Abstract
The instructive capabilities of extracellular matrix components in progenitor cell differentiation have recently generated significant interest in the development of bioinspired materials for regenerative applications. Previously, a correlation was described between the osteogenic capabilities of nanoparticulate mineralized collagen glycosaminoglycan scaffolds (MC-GAG) and an autogenous activation of small mothers against decapentaplegic ( Smad1/5) in the canonical bone morphogenetic protein receptor (BMPR) pathway with a diminished extracellular signal regulated kinase 1/2 (ERK1/2) activation when compared to nonmineralized collagen glycosaminoglycan scaffolds (Col-GAG). This work utilizes a canonical BMPR inhibitor (dorsomorphin homologue 1, DMH1) and an inhibitor of the mitogen activated protein kinase/ERK kinase (MEK)/(ERK) cascade (PD98059) to characterize the necessity of each pathway for osteogenesis. While DMH1 inhibits runt-related transcription factor 2 (Runx2) and bone sialoprotein II (BSPII) gene expression of primary human mesenchymal stem cells (hMSCs) on MC-GAG, PD98059 inhibits BSPII expression on Col-GAG independent of Runx2 expression. DMH1 inhibits mineralization on both Col-GAG and MC-GAG, however, PD98059 only inhibits mineralization on Col-GAG. DMH1 inhibits both Smad1/5 phosphorylation and Runx2 protein expression, whereas PD98059 inhibits ERK1/2 and c-Jun amino-terminal kinase 1/2 (JNK1/2) phosphorylation without affecting Runx2. Thus, activation of the canonical BMPR signaling is necessary for osteogenic differentiation and mineralization of hMSCs on Col-GAG or MC-GAG. The MEK/ERK cascade, intimately tied to JNK activation, is necessary for Runx2-independent osteogenesis on Col-GAG, while completely dispensable in osteogenesis on MC-GAG.
Keywords: bone regeneration, nanoparticulate mineralized collagen glycosaminoglycan scaffold
1. Introduction
The current paradigm for bone regenerative strategies integrates three elements: biomaterials, osteogenic cells, and growth factors. However, this paradigm has a few significant clinical limitations. First, the procurement of progenitor cells usually requires an additional surgical procedure which may incur a certain amount of morbidity. Second, the requirement for ex vivo progenitor cell expansion is both time-consuming and costly. Third, in order to proliferate and differentiate progenitor cells, exogenous growth factors are frequently utilized either in the ex vivo expansion stage or simultaneously with implantation. Such deficiencies reduce the practicality of this strategy in clinical translation due to the increased morbidity, excessive amount of time for cultures, cost, and the unpredictable side effects of growth factor stimulation. With the understanding that the extracellular matrix (ECM) can serve an instructive role on endogenous progenitor cell differentiation, the impetus to generate modular ECM-inspired materials has become increasingly attractive.[1] Characterization of the mechanistic effects of ECM-based materials on cell biology is, thus, essential for understanding and refinement of materials in preparation for translation.
Previously, we described efficient osteogenic differentiation and matrix mineralization of human mesenchymal stem cells (hMSCs) and rabbit bone marrow stromal cells (rBMSCs) on nanoparticulate mineralized collagen glycosaminoglycan scaffolds (MC-GAG) when compared to nonmineralized collagen glycosaminoglycan scaffolds (Col-GAG).[2,3] In a rabbit cranial defect mode, MC-GAG was subsequently demonstrated to effect bone healing which approached 60% of the density and resistance to fracture of the adjacent native calvarium.[3] The disparities of mineralization between MC-GAG and Col-GAG were correlated to differences in the activation of two intracellular signaling molecules, small mothers against decapentaplegic (Smad1/5) and extracellular signal regulated kinase 1/2 (ERK1/2), as well as differential gene expression of bone morphogenetic protein (BMP)-2, -7, -9, and -4. In combination, these data suggested an autogenous, albeit differential, activation of the canonical or noncanonical bone morphogenetic protein receptor (BMPR) signaling pathways by MC-GAG or Col-GAG, respectively.
Smad1/5 are members of an evolutionarily conserved family of intracellular signaling molecules downstream of the transforming growth factor-β receptor superfamily.[4] Smad1/5/8 is specific for BMPR signaling and constitutes the well-characterized canonical signaling pathway. Alternatively, BMPRs can also signal using several noncanonical pathways such as mitogen-activated protein kinases (MAPKs) including the ERK1/2, p38, and c-Jun amino-terminal kinase 1/2 (JNK1/2) as well as phos-phatidyl-inositol-3-kinase (PI3K) and Akt.[5]
While the canonical BMPR signaling pathway is well understood, there are significantly more vagaries of the noncanonical pathways. Unlike the Smads, the MAPK cascades are frequently activated by a variety of stimuli including growth factors and stress.[5] Mechanistically, MAPK signaling is organized in a hierarchical structure consisting of three levels of protein kinases with increasing specificities. For each cascade, a mitogen-activated protein kinase kinase kinase (MAPKKK) (such as Rat sarcoma (Ras) or transforming growth factor-beta activated kinase (TAK-1)) activates a MAPKK (such as mitogen activated protein kinase/ERK kinase (MEK) MEK1) which then activates a MAPK (such as ERK1/2, JNK1/2, or p38). In non-canonical pathways, the exact mechanism for which BMPR binds to and activates upstream MAPKKKs is not completely elucidated. However, BMP-2 stimulation has been found to upregulate both Ras and TAK-1 phosphorylation, the MAP-KKKs for the ERK pathway and JNK pathways, respectively.[6–8] The activation of downstream MAPKs may be cell-type specific. While Gallea et al. detected both p38 and ERK cascade involvement in osteoblast differentiation of C2C12 cells in response to BMP-2,[9] Lai and Cheng found that p38 but not ERK was essential for osteogenic gene expression in MC3T3-E1.[7] BMP-9-mediated osteogenic differentiation was found to require p38 and JNK1/2 phosphorylation but not the MEK/ERK cascade for its osteogenic capabilities in various osteoprogenitor cell lines and primary cell cultures.[10] Using adipose stem cells, Lo et al. demonstrated that BMP-2 induced both Smad1/5 phosphorylation as well as ERK1/2 phosphorylation in a manner that could be augmented with the addition of stromal derived factor-1.[11] In periodontal ligament stem cells, Ren et al. demonstrated that cyclic mechanical tension mimicking occlusal force resulted in increased osteogenic differentiation via ERK1/2-mediated runt-related transcription factor 2 (Runx2) activation.[12]
Crosstalk between the canonical and noncanonical pathways exists at multiple levels in both the positive and negative manners. Abelson murine leukemia viral oncogene c-Abl, a nonreceptor tyrosine kinase, was found to be a major link between the canonical and noncanonical pathways activated by BMPR.[6] Upon tyrosine phosphorylation of BMPR1A by c-Abl, activation of the Smad pathway occurred while ERK1/2 activation was negatively regulated. ERK1/2-mediated phosphorylation has been reported to target Smads for ubiquitination by Smad ubiquitin regulatory factors (Smurf1) and proteasomal degradation.[13] Despite the apparent inverse relationship between BMPR-mediated Smad and ERK signaling, both have been reported to contribute to osteogenic differentiation.
In this work, we investigate the mechanisms contributing to differential osteogenic differentiation of human mesenchymal stem cells on Col-GAG and MC-GAG scaffolds utilizing two small molecular inhibitors, dorsomorphin homologue 1 (DMH1) and PD98059, specific for inhibition of the canonical BMP receptor signaling pathway and the MEK1/ERK1/2 MAPK signaling pathway, respectively.[14]
2. Results
2.1. Expression of Osteogenic Genes of hMSCs on Col-GAG and MC-GAG Scaffolds Treated with DMH1 and PD98050
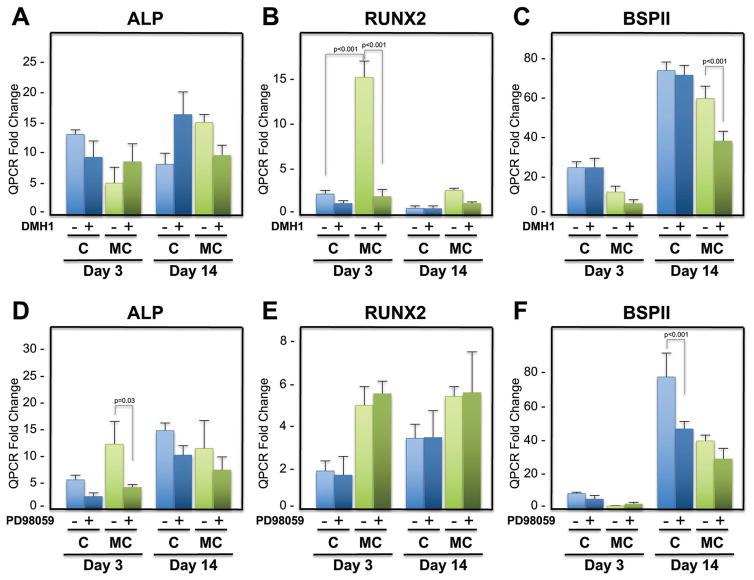
Our previous work demonstrated that hMSCs and rBMSCs undergo osteogenic differentiation on MC-GAG scaffolds in a manner that correlated to a significant upregulation of Smad1/5 phosphorylation and delayed ERK1/2 phosphorylation.[2,3] In addition, this activation was also coincident with activation of BMP-2, -9, and -4 gene expressions in a temporally dependent manner, suggestive that the BMP receptor signaling pathway was activated in an autogenous manner. To evaluate the necessity of the canonical BMP receptor signaling pathway in osteogenic differentiation on Col-GAG and MC-GAG scaffolds, bone marrow-derived hMSCs (CD105+CD166+CD29+CD44+CD14−CD34−CD45−) were cultured in osteogenic differentiation medium and treated with and without DMH1, a selective small molecule inhibitor for type I BMP receptors specific for the canonical pathway.[14,15] In the absence or presence of DMH1, no statistically significant differences in alkaline phosphatase (ALP) expression were noted for either Col-GAG or MC-GAG on day 3 or 14 (Figure 1A). Unlike ALP, Runx2 was significantly elevated in MC-GAG scaffolds in comparison to Col-GAG scaffolds (Figure 1B). In the presence of DMH1, Runx2 expression was significantly inhibited (p < 0.001) in MC-GAG scaffolds, whereas no significant differences occurred in the Col-GAG scaffolds. As a late osteogenic marker, bone sialoprotein II (BSPII) was evaluated (Figure 1C). While no statistically significant differences were noted between Col-GAG and MC-GAG scaffolds, treatment of MC-GAG scaffolds with DMH1 resulted in a statistically significant decrease in BSPII expression while DMH1 had no effect on Col-GAG scaffolds.
Figure 1.
Relative mRNA expression of alkaline phosphatase (ALP), runt-related transcription factor 2 (Runx2), and bone sialoprotein II (BSPII) in hMSCs in response to DMH1 and PD98059 on Col-GAG and MC-GAG scaffolds. Quantitative RT-PCR analysis of A) ALP, B) Runx2, and C) BSPII expression of hMSCs with and without DMH1 (50 μm) in Col-GAG and MC-GAG scaffolds at days 3 and 14 of culture. D) ALP, E) Runx2, and F) BSPII expression of hMSCs with and without PD98059 (50 μm) in Col-GAG and MC-GAG scaffolds at days 3 and 14. Data are expressed as the mean ± SD of three independent experiments. p values are indicated.
To evaluate the necessity of ERK1/2 phosphorylation on osteogenic gene expression, PD98059, a selective MEK1/2 inhibitor was used to treat hMSCs cultured on Col-GAG and MC-GAG and quantitative reverse-transcriptase polymerase chain reactions (RT-PCR) was performed in the same manner as the DMH1 experiment (Figure 1D–F). Although PD98059 treatment did inhibit ALP expression at day 3 in both Col-GAG and MC-GAG, this effect was no longer present by day 14 (Figure 1D). Runx2 expression was unaffected by PD98059 on either scaffold at either timepoint (Figure 1E). However, unlike DMH1, BSPII expression decreased on Col-GAG scaffolds in the presence of PD98059 (p < 0.001), whereas no effect was seen on MC-GAG scaffolds (Figure 1F). Differences between the expression levels of each gene between the DMH1 and PD98059 results were due to both different experiments as well as hMSC passage numbers. Taken together, osteogenic gene expression on Col-GAG and MC-GAG was differentially inhibited by PD98059 and DMH1, respectively. Interestingly, BSPII inhibition on Col-GAG scaffolds occurred in a manner independent of Runx2 expression.
2.2. Viability of hMSCs on Col-GAG and MC-GAG Scaffolds Treated with DMH1 and PD98059
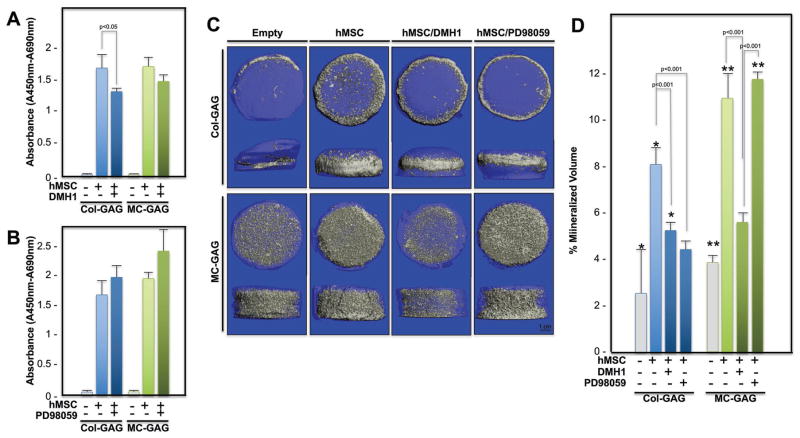
Prior to analysis of long term cultures for evaluation of matrix mineralization, the viability of hMSCs cultured on Col-GAG and MC-GAG scaffolds in the absence and presence of DMH1 or PD98049 was assessed by measuring the mitochondrial dehydrogenase activity using the water soluble tetrazolium-1 (WST-1) tetrazolium salt colorimetric assay (Figure 2A,B). hMSCs were cultured on Col-GAG or MC-GAG for 8 weeks with or without 50 μm DMH1, treated with the WST-1 reagent, and evaluated using spectrophotometry. A statistically significant difference was seen between empty scaffolds and hMSC cultured scaffolds when compared with ANOVA [F(5,12) = 163.83, p < 0.001]. While MC-GAG demonstrated no statistically significant difference in absorbance at 450–690 nm between scaffolds treated with or without DMH1, Col-GAG scaffolds showed a small reduction in viability that was statistically significant (p < 0.05).
Figure 2.
WST-1 assay and microCT scanning of hMSCs cultured on Col-GAG and MC-GAG scaffolds with or without DMH1 and PD98059 at 8 weeks of culture. Absorbance (A450–690 nm) of empty, hMSC-cultured, and hMSC-cultured with A) DMH1 (50 μm) or B) PD98059 (50 μm) Col-GAG and MC-GAG scaffolds incubated with the WST-1 substrate. C) Representative 3D reconstructed microCT scans of hMSCs cultured in osteogenic medium in the presence or absence of DMH1 (50 μm) and PD98059 (50 μm) for 8 weeks on Col-GAG and MC-GAG scaffolds. D) Quantification of mineralized volume on microCT scans. *p < 0.01 between empty Col-GAG and scaffolds with hMSCs; **p < 0.001 between empty MC-GAG and scaffolds with hMSCs.
Comparison of viability in empty Col-GAG or MC-GAG scaffolds and scaffolds cultured with hMSCs treated or untreated with PD98059 also demonstrated a statistically significant difference in absorbance when analyzed with ANOVA [F(5,12) = 95.29, p < 0.001] (Figure 2B). However, both Col-GAG and MC-GAG did not demonstrate any differences in viability in the absence or presence of 50 μm PD98059.
2.3. Matrix Mineralization of hMSCs on Col-GAG and MC-GAG Scaffolds Treated with DMH1 and PD98059
To determine the contributions of the canonical BMP receptor signaling or MEK/ERK pathways to hMSC mineralization on Col-GAG or MC-GAG scaffolds, quantification of mineralized content was evaluated with microcomputed tomography (microCT) scanning and subjected to ANOVA followed by post hoc analyses using the Tukey criterion for statistical analysis (Figure 2C,D). Statistically significant differences were found between the different conditions [F(7,23) = 60.25, p < 0.001]. Following 8 weeks of culture, mineral content was significantly higher in both Col-GAG and MC-GAG scaffolds cultured with hMSCs in comparison to empty scaffolds (p < 0.01 and p < 0.001, respectively). Mineralization was significantly reduced in both Col-GAG and MC-GAG scaffolds in the presence of DMH1 (p < 0.001). Interestingly, while PD98059 did not affect mineralization in MC-GAG scaffolds, mineralization on Col-GAG scaffolds was significantly inhibited (p < 0.001).
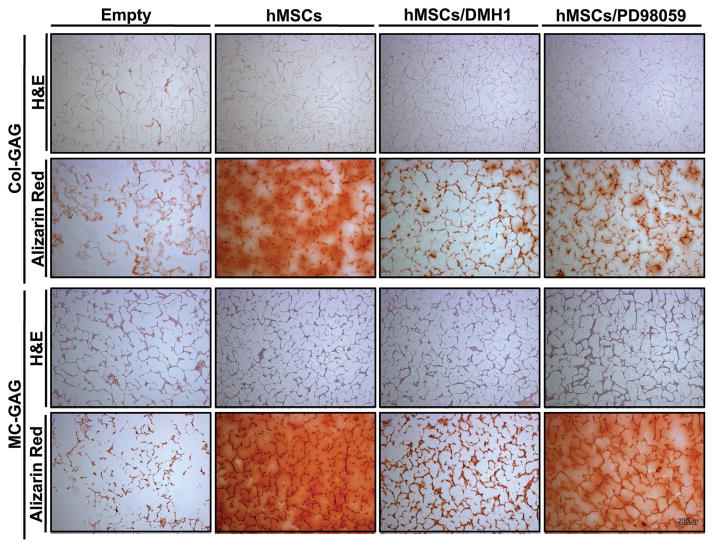
To confirm that both cellular content and mineral content were present for each scaffold and condition, histologic analyses were performed at 8 weeks of culture (Figure 3). Cellular content was noted in both Col-GAG and MC-GAG scaffolds seeded with hMSCs in the absence or presence of either inhibitor. Similar to the microCT data, mineralization was detected in all scaffolds via Alizarin red staining at 8 weeks which was inhibited in both scaffolds with DMH1 treatment. By contrast, PD98059 only inhibited mineralization on the Col-GAG scaffold with minimal to no differences on MC-GAG scaffolds.
Figure 3.
Histologic analysis of Col-GAG and MC-GAG scaffolds cultured with hMSCs in osteogenic medium with or without DMH1 (50 μm) and PD98059 (50 μm) for 8 weeks using H&E or Alizarin Red staining (Magnification, 4×).
2.4. Activation of Intracellular Signaling Molecules on Col-GAG and MC-GAG in the Absence and Presence of DMH1 or PD98059
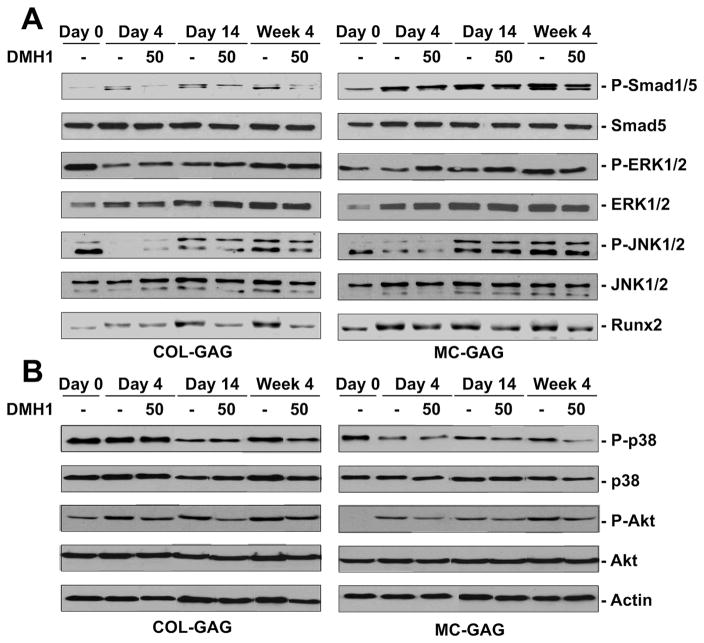
To elucidate the mechanism behind the canonical BMP receptor mediated osteogenic differentiation in both Col-GAG and MC-GAG scaffolds, activation of intracellular signaling molecules was investigated at days 0, 4, 14, and week 4 in the absence or presence of DMH1 (Figure 4). In both Col-GAG and MC-GAG scaffolds, phosphorylated Smad1/5 (p-Smad1/5) was decreased in the presence of DMH1 at all timepoints. In comparison to Col-GAG, MC-GAG demonstrated levels of p-Smad1/5 that were significantly higher, consistent with our previous results.[2,3] Additionally, both Col-GAG and MC-GAG demonstrated decreased Runx2 protein expression in the presence of DMH1. In both scaffolds, a number of other intracellular signaling molecules were unaffected by DMH1 treatment including p-ERK1/2, p-JNK1/2, p-p38, and phosphorylated Akt (p-Akt) suggesting that the MAP kinase and PI3K pathways were not affected by DMH1.
Figure 4.
Western blot of intracellular signaling molecules expressed by hMSCs cultured on Col-GAG and MC-GAG in the absence and presence of DMH1. Western blot of A) phosphorylated Smad1/5 (P-Smad1/5) and total Smad (Smad5), phosphorylated ERK1/2 (P-ERK1/2) and total ERK1/2 (ERK1/2), phosphorylated JNK1/2 (P-JNK1/2) and total JNK1/2 (JNK1/2), Runx2 and B) phosphorylated p38 (p-p38) and total p38, phosphorylated Akt (p-Akt) and total Akt, and actin in hMSCs cultured on Col-GAG and MC-GAG at days 0, 4, 14, and week 4 of culture with or without 50 μm DMH1.
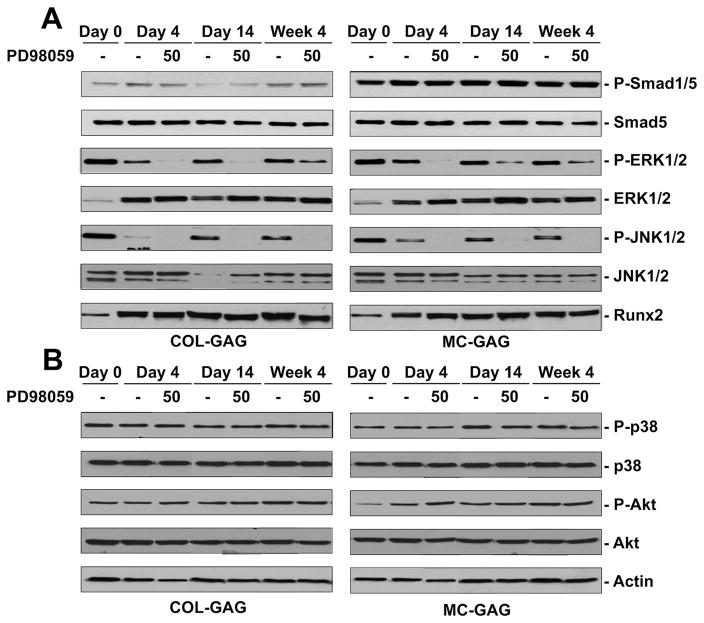
To elucidate the mechanism behind the MAPK-mediated osteogenic differentiation in both Col-GAG and MC-GAG scaffolds, activation of intracellular signaling molecules was investigated at days 0, 4, 14, and week 4 in the absence and presence of PD98059 (Figure 5). In both Col-GAG and MC-GAG scaffolds, p-ERK1/2 was decreased in the presence of PD98059 at all timepoints. Interestingly, p-JNK1/2 was also decreased in the presence of PD98059 at all timepoints. ERK1/2 inhibition with PD98059 did not have an effect on Runx2 expression in either Col-GAG or MC-GAG scaffolds. Additionally, PD98059 had no effect on p-Smad1/5, p-p38, or p-Akt suggesting that the canonical BMP receptor, p38 MAPK, and PI3K signaling pathways were not affected by PD98059.
Figure 5.
Western blot of intracellular signaling molecules expressed by hMSCs cultured on Col-GAG and MC-GAG in the absence and presence of PD98059. Western blot of A) phosphorylated Smad1/5 (P-Smad1/5) and total Smad (Smad5), phosphorylated ERK1/2 (P-ERK1/2) and total ERK1/2 (ERK1/2), phosphorylated JNK1/2 (P-JNK1/2) and total JNK1/2 (JNK1/2), Runx2 and B) phosphorylated p38 (p-p38) and total p38, phosphorylated Akt (p-Akt) and total Akt, and actin in hMSCs cultured on Col-GAG and MC-GAG at days 0, 4, 14, and week 4 of culture with and without 50 μm PD98059.
3. Discussion
In this work, we investigated the mechanism of osteogenic differentiation of hMSCs on Col-GAG and MC-GAG scaffolds. Using DMH1, a small molecule inhibitor for the canonical BMP receptor signaling pathway, our data demonstrated that DMH1 inhibited the expressions of Runx2 and BSPII on hMSCs differentiated on MC-GAG scaffolds but not Col-GAG scaffolds. When mineralization was evaluated, both Col-GAG and MC-GAG scaffolds exhibited decreased mineralization in a quantifiable manner on microCT scanning, as well as on histologic staining with Alizarin red in the presence of DMH1. Of note, DMH1 had no effect on the viability of hMSCs on MC-GAG scaffolds but there was a slight decrease in viability on Col-GAG scaffolds after 8 weeks of culture. When protein expression was evaluated, DMH1 treatment resulted in decreases in p-Smad1/5 and Runx2 protein. By contrast, treatment with PD98059, a small molecule inhibitor specific for MEK1, hMSCs cultured on Col-GAG resulted in decreased BSPII expression, whereas no differences were noted on MC-GAG. Similarly, when mineralization was evaluated, PD98059 significantly decreased matrix mineralization of hMSCs on Col-GAG without any effect on MC-GAG as demonstrated in microCT scanning, as well as histology. For both scaffolds, no differences in cell viability were noted after 8 weeks of culture with PD98059. When protein expression was evaluated, PD98059 decreased both p-ERK1/2 and p-JNK1/2 without any decreases in p-Smad1/5 or Runx2. Taken together these results suggest several conclusions: (1) the canonical BMPR signaling pathway is necessary for osteogenic differentiation and mineralization of hMSCs on Col-GAG or MC-GAG scaffolds, (2) the canonical BMPR signaling pathway is necessary for augmentation of Runx2 expression, (3) ERK1/2 phosphorylation is necessary for osteogenic differentiation and mineralization of hMSCs on Col-GAG but not MC-GAG scaffolds, (4) ERK1/2 and JNK1/2 activations are intimately tied in hMSCs undergoing osteogenic differentiation, and (5) Runx2 expression is not regulated by ERK1/2 phosphorylation or JNK1/2 phosphorylation.
The idea of an autogenous or autocrine activation of the BMPR signaling pathway on MC-GAG scaffolds was suggested in our previous reports with the detection of a massive difference in p-Smad1/5 expression in comparison to Col-GAG, as well as the elevated expression of a panel of BMP ligands.[2,3] In this work, the necessity of BMPR signaling is now confirmed in that mineralization is significantly decreased in the presence of DMH1 in a manner that correlated to a reduction in Runx2 expression. Interestingly, a decrease in mineralization was also found in Col-GAG which suggests that an autocrine BMPR activation mechanism is required in hMSCs differentiated on either scaffold. However, unlike MC-GAG, DMH1 treatment slightly decreased the viability of hMSCs on Col-GAG scaffolds after 8 weeks of culture (Figure 2A). Unlike Col-GAG, the massive amounts of Smad1/5 phosphorylation on MC-GAG could not be completely inhibited by DMH1. Thus, long term inhibition of canonical BMPR signaling may be either inhibitory to cell growth or toxic.
Conversely, our previous work also demonstrated that ERK1/2 phosphorylation was slightly diminished or delayed in activation on MC-GAG scaffolds in comparison to Col-GAG scaffolds for both hMSCs and rBMSCs, whereas no other known noncanonical signaling molecules were affected.[2,3] Our present work now highlights the importance of ERK1/2 phosphorylation in mineralization on Col-GAG scaffolds and that this intracellular signaling pathway is largely inconsequential in osteogenic differentiation and mineralization on MC-GAG scaffolds. From the PD98059 treated scaffolds, p-JNK1/2 was noted to be decreased in a similar manner as ERK1/2. The specificity of the inhibitor for MEK1/2 was considered and a more specific ERK1/2 inhibitor (FR180204) was evaluated in the same system and found to also decrease p-JNK1/2 (unpublished data, JCL). Thus, ERK1/2 and JNK1/2 phosphorylation are intimately tied in hMSCs undergoing osteogenesis on either scaffold such that JNK1/2 cannot be activated without ERK1/2 phosphorylation. Although the MAPK cascades are generally considered to be separate pathways for activation, the crosstalk between MEK/ERK and JNK is reminiscent of several reports in the literature. In Xenopus oocytes, a complex of ras-p21, Raf, MEK1, ERK, and JNK could be co-immunoprecitated in the presence of the onco-protein (Val12)-ras-p21.[16] Similarly, Kitanaka et al. evaluated COX-2 expression following IL-1β induction in feline synovial fibroblasts and demonstrated that activated and total JNK1/2, MEK, and ERK1/2 coprecipitated in the presence of IL-1β but not in the absence.[17] In both reports, JNK1/2 phosphorylation preceded ERK1/2 activation and phosphorylated JNK1/2 regulated ERK activation by phosphorylating Raf, the MAPKKK in the ERK axis. The former group also observed that MEK1 had the ability to phosphorylate JNK1/2, although this activity was not absolutely required for JNK activation.[16] Bavaria et al. reported in the apoptosis literature that MEK1/ERK1/2 negatively regulated JNK1/2 phosphorylation via induction of MAP kinase phosphatase-1 (MKP-1).[18]
Mineralization through the ERK1/2 pathway appeared to be independent of Runx2 expression. Although Runx2 is considered to be a master transcriptional regulator for chondro-osseous differentiation and matrix mineralization,[19] a number of investigators have reported findings that challenge the absolute necessity of Runx2 in osteogenesis. Lee et al. demonstrated that Runx2 is not necessary for the expression of Osterix (Osx), which is essential for differentiation to osteocytes.[20] Felthaus et al. have shown that ZBTB16, zinc finger and Broad-complex, Tramtrack, and Bric-a-brac (BTB) domain-containing protein 16 transcription factor, induced the expression of osteogenic differentiation markers independently of Runx2.[21] In low-density lipoprotein receptor-related protein (Lrp)-5 knockout mice, decreased osteoblast proliferation and bone formation was present despite comparable expression of Runx2 when compared to wildtype mice. Thus, an additional, evolutionarily conserved, osteogenic differentiation pathway independent of Runx2 exists.[22]
Unlike Smad1/5, MAPK cascades are not specifically downstream of BMPR signaling. Although our current study is focused primarily on the autocrine activation of the BMP receptor, one cannot ignore that other osteogenic pathways, such as the Wnt/β-catenin pathway, may be significantly affected by MEK1/ERK1/2 downregulation. As there does not exist a small molecule inhibitor that may downregulate the noncanonical activities of BMPR, future work with receptor knockouts or mutations may be necessary for further clarification of the pathways. Additionally, the specific trigger for magnified activation of the canonical BMPR signaling pathway in MC-GAG remains unclear. As both scaffolds require Smad1/5 phosphorylation for mineralization, both have the ability to autogenously activate the BMPR signaling pathway, albeit decreased in Col-GAG. However, as the only difference between Col-GAG and MC-GAG is the mineral content, it is likely that inorganic ion signaling or the mechanical properties imparted by the addition of mineral content is the reason for the improved differentiation. Further investigation on the exact contributions of inorganic ion signaling on this material is currently underway. Although Col-GAG is not an efficient scaffold for osteogenesis, the ability to pinpoint the signaling pathway for which hMSCs become mineralized on Col-GAG scaffolds may be potentially harnessed for optimization of other regenerative applications such as cartilage or tendon regeneration.
4. Conclusions
With the improved understanding of the instructive capabilities of extracellular matrix components in directing progenitor cell differentiation, the paradigm of material/stem cell/growth factor cocktail for regeneration has shifted significantly. Our current work details the necessary and sufficient signaling pathways induced by MC-GAG and Col-GAG in osteogenesis of primary human mesenchymal stem cells. Our findings that differential activation of intracellular signaling molecules is dependent on the composition of extracellular matrix-inspired materials contribute novel methods for further modulating regenerative materials. We suggest that the future in regenerative investigation requires not only demonstration of function but a rigorous understanding of mechanistic interactions between materials and biology.
5. Experimental Section
Fabrication and Chemical Crosslinking of Nonmineralized and Mineralized Collagen Scaffolds
Col-GAG and MC-GAG scaffolds were prepared using lyophilization as described previously.[23] Briefly, microfibrillar, type I collagen (Collagen Matrix, Oakland, NJ) and chondroitin-6-sulfate (Sigma-Aldrich, St. Louis, MO) were combined in suspension in the absence and presence of calcium salts (calcium nitrate hydrate: Ca(NO3)2·4H2O; calcium hydroxide: Ca(OH)2, Sigma-Aldrich, St. Louis, MO) in an acetic acid (Col-GAG) or phosphoric acid (MC-GAG) solution. Using a constant cooling rate technique at a rate of 1 °C min−1, the solution was frozen from room temperature to −10 °C using a freeze dryer (Genesis, VirTis). Following sublimation of the ice phase, scaffolds were sterilized via ethylene oxide and cut into 8 mm disks for culture.
Crosslinking of scaffolds was performed after rehydration in phosphate buffered saline (PBS) overnight using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDAC, Sigma-Aldrich) and N-hydroxysuccinimide (NHS, Sigma Aldrich) at a molar ratio of 5:2:1 EDC:NHS:COOH where COOH represents the amount of collagen in the scaffold as we previously described.[24] Scaffolds were washed with PBS to remove any of the residual chemical.
Cell Culture
hMSCs (Lonza, Inc., Allendale, NJ) were expanded in Dulbecco’s modified Eagle’s medium (Corning Cellgro, Manassas, VT) supplemented with 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 2 × 10−3 M L-glutamine (Life Technologies, Carlsbad, CA), 100 IU mL−1 penicillin/100 μg mL−1 streptomycin (Life Technologies). 3 × 105 hMSCs were seeded onto 8 mm Col-GAG and MC-GAG scaffolds in proliferation media. 24 h after seeding, media was switched to osteogenic differentiation media consisting of 10 × 10−3 M β-glycerophosphate, 50 μg mL−1 ascorbic acid, and 0.1 × 10−6 M dexamethasone. Scaffolds were treated or untreated with DMH1 (Sigma-Aldrich) and PD98059 (Cell Signaling Technologies, Beverly, MA) separately, all at a concentration of 50 μm. Fresh DMH1 and PD98059 were added to each media change every 3 days.
Quantitative Real-Time Reverse-Transcriptase Polymerase Chain Reaction
Total RNA was extracted using the RNeasy kit (Qiagen, Valencia, CA) at 0, 3, and 14 d of culture. Gene sequences for 18S, ALP, Runx2, and BSPII were obtained from the National Center for Biotechnology Information gene database and primers were designed (Table 1). Quantitative real-time RT-PCR were performed on the Opticon Continuous Fluorescence System (Bio-Rad Laboratories, Inc., Hercules, CA) using the QuantiTect SYBR Green RT-PCR kit (Qiagen). Cycle conditions were as follows: reverse transcription at 50 °C (30 min); activation of HotStarTaq DNA polymerase/inactivation of reverse transcriptase at 95 °C (15 min); and 45 cycles of 94 °C for 15 s, 58 °C for 30 s, and 72 °C for 45 s. Results were analyzed and presented as representative graphs of triplicate experiments.
Table 1.
Primer sequences. ALP: Alkaline phosphatase. Runx2: Runt-related transcription factor 2. BSPII: Bone sialoprotein II.
| Genes | Oligonucleotide sequence |
|---|---|
| 18S sense | 5′-TAGAGTGTTCAAAGCAGGCCCG-3′ |
| 18S antisense | 5′-TCCCTCTTAATCATGGCCTCAG-3′ |
| ALP sense | 5′-AAGCCGGTGCCTGGGTGGCCAT-3′ |
| ALP antisense | 5′-ACAGGAGAGTCGCTTCAGAG-3′ |
| Runx2 sense | 5′-TCGGAGAGGTACCAGATGGG-3′ |
| Runx2 antisense | 5′-AACTCTTGCCTCGTCCACTC-3′ |
| BSPII sense | 5′-GGACTGCCAGAGGAAGCAAT-3′ |
| BSPII antisense | 5′-GGCCTGTACTTAAAGACCCCA-3′ |
Cell Activity
The mitochondrial dehydrogenase activity of hMSCs treated with or without DMH1 or PD98059 on the Col-GAG and MC-GAG scaffolds were assessed with cell proliferation reagent WST-1 (Roche, Germany) after 8 weeks of culture. The scaffolds (n = 3, for each type) were incubated for 4 h in a 10% WST-1 solution (v/v) at 37 °C in a humidified atmosphere with 5% CO2. Absorbance of the incubation medium was measured at 450 and 690 mm (Epoch spectrophotometer, BioTek, Winooski, VT). The WST-1 assay was also applied to the synthetic scaffolds without hMSCs, which served as controls.
Histology and Immunohistochemistry
Histology was performed on scaffolds at 8 weeks of culture. Scaffolds were fixed in 10% formalin, paraffin-embedded, and sectioned at 4 μm. Following deparaffinization, the sections were stained with hematoxylin and eosin or alizarin red (Cell Signaling Technologies, Beverly, MA) and processed with the Dako automated FLEX system (Dako, Carpinteria, CA). Slides were analyzed qualitatively using a standard microscope and digitally photographed.
Microcomputed Tomographic Imaging
Scaffolds were fixed using 10% formalin and mineralization was quantified by microcomputed tomographic imaging (microCT) using the Scanco microCT 35 (Scanco Medical AG, Bruttisellen, Switzerland) at 8 weeks in triplicate for each timepoint. Scans were performed at medium resolution with a source voltage of 70 E (kVp) and I (μA) of 114. The images had a final element size of 12.5 μm. Images were analyzed using software supplied from Scanco (Image Processing Language version 5.6) and reconstructed into 3D volumes of interest. Optimum arbitrary threshold values of 20 (containing scaffold and mineralization) and 80 (containing mineralization alone) were used uniformly for all specimens to quantify mineralized areas from surrounding unmineralized scaffold.
Analysis of 3D reconstructions was performed using Scanco Evaluation script #2 (3D segmentation of two volumes of interest: solid dense in transparent low-density object) and script #6 (bone volume/density only bone evaluation) for volume determinations.
Western Blot
Lysates were prepared from scaffolds at 0, 3, 14, and 24 d of culture using SDS sample buffer and equal amounts were subjected to 4–20% SDS-PAGE (Bio-Rad, Hercules, CA). Western blot analysis was carried out with antibodies against phosphorylated Smad1/5 (p-Smad1/5), total Smad5, phosphorylated ERK1/2 (p-ERK1/2), total ERK1/2, phosphorylated JNK1/2 (p-JNK1/2), total JNK1/2, phosphorylated p38 (p-p38), total p38, p-Akt, total Akt, and β-actin followed by 1:4000 dilutions of horseradish peroxidase-conjugated IgG antibodies (Bio-Rad, Hercules, CA) and an enhanced chemiluminescent substrate (Thermo Scientific, Rockford, IL). For detection of p-Smad1/5 and total Smad5, 10 μg of lysate was loaded per lane. For detection of p-ERK1/2 and total ERK1/2, p-JNK1/2, total JNK1/2, p-p38, total p38, p-Akt, total Akt, 20 μg of lysate was loaded per lane. All primary phospho-antibodies and primary full length antibodies were obtained from Cell Signaling Technologies (Beverly, MA). β-actin antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Imaging analysis was carried out using ImageJ (NIH, Bethesda, MD).
Statistical Analysis
All statistical analyses were performed using SPSS Version 24 (Chicago, IL). Data points were composed of duplicates of at least three independent experiments, unless otherwise indicated. Mean measurements of mRNA expression were analyzed for statistical significance by analyses of variance (ANOVA) followed by post hoc tests using the Tukey criterion. A value of p < 0.05 was considered significant.
Acknowledgments
This work was supported by the US Department of Veterans Affairs under award number IK2 BX002442-01A2 (J.C.L.), the Aramont Foundation (T.A.M.), and the Jean Perkins Foundation (J.C.L.). Research reported in this publication was also supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R21 AR063331 (B.A.C.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by project number S-14-54H by AO Foundation, Switzerland (B.A.C.H.). D.W.W. was funded at UIUC from National Science Foundation (NSF) Grant 0965918 IGERT: Training the Next Generation of Researchers in Cellular & Molecular Mechanics and BioNanotechnology.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Contributor Information
Dr. Qi Zhou, Division of Plastic and Reconstructive Surgery, UCLA David Geffen School of Medicine, Los Angeles, CA 90095, USA. Research Service, Greater Los Angeles VA Healthcare System, Los Angeles, CA 90073, USA. Department of Periodontolology School of Stomatology Shandong University Jinan 250012, China
Dr. Xiaoyan Ren, Division of Plastic and Reconstructive Surgery, UCLA David Geffen School of Medicine, Los Angeles, CA 90095, USA. Research Service, Greater Los Angeles VA Healthcare System, Los Angeles, CA 90073, USA
Dr. David Bischoff, Research Service, Greater Los Angeles VA Healthcare System, Los Angeles, CA 90073, USA
Dr. Daniel W. Weisgerber, Department of Chemical and Biomolecular Engineering Institute for Genomic Biology University of Illinois at Urbana-Champaign Urbana, IL 61801, USA
Prof. Dean T. Yamaguchi, Research Service, Greater Los Angeles VA Healthcare System, Los Angeles, CA 90073, USA
Prof. Timothy A. Miller, Division of Plastic and Reconstructive Surgery, UCLA David Geffen School of Medicine, Los Angeles, CA 90095, USA. Research Service, Greater Los Angeles VA Healthcare System, Los Angeles, CA 90073, USA
Prof. Brendan A. C. Harley, Department of Chemical and Biomolecular Engineering Institute for Genomic Biology University of Illinois at Urbana-Champaign Urbana, IL 61801, USA
Prof. Justine C. Lee, Division of Plastic and Reconstructive Surgery, UCLA David Geffen School of Medicine, Los Angeles, CA 90095, USA. Research Service, Greater Los Angeles VA Healthcare System, Los Angeles, CA 90073, USA
References
- 1.Szpalski C, Wetterau M, Barr J, Warren SM. Tissue Eng, Part B. 2012;18:246. doi: 10.1089/ten.TEB.2011.0427. [DOI] [PubMed] [Google Scholar]
- 2.Ren X, Bischoff D, Weisgerber DW, Lewis MS, Tu V, Yamaguchi DT, Miller TA, Harley BA, Lee JC. Biomaterials. 2015;50:107. doi: 10.1016/j.biomaterials.2015.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ren X, Tu V, Bischoff D, Weisgerber DW, Lewis MS, Yamaguchi DT, Miller TA, Harley BA, Lee JC. Biomaterials. 2016;89:67. doi: 10.1016/j.biomaterials.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song B, Estrada KD, Lyons KM. Cytokine Growth Factor Rev. 2009;20:379. doi: 10.1016/j.cytogfr.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YE. Cold Spring Harbor Perspect Biol. 2017;9:a022129. doi: 10.1101/cshperspect.a022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kua HY, Liu H, Leong WF, Li L, Jia D, Ma G, Hu Y, Wang X, Chau JF, Chen YG, Mishina Y, Boast S, Yeh J, Xia L, Chen GQ, He L, Goff SP, Li B. Nat Cell Biol. 2012;14:727. doi: 10.1038/ncb2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai CF, Cheng SL. J Biol Chem. 2002;277:15514. doi: 10.1074/jbc.M200794200. [DOI] [PubMed] [Google Scholar]
- 8.Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, Matsumoto K, Nishida E. J Biol Chem. 1997;272:8141. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- 9.Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S. Bone. 2001;28:491. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- 10.a) Zhao YF, Xu J, Wang WJ, Wang J, He JW, Li L, Dong Q, Xiao Y, Duan XL, Yang X, Liang YW, Song T, Tang M, Zhao D, Luo JY. BMB Rep. 2013;46:422. doi: 10.5483/BMBRep.2013.46.8.266. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhao Y, Song T, Wang W, Wang J, He J, Wu N, Tang M, He B, Luo J. PLoS One. 2012;7:e43383. doi: 10.1371/journal.pone.0043383. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Lo SC, Li KC, Chang YH, Hsu MN, Sung LY, Vu TA, Hu YC. Biomaterials. 2017;124:1. doi: 10.1016/j.biomaterials.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 12.Ren D, Wei F, Hu L, Yang S, Wang C, Yuan X. J Cell Physiol. 2015;230:2426. doi: 10.1002/jcp.24972. [DOI] [PubMed] [Google Scholar]
- 13.Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Cell. 2007;131:980. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. ACS Chem Biol. 2010;5:245. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varas A, Valencia J, Lavocat F, Martínez VG, Thiam NN, Hidalgo L, Fernández-Sevilla LM, Sacedón R, Vicente A, Miossec P. Arthritis Res Ther. 2015;17:192. doi: 10.1186/s13075-015-0710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler V, Qu Y, Smith SJ, Izotova L, Pestka S, Kung HF, Lin M, Friedman FK, Chie L, Chung D, Boutjdir M, Pincus MR. Biochemistry. 2005;44:10784. doi: 10.1021/bi050619j. [DOI] [PubMed] [Google Scholar]
- 17.Kitanaka T, Nakano R, Kitanaka N, Kimura T, Okabayashi K, Narita T, Sugiya H. Sci Rep. 2017;7:39914. doi: 10.1038/srep39914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bavaria MN, Jin S, Ray RM, Johnson LR. Apoptosis. 2014;19:467. doi: 10.1007/s10495-013-0944-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a) Ducy P, Schinke T, Karsenty G. Science. 2000;289:1501. doi: 10.1126/science.289.5484.1501. [DOI] [PubMed] [Google Scholar]; b) Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cell. 1997;89:765. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 20.Lee MH, Kwon TG, Park HS, Wozney JM, Ryoo HM. Biochem Biophys Res Commun. 2003;309:689. doi: 10.1016/j.bbrc.2003.08.058. [DOI] [PubMed] [Google Scholar]
- 21.Felthaus O, Gosau M, Morsczeck C. J Periodontol. 2014;85:e144. doi: 10.1902/jop.2013.130445. [DOI] [PubMed] [Google Scholar]
- 22.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. J Cell Biol. 2002;157:303. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.a) Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. J Biomed Mater Res, Part A. 2010;92:1066. doi: 10.1002/jbm.a.32361. [DOI] [PubMed] [Google Scholar]; b) Harley BA, Leung JH, Silva EC, Gibson LJ. Acta Biomater. 2007;3:463. doi: 10.1016/j.actbio.2006.12.009. [DOI] [PubMed] [Google Scholar]; c) Weisgerber DW, Kelkhoff DO, Caliari SR, Harley BA. J Mech Behav Biomed Mater. 2013;28:26. doi: 10.1016/j.jmbbm.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olde Damink LH, Dijkstra PJ, van Luyn MJ, van Wachem PB, Nieuwenhuis P, Feijen J. Biomaterials. 1996;17:765. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]