Abstract
Perfluoroalkyl acids (PFAAs) are highly stable compounds that have been associated with immunotoxicity in epidemiologic studies and experimental rodent models. Lengthy half-lives and resistance to environmental degradation result in bioaccumulation of PFAAs in humans and wildlife. Perfluorooctane sulfonate (PFOS), the most prevalent PFAA detected within the environment, is found at high levels in occupationally exposed humans. We have monitored the environmental exposure of dolphins in the Charleston, SC region for over 10 years and levels of PFAAs, and PFOS in particular, were significantly elevated. As dolphins may serve as large mammal sentinels to identify the impact of environmental chemical exposure on human disease, we sought to assess the effect of environmental PFAAs on the cellular immune system in highly exposed dolphins. Herein, we utilized a novel flow cytometry-based assay to examine T cell-specific responses to environmental PFAA exposure ex vivo and to exogenous PFOS exposure in vitro. Baseline PFOS concentrations were associated with significantly increased CD4+ and CD8+ T cell proliferation from a heterogeneous resident dolphin population. Further analysis demonstrated that in vitro exposure to environmentally relevant levels of PFOS promoted proinflammatory cytokine production and proliferation in a dose-dependent manner. Collectively, these findings indicate that PFOS is capable of inducing proinflammatory interferon-gamma, but not immunoregulatory interleukin-4 production in T cells, which may establish a state of chronic immune activation known to be associated with susceptibility to disease. These findings suggest that PFOS directly dysregulates the dolphin cellular immune system and has implications for health hazards.
Keywords: perfluorooctane sulfonate (PFOS), perfluoroalkyl acids, immunology, bottlenose dolphin (Tursiops truncatus), CD4+ and CD8+, T cell activation, interferon-gamma (IFNγ)
Introduction
Perfluorooctane sulfonate (PFOS) is a persistent organic pollutant consisting of an eight-carbon chain perfluoroalkyl acid (PFAA). PFOS is the principal and final degradation product of several commercial per/polyfluoroalkyl substances (Hansen et al., 2002; Lau et al., 2004). While the production of PFOS and several PFAAs have ceased in the USA and elsewhere, progress on global emission reductions are lagging due to continued production in developing regions and their slow degradation (Lindstrom et al., 2011). An important environmental concern is that PFOS can bioaccumulate and biomagnify in food chains and has been reported in aquatic wildlife and humans (Houde et al., 2006a; Kannan et al., 2004; Olsen et al., 2003). As such, PFOS is the dominant PFAA found in human and wildlife blood (Houde et al., 2011; Lau et al., 2007). Despite reduced production, PFOS continues to be detected globally in biota and the environment and has generated concerns regarding the potential consequences of chronic exposure to wildlife and human health.
PFAA sediment levels in Charleston (SC, USA) were recently found to be higher than any other urban US area with 52% of 36 sites exceeding the median global PFOS sediment concentration (White et al., 2015). Not surprisingly, higher body burdens of specific PFAA compounds are found in Charleston dolphins inhabiting areas with greater developed land use (Adams et al., 2008). Fish are an important dietary source of PFAAs for wildlife and humans (Haug et al., 2010; D’Hollander et al., 2010; Hölzer et al., 2011). As such, a dolphin food web study conducted in 2006 reported significantly higher PFAA concentrations in the Charleston dolphin food web compared to Florida (Houde et al., 2006b). High levels of PFAA have been detected in plasma of resident dolphins inhabiting the estuarine waters of Charleston and in fact, are on the same order of magnitude as that of occupationally exposed humans (Fair et al., 2013; Houde et al., 2005). The Charleston dolphins with their high PFAA levels have served as important sentinels prompting a comprehensive survey of PFAAs in their environment. Dolphins are long-lived, top-level predators in coastal waters where they are exposed to ecosystem perturbations, including anthropogenic contaminants and natural toxins, through both direct exposure and food web magnification. As environmental sentinels, they may provide insight on the health of coastal marine ecosystems and serve as a proxy for human health.
Numerous reports have focused on the occurrence of PFOS along with other PFAAs and their toxic effects including hepatotoxic, carcinogenic, adverse reproductive and developmental, immune- and neurotoxic effects (Lau et al., 2004, 2007; Kennedy et al., 2004; Stahl et al., 2011). Compared to other toxicological endpoints, the immune system appears to be particularly sensitive to exposure of PFOS and other PFAAs as noted in recent reviews (Corsini et al., 2014; DeWitt et al., 2012). Epidemiology and laboratory studies indicate both cell-mediated and humoral immunotoxic effects, including altered cytokine profiles and reduced antibody production (Corsini et al., 2011, 2012; DeWitt et al., 2009; Dong et al., 2009, 2011; Fair et al., 2011, 2013; Granum et al., 2013; Lv et al., 2015; Peden-Adams et al., 2008). Studies in mouse splenocytes demonstrate that PFOS exposure results in cell cycle dysregulation, NRF2-mediated oxidative stress response and activation of T cell receptor and calcium signaling pathways (Lv et al., 2015). The disruption of both T cell-independent and -dependent IgM antibody responses in mice suggest that B cells, macrophages, or both may be specific targets of PFAAs and that additional studies are needed to clarify mechanisms involved with this effect (Corsini et al., 2014). Although in vitro studies have demonstrated that PFAA toxicity occurs, in part, via ligation of peroxisome proliferator-activated receptor alpha (PPARα), few studies have evaluated the ability of PFAAs to modulate directly the inflammatory responses and the specific effects upon T cell activation (Takacs et al., 2007). Mounting evidence suggests that immune effects in laboratory models occur below and within the range of reported levels for highly exposed humans and wildlife (Corsini et al., 2014; DeWitt et al., 2012). In fact, the bioaccumulation of PFAA contaminants in the Charleston dolphins was associated with modulation of their immune responses and thus, may pose a threat to the health of these populations (Fair et al., 2013). Similarly, PFAA exposure has been associated with reduced survival after influenza infection in mice, elevated rates of disease in sea otters, and immune and hematopoietic alterations in dolphin populations (Fair et al., 2013; Guruge et al., 2009; Kannan et al., 2006).
Owing to the limited availability of commercial reagents, direct evaluation of T cell function in dolphins has yet to be examined. Herein, we utilized fluorochrome conjugation methods to label monoclonal antibodies specific for bottlenose dolphin CD4+ and CD8+ T cells appropriate for multiparametric flow cytometry. These reagents were then used to examine T cell responses, including proliferation and cytokine production, from a cohort of native dolphins residing in the estuarine coastal waterways surrounding Charleston. Utilizing flow cytometry for single-cell analysis, we investigated the effects of both baseline toxicant loading and in vitro exposure to environmentally relevant concentrations of PFOS on T cell function.
Materials and methods
Sample collections
Blood samples were collected from free-ranging Atlantic bottlenose dolphins (Tursiops truncatus) during the Dolphin Health and Risk Assessment Project conducted in August 2013. Dolphins were temporarily captured and released in the estuarine waters of Charleston (SC, USA) to assess their clinical and immune status, disease and contaminant exposure. This study was carried out in strict accordance under National Marine Fisheries permit no. 14352–02 issued to G.D.B. and approved by the Florida Atlantic University Institutional Animal Care and Use Committee. Detailed information pertaining to the study site, methods for capture, sampling and release are described elsewhere (Fair et al., 2005). During the sampling process, each animal received a physical examination, including full body photo-documentation, diagnostic ultrasound, blood and urine collection, blubber and lesion biopsies, and microbiologic and cytologic sampling. Once restrained, blood samples were collected from the periarterial venous rete in the flukes using a 19-gauge needle, 1.9 cm butterfly catheter with a vacutainer attachment. Whole blood was collected in 10 ml ethylenediaminetetraacetic acid vacutainers. Peripheral blood leukocytes (PBL) were isolated following lysis of red blood cells after treatment with Becton Dickinson (BD, San Jose, CA, USA) Pharm Lyse solution as per the manufacturer’s instructions. PBLs were isolated within 12 h of sample collection and cryopreserved in freezing media (90% fetal bovine serum, 10% dimethyl sulfoxide [DMSO]). Plasma for PFAA analysis was isolated from whole blood collected in 10 ml sodium heparin tubes. Blood was immediately centrifuged and plasma transferred into polypropylene cryovials, placed in liquid nitrogen Dewar and stored at −80°C until analysis. A tooth was extracted under local anesthesia and sectioned according to established protocols to determine age (Hohn et al., 1989).
Sample perfluoroalkyl acid extraction/instrument analysis
Concentrations of PFAAs in plasma were determined at the Environment Canada’s Laboratories (Burlington, ON, Canada). Plasma samples were stored at −80°C. Sample extraction, analysis and quality control procedures have been previously described in detail (Houde et al., 2005). In brief, plasma (0.25 g) was extracted with acetonitrile, followed by cleanup on a Supelco© graphite carbon solid phase extraction column as described by De Silva et al. (2011) and Reiner et al. (2012). PFAAs were quantified using high-performance liquid chromatography with negative electrospray tandem mass spectrometry. Sixteen PFAA analytes were determined including C6–C14 perfluorocarboxylates, perfluorobutane sulfonate, perfluorohexane sulfonate, perfluorodecane sulfonate, PFOS and perfluorooctane sulfonamide. A detailed list is provided in the supporting information (Tables S1 and S2). Data quality assurance and control measures (Table S2) included both field and laboratory blanks, matrix spikes and a standard reference material injection every 10 samples. Instrumentation consisted of an Agilent 1200 (Santa Clara, CA) series liquid chromatograph coupled to an AB SCIEX© 4000 Q Trap™ tandem mass spectrometer operated in negative electrospray ionization multiple reaction monitoring with a pentafluorophenyl stationary phase (Hypersil Gold PFP 150 × 3 mm, 3 µm; ThermoFisher Scientific, Waltham, MA, USA). Analytes were quantified using C13-labeled surrogates via internal standard calibration curves. The method detection limit was defined as 3× standard deviation the blanks (ng g−1) assuming average sample weight of 0.25 g. If the blanks had non-detect PFAAs then the instrument detection limit at S/N= 10 was used. Results for National Standards and Technology (NIST) 1946 Standard Reference Material (SRM) for PFOS were within 26% of the certified value, while results for other PFAA were generally within the range of consensus values reported by Reiner et al. (2012) (Supporting Information, Table S2).
Cell culture and in vitro perfluorooctane sulfonate treatment
Cryopreserved PBLs were rapidly thawed, washed and plated at 1–5 × 106 cells in supplemented Roswell Park Memorial Institute medium (RPMI-1640 with l-glutamine, 10% fetal bovine serum, 1% non-essential amino acids, 1% sodium pyruvate, 10 mm HEPES, 1% antibiotic/antimycotic [10 000 units ml−1 penicillin, 10 000 µg ml−1 streptomycin and 25 µg ml−1 Gibco (ThermoFisher Scientific) amphotericin B] and 10 µm 2-mercaptoethanol, pH7.4). PBL cultures were exposed to PFOS (perfluorooctane sulfonic acid potassium salt [stated purity >98%]) at concentrations of 0, 0.5 and 5.0 µg ml−1. Exposure concentrations are environmentally relevant levels representing concentrations reported in plasma from Charleston dolphins ranging from 0.5 to 3.1 µg ml−1 (Houde et al., 2005) and 0.3 to 6.3 µg ml−1 (Fair et al., 2012). As a vehicle control, DMSO was used as in all treatments ensuring all cells were exposed to 0.1% DMSO. Cells were incubated at 37°C, 5% CO2 for 0, 48 or 96 h for each assay. PFOS and DMSO were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Characterization of dolphin T cells, proliferation and cytokine production by flow cytometry
Phenotypic characterization of CD4+ and CD8+ T cells was performed by flow cytometry as previously described with minor modifications (Soloff et al., 2014). Monoclonal antibodies with specificity against bottlenose dolphin CD4 and CD8 proteins were generated in mice and kindly provided by Dr. Tracy Romano (Mystic Aquarium, Mystic, CT, USA) (Romano et al., 1999). CD4-and CD8-specific antibodies were fluorescently labeled at the Flow Cytometry and Cell Sorting Shared Resource of the Hollings Cancer Center, Medical University of South Carolina (Charleston, SC, USA) using PE-Cy5.5 and APC-Cy7 fluorochrome conjugation kits, respectively, as per manufacturer’s instructions (Abcam, Cambridge, MA, USA). In all subsequent flow cytometry assays cultured PBL were labeled to identify dead cells by incubation with amine-reactive LiveDead dye (Blue or Aqua; ThermoFisher Scientific, Waltham, MA, USA) for 15 min at 4°C in PBS and then stained for extracellular expression of CD4 or CD8 for 30 min at 4°C in fluorescence-activated cell sorting staining buffer. To measure T cell proliferation, 1–5 × 106 PBL were labeled with 1 µm carboxyfluorescein succinimidyl ester (CFSE) (BioLegend, San Diego, CA, USA) at 37°C for 7 min then thoroughly washed with fluorescence-activated cell sorting staining buffer before in vitro culture with or without PFOS as above. Cells were then sequentially stained with LiveDead, then CD4 and CD8 antibodies were immediately analyzed. To detect intracellular cytokine expression, 1–5 × 106 PBL were cultured in the presence of 0, 0.5 or 5.0 µg ml−1 PFOS with 10 µg ml−1 brefeldin A included for the last 12 h. No additional mitogen was included. Cultured cells were subsequently labeled with LiveDead dye followed by staining for extracellular CD4 and CD8 expression. Cells were then fixed and permeabilized by treatment with BD Cytofix/Cytoperm solution (BD Pharmingen, San Jose, CA, USA) followed by labeling with monoclonal antibodies specific for interferon (IFN) γ (clone CC302, AlexaFluor647; AbD Serotec, Raleigh, NC, USA) and interleukin (IL)-4 (clone CC303, RPE; AbD Serotec) for 30 min at 4°C in the presence of permeabilizing staining buffer. Population gating was performed using a fluorescence minus 1 strategy for all populations. Data were acquired using an LSR Fortessa flow cytometer (BD, San Jose, CA, USA) collecting a minimum of 200 000 events and analyzed using FlowJo V10 (Tree Star, Inc., Ashland, OR, USA).
Statistical analysis
Descriptive statistics were calculated for all demographics and baseline plasma data. PFAA associations between baseline plasma levels of each of the PFAAs and proliferation and cytokine production by cell type over time was evaluated using a series of generalized linear mixed models (GLMMs) assuming a beta distribution with a logit link. The beta distribution was selected, as the outcomes were proportions ranging between 0 and 1. Similarly, the immune parameters for low and high PFOS doses were evaluated using a series of GLMMs assuming a beta distribution and a logit link. All models examining the impact of PFOS perturbation included fixed effects for baseline serum PFOS levels, baseline immune parameters, PFOS treatment level, time and treatment × time interaction. All GLMMs included a random subject effect to account for repeated measurements taken on the same dolphin plasma sample. Model assumptions were checked graphically and transformations and/or quadratic terms were considered in models where linearity assumption did not appear to hold. Using a regression model, we also compared IFNγ production from CD8+ T cells of dolphins considered diseased (n = 7) to those classified as possibly diseased (n = 8) and healthy (n = 4). The health classification was based on Reif et al. (2008), and in our study group, the diseased group included two dolphins with genital papillomatosis and five dolphins with several abnormal hematologic and serum chemistry values. The possibly diseased group had less concerning hematologic and serum chemistry alterations but further diagnostic evaluation was recommended. All analyses were conducted in SAS v. 9.3.1 (IBM Corp. 2011, Armonk, NY, USA).
Results
Population demographics and perfluoroalkyl acid exposure
Samples from 19 animals ranging in age from 8 to 35 years with a mean of 24 years were collected during the dolphin capture–release health assessment studies conducted in 2013. Of the animals sampled, 68% were male and 79% were adults. Previously reported, juvenile dolphins have higher concentrations of PFAAs compared to adults (Fair et al., 2012). We were unable to account for age as a possible confounder; however, as juveniles comprised a small portion of the samples (n = 4) in this study they likely were not a major contributing factor to the overall outcome. Thus, PFAA concentrations in plasma reported in Table 1 are for all dolphins (n = 19).
Table 1.
Plasma perfluoroalkyl acids concentration (ng g−1 wet wt) in dolphins (n = 19) represented as median and range
| Chemical | Chemical name | Median concentrations (ng g−1 wet wt) |
|---|---|---|
| PFHxA | Perfluorohexanoate | 0.14 (0.04–0.41) |
| PFHpA | Perfluoroheptanoate | 6.69 (0.93–38.6) |
| PFOA | Perfluorooctanoate | 23.3 (6.34–55.4) |
| PFNA | Perfluorononanoate | 59.9 (18.6–113) |
| PFDA | Perfluorodecanoate | 70.8 (41.4–110) |
| PFUnA | Perfluoroundecanoate | 25.6 (14.8–69.2) |
| PFDoA | Perfluorododecanoate | 7.16 (3.02–36.4) |
| PFTriA | Perfluorotridecanoate | 4.27 (2.02–11.7) |
| PFTA | Perfluorotetradecanoate | 0.70 (0.31–1.93) |
| PFBS | Perfluorobutane sulfonate | 0.04 (0.04–0.18) |
| PFHxS | Perfluoroheptane sulfonate | 11.8 (4.14–45.4) |
| PFHpS | Perfluorohexane sulfonate | 9.47 (2.22–47.6) |
| PFOS (total) | Perfluorooctane sulfonate | 571 (288–1833) |
| PFOS (linear) | Perfluorooctane sulfonate | 535 (262–1409) |
| PFDS | Perfluorodecane sulfonate | 3.85 (2.03–24.0) |
| PFSOA | Perfluorooctanesulfonamide | 5.61 (2.44–14.6) |
| PFECHS | Perfluoroethylcyclohexane sulfonate | 9.90 (3.29–33.5) |
Environmental perfluoroalkyl acid exposure is associated with enhanced T cell proliferation
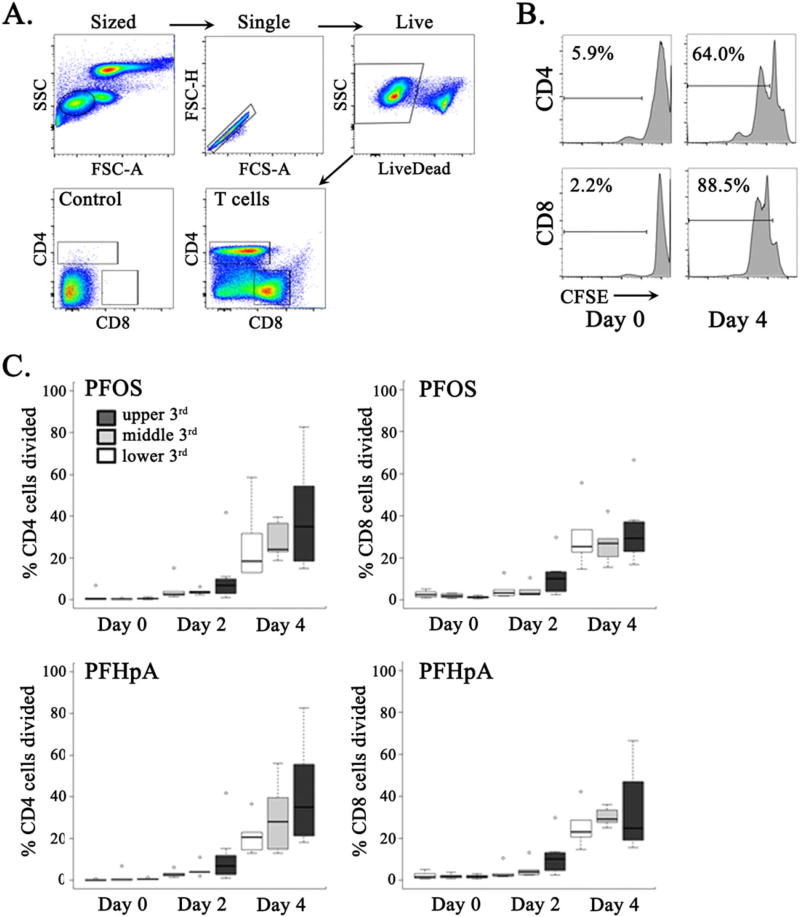
We have previously identified an association between increased perfluoroalkyl compounds and altered immune function in the Charleston dolphin population (Fair et al., 2013). To assess the impact of environmental PFAA exposure on the dolphin T cell compartment ex vivo, we designed multiparametric flow cytometry-based assays utilizing custom-conjugated monoclonal antibodies specific for dolphin CD4 and CD8 receptors. Flow cytometry-based assays allow for direct measurement of CD4+ and CD8+ T cell responses to stimuli at the single-cell level. Herein, we examined the CD4+ and CD8+ T cell division following culture of the PBLs in the absence of mitogenic stimulation, representing baseline proliferation from PFAA-exposed dolphins. T cell division, detected by flow cytometry via CFSE dilution, was evaluated at day 0 and again at 2 and 4 days following sample culture (Quah et al., 2007). Our initial analysis identified a significant correlation between increased plasma levels of PFOS and increased CD8+ T cell division following 2 and 4 days of culture (Supporting Information, Fig. S1). Similarly, elevated perfluoroheptanoate levels were associated with increased CD8+ T cell division after 2 days in culture (Supporting Information, Fig. S1). Although these findings suggest that elevated PFAA levels affect CD8+ T cell proliferation over time, correlative analysis does not control for repeatedly measuring the same PBL sample at different times. As such, animals were stratified into lower, middle and upper tertiles based on plasma PFAA concentrations. Tests for univariate associations between the T cell division and plasma concentrations for 16 PFAAs found a significant association between increased proliferation among both CD4+ and CD8+ T cells and plasma PFOS and perfluoroheptanoate levels (Fig. 1). Additionally, there was a trend towards greater proliferation with increasing plasma levels of perfluoroundecanoate, in both CD4+ and CD8+ T cells, with increasing levels of perfluorodecane sulfonate in CD4+ cells, and with increasing levels of perfluorodecanoate in CD8+ cells, although none of these relationships achieved statistical significance (data not shown).
Figure 1.
Environmental PFOS exposure promotes dolphin T cell division. (A) Representative dot plots depicting the gating strategy used to identify sized (SSC vs. FSC-A), single (FSC-H vs. FSC-A), live (LiveDeadneg), CD4+ and CD8+ T cells in dolphin peripheral blood leukocytes. (B) Illustration of CFSE staining profile of CD4+ and CD8+ T cells from peripheral blood leukocytes at day 0 and following 4 days incubation with exogenous PFOS. Numbers represent the percentage of cells in each gate. (C) CD4+ and CD8+ T cell proliferation following 4 days culture in the absence of exogenous PFOS. Extent of CD4+ and CD8+ T cell division is shown in groups of dolphins segregated by tertiles of plasma PFOS (upper graphs) and PFHpA (lower graphs). CFSE, carboxyfluorescein succinimidyl ester; FSC-A, forward scatter-area; FSC-H, forward scatter-height; PFHpA, perfluoroheptanoate; PFOS, perfluorooctane sulfonate; SSC, side scatter. [Colour figure can be viewed at wileyonlinelibrary.com]
Perfluorooctane sulfonate exposure induces profound T cell division
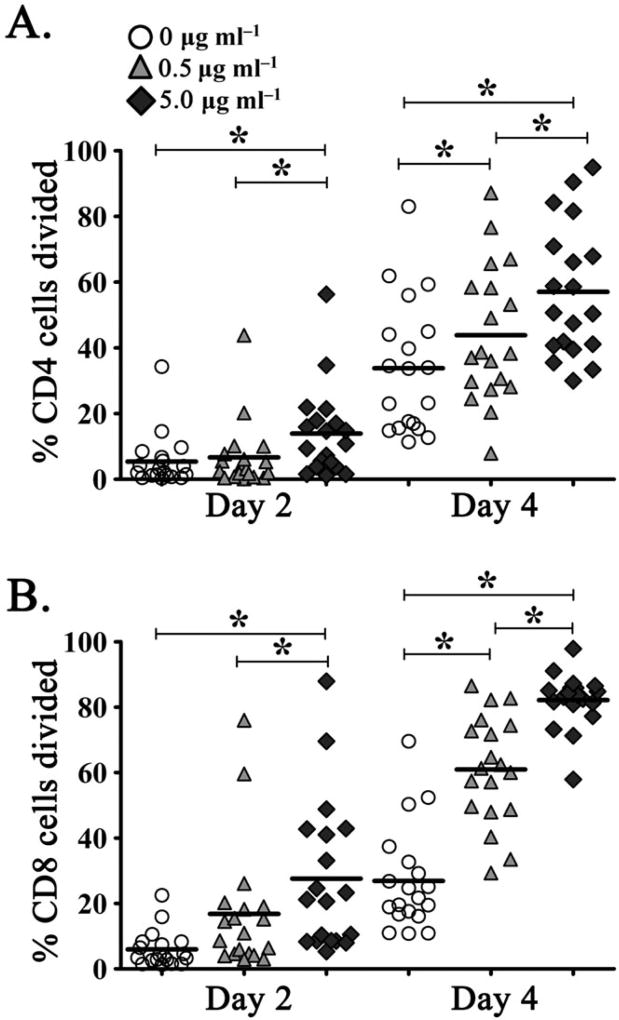
Given the finding that elevated plasma PFAA concentrations in coastal dolphins were associated with increased T cell division ex vivo, we next sought to define the role of PFAA exposure on T cell function in vitro. As plasma concentrations of PFOS are consistently among the highest PFAAs detected in humans and dolphins, the immunotoxicity of PFOS was evaluated. Furthermore, we have previously observed that PFOS exposure in vitro enhances dolphin lymphocyte proliferation in response to mitogenic stimulation, an effect that was positively associated with elevated plasma PFOS levels in wild dolphins and thus levels of environmental exposure (Wirth et al., 2014). Herein, PBLs were cultured in the presence of 0, 0.5 or 5.0 µg ml−1 PFOS, without mitogenic stimulation and the rate of CD4+ and CD8+ T cell division was determined via CFSE dilution following 2 and 4 days exposure. We observed robust proliferation in response to PFOS exposure, with 57% ± 4.6% SEM and 82% ± 1.9% SEM cells dividing within the CD4+ and CD8+ T cell subsets, respectively, following 4 days, high-dose co-culture (Fig. 2). To account for variable levels of environmental PFAA exposure in study subjects, T cell division was analyzed using a multivariable GLM regression model, which included day 0 cell division, plasma concentration of PFOS, concentration of PFOS exposure in vitro, time post-exposure and exposure level by time interaction (McCulloch et al., 2008). Additionally, this model included a random sample effect to control for the correlation due to repeatedly measuring the same plasma sample under different PFOS exposure levels and times (McCulloch et al., 2008). Notably, the amount of PFOS exposure, time post-PFOS exposure and the interaction between time and PFOS exposure levels were significantly associated in the final model of cellular division for both CD4+ and CD8+ T cells. We detected a significant dose-dependent effect on lymphocyte proliferation with increased CD4+ and CD8+ T cell division associated with greater exposure to PFOS (P < 0.001). Similarly, this effect was enhanced with time, with longer PFOS co-culture resulting in significantly elevated cell division (P < 0.001). Additionally, we observed a significant interaction between the level of PFOS exposure and time, collectively, such that cells exposed to higher doses of PFOS had greater proliferative with increasing time relative to samples that received a lower dose of PFOS (Fig. 2). Interestingly, exposure to PFOS had no significant effect on T cell apoptosis, irrespective of PFOS dose or duration of exposure, as determined by amine-reactive viability staining (data not shown).
Figure 2.
In vitro exposure to perfluorooctane sulfonate induces dose-dependent CD4+ and CD8+ T cell division in the dolphin. Percentage of (A) CD4+ and (B) CD8+ T cells divided as determined by carboxyfluorescein succinimidyl ester dilution at 2 and 4 days post-culture with 0 µg ml−1 (open circles), 0.5 µg ml−1 (gray triangles) and 5.0 µg ml−1 (dark diamonds) perfluorooctane sulfonate. *P ≤ 0.001.
Perfluorooctane sulfonate promotes a proinflammatory cytokine response in T cells
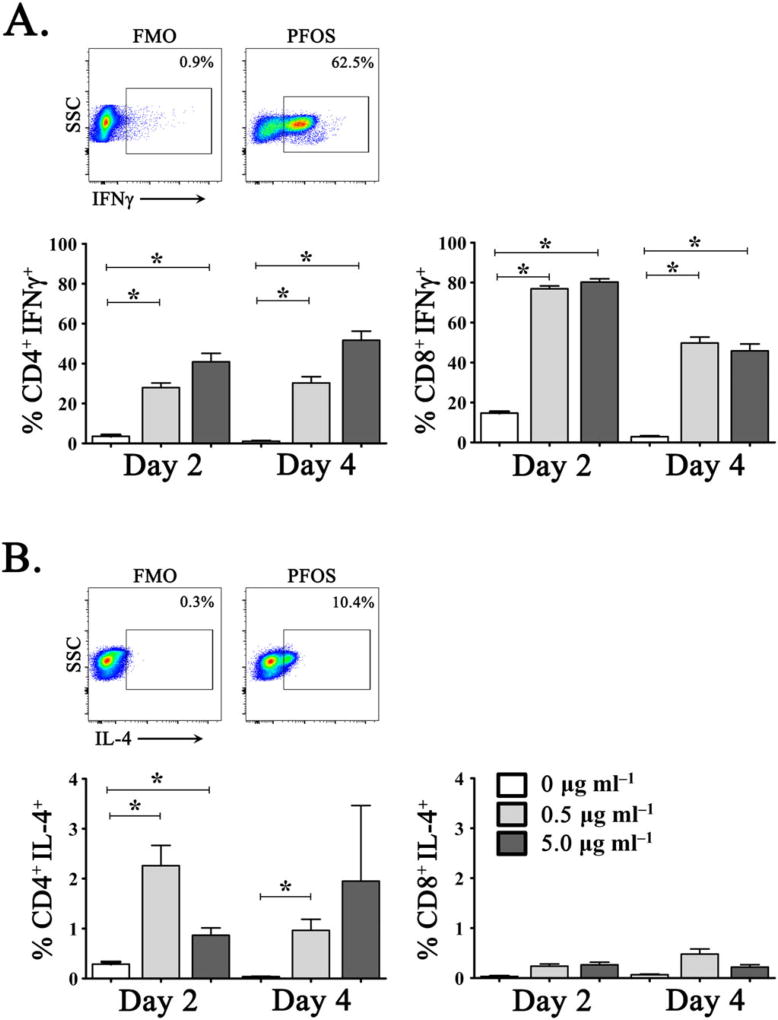
To assess further the effects of PFOS exposure on T cell function, we examined the ability of exogenous PFOS to induce T cell production of the archetypical T-helper (TH)1 and TH2 cytokines, IFNγ and IL-4, respectively. Dolphin PBLs were co-cultured with 0, 0.5 or 5.0 µg ml−1 PFOS, and cytokine production was determined via intracellular flow cytometry after 0, 2 and 4 days. As above, results were analyzed using a multivariable GLM including the percentage of CD4+ and CD8+ T cells that produced IFNγ or IL-4 as the outcome, and independent variables were the dose of PFOS exposure and time of post-PFOS exposure in addition to an exposure level by time interaction. Notably, co-culture with exogenous PFOS induced significant production of IFNγ, but only modest IL-4 from both CD4+ and CD8+ T cells compared to cytokine levels detected from PBLs following exposure to media alone (Fig. 3). Within the CD4+ T cell subset, we detected a dose-dependent effect of PFOS exposure upon IFNγ production with increased PFOS levels associated with greater cytokine production at 2 and 4 days co-culture (P < 0.001) (Fig. 3). Interestingly, exposure to either low- or high-dose PFOS resulted in a rapid and robust IFNγ response from CD8+ T cells at 2 days co-culture (80.2% ± 1.6% high; 76.8% ± 1.4% low), which was significantly reduced by 4 days post-exposure (45.8% ± 3.4% high; 49.7% ± 2.9% low) (Fig. 3). Nevertheless, peak levels of IFNγ produced by CD8+ T cells were nearly twice that of CD4+ T cells, suggesting that the CD8+ T cell compartment may be more susceptible to the effects of PFOS exposure. As such, we examined the ability of environmentally PFAA-exposed CD4+ and CD8+ T cells to produce IFNγ following polyclonal activation with phorbol myristate acetate (50 ng ml−1) and ionomycin (1 µgml−1) during 6 h culture without additional PFOS exposure. Although the group size for the dolphin health categories was small, it was interesting, when measured at day 0 in the absence of further PFOS exposure, CD8+ T cells from diseased animals produced significantly more IFNγ following polyclonal activation than healthy animals based on beta regression model comparisons (mean 45.0% healthy, 52.4% possible disease, 62.4% diseased) (P = 0.042) (data not shown).
Figure 3.
In vitro exposure to PFOS induces potent IFNγ production from dolphin CD4+ and CD8+ T cells. Production of (A) IFNγ and (B) IL-4 in CD4+ (left) and CD8+ (right) T cells determined by intracellular cytokine staining following in vitro culture with 0 µgml−1 (open bars), 5 µgml−1 (gray bar), or 50 µgml−1 (dark bars) exogenous PFOS. Representative images of dolphin peripheral blood T cell production of IFNγ (CD8+) and IL-4 (CD4+) following in vitro stimulation with exogenous PFOS. Representative FMO-negative gating controls are illustrated for IFNγ and IL-4 staining. *P ≤ 0.001. FMO, fluorescence minus 1; IFNγ, interferon-γ; IL-4, interleukin-4; PFOS, perfluorooctane sulfonate; SSC, side scatter. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Free-ranging dolphins are large mammal species that share a marine diet with human coastal inhabitants, representing a unique sentinel population to monitor the effects of environmental contaminant exposure on health outcomes. Owing to the lack of species-specific reagents suitable for the analysis of dolphin leukocytes, our understanding of the cellular immune response in these animals has been limited. In the present study, we utilized multiparametric flow cytometry to examine the effects of PFOS exposure specifically upon the CD4+ and CD8+ T cell populations present in the peripheral blood of native dolphins. These experiments were made possible by advances in fluorochrome conjugation methods, allowing for in-house fluorescence labeling of dolphin-specific antibodies. Flow cytometry provides the ability to examine rapidly large quantities of cells, and importantly, delivers phenotypic and functional data at the single cell level. As such, this study represents the first report detailing the immunotoxic effects of PFOS exposure specifically upon the CD4+ and CD8+ T cell subsets from the dolphin.
Our findings suggest that environmental PFAA exposure dysregulates the cellular immune compartment through driving aberrant T cell activation in the dolphin. Notably, we observed these effects in the absence of antigenic stimulation; thus, measuring a putative major histocompatibility complex independent mechanism. Yet, these data are difficult to interpret in the context of the experimental rodent and in vitro human models of PFAA exposure. Signaling via the PPAR family of nuclear receptor proteins has been implicated in regulating the immunotoxic effects of PFAAs, including PFOS (Corsini et al., 2014). PPAR-mediated pathways regulate lipid metabolism and inflammation, and may affect immunity via signaling of the PPAR receptors expressed by T cells or through bystander activation of PPAR pathways in non-leukocyte populations (Clarke, 2002). Species-specific variation of PPAR receptor expression has been documented, with rodents expressing 10-fold greater levels of PPARα mRNA levels in the liver than humans (Kennedy et al., 2004). In addition, mouse and human PPAR receptors exhibit differential activation following PFOS and perfluorooctanoate stimulation, highlighting species-specific discrepancies of PFAA-induced immunoregulation (Takacs et al., 2007). To date, the role of PPAR activation in the dolphin in response to PFAAs has yet to be examined. In conjunction, PFOS exposure increases protein expression of Themis, CD3G and CAMK4 in mouse splenocytes, associated with upregulation of genes mediating T cell receptor signaling and calcium influx necessary for T cell activation (Lv et al., 2015). Accordingly, in vitro PFOS exposure in this model increased intracellular calcium levels in splenocytes in a dose-dependent manner (Lv et al., 2015). Collectively, our findings in conjunction with alternate experimental models suggest that PFOS may directly stimulate T cell responses through multiple, albeit incompletely defined, mechanisms that may vary among species.
Cytokine production by T cells is a highly ordered process whereby the host elicits a tailored response suitable to eliminate pathogenic or malignant threats, or re-establish homeostasis upon inflammatory resolution. Herein, we have demonstrated that PFOS exposure is sufficient to induce robust IFNγ production from both CD4+ and CD8+ dolphin T cell populations in the absence of significant quantities of IL-4. It is possible that such a TH1-dominant cytokine profile will be the result of non-specific PFOS activation of T cells present within an outbred population of free ranging dolphins. Alternatively, PFOS may directly promote a proinflammatory response in dolphin CD4+ and CD8+ T cells via PPAR and/or yet unknown signaling mechanisms as described above. Interestingly, we observed robust IFNγ production in the CD8+ T cell subset in response to PFOS exposure, which decreased over time (Fig. 3). It is possible that high-level activation rendered the CD8+ T cell compartment refractory to continued PFOS stimulation. Notably, chronic hyperactivation results in T cell exhaustion and cellular immune dysfunction as seen during progressive human immunodeficiency virus infection (Appay & Rowland-Jones, 2002; Day et al., 2006). Although a similar reduction in IFNγ-producing CD4+ T cells was not observed following continued PFOS stimulation, IFNγ responses among CD4+ T cells were markedly lower compared to the CD8+ counterparts, suggesting that CD4+ T cells may be less susceptible to PFOS-mediated activation. These results are in contrast to previous PFOS exposure studies in mice, which have described the development of a TH2-skewed cytokine profile, with increased IL-4 and decreased IFNγ production from bulk splenocyte cultures (Dong et al., 2011; Zheng et al., 2011). Although these studies did not look at T cells directly, we believe it is likely that species-specific variation in PFOS susceptibility and outbred nature of study populations may account for these differences.
T cell regulation is a critical component to maintaining homeostasis and for proper response to, and resolution of disease. The HIV-mediated elimination of CD4+ T cells results in immunodeficiency, whereas CD8+ T cell recruitment and proliferation have been associated with active multiple sclerosis lesions (Hohlfeld et al., 2016). Yet, reports describing the effects of PFOS on leukocyte proliferation and apoptosis across species remain inconclusive. Whereas oral PFOS exposure resulted in a decreased cell count in the spleens and lymph nodes of mice (Dong et al., 2012), exposure of human CD4+ T cells to PFOS in vitro failed to elicit cell death (Midgett et al., 2015). Additionally, we have found that treatment of bulk dolphin PBLs with PFOS in vitro further enhanced mitogen-activated T- and B-cell proliferation, with B cells showing statistical association (Wirth et al., 2014). Notably, our previous studies observed a significant positive association between PFOS levels and increased CD2+ total T cells and CD4+ T-helper cell populations in the peripheral blood of wild dolphins (Aheng et al., 2011; Fair et al., 2013). The finding that PFOS promotes T cell proliferation in vitro suggests that environmental PFOS exposure may result in aberrant immune activation and dysregulation, resulting in increased susceptibility to disease.
Although there is strong epidemiologic evidence of PFAA immunotoxicity in humans, the specific effects of PFAA immune regulation remain uncertain. Prenatal PFAA exposure was associated with dramatic inhibition of vaccine-induced immunity, with a twofold increase in PFOS exposure resulting in a roughly 39% decrease in diphtheria-specific antibody concentrations in children post-immunization (Grandjean et al., 2012). Similar prospective studies observed an increase in cases of the common cold and gastroenteritis, but not allergy in children associated with high levels of prenatal PFAA exposure (Granum et al., 2013). Studies in mice suggest that ingestion of PFOS at levels relevant to those identified in human populations is capable of inducing immunotoxicity characterized by reduced antigen-specific IgM production and increasing susceptibility to influenza A infection (Guruge et al., 2009; Peden-Adams et al., 2008). In the current study, we describe a state of heightened T cell activation in environmentally PFOS-exposed dolphins, characterized by increased proliferation ex vivo and robust proinflammatory IFNγ production and proliferation following exogenous PFOS exposure in vitro. Notably, such chronic activation can lead to T cell exhaustion and state of “frustrated resolution” marked by the inability to resolve chronic inflammation (Fullerton & Gilroy, 2016). Sustained low-level inflammation, termed smoldering inflammation, such as that identified in the current study for our dolphin population is associated with the development of cancer, autoimmune disease and increased susceptibility to infectious disease in humans (Fullerton & Gilroy, 2016; Hinks, 2016; Shalapour & Karin, 2015). In conclusion, the findings reported here suggest that PFOS exposure significantly dysregulates the cellular immune system in wild dolphins, and has consequences for dolphin health. Dolphins represent a worst case model for human health risk assessment due to their constant exposure to contaminants of emerging concern. They represent a prime sentinel species that can be exploited to provide spatial and temporal trends in contaminant exposures and afford early warnings of the potential hazards of environmental exposure to these chemicals. Therefore, the findings reported here suggest that the long-term effects of PFAA exposure on rates of diseases of chronic inflammation in high-risk wildlife and human populations should be examined.
Supplementary Material
Acknowledgments
We would like to thank the numerous researchers who participated in the dolphin capture and release studies in South Carolina. We are particularly grateful to B. Joseph, L. Hansen, S. McCulloch, the NOAA and GA staff, the collaborators and veterinarians who provided their expertise, and the many volunteers whose help made the health assessment studies possible. We thank Mary Williamson (Environment Canada, Burlington, ON) for PFAA analysis and Wayne McFee (NOAA) for the age assessment on the dolphin teeth. We thank Zach McPherson (MUSC) for thoughtful discussions and insight on flow cytometry techniques. The Soloff Lab is supported by research funding provided by the Susan G. Komen Foundation’s Career Catalyst Research Grant (CCR15329745) and by pilot research funding from an American Cancer Society Institutional Research Grant (IRG-97-219-14) awarded to the Hollings Cancer Center. This work was supported in part by the Cell Evaluation & Therapy Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313). Dolphin cells were utilized under Permit No. 16305–00 held by Dr. John Wise.
Footnotes
Conflict of interest
The authors did not report any conflict of interest.
Additional Supporting Information may be found online in the supporting information tab for this article.
References
- Adams J, Houde M, Muir D, Bossart G, Fair PA. Land use and the spatial distribution of perfluoroalkyl compounds as measured in the plasma of bottlenose dolphins (Tursiops truncatus) Mar. Environ. Res. 2008;66:430–437. doi: 10.1016/j.marenvres.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Appay V, Rowland-Jones SL. Premature ageing of the immune system: the cause of AIDS? Trends Immunol. 2002;23:580–585. doi: 10.1016/s1471-4906(02)02338-4. [DOI] [PubMed] [Google Scholar]
- Clark RB. The role of PPARs in inflammation and immunity. J. Leukoc. Biol. 2002;71:388–400. [PubMed] [Google Scholar]
- Corsini E, Avogadroa A, Galbiatia V, dell’Aglib M, Marinovicha M, Gallia CL, dell’Aglib M, Germolec DR. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs) Toxicol. Appl. Pharmacol. 2011;250:108–116. doi: 10.1016/j.taap.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Corsini E, Sangiovannib E, Avogadroa A, Galbiatia V, Viviania B, Marinovicha M, Galli CL, Dell’Agli M, Germolec DR. In vitro characterization of the immunotoxic potential of several perfluorinated compounds (PFCs) Toxicol. Appl. Pharmacol. 2012;258:248–255. doi: 10.1016/j.taap.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Corsini E, Luebke RW, Germolec DR, DeWitt JC. Perfluorinated compounds: emerging POPs with potential immunotoxicity. Toxicol. Lett. 2014;230:263–270. doi: 10.1016/j.toxlet.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- De Silva AO, Spencer C, Scott BF, Backus S, Muir DCG. Detection of a cyclic perfluorinated acid, perfluoroethylcyclohexane sulfonate, in the Great Lakes of North America. Environ. Sci. Technol. 2011;45:8060–8066. doi: 10.1021/es200135c. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Copeland CB, Luebke RW. Suppression of humoral immunity by perfluorooctanoic acid is independent of elevated serum corticosterone concentration in mice. Toxicol. Sci. 2009;109:106–112. doi: 10.1093/toxsci/kfp040. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: Recent developments. Toxicol. Pathol. 2012;40:300–311. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- D’Hollander W, de Voogt P, De Coen W, Bervoets L. Reviews of Environmental Contamination and Toxicology. Vol. 208. Springer; New York: 2010. pp. 179–215. [DOI] [PubMed] [Google Scholar]
- Dong G-H, Zhang Y-H, Zheng L, Liu W, Jin Y-H, He Q-C. Chronic effects of perfluorooctanesulfonate exposure on immunotoxicity in adult male C57BL/6 mice. Arch. Toxicol. 2009;83:805–815. doi: 10.1007/s00204-009-0424-0. [DOI] [PubMed] [Google Scholar]
- Dong G-H, Liu M-M, Wang D, Zheng L, Liang ZF, Jin Y-H. Sub-chronic effect of perfluorooctanesulfonate (PFOS) on the balance of type 1 and type 2 cytokine in adult C57BL6 mice. Arch. Toxicol. 2011;85:1235–1244. doi: 10.1007/s00204-011-0661-x. [DOI] [PubMed] [Google Scholar]
- Dong G-H, Wang J, Zhang YH, Liu MM, Wang D, Zheng L, Jin YH. Induction of p53-mediated apoptosis in splenocytes and thymocytes of C57BL/6 mice exposed to perfluorooctane sulfonate (PFOS) Toxicol. Appl. Pharmacol. 2012;264:292–299. doi: 10.1016/j.taap.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Fair PA, Adams JD, Zolman E, McCulloch SD, Goldstein JD, Murdoch ME, Varela R, Hansen L, Townsend F, Kucklick J, Bryan C, Christopher S, Pugh R, Bossart GD. Protocols for conducting dolphin capture-release health assessment studies. NOAA Technical Memorandum NOS NCCOS. 2005;49:1–83. [Google Scholar]
- Fair PA, Driscoll E, Mollenhauer MAM, Bradshaw SG, Hun Yun S, Kannan K. Effects of environmentally-relevant levels of perfluorooctane sulfonate on clinical parameters and immunological functions in B6C3F1 mice. J. Immunotoxicol. 2011;8:17–29. doi: 10.3109/1547691X.2010.527868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair PA, Houde M, Hulsey TC, Bossart GD, Adams J, Balthis L, Muir DC. Assessment of perfluorinated compounds (PFCs) in plasma of bottlenose dolphins from two southeast US estuarine areas: relationship with age, sex and geographic locations. Mar. Pollut. Bull. 2012;64:66–74. doi: 10.1016/j.marpolbul.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Fair PA, Romano TA, Reif JS, Schaefer AM, Hulsey TC, Bossart GB. Associations between perfluoroalkyl compounds and immune and clinical chemistry parameters in highly exposed bottlenose dolphins (Tursiops truncatus) Environ. Toxicol. Chem. 2013;32:736–746. doi: 10.1002/etc.2122. [DOI] [PubMed] [Google Scholar]
- Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat. Rev. Drug Discov. 2016;15:551–567. doi: 10.1038/nrd.2016.39. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Andersen E, Budtz-Jørgensen E, Nielsen F, Mølbak K, Weihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–397. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, van Loveren H, Løvik M, Nygaard UC. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol. 2013;10:373–379. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- Guruge KS, Hikono H, Shimada N, Murakami K, Hasegawa J, Yeung LWY, Yamanaka N, Yamashita N. Effect of perfluorooctane sulfonate (PFOS) on influenza A virus-induced mortality in female B6C3F1 mice. J. Toxicol. Sci. 2009;34:687–691. doi: 10.2131/jts.34.687. [DOI] [PubMed] [Google Scholar]
- Hansen KJ, Johnson HO, Eldridge JS, Butenhoff JL, Dick LA. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ. Sci. Technol. 2002;36:1681–1685. doi: 10.1021/es010780r. [DOI] [PubMed] [Google Scholar]
- Haug LS, Thomsen C, Brantsaeter AL, Kvalem HE, Haugen M, Becher G. Diet and particularly seafood are major sources of perfluorinated compounds in humans. Environ. Int. 2010;36:772–1778. doi: 10.1016/j.envint.2010.05.016. [DOI] [PubMed] [Google Scholar]
- Hinks TSC. Mucosal-associated invariant T cells in autoimmunity, immune-mediated diseases and airways disease. Immunology. 2016;148:1–12. doi: 10.1111/imm.12582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R, Dornmair K, Meinl E, Wekerle H. The search for the target antigens of multiple sclerosis, part 2: CD8+ T cells, B cells, and antibodies in the focus of reverse-translational research. Lancet Neurol. 2016;15:317–331. doi: 10.1016/S1474-4422(15)00313-0. [DOI] [PubMed] [Google Scholar]
- Hohn AA, Scott MD, Wells RS, Sweeney JC, Irvine AB. Growth layers in teeth from known-age, free-ranging bottlenose dolphins. Mar. Mamm. Sci. 1989;5:315–342. [Google Scholar]
- Hölzer J, Göen T, Just P, Reupert R, Rauchfuss K, Kraft M. Perfluorinated compounds in fish and blood of anglers at Lake Möhne, Sauerland Area, Germany. Environ. Sci. Technol. 2011;45:8046–8052. doi: 10.1021/es104391z. [DOI] [PubMed] [Google Scholar]
- Houde M, Wells RS, Fair PA, Bossart GD, Hohn AA, Rowles TK, Sweeney JC, Solomon KR, Muir DCG. Polyfluoroalkyl compounds in free-ranging bottlenose dolphins (Tursiops truncatus) from the Gulf of Mexico and the Atlantic Ocean. Environ. Sci. Technol. 2005;39:6591–6598. doi: 10.1021/es0506556. [DOI] [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DCG. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006a;40:3463–3473. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- Houde M, Bujas TAD, Small J, Wells R, Fair PA, Bossart G, Solomon KR, Muir DCG. Biomagnification of perfluoroalkyl compounds in the bottlenose dolphin (Tursiops truncatus) food web. Environ. Sci. Technol. 2006b;40:4138–4144. doi: 10.1021/es060233b. [DOI] [PubMed] [Google Scholar]
- Houde M, De Silva AO, Muir DCG, Letcher RJ. Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ. Sci. Technol. 2011;45:7962–7973. doi: 10.1021/es104326w. [DOI] [PubMed] [Google Scholar]
- Kannan K, Corsolini S, Falandysz J, Fillmann G, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH, Aldoust KM. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ. Sci. Technol. 2004;38:4489–4495. doi: 10.1021/es0493446. [DOI] [PubMed] [Google Scholar]
- Kannan K, Perrotta E, Thomas NJ. Association between perfluorinated compounds and pathological conditions in Southern sea otters. Environ. Sci. Technol. 2006;40:4943–4948. doi: 10.1021/es060932o. [DOI] [PubMed] [Google Scholar]
- Kennedy GL, Butenhoff JL, Olsen GW, O’Connor JC, Seacat AW, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit. Rev. Toxicol. 2004;34:351–384. doi: 10.1080/10408440490464705. [DOI] [PubMed] [Google Scholar]
- Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmacol. 2004;198:231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Lau C, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: Past, present, and future. Environ. Sci. Technol. 2011;45:7954–7961. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- Lv Q-Y, Wan B, Guo LH, Yang Y, Ren XM, Zhang H, et al. In vivo immunotoxicity of perfluorooctane sulfonate in BALB/c mice: Identification of T cell receptor and calcium-mediated signaling pathway disruption through gene expression profiling of the spleen. Chem. Biol. Interact. 2015;240:84–93. doi: 10.1016/j.cbi.2015.07.015. [DOI] [PubMed] [Google Scholar]
- McCulloch CE, Searle SR, Neuhaus JM. Generalized, Linear, and Mixed Models. Wiley Publishers; Hoboken, NJ: 2008. [Google Scholar]
- Midgett K, Peden-Adams MM, Gilkeson GS, Kamen DL. In vitro evaluation of the effects of perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) on IL-2 production in human T cells. J. Appl. Toxicol. 2015;35:459–465. doi: 10.1002/jat.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, Armitage JB, Herron RM, Medhdizadehkashi Z, Nobiletti JB, O’Neill M, Mandel JH, Zobel LR. Perfluorooctanesulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ. Health Perspect. 2003;111:1892–1901. doi: 10.1289/ehp.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden-Adams MM, Keller JM, Eudaly JG, Berger J, Gilkeson GS, Keil DE. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci. 2008;104:144–154. doi: 10.1093/toxsci/kfn059. [DOI] [PubMed] [Google Scholar]
- Quah BJC, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat. Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- Reif JS, Fair PA, Adams J, Joseph B, Kilpatrick DK, Sanchez R, Goldstein JD, Townsend FI, McCulloch SD, Mazzoil M, Zolman E, Hansen LJ, Bossart GD. Health status of bottlenose dolphins (Tursiops truncatus) from the Indian River Lagoon, FL and Charleston, SC. J. Am. Vet. Med. Assoc. 2008;233:299–307. doi: 10.2460/javma.233.2.299. [DOI] [PubMed] [Google Scholar]
- Reiner JL, O’Connell SG, Butt CM, Mabury SA, Small JM, De Silva AO, Muir DC, Delinsky AD, Strynar MJ, Lindstrom AB, Reagen WK, Malinsky M, Schäfer S, Kwadijk CJ, Schantz MM, Keller JM. Determination of perfluorinated alkyl acid concentrations in biological standard reference materials. Anal. Bioanal. Chem. 2012;404:2683–2692. doi: 10.1007/s00216-012-5943-5. [DOI] [PubMed] [Google Scholar]
- Romano TA, Ridgway SH, Felten DL, Quaranta V. Molecular cloning and characterization of CD4 in an aquatic mammal, the white whale Delphinapterus leucas. Immunogenetics. 1999;49:376–383. doi: 10.1007/s002510050510. [DOI] [PubMed] [Google Scholar]
- Shalapour S, Karin M. Immunity, inflammation, and cancer: an eternal fight between good and evil. J. Clin. Invest. 2015;125:3347–3355. doi: 10.1172/JCI80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soloff AC, Bissel SJ, Junecko BF, Giles BM, Reinhart TA, Ross TM, Barratt-Boyes SM. Massive mobilization of dendritic cells during influenza A virus subtype H5N1 infection of nonhuman primates. J. Infect. Dis. 2014;209:2014. doi: 10.1093/infdis/jiu009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl T, Mattern D, Brunn H. Toxicology of perfluorinated compounds. Environ. Sci. Eur. 2011;23:1–52. [Google Scholar]
- Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator–activated receptors (α, β/δ, γ) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 2007;95:108–117. doi: 10.1093/toxsci/kfl135. [DOI] [PubMed] [Google Scholar]
- White ND, Balthis L, Kannan K, De Silva AO, Wu Q, French KM, Daugomah J, Spencer C, Fair PA. Elevated levels of perfluoroalkyl substances in estuarine sediments of Charleston, SC. Sci. Total Environ. 2015;521–522:79–89. doi: 10.1016/j.scitotenv.2015.03.078. [DOI] [PubMed] [Google Scholar]
- Wirth JR, Peden-Adams MM, White ND, Bossart GD, Fair PA. In vitro PFOS exposure on immune endpoints in bottlenose dolphins (Tursiops truncatus) and mice. J. Appl. Toxicol. 2014;34:658–666. doi: 10.1002/jat.2891. [DOI] [PubMed] [Google Scholar]
- Zheng L, Dong GH, Zhang YH, Liang ZF, Jin YH, He QC. Type 1 and Type 2 cytokines imbalance in adult male C57BL/6 mice following a 7-day oral exposure to perfluorooctanesulfonate (PFOS) J. Immunotoxicol. 2011;8:30–38. doi: 10.3109/1547691X.2010.537287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.