Abstract
Hepatorenal Syndrome (HRS) continues to be one of the major complications of decompensated cirrhosis leading to death in the absence of liver transplantation. Challenges in precisely evaluating renal function in the patient with cirrhosis remain due to the limitations of serum creatinine (Cr) alone in estimating glomerular filtration rate (GFR); current GFR estimating models appear to underestimate renal dysfunction. Newer models incorporating renal biomarkers, such as the Cr-Cystatin C GFR Equation for Cirrhosis appear to estimate measured GFR more accurately. A major change in the diagnostic criteria for HRS based on dynamic serial changes in serum Cr which regard HRS type 1 as a special form of acute kidney injury (AKI) promises the possibility of earlier identification of renal dysfunction in patients with cirrhosis. The diagnostic criteria of HRS still include the exclusion of other causes of kidney injury. Renal biomarkers have been disappointing in assisting with the differentiation of HRS from pre-renal azotemia and other kidney disorders. Serum metabolomic profiling may be a more powerful tool to assess renal dysfunction although the practical clinical significance of this remains unclear. As a result of the difficulties of assessing renal function in cirrhosis and the varying HRS diagnostic criteria and the rigor with which they are applied, the precise incidence and prevalence of HRS is unknown but it is likely that HRS occurs more commonly than expected. The pathophysiology of HRS is firmly rooted in the setting of progressive reduction in renal blood flow (RBF) as a result of portal hypertension and splanchnic vasodilation. Progressive, marked renal cortical ischemia in patients with cirrhosis parallels the evolution of diuretic-sensitive ascites to diuretic-refractory ascites and HRS, a recognized continuum of renal dysfunction in cirrhosis. Alterations in nitrous oxide (NO) production, both increased and decreased may play a major role in the pathophysiology of this evolution. The inflammatory cascade triggered by bacterial translocation and endotoxemia, increasingly recognized as important in the manifestations of acute on chronic liver failure, may also play a significant role in the pathophysiology of HRS. The mainstay of treatment remains vasopressor therapy with albumin in an attempt to reverse splanchnic vasodilation and improve RBF. Several meta-analyses confirm the value of vasopressors, chiefly terlipressin and noradrenaline, in improving renal function and reversing HRS type 1. Other interventions such as renal replacement therapy, transjugular intrahepatic porto-systemic shunt (TIPS), and artificial liver support systems have a very limited role to improve outcomes in HRS. Liver transplantation remains the definitive treatment for HRS. The frequency of simultaneous liver-kidney transplantation (SLKT) has increased dramatically in the Model for End-stage Liver Disease era, with changes in organ allocation policies. This has resulted in a more urgent need to accurately predict native kidney recovery from HRS after liver transplantation alone, to avoid unnecessary SLKT.
Keywords: Hepatorenal Syndrome, cirrhosis, terlipressin, meta-analysis, cystatin C
Hepatorenal Syndrome (HRS) is characterized as renal dysfunction secondary to reduction in renal blood flow (RBF) occurring in the setting of underlying cirrhosis and portal hypertension.1 It is classified as either rapidly developing acute kidney injury (AKI), HRS type 1 or slowly progressive chronic kidney disease (CKD), HRS type 2.2 Both types of HRS are associated with a decline in RBF and glomerular filtration rate (GFR). HRS is a frequently fatal complication of cirrhosis.3 The diagnosis conveys a poor prognosis; median survival for HRS Type 1 and 2 is approximately 1 and 6.7 months, respectively.3 Challenges for the study of HRS include establishing when renal dysfunction in patients with cirrhosis occurs, largely related to the limitations of serum creatinine (Cr), evolving criteria for the diagnosis of HRS, the uncertain value of renal biomarkers, and the limited availability of pharmacologic and other therapies to address the fundamental underlying pathophysiology. In this review, we summarize new concepts and developments in the diagnosis, epidemiology, pathophysiology, and treatment of HRS, chiefly focusing on HRS type 1.
Diagnosis of Renal Dysfunction in Patients with Cirrhosis
It is well-established that serum Cr is not an accurate marker of renal dysfunction in cirrhosis.4-8 Multiple factors contribute to lower serum Cr concentrations in cirrhosis, reducing the sensitivity of serum Cr for the detection of renal dysfunction and resulting in an overestimation of renal function, misclassification of kidney disease stage and delays in management and treatment of kidney disease in patients with cirrhosis.4-8 The production of creatine, the precursor of serum Cr is impaired in hepatic dysfunction.6, 7 Patients with decompensated cirrhosis have reduced muscle mass and increased tubular secretion of Cr.4-8 Collectively, all these factors reduce serum Cr concentration making it an insufficiently accurate marker of renal function in cirrhosis.4-8 Assessing renal function by measuring GFR (e.g. inulin clearance, iothalamate clearance) is the most reliable and accurate method, but it is expensive, time consuming and labor intensive. Additionally, some of the exogenous markers used in GFR measurement are radioactive. Directly measuring Cr clearance is an alternative method to measuring GFR. However, the Cr clearance method is time-intensive, prone to logistical errors, and impractical in critically ill patients with end-stage liver disease and low or no urine output. In subjects with cirrhosis, Cr clearance has been shown to have poor accuracy in predicting measured GFR.9 Hyperbilirubinemia and hemolysis in patients with cirrhosis may produce spuriously low levels of Cr.10 Serum Cr varies with sex and age, distinct from changes in GFR.8 Even after controlling for age, race, weight, height and measured GFR, female gender was shown to be an independent predictor of serum Cr in patients with cirrhosis.11
Table 1 shows original development12-22 and validation studies9, 15, 23-26 of conventional Cr clearance and GFR-estimating and new GFR-estimating equations in patients with cirrhosis. Cr-based GFR-estimating equations have major limitations in patients with cirrhosis.9, 25, 26 Additionally, the majority of Cr-based GFR- and Cr clearance-estimating equations used in clinical practice were derived either from patients with CKD or patients without cirrhosis.12, 17-20 A Cr-based GFR-estimating equation derived from 469 patients with cirrhosis, validated both internally and externally in 174 and 82 patients with cirrhosis, respectively has recently been described.15 Although this Cr-based GFR model suggested improved performance in predicting measured GFR compared to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) Cr and Modification of Diet In Renal Disease (MDRD) equations15, prospective, independent assessments of differences in accuracy between GFR estimates by the new equation, MDRD and CKD-EPI Cr equations are not yet available. Blood urea and degree of ascites (moderate vs. severe) are two of the parameters used in this new model.15 Elevated blood urea levels in the setting of gastrointestinal bleeding and the subjective nature of assessing the degree of ascites among clinicians may limit the value of this model15 and further validation is required.
Table 1.
GFR and Cr Clearance Estimating Equations Validated in Patients with Cirrhosis
| GFR and Cr Clearance Estimating Equations | Original GFR Equation Development Studies | Renal Marker Used | Population from which the GFR Equation was Derived | Most Recent External Validation Studies in Patients with Cirrhosis | |
|---|---|---|---|---|---|
| Cr Equations | Cockcroft-Gault12 | Cockcroft and Gault (1976)12 | Cr | Patients from Queen Mary Veterans’ Hospital | Francoz (2010)26, Mindikoglu (2014)9 |
| MDRD-417-19 | Levey (1999)17, Levey (2006)18, Levey (2007)19 | Cr | CKD | Francoz (2010)26, Francoz (2014)25, De Souza (2014)24, Mindikoglu (2014)9, Cholongitas (2017)23, Kalafateli (2017)15 | |
| MDRD-617-19 | Levey (1999)17, Levey (2006)18, Levey (2007)19 | Cr | CKD | Francoz (2014)25, De Souza (2014)24, Mindikoglu (2014)9, Kalafateli (2017)15 | |
| CKD-EPI Cr 2020 | Levey (2009)20 | Cr | With and without CKD | Francoz (2010)26, Francoz (2014)25, De Souza (2014)24, Mindikoglu (2014)9, Cholongitas (2017)23, Kalafateli (2017)15 | |
| Royal Free Hospital Cirrhosis GFR15 | Kalafateli (2017)15 | Cr | Cirrhosis | Kalafateli (2017)15 | |
| Cystatin C Equations | LARSSON16 | Larsson (2004)16 | Cystatin C | University Hospital MAS | Mindikoglu (2014)9, Cholongitas (2017)23 |
| HOEK13 | Hoek (2003)13 | Cystatin C | With suspected or established renal dysfunction | De Souza (2014)24, Mindikoglu (2014)9, Cholongitas (2017)23 | |
| CKD-EPI Cystatin C (2012)14 | Inker (2012)14 | Cystatin C | With and without CKD | De Souza (2014)24, Mindikoglu (2014)9, Cholongitas (2017)23, Kalafateli (2017)15 | |
| Cr-Cystatin C Equations | STEVENS22 | Stevens (2008)22 | Combined Cr and cystatin C | CKD | Mindikoglu (2014)9 |
| CKD-EPI Cr-Cystatin C (2012)14 | Inker (2012)14 | Combined Cr and cystatin C | With and without CKD | De Souza (2014)24, Mindikoglu (2014)9, Cholongitas (2017)23, Kalafateli (2017)15 | |
| Cr-Cystatin C GFR Equation for Cirrhosis21 | Mindikoglu (2016)21 | Combined Cr and cystatin C | Cirrhosis | Cholongitas (2017)23 |
Serum Cr is used not only in determining renal function and mortality in patients with cirrhosis on the liver transplant waiting list, but also in making decisions on whether to proceed with liver transplant alone (LTA) or simultaneous liver–kidney transplantation (SLKT) for patients with severe renal dysfunction in the setting of HRS. According to SLKT guidelines, GFR is estimated using the MDRD-6 equation, a Cr-based formula.27 However, the MDRD-6 formula was shown to underestimate measured GFR when the measured GFR was > 30 ml/min/1.73m2 in patients with cirrhosis, and therefore can potentially lead to listing a patient for an unnecessary SLKT.25 Conversely, the overestimation of measured GFR can result in increased mortality after liver transplantation due to severe renal dysfunction underscoring the need for a practical, accurate assessment of GFR.25, 28
The recent development of GFR equations supplementing serum Cr with renal biomarker, cystatin C measurements appear promising. In contrast to serum Cr, cystatin C is independent of hepatic function5, 29, gender-neutral11 and sensitive to GFR reductions in the range which GFR equations using serum Cr alone may not detect GFR reductions.29 Studies showed that Cr-Cystatin C combined GFR-equations were more accurate compared to Cr-based GFR equations in estimating measured GFR in patients with cirrhosis.9, 21, 23 The advantage of using serum Cr and cystatin C in combination is that cystatin C is a Cr-blind range marker16 and increases the performance of the GFR equation.21 The new Cr-Cystatin C GFR equation for Cirrhosis estimates GFR with greater accuracy than the CKD-EPI cystatin C (2012)14 and Cr-Cystatin C (2012)14 equations in patients with cirrhosis and diuretic-refractory ascites.21 Cr-Cystatin C GFR Equation for Cirrhosis has been recently validated in an independent cohort of 129 patients with decompensated cirrhosis in Europe.23 Compared with CKD-EPI cystatin C (2012)14 and Cr-Cystatin C (2012)14 equations, the Cr-Cystatin C GFR Equation for Cirrhosis21 had significantly higher accuracy and showed the best performance to discriminate patients with cirrhosis who had GFR < 60ml/min with an area under the concentration-time curve (AUC) of 0.91 compared with MDRD-417-19, CKD-EPI Cr (2009)20 and GFR equation developed by Cholongitas et al.23 These developments may become relevant to establishing the diagnosis of HRS more precisely and selecting the most appropriate therapy and its timing, be it vasopressors, LTA or SLKT.
New Diagnosis of HRS in Patients with Cirrhosis
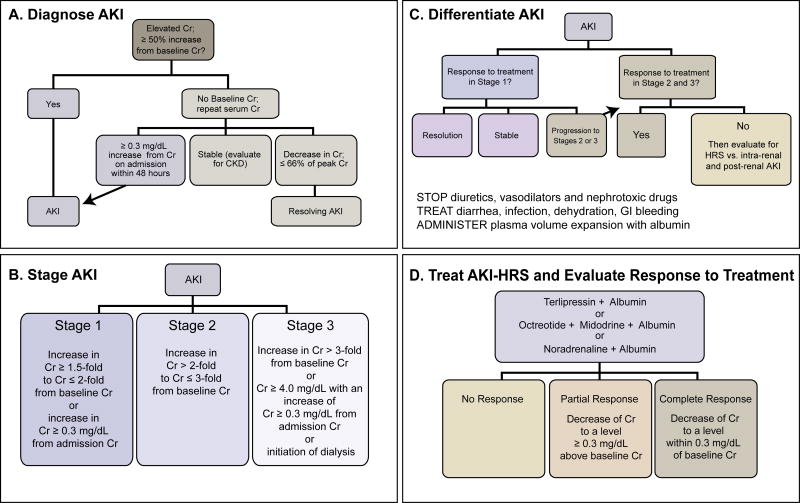
One of the most important developments in HRS is the move away from a diagnosis based on a single level of serum Cr to one based on dynamic serial changes in serum Cr such as the recently revised recommendations of International Ascites Club (IAC) for hepatorenal disorders in cirrhosis.30 This change has been stimulated by a perceived need to more precisely establish an early diagnosis of HRS allowing the start of earlier therapy. The key difference between the prior criteria31 and the newly proposed classification system to diagnose HRS30 is that the diagnosis of HRS is not based on an increase in serum Cr from a single serum Cr value to a fixed value of serum Cr (i.e. 2.5 mg/dL for HRS type 131) but rather on an amount of increase in serum Cr from baseline serum Cr value comporting with the Acute Kidney Injury Network (AKIN)32, Kidney Disease Improving Outcomes (KDIGO) clinical practice guidelines for AKI33, Acute Dialysis Quality Initiative (ADQI) and International Ascites Club (IAC)2 classification systems and revised recommendations of IAC.30 In this new classification system, the diagnosis of AKI precedes the diagnosis of HRS (Figure 1A).30, 34, 35 Once AKI is diagnosed, the stage of AKI should be identified (Figure 1B) as progression to subsequent AKI stages has higher mortality in patients with cirrhosis.30, 34-36 Since the diagnosis of HRS is one of exclusion, adjudication of the etiology of AKI should begin by discontinuation of diuretics, vasodilators and nephrotoxic drugs, treatment of all etiologies that may be culprit of volume depletion and administration of intravenous albumin (1 g/kg/day for 2 days; maximum 100 g a day) (Figure 1C).30, 34, 35 Patients responding to volume replacement therapy can be considered to have pre-renal azotemia.30, 34, 35 Patients who do not respond to volume replacement therapy should be evaluated for etiologies including HRS, intra-renal (e.g. acute tubular necrosis [ATN], glomerulonephritis, interstitial nephritis) and post-renal (e.g. urinary obstruction) AKI.30, 34, 35 Presence of granular casts in urine sediment and urine osmolality equal to plasma osmolality are suggestive findings of ATN.37 Proteinuria and microhematuria should warrant further investigation for an intra-renal cause of AKI.30 Renal ultrasound should be obtained to assess structural changes in the kidneys and rule out urinary obstruction. Increased renal resistive indices (RI)s in hilar, medullary and cortical areas on Duplex Doppler Ultrasonography and disappearance of gap between interlobar and cortical RIs can be an indicator of reduction in RBF and the possibility of HRS type 1 or HRS type 2 depending on how quickly renal dysfunction develops (Figure 2).1, 38-41. HRS can be superimposed on pre-renal azotemia or CKD.2, 42 Similarly, HRS can progress to ATN if renal vasoconstriction is prolonged or severe.42 Two or more AKI types can occur simultaneously in a patient with cirrhosis making the differentiation challenging. These possibilities should be taken into account when evaluating patients with HRS. Although several new blood and urinary AKI markers have been recently identified, no specific biomarker(s) is available to diagnose HRS superimposed on other AKI etiologies (e.g. pre-renal azotemia, ATN). It remains to be seen whether application of this new classification of HRS to treatment selection and earlier tratment will improve outcomes.
Figure 1.
Algorithm in the diagnosis and treatment of AKI-HRS for hepatorenal disorders in cirrhosis.30, 32-35
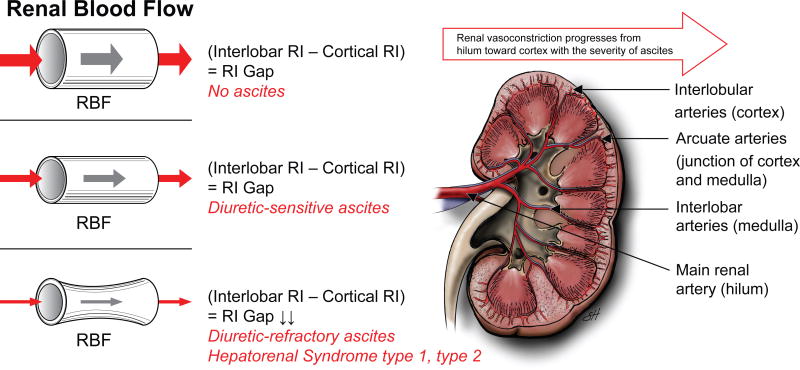
Figure 2.
Renal vasoconstriction in cirrhosis progresses from main renal artery (hilum), toward interlobar arteries (renal medulla) and finally affects arcuate (junction of renal medulla and cortex) and interlobular arteries (renal cortex).40 There is an inverse relationship between renal blood flow (RBF) and renal resistive index (RI).39, 40 When RBF decreases, renal RI increases. 39, 40 While patients without ascites and with diuretic-sensitive ascites preserve cortical renal blood, those with diuretic-refractory ascites have a substantial reduction in cortical renal blood flow. 39, 40 Therefore, while there is a renal RI gap between interlobar and cortical arteries in patients with cirrhosis without ascites and with diuretic-sensitive ascites, this RI gap disappears due to an increase in both interlobar and cortical RIs in patients with cirrhosis and diuretic-refractory ascites.40 Cortical ischemia is considered to be the landmark feature of cirrhosis and diuretic-refractory ascites and HRS.1, 38-41 (Used with permission of Baylor College of Medicine).
AKI and Renal Biomarkers in Patients with Cirrhosis
In recent years, several urinary AKI markers that played a role in trying to determine the etiology of AKI in patients with cirrhosis were reported. Fagundes et al.43 reported that urinary neutrophil gelatinase-associated lipocalin (NGAL) levels were significantly elevated in patients with cirrhosis and ATN compared with those who had pre-renal azotemia and HRS. They reported that the cut-off value of 194 microgram/g Cr for urinary NGAL differentiated HRS from ATN with 91% and 82% sensitivity and specificity, respectively.43 A major limitation of urinary NGAL is that it increases in patients with urinary tract infection and can give false positive results.43 Belcher et al.44 conducted a multicenter, prospective study in inpatients with cirrhosis and AKI and showed that a panel of urinary AKI biomarkers including NGAL, interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), liver-type fatty acid binding protein (L-FABP), and albumin differentiated patients with ATN from those with pre-renal azotemia or HRS. While of some value, the clinician is often faced with the more difficult challenge of differentiating between volume contraction and HRS.
Several renal blood biomarkers have been evaluated rigorously in patients with cirrhosis to estimate GFR, renal plasma flow and renal RIs.21, 39 The results of a study conducted in patients with cirrhosis showed that the mean serum concentrations of Cr, cystatin C, beta-trace protein, beta-2 microglobulin and dimethylarginines including asymmetric (ASMA) and symmetric dimethylarginine (SDMA) were significantly elevated in patients with diuretic-refractory ascites compared to patients with no ascites and diuretic-sensitive ascites.21 In this study, serum Cr and cystatin C significantly predicted measured GFR in patients with cirrhosis.21 GFR markers other than Cr and cystatin C including beta-trace protein, beta-2 microglobulin, and dimethylarginines were also tested in combinations.21 However, the additional proportion of variance explained by adding these GFR markers to the Cr and cystatin C model was not statistically or clinically significant.21
A pilot study that evaluated altered renal hemodynamics in patients with cirrhosis showed that while cystatin C (R-Square=0.43, P=0.038) and beta-2 microglobulin (R-Square=0.46, P=0.030) performed better compared with serum Cr in estimating renal plasma flow (RPF) measured by para-aminohippurate clearance; beta-trace protein (R-Square=0.52, P=0.018) and SDMA (R-Square=0.44, P=0.038) performed better in estimating renal arcuate artery RI, a surrogate marker for cortical blood flow.39
Serum metabolomic profiling may be a powerful non-invasive tool to discover renal biomarkers of renal dysfunction in patients with cirrhosis allowing early diagnosis of HRS, prediction of response to HRS treatment and native kidney recovery after liver transplantation, an important goal to avoid unnecessary SLKT.45 A non-targeted global plasma metabolomic profiling in 103 patients with cirrhosis identified a robust metabolomic signature of hepatic and renal dysfunction consisting of 17 metabolites that were significantly associated with pyrimidine, nicotinate/nicotinamide, purine, inositol phosphate, DNA repair, glycolysis, IL-2/STAT5 signaling and lipid metabolism pathways.45 4-acetamidobutanoate, trans-aconitate, cytidine, myo-inositol, N4-acetylcystidine, N6-carbamoylthreonyladenosine, erythronate, N-acetylserine, pseudouridine, and N2,N2-dimethylguanosine were the 10 most increased metabolites when subjects with high severity of hepatorenal dysfunction were compared to those with low severity hepatorenal dysfunction.45 The practical clinical significance of this metabolomics signature remains unclear but it appears further study in this area is warranted.
As suggested earlier, gender differences exist for serum Cr levels. Cr production rate was reported to be 10% lower in healthy females compared with healthy males who had the same age and weight.46 The Model for End-Stage Liver Disease (MELD) score, which is used to identify patients with cirrhosis with the greatest need for liver transplant consists of four laboratory parameters, one of which is serum Cr.47-49 Lower serum Cr levels in female patients with cirrhosis result in lower MELD scores and in turn, reduced access to liver transplantation and significantly higher mortality on the liver transplant waiting list compared with men with comparable hepatic dysfunction.5, 50 A study conducted in 103 patients with cirrhosis showed that the mean Cr production rate estimated using the Mitch and Walser formula51 (14.50 ± 1.36 vs. 17.12 ±1.90 mg/kg/day, P<0.0001) and mean serum Cr level (0.82 vs 0.97 mg/dL, P=0.023) were significantly lower in women than in men.11 Although the estimated Cr production rate and mean serum Cr level were lower in women, the mean measured GFR was not significantly different between women and men, indicating that the MELD-Na score underestimated renal dysfunction in women.11 There was no significant difference in the mean cystatin C, beta-trace protein, beta-2 microglobulin and SDMA levels between men and women.11 Female sex remained an independent predictor of serum Cr (P=0.003), even after controlling for measured GFR, age, race, height and weight. However, female sex was not a predictor of other GFR markers including cystatin C (P=0.169), beta-trace protein (P=0.463), beta-2 microglobulin (P=0.161), and SDMA (P=0.184).11 Given the results of this study, the revision of the MELD score using either a more accurate estimate of GFR (e.g., new Cr-Cystatin C GFR equation for Cirrhosis21) or gender-neutral biomarkers of renal function alternative to serum Cr (e.g., cystatin C) may eliminate this disadvantage for women on the liver transplant waiting list. Further studies are warranted to eliminate gender disparity on the liver transplant waiting list.
Prevalence of Acute Kidney Injury (AKI) and HRS in Patients with Cirrhosis
The prevalence of AKI and HRS in cirrhosis in published studies show significant variations due to the definition of HRS employed and how rigidly inclusion/exclusion criteria are applied. Reflecting the difficulty in the application of even generally accepted diagnostic criteria, Salerno and colleagues52 reported that they presumed the diagnosis of HRS in 36% of patients as they did not meet all the consensus diagnostic criteria prevailing at that time. In a prospective study conducted by Planas and colleagues53 among 263 patients with decompensated cirrhosis and moderate to severe ascites, 8% of patients developed HRS (3% type 1 and 5% type 2 HRS) during a mean follow-up of 41 months. According to one review where the number of patients with AKI was reported by adding up from multiple references, 19% of hospitalized patients with cirrhosis had AKI/acute renal failure; and among those with acute renal failure, about 17% had HRS54. In a multicenter prospective study of 188 inpatients with cirrhosis and AKI diagnosed by criteria proposed by ADQI and IAC2, about 9% and 21% of subjects were diagnosed with HRS and ATN, respectively.44 In a single study that was conducted among 283 patients with cirrhosis, 42% of patients had AKI defined based on risk, injury, failure, loss and end-stage renal disease (RIFLE)55 criteria.56 Among these 283 patients with cirrhosis, 12% and 11% were diagnosed with HRS and ATN, respectively.56 A prospective study conducted among 90 outpatients with cirrhosis and ascites, 54% of patients developed AKI diagnosed by criteria proposed by ADQI and IAC2 and had 82 episodes of AKI during an average of 14 months of follow-up.57 Huelin et al.58 found that based on the new AKI criteria30, 33, over half of the patients (290 out of 547 patients) with cirrhosis admitted to the hospital had AKI. Not surprisingly, HRS and ATN were more common in patients with stage 2 and 3 AKI compared to patients with stage 1 AKI.58 As expected, the prevalence of HRS in patients with cirrhosis varies most dramatically based on the diagnostic criteria employed and the rigor with which it is applied. At this time, a large cohort study is needed to determine the precise incidence and prevalence of HRS based on the new criteria proposed by the IAC.30
Pathophysiology of HRS
Reduction in Cortical Renal Blood Flow is a Landmark Feature of HRS
The hallmark of HRS is renal dysfunction secondary to renal vasoconstriction in patients with cirrhosis and portal hypertension.1 Rivolta et al.40 assessed RBF by measuring renal RIs using Doppler Ultrasonography. The results of this study showed that in patients with cirrhosis without ascites and diuretic-sensitive ascites, renal RIs showed a gradual decrease starting from the main renal artery, followed by interlobar arteries toward cortical arteries.40 In contrast, in patients cirrhosis and diuretic-refractory ascites, this gradual decrease disappeared as renal RIs were almost equally elevated, not only in the main renal and interlobar arteries but also in the cortical arteries.40 These findings suggest that reduction in RBF in cirrhosis progresses from the renal hilum toward the renal cortex with the severity of ascites, the latter being a surrogate marker for portal hypertension.40 Eventually, the gap between interlobar and cortical artery RIs disappear in patients with diuretic-refractory ascites (Figure 2).40 Similar findings were reported when renal RIs in the renal arcuate arteries were measured to evaluate the cortical RBF in patients with cirrhosis (Figure 2).39 The results of this study showed that patients with cirrhosis and diuretic-refractory ascites had lower filtration fraction and higher kidney arcuate artery resistive index compared to those without ascites.39 This negative correlation between kidney arcuate artery RI and filtration fraction suggests that the inability of kidneys to increase filtration fraction was associated with reductions in renal cortical blood flow in advanced cirrhosis.39 Kew et al.38 demonstrated that there was a significant correlation between cortical blood flow and Cr clearance in patients with cirrhosis. Similarly, Epstein et al.1, 41 showed that in HRS type 1, there was severe vasoconstriction involving cortical renal arteries. While patients without ascites and with diuretic-sensitive ascites preserve cortical renal blood, those with diuretic-refractory ascites and HRS have substantial reduction in RBF in renal cortical arterial flow.39, 40 Marked cortical ischemia is considered to be a landmark feature of cirrhosis and diuretic-refractory ascites and in particular, HRS. 1, 38-41
Nitric Oxide (NO) Dysfunction
Patients with compensated cirrhosis without a baseline CKD may have normal GFR despite mild to moderate reduction in RPF for prolonged periods.38 This is due to increased filtration fraction (normal GFR= [increased filtration fraction]*[reduced RPF])59 compensating for mild to moderate reductions in RPF.39 Filtration fraction is increased by vasoconstrictor effect of angiotensin II on efferent renal arterioles and vasodilator effect of prostaglandins on afferent renal arterioles; thereby preserving adequate pressure in the glomeruli to maintain GFR despite mild to moderate reduction in renal blood flow.59 In advanced stages of cirrhosis however, GFR decreases substantially as the more severe reduction in RPF cannot be compensated by increases in filtration fraction.39, 42 Drugs (e.g. angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, non-steroidal anti-inflammatory drugs) that blunt this compensatory mechanism of kidneys for reduced RBF can further reduce GFR, and thereby result in AKI.59 Several studies suggest that RBF may be reduced at earlier stages of cirrhosis with a progressive reduction in RBF and GFR associated with progressive portal hypertension manifested by increasing severity of ascites.21, 38-40, 60, 61
In cirrhosis, reduction in RBF is attributed to either excessive or insufficient production of NO.62-65 Excessive NO production results in splanchnic vasodilation, reduced effective arterial blood volume, activation of the renin-angiotensin-aldosterone system (RAAS), sympathetic nervous system, renal vasoconstriction and thereby reduced RBF.62-65 While excessive NO production leads to reduced RBF, several investigators have demonstrated that, reduced NO production also causes reduced RBF; and elevated levels of dimethylarginines including SDMA and ADMA are associated with reduced NO production.62-64, 66, 67 NO synthesis from L-arginine is catalyzed by nitric oxide synthase (NOS).68, 69 ADMA, an endogenous inhibitor of NOS62, 68, is hydrolyzed by dimethylarginine dimethylaminohydrolase (DDAH).62, 67, 70-75 Because DDAH activity requires intact liver function64, ADMA levels are elevated in advanced liver disease21, 62, 67, 70-75, thereby inhibiting NOS, reducing NO production and compromising RBF.62-64, 66 In this setting, plasma levels of SDMA, an ADMA isomer, are also increased due to impaired hepatic and renal clearance21, 62, 71. High levels of SDMA compete with L-arginine for endothelial transport71 and further reduce NO production, leading to reduced RBF; it has been suggested that SDMA is a potential marker of HRS.62 Another study has shown that dimethylarginines including SDMA and ADMA, were independent predictors of measured GFR in patients with cirrhosis.21 HRS is clearly a dynamic process, and in the presence of severe, or prolonged reduction in RBF due to altered NO production, may be the mechanism by which HRS can progress to ischemic ATN.42
The Association of HRS with Infections, Systemic Inflammatory Response Syndrome (SIRS), Bile Cast Nephropathy, and Proximal Tubulopathy
Despite currently available treatments, HRS is associated with high mortality.3, 76 Multiple factors including infections, bile cast nephropathy, proximal tubulopathy and progression of HRS to ATN can be associated with poor outcomes and non-response to HRS treatment in patients with cirrhosis.
HRS is often precipitated by infections.77, 78 Barreto et al.77 showed that nearly 70% of patients with infection-related HRS could not recover from HRS even after adequate treatment of infection. It is noted however that in that study, patients received therapy for HRS (terlipressin and albumin) only after the infection was treated; it is possible that earlier simultaneous treatment of infection and HRS may be associated with improved outcomes.77 Age, presence of a nosocomial infection and serum bilirubin level at the time of diagnosis were independent predictors of irreversibility of HRS type 1.77 Similarly, Nazar et al.79 showed that the proportion of patients with HRS type 1 and serum bilirubin levels equal or greater than 10 mg/dL who responded to terlipressin and albumin treatment was significantly lower compared with patients whose serum bilirubin levels were lower than 10 mg/dL (13% vs. 67%, P=0.001). The irreversibility of HRS in patients with cirrhosis and markedly elevated bilirubin levels may be explained by bile cast nephropathy and proximal tubulopathy superimposed on HRS80-82. Van Slambrouck et al.81 reported that 85% of patients with HRS had bile cast nephropathy that was diagnosed by microscopic demonstration of intratubular bile casts positively stained by Hall histochemical staining. Besides bile cast nephropathy superimposed on HRS, patients with cirrhosis and jaundice can develop proximal tubulopathy mimicking Fanconi syndrome and present with low serum uric acid and phosphate and elevated bile acid levels.81-83 While serum bilirubin has commonly been shown to be an independent predictor of response to therapy, this has not been uniformly observed84 and the precise role of bile cast nephropathy in HRS remains unclear.
HRS can also be associated with SIRS with or without infection.78, 85 A multicenter prospective study showed that SIRS was an independent predictor of mortality in patients with cirrhosis and acute functional renal failure.78 Results of this study showed that 59% of patients with cirrhosis and HRS developed SIRS; 50% of those who developed HRS associated with SIRS had an infection.78 A recent retrospective study conducted among 58 patients with HRS associated with SIRS showed that the proportion of patients who had HRS reversal was significantly higher in the terlipressin plus albumin group compared to those who were treated with albumin and placebo.85 No significant improvement in renal function was observed when terlipressin and albumin was administered to patients with HRS without SIRS.85 At first glance, this study outcome may seem implausible; terlipressin, -via its splanchnic and systemic vasoconstrictive effect would be expected to improve renal function better in HRS patients without SIRS compared to those with SIRS.85 However, the observed outcome may be attributed to the indirect anti-inflammatory effect of terlipressin in the presence of SIRS.85 Terlipressin, or other splanchnic vasoconstrictors, by decreasing portal venous pressure may prevent gut bacterial translocation, thereby decreasing the synthesis of pro-inflammatory cytokines (i.e. interleukin-6, tumor necrosis factor-alpha) and endotoxins.85 These findings are in line with studies that showed beneficial effect of pentoxifylline, a tumor necrosis factor-alpha synthesis inhibitor, in reversing HRS in patients with severe alcoholic hepatitis86 and preventing HRS87 and renal insufficiency88 in those with cirrhosis.85 Like other manifestations of acute on chronic liver failure, proinflammatory mechanisms may play a significant role in the pathogenesis of HRS.
Treatment of HRS
Vasoconstrictor Drug Treatment
Once the diagnosis of AKI-HRS is established, the vasopressin analogue terlipressin is considered the first-line vasoconstrictor drug in the treatment of HRS where available89, 90; it is not available in the US (Figure 1D). Terlipressin is generally administered initially at a dose of 0.5-1 mg intravenous (IV) bolus, every 4 to 6 hours (h); the dose can be increased to 2 mg IV bolus every 4 to 6 h if there is less than 25% reduction in serum Cr after 3 days and no side effects occur.35, 89-91 Increasing experience with continuous infusion terlipressin has accumulated suggesting it may be the preferred dosing regimen.92 Terlipressin should be discontinued after a maximum 14 days of treatment if there is no improvement in renal function.35, 89, 91 Where terlipressin is not available, octreotide, a somatostatin analogue in combination with midodrine, an alpha-adrenergic agonist is the recommended drug regimen for the treatment of HRS type I.93, 94 Octreotide is administered as 100 micrograms to 200 micrograms subcutaneously every 8 h. 35, 89-91, 93, 94 Midodrine is administered as 7.5 mg orally three times a day up to 12.5 mg orally three times a day; the dose should be titrated to achieve an increase of 15 mm Hg in mean arterial pressure.35, 89-91, 93, 94 Noradrenaline, an alpha-adrenergic agonist may be used for the treatment of HRS type 1; cardiac monitoring in an intensive care unit is required. 35, 89-91, 93, 94 Noradrenaline is administered at 0.5-3 mg/h continuous IV infusion, titrating dosing to achieve an increase of 10 mm Hg in mean arterial pressure.35, 89-91, 93, 94 Albumin should be given in combination with any vasoconstrictor drug regimens. 35, 89-91, 93, 94 The recommended dose is generally 20 to 40 g IV once daily after the initial dose of albumin is administered as 1 g/kg/day for 2 days.35, 89-91, 93, 94
Several meta-analyses95-102 have evaluated the effectiveness of vasoconstrictors92, 103-117 for reversal of HRS (Table 2). All these studies showed that terlipressin was significantly superior to placebo with or without albumin.95-100, 102 Comparisons of terlipressin to noradrenaline and noradrenaline to octreotide plus midodrine did not show any significant difference in reversing HRS.95, 97, 98, 101 Terlipressin was significantly more efficacious in reversing HRS compared to octreotide plus midodrine.97, 104 A pooled analysis of the two large placebo-controlled, randomized studies in patients with HRS type 1 showed that terlipressin plus albumin was significantly more effective then placebo plus albumin.118
Table 2.
Meta-Analyses of Randomized Controlled Trials of Vasoactive Drugs for Reversal of HRS
| Meta- Analysis Studies |
Number of Studies |
Drug Combinations | Odds Ratio (OR) or Risk Ratio (RR) for HRS Reversal (95% Confidence Interval) |
Heterogeneity (I2) |
Test for Overall Effect (P Value) |
Studies Included in the Meta-Analysis |
|---|---|---|---|---|---|---|
| Fabrizi (2009)96 | 5 | Terlipressin vs. placebo | OR=8.09 [3.52, 18.59] | 41% | 0.0001 | Hadengue (1998)106, Solanki (2003)112, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108 |
| Gluud (2010)99 | 4 | Terlipressin alone or with albumin vs. no intervention or albumin | RR=3.76 [2.21, 6.39] | 0% | Not reported | Solanki (2003)112, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108 |
| Sagi (2010)102 | 4 | Terlipressin vs. placebo | RR=3.66 [2.15, 6.23] | 0% | <0.00001 | Solanki (2003)112, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108 |
| Dobre (2011)95 | 4 | Terlipressin vs. placebo | OR=7.47 [3.17, 17.59] | 24% | <0.00001 | Solanki (2003)112, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108 |
| 2 | Terlipressin vs. noradrenaline | OR=1.23 [0.43, 3.54] | 0% | 0.70 | Alessandria (2007)103,Sharma (2008)110 | |
| 6 | Terlipressin vs. placebo/terlipressin vs. noradrenaline | OR=4.49 [1.75, 11.56] | 56% | 0.002 | Solanki (2003)112, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108, Alessandria (2007)103, Sharma (2008)110 | |
| Gluud (2012)100 | 4 | Terlipressin alone or with albumin vs. no intervention or albumin | RR=3.76 [2.21, 6.39] | 0% | <0.00001 | Solanki (2003)112, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108 |
| Mattos (2016)101 | 4 | Terlipressin vs. noradrenaline | RR=1.03 [0.81, 1.31] | 0% | 0.80 | Alessandria (2007)103, Sharma (2008)110, Singh (2012)111, Ghosh (2013)105 |
| Gifford (2017)98 | 5 | Terlipressin+/-albumin vs. no intervention/placebo+/-albumin | RR=2.54 [1.51, 4.26] | 52% | 0.0004 | Solanki (2003)112, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108, Boyer (2016)117 |
| 1 | Terlipressin infusion vs. terlipressin bolus | RR=1.22 [0.77, 1.93] | Not applicable | 0.40 | Cavallin (2016)92 | |
| 3 | Terlipressin vs. noradrenaline | RR=0.99 [0.67, 1.45] | 0% | 0.94 | Alessandria (2007)103, Sharma (2008)110, Singh (2012)111 | |
| 1 | Terlipressin+albumin vs. dopamine+standard care | RR=2.00 [1.14, 3.52] | Not applicable | 0.02 | Silawat (2011)114 | |
| 1 | Noradrenaline+albumin vs. octreotide+midodrine+albumin | RR=1.25 [0.70, 2.24] | Not applicable | 0.45 | Tavakkoli (2012)113 | |
| Facciorusso (2017)97 | 5 | Terlipressin vs. placebo | OR=4.48 [1.88, 10.67] | 60% | 0.0007 | Sanyal (2008)109, Martin- Llahi (2008)107, Neri (2008)108, Zafar (2012)116, Boyer (2016)117 |
| 4 | Terlipressin vs. noradrenaline | OR=0.89 [0.47, 1.69] | 0% | 0.72 | Alessandria (2007)103, Sharma (2008)110, Singh (2012)111, Indrabi (2013)115 | |
| 1 | Terlipressin vs. octreotide+midodrine | OR=26.25 [3.07, 224.21] | Not applicable | 0.003 | Cavallin (2015)104 | |
| 1 | Noradrenaline vs. octreotide+midodrine | OR=2.50 [0.19, 32.19] | Not applicable | 0.48 | Tavakkoli (2012)113 |
Meta-analyses96-102 have also evaluated drug therapies92, 103-117, 119-121 for mortality reduction without liver transplantation (Table 3). Meta-analyses of terlipressin versus noradrenaline, dopamine plus furosemide, and octreotide plus midodrine did not show any significant reduction in mortality.97, 98, 101 Similarly, meta-analyses of noradrenaline versus octreotide plus midodrine did not significantly reduce mortality.97, 98 Although meta-analyses showed that there was no survival superiority of terlipressin over noradrenaline97, 98, 101, terlipressin was shown to be more economical compared to noradrenaline in the treatment of HRS.101 While these results are disappointing, it must be noted that interventions to improve renal function do not affect the underlying liver disease in these patients. Any intervention should not be expected to have more than a modest effect on survival which would be difficult to demonstrate even in very large studies.
Table 3.
Meta-Analyses of Randomized Controlled Trials of Vasoactive Drugs for Reduction of Mortality
| Meta-Analysis Studies | Number of Studies | Drug Combinations | Odds Ratio (OR) or Risk Ratio (RR) for All-Cause Mortality or Survival (95% Confidence Interval) | Heterogeneity (I2) | Test for Overall Effect (P Value) | Studies Included in the Meta-Analysis |
|---|---|---|---|---|---|---|
| Fabrizi (2009)96 | 5 | Terlipressin vs. placebo | OR=2.06 [0.94, 4.54]* | 55% | 0.07 | Hadengue (1998)106, Solanki (2003)112, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108 |
| Gluud (2010)99 | 6 | Vasoconstrictor drug alone or with albumin vs. no intervention or albumin | RR=0.82 [0.70, 0.96] | 0 | Not reported | Yang (2001)119, Solanki (2003)112,Pomier-Lyrargues (2003)120, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108 |
| Not reported | Terlipressin alone or with albumin vs. no intervention or albumin | RR=0.80 [0.66, 0.97] | Not reported | Not reported | Not reported | |
| Not reported | Terlipressin+albumin vs. albumin | RR=0.81 [0.68, 0.97] | Not reported | Not reported | Not reported | |
| Not reported | Terlipressin vs. no intervention | RR=0.13 [0.01, 2.10] | Not reported | Not reported | Not reported | |
| Not reported | Octreotide+albumin vs. albumin | RR=0.86 [0.58, 1.30] | Not reported | Not reported | Not reported | |
| Sagi (2010)102 | 3 | Terlipressin vs. control/placebo | RR=1.85 [1.00, 3.41]* | 0% | 0.05 | Sanyal (2008) 109, Martin-Llahi (2008)107, Neri (2008)108 |
| Gluud (2012)100 | 5 | Terlipressin alone or with albumin vs. no intervention or albumin | RR=0.75 [0.59, 0.97] | 39% | 0.028 | Yang (2001)119, Solanki (2003)112,Sanyal (2008) 109, Martin-Llahi (2008)107, Neri (2008)108 |
| Mattos (2016)101 | 4 | Terlipressin vs. noradrenaline | RR=1.04 [0.84, 1.30]* | 0% | 0.70 | Alessandria (2007)103, Sharma (2008)110, Singh (2012)111, Ghosh (2013)105 |
| Gifford (2017)98 | 4 | Terlipressin+/-albumin vs. no intervention/placebo+/-albumin | RR=0.79 [0.63, 1.01] | 53% | 0.06 | Solanki (2003)112, Sanyal (2008)109, Neri (2008)108, Boyer (2016)117 |
| 1 | Terlipressin infusion vs. terlipressin bolus | RR=1.58 [0.86, 2.91] | Not applicable | 0.14 | Cavallin (2016)92 | |
| 3 | Terlipressin vs. noradrenaline | RR=1.04 [0.74, 1.47] | 0% | 0.81 | Alessandria (2007)103, Sharma (2008)110, Singh (2012)111 | |
| 2 | Terlipressin+albumin vs. dopamine+standard care | RR=0.98 [0.76, 1.26] | 0% | 0.87 | Silawat (2011)114, Srivastava (2015)121 | |
| 1 | Noradrenaline+albumin vs. octreotide+midodrine+albumin | RR=1.50 [0.60, 3.78] | Not applicable | 0.39 | Tavakkoli (2012)113 | |
| Facciorusso (2017)97 | 6 | Terlipressin vs. placebo | OR=0.65 [0.41, 1.05] | 20% | 0.08 | Solanki (2003)109, Sanyal (2008)109, Martin-Llahi (2008)107, Neri (2008)108, Zafar (2012)116, Boyer (2016)117 |
| 4 | Terlipressin vs. noradrenaline | OR=1.02 [0.46, 2.28] | 0% | 0.95 | Alessandria (2007)103, Sharma (2008)110, Singh (2012)111, Indrabi (2013)115 | |
| 1 | Terlipressin vs. dopamine+furosemide | OR=1.00 [0.18-5.67] | Not applicable | 1.00 | Srivastava (2015)121 | |
| 1 | Terlipressin vs. octreotide+midodrine | OR=0.90 [0.27, 3.05] | Not applicable | 0.87 | Cavallin (2015)104 | |
| 1 | Noradrenaline vs. octreotide+midodrine | OR=2.50 [0.29, 21.40] | Not applicable | 0.40 | Tavakkoli (2012)113 |
Survival was reported instead of mortality.
Renal Replacement Therapy (RRT), Transjugular Intrahepatic Porto-Systemic Shunt (TIPS), and Molecular Adsorbent Recirculating System (MARS)
Non-vasoconstrictor treatment of HRS include RRT, MARS and TIPS. In patients with irreversible HRS with no response to vasoconstrictor drugs, RRT either in the form of hemodialysis or continuous veno-venous hemofiltration should be considered; particularly in the presence of intractable fluid overload and acidosis, uremic symptoms and electrolyte abnormalities (i.e. hyperkalemia, hyponatremia, hypercalcemia).35, 89, 93, 94, 122-124 In a randomized trial of 189 patients with acute on chronic liver failure, MARS significantly decreased serum Cr at day 4 compared to standard medical therapy.125 However, there was no significant difference in 28-day mortality rate between patients with HRS who had MARS compared to those who had standard medical therapy.125 A prospective, randomized, controlled trial showed that patients with HRS type 1 who were treated with MARS, standard medical treatment and hemodiafiltration had significant reduction in serum Cr and mortality compared to those who were treated with standard medical treatment and hemodiafiltration.126 While TIPS is generally contraindicated in patients with unresolved HRS type 1, it was shown to reduce the risk of HRS in patients with cirrhosis and diuretic-refractory ascites.127
Liver Transplantation Alone (LTA) vs. Simultaneous Liver-Kidney Transplantation (SLKT)
Liver transplantation, when available and possible, is clearly the optimal treatment for HRS type 1.93, 94 SLKT is the procedure of choice if native kidney recovery is not expected after LTA.27, 35 Identifying patients who will require SLKT versus LTA remains a major challenge. According to SLKT Summit Consensus recommendations published in 2012, liver transplant candidates with AKI used to be qualified for SLKT if they had a stage 3 AKI for 4 weeks or GFR measured by iothalamate clearance ≤ 25 ml/min or GFR estimated by MDRD-6 equation ≤ 35 ml/min for 4 weeks.27 Candidates with CKD used to be qualified if they had GFR measured by iothalamate clearance ≤ 30 ml/min or GFR estimated by MDRD-6 equation ≤ 40 ml/min, or proteinuria ≥ 2 g/day, greater than 30% global glomerulosclerosis or interstitial fibrosis or metabolic disease for at least 3 months.27 These eligibility criteria did not appear to be strictly applied and showed large variations among liver transplant centers in the US.27, 35 Organ Procurement and Transplantation Network (OPTN) data show that the percentage of adult SLKTs among all adult deceased donor liver transplants in the US increased by 150%; from 4% in 2002 to 10% in 2016128 (Figure 3). The cause for this increase is unclear. Table 4 shows studies that reported potential predictors56, 129-133 and percent native kidney recovery56, 129-134 after SLKT or LTA. Although multiple factors (renal ultrasound findings130, warm ischemia129, severity of AKI56, plasma protein markers131, diabetes131, 132, age131, 132, duration of dialysis132, 133, retransplantation132) have been suggested to be associated with native kidney recovery (Table 4), lack of reliable biomarkers of native kidney recovery after liver transplantation validated in large cohorts appears to be a major contributor to the dramatic increase in the percentage of adult SLKTs among adult deceased liver transplantations. Additionally, under the Share 35 policy implemented in 2013, more patients with cirrhosis and renal dysfunction receive transplants because of the heavily weighted serum Cr in the MELD score. The Share 35 policy assigns a higher priority for liver transplantation to regional waitlist candidates who have MELD scores ≥ 35 than local liver transplant candidates who have MELD score < 35.135 In their recent analysis of Organ Procurement and Transplantation Network data, Formica et al.136 showed that approximately 50% of donor kidneys implanted in liver transplant recipients had a low kidney donor profile index (kidneys with low Kidney donor profile index values have increased donor quality and low risk of graft failure after a kidney transplant137). Originally, these were prioritized for children and other selected patients on the kidney transplant waiting list and this would correspond to about 250 donor kidneys per year on the kidney transplant list.136 In order to optimize the use of donor kidneys, while increasing the availability of kidneys for prioritized kidney transplant candidates, Organ Procurement and Transplantation Network published new guidelines for SLKT to be effective August 10, 2017.138
Figure 3.
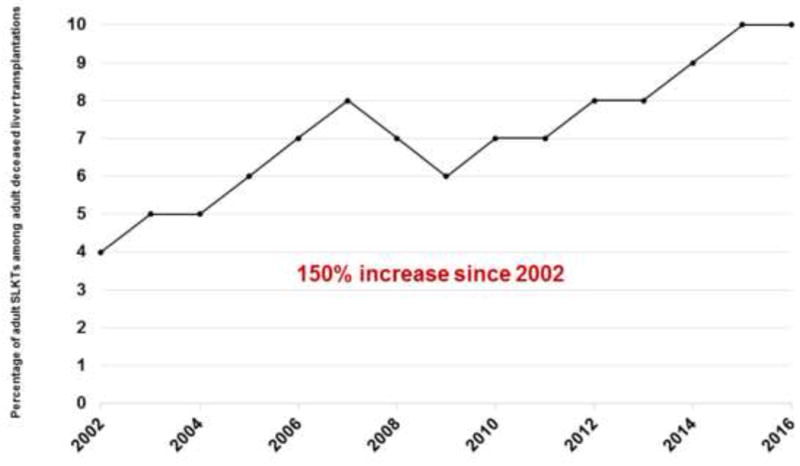
The percentage of adult simultaneous liver-kidney transplants (SLKT) among all adult deceased liver transplants in the US. Since the implementation of MELD score in 2002, there has been a 150% increase in the percentage of adult SLKTs among all adult deceased donor liver transplants in the US; from 4% in 2002 to 10% in 2016. Data are based on Organ Procurement and Transplantation Network data as of January 23, 2017.128
Table 4.
Predictors and Percent Native Kidney Recovery after Simultaneous Liver-Kidney Transplantation (SLKT) or Liver Transplantation Alone (LTA)
| Study | Type of Liver Transplant | Predictors | Number of Subjects | % Recovery | Definition of Native Kidney Recovery | Method used to Evaluate Native Kidney Recovery |
|---|---|---|---|---|---|---|
| Levitsky (2012)130 | SLKT | Renal ultrasound findings | 78 | 51% | > 20 ml/min | GFR measured by Tc-99m DTPA renal scan |
| 27% | > 30 ml/min | |||||
| 17% | > 40 ml/min | |||||
| Francis (2012)134 | SLKT | N/A | 13 | 38% | > 40% of total native kidney function | GFR measured by MAG3 renal scan |
| Nadim (2012)56 | LTA | Etiology of AKI based on RIFLE classification | 118 | ATN group 71% | < 50% increase in serum Cr | Serum Cr |
| Risk group 94% | ||||||
| HRS group 88% | ||||||
| Sharma (2013)132 | LTA | Diabetes, re-transplant, age, duration of dialysis | 2112 | 91% | Not on dialysis, not listed for kidney transplant, did not receive kidney transplant after LTA | Not applicable |
| Levitsky (2014)131 | LTA | Biomarkers (osteopontin, tissue inhibitor of metalloproteinase-1 diabetes, age | 16 test | 58% | Estimated GFR > 50 ml/min | GFR estimated by MDRD-4 equation |
| 46 validation | ||||||
| Wong (2015)133 | LTA | Duration of dialysis | 62 | 76% | HRS reversal defined as serum Cr < 1.5 mg/dL | Serum Cr |
| Laskey (2016)129 | LTA | Liver graft warm ischemia time | 40 | 65% | Not on dialysis, not listed for kidney transplant, serum Cr < 2 mg/dL after LTA | Serum Cr |
DTPA=Diethylene triamine pentaacetic acid; MAG3=mercaptoacetyltriglycine
Conclusions
Recent developments in HRS have created a state of flux in this already somewhat confusing and very challenging diagnostic and therapeutic arena. It still remains unclear as to how to best practically assess GFR in patients with cirrhosis. Serum Cr alone is limited but still remains the best practical assessment of renal function. While newer models such as the Cr-Cystatin GFR equation for Cirrhosis21 are promising, it remains to be seen whether they will find widespread acceptance and application. The clinical implications of the newly proposed diagnostic criteria based on the dynamic, serial changes in serum Cr and the AKIN32, KDIGO clinical practice guidelines for AKI33, ADQI and IAC2 classification systems and revised recommendations of IAC30 remain unclear. These new diagnostic criteria will certainly result in more hospitalized patients with cirrhosis deemed to have AKI, but whether this will result in earlier treatment and improved outcomes is not known. Renal biomarkers, particularly associated with metabolomic profiling, may ultimately prove to be helpful in more precisely establishing a diagnosis of HRS, but they currently appear to be of limited, practical clinical value. Current drug therapy with vasopressors, such as terlipressin and noradrenaline, are effective in improving renal function, although clearly more effective therapy to increase the rates of HRS reversal is needed. The increasing recognition of the roles of NO and the “ACLF inflammatory cascade” should allow the development of non-vasopressor interventions in combination therapies. Liver transplantation remains the definitive treatment for HRS; the current main challenge is the accurate identification of those patients who require SLKT versus LTA. A practical, robust, evidence-based algorithm to predict native kidney recovery after LTA remains an unmet medical need.
Acknowledgments
Funding
This project was supported in part by NIH Public Health Service grant P30DK056338, which funds the Texas Medical Center Digestive Diseases Center and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the NIH.
The authors thank Scott C. Holmes, CMI, a member of the Michael E. DeBakey Department of Surgery Research Core at Baylor College of Medicine, for his assistance during the preparation of Figures 1 and 2
Footnotes
Author Contributions:
Ayse L. Mindikoglu, M.D., M.P.H. conceptualized, designed and drafted the manuscript, tables and figures and performed critical review of the manuscript for important intellectual content. Stephen C. Pappas, M.D. J.D., FCLM, FAASLD conceptualized, designed and critically reviewed the manuscript for important intellectual content.
Disclosures:
Ayse L. Mindikoglu, M.D., M.P.H. A provisional patent application (serial no: 62/442,479) is filed with the US patent office on 01/05/2017 (Metabolomic Markers to Predict Mortality in Patients with Cirrhosis).
Stephen C. Pappas, M.D. J.D., FCLM, FAASLD is a consultant for Orphan Therapeutics LLC and Ikaria Inc., a Mallinckrodt Company.
References
- 1.Epstein M. Hepatorenal syndrome: emerging perspectives of pathophysiology and therapy. J Am Soc Nephrol. 1994;4:1735–53. doi: 10.1681/ASN.V4101735. [DOI] [PubMed] [Google Scholar]
- 2.Wong F, Nadim MK, Kellum JA, et al. Working Party proposal for a revised classification system of renal dysfunction in patients with cirrhosis. Gut. 2011;60:702–9. doi: 10.1136/gut.2010.236133. [DOI] [PubMed] [Google Scholar]
- 3.Alessandria C, Ozdogan O, Guevara M, et al. MELD score and clinical type predict prognosis in hepatorenal syndrome: relevance to liver transplantation. Hepatology. 2005;41:1282–9. doi: 10.1002/hep.20687. [DOI] [PubMed] [Google Scholar]
- 4.Cocchetto DM, Tschanz C, Bjornsson TD. Decreased rate of creatinine production in patients with hepatic disease: implications for estimation of creatinine clearance. Ther Drug Monit. 1983;5:161–8. doi: 10.1097/00007691-198306000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Mindikoglu AL, Regev A, Seliger SL, et al. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl. 2010;16:1147–57. doi: 10.1002/lt.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadakis MA, Arieff AI. Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study Am J Med. 1987;82:945–52. doi: 10.1016/0002-9343(87)90156-2. [DOI] [PubMed] [Google Scholar]
- 7.Sherman DS, Fish DN, Teitelbaum I. Assessing renal function in cirrhotic patients: problems and pitfalls. Am J Kidney Dis. 2003;41:269–78. doi: 10.1053/ajkd.2003.50035. [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 9.Mindikoglu AL, Dowling TC, Weir MR, et al. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatology. 2014;59:1532–42. doi: 10.1002/hep.26556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daugherty NA, Hammond KB, Osberg IM. Bilirubin interference with the kinetic Jaffé method for serum creatinine. Clin Chem. 1978;24:392–3. [PubMed] [Google Scholar]
- 11.Mindikoglu AL, Opekun AR, Mitch WE, et al. Serum Creatinine in Female Patients with Cirrhosis Unfairly Bias Liver Transplant Wait List Ranking: Implications for Elimination of Gender Disparities in Access to Orthotopic Liver Transplantation. Gastroenterology. 2017;152(5):S1120. [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Hoek FJ, Kemperman FA, Krediet RT. A comparison between cystatin C, plasma creatinine and the Cockcroft and Gault formula for the estimation of glomerular filtration rate. Nephrol Dial Transplant. 2003;18:2024–31. doi: 10.1093/ndt/gfg349. [DOI] [PubMed] [Google Scholar]
- 14.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalafateli M, Wickham F, Burniston M, et al. Development and validation of a mathematical equation to estimate glomerular filtration rate in cirrhosis: The royal free hospital cirrhosis glomerular filtration rate. Hepatology. 2017;65:582–591. doi: 10.1002/hep.28891. [DOI] [PubMed] [Google Scholar]
- 16.Larsson A, Malm J, Grubb A, et al. Calculation of glomerular filtration rate expressed in mL/min from plasma cystatin C values in mg/L. Scand J Clin Lab Invest. 2004;64:25–30. doi: 10.1080/00365510410003723. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mindikoglu AL, Dowling TC, Magder LS, et al. Estimation of Glomerular Filtration Rate in Patients With Cirrhosis by Using New and Conventional Filtration Markers and Dimethylarginines. Clin Gastroenterol Hepatol. 2016;14:624–632. e2. doi: 10.1016/j.cgh.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cholongitas E, Ioannidou M, Goulis I, et al. Comparison of creatinine and cystatin formulae with 51 Chromium-ethylenediaminetetraacetic acid glomerular filtration rate in patients with decompensated cirrhosis. J Gastroenterol Hepatol. 2017;32:191–198. doi: 10.1111/jgh.13446. [DOI] [PubMed] [Google Scholar]
- 24.De Souza V, Hadj-Aissa A, Dolomanova O, et al. Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis. Hepatology. 2014;59:1522–31. doi: 10.1002/hep.26886. [DOI] [PubMed] [Google Scholar]
- 25.Francoz C, Nadim MK, Baron A, et al. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: validation of current recommendations. Hepatology. 2014;59:1514–21. doi: 10.1002/hep.26704. [DOI] [PubMed] [Google Scholar]
- 26.Francoz C, Prie D, Abdelrazek W, et al. Inaccuracies of creatinine and creatinine-based equations in candidates for liver transplantation with low creatinine: impact on the model for end-stage liver disease score. Liver Transpl. 2010;16:1169–77. doi: 10.1002/lt.22128. [DOI] [PubMed] [Google Scholar]
- 27.Nadim MK, Sung RS, Davis CL, et al. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12:2901–8. doi: 10.1111/j.1600-6143.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- 28.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179–85. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 29.Filler G, Bokenkamp A, Hofmann W, et al. Cystatin C as a marker of GFR--history, indications, and future research. Clin Biochem. 2005;38:1–8. doi: 10.1016/j.clinbiochem.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968–74. doi: 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 31.Salerno F, Gerbes A, Ginés P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–8. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter Suppl. 2012;2:1–138. [Google Scholar]
- 34.Nadim MK, Durand F, Kellum JA, et al. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J Hepatol. 2016;64:717–35. doi: 10.1016/j.jhep.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 35.O’Leary JG, Levitsky J, Wong F, et al. Protecting the kidney in liver transplant candidates Practice-Based Recommendations from the American Society of Transplantation Liver and Intestine Community of Practice. Am J Transplant. 2016 doi: 10.1111/ajt.13790. [DOI] [PubMed] [Google Scholar]
- 36.Belcher JM, Garcia-Tsao G, Sanyal AJ, et al. Association of AKI with mortality and complications in hospitalized patients with cirrhosis. Hepatology. 2013;57:753–62. doi: 10.1002/hep.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epstein M. Hepatorenal Syndrome. In: Epstein M, editor. The Kidney in Liver Disease. 4th ed. Philadelphia: Hanley & Belfus, Inc; 1996. pp. 75–108. [Google Scholar]
- 38.Kew MC, Brunt PW, Varma RR, et al. Renal and intrarenal blood-flow in cirrhosis of the liver. Lancet. 1971;2:504–10. doi: 10.1016/s0140-6736(71)90435-1. [DOI] [PubMed] [Google Scholar]
- 39.Mindikoglu AL, Dowling TC, Wong-You-Cheong JJ, et al. A pilot study to evaluate renal hemodynamics in cirrhosis by simultaneous glomerular filtration rate, renal plasma flow, renal resistive indices and biomarkers measurements. Am J Nephrol. 2014;39:543–52. doi: 10.1159/000363584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rivolta R, Maggi A, Cazzaniga M, et al. Reduction of renal cortical blood flow assessed by Doppler in cirrhotic patients with refractory ascites. Hepatology. 1998;28:1235–40. doi: 10.1002/hep.510280510. [DOI] [PubMed] [Google Scholar]
- 41.Epstein M, Berk DP, Hollenberg NK, et al. Renal failure in the patient with cirrhosis. The role of active vasoconstriction Am J Med. 1970;49:175–85. doi: 10.1016/s0002-9343(70)80073-0. [DOI] [PubMed] [Google Scholar]
- 42.Mindikoglu AL, Weir MR. Current concepts in the diagnosis and classification of renal dysfunction in cirrhosis. Am J Nephrol. 2013;38:345–54. doi: 10.1159/000355540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fagundes C, Pepin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57:267–73. doi: 10.1016/j.jhep.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Belcher JM, Sanyal AJ, Peixoto AJ, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60:622–32. doi: 10.1002/hep.26980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mindikoglu AL, Opekun AR, Coarfa C, et al. Robust Metabolomic Signature is Associated with Altered Renal Hemodynamics in Patients with Cirrhosis. Gastroenterology. 2017;152(5):S1044. [Google Scholar]
- 46.Bjornsson TD. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet. 1979;4:200–22. doi: 10.2165/00003088-197904030-00003. [DOI] [PubMed] [Google Scholar]
- 47. [January 1, 2017];Organ Procurement and Transplantation Network Policies. Available at: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_09.
- 48.Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359:1018–26. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malinchoc M, Kamath PS, Gordon FD, et al. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–71. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
- 50.Cholongitas E, Marelli L, Kerry A, et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant. 2007;7:685–92. doi: 10.1111/j.1600-6143.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- 51.Mitch WE, Walser M. A proposed mechanism for reduced creatinine excretion in severe chronic renal failure. Nephron. 1978;21:248–54. doi: 10.1159/000181400. [DOI] [PubMed] [Google Scholar]
- 52.Salerno F, Cazzaniga M, Merli M, et al. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J Hepatol. 2011;55:1241–8. doi: 10.1016/j.jhep.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 53.Planas R, Montoliu S, Balleste B, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–94. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064–77. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 55.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nadim MK, Genyk YS, Tokin C, et al. Impact of the etiology of acute kidney injury on outcomes following liver transplantation: acute tubular necrosis versus hepatorenal syndrome. Liver Transpl. 2012;18:539–48. doi: 10.1002/lt.23384. [DOI] [PubMed] [Google Scholar]
- 57.Tsien CD, Rabie R, Wong F. Acute kidney injury in decompensated cirrhosis. Gut. 2013;62:131–7. doi: 10.1136/gutjnl-2011-301255. [DOI] [PubMed] [Google Scholar]
- 58.Huelin P, Piano S, Sola E, et al. Validation of a Staging System for Acute Kidney Injury in Patients With Cirrhosis and Association With Acute-on-Chronic Liver Failure. Clin Gastroenterol Hepatol. 2017;15:438–445. e5. doi: 10.1016/j.cgh.2016.09.156. [DOI] [PubMed] [Google Scholar]
- 59.Koeppen BM, Stanton BA. In: Renal Physiology. 4. Koeppen BM, Stanton BA, editors. Philadelphia: Mosby; 2007. pp. 31–46. [Google Scholar]
- 60.Celebi H, Donder E, Celiker H. Renal blood flow detection with Doppler ultrasonography in patients with hepatic cirrhosis. Arch Intern Med. 1997;157:564–6. doi: 10.1001/archinte.157.5.564. [DOI] [PubMed] [Google Scholar]
- 61.Leslie SH, Johnston B, Ralli EP. Renal function as a factor in fluid retention in patients with cirrhosis of the liver. J Clin Invest. 1951;30:1200–7. doi: 10.1172/JCI102539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lluch P, Mauricio MD, Vila JM, et al. Accumulation of symmetric dimethylarginine in hepatorenal syndrome. Exp Biol Med (Maywood) 2006;231:70–5. doi: 10.1177/153537020623100108. [DOI] [PubMed] [Google Scholar]
- 63.Nijveldt RJ, Teerlink T, Siroen MP, et al. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA) Clin Nutr. 2003;22:17–22. doi: 10.1054/clnu.2002.0612. [DOI] [PubMed] [Google Scholar]
- 64.Nijveldt RJ, Teerlink T, van Leeuwen PA. The asymmetrical dimethylarginine (ADMA)-multiple organ failure hypothesis. Clin Nutr. 2003;22:99–104. doi: 10.1054/clnu.2002.0614. [DOI] [PubMed] [Google Scholar]
- 65.Martin PY, Gines P, Schrier RW. Nitric oxide as a mediator of hemodynamic abnormalities and sodium and water retention in cirrhosis. N Engl J Med. 1998;339:533–41. doi: 10.1056/NEJM199808203390807. [DOI] [PubMed] [Google Scholar]
- 66.Nijveldt RJ, Teerlink T, Van Der Hoven B, et al. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr. 2003;22:23–30. doi: 10.1054/clnu.2002.0613. [DOI] [PubMed] [Google Scholar]
- 67.Vallance P, Leone A, Calver A, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–5. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 68.Vallance P, Leone A, Calver A, et al. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20(Suppl 12):S60–2. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 69.Vallance P. Endothelial regulation of vascular tone. Postgrad Med J. 1992;68:697–701. doi: 10.1136/pgmj.68.803.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lluch P, Torondel B, Medina P, et al. Plasma concentrations of nitric oxide and asymmetric dimethylarginine in human alcoholic cirrhosis. J Hepatol. 2004;41:55–9. doi: 10.1016/j.jhep.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 71.Siroen MP, van der Sijp JR, Teerlink T, et al. The human liver clears both asymmetric and symmetric dimethylarginine. Hepatology. 2005;41:559–65. doi: 10.1002/hep.20579. [DOI] [PubMed] [Google Scholar]
- 72.Siroen MP, Wiest R, Richir MC, et al. Transjugular intrahepatic portosystemic shunt-placement increases arginine/asymmetric dimethylarginine ratio in cirrhotic patients. World J Gastroenterol. 2008;14:7214–9. doi: 10.3748/wjg.14.7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richir MC, Bouwman RH, Teerlink T, et al. The prominent role of the liver in the elimination of asymmetric dimethylarginine (ADMA) and the consequences of impaired hepatic function. JPEN J Parenter Enteral Nutr. 2008;32:613–21. doi: 10.1177/0148607108321702. [DOI] [PubMed] [Google Scholar]
- 74.Vizzutti F, Romanelli RG, Arena U, et al. ADMA correlates with portal pressure in patients with compensated cirrhosis. Eur J Clin Invest. 2007;37:509–15. doi: 10.1111/j.1365-2362.2007.01814.x. [DOI] [PubMed] [Google Scholar]
- 75.Mookerjee RP, Malaki M, Davies NA, et al. Increasing dimethylarginine levels are associated with adverse clinical outcome in severe alcoholic hepatitis. Hepatology. 2007;45:62–71. doi: 10.1002/hep.21491. [DOI] [PubMed] [Google Scholar]
- 76.Cholongitas E, Senzolo M, Patch D, et al. Cirrhotics admitted to intensive care unit: the impact of acute renal failure on mortality. Eur J Gastroenterol Hepatol. 2009;21:744–50. doi: 10.1097/MEG.0b013e328308bb9c. [DOI] [PubMed] [Google Scholar]
- 77.Barreto R, Fagundes C, Guevara M, et al. Type-1 hepatorenal syndrome associated with infections in cirrhosis: natural history, outcome of kidney function, and survival. Hepatology. 2014;59:1505–13. doi: 10.1002/hep.26687. [DOI] [PubMed] [Google Scholar]
- 78.Thabut D, Massard J, Gangloff A, et al. Model for end-stage liver disease score and systemic inflammatory response are major prognostic factors in patients with cirrhosis and acute functional renal failure. Hepatology. 2007;46:1872–82. doi: 10.1002/hep.21920. [DOI] [PubMed] [Google Scholar]
- 79.Nazar A, Pereira GH, Guevara M, et al. Predictors of response to therapy with terlipressin and albumin in patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2010;51:219–26. doi: 10.1002/hep.23283. [DOI] [PubMed] [Google Scholar]
- 80.Adebayo D, Morabito V, Davenport A, et al. Renal dysfunction in cirrhosis is not just a vasomotor nephropathy. Kidney Int. 2015;87:509–15. doi: 10.1038/ki.2014.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Slambrouck CM, Salem F, Meehan SM, et al. Bile cast nephropathy is a common pathologic finding for kidney injury associated with severe liver dysfunction. Kidney Int. 2013;84:192–7. doi: 10.1038/ki.2013.78. [DOI] [PubMed] [Google Scholar]
- 82.Bairaktari E, Liamis G, Tsolas O, et al. Partially reversible renal tubular damage in patients with obstructive jaundice. Hepatology. 2001;33:1365–9. doi: 10.1053/jhep.2001.25089. [DOI] [PubMed] [Google Scholar]
- 83.Moutzouri E, Liberopoulos EN, Elisaf M. Life-threatening hypophosphataemia in a cirrhotic patient with jaundice. Arch Med Sci. 2011;7:736–9. doi: 10.5114/aoms.2011.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boyer TD, Sanyal AJ, Garcia-Tsao G, et al. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: relationship of serum creatinine to hemodynamics. J Hepatol. 2011;55:315–21. doi: 10.1016/j.jhep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong F, Pappas SC, Boyer TD, et al. Terlipressin Improves Renal Function and Reverses Hepatorenal Syndrome in Patients With Systemic Inflammatory Response Syndrome. Clin Gastroenterol Hepatol. 2017;15:266–272. e1. doi: 10.1016/j.cgh.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 86.Akriviadis E, Botla R, Briggs W, et al. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–48. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 87.Tyagi P, Sharma P, Sharma BC, et al. Prevention of hepatorenal syndrome in patients with cirrhosis and ascites: a pilot randomized control trial between pentoxifylline and placebo. Eur J Gastroenterol Hepatol. 2011;23:210–7. doi: 10.1097/MEG.0b013e3283435d76. [DOI] [PubMed] [Google Scholar]
- 88.Lebrec D, Thabut D, Oberti F, et al. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755–62. doi: 10.1053/j.gastro.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 89.Ginès PAP, Lenz K, Møller S, Moore K, Moreau R, Merkel C, Ring-Larsen H, Bernardi M. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 90.Fagundes C, Gines P. Hepatorenal syndrome: a severe, but treatable, cause of kidney failure in cirrhosis. Am J Kidney Dis. 2012;59:874–85. doi: 10.1053/j.ajkd.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 91.Durand F, Graupera I, Gines P, et al. Pathogenesis of Hepatorenal Syndrome: Implications for Therapy. Am J Kidney Dis. 2016;67:318–28. doi: 10.1053/j.ajkd.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 92.Cavallin M, Piano S, Romano A, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: A randomized controlled study. Hepatology. 2016;63:983–92. doi: 10.1002/hep.28396. [DOI] [PubMed] [Google Scholar]
- 93.Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 94.Runyon BA, Aasld Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651–3. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 95.Dobre M, Demirjian S, Sehgal AR, et al. Terlipressin in hepatorenal syndrome: a systematic review and meta-analysis. Int Urol Nephrol. 2011;43:175–84. doi: 10.1007/s11255-010-9725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fabrizi F, Dixit V, Messa P, et al. Terlipressin for hepatorenal syndrome: A meta-analysis of randomized trials. Int J Artif Organs. 2009;32:133–40. [PubMed] [Google Scholar]
- 97.Facciorusso ACA, Murad MH, Prokop LJ, Muscatiello N, Kamath PS, Singh S. Comparative effi cacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:94–102. doi: 10.1016/S2468-1253(16)30157-1. [DOI] [PubMed] [Google Scholar]
- 98.Gifford FJ, Morling JR, Fallowfield JA. Systematic review with meta-analysis: vasoactive drugs for the treatment of hepatorenal syndrome type 1. Aliment Pharmacol Ther. 2017;45:593–603. doi: 10.1111/apt.13912. [DOI] [PubMed] [Google Scholar]
- 99.Gluud LL, Christensen K, Christensen E, et al. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576–84. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 100.Gluud LL, Christensen K, Christensen E, et al. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD005162.pub3. CD005162. [DOI] [PubMed] [Google Scholar]
- 101.Mattos AZ, Mattos AA, Ribeiro RA. Terlipressin versus noradrenaline in the treatment of hepatorenal syndrome: systematic review with meta-analysis and full economic evaluation. Eur J Gastroenterol Hepatol. 2016;28:345–51. doi: 10.1097/MEG.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 102.Sagi SV, Mittal S, Kasturi KS, et al. Terlipressin therapy for reversal of type 1 hepatorenal syndrome: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2010;25:880–5. doi: 10.1111/j.1440-1746.2009.06132.x. [DOI] [PubMed] [Google Scholar]
- 103.Alessandria C, Ottobrelli A, Debernardi-Venon W, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 104.Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology. 2015;62:567–74. doi: 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- 105.Ghosh S, Choudhary NS, Sharma AK, et al. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: a randomized pilot study. Liver Int. 2013;33:1187–93. doi: 10.1111/liv.12179. [DOI] [PubMed] [Google Scholar]
- 106.Hadengue A, Gadano A, Moreau R, et al. Beneficial effects of the 2-day administration of terlipressin in patients with cirrhosis and hepatorenal syndrome. J Hepatol. 1998;29:565–70. doi: 10.1016/s0168-8278(98)80151-7. [DOI] [PubMed] [Google Scholar]
- 107.Martin-Llahi M, Pepin MN, Guevara M, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–9. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 108.Neri S, Pulvirenti D, Malaguarnera M, et al. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci. 2008;53:830–5. doi: 10.1007/s10620-007-9919-9. [DOI] [PubMed] [Google Scholar]
- 109.Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–8. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sharma P, Kumar A, Shrama BC, et al. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. Am J Gastroenterol. 2008;103:1689–97. doi: 10.1111/j.1572-0241.2008.01828.x. [DOI] [PubMed] [Google Scholar]
- 111.Singh V, Ghosh S, Singh B, et al. Noradrenaline vs terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293–8. doi: 10.1016/j.jhep.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 112.Solanki P, Chawla A, Garg R, et al. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152–6. doi: 10.1046/j.1440-1746.2003.02934.x. [DOI] [PubMed] [Google Scholar]
- 113.Tavakkoli H, Yazdanpanah K, Mansourian M. Noradrenalin versus the combination of midodrine and octreotide in patients with hepatorenal syndrome: randomized clinical trial. Int J Prev Med. 2012;3:764–9. [PMC free article] [PubMed] [Google Scholar]
- 114.Silawat FNS, Shaikh MK, Lohana RK, et al. Efficacy of Terlipressin and Albumin in the Treatment of Hepatorenal Syndrome. World Appl Sci J. 2011;12:1946–50. [Google Scholar]
- 115.Indrabi RA, Javid G, Zargar SA, et al. Noradrenaline is equally effective as terlipressin in reversal of type 1 hepatorenal syndrome: a randomized prospective study. J Clin Exp Hepatol. 2013;1:S97. [Google Scholar]
- 116.Zafar S, Haque I, Tayyab GU, et al. Role of terlipressin and albumin combination versus albumin alone in hepatorenal syndrome. Am J Gastroentreology. 2012;107:S175–S76. [Google Scholar]
- 117.Boyer TD, Sanyal AJ, Wong F, et al. Terlipressin Plus Albumin Is More Effective Than Albumin Alone in Improving Renal Function in Patients With Cirrhosis and Hepatorenal Syndrome Type 1. Gastroenterology. 2016;150:1579–1589. e2. doi: 10.1053/j.gastro.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 118.Sanyal AJ, Boyer TD, Frederick RT, et al. Reversal of hepatorenal syndrome type 1 with terlipressin plus albumin vs placebo plus albumin in a pooled analysis of the OT-0401 and REVERSE randomised clinical studies. Aliment Pharmacol Ther. 2017 doi: 10.1111/apt.14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yang YZ, Dan ZL, Liu NZ, et al. Efficacy of terlopressin in treatment of liver cirrhosis with hepatorenal syndrome. Journal of Internal Intensive Medicine. 2001;7:123–5. [Google Scholar]
- 120.Pomier-Layrargues G, Paquin SC, Hassoun Z, et al. Octreotide in hepatorenal syndrome: a randomized, double-blind, placebo-controlled, crossover study. Hepatology. 2003;38:238–43. doi: 10.1053/jhep.2003.50276. [DOI] [PubMed] [Google Scholar]
- 121.Srivastava S, Shalimar, Vishnubhatla S, et al. Randomized Controlled Trial Comparing the Efficacy of Terlipressin and Albumin with a Combination of Concurrent Dopamine, Furosemide, and Albumin in Hepatorenal Syndrome. J Clin Exp Hepatol. 2015;5:276–85. doi: 10.1016/j.jceh.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Capling RK, Bastani B. The clinical course of patients with type 1 hepatorenal syndrome maintained on hemodialysis. Ren Fail. 2004;26:563–8. doi: 10.1081/jdi-200035988. [DOI] [PubMed] [Google Scholar]
- 123.Keller F, Heinze H, Jochimsen F, et al. Risk factors and outcome of 107 patients with decompensated liver disease and acute renal failure (including 26 patients with hepatorenal syndrome): the role of hemodialysis. Ren Fail. 1995;17:135–46. doi: 10.3109/08860229509026250. [DOI] [PubMed] [Google Scholar]
- 124.Witzke O, Baumann M, Patschan D, et al. Which patients benefit from hemodialysis therapy in hepatorenal syndrome? J Gastroenterol Hepatol. 2004;19:1369–73. doi: 10.1111/j.1440-1746.2004.03471.x. [DOI] [PubMed] [Google Scholar]
- 125.Banares R, Nevens F, Larsen FS, et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology. 2013;57:1153–62. doi: 10.1002/hep.26185. [DOI] [PubMed] [Google Scholar]
- 126.Mitzner SR, Stange J, Klammt S, et al. Improvement of hepatorenal syndrome with extracorporeal albumin dialysis MARS: results of a prospective, randomized, controlled clinical trial. Liver Transpl. 2000;6:277–86. doi: 10.1002/lt.500060326. [DOI] [PubMed] [Google Scholar]
- 127.Gines P, Uriz J, Calahorra B, et al. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839–47. doi: 10.1053/gast.2002.37073. [DOI] [PubMed] [Google Scholar]
- 128.Organ Procurement and Transplantation Network. [January 23, 2017];Data. Available at: https://optn.transplant.hrsa.gov/data/
- 129.Laskey HL, Schomaker N, Hung KW, et al. Predicting renal recovery after liver transplant with severe pretransplant subacute kidney injury: The impact of warm ischemia time. Liver Transpl. 2016;22:1085–91. doi: 10.1002/lt.24488. [DOI] [PubMed] [Google Scholar]
- 130.Levitsky J, Baker T, Ahya SN, et al. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant. 2012;12:2949–57. doi: 10.1111/j.1600-6143.2012.04182.x. [DOI] [PubMed] [Google Scholar]
- 131.Levitsky J, Baker TB, Jie C, et al. Plasma protein biomarkers enhance the clinical prediction of kidney injury recovery in patients undergoing liver transplantation. Hepatology. 2014;60:2017–26. doi: 10.1002/hep.27346. [DOI] [PubMed] [Google Scholar]
- 132.Sharma P, Goodrich NP, Zhang M, et al. Short-term pretransplant renal replacement therapy and renal nonrecovery after liver transplantation alone. Clin J Am Soc Nephrol. 2013;8:1135–42. doi: 10.2215/CJN.09600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wong F, Leung W, Al Beshir M, et al. The outcome of patients with cirrhosis and Type-1 hepatorenal sydrome treated with liver transplantation. Liver Transpl. 2015;21:300–7. doi: 10.1002/lt.24049. [DOI] [PubMed] [Google Scholar]
- 134.Francis JM, Palmer MR, Donohoe K, et al. Evaluation of native kidney recovery after simultaneous liver-kidney transplantation. Transplantation. 2012;93:530–5. doi: 10.1097/TP.0b013e3182449161. [DOI] [PubMed] [Google Scholar]
- 135.Feng S, O’Grady J. Share 35: a liver in time saves lives? Am J Transplant. 2015;15:581–2. doi: 10.1111/ajt.13102. [DOI] [PubMed] [Google Scholar]
- 136.Formica RN, Aeder M, Boyle G, et al. Simultaneous Liver-Kidney Allocation Policy: A Proposal to Optimize Appropriate Utilization of Scarce Resources. Am J Transplant. 2016;16:758–66. doi: 10.1111/ajt.13631. [DOI] [PubMed] [Google Scholar]
- 137. [January 21, 2017];A Guide to Calculating and Interpreting the Kidney Donor Profile Index (KDPI) Available at https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf.
- 138.Organ Procurement and Transplantation Network. Policies. [August 19, 2017];Liver-Kidney Allocation. Available at: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_09.