Abstract
This study examines the proportion of men treated primarily with active surveillance across practices and among urologists in the Michigan Urological Surgery Improvement Collaborative.
Owing to concerns about overtreatment, urologists are increasingly using active surveillance (AS) as the initial management for men with low-risk prostate cancer. Nonetheless, additional progress in this area requires a deeper understanding of the well-established and wide variation in use of AS. Of particular interest from a quality improvement perspective is whether practice patterns tend to vary widely even among urologists in the same practice and/or based on her or his panel size (ie, the volume of men with low-risk prostate cancer a given urologist manages). In the context of limited resources, the availability of such information may be used to develop efficient improvement interventions aimed at optimizing the implementation of AS among diverse urologists and practice settings.
Methods
The Michigan Urological Surgery Improvement Collaborative is a consortium of 43 academic and community urology practices in Michigan that maintains a prospective clinical registry with detailed and validated clinical information for men newly diagnosed as having prostate cancer seen in participating practices. For this analysis, we identified all Michigan Urological Surgery Improvement Collaborative practices with at least 5 urologists who each managed 5 or more men with low-risk prostate cancer from January 2012 through July 2016. We then examined the proportion of men managed primarily with AS across practices and among urologists within each practice, adjusting for differences in patient age and comorbidity. Finally, we fit a linear regression model to estimate the association between the proportion of patients entering AS and urologist panel size. Two-sided testing was performed, with P < .05 considered significant (StataCorp).
Each practice obtained institutional review board approval of not-regulated or exempt status or had an expedited review for collaborative participation. As a part of the institutional review board process at all participating sites, it was determined that given the quality improvement focus of the Michigan Urological Surgery Improvement Collaborative and the fact that the data it houses are (1) collected for quality improvement and not human participants research and (2) is collected during routine care of patients (eg, does not require any changes or burdens beyond routine care processes), informed consent was not necessary.
Results
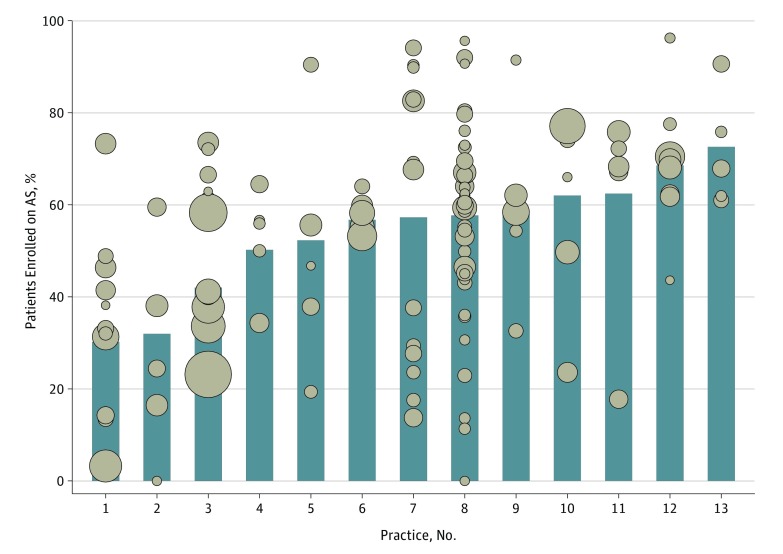
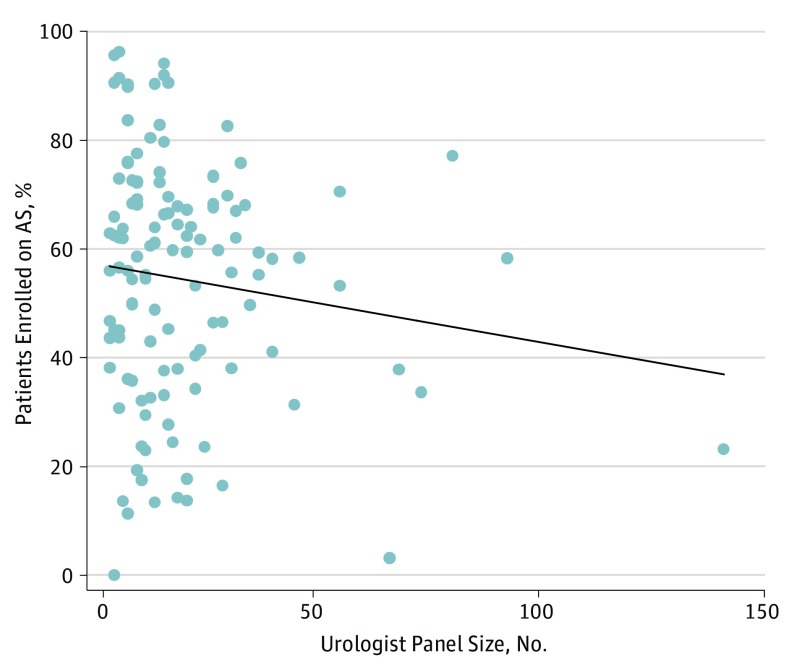
We identified 124 urologists from 13 practices who managed 2643 men (median age, 64 years) diagnosed as having low-risk prostate cancer during the interval of interest. The median practice and urologist panel size was 165 patients (range, 70-524) and 16 patients (range, 5-141), respectively. The adjusted proportion of men entering initial AS varied significantly across practices (median, 57.3%; range, 30.2%-72.6%; P < .001) (Figure 1). In almost all practices, urologist-specific use of AS also varied widely; in 1 practice, for instance, the adjusted rates varied from 0% to 95.6% among more than 30 urologists (Figure 1). We identified no significant association between a urologist’s panel size and rates of AS use (R2 = 0.02; P = .17) (Figure 2).
Figure 1. Variation in Adjusted Rates of Active Surveillance (AS) for Men With Low-Risk Prostate Cancer by Practice (Bars) and by Urologist Within Each Practice (Dots).
Median patient age was 64 years; median prostate-specific antigen level was 5.0 ng/mL; and Charlson comorbidity score was 0 for 1868 men, 1 for 415 men, and 2 or higher for 360 men. Adjusted AS rates for each urologist and practice were estimated using a multivariable logistic regression model fit to account for differences in patient age and comorbidity among urologists. In the model, age was a continuous predictor, and Charlson comorbidity was a categorical predictor (categories = 0, 1, and ≥2). We defined low-risk according to criteria from the National Comprehensive Cancer Network (ie, clinical stage ≤T2a, prostate-specific antigen <10 ng/mL, and biopsy Gleason score ≤6). The number of urologists per practice varies from 5 to 38. The size of each dot is scaled to represent individual clinician panel size (range, 5-141).
Figure 2. Relationship Between Urologist Panel Size and Percentage of Men Enrolled on Active Surveillance (AS) Within the Evaluated 4.5-Year Perioda.
aUrologist panel size = number of men with National Comprehensive Cancer Network low-risk prostate cancer a given urologist primarily treated.
Discussion
The use of AS for primary management of men with low-risk prostate cancer varies widely both across and within urology practices in Michigan; moreover, the propensity to use AS does not appear to correlate with a urologist’s low-risk prostate cancer panel size. Although our analysis is limited by small sample size for some urologists and a lack of insight on patient preferences, our findings nonetheless provide evidence that individual urologists develop treatment patterns for men with low-risk prostate cancer that differ markedly from both others in the same group practice and from colleagues with similar experience managing men with prostate cancer.
Collectively, these data indicate that quality improvement activities focused only on specific practices or on urologists who see a high or low volume of patients with prostate cancer may be less effective at addressing variation in use of AS. Instead, such activities should be tailored to individual physicians. In Michigan, these findings are guiding our interventions to improve AS including dissemination of a clinical roadmap for men with favorable-risk prostate cancer that aims to achieve more consistent practice patterns as urologists consider the appropriateness and implementation of AS. The roadmap is being delivered via personalized outreach that also includes clinician-level performance measures that allow individuals to understand their own practice relative to patterns of care for other urologists within their group and across Michigan.
References
- 1.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990-2013. JAMA. 2015;314(1):80-82. [DOI] [PubMed] [Google Scholar]
- 2.Loeb S, Folkvaljon Y, Curnyn C, Robinson D, Bratt O, Stattin P. Uptake of active surveillance for very-low-risk prostate cancer in Sweden [published online October 20, 2016]. JAMA Oncol. 2016. doi: 10.1001/jamaoncol.2016.3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman KE, Niu J, Shen Y, et al. Physician variation in management of low-risk prostate cancer: a population-based cohort study. JAMA Intern Med. 2014;174(9):1450-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC; Michigan Urological Surgery Improvement Collaborative . Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67(1):44-50. [DOI] [PubMed] [Google Scholar]
- 5.Cher ML, Dhir A, Auffenberg GB, et al. ; Michigan Urological Surgery Improvement Collaborative . Appropriateness criteria for active surveillance of prostate cancer. J Urol. 2017;197(1):67-74. [DOI] [PubMed] [Google Scholar]
- 6.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14(1):19-30. [DOI] [PubMed] [Google Scholar]