Abstract
It has been recognized that cancer-associated mortality is more of a result of the disrupted physiological functions in multiple organs following metastatic dissemination of cancer cells, rather than the presence and growth of the primary tumor. Despite advances in our understanding of the events leading to cancer initiation, growth, and acquisition of invasive properties, we are still unable to effectively treat metastatic disease. It is now being accepted that the secretion of extracellular vesicles, such as exosomes from cancer cells, has a profound impact on the initiation and propagation of metastatic breast cancer. These cancer-secreted vesicles differ from other means of cellular communication due to their capability of bulk delivery and organotropism. Here we provide an overview of the role of extracellular vesicles in breast cancer metastasis and discuss key areas that may facilitate our understanding of metastatic breast cancer to guide our efforts towards providing better therapies.
Keywords: Breast cancer, Extracellular vesicles, Exosomes, Premetastatic niche, Metastasis
Exosomes and extracellular vesicles: a means of delivering cellular messages in bulk
Breast cancer patients typically die as a result of complications caused by the spread of cells from the primary tumor to distant parts of the body in a process termed metastasis. There are no effective means to treat metastatic breast cancer highlighting the need for further research to understand how and why breast cancer metastasizes to certain organs. Increasing evidence has indicated that extracellular vesicles (EVs), through their DNA, RNA, and protein contents, play an intimate role in preparing for and inducing metastasis. EVs are a diverse population of secreted vesicles that include exosomes, microvesicles, and apoptotic bodies. Most of the effects of EVs have been attributed to exosomes, although increasing evidence indicates that the larger microvesicles also play an important role in cellular communication. Exosomes are characterized by their small size (30–100 nm) and endocytic origin. Exosomes are traditionally isolated by differential ultracentrifugation with a final isolation speed of over 100,000 ×g, however these preparations often contain other larger vesicles such as microvesicles. Further purification can be done by using methods such as density gradients to separate exosomes from larger vesicles. Microvesicles are much larger vesicles (>200 nm) secreted by cells through outward budding of the plasma membrane. These larger vesicles are typically pelleted by a 10,000–20,000 ×g centrifugation spin, although this pellet can also contain exosomes that have bound to other pelleted materials. EV-mediated communication is of particular interest in cancer as cancer cells secrete notably more EVs than normal cells, resulting in a substantial increase in detectable EVs circulating in the blood. Furthermore these EVs contain distinct cargo from their non-cancerous counterparts, allowing them to alter both neighboring and distant cells to promote metastasis.
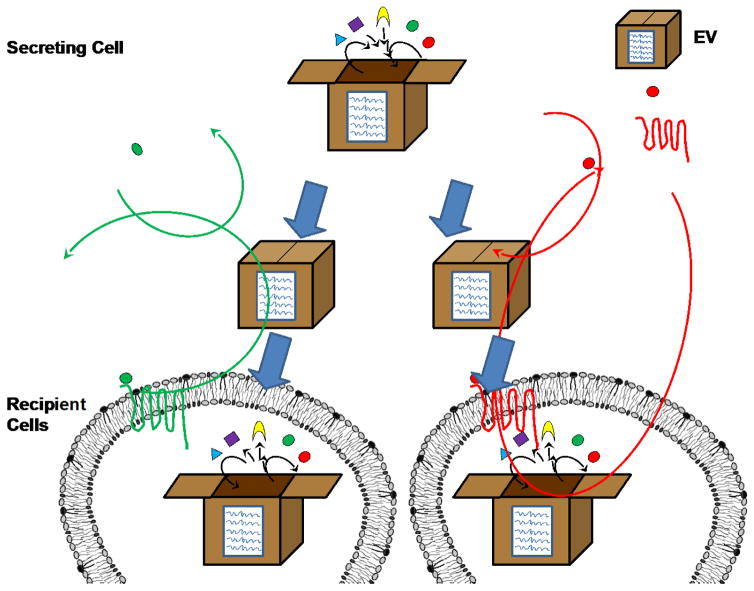
One key underappreciated advantage of EVs over traditional secretion is their ability to transfer a suite of signaling molecules to influence multiple pathways that are all controlled through mechanisms involving uptake of the EV itself (Fig. 1), which have been described in detail elsewhere (Villarroya-Beltri, Baixauli et al. 2014). This is similar to how it is more efficient to ship cargo in bulk rather than to transfer each item individually. As such, one set of instructions mediates the delivery of all the cargo, establishing a multi-pathway control above each individual cargo signaling pathway. This is even more important in vivo as there are many cells expressing the same receptors, so getting different molecules into the same cell at a given ratio can be challenging if they are not being secreted at high concentrations. These EVs can have distinct organotropisms, just as packages have shipping labels, allowing for specific communication with distant niche sites (Hoshino, Costa-Silva et al. 2015). Aside from destination control, EV-mediated communication allows for uncoupling and redesignation of the function-destination relationship of traditional secreted factors. When cytokines are secreted, they bind to cells that express their corresponding receptors to initiate cellular functions. Thus the cytokines may only influence cells that are programmed to receive that specific signal. In contrast, many of the effects of EVs rely on their internal contents, which are separated from EV delivery that is mediated by factors on the EV exterior. In other words, cytokine-mediated communication requires knowledge of signal being sent, whereas EV-mediated communication only requires knowledge of parcel uptake and does not rely on knowledge of the cargo. Here we summarize the involvement of EVs in breast cancer metastasis.
Fig. 1.
EV bulk packaging. In traditional cytokine-mediated cell signaling the cell secretes a cytokine that can only activate downstream signaling in cells expressing its corresponding receptor, thus linking signal reception to function. In contrast, EV-mediated signaling does not have a means of content screening, allowing for downstream signaling to occur as long as the cell can take up EVs and has the appropriate internal machinery necessary for signal transduction. Furthermore, EVs allow the delivery of multiple signaling molecules into the same cell, allowing for simultaneous activation of multiple pathways.
Delivery to thy neighbor: communication within the primary tumor
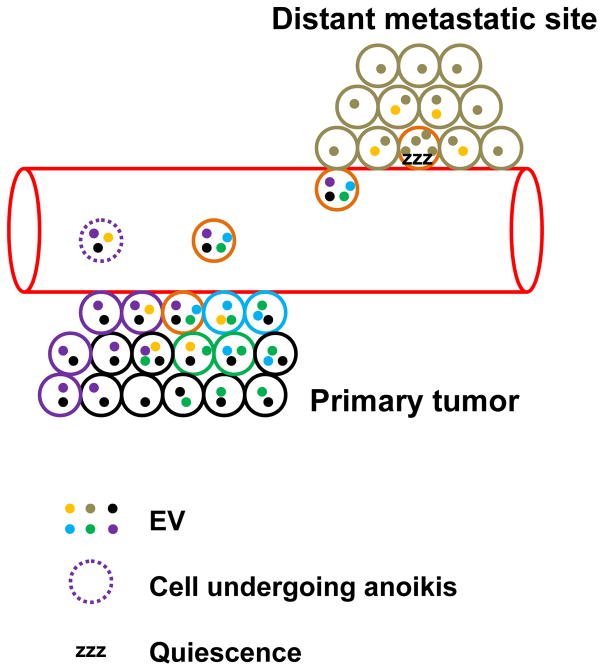
Solid tumors are remarkably heterogeneous resulting in the secretion of tumor EVs with diverse effects. Alarmingly these tumor-secreted EVs have been demonstrated to transfer partial phenotypic identity to other cancer cells, altering the recipient cell’s migration, invasion, stress-sensitivity (McCready, Sims et al. 2010; O’Brien, Rani et al. 2013; Melo, Sugimoto et al. 2014; Singh, Pochampally et al. 2014; Harris, Patel et al. 2015), and tissue-tropism (Hoshino, Costa-Silva et al. 2015) towards that of the secreting cell, granting the tumor entity a greater overall malignancy than the sum of its parts. EVs secreted by a highly invasive variant of the Hs578T triple negative breast cancer cells increase the proliferation, migration, invasion, and sensitivity to anoikis of other cancer cells in comparison to EVs isolated from the less invasive parental Hs578T (O’Brien, Rani et al. 2013), however the mechanism behind these alterations is still unknown. Recent studies have reported similar results in which EVs from MCF7, MCF7 overexpressing Rab27b, and MDA-MB-231 cells increase the migration of target breast cancer cells proportionate to the metastatic potential of the producer cells (Harris, Patel et al. 2015). The enhanced migration induced by metastatic cell-derived EVs can be mediated by EV-associated Hsp90α which promotes cancer cell migration through tissue plasminogen activator-mediated activation of the extracellular protease plasmin (McCready, Sims et al. 2010). Likewise EV-associated miR-10b increases invasion of other cancer cells possibly though targeting Hoxd10 and Klf4 (Singh, Pochampally et al. 2014). EV-associated miR-200 family members are secreted by epithelial breast cancer cell lines and enhance metastasis, potentially by inducing a mesenchymal-to-epithelial transition in metastatic cells (Le, Hamar et al. 2014). Together these findings demonstrate that a metastatic subpopulation of cancer cells can enhance the metastatic potential of the tumor as a whole. This phenotypic transfer of metastatic traits to other cancer cells may contribute to explaining why circulating tumor cells (CTCs) can be found very early during tumor progression (Fig. 2). It is possible that CTCs may originate from cells in the primary tumor that are surrounded by cells that have some but not all of the traits necessary for intravasation and survival in circulation. These traits may be transferred to these central cells giving them all the traits necessary to become CTCs, however since these traits are endowed by EVs rather than innately presented, these cells do not have the capacity to form metastases without continued EV support from their original neighbors. Future studies focusing on the EV-mediated, stepwise acquisition of metastatic traits by primary tumor cells are needed to test this model. Nevertheless, it is well supported that different conditions/stresses within the tumor influence EV secretion resulting in numerous subpopulations of EVs secreted within the tumor (as discussed later). Given this, there is also likely remarkable heterogeneity within the tumor-derived EVs secreted into the blood stream. Several new technologies have been developed that allow for the detection and assessment of individual EVs (Smith, Lee et al. 2015; Su 2015). These technologies will likely reveal that EV-mediated communication is much more complex than we initially thought. It has long been shown that polarized epithelial cells secrete different populations of EVs (Tauro, Greening et al. 2013), but now we are beginning to understand that cancer cells also secrete multiple populations of EVs as well (Smith, Lee et al. 2015; Willms, Johansson et al. 2016).
Fig. 2.
EV secretion may lead to the early acquisition of CTCs. Early CTCs (orange) may receive EVs from neighboring tumor cells that give it different traits necessary to intravasate and survive in the circulation. Transferred traits may include: enhanced invasion through the ECM (purple), resistance to anoikis (blue), protection from immune cells (may be mediated by recruitment of platelets, green), adhesion at the premetastatic niche (black). Once these cells arrive at a premetastatic site they may enter quiescence due to exhaustion of EV signals from the primary tumor, as well as EV-dependent and -independent metastasis suppression mechanisms by stromal cells (brown) in the premetastatic niche.
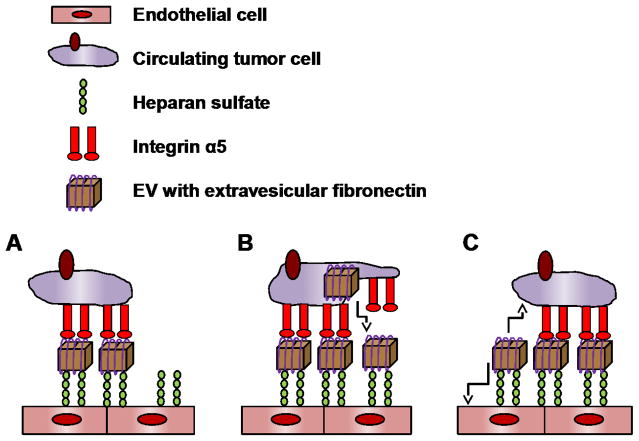
Several in vitro studies indicate that EVs may also act as both a guide post and stepping stone for migrating cells. When cells detach from the extracellular matrix (ECM) they release a burst of EVs, which help them adhere to plastic and ECM substrates such as fibronectin and laminin (Koumangoye, Sakwe et al. 2011). Other labs have shown that these EVs promote adhesion and enhance directional trafficking of the secreting cell (Sung, Ketova et al. 2015). Furthermore migrating cells can secrete EVs from their leading edge (Sung, Ketova et al. 2015) through invadopodia to facilitate ECM degradation during cellular invasion (Hoshino, Kirkbride et al. 2013). These EVs have an outer coating of fibronectin which is bound to the EVs through integrin α5. This fibronectin coat facilitates the interaction between the EVs and target cells through heparan sulfate (Purushothaman, Bandari et al. 2016). These fibronectin-containing EVs may anchor themselves to both the cancer cell and the endothelial wall, allowing for the cancer cell to undergo directional migration on the inner surface of blood vessels (Fig. 3).
Fig. 3.
Proposed role of EVs as stepping stones during migration. Cancer cells may rely on EVs to adhere to the endothelial cell walls during migration on the inner surface of blood vessels. This adhesion may be mediated by the binding of EVs containing extravesicular fibronectin to the endothelial cells through heparan sulfate and to the cancer cells through interactions with integrin α5 (A). As the cancer cell migrates it induces the secretion of EVs from the leading edge to facilitate adhesion (B). After advancing EVs from the lagging edge may be absorbed by either the cancer cell or the endothelial cell (C).
Cancer cells are also able to reprogram stromal cells to secrete tumor promoting EVs. MCF7 and MDA-MB-231 cells secrete EVs that induce the differentiation of adipose-derived mesenchymal stem cells into myofibroblasts, increasing their secretion of factors such as SDF-1, VEGF, CCL5, and TGFβ which regulate tumor growth, angiogenesis, and metastasis (Cho, Park et al. 2012). Cancer-associated fibroblasts also secrete CD81+ EVs which increase the motility of the primary tumor resulting in increased metastasis through inducing autocrine planar cell polarity signaling (Luga, Zhang et al. 2012). Breast cancer EVs can also induce autophagy in breast epithelial cells through induction of reactive oxygen species, causing these cells to acquire the senescence-associated secretory phenotype which supports tumor growth (Dutta, Warshall et al. 2014). EVs secreted by IL4-activated macrophages transfer miR-223 into breast cancer cells to increase their invasive potential (Yang, Chen et al. 2011). Thus EVs secreted by breast cancer cells reprogram both cancer cells and stromal cells in the primary tumor to enhance metastatic growth.
Message in a bottle: preparation of a premetastatic niche
Metastasis not only requires the acquisition of invasive traits by cells within the primary tumor, but also relies upon the generation of a permissive microenvironment at distant metastatic sites termed the premetastatic niche. EVs secreted by cancer cells contain the same organotropism as the cells they originate from (Hoshino, Costa-Silva et al. 2015). Mice pre-treated with lung-tropic EVs can shift the metastatic preference of brain-tropic breast cancer cells to the lungs, suggesting that EVs may be the initiators in organ-specific premetastatic niche formation. EV homing to the lungs and liver has been shown to be mediated by ITGβ4 and ITGαV respectably. However, metastasis is not limited to these two organs; EVs from breast cancer cells metastasizing to the brain do not show a unique integrin profile, highlighting the importance of studying other EV surface proteins. Other recent studies have indicated that tissue inhibitor of metalloproteinases-1 (TIMP-1) specifically confers liver homing to cell lines that don’t normally home to the liver, without affecting the metastatic capabilities of the cells (Seubert, Grunwald et al. 2015). Interestingly TIMP-1 can also be found within in EVs (Skog, Wurdinger et al. 2008), however whether it is located internally or externally on EVs has not been determined. If TIMP-1 is located on the exterior of the EVs then it may mediate EV homing by co-localizing with ITGβ1 and maintaining it in an active form with the help of CD63 (Jung, Liu et al. 2006).
Once EVs arrive at the pre-metastatic niche they reprogram niche cells to support future metastatic growth and entry. EV-associated miR-105 is secreted from cancer cells to disrupt tight junctions in the premetastatic niche, resulting in enhanced vascular permeability to facilitate cancer cell extravasation (Zhou, Fong et al. 2014). Similarly EV miR-181c can disrupt the blood brain barrier to facilitate entry into the brain (Tominaga, Kosaka et al. 2015). Once cancer cells arrive at the premetastatic niche they must compete with other niche cells for the nutrients they need to establish a metastatic colony. Cancer cells secrete EV-encapsulated miR-122 to inhibit the glucose uptake of premetastatic niche cells, leaving more glucose available for metastatic cancer cells (Fong, Zhou et al. 2015). EV-mediated communication is a bi-directional process as EVs released by niche cells are also able to influence cancer cells. Astrocytes in the metastatic brain niche secrete EV-encapsulated miR-19a which decreases PTEN expression in breast cancer cells metastasized to the brain resulting in increased survival of the metastatic cancer cells (Zhang, Zhang et al. 2015).
Corrupting the policemen: immune modulation
Cancer-secreted EVs can induce the transformation of recruited immune cells towards a tumor promoting phenotype. Breast cancer EV-encapsulated prostaglandin E2 and TGF-β induce the differentiation of bone marrow derived cells towards tumor-promoting Gr-1+CD11b+ MDSCs resulting in the accumulation of MDSCs in the tumor and lungs to promote tumor growth and possibly metastases (Xiang, Poliakov et al. 2009). Protein palmitoylation on the surface of breast cancer EVs induces the secretion of inflammatory cytokines such as IL6 in macrophages through Toll-like receptor 2 to promote metastasis (Chow, Zhou et al. 2014). In turn, EVs secreted by IL4-activated macrophages transfer miR-223 into breast cancer cells to increase their invasive potential (Yang, Chen et al. 2011).
Breast cancer-secreted EVs can also inhibit the tumor suppressive functions of immune cells. IL6 in EVs from breast cancer cells shifts the differentiation of myeloid progenitors towards tumor-promoting macrophages, away from dendritic cells (Yu, Liu et al. 2007). The remaining dendritic cells after treatment with breast cancer EVs are defective in their capacity to induce T cell proliferation, hindering the tumoricidal immune response. Similarly, CD39 and CD73 on cancer EVs can catalyze the generation of adenosine from ATP and AMP to decrease tumoricidal CD3+ T cell activity (Clayton, Al-Taei et al. 2011). Cancer EVs also decrease the cytotoxicity of DX5+ NK cells and inhibit NK cell proliferation through inhibition of Jak3 resulting in increased tumor growth (Liu, Yu et al. 2006). Therefore cancer-secreted EVs educate immune cells to aid in the generation of a tumorigenic microenvironment.
Angiogenesis and metabolism
In order to metastasize, tumor cells must disrupt the endothelial cell barrier at both the primary tumor and distant metastatic sites. Hypoxia potently induces angiogenesis through several mechanisms including EV secretion. Neutral sphingomyelinase 2 (nSMase2), which mediates miRNA secretion in EVs (Kosaka, Iguchi et al. 2013), can be induced by hypoxia (Cogolludo, Moreno et al. 2009) leading to the secretion of angiogenic miRNAs such as miR-210 to facilitate tumor angiogenesis and metastasis (King, Michael et al. 2012; Kosaka, Iguchi et al. 2013). Since hypoxia is also involved in the acquisition of invasive traits by cancer cells (Wilson and Hay 2011), this may explain why more invasive cancer cells produce more potent angiogenic EVs (O’Brien, Rani et al. 2013). Perhaps the greatest advantage of EV-mediated communication over traditional cytokine communication is the ability of EVs to specifically communicate to distant metastatic sites. Metastatic cancer cells secrete EV-associated miR-105 which targets zonula occludens 1 in endothelial cells at metastatic sites such as the lungs and the brain to disrupt tight junctions and increase vascular permeability resulting in increased metastasis (Zhou, Fong et al. 2014). Furthermore serum levels of miR-105 serve as a predictive marker for breast cancer metastasis. Breast cancer EV-encapsulated miR-181c alters the localization of actin and N-cadherin through targeting 3-phosphoinositide-dependent protein kinase-1 to disrupt the blood brain barrier resulting in increased brain metastases (Tominaga, Kosaka et al. 2015). Breast cancer patients with brain metastases had increased levels of miR-181c in their serum, but it remains to be seen whether miR-181c can be used as a prognostic indicator in early-stage patients before brain metastases occur.
Once metastatic cells successfully extravasate into a distant metastatic site they need to compete with the resident niche cells for the nutrients they need to survive and proliferate. Cancer cells can shift the balance in their favor through the secretion of EV-associated miR-122 which targets niche cells such as lung fibroblasts and brain astrocytes resulting in decreased glucose consumption through targeting pyruvate kinase (Fong, Zhou et al. 2015). As a result more glucose is available for cancer cells allowing for increased proliferation and metastasis. EVs secreted by activated stromal cells can also alter cancer cell metabolism. Prostate cancer-associated fibroblasts (CAFs) secrete EVs containing miR-22, let-7a and miR-125b that target the oxidative phosphorylation pathway resulting in decreased basal oxidative phosphorylation and increased prostate cancer glycolysis and glutamine entry into the TCA cycle (Zhao, Yang et al. 2016). Furthermore these CAF EVs can feed cancer cells with TCA metabolites and amino acids to facilitate their growth. Whether this can also occur in metastatic sites in breast cancer remains to be seen.
Parcel delivery: cancer EV secretion
For a more comprehensive overview of the mechanisms involved in EV secretion and packaging we refer you to a broader review on this topic (Villarroya-Beltri, Baixauli et al. 2014). Here we will discuss mechanisms by which EV secretion can be altered in breast cancer. Cancer cells secrete considerably more EVs than healthy epithelial cells, due to cellular stresses and overexpression of secretion related genes. Cellular stresses such as hypoxia induce alterations in EV secretion and loading. Stabilization of HIF-1α under hypoxia increases EV secretion without altering vesicle size and specifically increases the loading of a set of miRNAs into EVs including miR-210 (King, Michael et al. 2012). This induction of EV secretion may be mediated by increased expression of nSMase2 and Rab22a under hypoxia (Kosaka, Iguchi et al. 2013; Wang, Gilkes et al. 2014). Tumors are more acidic than normal tissue. Cancer cells secrete and take up more EVs at pH 6 than at pH 7.4, which may be due to the enhanced membrane rigidity of acidic EVs due to the increased incorporation of the exosomal lipids sphingomyelin and GM3 (Parolini, Federici et al. 2009). Furthermore acidic EVs also had increased Caveolin-1 and Lamp-2 indicating that acidity can alter exosomal loading. Increased EV secretion in acidic conditions has also been shown in HEK293 cells hinting that this is not a cell line-specific effect (Ban, Lee et al. 2015). Together these studies indicate that different cellular stresses within the tumor induce alterations in both the number and contents of EVs secreted by cancer cells, thus different areas of the tumor likely have remarkably different EV secretion.
Aside from cellular stresses the increased EV secretion by cancer cells may also be explained by overexpression of EV secretion related genes. Both Rab27a and Rab27b are implicated in EV secretion and control different pathways with different downstream effectors (Ostrowski, Carmo et al. 2010). Rab27a is highly expressed in several breast cancer cell lines and knockdown of Rab27a but not Rab27b reduced EV secretion from 4T1 and TS/A cells (Bobrie, Krumeich et al. 2012). Furthermore Rab27a knockdown decreases lung metastases and mobilization of neutrophils to the tumor and spleen in the 4T1 tumor model, however this effect may be mediated in part by non-EV Rab27a signaling. Rab27b induces the loading of Vacuolar H1 ATPase (V-ATPase) into EVs (Hendrix, Sormunen et al. 2013). Inhibiting V-ATPase alters the localization of Rab27b vesicles and decreases breast cancer proliferation and invasion. However whether the effects of V-ATPase inhibition are due to inhibition of vesicular release or to non-vesicular effects is not clear. Rab27b is increased in some breast cancers and inversely correlated with patient survival (Zhang, Huang et al. 2012). It is also correlated with the expression of mesenchymal markers and inversely correlated with epithelial markers in the primary tumor, suggesting its involvement in epithelial–mesenchymal transition (EMT). Furthermore cells that have undergone EMT have increased secretion of EVs as well as increased tumorigenic exosomal contents (Garnier, Magnus et al. 2012; Tauro, Mathias et al. 2013; Gopal, Greening et al. 2016). nSMase2 is overexpressed in some breast cancers where it controls the secretion of EV-encapsulated small RNAs but not proteins; inhibition of nSMase2 decreases lung metastases in the 4T1 tumor model (Kosaka, Iguchi et al. 2013). Overexpression of nSMase2 in 4T1 cells does not alter cell proliferation, migration, or invasion in vitro, nor does it alter primary tumor growth in vivo, indicating that nSMase2 primarily regulates EV-mediated communication with distant metastatic niches rather than communication within the primary tumor. Heparanase is increased in many cancers and enhances EV secretion as well as EV loading of syndecan-1, VEGF, and HGF to increase cell spreading and trans-endothelial migration (Thompson, Purushothaman et al. 2013). Interestingly recent studies have indicated that heparanase can inhibit the interaction between EVs and target cells due to disruption of heparan sulfate on target cells with EV-associated fibronectin (Purushothaman, Bandari et al. 2016). Thus the increased expression of heparanase in cancer cells may serve to prevent cancer EVs from signaling back to the producer cells. Together these studies indicate that there are multiple pathways which regulate EV secretion, and different cancer models have variable reliance on each pathway.
Clinical implications
Due to the role of cancer-secreted EVs in enhancing metastasis through interactions with cells in the primary tumor and distant metastatic niches, the prevention of cancer EV secretion may contain the tumor in the primary site to reduce metastasis-related mortality. Several groups have demonstrated that tumors formed from cancer cells with EV secretion defects such as RAB27a, RAB27b, and nSMase2 knockdown have greatly hindered metastatic capabilities (Hendrix, Maynard et al. 2010; Bobrie, Krumeich et al. 2012; Peinado, Aleckovic et al. 2012; Kosaka, Iguchi et al. 2013). Furthermore intracranial injection of RAB27a and RAB27b shRNAs decreases brain metastases (Zhang, Zhang et al. 2015), indicating that blocking EV secretion at metastatic sites can inhibit metastatic growth. However it is unclear whether inhibiting EV-mediated secretion may reduce metastatic growth in patients who have already developed metastatic disease. Furthermore, general inhibition of EV secretion may have unexpected consequences. EV secretion does not solely occur during disease states and the function of epithelial EVs in healthy individuals is largely unstudied. Mammary-derived EVs secreted into the milk are known to have anti-microbial functions that support the nursing baby’s developing immune system (Admyre, Johansson et al. 2007) and non-cancerous breast epithelial cells also secrete EVs into the circulation. The presence of occult quiescent tumors in several organs is surprisingly common in individuals as young as 20, however the majority of these occult tumors are kept in check by the microenvironment (Bissell and Hines 2011). Little work has been done to characterize the potential effect of EVs from non-transformed niche cells on metastatic cancer cells, however there is evidence that EVs secreted by these cells may play a role in suppressing these occult tumors (Lim, Bliss et al. 2011). Bone marrow stromal cells are able to decrease the proliferation and induce quiescence in T47D and MDA-MB-231 breast cancer cells which is partly mediated through EV communication. Although in this study the effects of EV-containing media are considerably weaker than direct co-culture, the continual secretion of EVs should be taken into account. Cells are constantly secreting and taking up EVs from the media and the collection of EVs from a single time point is just a snap shot of its current equilibrium. Thus the EVs contained in media collected after 48 hrs is much lower than the total amount of EVs that were secreted by the cells. Given this it is likely that the role of EVs in inducing quiescence has been underestimated. In support of this, EVs isolated from non-transformed primary bone marrow mesenchymal stem cells induce quiescence in bone-metastatic breast cancer cells (Ono, Kosaka et al. 2014). Whether other non-transformed stromal cell-derived EVs also have this effect remains to be seen. This may be the result of phenotypic transference as non-transformed stromal cells as a whole are not very proliferative in the absence of stimulatory factors. Therefore further work needs to be done to determine the potential tumor-suppressing role of non-transformed stromal EVs. If this is the case, general inhibition of EVs may lead to the activation of occult tumor cells, inducing metastatic disease. On the other hand, it is critical to have a comprehensive understanding of cancer-specific molecules/pathways that control EV production, in order to specifically target cancer secretion of EVs as a more feasible therapeutic approach.
Recently developed technologies that allow for the detection and assessment of individual EVs (Smith, Lee et al. 2015; Su 2015) will facilitate further characterization of the complexity and heterogeneity in EV secretion and action. Much work remains to be done to characterize and identify the functional significance of different EV subpopulations, as well as identify which subpopulations have potential as therapeutic targets or diagnostic biomarkers. Thus the EVs isolated from biofluids represent both cellular heterogeneity of the cells of origin, as well as EVs heterogeneity secreted by those cells. This double layer of heterogeneity will likely hamper the efficacy of traditional bulk exosomal analyses from biofluids, highlighting the need for further development and use of single EV analyses.
Conclusions/key unanswered questions
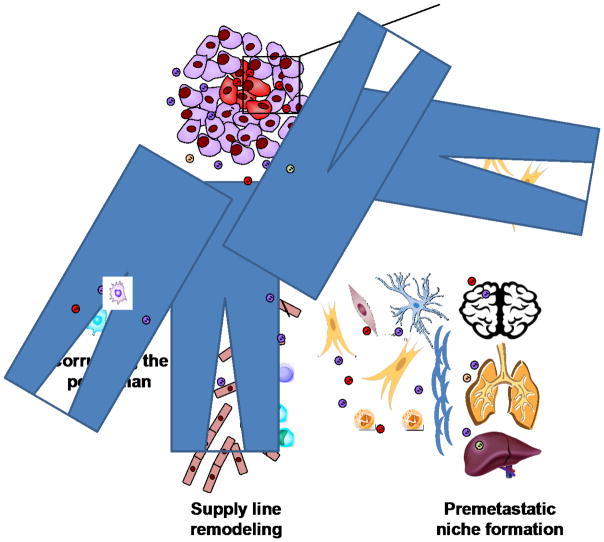
Cancer secreted EVs facilitate the generation of a pro-tumorigenic microenvironment in both the primary tumor and the pre-metastatic niche through the reprogramming of tumor cells, stromal cells, and immune cells (Fig. 4). Cellular stresses such as metabolic stress and hypoxia enhance EV secretion and may be responsible for the increased abundance of EVs isolated from cancer patients. Although this seems to suggest that inhibition of EV secretion would inhibit metastatic growth, further work needs to be done to determine the potential role of non-transformed stromal EVs in inhibiting tumor growth as well as cancer-specific control of EV secretion. One of the biggest differences between EV-mediated communication and cytokine secretion is the capability of bulk delivery, however most EV research has focused on the effects of single molecules contained within EVs, highlighting the need to expand our views and inspect how these cancer-secreted EVs may be regulating multiple pathways through simultaneous delivery of EV cargo. Lastly due to the heterogeneity of EVs, further work needs to be done to characterize and analyze subpopulations of exosomes/microvesicles that may be responsible for the effects that may have been misattributed to the bulk EV population.
Fig. 4.
Intra- and extra-tumoral functions of EVs. Exchange of EVs within the tumor induces phenotypic transfer, spreading metastatic traits to neighboring cells. This allows small subpopulations of cells within the tumor to exert a much larger effect. EVs secreted by tumor cells can also travel to and influence other cell types including endothelial cells, immune cells, and fibroblasts. EVs can be organotropic, allowing them to form a premetastatic niche in specific organs to facilitate cancer metastatic spread. Together these effects can create a permissive environment for cancer metastasis.
Acknowledgments
We apologize to the authors of those excellent studies we are not able to include in this review due to the space limit. This work was supported by the National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R01CA166020 (SEW) and R01CA163586 (SEW), California Breast Cancer Research Program grant 20IB-0118 (SEW), and Breast Cancer Research Foundation-AACR grant 12-60-26-WANG (SEW).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- Admyre C, Johansson SM, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179(3):1969–1978. doi: 10.4049/jimmunol.179.3.1969. [DOI] [PubMed] [Google Scholar]
- Ban JJ, Lee M, et al. Low pH increases the yield of exosome isolation. Biochem Biophys Res Commun. 2015;461(1):76–79. doi: 10.1016/j.bbrc.2015.03.172. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17(3):320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrie A, Krumeich S, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72(19):4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- Cho JA, Park H, et al. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40(1):130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- Chow A, Zhou W, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci Rep. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Al-Taei S, et al. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187(2):676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- Cogolludo A, Moreno L, et al. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res. 2009;82(2):296–302. doi: 10.1093/cvr/cvn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S, Warshall C, et al. Interactions between exosomes from breast cancer cells and primary mammary epithelial cells leads to generation of reactive oxygen species which induce DNA damage response, stabilization of p53 and autophagy in epithelial cells. PLoS One. 2014;9(5):e97580. doi: 10.1371/journal.pone.0097580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong MY, Zhou W, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier D, Magnus N, et al. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287(52):43565–43572. doi: 10.1074/jbc.M112.401760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal SK, Greening DW, et al. Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells. Oncotarget. 2016 doi: 10.18632/oncotarget.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DA, Patel SH, et al. Exosomes released from breast cancer carcinomas stimulate cell movement. PLoS One. 2015;10(3):e0117495. doi: 10.1371/journal.pone.0117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix A, Maynard D, et al. Effect of the secretory small GTPase Rab27B on breast cancer growth, invasion, and metastasis. J Natl Cancer Inst. 2010;102(12):866–880. doi: 10.1093/jnci/djq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix A, Sormunen R, et al. Vacuolar H+ ATPase expression and activity is required for Rab27B-dependent invasive growth and metastasis of breast cancer. Int J Cancer. 2013;133(4):843–854. doi: 10.1002/ijc.28079. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino D, Kirkbride KC, et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5(5):1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KK, Liu XW, et al. Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein. EMBO J. 2006;25(17):3934–3942. doi: 10.1038/sj.emboj.7601281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HW, Michael MZ, et al. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, et al. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J Biol Chem. 2013;288(15):10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koumangoye RB, Sakwe AM, et al. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS One. 2011;6(9):e24234. doi: 10.1371/journal.pone.0024234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le MT, Hamar P, et al. miR-200-containing extracellular vesicles promote breast cancer cell metastasis. J Clin Invest. 2014;124(12):5109–5128. doi: 10.1172/JCI75695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PK, Bliss SA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71(5):1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu S, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375–1385. doi: 10.4049/jimmunol.176.3.1375. [DOI] [PubMed] [Google Scholar]
- Luga V, Zhang L, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- McCready J, Sims JD, et al. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: a role for plasminogen activation. BMC Cancer. 2010;10:294. doi: 10.1186/1471-2407-10-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo SA, Sugimoto H, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien K, Rani S, et al. Exosomes from triple-negative breast cancer cells can transfer phenotypic traits representing their cells of origin to secondary cells. Eur J Cancer. 2013;49(8):1845–1859. doi: 10.1016/j.ejca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- Ono M, Kosaka N, et al. Exosomes from bone marrow mesenchymal stem cells contain a microRNA that promotes dormancy in metastatic breast cancer cells. Sci Signal. 2014;7(332):ra63. doi: 10.1126/scisignal.2005231. [DOI] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. 11–13. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- Parolini I, Federici C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purushothaman A, Bandari SK, et al. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J Biol Chem. 2016;291(4):1652–1663. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert B, Grunwald B, et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015;61(1):238–248. doi: 10.1002/hep.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Pochampally R, et al. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ZJ, Lee C, et al. Single exosome study reveals subpopulations distributed among cell lines with variability related to membrane content. J Extracell Vesicles. 2015;4:28533. doi: 10.3402/jev.v4.28533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. Label-free Single Molecule Detection Using Microtoroid Optical Resonators. J Vis Exp. 2015;(106):e53180. doi: 10.3791/53180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, Ketova T, et al. Directional cell movement through tissues is controlled by exosome secretion. Nat Commun. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12(3):587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauro BJ, Mathias RA, et al. Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition. Mol Cell Proteomics. 2013;12(8):2148–2159. doi: 10.1074/mcp.M112.027086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CA, Purushothaman A, et al. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288(14):10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga N, Kosaka N, et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat Commun. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C, Baixauli F, et al. Sorting it out: regulation of exosome loading. Semin Cancer Biol. 2014;28:3–13. doi: 10.1016/j.semcancer.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Gilkes DM, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111(31):E3234–3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms E, Johansson HJ, et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 2016;6:22519. doi: 10.1038/srep22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11(6):393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- Xiang X, Poliakov A, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Chen J, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Liu C, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- Zhang JX, Huang XX, et al. Overexpression of the secretory small GTPase Rab27B in human breast cancer correlates closely with lymph node metastasis and predicts poor prognosis. J Transl Med. 2012;10:242. doi: 10.1186/1479-5876-10-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang S, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang L, et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5 doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Fong MY, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]