Abstract
Introduction
Although registries can rapidly identify clinical study participants, it is unknown which follow up methods for recruiting are most effective. We examined the efficacy of three communication strategies for recruiting and enrolling patients who were identified via a contact registry (i.e., registry linked to a consent to re-contact program) into a clinical study.
Methods
Patients who met the study criteria were identified via the contact registry and targeted for recruitment. In condition 1, patients established in the university hepatology specialty clinics were contacted one time via phone call by the study coordinator and asked to participate (C1). In condition 2, non-established specialty clinic patients were mailed an IRB-approved letter with study information and instructions for calling the study coordinator to participate (C2). Condition 2A included patients who called within two weeks of receiving the letter (C2A); condition 2B included patients who did not call after receiving the letter but were subsequently contacted via phone call.
Results
A registry identified 1060 patients, of which 661 were eligible and targeted for recruiting. All 37 patients were reached in C1 and 17 (45.9%) were recruited. Nineteen of the 624 patients in C2A were reached and 10 were recruited whereas 120 of the 605 patients in C2B were reached and 53 (8.7%) were recruited. Seventy patients enrolled with C2B being the most effective (total, cost) recruitment strategy (n = 50) (p < .001).
Conclusion
The efficacy of enrolling patients identified via a contact registry into clinical trials varies based on the communication strategies used for recruiting.
Keywords: Patient recruitment, Communication, Registry, Broad consent
1. Introduction
Recruiting and enrolling individuals at risk for rare diseases into clinical trials can be critical to their diagnosis and to avoiding early mortality; however, identifying participant cohorts can be challenging. One strategy for rare disease recruiting is to identify those at risk using computable phenotypes in an integrated data repository (IDR) or a research registry. A registry is a clinical data warehouse that integrates numerous sources of data to support queries for a range of research-like functions [1], including efficiently querying large amounts of data to identify patients who meet certain inclusion and exclusion criteria [2], determining cohort feasibility prior to developing a protocol [3], and connecting investigators with prospective participants[4], [5]. Because registries leverage multiple sources of patient information, institutions are increasingly adopting them into clinical research [6].
In order to achieve recruiting and accrual goals, without draining study resources, researchers must think strategically about how to communicate with their prospective cohorts after they have been identified. Even with registries, challenges to recruiting remain; registries rely on one or more follow-up strategies for contacting and recruiting participants [2], which can dramatically increase costs [7], even for well-funded studies. Effective communication with participants after they have been identified is critical to recruiting and enrolling prospective participants into clinical studies. One commonly used strategy is to have study staff mail information to potential participants. This passive strategy has wide reach [8] and can reduce costs [9], but may be perceived as less personal than active recruiting methods and have lower return. A second strategy is to have a coordinator call prospective participants on the telephone and ask them to participate. The benefit of using this strategy is that study staff (i.e., study coordinators, nurses) can use nonverbal communication behaviors to establish a connection with prospective participants and adapt their communication (i.e., rate, volume, tone) to match the participant [10]. These methods are more effective than mailers [11], but may result in high costs and participant refusals [12]. A third option is to use a combination of strategies for recruiting. Combination strategies [13], [14] (e.g., letter and phone call), particularly those that incorporate study coordinators into recruiting, have been shown to increase clinical study enrollment [15]. Although individuals enroll in registries knowing they may be contacted to participate in research, it is unknown which communication strategies are most effective for recruiting prospective cohorts identified via registries. Thus, our goal is examine best practices for recruiting and enrolling prospective participants into clinical studies after identifying them via a contact registry (i.e., traditional registry + Consent2Share program) [5] and recruiting them using three communication strategies.
2. Material and methods
Recruitment occurred as part of a larger, multi-site study to identify a cohort who may be at risk for Lysosomal Acid Lipase Deficiency (LAL-D). LAL-D is a rare autosomal recessive condition, affects major organ functioning [16], is often under-recognized and misdiagnosed [16], [17], [18], and can lead to premature death [16]. Each site was responsible for enrolling 50 patients into the LAL-D clinical trial. Subjects who met screening criteria were asked to come to the University Clinical Research Center (CRC) and have clinical and laboratory information collected, along with genetic testing. All procedures were approved by the Institutional Review Board prior to starting the study.
2.1. Cohort identification and study design
In March of 2015, a cohort query was used to identify eligible patients for the LAL-D study. Patients in a large university health system were eligible if they: (1) were > 2 years of age, (2) were enrolled in the Consent2Share program (i.e., registry of patients who have previously given consent to be re-contacted about future research studies for which they qualify) [5], and (3) met the required phenotype for screening (i.e., met at least one high risk segment). See Table 1 for high risk screening segments. Patients with a history of active viral hepatitis or other confirmed genetic liver diseases were ineligible for participation and excluded from the cohort search. See Table 1 for full search criteria.
Table 1.
Registry search criteria.
| Inclusion criteria |
| >2 years of age |
| Enrolled in Consent2Share |
| High risk segmentsa |
| Non-obesec with low-density lipoprotein (LDL) ≥ 160 mg/dL (≥4.1 mmol/L) |
| Non-obesec with high-density lipoprotein (HDL) ≤ 50 mg/dL (≤1.3 mmol/L) |
| Non-obesec with unexplained and persistently elevated liver transaminases, (i.e., two or more results of alanine aminotransferase [ALT] ≥ 50 U/L at least 2 months apart) |
| Non-obesec with hepatomegaly |
| Cryptogenic cirrhosis |
| Autosomal recessive hypercholesterolemia (other than homozygous FH) |
| Autosomal recessive low HDL levels (≤40 mg/dL [≤1.0 mmol/L]) of unknown etiology |
| Exclusion criteriab |
| Viral hepatitis |
| Hereditary hemochromatosis |
| Hemochromatosis due to repeated red blood cell transfusions |
| Other hemochromatosis |
| Other disorders of iron metabolism |
| Alpha-1-antitrypsin deficiency |
| Alpha-1-antitrypsin |
| Disorders of copper metabolism |
Note. Table excludes additional metrics used to screen medical records of registry-identified participants (e.g., Biopsy-proven microvesicular or mixed micro/macrovesicular steatosis with unknown etiology; Familial Hypercholesterolemia (FH) in which genetic analysis was performed for the genes encoding the low-density lipoprotein receptor (LDLR), Apo-B and PCSK9 genes and no disease-causing mutations; Presumed FH with unclear family history) and local area code used to refine the sample. Participants (N = 1060) identified via the registry, (n = 725) eligible after medical record screening, (n = 661) with local area code targeted for recruiting.
Participants were required to meet at least one high risk segment for inclusion in the study.
Participants who met any of the exclusion criteria in their medical history were ineligible.
Non-obese = (body mass index [BMI] ≤ 30 kg/m2 or for patients < 18 years, body mass index for age [BMIA] ≤ 95% percentile for weight, according to Center for Disease Control growth chart.
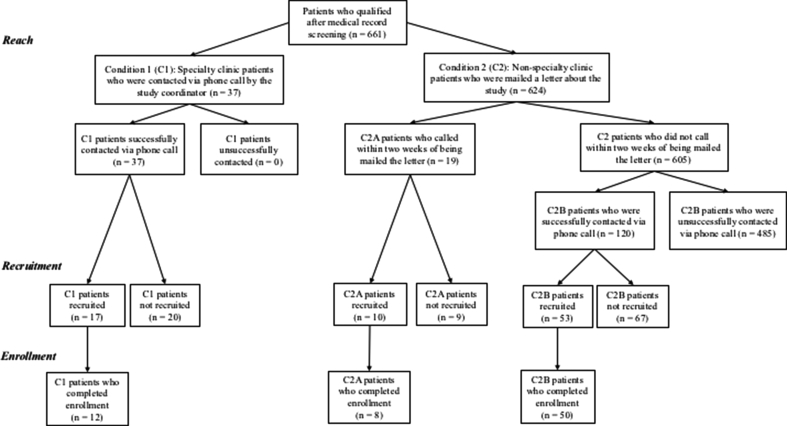
There were three recruitment arms in the study. Patients were assigned to condition based on clinical and behavioral characteristics. Participants in C1 were patients who had, at one time, scheduled an appointment in one of the university hepatology specialty clinics (clinics offer treatment, evaluation, and prevention screenings for patients presenting with liver diseases) as listed in medical record. Patients in condition 1 were contacted one time via phone call by the study coordinator and asked to participate in the study (C1). Non-specialty clinic patients were mailed an IRB-approved letter with information about the study, which included a phone number with instructions for contacting the study coordinator to participate (C2). Patients who received the letter and initiated contact with the coordinator via phone call to discuss the study were assigned to (C2A). After two weeks of no response, patients who were sent the letter via mail were contacted one time, via phone call [14], by the study coordinator and asked to participate (C2B). Patients recruited into the study (i.e., who agreed to participate) scheduled and completed one visit to the CRC to complete their enrollment (i.e., data capture and blood draw). See Fig. 1 for diagram of cohort identification and recruitment methods.
Fig. 1.
Process identifying, recruiting, and enrolling patients. Note: All patients were identified via the contact registry (i.e., IDR and Consent2Share). C1: Specialty clinic patients contacted via coordinator initiated phone call; C2A: Non-specialty clinic patients mailed a letter, patient initiated the phone call; C2B: Non-specialty clinic patients mailed a letter, contacted via coordinator initiated phone call after two weeks of no reply.
2.2. Study outcomes
Study outcomes pertain to using the contact registry for reach, recruitment, and enrollment. Reach included the total patients identified by the registry (i.e., who met the registry inclusion criteria) (cohort identification), the total patients eligible for participation (i.e., met the full study criteria) (potential reach), and the total patients who were in contact via phone call with the study coordinator to discuss the study (reach). Recruitment included total recruitment, recruitment response rate, total recruitment by condition, and recruitment response rate by condition. Recruitment total was the total patients who were recruited (i.e., agreed) to participate in the study whereas recruitment condition total was the total patients recruited to participate by condition. Recruitment response rate was calculated as the number of eligible patients recruited divided by the total eligible sample initially targeted for recruiting [19], [20], [21]. Recruitment response rate by condition was calculated as the number of eligible patients recruited by condition divided by the total eligible patients targeted for recruiting in that condition [19], [20], [21].
Enrollment included total enrollment, enrollment cost, and enrollment length. Total enrollment is the total patients who completed one visit to the Clinical Research Center (CRC) for data capture and blood draw. Enrollment cost refers to the amount spent (in USD), per patient who completed enrollment (i.e., identifying, recruiting, and enrolling them into the study). Enrollment length refers to the total length of time, in days, spent recruiting and enrolling patients into the study and was calculated by adding the total days beginning on the first date letters were sent via mail and extending until the date of the last patient CRC appointment. Enrollment cost was determined by condition and by adding the total amount spent identifying patients via the registry to the amount spent on follow-up recruiting strategies (i.e., letter, coordinator initiated phone calls) within each condition.
2.3. Statistical analysis
All analyses were conducted with SPSS 24.0 statistical software package. Frequency statistics were computed to calculate the number of patients in each condition and the number of patients who were recruited and enrolled in the study by condition. Chi-squares were calculated to determine whether patient recruitment and enrollment varied by condition. Descriptive statistics (i.e., mean) are reported and describe the enrollment cost by condition. P values ≤ .05 were considered significant.
3. Results
3.1. Reach
A registry cohort query identified 1060 patients who met the inclusion criteria, of which, 661 patients (62.4%) met the full study criteria (i.e., inclusion and exclusion) and were targeted for recruitment (potential reach). Of the 661 patients, 37 were identified as specialty clinic patients and were called by the study coordinator and asked to participate (C1). All 37 patients in C1 were reached (i.e., were successfully contacted via phone call by the study coordinator). The remaining 624 patients (94.4%) were mailed a letter with information about the study (C2), of which 19 letters (3%) were returned as undeliverable (i.e., sent back). Of these, 19 patients in C2A were reached (i.e., called the study coordinator to inquire about the study after receiving the mailer). After two weeks of no reply, 120 of the patients in C2B (n = 605)1 who were mailed the letter and did not call to inquire about the study were reached (i.e., were successfully contacted via phone call by the study coordinator and asked to participate). The remaining 485 patients were unreachable (i.e., study coordinator was unable to make contact with patient via phone call). Across conditions, 176 patients (26.6%) were reached. Table 2 reports the differences and percentages of the patients reached among those eligible within each condition.
Table 2.
Patients reached, recruited, and enrolled within condition.
| C1 n (%) | C2A n (%) | C2B n (%) | |
|---|---|---|---|
| Reach | |||
| Eligible | 37 | 624 | 605 |
| Contacted | 37 (100) | 19 (3)* | 120 (19.8)* |
| Recruitment | |||
| Eligible | 37 | 624 | 605 |
| Recruited | 17 (45.9) | 10 (1.6)* | 53 (8.7)* |
| Enrollment | |||
| Eligible | 17 | 10 | 53 |
| Enrolled | 12 (70.6) | 8 (80) | 50 (94.3)* |
Note. C1: Specialty clinic patients recruited via coordinator initiated phone call; C2A: Non-specialty clinic patients sent the letter with information about the study, patient initiated phone call to study coordinator; C2B: Non-specialty clinic patients sent the letter with information about the study, contacted via coordinator initiated phone call.
*Difference is significant at p < .001.
3.2. Recruitment
Among eligible patients for recruiting, there were significant differences in the proportion of patients recruited among the total eligible in C2A, χ2 (1, N = 624) = 584.641, p < .001 and C2B, χ2 (1, N = 605) = 411.572, p < .001. However, there was no difference in the proportion of patients recruited in C1, χ2 (1, N = 37) = 0.243, p > .62. In other words, a higher proportion of patients in C1 were recruited to participate than patients in C2A and C2B. See Table 2 for differences and percentages of the patients recruited within each condition. Recruitment response rate was 12.1% and a total of 80 patients were recruited (i.e., agreed) across conditions.
3.3. Enrollment
Of the total patients recruited, there was a significant difference in the proportion of patients who enrolled among the total patients recruited into C2B, χ2 (1, N = 53) = 41.679, p < .001. However, there were no differences in the percentage of patients who enrolled in C1, χ2 (1, N = 17) = 2.882, p > .09 or in C2A, χ2 (1, N = 10) = 3.600, p > .06. Thus, the highest proportion of patients who enrolled in the study were recruited from C2B. See Table 2 for differences and percentages of the patients who completed enrollment within each condition. A total of 70 patients enrolled (i.e., completed one visit to the CRC for data capture and blood draw) across conditions.
Enrollment length was 157 days, which includes the time spent recruiting and enrolling an additional 20 patients. Enrollment cost for using the registry was $2000; the total cost for all coordinator-initiated phone calls was $1500; and the total cost for the letters was $100. Coordinator-initiated phone calls were split across conditions 1 and 2B and by the total number of patients who were contacted via phone call by the study coordinator in each condition (i.e., C1, C2B) ($1500/157 patients = $9.55 cost/per time spent contacting patients). The overall cost per condition was divided by the total number of patients who completed enrollment in each condition (e.g., C1: Registry, one phone call from study coordinator (2000 + 353.35)/12; C2A: Registry, mailed letter (patient called) (2000 + 100)/8; C2B: Registry, mailed letter, one phone call from study coordinator (2000 + 1146+100)/50). Enrollment cost for each patient who completed enrollment was $196.11 per patient in C1, $262.50 per patient in C2A, and $64.92 per patient in C2B.
4. Discussion
The current study demonstrates that the effectiveness of enrolling patients into clinical studies varies based on the communication strategies used for recruiting. Several findings emerged that are important to recruiting and increasing participation in clinical studies. Consistent with other studies [6], results confirm that registries can facilitate rapid cohort identification and efficient screening of study participants. The contact registry, specifically Consent2Share (i.e., registry of patients who have previously given consent to be re-contacted about future research studies for which they qualify), enabled investigators to rapidly identify participants who were interested in participating in clinical research and had previously consented to be contacted about future studies. Identifying eligible and interested participants saved valuable study resources and is in line with patient preferences for providing broad consent and for being contacted to participate in clinical research studies [5]. The proportion of prospective participants who were in contact via phone call with the study coordinator but declined to participate demonstrates that a carefully regulated, broad consent program does not compromise patients' decisions to opt out of studies, despite their decision to enroll in a contact registry. This approach also facilitated successful identification and screening of an at risk patient cohort, a significantly challenging group to identify [6], increasing their likelihood of obtaining treatment and care as needed. Future studies should strive to identify practices for enrolling individuals with stigmatized illnesses (e.g., depression), who may be unlikely to seek medical care, into registries as a first step to increasing their participation in clinical care.
Another contribution of this study is that is shows promise for recruiting and enrolling participants identified via contact registries into clinical studies using a combination of traditional communication strategies. First, all participants in C1 (i.e., patients who had scheduled an appointment in one of the hepatology clinics) were reached and the greatest proportion of patients were recruited from this condition. This suggests that combining registries with high touch methods (e.g., phone call from a coordinator) may be important for identifying and recruiting patients to participate in rare disease studies. Second, the high touch, coordinator initiated phone call made to patients who did not respond within two weeks of receiving the letter (i.e., did not call to inquire about the study) resulted in the highest total recruitment and subsequent enrollment of patients. It appears that by sending the letter (low touch) and calling prospective participants via the phone call (high touch), study coordinators were able to establish contact with individuals, who were aware of the study and their eligibility, to discuss the trial and presumably, address participants' questions or concerns during this critical interaction. This confirms the benefits of using communication strategies that can establish a connection and maintain trust with prospective participants to increase recruitment [22] and accrual [10], [22], [23], it underscores the utility of approaching clinical recruiting similar to other persuasive communication encounters; in order to effectively recruit and enroll participants into clinical studies, recruiters must engage strategies that provide opportunities for framing study participation in terms of the participant's needs, rather than strictly providing (e.g., sending a letter) information about the study [23]. Future studies should evaluate participant's preferences for being recruited into clinical studies, specifically individuals who have enrolled in a registry, been contacted about research, and declined to participate. Not only do cohorts differ in their preferred methods for communication, engaging participant stakeholders could lead to the development of best practices for recruiting participants who were identified for research studies via registries.
C2B, the combination of low and high touch communication strategies, was also the most effective in terms of cost spent, per patient, on completed enrollment and was instrumental in exceeding the targeted accrual goal and in less than six months. Minimizing study costs without compromising study deadlines or targeted accrual goals is an optimal practice for clinical studies. Due to the high proportion of trials that fail to meet enrollment goals or complete enrollment on time [24], and experience an increase in costs [7], this finding is particularly noteworthy. It also suggests that strategically communicating with prospective participants, including those without an existing relationship with the study team (e.g., patients identified via a registry), benefits individual studies and contributes to national efforts aimed at increasing participation in clinical research.
As with any study, there are strengths and limitations. Strengths of this study include the innovative registry linked to a re-contact program (Consent2Share) to identify prospective patient cohorts and the emphasis on evaluating recruiting strategies to increase participation in clinical trials. The current study is limited by the non-randomized design and unequal proportion of patients recruited across conditions. Specifically, the low proportion of specialty clinic patients identified by the registry coupled with the absence of randomization and a control condition limits the generalizability of the findings.
5. Conclusion
This study provides evidence of an effective approach to reducing the barrier (i.e., cohort identification, recruiting, enrolling) of clinical trial recruitment. Using a registry linked to a consent to re-contact program to identify a prospective participant cohort who were interested in participating in clinical research was an important first step. However, effectively engaging participants and communicating with them via a combination of traditional, high and low touch strategies, was responsible for enrolling the highest proportion of participants into the study and at the lowest cost.
Funding
Research reported in this publication was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Award UL1TR001427. Publication of this article was funded in part by the University of Florida Open Access Publishing Fund.
Footnotes
The 605 patients in C2B were determined by subtracting the 19 patients who called to inquire about the study after receiving the letter about the study.
References
- 1.Wade T.D., Zelarney P.T., Hum R.C., McGee S., Batson D.H. Using patient lists to add value to integrated data repositories. J. Biomed. Inf. 2014;52:72–77. doi: 10.1016/j.jbi.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krieger J.L., Neil J.M. August 2016. Communication and Recruitment to Clinical Research Studies. [Google Scholar]
- 3.Nelson D.R., Conlon M., Baralt C., Johnson J.A., Clare-Salzler M.J., Rawley-Payne M. University of Florida clinical and translational science institute: transformation and translation in personalized medicine. Clin. Transl. Sci. 2011;4(6):400–402. doi: 10.1111/j.1752-8062.2011.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ResearchMatch. https://www.researchmatch.org/ (Accessed 2 February 2017).
- 5.Iafrate P., Lipori G.P., Harle C.A., Nelson D.R., Barnash T.J., Leebove P.T., Adams K.A., Montgomer D. Consent2Share: an integrated broad consenting process for re-contacting potential study subjects. J. Clin. Transl. Res. 2016;2(4) [PMC free article] [PubMed] [Google Scholar]
- 6.Tan M.H., Thomas M., MacEachern M.P. Using registries to recruit subjects for clinical trials. Contemp. Clin. Trials. 2015;41:31–38. doi: 10.1016/j.cct.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hapca A., Jennings C.G., Wei L., Wilson A., MacDonald T.M., Mackenzie I.S. Effectiveness of newspaper advertising for patient recruitment into a clinical trial. Br. J. Clin. Pharmacol. 2014;77(6):1064–1072. doi: 10.1111/bcp.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendrick D., Watson M., Dewey M., Woods A.J. Does sending a home safety questionnaire increase recruitment to an injury prevention trial? A randomised controlled trial. J. Epidemiol. Community Health. 2001;55(11):845–846. doi: 10.1136/jech.55.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valanis B., Blank J., Glass A. Mailing strategies and costs of recruiting heavy smokers in CARET, a large chemoprevention trial. Control Clin. Trials. 1998;19(1):25–38. doi: 10.1016/s0197-2456(97)00027-5. [DOI] [PubMed] [Google Scholar]
- 10.Morgan S.E., Occa A., Mouton A., Potter J. The role of nonverbal communication behaviors in clinical trial and research study recruitment. Health Commun. 2017;32(4):461–469. doi: 10.1080/10410236.2016.1140266. [DOI] [PubMed] [Google Scholar]
- 11.McDonald P.W. Population-based recruitment for quit-smoking programs: an analytic review of communication variables. Prev. Med. 1999;28(6):545–557. doi: 10.1006/pmed.1998.0479. [DOI] [PubMed] [Google Scholar]
- 12.Gul R.B., Ali P.A. Clinical trials: the challenge of recruitment and retention of participants. J. Clin. Nurs. 2010;19(1–2):227–233. doi: 10.1111/j.1365-2702.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 13.Nystuen P., Hagen K.B. Telephone reminders are effective in recruiting nonresponding patients to randomized controlled trials. J. Clin. Epidemiol. 2004;57(8):773–776. doi: 10.1016/j.jclinepi.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Harris T.J., Carey I.M., Victor C.R., Adams R., Cook D.G. Optimising recruitment into a study of physical activity in older people: a randomised controlled trial of different approaches. Age Ageing. 2008;37(6):659–665. doi: 10.1093/ageing/afn159. [DOI] [PubMed] [Google Scholar]
- 15.Swanson G.M., Ward A.J. Recruiting minorities into clinical trials: toward a participant-friendly system. J. Natl. Cancer Inst. 1995;87(23):1747–1759. doi: 10.1093/jnci/87.23.1747. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein D.L., Hülkova H., Bialer M.G., Desnick R.J. Cholesteryl ester storage disease: review of the findings in 135 reported patients with an underdiagnosed disease. J. Hepatol. 2013;58(6):1230–1243. doi: 10.1016/j.jhep.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Reiner Ž Guardamagna O., Nair D. Lysosomal acid lipase deficiency – an under-recognized cause of dyslipidaemia and liver dysfunction. Atherosclerosis. 2014;235(1):21–30. doi: 10.1016/j.atherosclerosis.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Hůlková H., Elleder M. Distinctive histopathological features that support a diagnosis of cholesterol ester storage disease in liver biopsy specimens. Histopathology. 2012;60(7):1107–1113. doi: 10.1111/j.1365-2559.2011.04164.x. [DOI] [PubMed] [Google Scholar]
- 19.Draugalis J.R., Coons S.J., Plaza C.M. Best practices for survey research reports: a synopsis for authors and reviewers. Am. J. Pharm. Educ. 2008;72(1) doi: 10.5688/aj720111. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2254236/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fincham J.E. Response rates and responsiveness for surveys, standards, and the journal. Am. J. Pharm. Educ. 2008;72(2) doi: 10.5688/aj720243. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2384218/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell R. Resources for the Future; Washington D.C: 1989. Using Surveys to Value Public Goods: the Contingent Valuation Method. [Google Scholar]
- 22.Morgan S.E., Occa A., Potter J., Mouton A., Peter M.E. “You need to Be a good listener”: recruiters' use of relational communication behaviors to enhance clinical trial and research study accrual. J. Health Commun. 2017;22(2):95–101. doi: 10.1080/10810730.2016.1256356. [DOI] [PubMed] [Google Scholar]
- 23.Albrecht T.L., Blanchard C., Ruckdeschel J.C., Coovert M., Strongbow R. Strategic physician communication and oncology clinical trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999;17(10):3324–3332. doi: 10.1200/JCO.1999.17.10.3324. [DOI] [PubMed] [Google Scholar]
- 24.Allison M. Can web 2.0 reboot clinical trials? Nat. Biotechnol. 2009;27(10):895–902. doi: 10.1038/nbt1009-895. [DOI] [PubMed] [Google Scholar]