Abstract
Prevention of amyloidogenic processing of amyloid precursor protein with the use of natural phytochemicals capable of enhancing alpha-secretase activity may be a therapeutic approach for treatment of neurodegenerative diseases including Alzheimer’s Disease (AD) and HIV-associated dementia (HAD). We have recently shown promising preclinical results with the use of green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) in mouse models of both diseases, however the translation into clinical use has been problematic primarily as a result of poor bioavailability and inefficient delivery to the central nervous system (CNS). While the antioxidant properties of EGCG are well known, we have shown that it is able to promote non-amyloidogenic processing of amyloid precursor protein (APP) by upregulating α-secretase, thus preventing brain beta amyloid plaque formation, a hallmark of AD pathology and common finding in HIV infection. In this preliminary study, we investigated the ability of one preformulation method to improve the oral bioavailability of EGCG. We found that forming nanolipidic EGCG particles improves the neuronal (SweAPP N2a cells) α-secretase enhancing ability in vitro by up to 91% (P<.001) and it’s oral bioavailability in vivo by more than two-fold over free EGCG.
Keywords: Nanoparticle, EGCG, Bioavailability, Alzheimer's Disease, Pharmacokinetics
1. Introduction
The deterioration, malfunction, or death of neurons is a common etiological factor in several diseases including Alzheimer’s Disease (AD) and HIV-associated dementia (HAD) [1, 2]. As the number of elderly individuals continues to rapidly increase, neurodegenerative disease, marked by progressive loss of mnemonic and higher cortical functions, has led to a massive socioeconomic burden which is projected to worsen [3]. Specifically, some 15% of the population greater than 65 years of age suffers from dementia [4]. Its presentation is heterogeneous as it is caused by multiple disorders. Alzheimer’s disease (AD) and vascular dementia (VaD) are the two main causes of dementia affecting between 25–45% and 15–35%, respectively, of all patients suffering from dementia [5]. Among dementias where brain infectious viruses are etiologic, human immunodeficiency virus type 1 (HIV-1) associated dementia (HAD) is the most common cause of dementia [6]. We have previously shown that modulation of apoptosis cascades [7, 8], and APP (amyloid precursor protein) processing [9–11] with the green tea polyphenol, (-)-epigallocatechin-3-gallate (EGCG) is a plausible therapy in mouse models of AD and HAD. In spite of these preclinical works, translating them to a human clinical trial has presented problems, primarily as a result of inefficient systemic delivery and bioavailability issues. To achieve maximum response of a neuroprotective agent, novel strategies are required to enhance the oral bioavailability of potentially useful agents. Self-assembled polymer micelles based on amphiphilic block copolymers have attracted substantial interest as delivery vehicles particularly for anti-cancer drugs [12–18]. Additionally, lipid carriers with incorporated drugs have been demonstrated to increase the absorption and circulation time in the body versus standalone compounds secondary to minimized renal clearance [17, 19]. This study investigated the ability of nanolipidic particle complexes for increasing the oral bioavailability of EGCG. These nanoparticles (NanoEGCG) differ from traditional liposomes because they do not require micelle formation. Rather, they are drug:lipid complexes. This enables the formation of smaller diameter particles that we hypothesized would be useful for increasing the oral bioavailability of EGCG.
2. Materials and Methods
Reagents and Materials
Green tea-derived EGCG (> 95% purity by HPLC) was purchased from www.herbs-tech.com. The BCA protein assay kit was purchased from Pierce Biotechnology (Rockford, IL, USA). Anti-human amyloid-β antibodies 4G8 and 6E10 were obtained from Signet Laboratories (Dedham, MA, USA) and Biosource International (Camarillo, CA, USA), respectively.
Preparation of Nanolipidic EGCG Particles (NanoEGCG)
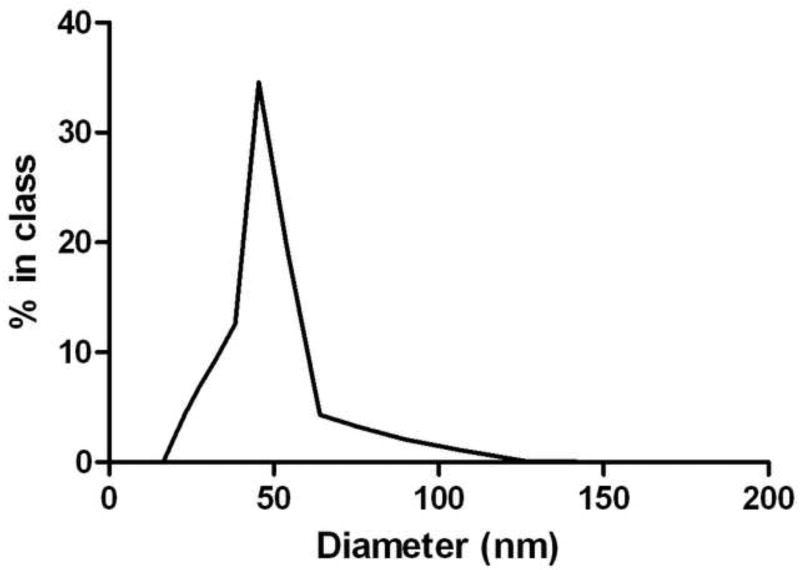
Nanolipidic particles (NanoEGCG) were prepared using a proprietary (US Provisional Patent Application #61/238,381) co-solubilization methodology involving use of monophasic liquid preparations developed by Nature’s Defense Systems, Tampa, Florida. These particles have a defined size range from 30 to 80nm. Six nanoparticle formulations were prepared for the study with various ratios of lipid carrier to EGCG. Formulations prepared for this study were 1:1, 1:2, 1:4, 1:8, 1:16, 1:32 (Nanocarrier material to EGCG on a mg/mg basis). To form NanoEGCG co-solubilization methodology involving use of monophasic liquid preparations were employed with proprietary starting materials. These materials are first solubilized into a water-in-ethanol solution (Step 1). Anhydrous EGCG was added to the materials in Step 1 and co-solubilized by mixing at room temperature (Step 2). NanoEGCG particles were formed by the addition of distilled water while mixing materials (Step 3). The final preparation of NanoEGCG particles was stirred for an additional 10 minutes prior to subjecting the preparation to sizing analysis with a Wyatt DynaPro Multiwell Reader (Wyatt Technology Corporation, Santa Barbara, California). Figure 1 shows a representative analysis of the size distribution of the 1:8 NanoEGCG formulation. The stock formulations were stored at 20°C and protected from light until needed.
Fig. 1.
Figure 1 is the dynamic light scattering data. A Wyatt DynaPro Multiwell Reader was used to characterize the diameter of the NanoEGCG particles. The data indicates a narrow size distribution, with a cumulants mean of 49.5nm and polydispersity of 0.052.
Neuronal sAPP-α ELISA
Murine neuroblastoma cells that were stably transfected with the human APP gene (APP; SweAPP N2a cells) were cultured in 24-well tissue-culture plates at 1×105 cells/well (n = 2 for each condition) with 0.5 mL of complete medium (MEM medium with 10% fetal calf serum). Prior to treatment, the MEM was aspirated and replaced with 0.5 mL of neurobasal media and differentiated with cAMP for 4 hours. Following differentiation, the cells were treated with various nanoparticle formulations and controls (25µM - 3µM) for 18 hours. Controls included two formulations of EGCG without a lipid carrier: one dissolved in water and another in ethanol and water at the same ratio that the nanoparticles were formed (described above). The conditioned media was collected and sAPP-α levels were quantified using a sAPP-α sandwich ELISA protocol as previously described [20]. High binding 96-well plates (Nunc, Denmark) were coated with monoclonal antibody 22C11 diluted in 100 µL (1 µg/mL) of carbonate buffer (pH 9.6) and incubated overnight at 4°C. The plate was washed five times with PBS-Tween buffer (0.05% Tween 20) and blocked with 300 µL of blocking buffer (1% BSA, 5% Horse Serum in PBS) for 2 hrs at 37°C. Synthetic sAPP-α protein (Abgent, San Diego, CA) was used as the positive control for this ELISA. All samples were analyzed in duplicate. 100 µL samples of conditioned media were added to each well of the plate. The plate was incubated for 2 hrs at 37°C. After washing 5 times, 100 µL of goat anti-human antibody 6E10 (Biosource; diluted 1:3,000 in reagent diluent) was added to each well of the plate. Following 2 hour-incubation at 37°C and 5-time washing, 100 µL of anti-goat IgG conjugated with HRP (1:1500) was added to each well of the plate. The plate was incubated for 1 hour at 37°C. Following 5-time washing, 100 µL of substrate solution (TMB) was added to each well and plate was incubated at room temperature. Twenty minutes later, 50 µL of stop solution (2 N H2SO4) was added to each well of the plate. The optical density was determined using a microplate reader at 450nm. Data were reported as ng of sAPP-α/mg of total intracellular protein produced per well. Total intracellular protein was quantified using a BCA kit (Pierce Biotechnology, Rockford, IL) in accordance with the manufacturer's instructions.
Pharmacokinetic Screening of EGCG Formulations in Rats
Male Sprague Dawley rats weighing 200–250 g were purchased from Harlan Laboratories (Indianapolis, IN). The rats were pre-cannulated by Harlan. The rounded tip catheters were surgically implanted into the jugular vein of the rats making multiple, precise blood draws painless to the animal. The rats were food (not water) deprived for 18 hours prior to the start of the experiment. The EGCG formulations were delivered via oral gavage at a dosage of 100 mg EGCG/kg body weight. Blood was collected at the following time points: 0, 5, 10, 30, 60, 120, 240, and 480 minutes. Because heparin was kept in the catheter lines to prevent clotting, a small amount of blood was drawn and discarded before collecting each sample. Approximately 300 µL of blood was collected in EDTA tubes for each time point. The samples were kept on ice to preserve their integrity, then centrifuged at 4000 rpm for 10 minutes, after which the plasma was transferred to sterile centrifuge tubes. A preservative solution was added to each plasma sample at 10% (v/v) concentration to ensure the integrity of the EGCG during storage [21]. This preservative was comprised of 20% ascorbic acid (to prevent oxidation of EGCG) and 0.1% EDTA (to scavenge any metal contaminants). The samples were stored at -80°C until they were analyzed for EGCG content.
Quantification of EGCG in Rat Plasma
The plasma samples were blinded and sent to be analyzed for EGCG content by the Burnham Institute for Medical Research Pharmacology Core (Orlando, FL). To accurately quantify the concentration of EGCG in the plasma, a previously described method was employed using liquid chromatography with tandem mass spectrometry [22–25].
Stock Preparation
Accurately prepared a 2.00mg/mL stock solution in DMSO of EGCG. The standard spiking solutions were prepared by diluting the stock solution to 1000 µg/mL and 100 µg/mL using acetonitrile:water (1:1, v:v). Both solutions were protected from light using amber vials and all solutions were stored at -20°C.
Standard Curve Preparation
For this analysis two standard curves were prepared one with a higher (10 µg/ml - 0.100 µg/ml) dynamic range the other a lower (1000 ng/ml - 10ng/ml). Both standard curves were prepared using the appropriate blank rat plasma containing the preservative. The results indicated that the standard curve performance was within acceptable range for bioanalytical method acceptance (R2> 0.99) [22, 23, 25].
Pharmacokinetic Calculations
Mean plasma EGCG concentrations ± the standard error in the mean (SEM) were calculated using GraphPad PRISM software (GraphPad Software, Inc.). Pharmacokinetic graphs and parameters were determined using GraphPad PRISM. Pharmacokinetic parameters included Cmax, Tmax, area under curve (AUC), and relative bioavailiblity. Relative bioavailibility was determined by dividing the AUC of each NanoEGCG formulation by the AUC of the control.
Statistical Analysis
sAPP-α ELISA
A Two-way ANOVA was performed using GraphPad PRISM software (GraphPad Software, Inc.). This was followed by Bonferonni post-tests to assess the significance of each NanoEGCG formulation versus the EGCG/10%EtOH/H2O Control at each concentration.
Pharmacokinetics
A Two-way ANOVA was performed using GraphPad PRISM software (GraphPad Software, Inc.). This was followed by Bonferonni post-tests to assess the significance of the 1:16 NanoEGCG formulation versus the EGCG/10%EtOH/H2O Control at each time point.
3. Results
Encapsulating EGCG increases sAPP-α generation in cultured SweAPP N2a cells
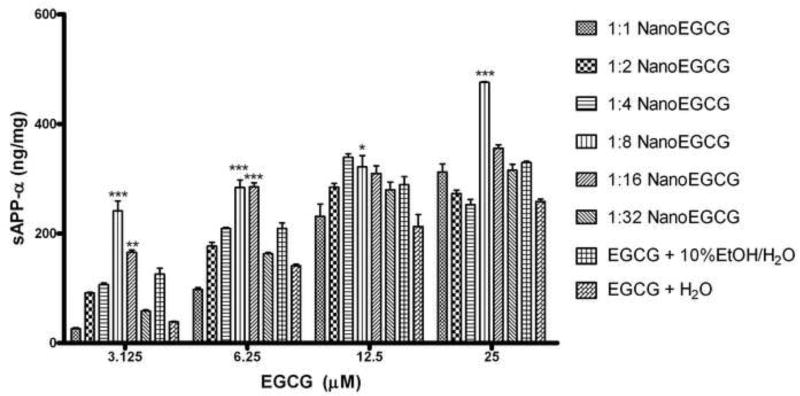
We utilized an in vitro model for Alzheimer’s disease to test the hypothesis that formation of nanoparticle complexes would increase the bioactivity of EGCG by promoting α-secretase activity in cultured SweAPP N2a cells. These cells overproduce human APP, making them ideal for screening compounds that modulate APP processing [9]. Additionally, we used this assay as a criterion to select the most effective nanoparticle formulations to carry through to the pharmacokinetic pilot study. Figure 2 shows the mean ng of sAPP-α per mg of total protein produced ± standard deviation for all EGCG formulations. Because ethanol was used to solubilize the lipid carrier and EGCG during the NanoEGCG productions process, it was appropriate to include a similarly formulated EGCG solution (10% EtOH solution v/v) to rule out any potential gains in α secretase activity being due to the alcohol content of the nanoparticle formulations.
Fig. 2.
Figure 2 is the estimated sAPP-α generation for each treatment group. The sAPP-α concentration (ng/ml) was normalized to the total protein content (mg/ml). Data is presented as mean ng of sAPP-α per mg of total protein produced ± standard deviation. The 1:8 and 1:16 formulations were superior to the other formulations, with the 1:8 showing 92% improvement in α secretase activity over the EtOH control at the 3µM concentration. The 1:8 NanoEGCG formulation was statistically higher than the control at all concentrations tested. The 1:16 NanoEGCG formulation was statistically higher at the lower two concentrations (*** P<.001, **P<.01, *P<.05).
From figure 2, not all NanoEGCG formulations were effective. In fact, the 1:1 and 1:2 formulations were outperformed by the EGCG and 10%EtOH/H2O control at all concentrations tested. The 1:8 and 1:16 NanoEGCG formulations were selected to be advanced to the pharmacokinetics phase of the study because they outperformed the control at all concentrations tested. The 1:8 formulation was statistically significant at all concentrations, whereas the 1:16 was only statistically significant at the lower two concentrations. Not only did these formulations show marked increases in sAPP-α generation but, perhaps more importantly, they continued to promote enhanced levels of α secretase activity even at the lowest EGCG concentration tested.
Encapsulation improves the bioavailability of EGCG in rats
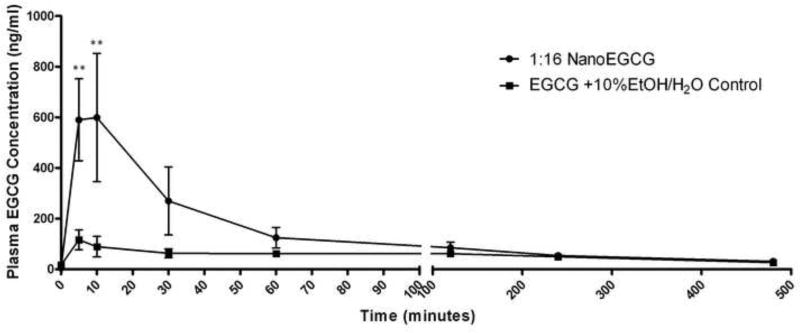
There have been numerous groups to report the poor oral bioavailability of EGCG [26–33]. Recent reports suggest that this poor oral bioavailability is mostly due to factors such as poor absorption and intestinal metabolism, rather than elimination via first pass metabolism [27]. Larger lipid-based bilayer delivery systems have been shown to increase the absorption of poorly permeable compounds [34]. This preliminary study evaluated the ability of proprietary lipid nanoparticle complexes to increase the oral bioavailability of EGCG in rats. Our results indicate that nanoparticles are highly effective at increasing the absorption of EGCG into systemic circulation. Figure 3 shows a compilation of the mean pharmacokinetic curves for the nanoparticle formulation tested and the control. Because EGCG is poorly water soluble, 10% EtOH was added to fully solubilize the EGCG at a concentration equivalent to the NanoEGCG stock (50mg/ml) and ensure accurate dosing in the control. Our data suggests that the nanoparticle formulations result in substantial increases in the absorption of EGCG. Although Figure 3 indicates only one NanoEGCG curve, both 1:8 and 1:16 formulations were tested. However, both nanoparticle formulations were similarly absorbed and not statistically different, so the 1:16 preperation was selected to represent the NanoEGCG pharmacokinetic curve. The control was very poorly absorbed in comparison to the NanoEGCG. Statistical significance (**P<.01) was observed at the 5 and 10-minute time points. Table 1 shows some important pharmacokinetic parameters: Cmax, Tmax, AUC, and relative bioavailiblity. The relative bioavailability (defined by the AUC) of the NanoEGCG was 2.31 and 2.50 for the 1:16 and 1:8 formulations, respectively, in comparison to the free EGCG in 10%EtOH solution (10%EtOH Control).
Fig. 3.
Figure 3 is the EGCG pharmacokinetic curve (mean plasma concentration ± SEM vs. time) for the 1:16 NanoEGCG formulation (n=3) and free EGCG in 10%EtOH solution (n=3). The nanoparticle formulation resulted in substantial increase in systemic EGCG absorption. Statistical significance (**P<.01) was observed at the 5 and 10-minute time points. The 10% EtOH control had very poor absorption, with plasma concentration peaking at 116.57 ng/ml. In comparison, the 1:16 NanoEGCG reached a maximum plasma concentration of 599.33 ng/ml.
Table 1.
Pharmacokinetic Parameters
| Treatment | Cmax | Tmax | AUC (0-240min) |
Relative Bioavailability |
|---|---|---|---|---|
| EGCG+10% EtOH (Control) | 116.57 | 5 | 14621 | 1 |
| NanoEGCG (1:16) | 599.33 | 10 | 33722 | 2.31 |
| NanoEGCG (1:8) | 704.67 | 5 | 36524 | 2.50 |
4. Discussion
Nanoparticles and larger liposomes have been investigated extensively for increasing the oral bioavailability of poorly absorbed compounds [35–39]. It has been recently reported that encapsulating EGCG into liposomes can improve its anti-cancer efficacy [18] and antioxidant capacity [40], probably by increasing its bioavailability. However, these studies utilized larger diameter particles (>100nm) and focused primarily on improved efficacy of EGCG for specific disease modifying parameters. Here, we have tested the ability of small diameter nanolipidic particle formation as a method for increasing not only the α-secretase inducing ability of EGCG, but also its oral bioavailability.
It has been shown that an oral dose of 800mg/70kg/day provides approximately 400ng/ml EGCG in human plasma [41]. Given that we have recently shown that 1000 - 2000ng/mL of free EGCG is necessary for promoting APP α-secretase cleavage in SweAPP N2a cells [10], using linear approximation, an oral dose of EGCG of 1800mg/70kg/day would be required to reach therapeutically effective plasma concentrations of EGCG. From a safety and practicality point of view, this dose might be unacceptable for clinical trials [42, 43]. Since the oral EGCG dosage in most clinical trials for cancer therapy is typically not more than 800 mg/day [41] regimens which enhance EGCG bioavailability, effecting reductions in neuropathology and cognitive decline at minimum doses, are very desirable. Thus, the bioavailability of EGCG is an important issue for oral administration of EGCG to clinical trials.
It has been previously reported that decreased bioavailability of EGCG is greatly associated with the glucuronidated form, which is largely present in the plasma of treated mice [44]. Additionally, it has been shown that piperine, an alkaloid derived from black pepper, enhances the bioavailability of EGCG by inhibiting glucuronidation [30]. Unfortunately the consumption of piperine also influences the metabolism of all other ingested food and drugs [45]. For example it increases the plasma concentration of phenytoin [46], propanalol, and theophylline in healthy volunteers [47] and plasma concentrations of rifamipicin (rifampin™) in patients with pulmonary tuberculosis [48]. By forming EGCG nanolipidic complexes as we have, it is possible to increase the oral bioavailability of EGCG as well as its AD and HAD preventative and therapeutic actions, without affecting the absorption of other ingested compounds. This may be an important factor to consider when bringing an EGCG therapeutic into the clinical setting.
In this study, we have modified EGCG such that it requires no co-administration of other drugs. Rather, it is co-solubilized with a lipid carrier using proprietary methodology to form 30–80nm diameter nanoparticle complexes. The importance of particle diameter for drug delivery is particularly important for delivery of drugs to the brain [49]. Previously, even smaller diameter liposomes (100nm) have had trouble penetrating the tight junctions between the endothelial cells of the blood brain barrier without osmotic disruption [50]. This highlights an important distinction between this nanoparticle technology and previous liposomal technologies, which require micelle formation. NanoEGCG does not involve encapsulating the EGCG into a micelle. Instead, lipid:EGCG complexes are formed. Because the EGCG is not fully encased in a micelle structure, it is possible to achieve smaller diameter particles without compromising the stability of the carrier. Although this preliminary study has demonstrated the ability of nanoparticles to increase the systemic absorption of EGCG taken orally, it is likely that the small diameter of these particles will also lead to improved blood brain barrier penetration. Further studies will be performed to investigate the possibility that nanolipidic particles can be used to enhance the delivery of poorly absorbed drugs to the brain.
This study provides important preliminary evidence that nanolipidic particles might be useful for safely translating EGCG into human clinical trials. Not only did NanoEGCG more than double the oral bioavailability of EGCG in rats (Figure 3) but also was more effective at promoting α-secretase activity in vitro, even at reduced concentrations (Figure 2). Taken together, it is possible that NanoEGCG will be therapeutically effective at doses that would be considered acceptable in the clinical setting.
Acknowledgments
B.G. is supported by an NIMH K08 Clinical Scientist Career Development Award (5K08MH082642-02) (K08). This work was also supported by an SBIR grant (R43AT004871) from the NCAAM (JT) and an R21 grant (R21AG031037) from the NIA (RDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The investigators have filed a patent application for this technology.
References
- 1.Alisky JM. The coming problem of HIV-associated Alzheimer's disease. Med Hypotheses. 2007;69(5):1140–3. doi: 10.1016/j.mehy.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 2.Wojtera M, Sikorska B, Sobow T, Liberski PP. Microglial cells in neurodegenerative disorders. Folia Neuropathol. 2005;43(4):311–21. [PubMed] [Google Scholar]
- 3.Tarkowski E, Liljeroth AM, Minthon L, Tarkowski A, Wallin A, Blennow K. Cerebral pattern of pro- and anti-inflammatory cytokines in dementias. Brain Res Bull. 2003 Aug 15;61(3):255–60. doi: 10.1016/s0361-9230(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 4.Meeuwsen EJ, German P, Melis RJ, Adang EM, Goluke Willemse GA, Krabbe PF, et al. Cost-effectiveness of plost-diagnosis treatment in dementia coordinated by multidisciplinary memory clinics in comparison to treatment coordinated by general practioners: an example of a pragmatic trial. J Nutr Health Aging. 2009 Mar;13(3):242–8. doi: 10.1007/s12603-009-0066-1. [DOI] [PubMed] [Google Scholar]
- 5.Burns A, Iliffe S. Dementia. BMJ. 2009;338:b75. doi: 10.1136/bmj.b75. [DOI] [PubMed] [Google Scholar]
- 6.Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giunta B, Obregon D, Hou H, Zeng J, Sun N, Nikolic V, et al. EGCG mitigates neurotoxicity mediated by HIV-1 proteins gp120 and Tat in the presence of IFN-gamma: role of JAK/STAT1 signaling and implications for HIV-associated dementia. Brain Res. 2006 Dec 6;1123(1):216–25. doi: 10.1016/j.brainres.2006.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giunta B, Zhou Y, Hou H, Rrapo E, Fernandez F, Tan J. HIV-1 TAT Inhibits Microglial Phagocytosis of Abeta Peptide. Int J Clin Exp Pathol. 2008;1(3):260–75. [PMC free article] [PubMed] [Google Scholar]
- 9.Obregon DF, Rezai-Zadeh K, Bai Y, Sun N, Hou H, Ehrhart J, et al. ADAM10 activation is required for green tea (-)-epigallocatechin-3-gallate-induced alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 2006 Jun 16;281(24):16419–27. doi: 10.1074/jbc.M600617200. [DOI] [PubMed] [Google Scholar]
- 10.Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005 Sep 21;25(38):8807–14. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rezai-Zadeh K, Shytle RD, Bai Y, Tian J, Hou H, Mori T, et al. Flavonoid-mediated presenilin-1 phosphorylation reduces Alzheimer's disease beta-amyloid production. J Cell Mol Med. 2008 Apr 9; doi: 10.1111/j.1582-4934.2008.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bontha S, Kabanov AV, Bronich TK. Polymer micelles with cross-linked ionic cores for delivery of anticancer drugs. J Control Release. 2006 Aug 28;114(2):163–74. doi: 10.1016/j.jconrel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Kim JO, Kabanov AV, Bronich TK. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J Control Release. 2009 Apr 20; doi: 10.1016/j.jconrel.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Lee H, Allen C. Formulation of drugs in block copolymer micelles: drug loading and release. Curr Pharm Des. 2006;12(36):4685–701. doi: 10.2174/138161206779026263. [DOI] [PubMed] [Google Scholar]
- 15.Tian Y, Bromberg L, Lin SN, Hatton TA, Tam KC. Complexation and release of doxorubicin from its complexes with pluronic P85-b-poly(acrylic acid) block copolymers. J Control Release. 2007 Aug 28;121(3):137–45. doi: 10.1016/j.jconrel.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama M, Fukushima S, Uehara R, Okamoto K, Kataoka K, Sakurai Y, et al. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J Control Release. 1998 Jan 2;50(1–3):79–92. doi: 10.1016/s0168-3659(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 17.Huynh NT, Passirani C, Saulnier P, Benoit JP. Lipid nanocapsules: a new platform for nanomedicine. Int J Pharm. 2009 Sep 11;379(2):201–9. doi: 10.1016/j.ijpharm.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, Khwaja SI, et al. Introducing nanochemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009 Mar 1;69(5):1712–6. doi: 10.1158/0008-5472.CAN-08-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 20.Bailey AR, Giunta BN, Obregon D, Nikolic WV, Tian J, Sanberg CD, et al. Peripheral biomarkers in Autism: secreted amyloid precursor protein-alpha as a probable key player in early diagnosis. Int J Clin Exp Med. 2008;1(4):338–44. [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, et al. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab Dispos. 2006 Jan;34(1):8–11. doi: 10.1124/dmd.104.003434. [DOI] [PubMed] [Google Scholar]
- 22.Sparidans RW, Lagas JS, Schinkel AH, Schellens JH, Beijnen JH. Liquid chromatography-tandem mass spectrometric assays for salinomycin in mouse plasma, liver, brain and small intestinal contents and in OptiMEM cell culture medium. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Aug 15;855(2):200–10. doi: 10.1016/j.jchromb.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Miksa IR. Multi-component plasma quantitation of anti-hyperglycemic pharmaceutical compounds using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007 Sep 1;856(1–2):318–27. doi: 10.1016/j.jchromb.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Tang Y, Gu J, Fawcett JP, Bai X. Rapid and sensitive liquid chromatography-tandem mass spectrometric method for the quantitation of metformin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Sep 5;808(2):215–9. doi: 10.1016/j.jchromb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Hop CE, Leung KH, Pang J. Determination of in vitro permeability of drug candidates through a caco-2 cell monolayer by liquid chromatography/tandem mass spectrometry. J Mass Spectrom. 2000 Jan;35(1):71–6. doi: 10.1002/(SICI)1096-9888(200001)35:1<71::AID-JMS915>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Feng WY. Metabolism of green tea catechins: an overview. Curr Drug Metab. 2006 Oct;7(7):755–809. doi: 10.2174/138920006778520552. [DOI] [PubMed] [Google Scholar]
- 27.Cai Y, Anavy ND, Chow HH. Contribution of presystemic hepatic extraction to the low oral bioavailability of green tea catechins in rats. Drug Metab Dispos. 2002 Nov;30(11):1246–9. doi: 10.1124/dmd.30.11.1246. [DOI] [PubMed] [Google Scholar]
- 28.Chan KY, Zhang L, Zuo Z. Intestinal efflux transport kinetics of green tea catechins in Caco-2 monolayer model. J Pharm Pharmacol. 2007 Mar;59(3):395–400. doi: 10.1211/jpp.59.3.0009. [DOI] [PubMed] [Google Scholar]
- 29.Henning SM, Choo JJ, Heber D. Nongallated compared with gallated flavan-3-ols in green and black tea are more bioavailable. J Nutr. 2008 Aug;138(8):1529S–34S. doi: 10.1093/jn/138.8.1529S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS. Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J Nutr. 2004 Aug;134(8):1948–52. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 31.Lambert JD, Sang S, Hong J, Kwon SJ, Lee MJ, Ho CT, et al. Peracetylation as a means of enhancing in vitro bioactivity and bioavailability of epigallocatechin-3-gallate. Drug Metab Dispos. 2006 Dec;34(12):2111–6. doi: 10.1124/dmd.106.011460. [DOI] [PubMed] [Google Scholar]
- 32.Lin LC, Wang MN, Tseng TY, Sung JS, Tsai TH. Pharmacokinetics of (-)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J Agric Food Chem. 2007 Feb 21;55(4):1517–24. doi: 10.1021/jf062816a. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Zheng Y, Chow MS, Zuo Z. Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int J Pharm. 2004 Dec 9;287(1–2):1–12. doi: 10.1016/j.ijpharm.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 34.Allen TM. Liposomal drug formulations. Rationale for development and what we can expect for the future. Drugs. 1998 Nov;56(5):747–56. doi: 10.2165/00003495-199856050-00001. [DOI] [PubMed] [Google Scholar]
- 35.Frezard F, Martins PS, Bahia AP, Le Moyec L, de Melo AL, Pimenta AM, et al. Enhanced oral delivery of antimony from meglumine antimoniate/beta-cyclodextrin nanoassemblies. Int J Pharm. 2008 Jan 22;347(1–2):102–8. doi: 10.1016/j.ijpharm.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 36.He W, Horn SW, Hussain MD. Improved bioavailability of orally administered mifepristone from PLGA nanoparticles. Int J Pharm. 2007 Apr 4;334(1–2):173–8. doi: 10.1016/j.ijpharm.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Kumar VV, Chandrasekar D, Ramakrishna S, Kishan V, Rao YM, Diwan PV. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: influence of wax and glyceride lipids on plasma pharmacokinetics. Int J Pharm. 2007 Apr 20;335(1–2):167–75. doi: 10.1016/j.ijpharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Pandey R, Ahmad Z, Sharma S, Khuller GK. Nano-encapsulation of azole antifungals: potential applications to improve oral drug delivery. Int J Pharm. 2005 Sep 14;301(1–2):268–76. doi: 10.1016/j.ijpharm.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 39.Rao SV, Yajurvedi K, Shao J. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of protein drugs: III. In vivo oral absorption study. Int J Pharm. 2008 Oct 1;362(1–2):16–9. doi: 10.1016/j.ijpharm.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 40.Italia JL, Datta P, Ankola DD, Kumar MNVR. Nanoparticles Enhance Per Oral Bioavailability of Poorly Available Molecules: Epigallocatechin Gallate Nanoparticles Ameliorates Cyclosporine Induced Nephrotoxicity in Rats at Three Times Lower Dose Than Oral Solution. Journal of Biomedical Nanotechnology. 2008 Sep;4(3):304–12. [Google Scholar]
- 41.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, et al. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001 Jan;10(1):53–8. [PubMed] [Google Scholar]
- 42.Ullmann U, Haller J, Decourt JD, Girault J, Spitzer V, Weber P. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int J Vitam Nutr Res. 2004 Jul;74(4):269–78. doi: 10.1024/0300-9831.74.4.269. [DOI] [PubMed] [Google Scholar]
- 43.Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, et al. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J Int Med Res. 2003 Mar-Apr;31(2):88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- 44.Lambert JD, Lee MJ, Lu H, Meng X, Hong JJ, Seril DN, et al. Epigallocatechin-3-gallate is absorbed but extensively glucuronidated following oral administration to mice. J Nutr. 2003 Dec;133(12):4172–7. doi: 10.1093/jn/133.12.4172. [DOI] [PubMed] [Google Scholar]
- 45.Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002 Apr;9(3):224–31. doi: 10.1078/0944-7113-00114. [DOI] [PubMed] [Google Scholar]
- 46.Pattanaik S, Hota D, Prabhakar S, Kharbanda P, Pandhi P. Effect of piperine on the steady-state pharmacokinetics of phenytoin in patients with epilepsy. Phytother Res. 2006 Aug;20(8):683–6. doi: 10.1002/ptr.1937. [DOI] [PubMed] [Google Scholar]
- 47.Bano G, Raina RK, Zutshi U, Bedi KL, Johri RK, Sharma SC. Effect of piperine on bioavailability and pharmacokinetics of propranolol and theophylline in healthy volunteers. Eur J Clin Pharmacol. 1991;41(6):615–7. doi: 10.1007/BF00314996. [DOI] [PubMed] [Google Scholar]
- 48.Zutshi RK, Singh R, Zutshi U, Johri RK, Atal CK. Influence of piperine on rifampicin blood levels in patients of pulmonary tuberculosis. J Assoc Physicians India. 1985 Mar;33(3):223–4. [PubMed] [Google Scholar]
- 49.Wissing SA, Kayser O, Muller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004 May 7;56(9):1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 50.Sakamoto A, Ido T. Liposome targeting to rat brain: effect of osmotic opening of the blood-brain barrier. Brain Res. 1993 Nov 26;629(1):171–5. doi: 10.1016/0006-8993(93)90499-d. [DOI] [PubMed] [Google Scholar]