This randomized clinical trial assesses the effect of postoperative incentive spirometry vs clinical observation on hypoxemia, oxygen saturation, and pulmonary complications among patients undergoing bariatric surgery.
Key Points
Question
What is the effect of postoperative incentive spirometry on hypoxemia, oxygen saturation, and pulmonary complications after bariatric surgery?
Findings
In this randomized noninferiority clinical trial of 224 patients undergoing bariatric surgery, no significant difference in the frequency of postoperative hypoxemia was found between patients who used incentive spirometry after surgery and those who did not. In addition, oxygen saturation levels and 30-day postoperative pulmonary complication rates did not differ between groups.
Meaning
In its current implementation, use of postoperative incentive spirometry after bariatric surgery does not appear to decrease hypoxemia or postoperative pulmonary complications.
Abstract
Importance
The combination of obesity and foregut surgery puts patients undergoing bariatric surgery at high risk for postoperative pulmonary complications. Postoperative incentive spirometry (IS) is a ubiquitous practice; however, little evidence exists on its effectiveness.
Objective
To determine the effect of postoperative IS on hypoxemia, arterial oxygen saturation (Sao2) level, and pulmonary complications after bariatric surgery.
Design, Setting, and Participants
A randomized noninferiority clinical trial enrolled patients undergoing bariatric surgery from May 1, 2015, to June 30, 2016. Patients were randomized to postoperative IS (control group) or clinical observation (test group) at a single-center tertiary referral teaching hospital. Analysis was based on the evaluable population.
Interventions
The controls received the standard of care with IS use 10 times every hour while awake. The test group did not receive an IS device or these orders.
Main Outcomes and Measures
The primary outcome was frequency of hypoxemia, defined as an Sao2 level of less than 92% without supplementation at 6, 12, and 24 postoperative hours. Secondary outcomes were Sao2 levels at these times and the rate of 30-day postoperative pulmonary complications.
Results
A total of 224 patients (50 men [22.3%] and 174 women [77.7%]; mean [SD] age, 45.6 [11.8] years) were enrolled, and 112 were randomized for each group. Baseline characteristics of the groups were similar. No significant differences in frequency of postoperative hypoxemia between the control and test groups were found at 6 (11.9% vs 10.4%; P = .72), 12 (5.4% vs 8.2%; P = .40), or 24 (3.7% vs 4.6%; P = .73) postoperative hours. No significant differences were observed in mean (SD) Sao2 level between the control and test groups at 6 (94.9% [3.2%] vs 94.9% [2.9%]; P = .99), 12 (95.4% [2.2%] vs 95.1% [2.5%]; P = .40), or 24 (95.7% [2.4%] vs 95.6% [2.4%]; P = .69) postoperative hours. Rates of 30-day postoperative pulmonary complications did not differ between groups (8 patients [7.1%] in the control group vs 4 [3.6%] in the test group; P = .24).
Conclusions and Relevance
Postoperative IS did not demonstrate any effect on postoperative hypoxemia, Sao2 level, or postoperative pulmonary complications. Based on these findings, the routine use of IS is not recommended after bariatric surgery in its current implementation.
Trial Registration
clinicaltrials.gov Identifier: NCT02431455
Introduction
At present, approximately 13% of the world population or 600 million adults worldwide are obese. This increase in obesity has been accompanied by a concurrent increase in bariatric surgery, with 468 609 cases being performed in 2013 alone. These morbidly obese patients are at a higher risk for postoperative pulmonary complications such as hypoxemia, atelectasis, pneumonia, laryngospasm, respiratory distress, or the potential need for reintubation.
Pulmonary complications after abdominal surgery are a major cause of morbidity and mortality. Foregut surgery in particular has high rates of pulmonary complications due to the close proximity to the diaphragm and the caudal location of the incision or port sites. Laparoscopic foregut operations also predispose patients to postoperative pulmonary complications due to altered pulmonary mechanics from pneumoperitoneum and patient positioning. By definition, bariatric surgery combines the risk factors of morbid obesity and foregut surgery, putting these patients at increased risk for postoperative pulmonary complications.
As a means to decrease postoperative pulmonary complications, Bartlett et al first described the incentive spirometry (IS) device in 1970. This device functions by encouraging patients to achieve maximal inspiration by providing visual feedback. Since its introduction, IS has gained widespread use in the postoperative period for the prophylaxis and treatment of respiratory complications. At present, postoperative IS is considered the standard of care and is incorporated into standardized bariatric surgery recovery protocols. However, despite the ubiquitous use of IS in the postoperative period, data on its efficacy is conflicting, and high-quality evidence is lacking.
The intrinsic combination of morbid obesity and laparoscopic foregut surgery puts patients undergoing bariatric surgery at higher risk for postoperative pulmonary complications. The purpose of this study was to determine the effect of postoperative IS on the clinical, relevant pulmonary outcome measure of postoperative hypoxemia. Secondary outcome measures of arterial oxygen saturation (Sao2) and 30-day postoperative pulmonary complications after bariatric surgery were also analyzed. We hypothesized that postoperative IS would have no effect on any of these outcomes. To answer this question, we conducted a randomized noninferiority clinical trial examining the effect of postoperative IS on these outcomes.
Methods
Study Design
This study was a single-center randomized clinical trial. All research and clinical care were performed at Lahey Hospital and Medical Center, Burlington, Massachusetts. The Surgical Weight Loss Center at Lahey Hospital is an American College of Surgeons Bariatric Surgery Center Network–accredited program that performs approximately 300 bariatric operations a year, with a bariatric surgery fellow or a general surgery resident assisting. Before the start of the project, the institutional review board of Lahey Hospital Medical Center approved all research (the full trial protocol is available in the Supplement), and all patients provided written informed consent.
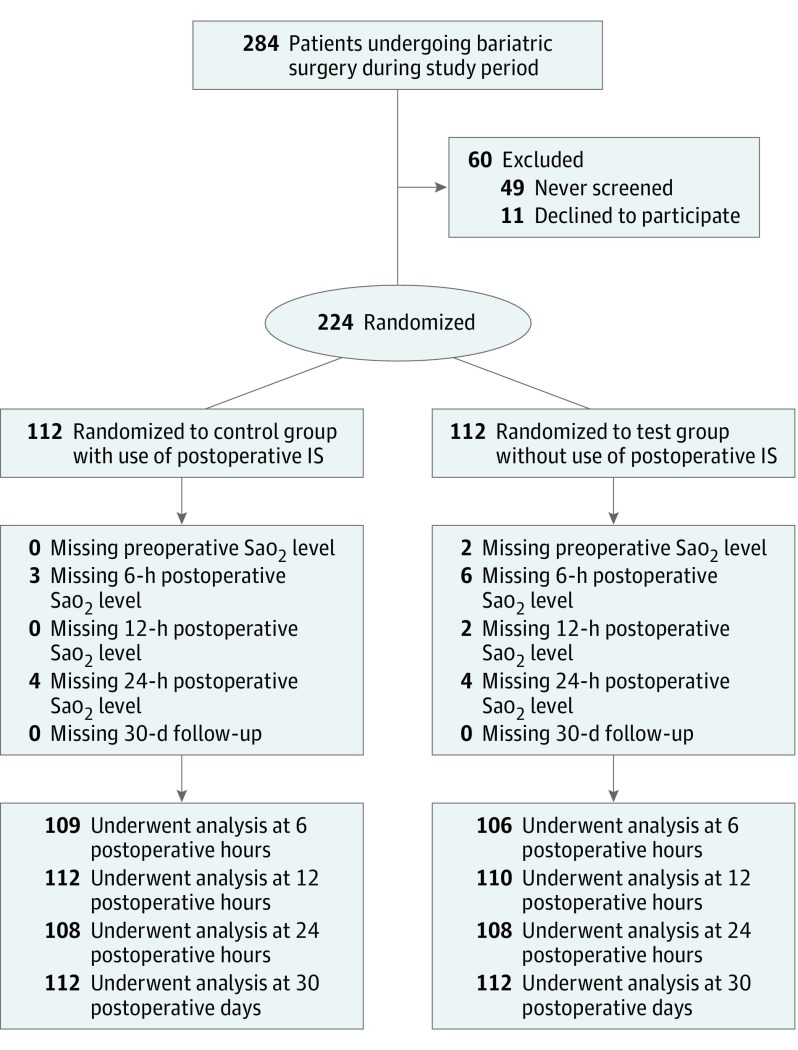
This study enrolled all consenting patients who underwent laparoscopic bariatric surgery from May 1, 2015, to June 30, 2016. No exclusion criteria other than failure to obtain consent were applied. We used a simple randomization scheme for group allocation. Before the start of the study, a computer-randomized list of group assignments was generated. After completion of the consent process, patients were randomized by sequentially accessing this list. Concealment of the next allocation and blinding during the consent processes was used (Figure).
Figure. Flow Diagram of the Study Design.
IS indicates incentive spirometry; Sao2, arterial oxygen saturation level.
Intervention and Control
Patients randomized to the control group received the current standard of care at our institution for postoperative use of IS. A postoperative nursing order was implemented to provide the patients with an IS device when the patient was “awake and alert” and for the patient to use the IS device “10 times every hour while awake.” In addition, preoperative teaching and postoperative coaching and prompting were performed by the nursing staff. Patients randomized to the test group were not provided with an IS device (Figure).
All patients received deep vein thrombosis prophylaxis with 5000 U of subcutaneous heparin sodium in the preoperative period. The anesthetic regimen was left to the discretion of the anesthesia team. Postoperatively, a standardized analgesia regimen was implemented, and no patient had epidural anesthesia. In addition to this analgesia protocol, a standardized recovery pathway of ambulation 3 times daily, diet advancement, and fluid management was implemented for both groups.
IS Use
To evaluate the frequency of IS use in the control group, a pilot study was conducted for 1 month before the start of this trial. Patients undergoing bariatric surgery received the same IS protocol used in the control group to record their device use. At the time of discharge, these logs were collected and analyzed.
Outcome Measures
The primary outcome measure of this study was the frequency of postoperative hypoxemia at 6, 12, and 24 postoperative hours. Hypoxemia was defined as an Sao2 level of less than 92%. Because no strict definition of hypoxemia exists based on oxygen saturation, with a mean pulse oximeter precision of 2% and less than 90% often used to represent hypoxemia in various studies, a value of less than 92% was used. Pulse oximetry measurements as opposed to arterial blood gas values were used because they were readily available, less costly, and less invasive. Data points needed to be measured within 1 hour before or after the determined time to be included. All Sao2 levels were measured with patients discontinuing any supplemental oxygen therapy or positive airway pressure ventilation for 5 minutes and while sitting up with the head of their bed at more than 45°. This interval without supplementation was based on Sao2 without supplemental oxygen reaching equilibration at 4.5 minutes and 95% of changes in Sao2 measurement to decreases in inspired oxygen occurring within 5 minutes. In addition, the interval when a decrease in positive pressure ventilation results in an Sao2 reaching equilibrium is about 5 minutes.
The secondary outcome measure of Sao2 as a continuous quantitative variable was examined from the values recorded in the above method. The secondary outcome measure of 30-day postoperative complications was defined as atelectasis, pneumonia, or the need for intubation. Atelectasis was defined as any radiology report mentioning pulmonary atelectatic changes regardless of clinical manifestations. Pneumonia was defined as radiographic or clinical documentation of pneumonia. Incidence of reintubation was obtained through the retrospective review of medical records.
Statistical Analysis
Before the start of the study, a noninferiority power calculation was performed to determine sample size. Review of the literature showed a 10% baseline proportion of patients after bariatric surgery with Sao2 levels less than 92%. A minimum of 112 patients per group provided a power of more than an 80% (β = 0.20) chance of rejecting a false null hypothesis. This level was based on detection of a clinically significant Δ statistic of 10% between groups with an expected SD of pulse oximetry values of 2%. We used 2-tailed paired t tests and χ2 tests to compare means of continuous variables and distributions of binary variables between both groups, respectively. We used Fisher exact tests to compare categorical variables between both groups. P < .05 was considered statistically significant.
Generalized linear models were used to evaluate outcomes over time with adjustment for multiple measures on each patient. A generalized linear mixed model with main effects for treatment and time (6, 12, and 24 hours) and the 2-factor interaction was used to evaluate the effect of IS use on postoperative hypoxemia. Because hypoxemia status was binary, the model assumed a binomial distribution for the outcome. The model adjusted for repeated measures over time on each person and used an autoregressive covariance structure.
We used a multivariable logistic regression model with a stepwise selection process to choose among candidate predictor variables that had unadjusted P < .15 for the outcome of any postoperative hypoxemia. This model included no IS as a candidate variable. The candidate variables included age, body mass index, asthma, chronic obstructive pulmonary disease (COPD), baseline Sao2 level, and no use of IS. For the variable selection process, limits were set to allow variables with adjusted P = .10 to enter and stay in the model. Power analysis and statistical analysis were performed by a blinded data assessor from the Tufts University Clinical and Translational Science Institute. All analyses were performed using SAS for Windows (version 9.4; SAS Institute Inc).
Results
Study Population
From May 1, 2015, to June 30, 2016, a total of 284 patients underwent laparoscopic bariatric surgery at our center. Of those, 224 (78.9%) provided informed consent and were enrolled (50 men [22.3%] and 174 women [77.7%]; mean [SD] age, 45.6 [11.8] years) (Figure). A total of 112 patients were randomized to the control group and 112 were randomized to the test group. Baseline characteristics of each group were similar, with no statistically significant differences in their demographics (Figure). The control group was noted to have more patients with COPD when compared with the test group (5 patients [4.5%] vs 0; P = .02). For all other preexisting pulmonary conditions, American Society of Anesthesiologists class, preoperative Sao2 level, operation types, and mean operative times, no differences were observed between groups (Table 1).
Table 1. Characteristics of Patients at Baseline.
| Characteristic | No. (%) of Patients | P Valuea | ||
|---|---|---|---|---|
| Full Cohort (N = 224) |
Incentive Spirometer Group | |||
| Control (n = 112) |
Test (n = 112) |
|||
| Age, mean (SD), y | 45.6 (11.8) | 46.0 (12.4) | 45.2 (11.2) | .62 |
| BMI, mean (SD) | 42.8 (5.2) | 42.4 (5.3) | 43.3 (5.1) | .21 |
| Male | 50 (22.3) | 24 (21.4) | 26 (23.2) | .75 |
| ASA class III vs II | 85 (37.9) | 37 (33.0) | 48 (42.9) | .13 |
| Obstructive sleep apnea | 106 (47.3) | 52 (46.4) | 54 (48.2) | .79 |
| Asthma | 62 (27.7) | 33 (29.5) | 29 (25.9) | .55 |
| Current or former smoker | 105 (46.9) | 52 (46.4) | 53 (47.3) | .89 |
| COPD | 5 (2.2) | 5 (4.5) | 0 | .02 |
| Operative time, mean (SD), min | 132.0 (37.4) | 130.9 (36.0) | 133.1 (38.8) | .66 |
| Operation | ||||
| Sleeve gastrectomy | 118 (52.7) | 59 (52.7) | 59 (52.7) | .38 |
| Gastric bypass | 83 (37.1) | 38 (33.9) | 45 (40.2) | |
| Gastric band to gastric bypass | 13 (5.8) | 8 (7.1) | 5 (4.5) | |
| Gastric band to sleeve gastrectomy | 6 (2.7) | 5 (4.5) | 1 (0.9) | |
| Duodenal switch | 3 (1.3) | 1 (0.9) | 2 (1.8) | |
| Sleeve gastrectomy to gastric bypass | 1 (0.4) | 1 (0.9) | 0 | |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); COPD, chronic obstructive pulmonary disease.
Calculated using the 2-tailed paired t test (for age, BMI, and operative time) or the χ2 or Fisher exact test (6 categories of operation).
IS Use
During the pilot study, a total of 16 patients underwent bariatric surgery. Of those 16 patients, log sheets on IS use were collected from 12. On postoperative day 1, the mean frequency of IS use was 4.1 times per day. On postoperative day 2, mean frequency of IS use was 10.4 times per day.
Postoperative Hypoxemia
We found no statistically significant differences in frequency of hypoxemia observed between groups for all points among patients with available measurements (Table 2). At 6 postoperative hours, 13 of 109 patients (11.9%) in the control group were hypoxemic compared with 11 of 106 patients (10.4%) in the test group (P = .72). At 12 postoperative hours, 6 of 112 patients (5.4%) in the control group were hypoxemic compared with 9 of 110 patients (8.2%) in the test group (P = .40). At 24 postoperative hours, 4 of 108 patients (3.7%) in the control group were hypoxemic compared with 5 of 108 patients (4.6%) in the test group (P = .73).
Table 2. Hypoxemia and Sao2 Levels Over Time.
| Variable | Full Cohort (N = 224) |
Incentive Spirometer Group |
P Valuea | |
|---|---|---|---|---|
| Control (n = 112) |
Test (n = 112) |
|||
| Baseline status | ||||
| Hypoxemia | 1/222 (0.5) | 1/112 (0.9) | 0/110 | .32 |
| Sao2 level, mean (SD), % [sample size] | 97.0 (1.8) [222] | 97.0 (1.7) [112] | 97.0 (1.9) [110] | .97 |
| Postoperative hypoxemia | .49b | |||
| 6 h | 24/215 (11.2) | 13/109 (11.9) | 11/106 (10.4) | .72 |
| 12 h | 15/222 (6.8) | 6/112 (5.4) | 9/110 (8.2) | .40 |
| 24 h | 9/216 (4.2) | 4/108 (3.7) | 5/108 (4.6) | .73 |
| Any | 33/224 (14.7) | 14/112 (12.5) | 19/112 (17.0) | .35 |
| Postoperative baseline Sao2 level, mean (SD), % [sample size] | .68b | |||
| 6 h | 94.9 (3.0) [215] | 94.9 (3.2) [109] | 94.9 (2.9) [106] | .99 |
| 12 h | 95.2 (2.4) [222] | 95.4 (2.2) [112] | 95.1 (2.5) [110] | .40 |
| 24 h | 95.7 (2.4) [216] | 95.7 (2.4) [108] | 95.6 (2.4) [108] | .69 |
| Minimum Sao2 among measurements | 93.9 (2.7) [224] | 93.9 (2.8) [112] | 93.8 (2.6) [112] | .71 |
Abbreviations: GLM, generalized linear model for comparison; Sao2, areterial oxygen saturation.
Calculated using the χ2 test for hypoxemia or 2-tailed paired t test for Sao2 data, presented as mean (SD) or number (percentage) of patients unless otherwise indicated.
Calculated using the generalized linear model for comparison of incentive spirometry vs non–incentive spirometry across all 3 postoperative points, with adjustment for baseline value and repeated measures.
Postoperative Sao2 Level
We observed no statistically significant differences in mean Sao2 levels between groups for all times (Table 2). At 6 postoperative hours, patients in the control group had a mean (SD) Sao2 level of 94.9% (3.2%) compared with a mean of 94.9% (2.9%) for patients in the test group (P = .99). At 12 postoperative hours, patients in the control group had a mean (SD) Sao2 level of 95.4% (2.2%) compared with a mean of 95.1% (2.5%) for patients in the test group (P = .40). At 24 postoperative hours, patients in the control group had a mean (SD) Sao2 level of 95.7% (2.4%) compared with 95.6% (2.4%) for patients in the test group (P = .69).
Postoperative Pulmonary Complications
We observed no differences between the rates of 30-day postoperative pulmonary complications between groups (Table 3). Eight of 112 patients (7.1%) in the control group had postoperative pulmonary complications, including 7 occurrences of atelectasis (6.%), 1 of pneumonia (0.9%), and no reintubations. For the test group, 4 of 112 patients (3.6%) had postoperative pulmonary complications, all consisting of atelectasis (P = .24 for the difference between groups).
Table 3. 30-Day Postoperative Pulmonary Complications.
| Postoperative Pulmonary Complication | No. (%) of Patients | P Valuea | ||
|---|---|---|---|---|
| Full Cohort (N = 224) |
Incentive Spirometer Group | |||
| Control (n = 112) |
Test (n = 112) |
|||
| Any | 12 (5.4) | 8 (7.1) | 4 (3.6) | .24 |
| Type | ||||
| None | 212 (94.6) | 104 (92.9) | 108 (96.4) | NA |
| Atelectasis | 11 (4.9) | 7 (6.2) | 4 (3.6) | |
| Pneumonia | 1 (0.4) | 1 (0.9) | 0 | |
Abbreviation: NA, not applicable.
Calculated using the χ2 test.
Univariate and Multivariate Logistic Regression Models
We found no significant interaction of treatment and time (P = .41), with no significant variation between the different evaluation points. We found no significant difference in hypoxemia between the control and test groups (P = .49; adjusted mean hypoxemia with or without IS, 5.8% vs 7.6%).
For the multivariate logistic regression model when the variable selection process was run, the only variable that reached significance was baseline Sao2 level. A single patient was hypoxemic preoperatively and hypoxemic at 6 and 24 postoperative hours. Using the unadjusted logistic regression model for each potential predictor variable, preoperative hypoxemia had a 100% specificity for postoperative hypoxemia based on this single patient. However, after controlling for this variable, none of the other variables had adjusted significant associations with hypoxemia with the outcome at the level of P < .10.
Discussion
We present findings from, to our knowledge, the first reported randomized clinical trial to evaluate the effect of IS use after bariatric surgery. We found no significant difference in the frequency of postoperative hypoxemia among patients using an IS device after surgery compared with those who did not. In addition, we did not observe a difference in Sao2 level or the rate of 30-day postoperative pulmonary complications between patients using an IS device after surgery compared with those who did not. These findings support our null hypothesis that postoperative IS use has no significant effect on hypoxemia or Sao2 after bariatric surgery. Based on these findings, we do not recommend the routine use of IS in its current implementation after bariatric surgery. In addition, we question its routine use for other laparoscopic foregut procedures.
These findings are consistent with the current published literature on IS use postoperatively. A 2007 Cochrane review of randomized clinical trials of IS in adult patients undergoing upper abdominal surgery found that participants receiving IS had the same rates of clinical or pulmonary complications as those who did not. In bariatric surgery specifically, preoperative IS use has been shown to have no effect on postoperative lung function. Despite the lack of evidence, use of IS is a common practice. At our institution, $33 491 was spent on purchasing these devices in the 2013-2014 fiscal year. In a setting where resources are limited or are being conserved, IS use has not been shown to be effective. Also, time is spent by health care workers in teaching, encouraging, and educating about these devices with little proven benefit. In addition to the time and monetary costs, these disposable devices go against many initiatives to reduce the amount of the environmental effect of health care.
By definition, bariatric operations combine the risk factors for postoperative complications of obesity and foregut surgery. This innate combination puts these patients at increased risk for postoperative pulmonary complications and hypothetically would make them ideal candidates for postoperative IS benefit. If no benefit is seen in this high-risk population, we postulate that the use of IS postoperatively in its current form for similar- or lower-risk operations would be of no benefit.
Limitations
This study has several limitations. Although postoperative hypoxemia and Sao2 level are both meaningful clinical measures, postoperative pulmonary complications are of more clinical significance. However, pulmonary complications after bariatric surgery are very rare, with the American College of Surgeons National Surgical Quality Improvement Project reporting a 0.2% pneumonia rate, 0.2% reintubation rate, and 0.1% requiring ventilation for more than 48 hours in 2014. Owing to the infrequency of these complications, this study was not powered to detect this difference; to power a study with postoperative pulmonary complications as a primary outcome, a very large sample size would be needed. Therefore, we opted to use hypoxemia as our primary outcome measure. At our institution, patients with Sao2 levels less than 92% receive supplemental oxygen therapy, thus making this outcome measure of clinical significance.
Another limitation is that patients with COPD were not stratified during randomization. As such, significantly more patients with COPD were in the control group (5 of 112) compared with the test group (0 of 112). Analysis of these patients with COPD revealed more frequent hypoxemia and lower Sao2 levels but without any 30-day postoperative pulmonary complications. However, when these patients were excluded from analysis, we found no significant difference between groups in any outcome measures. In addition, a multivariate logistic regression model did not identify COPD as having a significant association with hypoxemia. Thus, COPD is unlikely to have acted as a confounding variable; however, because few patients with COPD were enrolled, extrapolation of our results to patients with COPD is limited.
For patients in the control group, adherence to IS use was lower than protocol determined. The control group was ordered to use the IS device 10 times per hour based on our preexisting institutional policy. However, observed compliance was much lower, with a mean of 4.1 times per day on the first postoperative day and a mean of 10.4 times per day on the second day. This rate of adherence would appear to be poor; however, a standardized and evidence-based frequency of IS use is not defined. This lack of definition may have been attributable to a combination of factors, including variable length of hours in the first postoperative day because some operations were finished later in the day. Also, patients slept more on the first postoperative day; thus, IS was not used as frequently. For the purposes of this study, a frequency of 10 times per hour was used because this was a preexisting protocol at our institution, and our nurses were familiar with these instructions. This protocol was continued for the control group. However, review of the literature reveals a wide range of reported values from 4 times per day to every 6 minutes while awake or 10 times per hour. Although controls used IS less frequently than instructed by protocol, they still fell within the reported range of use and still represent an adequate control group for device use. However, the results of this study may be a consequence of that low adherence rate in the control group, with the possibility of increased adherence altering the results. Even in this study with structured teaching, adherence was difficult. This low rate of compliance may reflect a more universal problem with IS and may contribute to its lack of effectiveness.
The secondary outcome of 30-day postoperative pulmonary complications may have been confounded by patients seeking care at other facilities. After discharge, appointments are scheduled at 1 and 2 weeks from discharge; in our centers, annual rates of 30-day follow-up tracked by the American Society for Metabolic and Bariatric Surgery is more than 90%. We have no way of determining whether some patients presented to another facility with pulmonary complications, but we believe this occurrence to be a low number.
Conclusions
Our findings did not demonstrate any effect of IS on postoperative hypoxemia or Sao2 level. Based on these findings, we do not recommend the routine use of IS after bariatric surgery. With health care moving toward a more evidence-based, economically driven, and environmentally sustainable field, this study adds evidence to the concept that IS should not be universally used in all patients undergoing surgery and does not appear to be necessary in elective bariatric surgical procedures.
Trial Protocol
References
- 1.World Health Organization. Obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/. Udpated June 2016. Accessed December 8, 2016.
- 2.Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg. 2015;25(10):1822-1832. [DOI] [PubMed] [Google Scholar]
- 3.Pasulka PS, Bistrian BR, Benotti PN, Blackburn GL. The risks of surgery in obese patients. Ann Intern Med. 1986;104(4):540-546. [DOI] [PubMed] [Google Scholar]
- 4.Rothen HU, Sporre B, Engberg G, Wegenius G, Hedenstierna G. Airway closure, atelectasis and gas exchange during general anaesthesia. Br J Anaesth. 1998;81(5):681-686. [DOI] [PubMed] [Google Scholar]
- 5.Overend TJ, Anderson CM, Lucy SD, Bhatia C, Jonsson BI, Timmermans C. The effect of incentive spirometry on postoperative pulmonary complications: a systematic review. Chest. 2001;120(3):971-978. [DOI] [PubMed] [Google Scholar]
- 6.Sprung J, Whalley DG, Falcone T, Warner DO, Hubmayr RD, Hammel J. The impact of morbid obesity, pneumoperitoneum, and posture on respiratory system mechanics and oxygenation during laparoscopy. Anesth Analg. 2002;94(5):1345-1350. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett RH, Krop P, Hanson EL, Moore FD. Physiology of yawning and its application to postoperative care. Surg Forum. 1970;21:222-224. [PubMed] [Google Scholar]
- 8.O’Donohue WJ., Jr National survey of the usage of lung expansion modalities for the prevention and treatment of postoperative atelectasis following abdominal and thoracic surgery. Chest. 1985;87(1):76-80. [DOI] [PubMed] [Google Scholar]
- 9.Wattie J. Incentive spirometry following coronary artery bypass surgery. Physiotherapy. 1998;10(84):508-514. [Google Scholar]
- 10.Awad S, Carter S, Purkayastha S, et al. Enhanced Recovery After Bariatric Surgery (ERABS): clinical outcomes from a tertiary referral bariatric centre. Obes Surg. 2014;24(5):753-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freitas ER, Soares BG, Cardoso JR, Atallah AN. Incentive spirometry for preventing pulmonary complications after coronary artery bypass graft. Cochrane Database Syst Rev. 2007;(3):CD004466. [DOI] [PubMed] [Google Scholar]
- 12.Nickerson BG, Sarkisian C, Tremper K. Bias and precision of pulse oximeters and arterial oximeters. Chest. 1988;93(3):515-517. [DOI] [PubMed] [Google Scholar]
- 13.Majumdar SR, Eurich DT, Gamble JM, Senthilselvan A, Marrie TJ. Oxygen saturations less than 92% are associated with major adverse events in outpatients with pneumonia: a population-based cohort study. Clin Infect Dis. 2011;52(3):325-331. [DOI] [PubMed] [Google Scholar]
- 14.Gruber P, Kwiatkowski T, Silverman R, Flaster E, Auerbach C. Time to equilibration of oxygen saturation using pulse oximetry. Acad Emerg Med. 1995;2(9):810-815. [DOI] [PubMed] [Google Scholar]
- 15.Brown JT, Schur MS, McClain BC, Kafer ER. In vivo response time of transcutaneous oxygen measurement to changes in inspired oxygen in normal adults. Can Anaesth Soc J. 1984;31(1):91-96. [DOI] [PubMed] [Google Scholar]
- 16.Chiumello D, Coppola S, Froio S, et al. Time to reach a new steady state after changes of positive end expiratory pressure. Intensive Care Med. 2013;39(8):1377-1385. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad S, Nagle A, McCarthy RJ, Fitzgerald PC, Sullivan JT, Prystowsky J. Postoperative hypoxemia in morbidly obese patients with and without obstructive sleep apnea undergoing laparoscopic bariatric surgery. Anesth Analg. 2008;107(1):138-143. [DOI] [PubMed] [Google Scholar]
- 18.Zoremba M, Dette F, Gerlach L, Wolf U, Wulf H. Short-term respiratory physical therapy treatment in the PACU and influence on postoperative lung function in obese adults. Obes Surg. 2009;19(10):1346-1354. [DOI] [PubMed] [Google Scholar]
- 19.Sucandy I, Szomstein S, Rosenthal RJ. Understanding the reasons and significance of low oxygen saturation in the early postoperative period after laparoscopic Roux-en-Y gastric bypass. Am Surg. 2013;79(5):540-541. [PubMed] [Google Scholar]
- 20.Ralston AC, Webb RK, Runciman WB. Potential errors in pulse oximetry, I: pulse oximeter evaluation. Anaesthesia. 1991;46(3):202-206. [DOI] [PubMed] [Google Scholar]
- 21.Cattano D, Altamirano A, Vannucci A, Melnikov V, Cone C, Hagberg CA. Preoperative use of incentive spirometry does not affect postoperative lung function in bariatric surgery. Transl Res. 2010;156(5):265-272. [DOI] [PubMed] [Google Scholar]
- 22.Tyson AF, Kendig CE, Mabedi C, Cairns BA, Charles AG. The effect of incentive spirometry on postoperative pulmonary function following laparotomy: a randomized clinical trial. JAMA Surg. 2015;150(3):229-236. [DOI] [PubMed] [Google Scholar]
- 23.Spaniolas K, Kasten KR, Sippey ME, Pender JR, Chapman WH, Pories WJ. Pulmonary embolism and gastrointestinal leak following bariatric surgery: when do major complications occur? Surg Obes Relat Dis. 2016;12(2):379-383. [DOI] [PubMed] [Google Scholar]
- 24.Celli BR, Rodriguez KS, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry, and deep breathing exercises in preventing pulmonary complications after abdominal surgery. Am Rev Respir Dis. 1984;130(1):12-15. [DOI] [PubMed] [Google Scholar]
- 25.Craven JL, Evans GA, Davenport PJ, Williams RH. The evaluation of the incentive spirometer in the management of postoperative pulmonary complications. Br J Surg. 1974;61(10):793-797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol