Abstract
Cerebellar ataxia is one of the most frequent syndromes associated with autoantibodies against glutamic acid de-carboxylase (GAD-ab). Antibodies recognize the isoform GAD65, which is the standard biomarker, but additional immunoreactivity against GAD67 is found in high proportion of patients with GAD-ab-associated neurological disorders. We describe the case of a 59-year-old woman who presented with pancerebellar syndrome of subacute onset (9 weeks to nadir). In the etiological study, high titers of GAD-ab were found, but these only recognized the GAD67 isoform and not the GAD65. Screening of GAD67-ab should be considered in late-onset cerebellar ataxia when GAD65-ab are absent.
1. Introduction
Autoantibodies against glutamic acid decarboxylase (GAD-ab) associate with several neurologic disorders such as stiff-person syndrome, cerebellar ataxia, and limbic encephalitis (Saiz et al., 2008). GAD-ab recognize the smaller isoform of the enzyme, GAD65, localized at the intracellular presynaptic site of inhibitory synapses, and high titers of GAD65-ab are currently a biomarker of these disorders. The presence of antibodies against the larger isoform, GAD67, is rarely included in clinical testing despite it has been reported in a high proportion of patients with GAD-ab associated disorders, usually accompanying GAD65-ab (Meinck et al., 2001). It is unknown whether GAD67-ab alone associate with a specific neurological syndrome. We report here the case of a patient who developed cerebellar ataxia along with auto-antibodies restricted to the GAD67 isoform.
2. Case report
In November 2015, a 59-year-old woman presented with sudden onset of dizziness, vomiting and vertigo for 15 days. She denied sensory deficits and other focal neurologic or systemic symptoms, but complained of blurred vision. Neurological examination was remarkable for vertical nystagmus, horizontal diplopia, and mild right-arm dysmetria on finger-to-nose testing. Routine laboratory evaluation, brain MRI and MR-angiography were normal (Fig. 1). She was first treated for vertigo with betahistine and sulpiride, but during the ensuing 9 weeks she had a rapidly progressive neurological deterioration evolving to a pancerebellar syndrome. At examination, she had nystagmus on upward gaze, scanning speech, asymmetric dysmetria involving the 4 limbs, intention tremor, hypotonia, brisk deep tendon reflexes, right ankle clonus, and wide-based ataxic gait requiring bilateral assistance to walk. Cognition was normal. There were no other cranial nerve deficits, and she had normal strength. The score in ICARS scale was 61/100 (Trouillas et al., 1997).
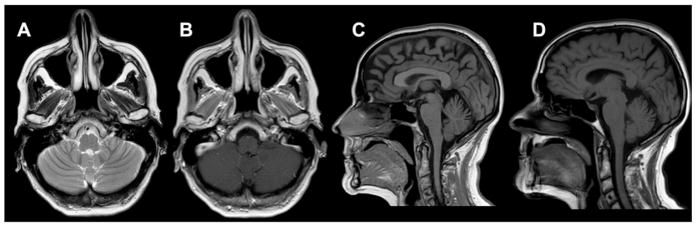
Fig. 1.
Brain MRI. At diagnosis axial T2 (A) and axial T1-gadolinium (B) did not reveal structural abnormalities or contrast enhancement. In sagittal slices, the volume of the cerebellar vermis was stable in a control (D) at 10 months from clinical onset, 6 weeks after the first neuroimaging (C).
Repeat brain MRI was normal (Fig. 1). Blood tests including blood count and renal function were normal. Specific studies for metabolic or nutritional deficits (copper, thyroid function, vitamins E and B12), autoimmune or inflammatory disorders (transglutaminase antibodies, antinuclear and Ro antibodies, angiotensin-converting enzyme) and tumor markers were unremarkable. Whole-body CT scan was normal. Two CSF studies were normal except for a CSF-restricted IgG oligoclonal band. Neuronal antibody studies using frozen rat brain tissue immunohistochemistry revealed a pattern of reactivity suggestive of GAD-ab in CSF and serum (Fig. 2). Immunoblot using 2 commercial kits containing GAD65 (Euroimmune and Ravo) were both negative in serum and CSF. Serum was also negative for GAD65 using a commercial RIA analysis. An in house cell-based assay using HEK293 cells transfected with recombinant human GAD65 or GAD67 (Gresa-Arribas et al., 2015) showed that both serum and CSF contained antibodies restricted to the GAD67 isoform. The titer of GAD-abs was evaluated by serial dilutions in immunohistochemistry until the specific immunoreactivity disappeared. Serum titer was 1:3600 and CSF titer 1:1200. The index of GAD-ab was calculated as previously described (Saiz et al., 2008) and it was 74.8, indicating a strong intrathecal synthesis.
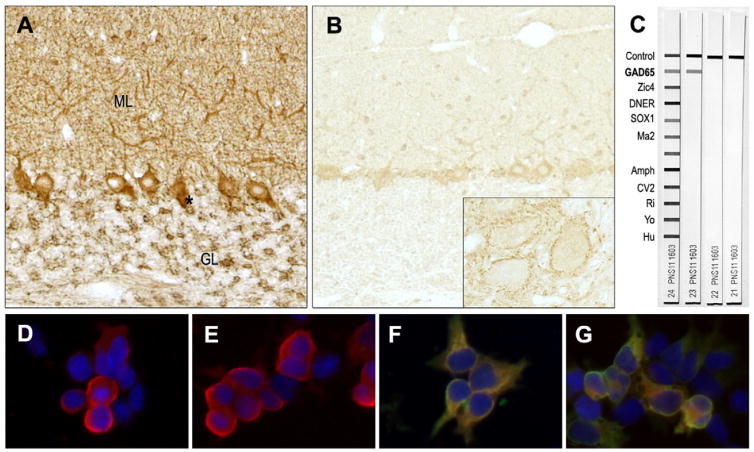
Fig. 2.
Immunoassays for GAD-ab detection. (A, B) Immunohistochemistry on rat cerebellar tissue shows the characteristic staining of the presynaptic inhibitory terminals, especially the axon hillock of Purkinje cells (*), the granular layer (GL) and dendritic tree of Purkinje cells penetrating the molecular layer (ML). At regular conditions for antibody screening this pattern was stronger in the CSF (A, dilution 1:2) compared with the serum (B, dilution 1:500), which had the immunostaining more apparent essentially in the cerebellar deep nuclei (inset, ×40). (C) A commercial immunoblot was negative for GAD65-ab in serum and CSF (strips 22 and 21 respectively), in contrast to the serum from another patient with cerebellar ataxia and GAD-ab (strip 23). (D–E) Merged images of a cell-based assay using HEK293 cells transfected with GAD65 (D, E) or GAD67 (F, G) showing selective recognition of the GAD67 isoform. The yellow signal shows the merged immunofluorescence of the commercial antibody and the patient’s antibody recognizing GAD67 in serum (F) and CSF (G). This contrasts to the GAD65 assay, which is only red due to the absence of reactivity in serum (D) and CSF (E).
The patient was treated with intravenous metilprednisolone (1 g per day during 3 days) and 3 pulses of IVIg (2 g/kg distributed along 5 days), with only mild clinical improvement. Subsequently, she received rituximab (2 cycles of 1 g separated by 15 days) and 1 month later 1 g of cyclophosphamide (the first of 3 cycles in a monthly basis). The patient has not developed diabetes mellitus. In the last follow-up, 8 months after symptom onset, the patient was slightly better; the ICARS was 48/100 and she was able to walk a few steps without assistance.
3. Discussion and conclusions
To our knowledge, this is the first report of cerebellar ataxia associated with antibodies exclusively against GAD67. The clinical picture consistent with late-onset rapidly progressive cerebellar degeneration in a woman close to her sixties, is undistinguishable from the phenotype associated with GAD65-ab (Ariño et al., 2014; Honnorat et al., 2001). High titer of GAD-ab and the demonstration of intrathecal synthesis of antibodies are key findings for the diagnosis of GAD-ab associated neurological disorders (Jarius et al., 2010; Saiz et al., 1997). This case satisfies both conditions. Other cases of neurological syndrome associated exclusively with GAD67-ab include 2 patients with stiff-person syndrome who presented GAD immunoreactivity in serum revealed by immunohistochemistry, negative by immunoblot but recognizing native GAD67 (Johnstone and Nussey, 1994).
Although the significance of GAD67-ab is poorly understood, a recent study focused in the systematic determination of GAD-ab in a large cohort of patients with neurological disorders showed that in patients with GAD65-ab, the study of CSF always showed the presence of GAD67-ab, despite 15% of paired sera were GAD67-ab negative, indicating an active antibody synthesis within the CNS (Gresa-Arribas et al., 2015).
The absence of GAD65-ab in our patient (and presumably in 2 previous patients with stiff-person syndrome) argues against the presence of GAD67-ab being the result of cross-reactivity or an epitope-spreading phenomenon secondary to a dominant epitope in GAD65. Moreover, it raises the question whether GAD65-ab are the most specific biomarker for neurological disorders or, in contrast, we should consider the routine screening of GAD67-ab or both in patients with suspected GAD-ab associated syndromes. It is unknown how antibodies against GAD65 or GAD67 reach their intracellular antigens, so the same concerns about the pathogenic role of GAD65-ab are applicable to GAD67-ab (Alexopoulos and Dalakas, 2010). Considering only their diagnostic value, this case illustrates the importance of GAD67-ab as autoimmune biomarker to identify patients that may respond to immunotherapy and the value of the antibody analysis by immunohistochemistry that detect antibodies against both GAD isoforms in contrast to the most common routine techniques (radioimmunoassay and immunoblot). The therapeutic strategy in patients with cerebellar ataxia and GAD-ab is not clear and the response is variable, but an early diagnosis is mandatory to increase the likelihood of a better outcome (Ariño et al., 2014). In summary, this study shows that patients with neurological symptoms suggestive of GAD-autoimmunity, but GAD65-ab negative, may have antibodies against GAD67.
Acknowledgments
We thank Mercè Albà and Eva Caballero for technical assistance. This study was supported in part by grant PI15/00377 Fondo de Investigaciones Sanitarias, Madrid, Spain; CIBERER (U764) and NIH ROIMH094741. These financial sources had no involvement in the study design, preparation or decision to submit the article for publication.
References
- Alexopoulos H, Dalakas MC. A critical update on the immunopathogenesis of Stiff Person Syndrome. Eur J Clin Investig. 2010;40:1018–1025. doi: 10.1111/j.1365-2362.2010.02340.x. http://dx.doi.org/10.1111/j.1365-2362.2010.02340. [DOI] [PubMed] [Google Scholar]
- Ariño H, Gresa-Arribas N, Blanco Y, Martínez-Hernández E, Sabater L, Petit-Pedrol M, Rouco I, Bataller L, Dalmau JO, Saiz A, Graus F. Cerebellar ataxia and glutamic acid decarboxylase antibodies: immunologic profile and long-term effect of immunotherapy. JAMA Neurol. 2014;71:1009–1016. doi: 10.1001/jamaneurol.2014.1011. http://dx.doi.org/10.1001/jamaneurol.2014.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresa-Arribas N, Ariño H, Martínez-Hernández E, Petit-Pedrol M, Sabater L, Saiz A, Dalmau J, Graus F. Antibodies to inhibitory synaptic proteins in neurological syndromes associated with glutamic acid decarboxylase autoimmunity. PLoS One. 2015;10:e0121364. doi: 10.1371/journal.pone.0121364. http://dx.doi.org/10.1371/journal.pone.0121364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnorat J, Saiz A, Giometto B, Vincent A, Brieva L, de Andres C, Maestre J, Fabien N, Vighetto A, Casamitjana R, Thivolet C, Tavolato B, Antoine J, Trouillas P, Graus F. Cerebellar ataxia with anti-glutamic acid decarboxylase antibodies: study of 14 patients. Arch Neurol. 2001;58:225–230. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- Jarius S, Stich O, Speck J, Rasiah C, Wildemann B, Meinck HM, Rauer S. Qualitative and quantitative evidence of anti-glutamic acid decarboxylase-specific intrathecal antibody synthesis in patients with stiff person syndrome. J Neuroimmunol. 2010;229:219–224. doi: 10.1016/j.jneuroim.2010.07.019. http://dx.doi.org/10.1016/j.jneuroim.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Johnstone AP, Nussey SS. Direct evidence for limited clonality of antibodies to glutamic acid decarboxylase (GAD) in stiff man syndrome using baculovirus expressed GAD. J Neurol Neurosurg Psychiatry. 1994;57:659. doi: 10.1136/jnnp.57.5.659-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinck HM, Faber L, Morgenthaler N, Seissler J, Maile S, Butler M, Solimena M, DeCamilli P, Scherbaum WA. Antibodies against glutamic acid decarboxylase: prevalence in neurological diseases. J Neurol Neurosurg Psychiatry. 2001;71:100–103. doi: 10.1136/jnnp.71.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz A, Arpa J, Sagasta A, Casamitjana R, Zarranz JJ, Tolosa E, Graus F. Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late-onset insulin-dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology. 1997;49:1026–1030. doi: 10.1212/wnl.49.4.1026. [DOI] [PubMed] [Google Scholar]
- Saiz A, Blanco Y, Sabater L, González F, Bataller L, Casamitjana R, Ramió-Torrentà L, Graus F. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–2563. doi: 10.1093/brain/awn183. http://dx.doi.org/10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, Bryer A, Diener HC, Massaquoi S, Gomez CM, Coutinho P, Ben Hamida M, Campanella G, Filla A, Schut L, Timann D, Honnorat J, Nighoghossian N, Manyam B. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]