Key Points
Question
Is incomplete radiotherapy in rectal cancer associated with worse outcomes?
Findings
This analysis of 17 600 adults with stage II to III rectal adenocarcinoma from the 2006-2012 National Cancer Database who received neoadjuvant chemoradiotherapy before surgical resection showed that a complete radiation dose significantly lowers the risk of long-term mortality vs an incomplete radiation dose. Patients receiving radiotherapy and surgery at different facilities were less likely to complete radiotherapy.
Meaning
In patients with rectal cancer, aligning multimodal care is associated with increased neoadjuvant radiotherapy completion, and completion is associated with increased survival.
Abstract
Importance
Failing to complete chemotherapy adversely affects survival in patients with colorectal cancer. However, the effect of incomplete delivery of neoadjuvant radiotherapy is unclear.
Objective
To determine whether incomplete radiotherapy delivery is associated with worse clinical outcomes and survival.
Design, Setting, and Participants
Data on 17 600 patients with stage II to III rectal adenocarcinoma from the 2006-2012 National Cancer Database who received neoadjuvant chemoradiotherapy followed by surgical resection were included. Multivariable regression methods were used to compare resection margin positivity, permanent colostomy rate, 30-day readmission, 90-day mortality, and overall survival between patients who received complete (45.0-50.4 Gy) and incomplete (<45.0 Gy) doses of radiation as preoperative therapy.
Main Outcomes and Measures
The primary outcome measure was overall survival; short-term perioperative and oncologic outcomes encompassing margin positivity, permanent ostomy rate, postoperative readmission, and postoperative mortality were also assessed.
Results
Among 17 600 patients included, 10 862 were men, with an overall median age of 59 years (range, 51-68 years). Of these, 874 patients (5.0%) received incomplete doses of neoadjuvant radiation. The median radiation dose received among those who did not achieve complete dosing was 34.2 Gy (interquartile range, 19.8-40.0 Gy). Female sex (adjusted odds ratio [OR] 0.69; 95% CI, 0.59-0.81; P < .001) and receiving radiotherapy at a different hospital than the one where surgery was performed (OR, 0.72; 95% CI, 0.62-0.85; P < .001) were independent predictors of failing to achieve complete dosing; private insurance status was predictive of completing radiotherapy (OR, 1.60; 95% CI, 1.16-2.21; P = .004). At 5-year follow-up, overall survival was improved among patients who received a complete course of radiotherapy (3086 [estimated survival probability, 73.2%] vs 133 [63.0%]; P < .001). After adjustment for demographic, clinical, and tumor characteristics, patients receiving a complete vs incomplete radiation dose had a similar resection margin positivity (OR, 0.99; 95% CI, 0.72-1.35; P = .92), permanent colostomy rate (OR, 0.96; 95% CI, 0.70-1.32; P = .81), 30-day readmission rate (OR, 0.92; 95% CI, 0.67-1.27; P = .62), and 90-day mortality (OR, 0.72; 95% CI, 0.33-1.54; P = .41). However, a complete radiation dose had a significantly lower risk of long-term mortality (adjusted hazard ratio, 0.70; 95% CI, 0.59-0.84; P < .001).
Conclusions and Relevance
Achieving a target radiation dose of 45.0 to 50.4 Gy is associated with a survival benefit in patients with locally advanced rectal cancer. Aligning all aspects of multimodal oncology care may increase the probability of completing neoadjuvant therapy.
This cohort study evaluates the outcomes of patients who receive complete vs incomplete doses of radiation following surgery for rectal adenocarcinoma.
Introduction
Despite advances in adjuvant therapies over the past 4 decades, rectal cancer remains highly morbid, with an estimated 39 220 new cases in the United States in 2016 and a 5-year overall survival rate of 64%. Early randomized trials in the late 1980s demonstrated significant local control and survival benefit for postoperative combined multimodality therapy over surgery alone in patients with resectable stage II and stage III colorectal cancers. In recent years, preoperative chemoradiotherapy with fluorouracil-based chemotherapy and at least 45.0 Gy of pelvic radiation have become the standard of care for patients with resectable stage II and stage III rectal adenocarcinoma in the United States. With these therapies, randomized data have consistently demonstrated marked improvements in tumor downstaging, sphincter preservation, and local control in patients receiving neoadjuvant therapy. Despite these benefits, some patients do not complete radiotherapy. For example, in the landmark German Rectal Cancer Study Group trial, 8% of patients did not receive the prescribed radiotherapy dose and 11% did not receive the complete dose of chemoradiotherapy. The effects of incomplete radiotherapy on perioperative outcomes and overall survival in rectal cancer have yet to be defined.
Although the impact of suboptimal therapy completion is unclear in rectal cancer, incomplete adjuvant or neoadjuvant therapy has been associated with poor clinical outcomes in other cancers. In colon cancer, survival rates were lower in patients who received incomplete adjuvant therapy than in those who received complete therapy. In breast cancer, incomplete neoadjuvant radiotherapy was associated with a 17.9% absolute decrease in 5-year survival and a 5.02-fold increased risk of local recurrence compared with adherent patients. Therefore, we aimed to evaluate the effect of incomplete neoadjuvant radiotherapy on patients with stage II and III rectal cancers with the hypothesis that incomplete therapy is associated with worse clinical outcomes and lower rates of survival.
Methods
Data Source
Jointly sponsored by the American College of Surgeons and the American Cancer Society, the National Cancer Database (NCDB) is a clinical oncology database that gathers data from more than 1500 Commission on Cancer–accredited centers. The database captures an estimated 70% of all new cancer diagnoses from the United States and Puerto Rico and contains more than 30 million patient records.
Study Design
All adults in the NCDB with stage II and III rectal adenocarcinoma diagnosed from 2006 to 2012 who received preoperative chemoradiotherapy and underwent definitive surgery were identified using histology codes from the International Classification of Diseases for Oncology, Third Edition. Any patients with missing radiotherapy dosing data, nonmalignant pathology, history of any other cancer, or those with clinically apparent metastatic disease before surgery were excluded. The Duke University Institutional Review Board approved this retrospective analysis from the NCDB with a waiver of informed consent.
Study Outcomes
The primary end point was overall survival. Secondary end points included short-term perioperative and oncologic outcomes, including margin positivity (defined as either distal or circumferential margin), permanent ostomy rate (defined as abdominoperineal resection or pelvic exenteration), 30-day postoperative readmission, and 90-day postoperative mortality.
Statistical Analysis
Patients were stratified based on whether they completed radiotherapy, defined as a 45.0- to 54.0-Gy total dose of radiation according to current National Comprehensive Cancer Network guidelines. Baseline characteristics and unadjusted outcomes were compared using the Kruskal-Wallis test for continuous variables and Pearson χ2 test for categorical variables. Multivariable logistic regression models were established to recognize factors independently associated with the use of incomplete and complete radiotherapy. Survival was plotted using the Kaplan-Meier method.
For short-term outcomes, multivariate linear and logistic regression models were used with adjustment for patient age, sex, race, insurance status, cancer stage, Charlson Comorbidity Index, hospital type and procedural volume, pathologic T and N stage, and extent of surgery (low anterior resection, abdominoperineal resection, and pelvic exenteration), and the Charlson-Deyo Index, which is the Charlson Comorbidity Index applied to International Classification of Diseases for Oncology, Ninth Edition codes. The Charlson-Deyo Index predicts 1-year mortality for patients with comorbid conditions and disease severity. Each comorbid condition is assigned a score of 1, 2, 3, or 6 depending on the mortality risk of that condition. The scores are then summed to predict total mortality. The scores range from 0 to a theoretically limitless total. We compared scores of 0, 1, and greater than 2. For long-term survival, a multivariate Cox proportional hazards regression model was created, adjusting for pathologic M stage and postoperative chemotherapy in addition to the covariates contained in the short-term outcome models.
A P value <.05 was deemed statistically significant. Statistical analysis was performed using R, version 3.1.2 (R Project for Statistical Computing, R Foundation).
Results
Among 17 600 patients who met our inclusion criteria, 874 (5.0%) received incomplete doses of neoadjuvant radiation. Baseline characteristics and unadjusted outcomes between complete and incomplete radiotherapy groups are reported in Table 1. Among patients who did not achieve complete dosing, the median radiation dose received was 34.2 Gy (interquartile range, 19.8-40.0 Gy). In addition, the group that did not complete radiotherapy had a slightly higher median age (61 vs 59 years; P < .001), a smaller percentage of males (52.4% vs 62.2%; P < .001), and a smaller percentage of privately insured patients (43.1% vs 53.5%; P < .001). Patients who did not complete treatment were more likely to receive radiotherapy at a different facility than where they underwent surgery (36.7% vs 29.9%; P < .001).
Table 1. Baseline Characteristics and Outcomes of Patients With Stage II and III Rectal Cancer Who Received Complete vs Incomplete Radiotherapy as Part of Neoadjuvant Chemoradiotherapy.
| Characteristic | Incomplete (n = 874) |
Complete (n = 16 726) |
Overall (n = 17 600) |
P Value |
|---|---|---|---|---|
| Age, median (range), y | 61 (51-72) | 59 (59-68) | 59 (51-68) | <.001 |
| Sex, No. (%) | ||||
| Male | 458 (52.4) | 10 404 (62.2) | 10 862 (61.7) | <.001 |
| Female | 416 (47.6) | 6322 (37.8) | 6738 (38.3) | |
| Race, No. (%) | ||||
| White | 770 (88.4) | 14 335 (86.2) | 15 105 (86.3) | .06 |
| Black | 71 (8.2) | 1428 (8.6) | 1499 (8.6) | |
| Other | 30 (3.4) | 860 (5.2) | 890 (5.1) | |
| Charlson-Deyo Comorbidity Index, No. (%)a | ||||
| 0 | 685 (78.4) | 13 164 (78.7) | 13 849 (78.7) | .97 |
| 1 | 154 (17.6) | 2890 (17.3) | 3044 (17.3) | |
| ≥2 | 35 (4.0) | 672 (4.0) | 707 (4.0) | |
| Hospital designation, No. (%) | ||||
| Community | 80 (9.2) | 1257 (7.5) | 1337 (7.6) | .19 |
| Comprehensive | 467 (53.5) | 9003 (53.9) | 9470 (53.9) | |
| Academic | 326 (37.3) | 6443 (38.6) | 6769 (38.5) | |
| Insurance status, No. (%) | ||||
| None | 60 (6.9) | 901 (5.4) | 961 (5.5) | <.001 |
| Private | 373 (43.1) | 8856 (53.5) | 9229 (53.0) | |
| Government | 432 (49.9) | 6792 (41.0) | 7224 (41.5) | |
| Income level, median, No. (%) | ||||
| Below | 269 (31.8) | 5156 (31.9) | 5425 (31.9) | .99 |
| Above | 576 (68.2) | 11 027 (68.1) | 11 603 (68.1) | |
| Educational level, median, No. (%) | ||||
| Below | 344 (40.7) | 6492 (40.1) | 6836 (40.1) | .73 |
| Above | 501 (59.3) | 9690 (59.9) | 10 191 (59.9) | |
| Travel distance, median (range), m | 10.85 (5.00-26.00) |
10.70 (4.80-24.80) |
10.70 (4.80-24.90) |
.49 |
| Year of diagnosis, No. (%) | ||||
| 2006 | 101 (11.6) | 1914 (11.4) | 2015 (11.4) | .58 |
| 2007 | 118 (13.5) | 2150 (12.9) | 2268 (12.9) | |
| 2008 | 119 (13.6) | 2211 (13.2) | 2330 (13.2) | |
| 2009 | 113 (12.9) | 2382 (14.2) | 2495 (14.2) | |
| 2010 | 142 (16.2) | 2578 (15.4) | 2720 (15.5) | |
| 2011 | 149 (17.0) | 2624 (15.7) | 2773 (15.8) | |
| 2012 | 132 (15.1) | 2867 (17.1) | 2999 (17.0) | |
| Stage of disease, No. (%) | ||||
| II | 340 (48.1) | 6288 (48.4) | 6628 (48.4) | .87 |
| III | 367 (51.9) | 6700 (51.6) | 7067 (51.6) | |
| Tumor size, cm, No. (%) | ||||
| <1.0 | 27 (3.9) | 613 (4.7) | 640 (4.6) | .33 |
| 1.0-1.9 | 86 (12.4) | 1373 (10.4) | 1459 (10.5) | |
| 2.0-4.9 | 353 (50.8) | 6668 (50.7) | 7021 (50.7) | |
| >4.9 | 229 (32.9) | 4488 (34.2) | 4717 (34.1) | |
| Tumor grade, No. (%) | ||||
| Well to moderately differentiated | 672 (86.6) | 12 643 (86.6) | 13 315 (86.6) | .98 |
| Poorly differentiated or undifferentiated | 104 (13.4) | 1950 (13.4) | 2054 (13.4) | |
| Extent of surgery, No. (%) | ||||
| Low anterior resection | 545 (4.7) | 11 110 (95.3) | 11 655 (69.2) | .03 |
| Abdominoperineal resection | 281 (5.4) | 4905 (94.6) | 5186 (30.8) | |
| Radiotherapy facility, No. (%) | ||||
| Same as surgery | 548 (63.3) | 11 698 (70.1) | 12 246 (69.8) | <.001 |
| Different from surgery | 318 (36.7) | 4986 (29.9) | 530 4 (30.2) | |
| Actual dose received, median (range), Gy b | 3420 (1980-4000) |
4500 (4500-5000) |
4500 (4500-4680) |
<.001 |
| Sphincter preservation, No. (%) | 545 (62.4) | 11 110 (66.4) | 11 655 (66.2) | |
| Sphincter loss, No. (%) | 281 (32.2) | 4905 (29.3) | 5186 (29.5) | |
| Unspecified, No. (%) | 48 (5.5) | 711 (4.3) | 759 (4.3) | |
| Postoperative, No. (%) | ||||
| Chemotherapy | 230 (26.3) | 5097 (30.5) | 5327 (30.3) | .009 |
| Radiotherapy | 14 (1.6) | 93 (0.6) | 107 (0.6) | <.001 |
| Positive distal or circumferential margin, No. (%) | 64 (7.3) | 1101 (6.6) | 1165 (6.6) | .40 |
| Lymph nodes examined, median (range), No. | 13 (7-17) | 13 (8-17) | 13 (8-17) | .54 |
| Hospital length of stay, median (range), d | 7 (5-9) | 6 (5-9) | 6 (5-9) | .32 |
| 30-d Unplanned readmission, No./Total No. (%) | 70/843 (8.3) |
1269/16 481 (7.7) |
1339/17 324 (7.8) |
.57 |
| Mortality, No./Total No. (%), d | ||||
| 30 | 8/737 (1.1) |
123/15 248 (0.8) |
131/14 466 (0.9) |
.60 |
| 90 | 19/729 (2.6) |
261/13 631 (1.9) |
280/14 360 (1.9) |
.19 |
Charlson Comorbidity Index applied to International Classification of Diseases for Oncology, Ninth Edition codes. The Charlson-Deyo Index predicts 1-year mortality for patients with comorbid conditions and disease severity. Each comorbid condition is assigned a score of 1, 2, 3, or 6 depending on the mortality risk of that condition. The scores are then summed to predict total mortality. The scores range from 0 to a theoretically limitless total. We compared scores of 0, 1, and greater than 2.
Low anterior resection was grouped within sphincter preservation, and pelvic exenteration and abdominoperineal resection were grouped as sphincter loss.
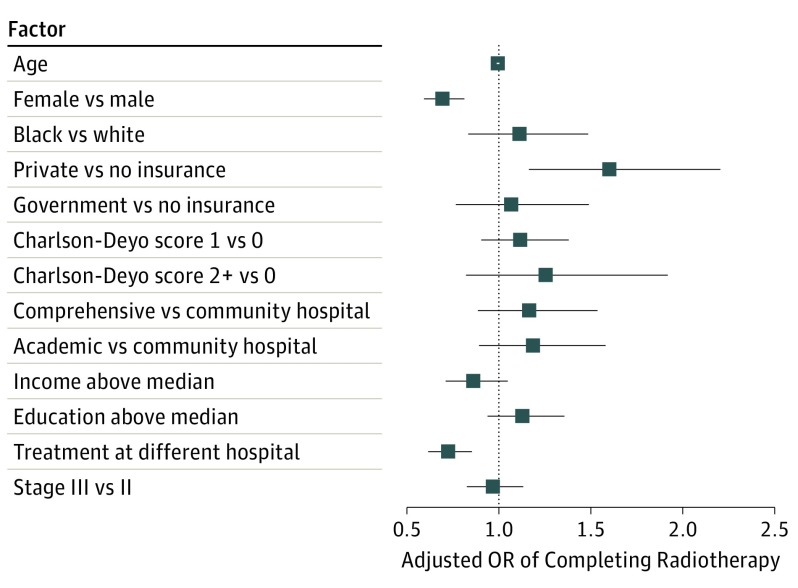
After adjustment for demographic, clinical, and hospital characteristics, women (odds ratio [OR], 0.69; 95% CI, 0.59-0.81; P < .001) and patients who received radiotherapy and surgery at different facilities (OR, 0.72; 95% CI, 0.62-0.85; P < .001) were less likely to complete radiotherapy (Figure 1). Patients who had private insurance were more likely to complete the treatment regimen than were patients with no insurance (OR, 1.60; 95% CI, 1.16-2.21; P = .004). Patients who held government insurance, however, did not show a difference in rates of treatment completion compared with patients without insurance (OR, 1.07; 95% CI, 0.77-1.42; P = .70). Age, race, cancer stage, Charlson-Deyo score, rurality, income level, and educational level were not significant predictors of radiotherapy completion (P > .05). In addition, the type of hospital did not affect the continuity of radiotherapy. Treatment at comprehensive cancer care centers vs community hospitals (OR, 1.17; 95% CI, 0.89-1.538; P = .29) and academic centers vs community hospitals (OR, 1.19; 95% CI, 0.89-1.58; P = .24) did not show a significant effect on continuance of radiotherapy.
Figure 1. Factors Associated With Likelihood of Completing Radiotherapy as Part of Neoadjuvant Chemoradiotherapy for Patients With Stage II and III Rectal Cancers.
Squares represent the odds ratios (ORs) for the independent association of each factor with likelihood of completing radiotherapy; 95% CI bounds are represented by the corresponding horizontal lines. Factors on the right of the dashed vertical line at 1.0 were associated with a higher likelihood of completing therapy.
Perioperative and Oncologic Outcomes
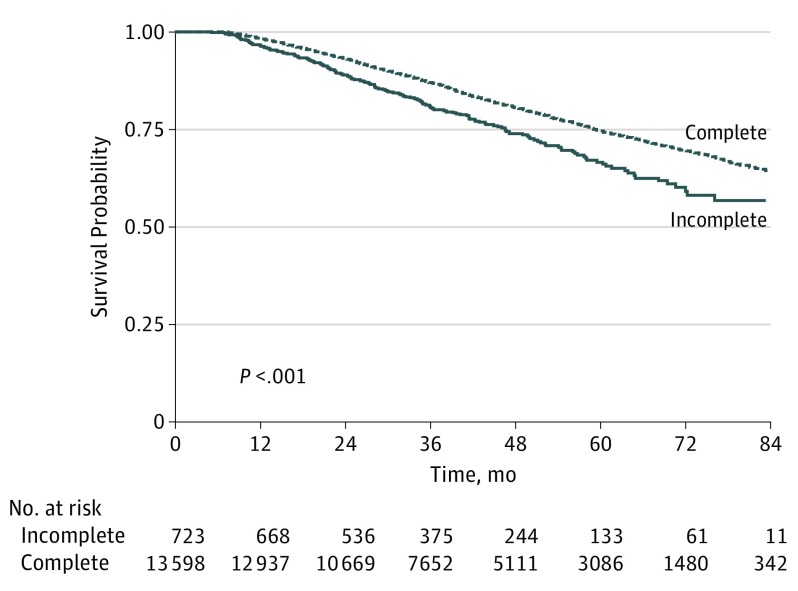
Before adjustment, patients who received incomplete radiotherapy had equivalent positive distal or circumferential margins (7.3% vs 6.6%; P = .40), mean (SD) length of hospital stay (8.09 [±6.70] vs 7.99 [±7.75] days; P = .32), 30-day readmission rate (70 [8.3%] vs 1269 [7.7%]; P = .57), 30-day mortality (8 [1.1%] vs 123 [0.8%]; P = .60), and 90-day mortality (2.6% vs 1.9%; P = .19). Patients who did not complete their course of radiotherapy were less likely to receive postoperative chemotherapy (230 [26.3%] vs 5097 [30.5%]; P = .009) but more likely to complete postoperative radiotherapy (14 [1.6%] vs 93 [0.6%]; P < .001). Overall survival rate at 5 years was significantly higher among patients who completed radiotherapy (3086 [estimated survival probability, 73.2%] vs 133 [63.0%]; P < .001) (Figure 2).
Figure 2. Overall Survival of Stage II and III Rectal Cancer Patients Who Received Neoadjuvant Chemoradiotherapy and Surgery, Stratified by Completed Radiotherapy Status.
Incomplete therapy, less than 45.0 Gy; complete therapy, 45.0 to 50.4 Gy.
After adjustment for demographic, clinical, and tumor characteristics, patients receiving a complete vs incomplete radiotherapy dose had a similar resection margin positivity (OR, 0.99; 95% CI, 0.72-1.35; P = .92), permanent colostomy rate (OR, 0.96; 95% CI, 0.70-1.32; P = .81), 30-day readmission rate (OR, 0.92; 95% CI, 0.67-1.27; P = .62), and 90-day mortality (OR, 0.72; 95% CI, 0.33-1.54; P = .41). After adjustment, patients who had a complete radiation dose had a significantly lower risk of long-term mortality (hazard ratio [HR], 0.70; 95% CI, 0.59-0.84; P < .001) (Table 2).
Table 2. Adjusted Outcomes of Complete vs Incomplete Radiotherapy as Part of Neoadjuvant Chemoradiotherapy for Patients With Stage II and III Rectal Cancera.
| Outcomeb | OR (95% CI) | P Value |
|---|---|---|
| Margin positivity | 0.99 (0.72-1.35) | .92 |
| Permanent ostomy | 0.96 (0.70-1.32) | .81 |
| 30-d Readmission | 0.92 (0.67-1.27) | .62 |
| 90-d Mortality | 0.72 (0.33-1.54) | .41 |
| Overall survival, HR (95% CI) | 0.70 (0.59-0.84) | <.001 |
Abbreviations: HR, hazard ratio; OR, odds ratio.
Adjusted variables included age, sex, race, insurance status, cancer stage, Charlson-Deyo Comorbidity Index (see Table 1 footnote for explanation), hospital type and procedural volume, pathologic T and N stage, and extent of surgery (low anterior resection, abdominoperineal resection, and pelvic exenteration).
Reference was incomplete radiotherapy.
Discussion
In what we believe to be the first population-level study examining the outcomes of patients with locally advanced rectal cancer who received an incomplete radiotherapy course, we found that completion of planned neoadjuvant radiotherapy as part of a neoadjuvant chemoradiotherapy regimen was associated with superior overall survival. However, complete dose delivery was not associated with differences in margin positivity or sphincter preservation. In addition, receiving radiotherapy at a different facility than where surgery was performed was associated with a higher likelihood of incomplete radiotherapy, suggesting that alignment of multidisciplinary therapy within 1 health system may be important for patient longevity.
Previous data have shown that incomplete or delayed radiotherapy has a negative effect on patient outcomes and tumor control in head and neck, lung, breast, and uterine cervical cancers. In patients with colorectal cancer, incomplete chemotherapy delivery has been associated with worse overall survival rates, but evidence concerning the effects of incomplete radiation delivery in patients with rectal cancer is lacking. Our study bridges this gap in knowledge and shows that patients with an incomplete radiotherapy dosage have a 10% lower 5-year overall survival rate and a higher risk of long-term mortality (HR, 0.70). This finding is in concordance with the results seen in other cancers when preoperative treatment was interrupted.
The evidence is unclear concerning what patient risk factors increase the rate of nonadherence to radiotherapy. Although this topic has not been studied in rectal cancer, incomplete radiotherapy treatment regimens in other cancers have been linked to lower socioeconomic status, age, sex, marital status, rehospitalization, and longer courses of therapy. One barrier to health care may be insurance status. Private insurance was a positive indication of radiotherapy treatment completion (OR, 1.60; 95% CI, 1.16-2.21; P = .004). Lack of insurance has been linked to worse outcomes in cancer patients. Issues such as lack of transportation or distance to the treatment facility could play a major part in completion of radiotherapy. The exact socioeconomic causation is unclear, especially since income level was not associated with completion of a radiotherapy course by patients with rectal cancer.
Receiving cancer care across multiple facilities may be another barrier to health care. Cancer care in the United States has become increasingly centralized, with a significant shift from many low-volume centers to few high-volume centers. Few studies have examined the effects of centralization in rectal cancer in the United States. In Europe, a Danish study found increased 5-year survival in patients with rectal cancer from 37% to 51% after instituting new standards and the creation of specialized and centralized colorectal surgical units covering populations of 350 000 to 500 000 people. A Swedish study also found an increase in overall survival after centralizing all rectal cancer surgeries in a single county into 1 colorectal unit. In both studies, centralization occurred concurrently with significant changes in protocol and technique. Our study showed that receiving radiotherapy at a different facility than where surgery was performed was associated with a higher likelihood of an incomplete radiotherapy course. Our findings support those seen in Europe and further illustrate that centralization of care can affect outcomes in patients with rectal cancer.
Cancer care is complex and necessitates coordination by multidisciplinary treatment teams. Historically, academic and community hospitals have been the setting for most patient cancer care. Specialized cancer centers espousing the theory that staff and facilities dedicated solely to oncologic care would improve patient outcomes have become prominent. Previously, institutions with a higher volume of rectal cancer surgery have been shown to be associated with improved perioperative outcomes. Similar to our results, the findings of a UK study showed that operative mortality and 5-year survival for colorectal cancer were equivalent between teaching and nonteaching hospital patients. Our study, however, found no significant difference in rectal cancer survival outcomes between academic, community, and specialized cancer centers (Figure 1). Instead, treatment at multiple hospitals is a predictive factor for worse outcomes (OR 0.72; P < .001). This finding suggests that continuity of care in one facility, regardless of facility type, should be emphasized in radiotherapy of patients with rectal cancer.
To our knowledge, this study is the only large series examining the effect of completion of radiotherapy on the survival of patients with rectal adenocarcinoma. We established that incomplete radiotherapy in patients with rectal cancer is associated with worse overall survival rates and a greater risk of long-term mortality. We also identified patient risk factors for not completing radiotherapy. Private insurance is associated with increased treatment completion. Notably, receiving cancer care in multiple facilities is associated with higher rates of incomplete therapy, but we found no significant difference in rectal cancer survival outcomes between academic, community, and specialized cancer centers.
Limitations
Our study has several limitations. Although the NCDB contains comprehensive and well-validated data, this retrospective analysis could potentially have selection bias for radiotherapy treatment. We hoped to minimize any bias by adjusting for tumor, clinical, and demographic characteristics in our multivariate analysis. Another limitation to our study is the absence of detailed surgical and tumor data in the NCDB. We cannot abstract more granular details regarding the treatment hospitals, such as center-of-excellence designations or cancer specializations, and were unable to delineate between patients receiving short-course vs long-course radiotherapy. Short-course radiotherapy by definition has a smaller number of fractions, making the treatment less expensive and more convenient for patients, which may improve adherence. More granular data regarding the delivery of radiotherapy (ie, radiation fields and specific regional dosing) would have allowed us to accurately evaluate the dose-response relationship. A detailed analysis of socioeconomic factors leading to incomplete radiotherapy treatment would be salient in this investigation; however, this analysis was impossible using NCDB data, limiting our ability to pinpoint the exact causes of incomplete radiotherapy on a patient-by-patient level. Similarly, we were unable to capture the completeness of chemotherapy and do not have data on toxic effects or reasons why patients did not complete radiotherapy.
Conclusions
Achieving a target radiation dose of 45.0 to 50.4 Gy is associated with survival benefit in patients with locally advanced rectal cancer. Aligning all aspects of multimodal oncology care may increase the probability of completing neoadjuvant therapy.
References
- 1.American Cancer Society Cancer facts and figures 2016. 2016:12-13. http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Published 2016. Accessed June 1, 2016.
- 2.Douglass HO Jr, Moertel CG, Mayer RJ, et al. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315(20):1294-1295. [DOI] [PubMed] [Google Scholar]
- 3.Gastrointestinal Tumor Study Group Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312(23):1465-1472. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network NCCN Guidelines, version 2. Rectal cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2016. [Google Scholar]
- 5.Wong RK, Tandan V, De Silva S, Figueredo A. Pre-operative radiotherapy and curative surgery for the management of localized rectal carcinoma. Cochrane Database Syst Rev. 2007;(2):CD002102. [DOI] [PubMed] [Google Scholar]
- 6.Krishnamurthi SS, Seo Y, Kinsella TJ. Adjuvant therapy for rectal cancer. Clin Colon Rectal Surg. 2007;20(3):167-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medical Research Council Rectal Cancer Working Party Randomised trial of surgery alone versus radiotherapy followed by surgery for potentially operable locally advanced rectal cancer. Lancet. 1996;348(9042):1605-1610. [PubMed] [Google Scholar]
- 8.Gérard A, Buyse M, Nordlinger B, et al. Preoperative radiotherapy as adjuvant treatment in rectal cancer: final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC). Ann Surg. 1988;208(5):606-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cedermark B, Johansson H, Rutqvist LE, Wilking N; Stockholm Colorectal Cancer Study Group . The Stockholm I trial of preoperative short term radiotherapy in operable rectal carcinoma: a prospective randomized trial. Cancer. 1995;75(9):2269-2275. [DOI] [PubMed] [Google Scholar]
- 10.Swedish Rectal Cancer Trial Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336(14):980-987. [DOI] [PubMed] [Google Scholar]
- 11.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23(24):5644-5650. [DOI] [PubMed] [Google Scholar]
- 12.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373(9666):811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926-1933. [DOI] [PubMed] [Google Scholar]
- 14.Ceelen WP, Van Nieuwenhove Y, Fierens K. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2009;(1):CD006041. [DOI] [PubMed] [Google Scholar]
- 15.Sauer R, Becker H, Hohenberger W, et al. ; German Rectal Cancer Study Group . Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731-1740. [DOI] [PubMed] [Google Scholar]
- 16.Dobie SA, Baldwin LM, Dominitz JA, Matthews B, Billingsley K, Barlow W. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98(9):610-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badakhshi H, Gruen A, Sehouli J, Budach V, Boehmer D. The impact of patient compliance with adjuvant radiotherapy: a comprehensive cohort study. Cancer Med. 2013;2(5):712-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American College of Surgeons National Cancer Data Base. https://www.facs.org/quality programs/cancer/ncdb. Accessed March 19, 2016.
- 19.Benson AB III, Venook AP, Bekaii-Saab T, et al. Rectal cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13(6):719-728. [DOI] [PubMed] [Google Scholar]
- 20.Bese NS, Hendry J, Jeremic B. Effects of prolongation of overall treatment time due to unplanned interruptions during radiotherapy of different tumor sites and practical methods for compensation. Int J Radiat Oncol Biol Phys. 2007;68(3):654-661. [DOI] [PubMed] [Google Scholar]
- 21.Gold HT, Do HT, Dick AW. Correlates and effect of suboptimal radiotherapy in women with ductal carcinoma in situ or early invasive breast cancer. Cancer. 2008;113(11):3108-3115. [DOI] [PubMed] [Google Scholar]
- 22.Ohri N, Rapkin BD, Guha D, et al. Predictors of radiation therapy noncompliance in an urban academic cancer center. Int J Radiat Oncol Biol Phys. 2015;91(1):232-238. [DOI] [PubMed] [Google Scholar]
- 23.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326-331. [DOI] [PubMed] [Google Scholar]
- 25.Robbins AS, Pavluck AL, Fedewa SA, Chen AY, Ward EM. Insurance status, comorbidity level, and survival among colorectal cancer patients age 18 to 64 years in the National Cancer Data Base from 2003 to 2005. J Clin Oncol. 2009;27(22):3627-3633. [DOI] [PubMed] [Google Scholar]
- 26.Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009;27(28):4671-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bülow S, Harling H, Iversen LH, Ladelund S; Danish Colorectal Cancer Group . Improved survival after rectal cancer in Denmark. Colorectal Dis. 2010;12(7 online):e37-e42. [DOI] [PubMed] [Google Scholar]
- 28.Khani MH, Smedh K. Centralization of rectal cancer surgery improves long-term survival. Colorectal Dis. 2010;12(9):874-879. [DOI] [PubMed] [Google Scholar]
- 29.Porter G. Surgeon-related factors and outcome in rectal cancer treatment. Int J Surg Investig. 1999;1(3):257-258. [PubMed] [Google Scholar]
- 30.Hodgson DC, Zhang W, Zaslavsky AM, Fuchs CS, Wright WE, Ayanian JZ. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst. 2003;95(10):708-716. [DOI] [PubMed] [Google Scholar]
- 31.Kingston RD, Walsh S, Jeacock J. Colorectal surgeons in district general hospitals produce similar survival outcomes to their teaching hospital colleagues: review of 5-year survivals in Manchester. J R Coll Surg Edinb. 1992;37(4):235-237. [PubMed] [Google Scholar]
- 32.Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72(1):15-24. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Sun P, Rong J, Weng HW, Dai QS, Ye S. Short course radiation in the treatment of localized rectal cancer: a systematic review and meta-analysis. Sci Rep. 2015;5:10953. [DOI] [PMC free article] [PubMed] [Google Scholar]