Our previous studies demonstrated a role for the zinc finger transcription factor Slug in ultraviolet radiation (UVR)-induced skin cancer and cutaneous wound healing consistent with its function in regulating epithelial-mesenchymal transition (Shirley et al., 2010). Unexpectedly, these studies also revealed that Slug knockout (KO) mice were resistant to sunburn, perhaps due to impaired release of proinflammatory cytokines from the UVR-exposed epidermis (Newkirk et al., 2008). The present studies expanded these findings to show conclusively that sunburn resistance in KO mice is due to altered UVR-induced cytokine release from the epidermis and not to altered inflammatory cell response to these cytokines. All animal studies were performed using protocols approved by the MD Anderson Cancer Center Institutional Animal Care and Use Committee.
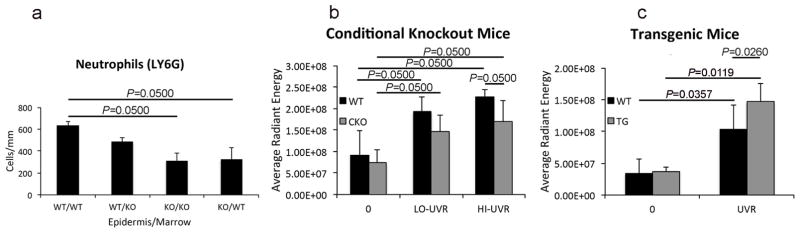
First, we performed reciprocal bone marrow grafts between wild type (WT) and Slug KO mice (see Supplementary Materials and Methods online). Fully chimeric mice were exposed to approximately three minimal erythemal doses (MED) of UVR, skin was harvested 48 hours later, immunohistochemistry was performed to identify inflammatory and immune cells, and cells were quantified. Figure 1a shows that mice with KO epidermis and either KO or WT bone marrow had significantly reduced dermal neutrophil influx compared to mice with WT epidermis and WT bone marrow. Significant differences between UVR-exposed KO and WT skin in numbers of T lymphocytes in the epidermis or dermis, mast cells in the dermis, or B cells in the dermis were not seen (data not shown). Second, we used fluorescent imaging techniques (described in Supplementary Materials and Methods online) to examine UVR-induced dermal inflammatory cell infiltration in mice with Slug deleted only in the epidermis (conditional Slug knockout, CKO) and in mice with expression of a Slug transgene targeted to the epidermis by the bovine keratin 5 (K5) promoter (transgenic Slug, TG) (see Supplementary Materials and Methods online for descriptions). Inflammatory cell influx was measured 24 hours after exposure to approximately two MED of UVR. UVR exposure significantly enhanced neutrophil influx in all genotypes; however, neutrophil influx was significantly lower in CKO compared to WT control mice and significantly higher in TG mice compared to WT mice (Figures 1b,c and S1). These results clearly illustrated that it was the genotype of the epidermis rather than that of the hematopoietic tissue that determined the extent of neutrophil influx into UVR-exposed skin. Moreover, neutrophil migration studies (see Supplementary Materials and Methods online) failed to demonstrate any difference in the migration of WT versus KO neutrophils in response to the chemotactic peptide WKYMVm (Trp-Lys-Tyr-Met-Val-D-Met-NH2) and to mouse serum (Figure S2), suggesting that both Slug KO and WT neutrophils were able to migrate normally in response to proinflammatory cytokines released from UVR-exposed epidermis.
Figure 1. Slug controls UVR-induced neutrophil influx into the skin.
(a) UVR induced significantly greater neutrophil influx in mice with WT epidermis and WT bone marrow (WT/WT) than in mice with KO epidermis and WT (KO/WT) or KO marrow (KO/KO). Data shown as means of four to six mice±SD (one-way ANOVA and Dunnett’s multiple comparison statistic). (b,c) Signal from a fluorescent neutrophil elastase probe was similar in unexposed CKO and TG mice and WT littermates. There was significant induction of neutrophil elastase activity by UVR; however, UVR-exposed CKO mice had significantly reduced and TG mice had significantly increased signal compared to UVR-exposed WT mice. Data shown as means of three to six adult mice per group ±SD (pairwise comparison with one-way Mann-Whitney statistic).
To identify those proinflammatory cytokines that might fail to be induced by UVR in KO epidermis, we performed microarray analysis of epidermal gene expression using a commercial inflammatory response PCR array (see Supplementary Materials and Methods online). Of the 370 mRNAs quantified, 131 appeared to be differentially induced by UVR in the KO compared to the WT epidermis (Table S1). Among these, we identified five genes encoding secreted proinflammatory cytokines that were very highly induced by UVR in WT epidermis, were not induced or induced to a much lower extent in the KO skin, and were chemotactic for neutrophils. These included CCL3, CCL4, CCrL2, interleukin-1β (IL-1β), and oncostatin (OSM). We examined induction of these genes in CKO versus WT epidermis at two different UVR doses. The higher UVR dose significantly enhanced expression of CCL3, CCL4, and OSM in WT but not in CKO epidermis (Figure S3). The lower UVR dose significantly enhanced expression of IL-1β; however, no significant UVR induction of these cytokines was seen for CKO epidermis (Figure S3). CCLr2 was not significantly induced in either genotype at either UVR dose (data not shown). This confirmed our microarray results for CCL3, CCL4, OSM, and IL-1β, indicating a role for Slug in regulating expression of these cytokine genes. It was not clear, however, if Slug modulated expression of these genes directly or through an indirect mechanism. The 3000 nucleotides upstream of the transcription start sites in these genes contain four Slug binding sites (CAGGTG, CACCTG) in CCL4 and two in OSM, but none in IL-1β or CCL3. Ingenuity Pathway Analysis (Figure S4), suggested that NF-κB was likely to play a central role in the differential gene expression identified by microarray analysis, and CCL3, CCL4, IL-1β, and OSM all have binding sites for NF-κB, a master regulator of inflammation (Widmer et al., 1993; Basagoudanavar et al., 2011; Eigenbrod et al., 2013; Chen et al., 2014).
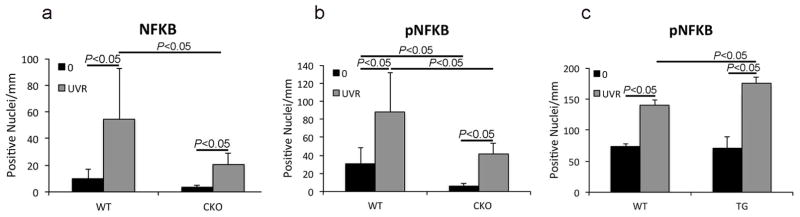
We used immunohistochemistry to determine expression of activated phospho-NF-κB, total NF-κB, and IKK-α, a kinase that phosphorylates inhibitors of NF-kappa-B leading to their inactivation, in the unexposed and UVR-exposed epidermis of WT and Slug KO mice. UVR exposure did not significantly enhance the number of IKK-α-positive nuclei in either genotype (data not shown). Numbers of total NF-κB-positive nuclei were similar in unexposed WT and CKO epidermis; UVR exposure significantly increased the number of positive nuclei for both genotypes, but there were significantly more NF-κB-positive nuclei in the epidermis of UVR-exposed WT mice than in CKO mice (Figures 2a and S5). The number of phospho-NF-κB-positive nuclei was significantly lower in the unexposed epidermis of CKO compared to WT mice (Figure 2b). Although UVR exposure significantly increased the number of phospho-NF-κB-positive nuclei in both WT and KO epidermis, this effect was significantly more pronounced in WT epidermis (Figures 2b and S5). Unexposed TG and WT epidermis had similar numbers of phospho-NF-κB-positive nuclei; UVR exposure significantly increased these numbers in both genotypes, but the numbers were significantly higher in TG versus WT epidermis (Figures 2c and S5).
Figure 2. Slug modulates NF-κB activation in response to UVR.
Mice were exposed to UVR and immunohistochemically positive epidermal nuclei were quantified. (a,b) UVR exposure significantly increased NF-κB and phospho-NF-κB-positive nuclei in WT mice but not CKO mice. Values shown as means of four to five mice ±SD (one-way ANOVA followed by Sidak’s multiple comparisons test). (c) Both WT and TG epidermis showed a significant increase in the number of phospho-NF-κB-positive nuclei after UVR exposure, but induction in TG mice was significantly higher than in WT mice. Values shown as means of three mice ±SD (one-way ANOVA followed by Tukey’s multiple comparisons test).
Taken together, our findings confirmed that Slug facilitated the sunburn response by enhancing the release of proinflammatory cytokines, including CCL3, CCL4, IL-1β, and OSM, from UVR-exposed keratinocytes. Our studies also suggested that Slug regulation of these cytokines occurred, at least in part, via NF-κB. This conclusion was consistent with the report that transgenic expression of Snail, a zinc finger transcription factor closely related to Slug, in keratinocytes results in activation of NF-κB and cutaneous inflammation (Du et al., 2010).
Supplementary Material
Acknowledgments
We thank the following excellent M.D. Anderson shared resources supported by DHHS/NCI Cancer Center Support Grant P30 CA016672: the Mutant Mouse Phenotyping Shared Resource, the Molecular Biology Core, the Histology Laboratory, the Transgenic Animal Core, and the Genetically Engineered Mouse Facility Core. This work was funded by the National Institutes of Health awards R21 AR054361 and R21 AR061641 and by Cancer Prevention and Research Institute of Texas Shared Instrumentation Award RP120997.
Abbreviations
- CKO
conditional knockout
- IL-1β
interleukin-1β
- KO
knockout
- MED
minimal erythemal dose
- OSM
oncostatin
- TG
transgenic
- UVR
ultraviolet radiation
- WT
wild type
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Basagoudanavar SH, Thapa RJ, Nogusa S, Wang J, Beg AA, Balachandran S. Distinct roles for the NF-kappa B RelA subunit during antiviral innate immune responses. J Virol. 2011;85:2599–610. doi: 10.1128/JVI.02213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Su CM, Huang YL, Tsai CH, Fuh LJ, Tang CH. CCN1 induces oncostatin M production in osteoblasts via integrin-dependent signal pathways. PLoS One. 2014;9:e106632. doi: 10.1371/journal.pone.0106632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du F, Nakamura Y, Tan TL, Lee P, Lee R, Yu B, et al. Expression of snail in epidermal keratinocytes promotes cutaneous inflammation and hyperplasia conducive to tumor formation. Cancer Res. 2010;70:10080–9. doi: 10.1158/0008-5472.CAN-10-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenbrod T, Bode KA, Dalpke AH. Early inhibition of IL-1β expression by IFN-γ is mediated by impaired binding of NF-κB to the IL-1β promoter but is independent of nitric oxide. J Immunol. 2013;190:6533–41. doi: 10.4049/jimmunol.1300324. [DOI] [PubMed] [Google Scholar]
- Newkirk KM, Duncan FJ, Brannick EM, Chandler HL, Parent AE, Kusewitt DF. The acute cutaneous inflammatory response is attenuated in Slug-knockout mice. Lab Invest. 2008;88:831–41. doi: 10.1038/labinvest.2008.37. [DOI] [PubMed] [Google Scholar]
- Shirley SH, Hudson LG, He J, Kusewitt DF. The skinny on Slug. Mol Carcinog. 2010;49:851–61. doi: 10.1002/mc.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer U, Manogue KR, Cerami A, Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J Immunol. 1993;150:4996–5012. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.