimates. Weights were calculated in 2 steps. First, an inclusion probability was computed as the facility size divided by the total size of the corresponding stratum. The sampling weight was then computed as the reciprocal of the inclusion probability. To generate the yearly estimates, multiplicative factors for high- (3 months per year/1.5 months of survey) and low-malaria periods (9 months per year/2 months of survey) were used to adjust the data. Cross-classified tables were generated using proper domain analysis to maintain design integrity. Analysis of the survey data was carried out using the SAS version 9.3 (SAS Institute, Cary, NC).
Ethical Considerations
The study protocol was reviewed and approved by the Tanzania National Institute of Medical Research, the Zanzibar Medical Research and Ethics Committee, and the Institutional Review Board at CDC.
Results
Survey of Blood Transfusion Requests
A total of 14 706 blood transfusion requests for 14 698 patients were submitted at the 42 sampled hospitals over 3.5 months, totaling 21 409 requested components. Of the 14 698 patients for whom blood products were requested, 767 (5.2%) died during the first 7 days of hospitalization. Pretransfusion hemoglobin level, patient age, and patient sex were missing in 14.2% (2090/14706), 1.2% (181/14706), and 0.3% (48/14706) of the requests, respectively. Of the 14 706 requests, for 526 (3.6%), it could not be determined if blood components were issued or not; these requests were adjusted for during analysis.
Of the 21 409 components requested, 17 163 (80.2%) were for WB, 2453 (11.5%) for adult PRBCs, 1165 (5.4%) for pediatric PRBCs, 321 (1.5%) for FFP, and 307 (1.4%) for platelets. The request for red blood cells was roughly equal for adults (97.5% of requested components for WB or PRBCs) and children (96.1%) and the same for males (96.9%) and females (97.1%).
Of the 14 706 requests, 3408 (23.2%) were submitted for children less than 5 years old, 1941 (13.2%) for children 5 to <18 years old, and 9176 (62.4%) for adults. Females accounted for 62.1% of the transfusion requests; women of reproductive age (18–39 years of age) accounted for 4718 (32.1%) of the requests.
Most of the issued components were issued by the TNBTS. Of the 16 728 components that were actually issued to patients, 11 004 (65.8%) were collected, tested, and released by TNBTS and 5412 (32.4%) by non-TNBTS blood banks. The proportion of components handled by TNBTS and non-TNBTS did not differ by medical unit (data not shown) but did differ by age of transfusion recipient and by hospital characteristics (size, type [government, private, faith based], and location [urban, rural]).
Annual National Estimates for 2013
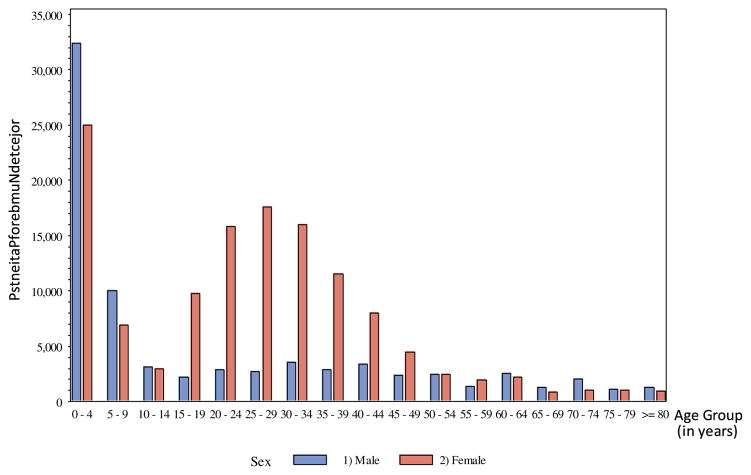
Based on the sample data collected from the 42 hospitals, we estimate that 209 052 transfusion orders for 278 371 blood components were submitted in 2013 in the 273 hospitals that perform transfusions in Tanzania (Table 1). Of the 278 371 component requests, 244 535 (87.8%) were met, 34 591 (12.4%) were available but not issued, and 6648 (2.4%) were unmet (Table 2). Of the estimated 278 371 components requested each year, 50 158 (18.0%) were submitted for boys, 43 183 (15.5%) for girls, 52 371 (18.8%) for men, and 132 657 (47.7%) for women (Fig 1). A mean number of 1.3 blood components units were requested per patient. Women of reproductive age (18–39 years of age) accounted for 34.8% of the component requests or 96 858 components in the year.
Table 1.
Patient demographics and hospital characteristics for annual national estimate of all requested blood components estimated from the survey of 42 representative Tanzanian hospitals, 2013
| Patient demographics | % | Requested components, n (SE) |
|---|---|---|
| Sex | ||
| Male | 35.7 | 99 305 (2550) |
| Female | 64 | 178 241 (3384) |
| Age | ||
| Adulta | 65.1 | 181 301 (3415) |
| Childb | 33.6 | 93 578 (2512) |
| Hospital characteristics | ||
| Hospital type | ||
| Faith based | 27.9 | 77 564 (2686) |
| Government | 65.7 | 182 864 (3413) |
| Private | 6.4 | 17 943 (937) |
| Hospital size | ||
| Large | 14.7 | 41 047 (2480) |
| Medium | 74.8 | 208 349 (4134) |
| Small | 10.4 | 28 975 (2172) |
| Hospital location | ||
| Rural | 26.6 | 74 177 (2691) |
| Urban | 73.4 | 204 194 (3918) |
Adult = 18+ years of age.
Child = <18 years of age.
Table 2.
Annual national estimate of number of patient transfusion orders, requested blood product units, and requests disposition estimated from the survey of 42 representative Tanzanian hospitals, 2013
| Patient age and sex | Transfusion orders | Components requested | Component disposition | ||
|---|---|---|---|---|---|
|
|
|
|
|||
| Issued | Not issueda | Not issued due to blood not available | |||
|
|
|
|
|
|
|
| n | n | n (%) | n (%) | n (%) | |
| All ages, both sexes | 209 052 | 278 371 | 244 535 (87.8) | 34 591 (12.4) | 6648 (2.4) |
| Males <18 y | 46 701 | 50 158 | 48 114 (95.9) | 2109 (4.2) | 296 (0.6) |
| Females <18 y | 39 101 | 43 183 | 41 142 (95.3) | 2036 (4.7) | 357 (0.8) |
| Males ≥18 y | 30 826 | 48 087 | 37 789 (78.6) | 10 434 (21.7) | 2456 (5.1) |
| Females ≥18 y | 89 314 | 132 657 | 113 697 (85.7) | 19 530 (14.7) | 3477 (2.6) |
| Females, reproductive age (18–39 y) | 66, 375 | 96 858 | 84 191 (86.9) | 13 099 (13.5) | 2185 (2.3) |
| Medical units | |||||
| Adult surgery | 18 656 | 29 445 | 20 009 (68) | 9515 (32.3) | 1580 (5.4) |
| Adult medical | 58 873 | 86 753 | 77 559 (89.4) | 9377 (10.8) | 2699 (3.1) |
| Pediatric medical | 70 387 | 74 499 | 72 399 (97.2) | 2126 (2.9) | 243 (0.3) |
| Pediatric surgical | 3028 | 3197 | 2510 (78.5) | 722 (22.6) | 91 (2.8) |
| Intensive care (pediatric or adult) | 2211 | 3759 | 2758 (73.4) | 1043 (27.7) | 248 (6.6%) |
| Obstetrics | 39 884 | 57 312 | 50 589 (88.3) | 6892 (12.0) | 1001 (1.7) |
| Gynecology | 14 337 | 21 760 | 17 283 (79.4) | 4689 (21.6) | 767 (3.5) |
| Orthopedic surgery | 442 | 733 | 647 (88.3) | 98 (13.4) | 15 (2.0) |
The percentages in columns issued and not issued may not add to 100% exactly because of rounding.
The column “Not issued due to blood not available” is included in the units “Not issued.”
Fig 1.
Projected number of transfused patients in Tanzania hospitals by age and sex, 2013.
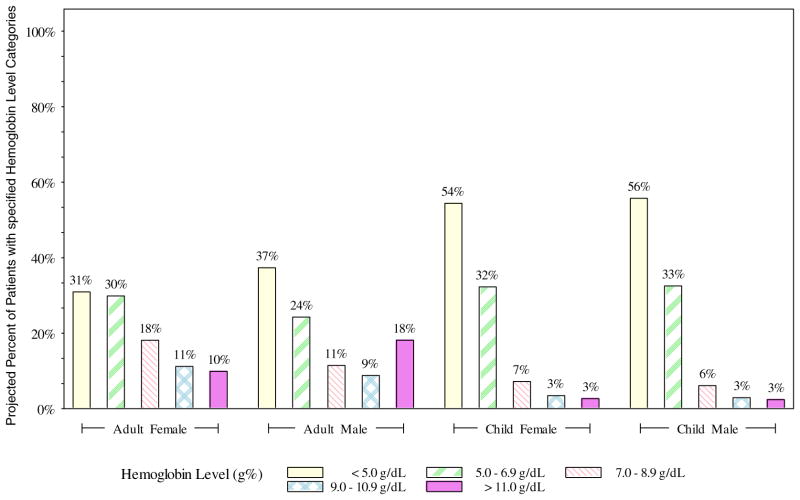
Of the estimated 209 052 component requests, 132 915 (63.6%) were noted to have a pretransfusion hemoglobin level less than 7 g/dL (Fig 2). The mean pretransfusion hemoglobin level for boys was 5.0 g/dL; for girls, 5.1 g/dL; for women, 6.7 g/dL; and for men, 7.0 g/dL.
Fig 2.
Pretransfusion hemoglobin levels for transfusion requests by age and sex (projected), Tanzania, 2013.
Three units accounted for 78.5% of the blood requested in Tanzanian hospitals (Table 2). Of the 278 371 components requested, 86 753 (31.2%) were requested by adult medical units, 74 499 (26.8%) by pediatric medical units, and 57 312 (20.6%) by obstetric units. In these units, unmet demand is lower than the national rate. Of the 218 564 components requested for these 3 units, 3887 (1.8%) were not issued because of nonavailability due to blood stockouts or lack of replacement donors,
Government hospitals requested most of the blood components in Tanzania (Table 3). Of the 278 371 component requests nationally, 182 864 (65.7%) were from government hospitals. These requests met 69.5% of this demand, non-TNTBS met most of the rest, and 2.2% of the requests went unfilled because of nonavailability. At private hospitals, almost twice as high a proportion, 4.1%, of demand was unmet, whereas at faith-based hospitals, the proportion unmet was about the same (2.5%). Regarding unmet demand associated with hospital size, 6.0% of requests were unmet at large hospitals, 2.0% at medium, and none at small hospitals.
Table 3.
Annual national estimate of number and source of requested, issued, and nonavailable (unmet demand) blood components for Tanzania by patient demographics and hospital characteristics estimated from a survey of 42 representative Tanzanian hospitals, 2013
| Patient demographics | Requested components | Issued components | Not issued due to blood not available, % | Source of issued componentsa | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| n (SE) | n | % | NBTS, n (SE) | % | Non-NBTS, n (SE) | % | ||
| Sex | ||||||||
| Male | 99 305 (2550) | 86 820 | 87.4 | 2.8 | 52 068 (2013) | 60.9 | 33 385 (1681) | 39.1 |
| Female | 178 241 (3384) | 157 029 | 88.1 | 2.2 | 95 930 (2635) | 62.3 | 58 040 (2484) | 37.7 |
| Age | ||||||||
| Adultb | 181 301 (3415) | 151 929 | 83.8 | 3.3 | 97 611 (2559) | 65.7 | 50 860 (2253) | 34.3 |
| Childc | 93 578 (2512) | 89 472 | 95.6 | 0.7 | 48 918 (2144) | 55.1 | 39 892 (1968) | 44.9 |
| Hospital characteristics | ||||||||
| Faith based | 77 564 (2686) | 70 073 | 90.3 | 2.5 | 29 066 (1853) | 42.6 | 39 210 (2340) | 57.4 |
| Government | 182 864 (3413) | 157 855 | 86.3 | 2.2 | 108 304 (3017) | 69.5 | 47 523 (2220) | 30.5 |
| Private | 17 943 (937) | 16 608 | 92.6 | 4.1 | 11 048 (866) | 69 | 4957 (478) | 31 |
| Hospital size | ||||||||
| Large | 41 047 (2480) | 25 140 | 61.2 | 6 | 18 559 (1186) | 76.2 | 5792 (529) | 23.8 |
| Medium | 208 349 (4134) | 190 642 | 91.5 | 2 | 118 829 (3395) | 63.5 | 68 446 (2643) | 36.5 |
| Small | 28 975 (2172) | 28 753 | 99.2 | 0 | 11 031 (1487) | 38.7 | 17 452 (1862) | 61.3 |
| Hospital location | ||||||||
| Rural | 74 177 (2691) | 67 487 | 91 | 2.3 | 28 354 (1731) | 42.3 | 38 688 (2203) | 57.7 |
| Urban | 204 194 (3918) | 177 048 | 86.7 | 2.4 | 120 064 (3339) | 69.4 | 53 003 (2409) | 30.6 |
Percent is of those components with documented blood supply; 1.8% did not have documented source.
Adult = 18+ years of age.
Child = <18 years of age.
Of the estimated 244 535 components issued to patients nationally, 148 418 (60.7%) were collected, tested, and released by TNBTS and 91 690 (37.5%) by non-TNBTS blood banks; the type of blood bank could not be determined for 4427 components (1.8%). A large proportion (44.9%) of non-TNBTS issued components was used by pediatric patients within hospitals. Hospital categories were also associated with non-TNBTS component use; 3 subsets of categories relied on non-TNBTS supplies for more than half of their issued units: faith-based hospitals (57.4%), small hospitals (61.3%), and rural hospitals (57.7%) (Table 3). However, all other hospital categories used between 23.8% and 36.5% non-TNBTS components.
Discussion
This study is important because it contributes to the limited data on national estimate of blood demand and use in an African country. Based on a representative sample of 42 hospitals, we estimate that roughly 278 000 blood components were requested in 2013 in Tanzania, more than 97% of which were WB or PRBC. Assuming that all of the issued platelets and FFP were coproduced from the same donations that resulted in the RBC components, it would take approximately 6.2 blood donations per 1000 population in Tanzania to accommodate the estimated demand, a little more than half the amount recommended in WHO guidelines [10]. Our estimate was based upon actual blood requests, and our sampling period attempted to control for high- vs low-need seasons due to malaria and high- vs low-donation seasons due to school holidays. The low rate of documented unmet demand (2.4%) may be attributed to several factors, but the finding that a high proportion of the available components came from blood banks not part of the TNBTS is a critical one to consider. We were able to determine the unmet demand for blood and identify the groups at greatest risk for not receiving requested blood products. The extent of requests for blood and documentation practices at transfusion facilities in low-income African countries was unknown. The results of this study will provide information about blood requests (demand) which can be used to develop a national plan to set realistic collection targets based upon requests and close the gap between supply and demand.
More than 37% of issued blood units were collected and screened by non-TNBTS blood banks. These non-TNBTS units were especially important in reducing the unmet demand in hospitals that were small, rural, or faith based (more than 50% of supply from non-TNBTS sources) and among children, almost half of whom received non-TNBTS components. To the degree that this blood may be less safe due to collection from paid or coerced donors or due to poor screening for blood-borne pathogens [27], people receiving blood components in these hospitals and children may be at a greater risk for transfusion-associated infections with HIV, HBV, HCV, or syphilis. However, because non-TNBTS blood is used in all hospital types, sizes, and locations studied, interventions should be taken to improve blood quality outside the NBTS system.
The unmet demand was not uniformly distributed over all patients or hospital wards or facilities. For patients in the obstetrics unit and the pediatric medical unit (where severe malaria is treated), the unmet demand was 1.7% and 0.3%, respectively. In contrast, unmet demand was greatest in the adult surgical units and the intensive care units, that is, 5.4% and 6.6%. Although the intensive care units represent a small proportion of the transfusion orders in Tanzanian hospitals, adult surgical units account for 10.5% of all the blood ordered. Regarding age and sex, adult men have the highest proportion of unmet demand, that is, 5.1%. There were differences in unmet demand between large and small hospitals. Large hospitals were mostly government and specialty hospitals that made most of the blood requests to the NBTS. A portion of the requests was not met due to limited stock of blood at the NBTS because of their heavy reliance on voluntary donations. This resulted in a higher percentage of unmet demand at the large hospitals compared with small hospitals. In case of small hospitals, less blood requests were made, which were filled through donations mostly outside the NBTS network.
Consistent with the results of our study, a study in a tertiary hospital in Kampala, Uganda, also showed a majority of blood issued as WB, although more red blood cells, platelets, and plasma were issued than in our study [28], as Tanzania is not yet routinely engaged in preparing other components. The Kampala study also found similar findings including a higher percentage of female transfusion recipients and obstetrics, gynecology, and medical wards as the top users of blood issued. Another Ugandan study from a regional referral hospital found a mean number of 1.7 units requested per recipient, similar to 1.3 units in our study, and also that most patients received WB transfusions [20]. Unlike our study, a study of blood transfusion services at a district hospital in Malawi during 1 month reported a higher percentage of pediatric transfusions (57%) compared with 34% in our study [29], which may be due to our more representative sample of hospitals and seasons.
Our study has a number of important strengths. Like a recent study in Namibia (population 2.1 million) reported by Pitman and colleagues [30], we based our review on clinical and demographic data, although derived from a much larger population with a larger and more complex health care system. Transfusion information was obtained from patients’ medical records while they were still hospitalized. Data collectors performed individual medical record reviews of the patients looking for specific information such as pretransfusion hemoglobin levels and documentation if the units were available and issued. Each individual blood request was followed for 7 days. Hence, we were able to determine if clinicians were ordering blood components to fill previously unfilled blood requests and eliminate double counting. Our approach used blood requests and medical record reviews to determine blood demand and whether demand was met or not. Most significantly, we captured blood components issued from both TNBTS and non-NTBTS sources.
The study has limitations that could suggest that the estimated unmet demand of 2.4% is an underestimate. The unmet demand could be higher if some of the 12.4% of requests listed as “available but not issued” were miscategorized and were actually not available. About 20% of records did not have a documented reason for unissued blood. Also, our study did not evaluate clinician ordering practices to determine if they were appropriate. Underordering may be common if clinicians assume lack of availability of blood. For instance, the study shows potentially low orders for blood components (average of 1.3 units per request), although orders were primarily for red blood cells and mean hemoglobin levels <7 g/dL. On the other hand, clinicians may overorder, recognizing that availability is limited and thus ordering for unpredictable needs like potential deterioration in a medical condition. Some of the “available but not issued” components may fall into this group, ordered prior to conclusive documentation of need. WHO has suggested that blood products “are often overprescribed in both developed and developing countries [3].”
We did not sample all facility types, such as the 4 military hospitals. Furthermore, concerning sampling of facilities by bed size, the official number of hospital beds may not be precisely accurate in the hospitals. In the generation of the national yearly estimate, we assumed 3 months of high malaria (May–July) and 9 months of low malaria (August–April) during the year. Any observed longer duration of high-malaria periods may have led to an underestimation of our national projection. We assumed that every unit of blood was derived from a different individual whereby donations are expressed in units for adult transfusions with variations between 450 and 500 mL; it is unclear if this assumption could slightly underestimate the total number of donations or blood donors to fill a blood request. Although extrapolation is based on statistical sampling theory and common in heath surveys, such an extrapolation may not be accurate and may be a potential limitation.
In conclusion, our study provides valuable information about estimation of blood demand among transfusing hospitals in Tanzania. This information can be used to set blood collection targets based upon documented requests. Documenting the substantial proportion of blood component requests filled by non-TNBTS also suggests that the TNBTS should institute quality control interventions that can be used by blood suppliers outside the national system while at the same time expanding the TNBTS blood supply and coverage to ensure overall safety of the blood supply. The study also raises questions about blood-requesting practices—either over- or underordering—that need to be further evaluated. The study results and the questions it raises can be applied to other developing countries and used for national collection planning to better match the blood supply with the clinical demand for its use and to better understand blood-requesting practices. In addition, the information presented here on current national transfusion practices may help in the development of standards for patient blood management.
Acknowledgments
Source of support: United States President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of Cooperative Agreement COAG# GH001613 and 939ZNHT. The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention
Dr Magdalena Lymo of AABB for supportive supervision during data collection coordinating the study, the procurement of supplies, equipment and hiring of study teams. We would also like to thank Dr Sridhar Basavaraju, CDC Atlanta, for assistance with the study protocol and project planning.
Footnotes
Conflict of Interest
None of the authors report a conflict of interest.
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.World Health Organization. Aide-memoire for national health programmes. World Health Organization; 2002. [Available at: http://www.who.int/bloodsafety/transfusion_services/en/Blood_Safety_Eng.pdf] [Google Scholar]
- 2.World Health Organization. The clinical use of blood handbook. Geneva: World Health Organization blood Transfusion Safety; 2003. [Google Scholar]
- 3.World Health Organization. Blood transfusion safety: safe and rational use. World Health Organization; 2006. [Available at http://www.who.int/bloodsafety/clinical_use/en] [Google Scholar]
- 4.World Health Organization. Availability, safety and quality of blood products; Executive board 125th session; 2009. Document EB125/5. [Google Scholar]
- 5.The United Republic of Tanzania. Guidelines on the clinical use of blood and blood products. Ministry of Health and Social Welfare; 2006. [Google Scholar]
- 6.CDC. Progress toward strengthening blood transfusion services—14 countries, 2003–2007. MMWR. 2008;57:1273–7. [PubMed] [Google Scholar]
- 7.CDC. Progress toward strengthening blood transfusion services—14 countries, 2008–2010. MMWR. 2011;57:1577–82. [PubMed] [Google Scholar]
- 8.World Health Organization. Global health observatory HIV/AIDS data, 2009. Geneva, Switzerland: World Health Organization; 2011. Global Database for Blood Safety. [Google Scholar]
- 9.Ministry of Health and Social Welfare. Annual health statistical abstract. Dar es Salaam: government of Tanzania; 2006. [Google Scholar]
- 10.World Health Organization Regional Office for Africa. Status of blood safety in the WHO African region: report of the 2006 survey. 2009. [Google Scholar]
- 11.Basavaraju SV, Mwangi J, Nyamnogo J, Zeh C, Kimani D, Shiraishi RW, et al. Reduced risk of transfusion-transmitted HIV in Kenya through centrally coordinated blood centres, stringent donor selection and effective p24 antigen-HIV antibody screening. Vox Sang. 2010;99(3):212–9. doi: 10.1111/j.1423-0410.2010.01340.x. [DOI] [PubMed] [Google Scholar]
- 12.Pitman JP, Basavaraju SV, Shiraishi RW, Wilkinson R, von Finckenstein B, Lowrance DW, et al. Namibia’s transition from whole blood–derived pooled platelets to single-donor apheresis platelet collections. Transfusion. 2015:1685–92. doi: 10.1111/trf.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed SG, Ibrahim UA, Hassan AW. Adequacy and pattern of blood donations in north eastern Nigeria: the implications for blood safety. Ann Trop Med Parasitol. 2007;101(8):725–31. doi: 10.1179/136485907X241442. [DOI] [PubMed] [Google Scholar]
- 14.Lackritz EM, Ruebush TK, Zucker JR, Adungosi JE, Were JB, Campbell CC. Blood transfusion practices and blood-banking services in a Kenyan hospital. AIDS. 1993;7(7):995999. doi: 10.1097/00002030-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Gumodoka B, Vos J, Kigadye FC, Asten HA, Dolmans WM, Borgdorff MW. Blood transfusion practices in Mwanza region, Tanzania. AIDS. 1993;7:387–92. doi: 10.1097/00002030-199303000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Vos J, Gumodoka B, Asten HA, Berege ZA, Dolmans WM, Borgdorff MW. Changes in blood transfusion practices after the introduction of consensus guidelines in Mwanza region, Tanzania. AIDS. 1994;8:1135–40. doi: 10.1097/00002030-199408000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Mosha D, Poulsen A, Reyburn H, Kituma E, Meti F, Bygbjerg IC. Quality of paediatric blood transfusions in two district hospitals in Tanzania: a cross-sectional hospital based study. BMC Pediatr. 2009;9(51):1–6. doi: 10.1186/1471-2431-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozumba BC, Ezegwui HU. Blood transfusion and caesarean section in a developing country. J Obstet Gynaecol. 2006;26(8):746–8. doi: 10.1080/01443610600955792. [DOI] [PubMed] [Google Scholar]
- 19.Desalu I, Dada OI, Ahmed RA, kin-Williams OO, Ogun HA, Kushimo OT. Transfusion trigger—how precise are we? Intraoperative blood transfusion practices in a tertiary centre in Nigeria. Transfus Med. 2008;18(4):211–5. doi: 10.1111/j.1365-3148.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 20.Natukunda B, Schonewille H, Smit Sibinga CT. Assessment of the clinical transfusion practice at a regional referral hospital in Uganda. Transfus Med. 2010;20(3):134–9. doi: 10.1111/j.1365-3148.2010.00992.x. [DOI] [PubMed] [Google Scholar]
- 21.Akinola OI, Fabamwo AO, Tayo AO, Rabiu KA, Oshodi YA, Onyekwere CA. Evaluation of blood reservation and use for caesarean sections in a tertiary maternity unit in south western Nigeria. BMC Pregnancy Childbirth. 2010;10(1):57. doi: 10.1186/1471-2393-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed SG, Ibrahim UA, Kagu MB. The burden of HIV and AIDS on blood bank reserves in northeast Nigeria. Trans R Soc Trop Med Hyg. 2007;101(6):618–20. doi: 10.1016/j.trstmh.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Basavaraju SV, Pitman JP, Henry N, McEwan C, Harry C, Hasbrouck L, et al. The need for computerized tracking systems for resource-limited settings: the example of Georgetown, Guyana. Transfus Med. 2009;19(3):149–51. doi: 10.1111/j.1365-3148.2009.00925.x. [DOI] [PubMed] [Google Scholar]
- 24.Fasola FA, Kotila TR, Shokunbi WA. Audit of the red cell units supply of a busy hospital blood bank in Nigeria. Niger J Clin Pract. 2009;12(2):165–8. [PubMed] [Google Scholar]
- 25.WHO and Ministry of Health and Social Welfare, National Malaria Control Program. Roll back malaria partnership: focus on mainland Tanzania program and impact series. Country report. 2011;3 [Google Scholar]
- 26.Zanzibar malaria epidemic detection system biannual reports, volumes 1–3; nos 1–2, September 2009, March 2010, October 2010, April 2011 and October 2011.
- 27.Kimani D, Mwangi J, Mwangi M, Bunnell R, Kellogg TA, Oluochi T, et al. Blood donors in Kenya: a comparison of voluntary and family replacement donors based on a population-based survey. Vox Sang. 2011;100:212–8. doi: 10.1111/j.1423-0410.2010.01376.x. [DOI] [PubMed] [Google Scholar]
- 28.Butler Elissa K, Hume H, Birungi I, Ainomugisha B, Namazzi R, Ddungu H, et al. Blood utilization at a national referral hospital in sub-Saharan Africa. Transfusion. 2015:1–9. doi: 10.1111/trf.13010. [DOI] [PubMed] [Google Scholar]
- 29.Bugge HF, Karlsen NC, Oydna E, Rake MM, Wexels N, Bendabenda J, et al. A study of blood transfusion services at a district hospital in Malawi. Vox Sang. 2013;104:37–45. doi: 10.1111/j.1423-0410.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 30.Pitman JP, Wilkinson R, Liu Y, von Finckenstein B, Sibinga CTS, Lowrance DW, et al. Blood component use in a sub-Saharan African country: results of a 4-year evaluation of diagnoses associated with transfusion orders in Namibia. Transfus Med Rev. 2015;29(1):45–51. doi: 10.1016/j.tmrv.2014.11.003. [DOI] [PubMed] [Google Scholar]