Key Points
Question
Does intraoperative dexmedetomidine reduce postoperative delirium?
Findings
Unlike its use as a sedative in the intensive care unit, intraoperative dexmedetomidine did not significantly reduce the incidence of delirium over saline placebo (12.2% vs 11.4%) in this randomized clinical trial.
Meaning
The administration of dexmedetomidine in the operating room does not prevent postoperative delirium, which may be due to the short-acting nature of the drug and loss of salutary effects after discontinuation of the infusion.
Abstract
Importance
Postoperative delirium occurs in 10% to 60% of elderly patients having major surgery and is associated with longer hospital stays, increased hospital costs, and 1-year mortality. Emerging literature suggests that dexmedetomidine sedation in critical care units is associated with reduced incidence of delirium. However, intraoperative use of dexmedetomidine for prevention of delirium has not been well studied.
Objective
To evaluate whether an intraoperative infusion of dexmedetomidine reduces postoperative delirium.
Design, Setting, and Participants
This study was a multicenter, double-blind, randomized, placebo-controlled trial that randomly assigned patients to dexmedetomidine or saline placebo infused during surgery and for 2 hours in the recovery room. Patients were assessed daily for postoperative delirium (primary outcome) and secondarily for postoperative cognitive decline. Participants were elderly (>68 years) patients undergoing major elective noncardiac surgery. The study dates were February 2008 to May 2014.
Interventions
Dexmedetomidine infusion (0.5 µg/kg/h) during surgery and up to 2 hours in the recovery room.
Main Outcomes and Measures
The primary hypothesis tested was that intraoperative dexmedetomidine administration would reduce postoperative delirium. Secondarily, the study examined the correlation between dexmedetomidine use and postoperative cognitive change.
Results
In total, 404 patients were randomized; 390 completed in-hospital delirium assessments (median [interquartile range] age, 74.0 [71.0-78.0] years; 51.3% [200 of 390] female). There was no difference in postoperative delirium between the dexmedetomidine and placebo groups (12.2% [23 of 189] vs 11.4% [23 of 201], P = .94). After adjustment for age and educational level, there was no difference in the postoperative cognitive performance between treatment groups at 3 months and 6 months. Adverse events were comparably distributed in the treatment groups.
Conclusions and Relevance
Intraoperative dexmedetomidine does not prevent postoperative delirium. The reduction in delirium previously demonstrated in numerous surgical intensive care unit studies was not observed, which underscores the importance of timing when administering the drug to prevent delirium.
Trial Registration
clinicaltrials.gov Identifier NCT00561678
This randomized clinical trial evaluates whether an intraoperative infusion of dexmedetomidine reduces postoperative delirium in elderly patients undergoing major elective noncardiac surgery.
Introduction
Postoperative delirium and postoperative cognitive dysfunction (POCD) are major complications that cause disability and distress for millions of patients annually. Delirium is an acute attentional deficit that typically occurs during the initial postoperative days and manifests with hyperactive, hypoactive, or mixed symptoms. Postoperative delirium has been reported to occur in 10% to 60% of elderly surgical patients, varying by surgical procedure. Assessed with neuropsychological tests and in the absence of delirium, POCD is a decline in cognitive ability occurring between 1 week and 1 year after surgery. The incidence of POCD is approximately 10% to 12% and varies with clinical, demographic, and surgical variables, as well as the interval between surgery and assessment. Both postoperative delirium and POCD are associated with longer hospital stay, functional decline, lower likelihood of return to independent living, and increased mortality.
The α2-adrenergic agonist dexmedetomidine has a range of effects that may be beneficial in the postoperative period, including opioid-sparing properties, decreased anesthetic requirements, and neuroprotective effects seen in animal models. For example, dexmedetomidine moderates the systemic stress response via the hypothalamic-pituitary-adrenal axis. Consistent with this finding, delirium is less common in intensive care unit (ICU) patients sedated with dexmedetomidine than with midazolam or propofol. Postoperative delirium may precede or be a risk factor for POCD. To date, no study has examined whether intraoperative dexmedetomidine can ameliorate postoperative delirium and POCD.
We hypothesized that intraoperative dexmedetomidine administration would reduce postoperative delirium and POCD. We aimed to investigate whether the reduction in delirium seen in the ICU with dexmedetomidine sedation could be replicated if only given during surgery and recovery room stay. Our primary objective was to evaluate the effect of intraoperative dexmedetomidine compared with standard perioperative management on postoperative delirium in patients older than 70 years undergoing major elective noncardiac surgery without a planned ICU stay. Secondarily, we tested whether dexmedetomidine use reduces cognitive change 3 months and 6 months after surgery.
Methods
This double-blind, randomized, parallel-group, placebo-controlled trial was conducted at 10 sites from February 2008 to May 2014. The lead site was The Mount Sinai Hospital in New York City. Institutional review board approval was obtained at The Mount Sinai Hospital and at each participating site, including Icahn School of Medicine at Mount Sinai (New York, New York), Cleveland Clinic (Cleveland, Ohio), Englewood Hospital and Medical Center (Englewood, New Jersey), Johns Hopkins School of Medicine (Baltimore, Maryland), Saint Louis University (St Louis, Missouri), Mayo Clinic School of Medicine (Rochester, Minnesota), University of Miami School of Medicine (Miami, Florida), The University of North Carolina at Chapel Hill, University of Maryland (College Park), and The Ohio State University (Columbus). Capacity of patients to give consent was determined by physician interview before enrollment in the study. All participants provided written informed consent, approved by the local institutional review boards. The trial was registered at clinicaltrials.gov (NCT00561678).
We included patients older than 70 years, which was amended to older than 68 years after difficulty identifying patients having at least a 2-day hospital stay, agreeing to interval home visits, and accepting a study drug. All included participants were undergoing major elective noncardiac surgery (including spine, thoracic, orthopedic, urologic, or general surgery) performed using general anesthesia. Major surgery was defined by a planned stay of at least 2 days. All patients were screened with a Mini-Mental State Examination (MMSE), and those with scores less than 20 were excluded to ensure that patients with frank dementia were not enrolled. The following exclusion criteria were applied: dementia, emergency surgery, intracardiac or intracranial surgery, planned postoperative intubation, severe visual or auditory handicap, illiteracy, Parkinson disease, life expectancy less than 6 months, renal failure requiring dialysis, sick sinus syndrome, second-degree or third-degree heart block or clinically significant sinus bradycardia, contraindication for use of an α2-adrenergic agonist, American Society of Anesthesiologists’ (ASA) classification IV or V at the time of enrollment, and hepatic dysfunction.
Protocol
The trial protocol is available in Supplement 1. An infusion of dexmedetomidine (0.5 µg/kg/h) or saline placebo was started in patients on entering the operating room and was continued until 2 hours into recovery. Randomization at 1:1 was based on a computer program (SAS PLAN; SAS Institute Inc) in blocks of 6 patients by center. Masked drug was provided by a pharmacy, thus concealing allocation from investigators and clinicians.
Anesthesiologists were instructed to avoid administering benzodiazepines and nitrous oxide. Any other induction agents were permissible. General anesthesia was maintained with propofol, sevoflurane, or both. Opioids and neuromuscular blocking agents were used per preference of the anesthesiologist, as were vasoactive medications. Patients were typically extubated at the end of surgery.
Measurements
Patients identified through the computerized scheduling system were approached by telephone between 1 day and 1 month before surgery. Those who consented were evaluated for study eligibility and completed a 1-hour cognitive battery (see below).
Delirium Battery
Our primary outcome was the presence of postoperative delirium determined by the confusion assessment method (CAM) while hospitalized or until day 5. Structured delirium assessment consisted of the Delirium Symptom Interview, abbreviated digit span, MMSE, CAM, and Memorial Delirium Assessment Scale. The Confusion Assessment Method for ICU Patients (CAM-ICU) was administered in the recovery room. The structured Delirium Symptom Interview allows trained lay interviewers to assess delirium and can capture whether delirium has occurred between assessments: the sensitivity is 0.90 and the specificity 0.80. The Delirium Symptom Interview and MMSE were administered concurrently for acute cognitive change. Delirium raters were masked to treatment; they received structured training on in-person mock encounters and completed a competency certification test. Quality of the assessments was monitored by monthly conferences.
Cognitive Battery
Cognition was assessed with the MMSE and the full Alzheimer’s Disease Centers’ Uniform Data Set. The latter is sensitive to mild cognitive impairment (MCI) and dementia and tests the following 5 domains: attention, processing speed, episodic memory, language, and executive function. Tests included Digit Span forward and backward, Digit Symbol, Trail-Making Test parts A and B, Logical Memory immediate and delayed recall, Category Fluency (animals) and Category Fluency (vegetables), and Boston Naming. Mild cognitive impairment at baseline was defined by the Logical Memory II delayed recall score relative to educational level and was used as a covariate. Two versions of the battery were available for use: one for in-person interview and one for use over the telephone when needed. Postoperative cognition, our secondary outcome, was measured by comparing baseline cognitive scores with scores at 3 months and 6 months after surgery.
Demographics and medical history were collected from patient interviews and confirmed by a physician. Need for vasopressor support and the occurrence of adverse events, such as intraoperative bradycardia, intraoperative hypotension, and intraoperative hypertension, were reported by the clinical anesthesiologist.
Statistical Analysis
Sample size was based on a 15% incidence of delirium among the placebo group and a 50% reduction in delirium incidence in the dexmedetomidine group and a 2-tailed test. We considered our assumption to be conservative because the incidence of delirium is described as 10% to 60%. Assuming an α error of 0.05, a total of 706 patients would provide 80% power for a 50% reduction in delirium incidence in the dexmedetomidine group.
Regarding stopping guidelines, 4 annual interim analyses were planned by the data safety monitoring board (DSMB) at their first meeting for safety and harm. The benefit was defined as a reduction in postoperative delirium, and the harm was defined as a higher-than-expected incidence of delirium or a severe adverse event in the dexmedetomidine group. We used 1-sided tests of significance in favor of the dexmedetomidine group (benefit) or the placebo group (harm). The overall α levels for delirium comparison (either benefit or harm) were 0.025 for benefit and 0.1 for harm. The boundary value for efficacy assumed 95% conditional power (ie, ≥95% confidence that the test statistic will be less than the specified boundary value at the end of the study). The choice for harm was 50%, reflecting a desire to identify the occurrence of these serious adverse events and the intervention. After slow recruitment and an extended time course, the DSMB thought that it would be difficult to continue to defend the risk-benefit ratio of administering a study drug. Therefore, they considered an analysis that examined the futility of treatment.
Conditional power analysis was used to determine the study power using the information observed before each interim analysis. The conditional power was calculated under the following 3 different assumptions: (1) the originally assumed effect, (2) the currently observed effect, and (3) a null effect.
Intent-to-treat analysis was performed throughout regardless of dropout status. χ2 Tests were used for categorical variables, and Wilcoxon rank sum tests were used for continuous variables because most were not normally distributed.
The primary analysis was a univariable logistic comparison of the incidence of delirium on postoperative days in the dexmedetomidine vs placebo groups. Planned secondary analyses were performed among all patients included in the primary analysis and examined demographic and clinical covariates associated with delirium. Covariates in the secondary analysis were those moderately associated with the outcome (P < .15), including educational level, body mass index, surgical time, MCI, surgical procedure, postanesthesia care unit delirium, study site, and history of cancer, myocardial infarction, diabetes, and hypertension. Under these criteria, treatment group was not part of the secondary analyses. The final logistic regression model was selected using the backward selection method (stay criterion, 0.1).
Cognition was measured with a normalized composite score defined from the cognitive battery. The means (SDs) of each of the 10 test scores were calculated. The individual test score was normalized by subtracting the mean of the baseline scores and dividing it by its SD. For lower subtest scores that reflect better test performance, the sign was reversed. Therefore, higher scores always signified better performance.
The composite score was the sum of the 10 standardized scores. We subtracted the baseline mean of 0.28 from this score and divided by the baseline SD of 5.91 to determine the composite z score.
Postoperative cognitive dysfunction was analyzed with generalized estimating equations. We compiled a list of covariates found to be moderately associated with the z score (P < .15) or having different effects between treatment groups (P < .10), including the following: treatment assignment, age, educational level, anesthesia time, sex, MCI, ASA status, hypertension, surgical procedure, amount of fentanyl citrate equivalents, intraoperative bradycardia, anesthetic depth, length of stay, and delirium. We performed sensitivity analyses of the missing data, including a mixed-effects linear model using all available data, multiple imputation under a missing-at-random assumption, pattern mixture models assuming nonignorable missing mechanism, and multiple imputation using distance-aided selection of donors.
All statistical procedures were performed using a software program (SAS for PC, version 9.3; SAS Institute Inc). Statistical significance was defined at the .05 level.
Results
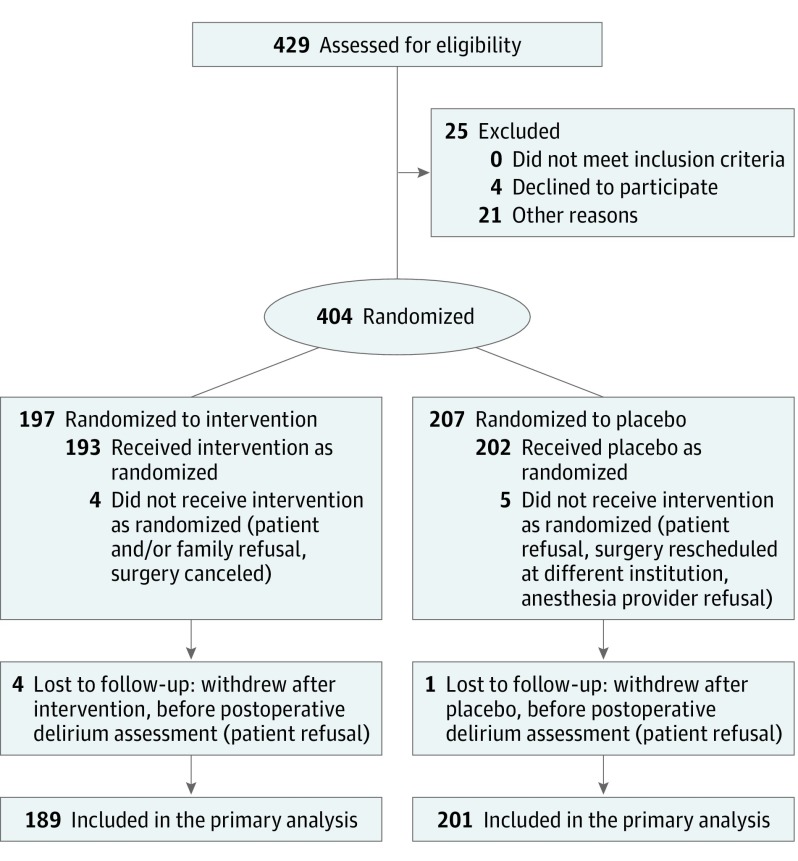
The study was stopped for futility in accord with the DSMB in January 2014 based on a planned interim analysis in the spring of 2013. The conditional power for concluding efficacy under the original assumption of treatment effect was 3% and under the observed trend and a null trend was less than 1% for both. The conditional power for concluding harm was less than 25% under the 3 different assumptions. At that point, 429 patients had been assessed for eligibility, and 25 were excluded before randomization because of patient withdrawal or surgeon or anesthesiologist preference. In total, 404 patients were randomized, 197 to dexmedetomidine and 207 to saline placebo. Four patients in the dexmedetomidine group and 5 patients in the placebo group did not receive the allocated treatment due to patient or clinician refusal or surgical cancellation. Therefore, 193 patients received dexmedetomidine, and 202 patients received placebo. Four patients dropped out after receiving dexmedetomidine and 1 patient dropped out after receiving placebo without completing the in-hospital portion of the protocol, including delirium assessment (Figure).
Figure. CONSORT Diagram.
CONSORT indicates Consolidated Standards of Reporting Trials.
At baseline, the median age of participants was 74.0 years (interquartile range, 71.0-78.0 years). The patients had a median of 16.0 years (interquartile range, 12.0-18.0 years) of education. There was no difference in patient characteristics between the dexmedetomidine and placebo groups (Table 1).
Table 1. Characteristics of the Patients at Baseline.
| Variable | Overall (N = 390) |
Intervention (n = 189) |
Placebo (n = 201) |
P Value |
|---|---|---|---|---|
| Age, median (IQR), y | 74.0 (71.0-78.0) | 74.0 (71.0-78.0) | 74.0 (71.0-78.0) | .76 |
| Educational level, median (IQR), y | 16.0 (12.0-18.0) | 16.0 (12.0-18.0) | 15.0 (12.0-17.0) | .15 |
| BMI, median (IQR) | 28.0 (24.1-31.9) | 28.1 (24.3-31.7) | 27.7 (24.0-32.3) | .89 |
| Anesthesia time, median (IQR), min | 253.0 (185.0-345.0) | 252.0 (173.0-348.0) | 254.0 (191.0-339.0) | .63 |
| Surgical time, median (IQR), min | 177.0 (121.0-238.0) | 174.0 (119.0-233.0) | 180.0 (123.0-249.0) | .36 |
| Female, No. (%) | 200 (51.3) | 97 (51.3) | 103 (51.2) | .99 |
| Mild cognitive impairment, No. (%) | 246 (63.1) | 124 (65.6) | 122 (60.7) | .18 |
| ASA status, No. (%) | ||||
| I-II | 136 (34.9) | 73 (38.6) | 63 (31.3) | .30 |
| III | 241 (61.8) | 108 (57.1) | 133 (66.2) | |
| IV | 13 (3.3) | 8 (4.2) | 5 (2.5) | |
| Coronary artery disease, No. (%) | 32 (8.2) | 14 (7.4) | 18 (9.0) | .58 |
| Hypertension, No. (%) | 240 (61.5) | 124 (65.6) | 116 (57.7) | .11 |
| Congestive heart failure, No. (%) | 1 (0.3) | 0 | 1 (0.5) | >.99 |
| Diabetes, No. (%) | 79 (20.3) | 39 (20.6) | 40 (19.9) | .86 |
| Cancer, No. (%) | 158 (40.5) | 81 (42.9) | 77 (38.3) | .36 |
| Surgical procedure, No. (%) | ||||
| Spine | 147 (37.7) | 70 (37.0) | 77 (38.3) | .13 |
| Thoracic | 11 (2.8) | 5 (2.6) | 6 (3.0) | |
| Orthopedic | 90 (23.1) | 54 (28.6) | 36 (17.9) | |
| Urologic | 49 (12.6) | 20 (10.6) | 29 (14.4) | |
| General | 93 (23.8) | 40 (21.2) | 53 (26.4) | |
| Fentanyl citrate, median (IQR), µg | 250.0 (175.0-375.0) | 250.0 (150.0-350.0) | 250.0 (200.0-400.0) | .27 |
| Propofol, median (IQR), mg | 165.0 (120.0-300.0) | 170.0 (120.0-360.0) | 160.0 (120.0-220.0) | .54 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range.
Primary Outcome of Postoperative Delirium
A total of 390 patients completed in-hospital delirium assessments. Among these patients, 46 (11.8%) developed postoperative delirium, including 23 in the dexmedetomidine group (12.2%) and 23 (11.4%) in the placebo group (risk ratio, 1.06; 95% CI, 0.79-1.41; P = .77) (Table 1). Among patients who developed delirium, there was no difference in severity (mild, moderate, or severe) by treatment group or by difference in subtype (hyperactive vs hypoactive motor features).
Baseline and surgical factors statistically significantly associated with delirium were educational level, baseline MCI, surgical procedure, and surgical time (Table 2). We conducted a sensitivity analysis using study sites as random effects using a generalized linear mixed model, and the results were unchanged (odds ratio, 1.08; 95% CI, 0.59-2.01; P = .80). Patients who did not complete high school, manifested baseline MCI, or had longer surgical times were at increased risk of delirium. Compared with general surgery, orthopedic, spine, and urologic surgical procedures were associated with greater odds of delirium.
Table 2. Multivariable Logistic Regression Model for Postoperative Delirium.
| Variable | Odds Ratio (95% CI) | P Value |
|---|---|---|
| Educational level above high school | 0.31 (0.15-0.62) | .001 |
| Baseline mild cognitive impairment | 2.42 (1.15-5.10) | .02 |
| Surgical procedure | ||
| General | 1 [Reference] | NA |
| Orthopedic | 6.23 (2.02-19.19) | .001 |
| Spine | 3.70 (1.25-10.95) | .02 |
| Thoracic | 5.31 (0.84-33.34) | .08 |
| Urologic | 4.03 (1.16-14.07) | .03 |
| Surgical time >4 h | 2.10 (1.01-4.40) | .048 |
| Presence of PACU delirium | 3.56 (1.35-9.31) | .01 |
Abbreviations: NA, not applicable; PACU, postanesthesia care unit.
Secondary Outcome of Cognition
A total of 330 patients were eligible to complete the cognitive battery at 3 months, and 298 patients were eligible at 6 months. Among them, 228 completed the 3-month postoperative cognitive battery, and 204 completed the 6-month battery. At 3 months, complete z scores were available for 113 patients in the dexmedetomidine group and 115 patients in the placebo group. At 6 months, complete z scores were available for 97 patients in the dexmedetomidine group and 107 patients in the placebo group. Baseline cognitive scores were similar between patients who did and did not remain in the study, and the fraction of patients who dropped out was similar in each group. The final model included age, educational level, baseline MCI, and ASA status as well as perioperative variables, such as anesthesia time, surgical time, and surgical procedure.
z Scores at baseline, 3 months, and 6 months are shown in the eFigure in Supplement 2. The median z score in the dexmedetomidine group was greater at each time point and was statistically significant at 6 months after surgery. The median z scores in the dexmedetomidine and placebo groups had improved at 3 months and 6 months after surgery, suggesting a learning effect. However, in the generalized estimating equations model (adjusted for covariates), the interaction of dexmedetomidine use with time (3 months and 6 months) was not statistically significant. Although we had dropouts at 3 months and 6 months, there was no evidence of any treatment effect of dexmedetomidine on any neurocognitive measure in our extensive analysis of missing data.
Safety
The incidence of adverse events was similar with dexmedetomidine and placebo (Table 3), with the exception that more intraoperative bradycardia was seen with dexmedetomidine, an expected consequence of an α2-adrenergic agonist (Table 4). Infections were more common in patients who received dexmedetomidine, although only 14 total infections were observed.
Table 3. Postoperative Adverse Events.
| Variable | No. (%) | P Valuea | ||
|---|---|---|---|---|
| Overall (N = 304) |
Intervention (n = 147) |
Placebo (n = 157) |
||
| Myocardial infarction | 1 (0.3) | 1 (0.7) | 0 | .48 |
| Unstable angina | 1 (0.3) | 1 (0.7) | 0 | .48 |
| New arrhythmia | 6 (2.0) | 2 (1.4) | 4 (2.5) | .69 |
| Pulmonary edema | 1 (0.3) | 1 (0.7) | 0 | .48 |
| Heart failure | 2 (0.7) | 1 (0.7) | 1 (0.6) | >.99 |
| Respiratory failure | 3 (1.0) | 2 (1.4) | 1 (0.6) | .61 |
| Pneumonia | 4 (1.3) | 3 (2.0) | 1 (0.6) | .36 |
| Stroke | 1 (0.3) | 0 | 1 (0.6) | >.99 |
| Venous thrombosis | 4 (1.3) | 2 (1.4) | 2 (1.3) | >.99 |
| Pulmonary embolus | 2 (0.7) | 1 (0.7) | 1 (0.6) | >.99 |
| Renal failure | 0 | 0 | 0 | >.99 |
| Gastrointestinal bleed | 2 (0.7) | 1 (0.7) | 1 (0.6) | >.99 |
| Infection | 14 (4.6) | 12 (8.2) | 2 (1.3) | .005 |
By Fisher exact test.
Table 4. Clinical Outcomes and Intraoperative Serious Events.
| Variable | Overall (N = 390) |
Intervention (n = 189) |
Placebo (n = 201) |
P Value |
|---|---|---|---|---|
| Delirium, No. (%) | ||||
| Postoperative delirium | 46 (11.8) | 23 (12.2) | 23 (11.4) | .94 |
| PACU delirium | 14 (3.6) | 6 (3.2) | 8 (4.0) | |
| Both | 9 (2.3) | 5 (2.6) | 4 (2.0) | |
| None | 321 (82.3) | 155 (82.0) | 166 (82.6) | |
| Intraoperative bradycardia, No. (%) | ||||
| Requiring treatment | 55 (14.1) | 35 (18.5) | 20 (10.0) | .06 |
| Requiring interruption of the drug | 4 (1.0) | 2 (1.1) | 2 (1.0) | |
| Resolved spontaneously | 87 (22.3) | 45 (23.8) | 42 (20.9) | |
| Did not occur | 244 (62.6) | 107 (56.6) | 137 (68.2) | |
| Intraoperative hypotension, No. (%) | ||||
| Requiring treatment | 150 (38.5) | 81 (42.9) | 69 (34.3) | .36 |
| Requiring interruption of the drug | 8 (2.1) | 3 (1.6) | 5 (2.5) | |
| Resolved spontaneously | 39 (10.0) | 18 (9.5) | 21 (10.4) | |
| Did not occur | 193 (49.5) | 87 (46.0) | 106 (52.7) | |
| Intraoperative hypertension, No. (%) | ||||
| Requiring treatment | 62 (15.9) | 26 (13.8) | 36 (17.9) | .10 |
| Requiring interruption of the drug | 1 (0.3) | 0 | 1 (0.5) | |
| Resolved spontaneously | 47 (12.1) | 17 (9.0) | 30 (14.9) | |
| Did not occur | 280 (71.8) | 146 (77.2) | 134 (66.7) | |
| Death, No. (%) | 4 (1.0) | 1 (0.5) | 3 (1.5) | .35 |
| Serious adverse event, No. (%) | 22 (5.6) | 13 (6.9) | 9 (4.5) | .30 |
| Length of stay, median (IQR), d | 4.0 (3.0-6.0) | 4.0 (3.0-6.0) | 4.0 (3.0-6.0) | .27 |
Abbreviations: IQR, interquartile range; PACU, postanesthesia care unit.
Discussion
In this article, we describe intraoperative use of dexmedetomidine in older adults to prevent postoperative delirium. We found that dexmedetomidine did not reduce the incidence of delirium over saline placebo. In total, 12.2% (23 of 189) of patients who received dexmedetomidine and 11.4% (23 of 201) of patients who received placebo experienced delirium, which is not a clinically or statistically significant difference. Dexmedetomidine also had no effect on cognitive change at 3 months and 6 months after surgery. Statistically significant associations of postoperative delirium were educational level, surgical procedure, and surgical time.
Our results contrast with previous reports in which sedation with dexmedetomidine in the cardiac ICU was associated with a lower incidence of delirium compared with propofol and benzodiazepines. Djaiani and colleagues randomized critical care patients recovering from cardiac surgery to sedation with dexmedetomidine (bolus of 0.4 µg/kg/h, followed by infusion of 0.2-0.7 µg/kg/h) or propofol and found that the incidence, duration, and severity of delirium were all substantially reduced. However, our method was different: we restricted dexmedetomidine administration to the intraoperative period and 2 subsequent hours rather than infusing the drug over a prolonged postoperative period. Therefore, it is possible that intraoperative dexmedetomidine has no effect in the setting of the neurochemical milieu of a general anesthetic. This conclusion would be consistent with a recent study of an elderly noncardiac surgery population in China that randomized patients to receive dexmedetomidine through postoperative day 1 and observed a reduction in delirium. In addition, another previous study demonstrated that use of intraoperative epidural and spinal analgesic agents as adjuncts to general anesthesia have only temporary effects on the hypothalamic-pituitary-adrenal axis response to surgical stress.
Delirium was associated with lower educational level and baseline MCI, which is consistent with previous findings. Intraoperative factors, including hypotension or desaturation, were not statistically significantly related to delirium. Postoperative cognitive dysfunction was not associated with dexmedetomidine administration, educational level, or baseline MCI. In fact, cognition improved at 3 months and 6 months in both groups, which is consistent with a meta-analysis showing that cognition is stable or improves within the first year after cardiac surgery. This result is also consistent with a study by Avidan et al, who found no correlation between illness, major surgery, and cognitive trajectory. A large proportion of our population had presurgical cognitive impairment (63.1% [246 of 390]), which might have been mitigated after surgery by a practice effect or relief of the presurgical condition or related to a preexisting downward trajectory. In any case, the inclusion of patients with MCI is a strength of the present study because they are at highest risk for further decline.
Our overall incidence of postoperative delirium was 46 patients (11.8%), including 23 in the dexmedetomidine group (12.2%) and 23 in the placebo group (11.4%). While this percentage is lower than that in older reports, it is in line with more recent studies in noncardiac patients. The postanesthesia care unit delirium rate was low in our study, likely due to more limited data collection (CAM-ICU only). We conducted a single delirium assessment each day during work hours, which means that delirium in the evening may have been undetected. However, we interviewed families and nurses to learn about the course of the patient in the interim. The dropout rate for the primary outcome was low. Almost all patients who were randomized completed in-hospital delirium assessments. Completion of recruitment exceeded the original time line (7 years vs 4 years); slow recruitment was related to identifying patients who were older than 68 years with at least a planned 2-day hospital stay, agreed to interval home visits, and accepted a study drug.
Limitations and Strengths
Limitations of our study design include not collecting data regarding the number of delirium episodes, the duration of delirium, or the potential consequences of delirium, such as discharge diagnosis to home or short-term rehabilitation. However, we chose to exclude sicker patients who were ASA status IV or had a planned ICU stay, which provided a homogeneous group for which we can make conclusive statements. The cognitive battery was performed at short intervals (months), maximizing the practice effect. A strength of our method is that we used z scores as a continuous variable, which avoids floor or ceiling effects and allows us to see differences in practice effects (should they occur) as well as deficits. Although practice effects across studies are difficult to estimate (because of heterogeneous batteries and testing intervals), we can report that there was no difference in change between the dexmedetomidine and placebo groups. Use of a continuous composite score also avoids choosing a cut point to define dysfunction: cut points make it more difficult to detect problems in low scorers at baseline (who cannot decline much further) or in high scorers (who have significant reserve). Generally, we saw improvement in cognitive scores over time. Indeed, it is possible that patients who did not improve actually had some unrelated impairment.
Conclusions
Intraoperative infusion of dexmedetomidine does not decrease postoperative delirium or affect postoperative cognition in elderly patients undergoing major elective noncardiac surgery. Specifically, we did not observe the reduction in delirium demonstrated previously in numerous surgical ICU studies. This result may be due to the short-acting nature of the drug and loss of salutary effects after discontinuation of the infusion, which underscores the importance of timing when administering the drug to prevent delirium. Future studies should focus on preoperative risk stratification and efforts to focus on the higher-risk cohort with low educational level and baseline MCI to prevent or decrease postoperative delirium.
Trial Protocol
eFigure. Plot of Unadjusted z Scores Over Time
References
- 1.Müller A, Lachmann G, Wolf A, Mörgeli R, Weiss B, Spies C. Peri- and postoperative cognitive and consecutive functional problems of elderly patients. Curr Opin Crit Care. 2016;22(4):406-411. [DOI] [PubMed] [Google Scholar]
- 2.Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165. [DOI] [PubMed] [Google Scholar]
- 3.Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin. 2015;33(3):505-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J Am Coll Surg. 2015;220(2):136-148.e1. [DOI] [PubMed] [Google Scholar]
- 5.Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179-1185. [DOI] [PubMed] [Google Scholar]
- 6.Moller JT, Cluitmans P, Rasmussen LS, et al. ; ISPOCD Investigators. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study: International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857-861. [DOI] [PubMed] [Google Scholar]
- 7.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100(1):4-10. [DOI] [PubMed] [Google Scholar]
- 8.Brown CH IV, Laflam A, Max L, et al. . The impact of delirium after cardiac surgical procedures on postoperative resource use. Ann Thorac Surg. 2016;101(5):1663-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mo Y, Zimmermann AE. Role of dexmedetomidine for the prevention and treatment of delirium in intensive care unit patients. Ann Pharmacother. 2013;47(6):869-876. [DOI] [PubMed] [Google Scholar]
- 10.Djaiani G, Silverton N, Fedorko L, et al. . Dexmedetomidine vs propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124(2):362-368. [DOI] [PubMed] [Google Scholar]
- 11.Hudetz JA, Patterson KM, Byrne AJ, Pagel PS, Warltier DC. Postoperative delirium is associated with postoperative cognitive dysfunction at one week after cardiac surgery with cardiopulmonary bypass. Psychol Rep. 2009;105(3, pt 1):921-932. [DOI] [PubMed] [Google Scholar]
- 12.Bryson GL, Wyand A, Wozny D, Rees L, Taljaard M, Nathan H. A prospective cohort study evaluating associations among delirium, postoperative cognitive dysfunction, and apolipoprotein E genotype following open aortic repair. Can J Anaesth. 2011;58(3):246-255. [DOI] [PubMed] [Google Scholar]
- 13.Saczynski JS, Marcantonio ER, Quach L, et al. . Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 15.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. [DOI] [PubMed] [Google Scholar]
- 16.Ely EW, Inouye SK, Bernard GR, et al. . Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA. 2001;286(21):2703-2710. [DOI] [PubMed] [Google Scholar]
- 17.Albert MS, Levkoff SE, Reilly C, et al. . The delirium symptom interview: an interview for the detection of delirium symptoms in hospitalized patients. J Geriatr Psychiatry Neurol. 1992;5(1):14-21. [DOI] [PubMed] [Google Scholar]
- 18.Weintraub S, Salmon D, Mercaldo N, et al. . The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riker RR, Shehabi Y, Bokesch PM, et al. ; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group . Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301(5):489-499. [DOI] [PubMed] [Google Scholar]
- 20.Shehabi Y, Grant P, Wolfenden H, et al. . Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (Dexmedetomidine Compared to Morphine–DEXCOM Study). Anesthesiology. 2009;111(5):1075-1084. [DOI] [PubMed] [Google Scholar]
- 21.Hipp DM, Ely EW. Pharmacological and nonpharmacological management of delirium in critically ill patients. Neurotherapeutics. 2012;9(1):158-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(suppl 1):i41-i46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su X, Meng ZT, Wu XH, et al. . Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893-1902. [DOI] [PubMed] [Google Scholar]
- 24.Mann C, Pouzeratte Y, Boccara G, et al. . Comparison of intravenous or epidural patient-controlled analgesia in the elderly after major abdominal surgery. Anesthesiology. 2000;92(2):433-441. [DOI] [PubMed] [Google Scholar]
- 25.Monk TG, Weldon BC, Garvan CW, et al. . Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18-30. [DOI] [PubMed] [Google Scholar]
- 26.Selnes OA, Gottesman RF, Grega MA, Baumgartner WA, Zeger SL, McKhann GM. Cognitive and neurologic outcomes after coronary-artery bypass surgery. N Engl J Med. 2012;366(3):250-257. [DOI] [PubMed] [Google Scholar]
- 27.Cormack F, Shipolini A, Awad WI, et al. . A meta-analysis of cognitive outcome following coronary artery bypass graft surgery. Neurosci Biobehav Rev. 2012;36(9):2118-2129. [DOI] [PubMed] [Google Scholar]
- 28.Avidan MS, Searleman AC, Storandt M, et al. . Long-term cognitive decline in older subjects was not attributable to noncardiac surgery or major illness. Anesthesiology. 2009;111(5):964-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Plot of Unadjusted z Scores Over Time