Abstract
Background
Exposure to ambient fine particulate matter (PM2.5) is associated with increased cardiometabolic morbidity and mortality. This is widely believed to be attributable to PM2.5 exposure-induced pulmonary and subsequent systemic inflammation. Tumor necrosis factor alpha (TNFα), lymphotoxin α (LTα), and lymphotoxin β (LTβ) are three homologous pro-inflammatory cytokines, each with both unique and redundant activities in inflammation. Their role in PM2.5 exposure-induced inflammation and adverse cardiometabolic effects has to be determined.
Methods and results
LTα/TNFα/LTβ triple-knockout (TNF/LT KO) and wildtype (WT) mice were exposed to concentrated ambient PM2.5 (CAP) for 5 months. Lung pathological analysis revealed that TNF/LT deficiency reduced CAP exposure-induced pulmonary inflammation. However, glucose homeostasis assessments showed that TNF/LT deficiency significantly aggravated CAP exposure-induced glucose intolerance and insulin resistance. Consistent with glucose homeostasis assessments, CAP exposure significantly increased the body weight and adiposity of TNF/LT KO but not WT mice. In contrast to its body weight effects, CAP exposure reduced food intake of WT but not TNF/LT KO mice. On the other hand, CAP exposure induced marked fat droplet accumulation in brown adipose tissues of WT mice and significantly decreased their uncoupling protein 1 (UCP1) expression, and these effects were markedly exacerbated in TNF/LT KO mice.
Conclusion
The present study suggests that TNF/LT deficiency influences PM2.5 exposure-induced response of energy metabolism through alterations in both food intake and energy expenditure.
Keywords: Ambient PM2.5, Tumor necrosis factor, Lymphotoxin, Obesity, Inflammation
1. Introduction
Exposure to ambient particulate matter with a diameter ≤2.5 μm (PM2.5) is associated with increased premature mortality (Lepeule et al., 2012), which is attributed primarily to PM2.5 exposure-induced cardiometabolic abnormalities such as endothelial dysfunction, insulin resistance, and hypertension. The majority of inhaled PM2.5 deposit in the airway and do not enter the systemic circulation (www.epa.gov/ncea/isa/pm.htm). As such, how PM2.5 in the airway cause those cardiometabolic abnormalities comes out to be a primary scientific question in the toxicology of PM2.5 pollution. By far, numerous studies have demonstrated that ambient PM2.5 inhalation induces both pulmonary and systemic inflammations (Brook et al., 2010). And increasing evidences indicate these pulmonary and systemic inflammations may play a crucial role in the development of cardiometabolic abnormalities due to exposure to PM2.5. Furthermore, a time course study has revealed the precedence of pulmonary inflammation over extra-pulmonary inflammation (Brook et al., 2010), suggesting that PM2.5 exposure-related extra-pulmonary inflammation may be consequent to pulmonary inflammation.
Inflammation is a complicated biological process involving diverse immune cells, blood vessels, and molecular mediators. Tumor necrosis factor alpha (TNFα) is one of the best known pro-inflammatory cytokine that has been shown to link inflammation to cardiometabolic abnormalities (Esser et al., 2015). Many studies have demonstrated that exposure to PM2.5 significantly increases pulmonary and circulating TNFα levels (Kennedy et al., 1998; Quay et al., 1998; van Eeden et al., 2001; Vogel et al., 2005), making it one of the most likely mediators for PM2.5 exposure-induced extra-pulmonary inflammation and cardiometabolic abnormalities. Lymphotoxin α (LTα) and lymphotoxin β (LTβ) are two pro-inflammatory cytokines closely homologous to TNFα. Their genes are in close proximity to the Tnfα gene in the major histocompatibility complex (MHC, the specific region is thus known as TNF/LT locus). Due to the proximity of these three genes on DNA, genetically modulating one of them will alter the expression of others (Kuprash et al., 2005). In addition, these homologous cytokines are functionally redundant in both developmental and inflammatory animal models. For example, both LTα and TNFα have been found to play a role in the control of Mycobacterium tuberculosis infection (Roach et al., 2001). Compared to single LTα, LTβ or TNFα knockout mice, the LTα/TNFα/LTβ triple-knockout (TNF/LT KO) mice demonstrated server conditions in the microarchitecture of spleen with disorder of lymphoid cell positioning and functional T- and B-cell compartmentalization (Kuprash et al., 2002). More importantly, deficiency of LTα or TNF/LT has been shown to markedly reduce ozone inhalation-induced increase in macrophages of bronchoalveolar lavage fluid (Bauer et al., 2010), suggesting that TNF/LT may play a critical role in the pulmonary response to inhalation of ambient pollutants. Therefore, in the present study, to delineate their role in mediating the health effects of PM2.5 exposure, we exposed TNF/LT KO and control mice to concentrated ambient PM2.5 (CAP) and assessed their inflammatory and metabolic responses.
2. Materials and methods
2.1. CAP exposure
The University of Maryland, Baltimore (UMB) is an AAALAC accredited institution. All procedures of this study were approved by the Institutional Animal Care and Use Committee at UMB, and all the animals were treated humanely and with regard for alleviation of suffering. TNF/LT KO mice were obtained from the Jackson Laboratories (Stock # 005108, Bar Harbor, ME) and bred in-house. Age-matched wildtype (C57BL/6J) mice were purchased from the Jackson Laboratories (Stock #000664) and were allowed to acclimate for two weeks in animal facilities at UMB before beginning inhalation exposure protocols. Animal exposure and the monitoring of exposure atmosphere and ambient aerosol were performed as previously described using a versatile aerosol concentration enrichment system that was modified for long-term exposures (Ying et al., 2014). Briefly, 10-week-old WT and KO mice were randomly grouped (N = 7–8/group, male only) and subjected to filtered air (FA) or CAP exposure from October 2015 to March 2016. The exposure protocol comprised exposures for 6 h/day, 5 days/week (no exposure took place during weekends). The measured PM2.5 concentrations during the exposure period were 7.6 ± 1.4 and 52.1 ± 25.1 μg/m3 in FA and CAP chambers, respectively. Thus the corresponding average PM2.5 concentrations (calculated using the measured values times 5/28 representing for 6 h/day and 5 days/week according to the exposure protocol) were 1.4 ± 0.25 and 9.3 ± 4.5 μg/m3 in FA and CAP chambers, respectively. This PM2.5 value is much lower than those in densely populated cities such as Beijing or Mexico City.
2.2. Lung histopathology
After 5-month exposure to FA/CAP, mice (around 7-month-old) were euthanized by overdose of isoflurane, the lung was harvested and either fixed with neutral-buffered formalin or snap-frozen in liquid nitrogen and then kept at −80 °C. To assess the inflammation in the lung, tissue blocks were embedded in paraffin and 5-μm thick sections were cut from the anterior surface, and the sections were subjected to hematoxylin and eosin (H & E) staining. Three consecutive sections per sample were used for histopathology. Images covering the entire tissue area were taken by one laboratory technician who was blind to the grouping, and all images were then sent to and quantified by the pathologist (blind to the grouping too). The pulmonary inflammation levels were scored according to the scoring system in Table 2, which was previously reported and used with some minor modifications (Curtis et al., 1991). A sum of all the indexes in Table 2 was calculated and demonstrated as the inflammatory score.
Table 2.
Pulmonary inflammation scoring system and results.
| Scoring standard | WT/FA | WT/CAP | KO/FA | KO/CAP | |
|---|---|---|---|---|---|
| Oedema | absence, 0; presence, 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Hyperaemia | absence, 0; presence, 1 | 0.87 ± 0.33 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| Haemorrhage | absence, 0; presence, 1 | 0.75 ± 0.43 | 1 ± 0 | 1 ± 0 | 1 ± 0 |
| Macrophage infiltration | presence of every 10% area = 1 | 0.17 ± 0.12 | 1.43 ± 0.57* | 0.53 ± 0.41*,# | 0.71 ± 0.39*,# |
| Interalveolar thickness | presence of every 10% area = 1 | 0.87 ± 0.35 | 2.14 ± 0.63* | 2.25 ± 0.43* | 1.85 ± 0.35* |
| Alveolar distortion | presence of every 10% area = 1 | 0.75 ± 0.43 | 1.71 ± 0.68* | 1.75 ± 0.66* | 2 ± 0.53* |
p < 0.05 versus WT/FA.
p < 0.05 versus WT/CAP; two way ANOVA.
2.3. Intraperitoneal glucose tolerance test (IPGTT)
IPGTT was performed after 4-month exposure to FA/CAP (5 weeks before the necropsy). Before testing, mice were fasted for 16 h. On the day of experiments, basal glucose level of tail vein blood was determined using an automatic glucometer (Contour, Bayer), and then mice were intraperitoneally injected with glucose (2 g/kg body weight). Blood glucose levels at 15, 30, 60, and 120 min after injection were measured as described above. Extra blood samples at every time point were collected from the tail veins, and plasma was isolated and immediately kept at −80 °C for insulin assessment.
2.4. Intraperitoneal insulin tolerance test (ITT)
ITT was performed 4 weeks before the necropsy. After 4-h fasting, baseline blood glucose of tail vein and body weights were measured. After intraperitoneal injection of 0.5 unit/kg body weight Novalin insulin (Novo Norkisk, Denmark), blood was collected by tail vein puncture and glucose was measured at 15, 30, 60, 90, 120 min by an automatic glucometer (Contour, Bayer).
2.5. Plasma insulin measurement and homeostasis model assessment-insulin resistance (HOMA-IR) calculation
Plasma insulin levels were determined using Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chemical) per manufacturer’s instruction. The HOMA-IR index of each mouse was calculated using the values of fasting plasma glucose (FPG) and plasma insulin (PI) as follows: HOMA-IR = FPG × PI × 172.1/22.5, with FPG expressed as mmol/L and PI as ng/ml.
2.6. Histological analysis of adipose tissues
Epididymal adipose tissue and brown adipose tissues (BAT) were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 5-μm sections, and stained with hematoxylin and eosin. The histology sections were viewed at 20× magnification, and images were obtained with a SPOT digital camera (Diagnostic Instruments, Sterling Heights, MI) by one person who was blind to the grouping. The total number and cross-sectional areas of adipocytes in epididymal adipose tissues were calculated as previously described (Chen and Farese, 2002). The fat droplet areas of BAT were obtained using Imagej software, and the results were expressed as the percentage of total area.
2.7. Real-time quantitative PCR (qPCR)
Total RNA was isolated from tissues with TRIzol reagent (Invitrogen, Carlsbad, CA, USA). 2 μg total RNA was reverse transcribed using random hexamers and the ThermoScript RT-PCR System (Invitrogen). qPCR was performed with the Lightcycler 480 using SYBER Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA). The sequences of primers were presented in Table 1. The relative expression level was obtained as described previously (Ying et al., 2009). Briefly, Ct values were acquired through analysis with software provided by the manufacturer, and differences of Ct value between target gene and GAPDH (ΔCt) and then 2ΔCt were calculated.
Table 1.
Primer sequences.
| Gene | Sequences | |
|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Sense | 5′-TGA ACG GGA AGC TCA CTG G-3′ |
| Antisense | 5′-TCC ACC ACC CTG TTG CTG TA-3′ | |
| Pro-opiomelanocortin (POMC) | Sense | 5′-GCC CTC CTG CTT CAG ACC TC-3′ |
| Antisense | 5′-CGT TGC CAG GAA ACA CGG-3′ | |
| Neuropeptide Y (NPY) | Sense | 5′-TAC CCC TCC AAG CCG GAC AA-3′ |
| Antisense | 5′-TTT CAT TTC CCA TCA CCA CAT G-3′ | |
| Agouti-related peptide (AgRP) | Sense | 5′-CGG AGG TGC TAG ATC CAC AGA-3′ |
| Antisense | 5′-AGG ACT CGT GCA GCC TTA CAC-3′ | |
| Interleukin-1β (IL-1β) | Sense | 5′-ACG GAC CCC AAA AGA TGA AG-3′ |
| Antisense | 5′-TTC TCC ACA GCC ACA ATG AG-3′ | |
| Interleukin-6 (IL-6) | Sense | 5′-ATC CAG TTG CCT TCT TGG GAC TGA-3′ |
| Antisense | 5′-TAA GCC TCC GAC TTG TGA AGT GGT-3′ | |
| Tumor necrosis factor alpha (TNFα) | Sense | 5′-TTC CGA ATT CAC TGG AGC CTC GAA-3′ |
| Antisense | 5′-TGC ACC TCA GGG AAG AAT CTG GAA-3′ | |
| Suppressor of cytokine signaling 3 (SOCS3) | Sense | 5′-GCG GGC ACC TTT CTT ATC C-3′ |
| Antisense | 5′-TCC CCG ACT GGG TCT TGA C-3′ | |
| Vascular cell adhesion molecule 1 (VCAM-1) | Sense | 5′-GGA GAC CTG TCA CTG TCA ACT G-3′ |
| Antisense | 5′-TCC ATT TCA CCA CTG TGT AAC C-3′ | |
| C-C motif chemokine ligand 2 (CCL-2) | Sense | 5′-GCA TTA GCT TCA GAT TTA CGG GT-3′ |
| Antisense | 5′-TTA AAA ACC TGG ATC GGA ACC AA-3′ | |
| Lymphotoxcin alpha (LTα) | Sense | 5′-AAC CTG CTG CTC ACC TTG TT-3′ |
| Antisense | 5′-CAG TGC AAA GGC TCC AAA GA-3′ | |
| Lymphotoxcin beta (LTβ) | Sense | 5′-TCG GGT TGA GAA GAT CAT TGG-3′ |
| Antisense | 5′-GCT CGT GTA CCA TAA CGA CC-3′ |
2.8. Western blotting
Animals were fasted overnight and i.p. injected with 10 U/kg insulin. After 20 min, mice were euthanized with overdose of isoflurane. Blood was collected from heart and centrifuged at 3000 rpm for 5 min. Plasma was immediately stored in dry ice and then −80 °C. All tissues were stored at −80 °C until further processing. Tissue lysates were prepared using RIPA buffer (Sigma, St. Louis, MO) supplemented with protease and phosphatase inhibitors (Sigma, St. Louis, MO). Protein samples were then separated by 10% SDS-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene fluoride membranes. Target proteins were detected by primary antibodies as follows: mouse anti-β-actin (Sigma, St. Louis, MO), rabbit anti-phospho-Akt (Thr308) (Cell Signaling, Boston, MA), rabbit anti-AKT (Santa Cruz, CA) and rabbit anti-UCP1 (Boster, CA). Secondary antibodies conjugated with horseradish peroxidase and chemiluminescence reagent (Amersham, Marlborough, MA) were used to visualize the target proteins. Densities of target protein bands were determined based on a previously published work with minor modifications (Gassmann et al., 2009). In brief, volume of the band (average OD of the band times its area, INT × mm2) of the expected molecular weight was collected using the Quantity One 4.4.1 (Bio-Rad, Hercules, CA) after the background correction process. The internal control, β-actin, was used to normalize loading variations.
2.9. Statistics
All data are expressed as means ± SEMs and all the data points were included unless noted otherwise. Statistical tests were performed using one-way or two-way analysis of variance (ANOVA) followed by Bonferroni correction or unpaired student’s t-test using GraphPad Prism (version 5; GraphPad Software, La Jolla, CA, USA). The significance level was set at p < 0.05.
3. Results
3.1. TNF/LT deficiency reduces CAP exposure-induced pulmonary inflammation
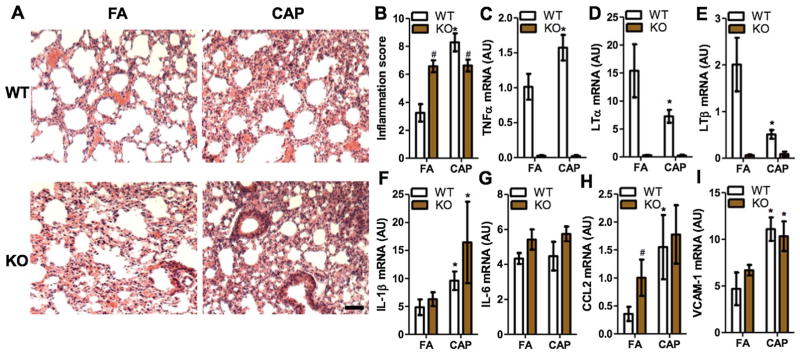
Lung is the primary target organ of PM2.5 exposure, and the resultant pulmonary inflammation is believed to be essential for the development of various adverse cardiometabolic effects. We therefore performed pathological analysis on the lungs of those FA- or CAP-exposed mice. Fig. 1A reveals that CAP exposure markedly induced pulmonary macrophage infiltration and interalveolar thickness (indexes of pulmonary inflammation in Table 2) in WT mice (FA/WT versus CAP/WT). TNF/LT deficiency alone increased interalveolar thickness (FA/WT versus FA/KO, Fig. 1A). However, TNF/LT deficiency markedly reduced CAP exposure-induced macrophage infiltration in the lung (CAP/WT versus CAP/KO, Fig. 1A). To quantitate the effects of TNF/LT deficiency on CAP exposure-induced pulmonary inflammation, we also performed semi-quantitative pathological analysis. Fig. 1B and Table 2 show that compared to WT controls, TNF/LT KO mice had a significantly higher pulmonary inflammation score in FA-exposed mice, but had a markedly lower pulmonary inflammation score in CAP-exposed groups. This reduction in pulmonary inflammation score in CAP-exposed mice appeared to be due to less macrophage infiltration in TNF/LT KO mice (Fig. 1A and Table 2). Pro-inflammatory cytokine expression assessments by RT-PCR demonstrated that CAP exposure significantly increased TNFα but decreased LTα and LTβ (Fig. 1C–E) in WT mice. Except for CCL2 in FA-exposed knockout mice, the expression levels of other tested pro-inflammatory genes were comparable between WT and KO mice (Fig. 1F–I).
Fig. 1.
TNF/LT deficiency reduces CAP exposure-induced pulmonary macrophage infiltration. A and B, Lungs from FA- and CAP-exposed mice were fixed and sectioned, and then subjected to H & E staining. The representative images (A) and the inflammatory score (a sum of all the inflammation indexes in Table 2) (B) are presented. Scale bar, 300 μm. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA. C–I, The mRNA expression of indicated pro-inflammatory genes in the lung were determined by real-time RT-PCR. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA.
3.2. TNF/LT deficiency aggravates CAP exposure-induced glucose intolerance
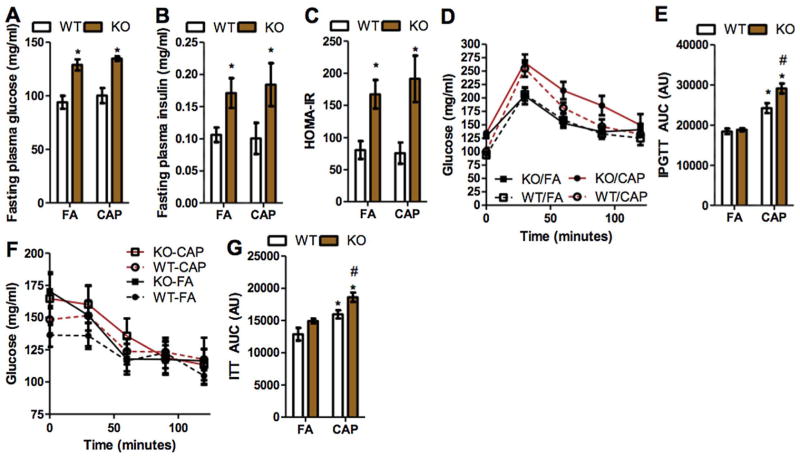
As increasing evidence demonstrates that exposure to ambient PM2.5 is associated with abnormalities in glucose metabolism, we examined the effects of TNF/LT deficiency on CAP exposure-induced glucose intolerance. Fig. 2A–C shows that TNF/LT deficiency significantly increased fasting plasma glucose, fasting plasma insulin, and HOMA-IR in both FA- and CAP-exposed animals. CAP exposure did not significantly impact these parameters in both WT and KO mice. To further document the effects of TNF/LT deficiency on CAP exposure-induced abnormalities in glucose metabolism, we performed IPGTT and ITT. Consistent with previous studies (Liu et al., 2014b), CAP exposure significantly induced glucose intolerance in WT mice (Fig. 2D and E). Notably, in contrast to its effects on pulmonary inflammation, TNF/LT deficiency did not impact glucose tolerance in FA-exposed animals, but significantly aggravated CAP exposure-induced glucose intolerance (Fig. 2D and E). Consistent with IPGTT results, Fig. 2F and G shows that CAP exposure significantly induced insulin resistance in WT mice, and TNF/LT deficiency aggravated CAP exposure-induced insulin resistance.
Fig. 2.
TNF/LT deficiency aggravates CAP exposure-induced glucose intolerance. A–C, mice were starved for 16 h and the glucose (A) and insulin (B) levels in plasma were assessed, and HOMA-IR (C) was calculated using these data. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA. D and E, mice were starved for 16 h, and subjected to i.p. injection with glucose (2 g/kg body weight). The response of plasma glucose (D) and area under the curve (E) are presented. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA. F and G, mice were starved for 4 h, and subjected to i.p. injection with insulin (0.5 unit/kg body weight). The response of plasma glucose (F) and area under the curve (G) are presented. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA.
3.3. CAP exposure increases adiposity in TNF/LT KO but not control mice
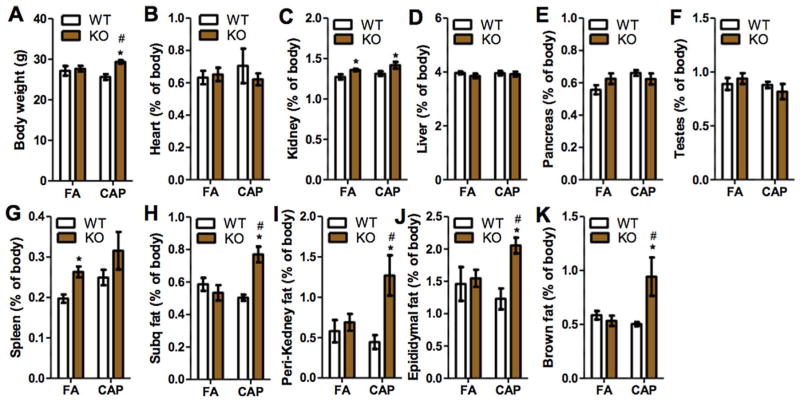
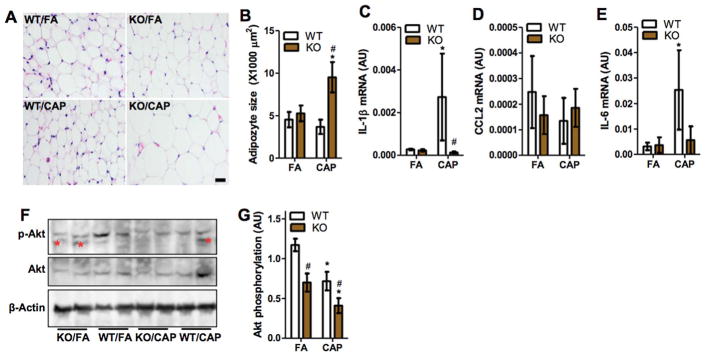
Obesity is one of the major risk factors for diabetes (Eckel et al., 2011), and body weight is a reflection of adiposity. Fig. 3A demonstrates that while CAP exposure did not significantly alter body weight in WT mice, it caused a significant weight gain in TNF/LT KO mice. To further document the effects of CAP exposure on energy metabolism, the main organs/tissues of mice were harvested and weighed. Fig. 3B–K shows that CAP exposure did not significantly change the weight of any organ/tissue in WT mice. TNF/LT deficiency alone (FA/WT versus FA/KO) significantly increased the weight of spleen (Fig. 3G) and kidney (Fig. 3C). CAP exposure did not have any significant effect on the weight of these organs in KO mice (FA/KO versus CAP/KO). In contrast, TNF/LT deficiency alone did not impact the weight of subcutaneous, peri-renal, epididymal, and brown adipose tissues, but CAP exposure significantly increased their weights in KO mice, strongly suggesting that TNF/LT locus may determine mouse metabolic response to CAP exposure. Morphological analysis revealed that CAP exposure markedly increased the adipocyte size of epididymal adipose tissues in TNF/LT KO but not WT mice (Fig. 4A and B), suggesting that the increased adiposity in CAP-exposed KO mice is subsequent to an adipose hypertrophy.
Fig. 3.
CAP exposure increases adiposity in TNF/LT KO but not control mice. Animals were euthanized and the indicated organs/tissues were carefully collected and weighed. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA.
Fig. 4.
TNF/LT deficiency decreases CAP exposure-induced adipose inflammation but aggravates glucose intolerance and insulin resistance. A and B, epididymal adipose tissues were sectioned and subjected to H & E staining. The representative images (A) and the quantitation of adipocyte size (B) are presented. Scale bar, 25 μm. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA. C–E, the mRNA expression of indicated pro-inflammatory genes in the epididymal adipose tissues were determined by real-time RT-PCR. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA. F and G, Akt phosphorylation levels in epididymal adipose tissues were determined by western blot, and the representative images (F) (the non-specific bands were marked out by *) and the quantitation of phosphorylation level (the Akt phosphorylation level was given by the p-Akt data normalized by Akt) (G) are presented. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA.
3.4. TNF/LT deficiency reduces CAP exposure-induced adipose inflammation but exacerbates its glucose intolerance and insulin resistance
CAP exposure has been shown to increase adipose inflammation, which may play a role in CAP exposure-induced insulin resistance (Xu et al., 2010). We therefore assessed the expression of pro-inflammatory cytokines in epididymal adipose tissues. Consistent with previous studies (Xu et al., 2011a), CAP exposure significantly increased adipose IL-1β and IL-6 mRNA expressions (Fig. 4C and E), and markedly reduced Akt phosphorylation levels (Fig. 4F and G) in WT mice. As expected, TNF/LT deficiency abolished the effect of CAP exposure on the expression of pro-inflammatory cytokines (Fig. 4C and E). However, compared to WT controls, it unexpectedly reduced adipose Akt phosphorylation level in both FA- and CAP-exposed animals (Fig. 4F and G). The Akt pathway is a well-known signal transduction pathway involved in the progression of inflammation response and the Akt phosphorylation level indicates the activation of Akt (Tang et al., 2016). These results suggested that TNF/LT may regulate adipose insulin sensitivity through inflammation-independent mechanisms.
3.5. CAP exposure reduces food intake in WT but not TNF/LT KO mice
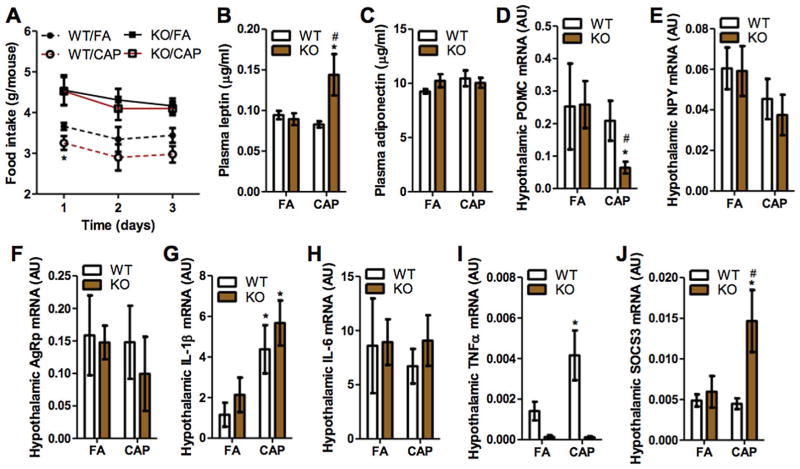
Energy balance is determined by energy intake and expenditure. To address why CAP exposure causes a positive energy balance in TNF/LT KO but not WT mice, we assessed mouse food intake. Fig. 5A reveals that CAP exposure significantly reduced food intake in WT mice and TNF/LT deficiency alone increased food intake in FA-exposed animals. In addition, comparisons of CAP-exposure effect on WT and TNF/LT KO mice indicated that TNF/LT deficiency also abolished the hypophagic effect of CAP exposure. The latter at least partly explained the difference between WT and TNF/LT KO mice in energy metabolic responses to CAP exposure. Leptin is one critical hormone regulating food intake, and its defective production (i.e., introduced by the leptin gene or leptin receptor gene defectivity) is associated with obesity (Chung et al., 1998). Fig. 5B shows that CAP exposure significantly increases plasma leptin level in TNF/LT KO but not WT mice, ruling out the possibility that defective leptin production mediates CAP exposure-induced obesity in TNF/LT KO mice. Leptin regulates food intake primarily through up-regulation of POMC and/or down-regulation of AgRp and NPY in the hypothalamus. In addition to defective leptin production, a lack of response of neuropeptides to leptin, known as central leptin resistance, also correlates to obesity. We therefore assessed the expression of those hypothalamic neuropeptides. Fig. 5D reveals that CAP exposure did not impact the expression of these neuropeptides in WT controls, but significantly decreased hypothalamic POMC expression in TNF/LT KO mice, suggesting a pronounced central leptin resistance in CAP-exposed KO mice that may account for their different food intake response to CAP exposure. SOCS3 is part of a negative feedback loop of leptin signaling pathway and plays a critical role in central leptin resistance (Lubis et al., 2008). Fig. 5J shows that CAP exposure did not impact the expression of SOCS3 in WT controls, but significantly increased its expression in KO mice, further supporting the central leptin resistance in CAP-exposed TNF/LT KO mice. CAP exposure has been shown to cause hypothalamic inflammation that may play a role in the pathogenesis of central leptin resistance (Ying et al., 2014). Fig. 5G shows that CAP exposure significantly increased hypothalamic IL-1β mRNA expression in both WT and KO mice, suggesting that TNF/LT are not involved in CAP exposure-induced hypothalamic inflammation and also that the central leptin resistance in CAP-exposed KO mice may not be subsequent to hypothalamic inflammation.
Fig. 5.
CAP exposure lowers food intake in wildtype but not TNF/LT knockout mice. A, mouse food intake (data was collected within the last three consecutive days of FA/CAP exposure period). *p < 0.05 versus FA, two way ANOVA. B and C, leptin (B) and adiponectin (C) in the fasting plasma were assessed using ELISA kits. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA. D–J, the mRNA expression of indicated genes in the mouse hypothalamus were determined by real-time RT-PCR. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA.
3.6. TNF/LT deficiency exacerbates CAP exposure-induced whitening of brown adipose tissue (BAT)
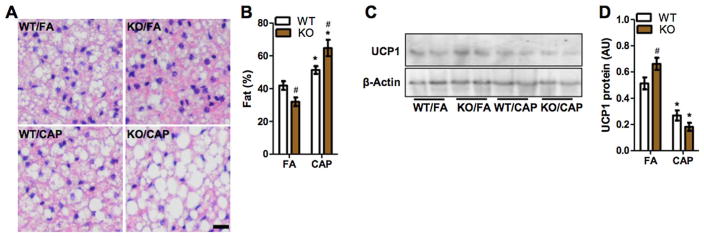
BAT is the key thermogenic tissue that regulates energy expenditure, and its whitening, characterized by the accumulation of large lipid droplets and mitochondrial dysfunction, is a reflective of decreased energy expenditure. Fig. 6A and B reveals that CAP exposure markedly induced accumulation of large lipid droplets in BAT in WT mice, strongly suggesting that CAP exposure may decrease their energy expenditure. Along with the above hypophagic effect, these counteracting energy metabolic effects of CAP exposure explained its failure to change energy balance in WT mice. Compared to FA-exposed WT mice, FA-exposed TNF/LT KO mice had slightly but significantly decreased lipid accumulation in BAT (Fig. 6A and B). This is in line with their increased food intake but normal body weight. Notably, TNF/LT deficiency markedly aggravated CAP exposure-induced accumulation of lipid droplets in BAT. Together with the lack of change in food intake, this provided an explanation for the positive energy balance of CAP-exposed KO mice. UCP1 is a mitochondrial protein that correlates to the heat-generating capacity of BAT. Fig. 6C and D shows that consistent with the results of morphological analysis, TNF/LT deficiency slightly increased UCP1 expression in FA-exposed animals, and compared to its effect in WT mice, CAP exposure induced much more reduction in UCP1 protein expression in KO mice.
Fig. 6.
TNF/LT deficiency enhances CAP exposure-induced BAT whitening. A and B, morphological analysis of mouse BAT. The representative images (A) and the quantitation of fat droplet area (B) are presented. Scale bar, 20 μm. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA. C and D, UCP1 protein levels in BAT were determined by western blot, and the representative images (C) and the quantitation of protein levels (D) are presented. *p < 0.05 versus FA and #p < 0.05 versus WT, two way ANOVA.
4. Discussion
Exposure to ambient PM2.5 induces pronounced pulmonary and extra-pulmonary inflammation, which is widely believed to play a critical role in the development of various adverse cardiometabolic effects due to exposure to PM2.5. TNFα, LTα, and LTβ are three homologous pro-inflammatory cytokines within TNF/LT locus that have both distinctive and overlapping biological functions. In the present study, to delineate their collective role in PM2.5 exposure-induced inflammation and metabolic abnormalities, we systemically investigated the effects of TNF/LT locus inactivation on the inflammatory and metabolic responses to CAP exposure by exposing WT and TNF/LT KO mice in FA/CAP environment for 5 months. When comparing WT and TNF/LT KO mice under FA exposure, a higher pulmonary inflammation score due to more infiltration of macrophages were observed in TNF/LT KO mice, which is consistent with the well-known pro-inflammation mediating effect of TNFα, LTα and LTβ. In addition, an increase food intake in line with a significant decrease of lipid accumulation in BAT in TNF/LT KO mice indicate that TNF/LT deficiency alone may contribute to alternations in energy metabolism.
Consistent with their long-known pro-inflammatory action (Etemadi et al., 2013; Remouchamps et al., 2011), the present data show that TNF/LT deficiency reduces CAP exposure-induced inflammation, as evidenced by the lower pulmonary inflammation scores and the reduction in the expression of adipose inflammatory markers in CAP-exposed TNF/LT KO mice. Notably, the decrease in the pulmonary inflammation score is primarily due to a decrease in macrophage infiltration in the lung. Furthermore, pro-inflammatory gene expression assessments in the lung showed that TNF/LT deficiency did not reduce the pulmonary expression of other pro-inflammatory genes, including IL-1β, IL-6, CCL2, and VCAM-1. These data collectively suggest that TNF/LT may play a limited role in the development of CAP exposure-induced pulmonary inflammation. This specific effect of TNF/LT deficiency on reducing CAP exposure-induced macrophage infiltration is consistent with previous findings on a critical role of TNF/LT stimulating macrophage activation (Goetz et al., 2004). These data also strongly suggest that CAP exposure may induce pulmonary inflammation through multiple independent pathways. This complex inflammation effect caused by PM2.5 may derive from their various chemical and morphological characterizations, multiple entrance routines as well as complicated PM-cell or PM-tissue interactions. Thus serious consideration must been taken when developing prevention/treatment for PM2.5 pollution-induced diseases in the future.
Increasing evidence has indicated that exposure to air pollutants such as PM2.5 is a risk factor for type II diabetes, and inflammation may be central in the development of PM2.5 exposure-associated diabetes (Brook et al., 2010). The present study replicates CAP exposure-induced inflammation and abnormalities in glucose homeostasis in WT mice. Interestingly, we also observed that despite that TNF/LT deficiency reduced CAP exposure-induced pulmonary and adipose inflammation, it however aggravated CAP exposure-induced insulin resistance and glucose intolerance. This disconnection between inflammation and abnormal glucose metabolism raises a question over the putative role of inflammation in the development of PM2.5 exposure-induced cardiometabolic abnormalities such as diabetes. This is consistent with our recent study showing that a 6-week withdrawal from CAP exposure is sufficient to resolve CAP exposure-induced hypertension and cardiac hypertrophy but not pulmonary inflammation (Ying et al., 2015). These results together may indicate potential development mechanisms of PM2.5 exposure-induced cardiometabolic abnormalities other than pulmonary inflammation. However, it should be noted that the present data meanwhile demonstrate that TNF/LT deficiency renders CAP exposure an obesogenic factor. As obesity has been well established to cause insulin resistance and glucose intolerance, the disconnection between inflammation and abnormal glucose metabolism in TNF/LT KO mice may be primarily due to their unique body weight response to CAP exposure.
Obesity is a major risk factor for cardiometabolic diseases (Eckel et al., 2011). Many studies have indicated that obesity may exaggerate the adverse health effects of PM2.5 pollution (McCormack et al., 2015). But how PM2.5 exposure influences the development of obesity has not yet been fully understood. In the present study, we demonstrate that CAP exposure leads to a significant weight gain in TNF/LT KO mice. This is in direct contrast to the non-significant weight loss in WT controls, which is consistent with previous studies (Xu et al., 2010). Our data further demonstrate that the increased body weight is expressed as an increased adiposity. These data are consistent with previous genetic linkage analysis showing significant and suggestive quantitative trait loci (QTLs) on TNF/LT locus (Ohtsuka et al., 2000). Together, the present study provides evidence that PM2.5 exposure promotes the development of obesity in a genetic context-dependent manner. To our knowledge, this is the first study showing a genetic determinant of energy metabolic response to ambient PM2.5 exposure. The present study thus also reaffirm the importance of gene-environment interactions in examination of the relationship between ambient exposures to PM2.5 and the development of adverse health effects.
Another important finding in the present study is the insight into CAP exposure-induced alterations in energy metabolism. Energy balance is determined by both energy intake and expenditure. The present data reveal that CAP exposure significantly decreases food intake and markedly induces BAT whitening in WT mice. These opposite effects of CAP exposure on energy balance suggest that CAP exposure may influence energy balance through multiple independent pathways and hence provide a basis for its modification by genetic contexts. Notably, the effect of CAP exposure on food intake may be mouse strain-dependent, as we did not observe this effect in KKAy mice (Liu et al., 2014a). In contrast, its effect on BAT whitening may be independent on mouse strain, as evidenced by the observations showing the same effects of exposure to CAP in ApoE−/− and KKAy mice (Liu et al., 2014a; Xu et al., 2011b).
In the present study, TNF/LT deficiency has been shown to abolish CAP exposure-induced hypophagia and exacerbate CAP exposure-associated BAT whitening. Both effects lead to a positive energy balance and thus can account for the unexpected weight gain of TNF/LT KO mice in response to CAP exposure. Consistent with the compelling evidence that TNFα signaling induces hypophagia (Romanatto et al., 2007, 2009), the present data support that CAP exposure-induced hypophagia may be mediated by TNFα. In contrast, the role of TNFα in BAT activity regulation remains controversial (Cawthorn and Sethi, 2008). It has been shown that TNFα treatment increases BAT whitening and knockout of its receptor decrease BAT whitening (Arruda et al., 2011; Romanatto et al., 2009). This is consistent with our results in FA-exposed animals. However, the present data also reveal that TNF/LT deficiency exacerbates CAP exposure-induced BAT whitening, together with a more significant decrease of UCP1 expression demonstrating a subdued heat-generating capacity of BAT compared to WT mice. These results suggested that either TNFα has an opposite activity in the presence of CAP exposure or LTs mediate the BAT effects of CAP exposure. Further studies are needed to test these possibilities.
Hypothalamus is the control center for energy balance, and hypothalamic inflammation has been shown to play an important role in regulation of energy metabolism. The present study replicates CAP exposure-induced hypothalamic inflammation in C57Bl/6J mice (Ying et al., 2014), and additionally demonstrates that TNF/LT deficiency does not impact this CAP exposure-induced hypothalamic inflammation. Hence, these data suggest that TNF/LT may not be implicated in CAP exposure-induced hypothalamic inflammation. Moreover, as TNF/LT deficiency reduces CAP exposure-induced pulmonary and adipose inflammations but exacerbates abnormalities in glucose homeostasis, the demonstration of TNF/LT deficiency having no effect on hypothalamic inflammation undermines its role in the mediation of PM2.5 exposure-induced abnormalities in glucose homeostasis.
5. Conclusion
The present study demonstrates an important and complicated role of TNF/LT in the pathophysiology caused by exposure to ambient PM2.5. Specifically, our results demonstrated that TNF/LT deficiency has: 1) significantly reduced CAP exposure-induced pulmonary inflammation and adipose inflammatory markers expression; 2) aggravated CAP exposure-induced glucose intolerance and insulin resistance; 3) rendered CAP exposure-induced weigh gain; 4) abolished CAP exposure-induced food intake decrease; 5) aggravated CAP exposure-induced BAT whitening. These present data not only extend our understanding of the genetics underlying the toxicity of exposure to ambient PM2.5 but also offer knowledge that may merit particular consideration when developing prevention/treatment for PM2.5 pollution in the future.
Acknowledgments
Funding
This work was supported by the National Institutes of Health (R01ES024516 to ZY), the American Heart Association (13SDG17070131 to ZY), Shanghai Pujiang Program (No. 17PJ1401300 to YX) and the National Natural Science Foundation of China (Grant No. 81270342 to ZY, 21407082 to XW, 81302452 to LQ, and 81500216 to MC).
Abbreviations
- TNF
tumor necrosis factor
- LT
lymphotoxin
- KO
knockout
- PM2.5
ambient fine particles
- CAP
concentrated ambient PM2.5
- BALF
bronchoalveolar lavage fluid
- UCP1
uncoupling protein 1
- MHC
the major histocompatibility complex
- IKK2
inhibitor κB kinase 2
- FA
filtered air
- IPGTT
intraperitoneal glucose tolerance test
- ITT
intraperitoneal insulin tolerance test
- HOMA-IR
homeostasis model assessment-insulin resistance
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- NPY
neuropeptide Y
- POMC
pro-opiomelanocortin
- AgRP
agouti-related peptide
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- SOCS3
suppressor of cytokine signaling 3
- VCAM-1
vascular cell adhesion molecule 1
- CCL-2
C-C motif chemokine ligand 2
Footnotes
Conflict of interest
The authors declare that their is no conflict of interest.
References
- Arruda AP, Milanski M, Coope A, Torsoni AS, Ropelle E, Carvalho DP, Carvalheira JB, Velloso LA. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology. 2011;152:1314–1326. doi: 10.1210/en.2010-0659. [DOI] [PubMed] [Google Scholar]
- Bauer AK, Travis EL, Malhotra SS, Rondini EA, Walker C, Cho HY, Trivedi S, Gladwell W, Reddy S, Kleeberger SR. Identification of novel susceptibility genes in ozone-induced inflammation in mice. Eur Respir J. 2010;36:428–437. doi: 10.1183/09031936.00145309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett. 2008;582:117–131. doi: 10.1016/j.febslet.2007.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Farese RV., Jr Determination of adipocyte size by computer image analysis. J Lipid Res. 2002;43:986–989. [PubMed] [Google Scholar]
- Chung WK, Belfi K, Chua M, Wiley J, Mackintosh R, Nicolson M, Boozer CN, Leibel RL. Heterozygosity for Lep(ob) or Lep(rdb) affects body composition and leptin homeostasis in adult mice. Am J Physiol. 1998;274:R985–990. doi: 10.1152/ajpregu.1998.274.4.R985. [DOI] [PubMed] [Google Scholar]
- Curtis JL, Byrd PK, Warnock ML, Kaltreider HB. Requirement of CD4-positive T cells for cellular recruitment to the lungs of mice in response to a particulate intratracheal antigen. J Clin Invest. 1991;88:1244–1254. doi: 10.1172/JCI115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel RH, Kahn SE, Ferrannini E, Goldfine AB, Nathan DM, Schwartz MW, Smith RJ, Smith SR. Obesity and type 2 diabetes: what can be unified and what needs to be individualized? Diab Care. 2011;34:1424–1430. doi: 10.2337/dc11-0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs. 2015;24:283–307. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- Etemadi N, Holien JK, Chau D, Dewson G, Murphy JM, Alexander WS, Parker MW, Silke J, Nachbur U. Lymphotoxin alpha induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J. 2013;280:5283–5297. doi: 10.1111/febs.12419. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Grenacher B, Rohde B, Vogel J. Quantifying Western blots: pitfalls of densitometry. Electrophoresis. 2009;30:1845–1855. doi: 10.1002/elps.200800720. [DOI] [PubMed] [Google Scholar]
- Goetz FW, Planas JV, MacKenzie S. Tumor necrosis factors. Dev Comp Immunol. 2004;28:487–497. doi: 10.1016/j.dci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Kennedy T, Ghio AJ, Reed W, Samet J, Zagorski J, Quay J, Carter J, Dailey L, Hoidal JR, Devlin RB. Copper-dependent inflammation and nuclear factor-kappa B activation by particulate air pollution. Am J Respir Cell Mol Biol. 1998;19:366–378. doi: 10.1165/ajrcmb.19.3.3042. [DOI] [PubMed] [Google Scholar]
- Kuprash DV, Alimzhanov MB, Tumanov AV, Grivennikov SI, Shakhov AN, Drutskaya LN, Marino MW, Turetskaya RL, Anderson AO, Rajewsky K, et al. Redundancy in tumor necrosis factor (TNF) and lymphotoxin (LT) signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single-knockout mice. Mol Cell Biol. 2002;22:8626–8634. doi: 10.1128/MCB.22.24.8626-8634.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuprash DV, Tumanov AV, Liepinsh DJ, Koroleva EP, Drutskaya MS, Kruglov AA, Shakhov AN, Southon E, Murphy WJ, Tessarollo L, et al. Novel tumor necrosis factor-knockout mice that lack Peyer’s patches. Eur J Immunol. 2005;35:1592–1600. doi: 10.1002/eji.200526119. [DOI] [PubMed] [Google Scholar]
- Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120:965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Fonken LK, Wang A, Maiseyeu A, Bai Y, Wang TY, Maurya S, Ko YA, Periasamy M, Dvonch T, et al. Central IKKbeta inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol. 2014a;11:53. doi: 10.1186/s12989-014-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Xu X, Bai Y, Wang TY, Rao X, Wang A, Sun L, Ying Z, Gushchina L, Maiseyeu A, et al. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect. 2014b;122:17–26. doi: 10.1289/ehp.1306841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubis AR, Widia F, Soegondo S, Setiawati A. The role of SOCS-3 protein in leptin resistance and obesity. Acta Med Indones. 2008;40:89–95. [PubMed] [Google Scholar]
- McCormack MC, Belli AJ, Kaji DA, Matsui EC, Brigham EP, Peng RD, Sellers C, Williams DL, Diette GB, Breysse PN, et al. Obesity as a susceptibility factor to indoor particulate matter health effects in COPD. Eur Respir J. 2015;45:1248–1257. doi: 10.1183/09031936.00081414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y, Brunson KJ, Jedlicka AE, Mitzner W, Clarke RW, Zhang LY, Eleff SM, Kleeberger SR. Genetic linkage analysis of susceptibility to particle exposure in mice. Am J Respir Cell Mol Biol. 2000;22:574–581. doi: 10.1165/ajrcmb.22.5.3895. [DOI] [PubMed] [Google Scholar]
- Quay JL, Reed W, Samet J, Devlin RB. Air pollution particles induce IL-6 gene expression in human airway epithelial cells via NF-kappa B activation. Am J Respir Cell Mol Biol. 1998;19:98–106. doi: 10.1165/ajrcmb.19.1.3132. [DOI] [PubMed] [Google Scholar]
- Remouchamps C, Boutaffala L, Ganeff C, Dejardin E. Biology and signal transduction pathways of the lymphotoxin-alphabeta/LTbetaR system. Cytokine Growth Factor Rev. 2011;22:301–310. doi: 10.1016/j.cytogfr.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Roach DR, Briscoe H, Saunders B, France MP, Riminton S, Britton WJ. Secreted lymphotoxin-alpha is essential for the control of an intracellular bacterial infection. J Exp Med. 2001;193:239–246. doi: 10.1084/jem.193.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanatto T, Cesquini M, Amaral ME, Roman EA, Moraes JC, Torsoni MA, Cruz-Neto AP, Velloso LA. TNF-alpha acts in the hypothalamus inhibiting food intake and increasing the respiratory quotient-effects on leptin and insulin signaling pathways. Peptides. 2007;28:1050–1058. doi: 10.1016/j.peptides.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Romanatto T, Roman EA, Arruda AP, Denis RG, Solon C, Milanski M, Moraes JC, Bonfleur ML, Degasperi GR, Picardi PK, et al. Deletion of tumor necrosis factor-alpha receptor 1 (TNFR1) protects against diet-induced obesity by means of increased thermogenesis. J Biol Chem. 2009;284:36213–36222. doi: 10.1074/jbc.M109.030874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tang B, Tang F, Wang Z, Qi G, Liang X, Li B, Yuan S, Liu J, Yu S, He S. Upregulation of Akt/NF-kappaB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: suppression by carnosic acid nanoparticle. Int J Nanomed. 2016;11:6401–6420. doi: 10.2147/IJN.S101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Vogel CF, Sciullo E, Wong P, Kuzmicky P, Kado N, Matsumura F. Induction of proinflammatory cytokines and C-reactive protein in human macrophage cell line U937 exposed to air pollution particulates. Environ Health Perspect. 2005;113:1536–1541. doi: 10.1289/ehp.8094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M, Chen LC, et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30:2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011a;124:88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, Bramble LA, Yang Y, Wang A, Harkema JR, et al. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol. 2011b;8:20. doi: 10.1186/1743-8977-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Kampfrath T, Thurston G, Farrar B, Lippmann M, Wang A, Sun Q, Chen LC, Rajagopalan S. Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol Sci. 2009;111:80–88. doi: 10.1093/toxsci/kfp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q, et al. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect. 2014;122:79–86. doi: 10.1289/ehp.1307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Z, Xie X, Bai Y, Chen M, Wang X, Zhang X, Morishita M, Sun Q, Rajagopalan S. Exposure to concentrated ambient particulate matter induces reversible increase of heart weight in spontaneously hypertensive rats. Part Fibre Toxicol. 2015;12:15. doi: 10.1186/s12989-015-0092-6. [DOI] [PMC free article] [PubMed] [Google Scholar]