Key Points
Question
What are the clinical outcomes of patients undergoing primary catheter drainage vs primary relaparotomy to manage pancreatic fistula after pancreatoduodenectomy?
Findings
In this propensity-matched analysis of 309 patients with clinically relevant postoperative pancreatic fistula, mortality was more than twice as high after primary relaparotomy compared with primary catheter drainage.
Meaning
The first step in management of clinically relevant pancreatic fistula should be through minimally invasive catheter drainage.
Abstract
Importance
Postoperative pancreatic fistula is a potentially life-threatening complication after pancreatoduodenectomy. Evidence for best management is lacking.
Objective
To evaluate the clinical outcome of patients undergoing catheter drainage compared with relaparotomy as primary treatment for pancreatic fistula after pancreatoduodenectomy.
Design, Setting, and Participants
A multicenter, retrospective, propensity-matched cohort study was conducted in 9 centers of the Dutch Pancreatic Cancer Group from January 1, 2005, to September 30, 2013. From a cohort of 2196 consecutive patients who underwent pancreatoduodenectomy, 309 patients with severe pancreatic fistula were included. Propensity score matching (based on sex, age, comorbidity, disease severity, and previous reinterventions) was used to minimize selection bias. Data analysis was performed from January to July 2016.
Exposures
First intervention for pancreatic fistula: catheter drainage or relaparotomy.
Main Outcomes and Measures
Primary end point was in-hospital mortality; secondary end points included new-onset organ failure.
Results
Of the 309 patients included in the analysis, 209 (67.6%) were men, and mean (SD) age was 64.6 (10.1) years. Overall in-hospital mortality was 17.8% (55 patients): 227 patients (73.5%) underwent primary catheter drainage and 82 patients (26.5%) underwent primary relaparotomy. Primary catheter drainage was successful (ie, survival without relaparotomy) in 175 patients (77.1%). With propensity score matching, 64 patients undergoing primary relaparotomy were matched to 64 patients undergoing primary catheter drainage. Mortality was lower after catheter drainage (14.1% vs 35.9%; P = .007; risk ratio, 0.39; 95% CI, 0.20-0.76). The rate of new-onset single-organ failure (4.7% vs 20.3%; P = .007; risk ratio, 0.15; 95% CI, 0.03-0.60) and new-onset multiple-organ failure (15.6% vs 39.1%; P = .008; risk ratio, 0.40; 95% CI, 0.20-0.77) were also lower after primary catheter drainage.
Conclusions and Relevance
In this propensity-matched cohort, catheter drainage as first intervention for severe pancreatic fistula after pancreatoduodenectomy was associated with a better clinical outcome, including lower mortality, compared with primary relaparotomy.
This cohort study compares the morbidity and mortality associated with primary catheter draining vs primary relaparotomy in patients with severe pancreatic fistula.
Introduction
Postoperative pancreatic fistula is a common and dreaded complication after pancreatoduodenectomy. This complication, as defined by the International Study Group for Pancreatic Fistula (ISGPF), can be divided into 2 major groups: biochemical, clinically irrelevant fistula (ie, grade A) and clinically relevant pancreatic fistula requiring a change in postoperative management (ie, grades B and C). In a recent systematic review of 40 studies reporting ISGPF-defined pancreatic fistula, clinically relevant pancreatic fistula occurred in 12% of patients after pancreatoduodenectomy and was associated with a mortality up to 39%. Major causes for mortality in these patients are multiple-organ failure and postpancreatectomy hemorrhage as a direct result of the pancreatic fistula.
Consensus on the optimal treatment strategy of clinically relevant pancreatic fistula is lacking. For decades, treatment was through direct relaparotomy. With this approach, surgical lavage and drainage and, if necessary, a completion pancreatectomy to entirely remove the source of sepsis can be performed. This invasive procedure is associated with high mortality. However, other studies have shown that completion pancreatectomy can be performed with a relatively good outcome (ie, low mortality), and the investigators argue that, in patients needing relaparotomy, the operation should be performed as soon as possible. Primary catheter drainage is a less invasive alternative to relaparotomy; it reduces tissue damage and the systemic inflammatory response otherwise induced by surgical stress in these already critically ill patients. In another group of critically ill patients with pancreatic disease (infected necrotizing pancreatitis), standard treatment is now a minimally invasive step-up approach consisting of percutaneous catheter drainage as a first step to be followed by surgical intervention if patients do not improve clinically. Several studies have shown a wide range (15%-50%) in the percentage of patients with pancreatic fistula treated with relaparotomy; however, relaparotomy might be needed in only a small selection of these patients. The aim of the present study was to evaluate the clinical outcome of patients undergoing catheter drainage compared with relaparotomy as the primary treatment for severe pancreatic fistula after pancreatoduodenectomy in 9 centers of the Dutch Pancreatic Cancer Group.
Methods
Design and Study Population
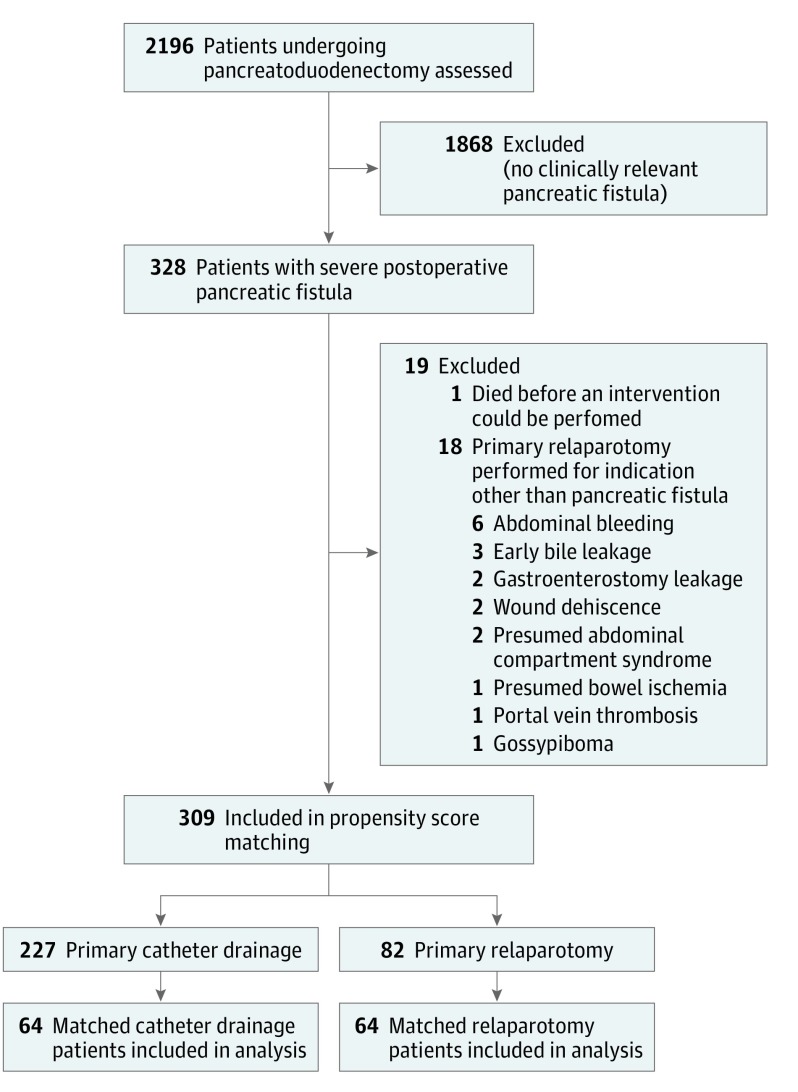
This was a multicenter, retrospective cohort study. All consecutive patients undergoing pancreatoduodenectomy for presumed cancer or precancerous condition ([pre-]malignancy) from January 1, 2005, to September 30, 2013, in 5 academic medical centers and 4 major teaching hospitals of the Dutch Pancreatic Cancer Group were evaluated. Included were patients with pancreatic fistula according to the ISGPF who underwent an invasive intervention to manage pancreatic fistula (ie, patients who were discharged with an intraoperatively placed drain in place and patients requiring additional catheter drainage or relaparotomy, defined as severe pancreatic fistula). We aimed to create an adequate sample of patients in whom pancreatic fistula could have been primarily managed through both relaparotomy and catheter drainage. Therefore, we excluded all patients with pancreatic fistula that was primarily managed with relaparotomy that was indicated by a complication that could not have been managed with catheter drainage (all indications listed in Figure 1). The indications for relaparotomy were assessed by 3 authors (F.J.S., H.C.v.S., and I.Q.M.) independently, and discrepancies were resolved in consensus.
Figure 1. Study Patient Selection.
Selection of patients included in the analyses.
Patients were identified using existing prospective databases from the individual hospitals and by systematic screening of patient files. This study was designed according to the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines and approved by the Medical Ethics Review Committee of the University Medical Center Utrecht, the Netherlands, with waiver of informed patient consent.
Data Collection and Outcomes
Using a predefined, standardized case-record form, we collected data on multiple patient factors, including age, sex, coexisting conditions, body mass index, weight loss, and preoperative cholestasis, as well as details on endoscopic retrograde cholangiopancreatography and pancreatoduodenectomy. In addition, data were obtained on American Society of Anesthesiologists (ASA) class (I, healthy status; II, mild systemic disease; and III, severe systemic disease) and the severity of illness 24 hours before the first intervention for pancreatic fistula as measured by the Acute Physiology and Chronic Health Evaluation II (APACHE II) scale (score ranges from 0 to 71, with higher scores indicating more severe disease); systemic inflammatory response syndrome, as defined by the American College of Chest Physicians and the Society of Critical Care Medicine; and the presence of single- or multiple-organ failure.
The primary outcome was in-hospital mortality. Secondary end points were major complications (ie, new-onset single- or multiple-organ failure or other complications requiring intervention), endocrine and exocrine pancreatic insufficiency, number and type of invasive interventions, length of hospital stay, need for admission to the intensive care unit (ICU), length of ICU stay, and duration of pancreatic fistula (ie, time to removal of last abdominal drain or completion pancreatectomy). Definitions are given in Table 1. Readmission within 10 days after discharge was considered to be the index admission, and follow-up was 90 days after discharge.
Table 1. Definitions of Outcomes.
| Outcome | Definition |
|---|---|
| New onset | Not present any time in 24 h before first intervention |
| Major complications | Single- or multiple-organ failure (eg, failure of ≥2 organ systems), bile leakage or gastroenterostomy leakage, or postpancreatectomy hemorrhage requiring intervention |
| Organ failure | |
| Pulmonary | Pao2 <60 mm Hg despite FiO2 of 0.3 or need for mechanical ventilation |
| Circulatory | Systolic blood pressure <90 mm Hg despite adequate fluid resuscitation or need for inotropic support |
| Renal | Creatinine level >2.0 mg/dL after rehydration or need for hemofiltration or hemodialysis |
| Postoperative pancreatic fistula | Amylase in drain fluid on or after postoperative day 3 of ≥3 times the upper level of normal serum amylase |
| Grade A | Requiring no change in postoperative management; hospital stay not prolonged |
| Grade B | Requiring change in postoperative management (ie, catheter drainage, discharge with intraoperatively placed drains in situ, or no relaparotomy); length of hospital stay might be prolonged |
| Grade C | Requiring relaparotomy and/or admission to ICU and/or pancreatic fistula leading to death; length of hospital stay prolonged |
| Severe pancreatic fistula | Requiring additional drainage or discharged with intraoperatively placed drain in place; requiring relaparotomy (ie, with surgical drainage or additional pancreatic resection) |
| Postoperative bile leakage | Bilirubin in drain fluid on or after postoperative day 3 of ≥3 times the upper level of normal serum bilirubin (adapted from Koch et al) |
| Delayed gastric emptying | Adapted from Wente et al |
| Grade A | Nasogastric tube on postoperative days 4-7 or need for replacement of tube after postoperative day 3; oral intake between days 7 and 14 |
| Grade B | Nasogastric tube on postoperative days 8-14 or need for replacement of tube after postoperative day 7; oral intake between days 14 and 21 |
| Grade C | Nasogastric tube after postoperative day 14 or need for replacement of tube after postoperative day 14; oral intake after day 14 |
| Gastroenteral leakage | Observed on abdominal imaging or during relaparotomy or secretion of fecal material from percutaneous drain or through surgical wound |
| Acute pancreatitis | Combination of abdominal pain, 3-fold increased amylase and lipase levels, or as observed on radiologic imaging |
| New-onset diabetes | Need for insulin or oral diabetic drugs within 3 mo after discharge; not present before pancreatoduodenectomy |
| Exocrine pancreatic insufficiency | Need for oral pancreatic enzyme supplementation within 3 mo after discharge; not present before pancreatoduodenectomy |
Abbreviations: Fio2, fraction of inspired oxygen; ICU, intensive care unit; Pao2, partial pressure of arterial oxygen.
SI conversion factor: To convert creatinine to micromoles per liter, multiply by 88.4.
Statistical Analysis
Patients were divided into 2 groups based on the first intervention for pancreatic fistula: catheter drainage or relaparotomy. These treatment groups were compared for baseline characteristics and outcomes. The Kolmogorov-Smirnov test was used to assess whether continuous data were normally distributed (P < .05). Normally distributed continuous data are presented as mean (SD), and skewed distributions are given as median (interquartile range [IQR]). Dichotomous data were compared using a χ2 test or Fisher exact test as appropriate. Continuous data were compared using the Mann-Whitney test. Length of ICU stay and hospital stay, as well as the duration of pancreatic fistula, were calculated in the survivors.
Propensity score matching was used to minimize the impact of selection bias. Predicted probabilities (ie, the propensity score) for relaparotomy as the first intervention were estimated for each patient using a logistic regression model. Patients undergoing primary relaparotomy were matched to patients undergoing primary catheter drainage with a similar score. All baseline variables possibly influencing the decision on primary treatment or mortality (based on literature and expert opinion) were included in the first model. The efficiency of this model was tested by evaluating the balance in baseline distribution. The optimal model (ie, smallest differences in baseline distribution) was achieved by including sex, age, ASA class, APACHE II score, organ failure 24 hours before the first intervention, and whether a patient underwent another intervention before the first intervention for pancreatic fistula. For practical reasons, patients were excluded if any of these data were missing. We used a 1:1 ratio in nearest-neighbor matching in a random order without replacement and with a caliper fixed to 0.2. Equal distribution of baseline characteristics was tested using standardized differences, defined as the mean difference between the groups divided by the SD of the treatment group. We aimed to reach the smallest standardized mean differences as possible for baseline characteristics, but always less than 0.25, to achieve the best balance. Matched dichotomous outcomes were compared using the McNemar test. Risk ratios (RRs) with 95% CIs were calculated by the method reported by Bonett and Price. Matched continuous outcomes were analyzed using the paired-samples, 2-tailed t test for normally distributed data or Wilcoxon signed rank test for skewed data. Median differences with 95% CIs were calculated using the method reported by Bonett and Price.
A predefined subgroup analysis for disease severity was performed within the entire cohort of patients. We divided patients undergoing primary relaparotomy into 3 subgroups based on the highest APACHE II score within 24 hours before the first intervention. Cutoff points (ie, <9, 9-12, and >12) were chosen so that the number of patients undergoing primary relaparotomy was equally distributed.
Data analysis was conducted from January to July 2016. Analyses were performed using SPSS, version 20.0 (SPSS Inc) and R, version 2.12. For the propensity score matching, the plugin designed by Thoemmes was used. A 2-sided P value <.05 was considered statistically significant.
Results
Study Population
From January 1, 2005, to September 30, 2013, a total of 2196 consecutive pancreatoduodenectomies were performed in the participating hospitals for patients with a presumed malignant or (pre-)malignancy neoplasm. Of these, 328 patients (14.9%) developed severe pancreatic fistula. Nineteen (5.8%) of these patients were excluded: 1 patient with pancreatic fistula who died before undergoing an intervention and 18 patients undergoing primary relaparotomy indicated for a complication that could not have been managed through catheter drainage. Details on patient inclusion are provided in Figure 1.
The final study cohort comprised 309 patients with severe pancreatic fistula after pancreatoduodenectomy; 209 patients (67.6%) were men, and mean (SD) age was 64.6 (10.1) years. In 10 patients, a pancreatogastrostomy was performed; all of the remaining patients underwent a pancreatojejunostomy. Overall in-hospital mortality was 17.8% (55 patients).
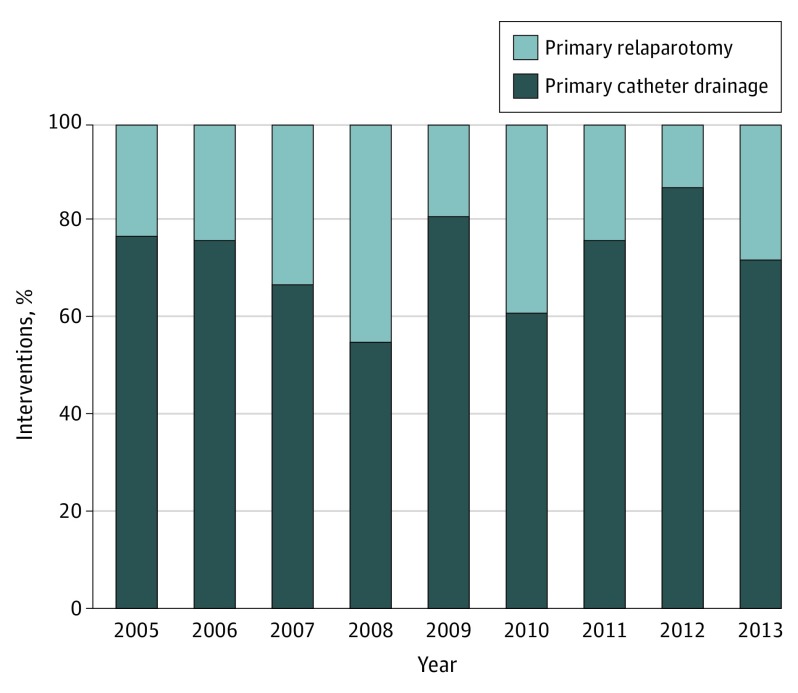
Of all 309 patients, 227 (73.5%) underwent catheter drainage and 82 patients (26.5%) underwent relaparotomy as the first intervention for pancreatic fistula. There was no tendency observed toward catheter drainage as the first intervention for severe pancreatic fistula over the years of inclusion (Figure 2). Primary catheter drainage was successful (ie, discharge without the need for relaparotomy) in 175 patients (77.1%).
Figure 2. First Intervention for Postoperative Pancreatic Fistula Over the Years.
Percentage of patients undergoing catheter drainage vs relaparotomy as primary intervention for pancreatic fistula.
There were important baseline differences observed between the 2 treatment groups in the full cohort of patients, including significantly more men undergoing primary relaparotomy, a higher incidence of cardiovascular disease, a higher ASA class, and more patients who were severely ill 24 hours before the first intervention (eTable 1 in the Supplement). With propensity score matching, 64 of 82 patients (78.0%) undergoing primary relaparotomy were successfully matched to 64 patients undergoing primary catheter drainage. In this matched cohort, there were no significant differences in baseline characteristics (Table 2).
Table 2. Baseline Characteristicsa.
| Characteristic | Catheter Drainage (n = 64) |
Relaparotomy (n = 64) |
Standardized Mean Difference, % | |
|---|---|---|---|---|
| Before Matching |
After Matching |
|||
| Age, median (IQR), y | 68 (57-73) | 66 (57-71) | 2.9 | 1.3 |
| Male sex, No. (%) | 50 (78.1) | 50 (78.1) | 25.1 | 0.0 |
| Coexisting condition, No. (%) | ||||
| Cardiovascular disease | 20 (31.3) | 21 (32.8) | 25.7 | 3.3 |
| Pulmonary disease | 6 (9.4) | 8 (12.5) | 15.3 | 9.4 |
| Chronic renal insufficiency | 2 (3.1) | 1 (1.6) | 4.4 | 12.5 |
| History of upper abdominal surgery | 13 (20.3) | 17 (26.6) | 1.7 | 14.0 |
| ASA class on admission (%)b | 30.2 | 4.6 | ||
| I | 8 (12.5) | 13 (20.3) | ||
| II | 43 (67.2) | 35 (54.7) | ||
| III | 13 (20.3) | 16 (25.0) | ||
| BMI, mean (SD)c | 26 (3.2) | 26 (3.7) | 8.1 | 3.1 |
| Weight loss, No. (%)d | 30 (49.2) | 30 (50.0) | 7.3 | 1.6 |
| Quantity, median (IQR), kge | 2 (0-7) | 1 (0-8) | 14.5 | 6.8 |
| Preoperative ERCPf | 9.7 | 0.3 | ||
| Without intervention | 14 (22.2) | 6 (9.5) | ||
| With stenting/papillotomy | 30 (47.6) | 35 (55.6) | ||
| Preoperative cholestasis, No. (%)g | 47 (73.4) | 43 (67.2) | 9.4 | 13.2 |
| Details on pancreatoduodenectomy | ||||
| Pylorus-preserving pancreatoduodenectomy | 49 (76.6) | 49 (76.6) | 6.2 | 0.0 |
| Reconstruction portal veinf | 1 (1.6) | 3 (4.8) | 2.7 | 14.8 |
| Additional organ resectionf | 3 (4.8) | 8 (12.7) | 23.6 | 23.6 |
| Abdominal drain | 63 (98.4) | 62 (96.9) | 14.7 | 8.9 |
| Pathology (pre-) malignant, No. (%) | 55 (85.9) | 56 (87.5) | 17.3 | 4.7 |
| Disease severity 24 h before first intervention | ||||
| APACHE II score, median (IQR)h | 8 (6-11) | 9 (6-13) | 53.1 | 3.9 |
| SIRS, No. (%)i | 32 (50.0) | 35 (54.7) | 29.0 | 9.3 |
| Organ failure, No. (%) | 22 (34.4) | 22 (34.4) | 71.0 | 0.0 |
| Single organ | 13 (20.3) | 15 (23.4) | 50.8 | 7.3 |
| Multiple organ | 9 (14.1) | 7 (10.9) | 32.3 | 9.9 |
| Previous reintervention, No. (%)j | 5 (7.8) | 7 (10.9) | 24.3 | 9.9 |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ERCP, endoscopic retrograde cholangiopancreatography; IQR, interquartile range; SIRS, systemic inflammatory response syndrome.
Propensity score matching was based on sex, age, ASA class on admission, APACHE II score, SIRS, organ failure, and previous reintervention.
Class I indicates healthy status; II, mild systemic disease; and III, severe systemic disease.
Determined in 60 patients in the catheter drainage group and 62 patients in the relaparotomy group.
Determined in 61 patients in the catheter drainage group and 60 patients in the relaparotomy group.
Determined in 60 patients in the primary catheter drainage group and 58 patients in the relaparotomy group.
Determined in 63 patients.
Defined as jaundice, elevated bilirubin level, and/or need for preoperative biliary drainage.
Scale ranges from 0 to 71, with higher scores indicating more severe disease.
As defined by the American College of Chest Physicians and the Society of Critical Care Medicine.
Represents the number of patients who underwent an intervention after pancreatoduodenectomy before first intervention for pancreatic fistula for any indication other than pancreatic fistula.
Primary Relaparotomy
The 64 matched patients underwent the following procedures during primary relaparotomy: 32 patients (50.0%) underwent extended lavage and drainage of the abdominal cavity, 17 patients (26.6%) received a direct completion pancreatectomy, the pancreatic anastomosis was revised in 12 patients (18.8%), and the pancreatic anastomosis was dismantled while pancreatic juice efflux was secured through a drain in the pancreatic duct in 3 patients (4.7%). Primary relaparotomy was performed a median of 8 (IQR, 5-11) days after pancreatoduodenectomy.
Primary Catheter Drainage
Of 64 matched patients undergoing primary catheter drainage, catheter drainage was performed percutaneously via interventional radiology in 60 patients (93.8%), endoscopic (transgastric) drainage was performed in 1 patient (1.6%), and 3 patients (4.7%) were discharged with an intraoperatively placed drain in place. Primary catheter drainage was performed a median of 9 (IQR, 7-11) days after pancreatoduodenectomy.
Primary and Secondary Outcomes
Clinical outcomes are given in Table 3. After primary relaparotomy, 23 of 64 patients (35.9%) died compared with 9 of 64 patients (14.1%) after primary catheter drainage (P = .007; RR, 0.39; 95% CI, 0.20-0.75).
Table 3. Matched Clinical End Pointsa.
| Outcome | Catheter Drainage (n = 64)b |
Relaparotomy (n = 64)b |
RR (95% CI) | P Value |
|---|---|---|---|---|
| Death, No. (%) | 9 (14.1) | 23 (35.9) | 0.39 (0.20-0.76) | .007 |
| Secondary end points | ||||
| Major complications after first intervention POPF, No. (%) | ||||
| New-onset single-organ failure | 3 (4.7) | 13 (20.3) | 0.15 (0.04-0.61) | .007 |
| New-onset multiple-organ failure | 10 (15.6) | 25 (39.1) | 0.40 (0.21-0.77) | .008 |
| Postpancreatectomy hemorrhageb | 14 (21.9) | 14 (21.9) | 1.00 (0.55-1.81) | >.99 |
| Gastroenterostomy leakageb | 4 (6.3) | 2 (3.1) | 2.00 (0.43-9.33) | .69 |
| Bile leakageb | 5 (7.8) | 8 (12.5) | 0.63 (0.22-1.82) | .58 |
| Other complications after first intervention POPF, No. (%) | ||||
| Delayed gastric emptyingc | .36 | |||
| Grade B | 3 (6.0) | 4 (8.0) | ||
| Grade C | 4 (8.0) | 9 (18.0) | ||
| Acute pancreatitisd | 2 (3.1) | 3 (4.7) | 0.66 (0.13-3.33) | >.99 |
| Long-term complications, No. (%) | ||||
| New-onset diabetesc,e | 6 (12.0) | 22 (44.0) | 0.27 (0.13-0.58) | <.001 |
| New-onset exocrine pancreatic insufficiencye,f | 16 (39.0) | 22 (53.7) | 0.72 (0.46-1.16) | .26 |
| Interventions | ||||
| Completion pancreatectomy, No. (%) | 2 (3.1) | 18 (28.1) | 0.11 (0.03-0.43) | <.001 |
| No. of additional relaparotomies | .006 | |||
| Median (range) per patient | 0 (0-5) | 0 (0-8) | ||
| Total per study group | 19 | 54 | ||
| No. (%) of patients | 14 (21.9) | 29 (45.3) | 0.48 (0.27-0.85) | .01 |
| No. of additional catheter drainages | .12 | |||
| Median (range) per patient | 1 (0-9) | 0 (0-6) | ||
| Total per study group | 90 | 57 | ||
| No. (%) of patients | 36 (56.3) | 28 (43.8) | 1.29 (0.91-1.82) | .22 |
| No. of interventions for pancreatic fistula | .18 | |||
| Total No. per study group | 150 | 127 | ||
| Median (range) per patient | 2 (0-9) | 2 (1-7) | ||
| No. of interventions during admission | .35 | |||
| Total No. per study group | 195 | 213 | ||
| Median (range) per patient | 2 (0-13) | 3 (1-13) | ||
| Hospitalization course | ||||
| New ICU admission after first intervention POPF, No. (%) | 24 (37.5) | 56 (87.5) | 0.43 (0.31-0.59) | <.001 |
| Length of ICU stay, median (IQR)g,h | 0 (0-3) | 6 (1-13) | .01 | |
| Length of hospital stay, median (IQR)h | 29 (19-45) | 55 (41-71) | .001 | |
| Duration of pancreatic fistula, median (IQR)h,i | 29 (17-62) | 37 (14-62) | .71 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range; POPF, postoperative pancreatic fistula; RR, risk ratio.
Length of ICU stay, hospital stay, and duration of pancreatic fistula were calculated over survivors.
Occurrence any time during admission after first intervention for pancreatic fistula, requiring intervention.
Calculated in 50 pairs of patients.
Defined as elevated serum amylase and lipase levels in combination with abdominal pain or as seen on computed tomography scan or during relaparotomy.
Occurrence within 90 days after date of admission.
Calculated in 41 patients.
After first intervention for pancreatic fistula.
Calculated in 36 pairs of survivors.
Time between pancreatoduodenectomy and removal of last abdominal drain or completion pancreatectomy.
New-onset organ failure occurred more often in the 64 patients undergoing primary relaparotomy vs 64 undergoing primary catheter drainage: single-organ failure in 13 (20.3%) vs 3 (4.7%) patients (P = .007; RR, 0.15; 95% CI, 0.03-0.60) and multiple-organ failure in 25 (39.1%) vs 10 (15.6%) patients (P = .008; RR, 0.40 (95% CI, 0.20-0.77). At 3 months’ follow-up in 50 patients, new-onset diabetes was observed in 22 (44.0%) patients after relaparotomy vs 6 (12.0%) patients after primary catheter drainage (P < .001; RR, 0.27; 95% CI, 0.12-0.57). There were no significant differences in other clinically relevant outcomes occurring after the first intervention for pancreatic fistula (ie, postpancreatectomy hemorrhage, gastroenterostomy leakage, bile leakage, delayed gastric emptying, acute pancreatitis, and new-onset exocrine pancreatic insufficiency) (Table 3).
During the index admission in the matched cohort of 128 patients, completion pancreatectomy was more frequently performed in patients undergoing primary relaparotomy (18 [28.1%]) compared with primary catheter drainage (2 [3.1%]) (P < .001; RR, 0.11; 95% CI, 0.03 to 0.43). After primary relaparotomy, more additional relaparotomies were performed: a total of 54 in 29 patients following primary relaparotomy vs a total of 19 in 14 patients following primary catheter drainage (median difference, 0; 95% CI, −0.46 to 0.46; P = .006). The number of additional catheter drainages was similar in both groups: 57 in 38 patients after relaparotomy vs 90 in 36 patients after catheter drainage (median difference, 1; 95% CI, 0.35 to 1.65; P = .12). The total number of interventions during admission was 213 after relaparotomy vs 195 after catheter drainage (median difference, 1; 95% CI, −2.03 to 0.03; P = .35); of these interventions, 127 vs 150 were indicated owing to pancreatic fistula (median difference, 0; 95% CI, −1.03 to 1.03; P = .17).
More patients were admitted to the ICU after relaparotomy than after primary catheter drainage: 56 (87.5%) vs 24 (37.5%) patients (P < .001; RR, 0.43; 95% CI, 0.31 to 0.59). Length of ICU stay was longer after relaparotomy (median [IQR], 6 [1-13] vs 0 [0-3] days; median difference, 6 days; 95% CI, 3.31 to 8.69; P = .01), as was length of hospital stay (median [IQR], 55 [41-71] vs 29 [19-45] days; median difference, 26 days; 95% CI, 17.44 to 34.56; P = .001). All patients were discharged to their home in good clinical condition. The duration of the pancreatic fistula (ie, time to removal of the last abdominal drain or completion pancreatectomy) was similar in both groups (median [IQR], 37 [14-62] days after relaparotomy vs 29 [17-62] days after primary catheter drainage; median difference, 8 days; 95% CI, −26.73 to 10.7 = 3; P = .71).
Subgroup Analysis Based on APACHE II Score
In each of the 3 subgroups based on APACHE II score (ie, <9, 9-12, and >12), mortality was higher in patients undergoing primary relaparotomy (8 [24.2% vs 14 [10.3%], P = .04; 6 [31.6%] vs 7 [9.5%], P = .02; and 16 [57.1%] vs 4 [23.5%], P = .04, respectively). There was also a significantly higher incidence of new-onset single- and multiple-organ failure in patients after relaparotomy (full details in eTable 2 in the Supplement).
Discussion
This multicenter matched cohort study showed that primary catheter drainage as the first intervention for severe pancreatic fistula after pancreatoduodenectomy is associated with a better clinical outcome, including lower mortality, less organ failure, fewer additional relaparotomies, and less new-onset diabetes compared with direct relaparotomy. From 2005 to 2013, one-fourth of the patients with severe pancreatic fistula were still treated with primary relaparotomy without a tendency toward a more conservative approach. Primary catheter drainage was successful (ie, survival without the need for relaparotomy) in 77.1% of the patients with severe pancreatic fistula.
To our knowledge, there have been no other studies comparing the first step in the treatment of severe pancreatic fistula. Several small, retrospective studies describe the general treatment of pancreatic fistula. Most of these studies indicate that minimally invasive catheter drainage should be the treatment of choice in these patients. However, the studies also report a relaparotomy rate varying from 15% to 50%, suggesting at least some hesitation to treat pancreatic fistula in a minimally invasive approach. On the contrary, relaparotomy can be performed with good outcomes and might prevent the need for additional interventions during admission. Previous studies contain a considerable selection bias that was not adjusted for in statistical analysis. To our knowledge, the present study is the largest data set of patients with severe pancreatic fistula that compares 2 management strategies in a matched cohort.
The success of catheter drainage can be explained by adhering to 2 main surgical principles: adequate source control and no further harm. Pancreatic fistula after pancreatoduodenectomy cause an intra-abdominal fluid collection filled with activated pancreatic juices. If drained adequately, even severe pancreatic fistula could resolve, as shown in 77.1% of the patients in the present study who were successfully treated with primary catheter drainage alone. In addition, catheter drainage is a minimally invasive procedure, which will provoke less surgical trauma (ie, tissue injury and systemic inflammatory response) compared with relaparotomy. Even a moderately small surgical trauma that induces a proinflammatory cytokine response can lead to organ failure in severely ill patients. In our unmatched cohort (eTable 1 in the Supplement), more severely ill patients underwent primary relaparotomy more frequently. These patients were more prone to developing organ failure due to the aforementioned cytokine response. However, even in the matched cohort, 39.1% of the patients developed new-onset multiple-organ failure after relaparotomy compared with just 15.6% of the patients after primary catheter drainage.
The obvious benefit of catheter drainage as the first intervention for severe pancreatic fistula is reduced mortality and prevention of major complications, such as new-onset organ failure. However, there are also other potential benefits from this treatment strategy. Patients in the present study who were treated primarily through relaparotomy more frequently underwent completion pancreatectomy. Consequently, new-onset diabetes was observed more often in patients undergoing primary relaparotomy. This type of diabetes tends to be difficult to control, leaving patients with a considerably elevated risk for severe hypoglycemia. The main implication of these findings is that, when possible, catheter drainage should be the primary step in management of severe pancreatic fistula. Relaparotomy should be reserved for patients who are not candidates for a minimally invasive intervention or whose condition is progressively worsening with catheter drainage.
Limitations
One limitation of this study is its retrospective design, causing an inevitable risk of selection bias and confounding. Propensity score matching was used to correct for this form of bias. This is the best statistical method to mimic a randomized design. However, the success of this matching method is limited by the presence of unknown confounders. We collected extensive data on baseline characteristics to determine the most accurate model for matching. The best matching was achieved by implementing patient characteristics combined with the severity of disease at the time of the first intervention. The matching was successful (ie, resulted in a well-balanced baseline) as presented in Table 2, most importantly with regard to disease severity 24 hours before the first intervention. However, the outcomes of this study should be interpreted with care, for there was no assessment of effect modification or interaction between the covariates included in the matching procedure, and no correction for multiple testing was performed. To ensure the success of matching, it was not possible to include all patients undergoing primary relaparotomy. We were able to match 64 of 82 patients: 2 were excluded because of missing essential data for matching and 16 could not be matched to an equivalent cohort undergoing primary catheter drainage. There is a chance that these excluded patients have a biologically different type of fistula that could not have been managed successfully through catheter drainage. To minimize the risk of missing a certain subgroup of patients with matching, we performed a predefined subgroup analysis of all 309 patients with severe pancreatic fistula based on their APACHE II score 24 hours before the first intervention. Mortality was significantly higher in all subgroups in patients undergoing primary relaparotomy (eTable 2 in the Supplement).
Our results should ideally be confirmed by a large, randomized clinical trial. However, we question whether there is justification for such a trial since minimally invasive treatments are gaining popularity and seem to have few downsides. Because patients seem to benefit from early catheter drainage and, therefore, from early standardized detection of pancreatic fistula, we believe that future studies should focus on a sufficiently aggressive diagnosis and minimally invasive treatment of pancreatic fistula.
Conclusions
In this multicenter study on a matched cohort of patients, catheter drainage was superior to relaparotomy as the primary intervention for pancreatic fistula after pancreatoduodenectomy because primary catheter drainage was associated with lower mortality. Therefore, when minimally invasive drainage is feasible, primary catheter drainage should be the first step in treatment of severe pancreatic fistula.
eTable 1. Crude Baseline Characteristics
eTable 2. Status Based on APACHE II Category
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362(17):1605-1617. [DOI] [PubMed] [Google Scholar]
- 2.Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138(12):1310-1314. [DOI] [PubMed] [Google Scholar]
- 3.de Castro SMM, Busch ORC, Van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg. 2005;92(9):1117-1123. [DOI] [PubMed] [Google Scholar]
- 4.Bassi C, Dervenis C, Butturini G, et al. ; International Study Group on Pancreatic Fistula Definition . Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138(1):8-13. [DOI] [PubMed] [Google Scholar]
- 5.Harnoss JC, Ulrich AB, Harnoss JM, Diener MK, Büchler MW, Welsch T. Use and results of consensus definitions in pancreatic surgery: a systematic review. Surgery. 2014;155(1):47-57. [DOI] [PubMed] [Google Scholar]
- 6.Fuks D, Piessen G, Huet E, et al. . Life-threatening postoperative pancreatic fistula (grade C) after pancreaticoduodenectomy: incidence, prognosis, and risk factors. Am J Surg. 2009;197(6):702-709. [DOI] [PubMed] [Google Scholar]
- 7.Dellaportas D, Tympa A, Nastos C, et al. . An ongoing dispute in the management of severe pancreatic fistula: pancreatospleenectomy or not? World J Gastrointest Surg. 2010;2(11):381-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malleo G, Pulvirenti A, Marchegiani G, Butturini G, Salvia R, Bassi C. Diagnosis and management of postoperative pancreatic fistula. Langenbecks Arch Surg. 2014;399(7):801-810. [DOI] [PubMed] [Google Scholar]
- 9.Hackert T, Werner J, Büchler MW. Postoperative pancreatic fistula. Surgeon. 2011;9(4):211-217. [DOI] [PubMed] [Google Scholar]
- 10.Tamijmarane A, Ahmed I, Bhati CS, et al. . Role of completion pancreatectomy as a damage control option for post-pancreatic surgical complications. Dig Surg. 2006;23(4):229-234. [DOI] [PubMed] [Google Scholar]
- 11.Farley DR, Schwall G, Trede M. Completion pancreatectomy for surgical complications after pancreaticoduodenectomy. Br J Surg. 1996;83(2):176-179. [PubMed] [Google Scholar]
- 12.Van Berge Henegouwen MI, De Wit LT, Van Gulik TM, Obertop H, Gouma DJ. Incidence, risk factors, and treatment of pancreatic leakage after pancreaticoduodenectomy: drainage versus resection of the pancreatic remnant. J Am Coll Surg. 1997;185(1):18-24. [DOI] [PubMed] [Google Scholar]
- 13.Gueroult S, Parc Y, Duron F, Paye F, Parc R. Completion pancreatectomy for postoperative peritonitis after pancreaticoduodenectomy: early and late outcome. Arch Surg. 2004;139(1):16-19. [DOI] [PubMed] [Google Scholar]
- 14.Van Santvoort HC, Besselink MG, Bakker OJ, et al. ; Dutch Pancreatitis Study Group . A step-up approach or open necrosectomy for necrotizing pancreatitis. N Engl J Med. 2010;362(16):1491-1502. [DOI] [PubMed] [Google Scholar]
- 15.Working Group IAP/APA Acute Pancreatitis Guidelines IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4)(suppl 2):e1-e15. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt CM, Choi J, Powell ES, et al. . Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg. 2009;2009:404520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad LBP, Scatton O, Randone B, et al. . Pancreatic fistula after pancreaticoduodenectomy: the conservative treatment of choice. HPB (Oxford). 2009;11(3):203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent TS, Callery MP, Vollmer CM Jr. The bridge stent technique for salvage of pancreaticojejunal anastomotic dehiscence. HPB (Oxford). 2010;12(8):577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gangl O, Fröschl U, Hofer W, Huber J, Sautner T, Függer R. Unplanned reoperation and reintervention after pancreatic resections: an analysis of risk factors. World J Surg. 2011;35(10):2306-2314. [DOI] [PubMed] [Google Scholar]
- 20.Balzano G, Pecorelli N, Piemonti L, et al. . Relaparotomy for a pancreatic fistula after a pancreaticoduodenectomy: a comparison of different surgical strategies. HPB (Oxford). 2014;16(1):40-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tol JAMG, Busch ORC, Van Delden OM, Van Lienden KP, Van Gulik TM, Gouma DJ. Shifting role of operative and nonoperative interventions in managing complications after pancreatoduodenectomy: what is the preferred intervention? Surgery. 2014;156(3):622-631. [DOI] [PubMed] [Google Scholar]
- 22.Sanjay P, Kellner M, Tait IS. The role of interventional radiology in the management of surgical complications after pancreatoduodenectomy. HPB (Oxford). 2012;14(12):812-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn TA, Yeo CJ, Cameron JL, et al. . Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointest Surg. 2003;7(2):209-219. [DOI] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499.25046131 [Google Scholar]
- 25.Bone RC, Balk RA, Cerra FB, et al. ; ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine . Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101(6):1644-1655. [DOI] [PubMed] [Google Scholar]
- 26.Koch M, Garden OJ, Padbury R, et al. . Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680-688. [DOI] [PubMed] [Google Scholar]
- 27.Wente MN, Bassi C, Dervenis C, et al. . Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761-768. [DOI] [PubMed] [Google Scholar]
- 28.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg. 2007;134(5):1128-1135. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cluver L, Boyes M, Orkin M, Pantelic M, Molwena T, Sherr L. Child-focused state cash transfers and adolescent risk of HIV infection in South Africa: a propensity-score-matched case-control study. Lancet Glob Health. 2013;1(6):e362-e370. [DOI] [PubMed] [Google Scholar]
- 31.Bonett DG, Price RM. Confidence intervals for a ratio of binomial proportions based on paired data. Stat Med. 2006;25(17):3039-3047. [DOI] [PubMed] [Google Scholar]
- 32.Bonett DG, Price RM. Statistical inference for a linear function of medians: confidence intervals, hypothesis testing, and sample size requirements. Psychol Methods. 2002;7(3):370-383. [DOI] [PubMed] [Google Scholar]
- 33.Core Team R R: the R Project for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.r-project.org/. Accessed July 1, 2015.
- 34.Thoemmes F. Propensity score matching in SPSS. http://arxiv.org/pdf/1201.6385. Published January 2012. Accessed July 1, 2015.
- 35.McMillan MT, Vollmer CM Jr, Asbun HJ, et al. . The characterization and prediction of ISGPF grade C fistulas following pancreatoduodenectomy. J Gastrointest Surg. 2016;20(2):262-276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Crude Baseline Characteristics
eTable 2. Status Based on APACHE II Category