Key Points
Question
Does remnant liver ischemia after resection of colorectal liver metastases have prognostic influence?
Findings
In this study of 202 patients based on data collected at The University of Texas MD Anderson Cancer Center, cancer-specific survival rates after hepatic resection were worse in patients with remnant liver ischemia grade 2 or higher vs grade 1 or lower. Remnant liver ischemia grade 2 or higher, which was significantly associated with nonanatomic resection, was an independent predictor of worse cancer-specific survival.
Meaning
High-quality anatomic surgery to minimize remnant liver ischemia after resection of colorectal liver metastases is essential.
Abstract
Importance
Ischemia-reperfusion injury during hepatic resection has been shown to accelerate progression of liver cancer. However, the prognostic relevance of remnant liver ischemia (RLI) after resection of colorectal liver metastases (CLMs) is unknown to date.
Objectives
To assess the prognostic influence of RLI after resection of CLMs and to identify correlates of greater extent of RLI.
Design, Setting, and Participants
This study was a retrospective analysis at The University of Texas MD Anderson Cancer Center based on prospectively collected data. The study identified 202 patients who underwent curative resection of CLMs between January 1, 2008, and December 31, 2014, and had enhanced computed tomographic images obtained within 30 days after surgery.
Main Outcomes and Measures
Remnant liver ischemia was defined as reduced or absent contrast enhancement during the portal phase. Postoperative RLI was classified as grade 0 (none), 1 (marginal), 2 (partial), 3 (segmental), or 4 (necrotic) as previously defined. Experienced members of the surgical team retrospectively performed imaging assessments. Team members were masked to the postoperative outcomes. Survival after resection was stratified by RLI grade. Predictors of RLI grade 2 or higher and survival were identified.
Results
Among 202 patients (median [range] age, 56 [27-87] years; 84 female), the RLI grades were as follows: grade 0 (105 patients), grade 1 (47 patients), grade 2 (45 patients), grade 3 (5 patients), and grade 4 (0 patients). Recurrence-free survival (RFS) and cancer-specific survival (CSS) rates after hepatic resection were worse in patients with RLI grade 2 or higher vs grade 1 or lower (RFS at 3 years, 6.4% [3 of 50] vs 39.2% [60 of 152]; P < .001 and CSS at 5 years, 20.7% [10 of 50] vs 63.7% [97 of 152]; P < .001). A largest metastasis at least 3 cm (OR, 2.74; 95% CI, 1.35-5.70; P = .005), multiple CLMs (OR, 2.51; 95% CI, 1.25-5.24; P = .009), and nonanatomic resection (odds ratio [OR], 3.29; 95% CI, 1.52-7.63; P = .002) were associated with RLI grade 2 or higher. A largest metastasis at least 3 cm (hazard ratio [HR], 1.70; 95% CI, 1.01-2.88; P = .045), mutant RAS (HR, 2.15; 95% CI, 1.27-3.64; P = .005), and RLI grade 2 or higher (HR, 2.90; 95% CI, 1.69-4.84; P < .001) were associated with worse CSS.
Conclusions and Relevance
In this study, remnant liver ischemia grade 2 or higher was associated with worse CSS after resection of CLMs. High-quality anatomic surgery to minimize RLI after resection is essential.
This study assesses the prognostic influence of remnant liver ischemia after resection of colorectal liver metastases and identifies correlates of greater extent of remnant liver ischemia.
Introduction
For patients with colorectal liver metastases (CLMs), the introduction of effective systemic chemotherapy and improvements in technique that have permitted more widespread use of hepatic resection have led to significant improvements in long-term survival. However, more than 30% of patients who undergo hepatic resection of CLMs experience recurrence within 1 year. Accurate prediction of prognosis in individual patients with CLMs undergoing hepatic resection remains an area of active research. Previously, prognosis in patients with CLMs undergoing hepatic resection has been correlated with clinical, radiologic, pathologic, and, more recently, molecular criteria (RAS indicating KRAS [OMIM 190070] and NRAS [OMIM 164790] mutation status).
Ischemia-reperfusion injury during liver surgery, which leads to hepatocyte dysfunction and elevations in proinflammatory cytokines and matrix metalloproteinases, has been shown to accelerate progression of colorectal carcinoma micrometastases in animal models. However, to our knowledge, there have been no reports on the prognostic influence of ischemia in the future liver remnant (remnant liver ischemia [RLI]) after hepatic resection in patients with CLMs undergoing curative resection. Remnant liver ischemia can be caused by either imprecise liver resection that leaves behind nonperfused liver tissue or excessive liver resection that results in unintentional damage to a segment’s inflow or outflow vessels.
Recently, it has been shown that parenchymal-sparing hepatic resection in patients with CLMs increases the likelihood of salvageability in case of recurrence. This finding has led to increased use of multiple nonanatomic resections rather than extended resection to achieve tumor clearance. However, anatomic resection (complete resection of the territory supplied by the respective glissonean pedicle) may be less likely to be associated with RLI. The deleterious influence of RLI of segment IV has been investigated in the context of conventional vs partial associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Whether devitalized liver tissue that remains behind after imprecise liver resection has an influence on prognosis has not been demonstrated to date.
The objective of the present study was 2-fold. We aimed to assess the prognostic influence of RLI in patients with CLMs undergoing curative hepatic resection and to identify predictors of greater extent of RLI.
Methods
Study Population
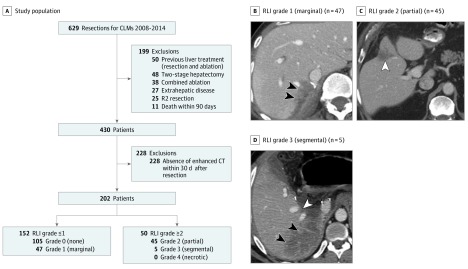
Institutional review board approval was obtained for this retrospective study based on prospectively collected data at The University of Texas MD Anderson Cancer, which waived the requirement for patient informed consent because of the nature of retrospective medical record review involving no therapeutic intervention. A hepatobiliary database at the Department of Surgical Oncology was reviewed, and 629 consecutive patients were identified who underwent curative resection of CLMs between January 1, 2008, and December 31, 2014. The following exclusion criteria were applied: (1) previous liver treatment, (2) two-stage hepatectomy, (3) concomitant ablation, (4) presence of extrahepatic disease, (5) R2 resection (macroscopic residual cancer), and (6) death within 90 days (Figure 1A). Among the remaining patients, we selected patients with available abdominal enhanced computed tomographic images obtained within 30 days after resection (n = 202) for inclusion.
Figure 1. Study Population and Representative Images of Different Grades of Remnant Liver Ischemia (RLI).
A, Study population. CLMs indicates colorectal liver metastases; CT, computed tomography. B, RLI grade 1 (limited to the margin). Black arrowheads indicate marginal hypoperfusion. C, RLI grade 2 (involving part of a hepatic segment). White arrowhead indicates partial hypoperfusion. D, RLI grade 3 (involving an entire segment VII). White arrowhead indicates right hepatic vein, and black arrowheads indicate segmental hypoperfusion without gas bubble. Grade 4 (necrotic tissue) is characterized by total absence of contrast and presence of intrahepatic gas bubbles on CT, but it was not observed in the present cohort.
The following data were recorded from the electronic medical record: sex, age, American Society of Anesthesiologists physical status classification, comorbidities, use of anticoagulant agents, primary tumor characteristics (location, depth of invasion, and associated lymph node metastases), prehepatic resection chemotherapy characteristics (number of cycles and regimens used), prehepatic resection laboratory values (serum carcinoembryonic antigen, aspartate aminotransferase, platelet count, potassium, lactate dehydrogenase, and neutrophil to lymphocyte ratio [NLR]), perioperative outcomes (presence or absence of Pringle maneuver, estimated blood loss, red blood cell transfusion, operative time, extent of surgical procedure [major resection was defined as hepatic resection that included ≥3 liver segments], and anatomic vs nonanatomic resection), CLM characteristics (synchronous vs metachronous, largest metastasis, tumor number, margin status [R1 was defined as a tumor-free margin <1 mm], and differentiation), length of stay, posthepatic resection chemotherapy, and NLR at 3 months after hepatic resection. The NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count; no patient had clinical signs of sepsis when blood samples were obtained for the NLR. An NLR exceeding 5 was defined as high in accord with the practice in previous studies.
Perioperative Management
After preoperative chemotherapy, restaging was performed. Colorectal liver metastases were deemed resectable when a negative-margin resection could be performed with preservation of 20% to 30% of the standardized total liver volume, together with preservation of vascular inflow and outflow and biliary drainage. Second-line chemotherapy was considered for patients with progression of disease or suboptimal tumor response after first-line chemotherapy. Pathologic response in CLMs to preoperative chemotherapy was defined as previously described: major response was 0% to 49% residual tumor cells, and minor response was at least 50% residual tumor cells. In patients with an anticipated insufficient future liver remnant, preoperative portal vein embolization was performed. Anatomic resection was defined as previously described by Shindoh et al: “complete removal of one Couinaud’s segment or a combination of contiguous territories of the ‘3rd-order’ sub-segmental portal venous branches smaller than one Couinaud’s segment.”(p595) Whether to perform anatomic or nonanatomic resection was decided by the surgeon on the basis of surgical findings or patient physical status. Postoperative chemotherapy was administered to complete a total of 12 cycles, including cycles administered before surgery. Postoperative complications were classified using the Clavien-Dindo criteria, with major complications defined as those classified as grade IIIa or higher.
Definition of RLI
All patients underwent multidetector row enhanced computed tomography with 4, 16, or 64 sections (Light-Speed; GE Healthcare) using a triphasic liver protocol or single-phase technique with section thickness of 2.5 to 5 mm. Previously described radiologic criteria for the assessment of liver hypoperfusion and necrosis on computed tomography were adopted. In brief, RLI was defined as reduced or absent contrast enhancement during the portal phase. The severity of RLI was graded as 0 (none), 1 (limited to the margin), 2 (involving part of a hepatic segment), 3 (involving an entire hepatic segment), or 4 (necrotic tissue). Patients were also divided into 2 groups according to the severity of RLI as grade 1 or lower vs grade 2 or higher. Members of the surgical team included one of us (hepatobiliary research fellow S.Y.) with experience in hepatobiliary surgery and another of us (attending diagnostic radiologist A.M.V.). The imaging assessment was performed independently. The diagnostic radiologist was masked to the (1) objective of the study, (2) postoperative outcomes, and (3) imaging assessment by Dr Yamashita. Low interobserver variability (<5%) in the sample sets was found.
RAS Mutation Profiling
RAS mutation profiling was performed as previously described. In brief, single mutations in KRAS codons 12, 13, 61, and 146 and NRAS codons 12, 13, and 61 were analyzed together by polymerase chain reaction and reported as RAS mutations.
Statistical Analysis
Continuous variables were compared using the Wilcoxon rank sum test, and categorical variables were compared using the χ2 test. For evaluation of risk factors for RLI grade 2 or higher and a high NLR (>5) at 3 months after hepatic resection, univariable and multivariable analyses were performed by logistic regression analysis. Recurrence-free survival (RFS) was measured from the date of hepatic resection to the date of radiographic detection of recurrence or last follow-up. Cancer-specific survival (CSS) was measured from the date of hepatic resection to the date of death from colorectal cancer or last follow-up. Survival curves were generated using the Kaplan-Meier method, and differences between curves were evaluated with the log-rank test. Univariable and multivariable analyses to identify predictors of survival were performed by Cox proportional hazards regression models. Variables with P < .10 in univariable analysis were entered into each multivariable analysis. Two-sided P < .05 was considered statistically significant in all analyses. Statistical analyses were performed with a software package (IBM SPSS, version 23.0; SPSS Inc).
Results
Patient Characteristics According to RLI
Of the 202 patients, 97 (48.0%) exhibited signs of RLI, which was grade 1 in 47 patients (23.3%), grade 2 in 45 patients (22.3%), and grade 3 in 5 patients (2.5%). Representative images of grade 1 to 3 RLI are shown in Figure 1B-D. Overall, 152 patients (75.2%) had RLI grade 1 or lower, and 50 (24.8%) had RLI grade 2 or higher (Figure 1A). Clinicopathologic and operative data for patients with RLI grade 1 or lower and RLI grade 2 or higher are summarized in Table 1. Compared with patients having RLI grade 1 or lower, patients having RLI grade 2 or higher were more likely to have nonanatomic resection, multiple CLMs, and major complications and had longer median length of stay. Almost all patients received preoperative (168 patients [83.2%]) and postoperative (184 [91.1%]) chemotherapy. Postoperative chemotherapy was used less often in patients with RLI grade 2 or higher than in those with RLI grade 1 or lower. Patients with RLI grade 2 or higher were more likely to have a high NLR (>5) at 3 months after hepatic resection. In multivariable analysis, the following 2 factors were independent predictors of a high NLR (>5) at 3 months after hepatic resection: major complication (Clavien-Dindo grade ≥IIIa) and RLI grade 2 or higher (eTable 1 in the Supplement). There were no significant differences between patients with RLI grade 1 or lower and RLI grade 2 or higher in terms of the other clinicopathologic and operative characteristics examined (Table 1).
Table 1. Patient Characteristics Overall and by Extent of Remnant Liver Ischemia (RLI).
| Characteristic | Total | RLI Grade ≤1 | RLI Grade ≥2 | P Value for RLI Grade ≤1 vs RLI Grade ≥2a |
|---|---|---|---|---|
| All patients | 202 | 152 | 50 | NA |
| Male:female | 118:84 | 88:64 | 30:20 | .79 |
| Age, median (range), y | 56 (27-87) | 55 (27-79) | 58 (33-87) | .12b |
| ASA I/II/III | 17/89/96 | 13/66/73 | 4/23/23 | .95 |
| Type 2 diabetes, No. (%) | 18 (8.9) | 16 (10.5) | 2 (4.0) | .16 |
| Hypertension, No. (%) | 95 (47.0) | 70 (46.1) | 25 (50.0) | .63 |
| Use of anticoagulant agent, No. (%) | 48 (23.8) | 36 (23.7) | 12 (24.0) | .96 |
| Primary tumor | ||||
| Colon-rectum | 161:41 | 120:32 | 41:9 | .64 |
| T1/T2-T3/T4 | 35:167 | 27:125 | 8:42 | .78 |
| Lymph node metastases, No. (%) | 125 (61.9) | 90 (59.2) | 35 (70.0) | .17 |
| Prehepatic resection chemotherapy, No. (%) | 168 (83.2) | 126 (82.9) | 42 (84.0) | .86 |
| ≤6 Cycles | 59 (35.1) | 42 (33.3) | 17 (40.5) | .40 |
| ≥2 Regimens | 15 (8.9) | 9 (7.1) | 6 (14.3) | .16 |
| Fluorouracil-based chemotherapy regimen | ||||
| Oxaliplatin | 134 (79.8) | 104 (82.5) | 30 (71.4) | .12 |
| Irinotecan hydrochloride | 29 (17.3) | 20 (15.9) | 9 (21.4) | .41 |
| Use of bevacizumab | 121 (72.0) | 94 (74.6) | 27 (64.3) | .20 |
| Use of anti-EGFR agent | 14 (8.3) | 10 (7.9) | 4 (9.5) | .75 |
| Major pathologic response | 84 (50.0) | 67 (53.2) | 17 (40.5) | .15 |
| Prehepatic resection laboratory value, median (range) | ||||
| Carcinoembryonic antigen, ng/mL | 3.8 (0.5-446) | 3.7 (0.5-446) | 4.2 (1.0-350) | .14b |
| Aspartate aminotransferase, U/L | 30 (12-99) | 31 (12-99) | 30 (14-99) | .63b |
| Platelet count, ×103/µL | 202 (93-690) | 204.5 (93-589) | 200.5 (111-690) | .93b |
| Potassium, mEq/L | 4.2 (3.5-5.7) | 4.2 (3.5-5.7) | 4.2 (3.5-5.4) | .91b |
| Lactate dehydrogenase, U/L | 485 (293-3155) | 483 (293-1431) | 492 (334-3155) | .32b |
| NLR before hepatic resection >5, No. (%) | 19 (9.4) | 14 (9.2) | 5 (10.0) | .87 |
| Portal vein embolization, No. (%) | 184 (91.1) | 138 (90.8) | 46 (92.0) | .79 |
| Hepatic resection | ||||
| Pringle maneuver, No. (%) | 163 (80.7) | 125 (82.2) | 38 (76.0) | .33 |
| Estimated blood loss, median (range), mL | 250 (45-3300) | 250 (50-3300) | 250 (45-1850) | .60b |
| Red blood cell transfusion, No. (%) | 17 (8.4) | 12 (7.9) | 5 (10.0) | .64 |
| Operative time, median (range), min | 235 (55-678) | 235 (55-678) | 263 (70-595) | .74b |
| Surgical procedure major-minor | 99:103 | 78:74 | 21:29 | .25 |
| Anatomic resection, No. (%) | 73 (36.1) | 61 (40.1) | 12 (24.0) | .04 |
| Liver metastases | ||||
| Synchronous- metachronous |
148:54 | 110:42 | 38:12 | .62 |
| Maximum tumor size, median (range), mm | 25 (5.0-120) | 24 (5.0-120) | 30 (6.0-105) | .16b |
| Solitary-multiple tumors, No. | 89:113 | 74:78 | 15:35 | .02 |
| Residual cancer R0-R1 | 185:17 | 137:15 | 48:2 | .20 |
| Well/moderately/poorly differentiated | 3/184/15 | 2/138/12 | 1/46/3 | .86 |
| Total morbidity, No. (%) | 125 (61.9) | 90 (59.2) | 35 (70.0) | .17 |
| Major complication with Clavien-Dindo grade ≥IIIa, No. (%) | 51 (25.2) | 33 (21.7) | 18 (36.0) | .04 |
| RAS status, No. (%) | ||||
| Wild type | 129 (63.9) | 101 (66.4) | 28 (56.0) | .18 |
| Mutant | 73 (36.1) | 51 (33.6) | 22 (44.0) | |
| Length of stay, median (range), d | 7 (3-37) | 7 (3-16) | 8 (4-37) | .006b |
| RLI grade, No. (%) | ||||
| 0, None | 105 (52.0) | 105 (69.1) | 0 | <.001 |
| 1, Marginal | 47 (23.3) | 47 (30.9) | 0 | |
| 2, Partial | 45 (22.3) | 0 | 45 (90.0) | |
| 3, Segmental | 5 (2.5) | 0 | 5 (10.0) | |
| 4, Necrotic | 0 | 0 | 0 | |
| Posthepatic resection chemotherapy, No. (%) | 184 (91.1) | 143 (94.1) | 41 (82.0) | .009 |
| Interval between hepatic resection and beginning of adjuvant chemotherapy, median (range), d | 56 (11-337) | 55 (11-337) | 60 (12-236) | .39b |
| NLR at 3 mo after hepatic resection >5 | 31 (15.3) | 16 (10.5) | 15 (30.0) | <.001 |
Abbreviations: ASA, American Society of Anesthesiologists’ physical status classification; EGFR, epidermal growth factor receptor; NA, not applicable; NLR, neutrophil to lymphocyte ratio.
SI conversion factors: To convert aspartate aminotransferase and lactate dehydrogenase levels to microkatals per liter, multiply by 0.0167; carcinoembryonic antigen level to micrograms per liter, multiply by 1.0; platelet count to ×109/µL, multiply by 1.0; and potassium level to millimoles per liter, multiply by 1.0.
By χ2 test unless indicated otherwise.
By Wilcoxon rank sum test.
Risk Factors for RLI Grade 2 or Higher
Table 2 lists the results of univariable analysis of risk factors for RLI grade 2 or higher after hepatic resection. In multivariable analysis, the following 3 factors were independent risk factors for RLI grade 2 or higher: a largest metastasis at least 3 cm, multiple CLMs, and nonanatomic resection (Table 3). There was no association between preoperative laboratory values (aspartate aminotransferase, platelet count, potassium, lactate dehydrogenase, and NLR) and RLI grade 2 or higher.
Table 2. Univariable Analysis of Remnant Liver Ischemia (RLI) Grade 2 or Higher After Hepatic Resection.
| Variable | No. | RLI Grade ≥2 After Hepatic Resection | |
|---|---|---|---|
| No. (%) | Univariable P Value | ||
| All patients | 202 | 50 (24.8) | NA |
| Sex | |||
| Male | 118 | 30 (25.4) | .79 |
| Female | 84 | 20 (23.8) | |
| Age at hepatic resection, y | |||
| <60 | 128 | 28 (21.9) | .21 |
| ≥60 | 74 | 22 (29.7) | |
| ASA | |||
| I/II | 106 | 27 (25.5) | .80 |
| III | 96 | 23 (24.0) | |
| Type 2 diabetes | |||
| Yes | 18 | 2 (11.1) | .16 |
| No | 184 | 48 (26.1) | |
| Hypertension | |||
| Yes | 95 | 25 (26.3) | .63 |
| No | 107 | 25 (23.4) | |
| Use of anticoagulant agent | |||
| Yes | 48 | 12 (25.0) | .96 |
| No | 154 | 38 (24.7) | |
| Prehepatic resection aspartate aminotransferase, U/La | |||
| ≥30 | 115 | 27 (23.5) | .63 |
| <30 | 87 | 23 (26.4) | |
| Prehepatic resection platelet count, ×103/µLa | |||
| ≥202 | 102 | 24 (23.5) | .68 |
| <202 | 100 | 26 (26.0) | |
| Prehepatic resection potassium, mEq/La | |||
| ≥4.2 | 112 | 26 (23.2) | .57 |
| <4.2 | 90 | 24 (26.7) | |
| Prehepatic resection lactate dehydrogenase, U/La | |||
| ≥485 | 101 | 26 (25.7) | .74 |
| <485 | 101 | 24 (23.8) | |
| NLR before hepatic resection | |||
| >5 | 19 | 5 (26.3) | .87 |
| ≤5 | 183 | 45 (24.6) | |
| Timing of diagnosis of primary tumor and CLMs | |||
| Synchronous | 148 | 38 (25.7) | .62 |
| Metachronous | 54 | 12 (22.2) | |
| Prehepatic resection chemotherapy | |||
| Yes | 168 | 42 (25.0) | .86 |
| No | 34 | 8 (23.5) | |
| Prehepatic resection chemotherapy cycles | |||
| ≤6 | 143 | 33 (23.1) | .39 |
| >6 | 59 | 17 (28.8) | |
| Multiple lines of prehepatic resection chemotherapy | |||
| Yes | 15 | 6 (40.0) | .16 |
| No | 187 | 44 (23.5) | |
| Fluorouracil-based chemotherapy regimen | |||
| Oxaliplatin | |||
| Yes | 134 | 30 (22.4) | .27 |
| No | 68 | 20 (29.4) | |
| Irinotecan hydrochloride | |||
| Yes | 29 | 9 (31.0) | .40 |
| No | 173 | 41 (23.7) | |
| Use of bevacizumab | |||
| Yes | 121 | 27 (22.3) | .33 |
| No | 81 | 23 (28.4) | |
| Use of anti-EGFR agent | |||
| Yes | 14 | 4 (28.6) | .73 |
| No | 188 | 46 (24.5) | |
| Prehepatic resection carcinoembryonic antigen, ng/mL | |||
| <5 | 118 | 27 (22.9) | .47 |
| ≥5 | 84 | 23 (27.4) | |
| Portal vein embolization | |||
| Yes | 18 | 4 (22.2) | .79 |
| No | 184 | 46 (25.0) | |
| Pringle maneuver | |||
| Yes | 163 | 38 (23.3) | .33 |
| No | 39 | 12 (30.8) | |
| Largest metastasis, cmb | |||
| ≥3 | 86 | 27 (31.4) | .06 |
| <3 | 116 | 23 (19.8) | |
| No. of CLMsb | |||
| Multiple | 113 | 35 (31.0) | .02 |
| Solitary | 89 | 15 (16.9) | |
| Residual tumor | |||
| R1 | 17 | 2 (11.8) | .20 |
| R0 | 185 | 48 (25.9) | |
| Operative time, min | |||
| >180 | 125 | 29 (23.2) | .52 |
| ≤180 | 77 | 21 (27.3) | |
| Estimated blood loss, mL | |||
| ≥500 | 37 | 8 (21.6) | .63 |
| <500 | 165 | 42 (25.5) | |
| Red blood cell transfusion | |||
| Yes | 17 | 5 (29.4) | .64 |
| No | 185 | 45 (24.3) | |
| Anatomic resectionb | |||
| No | 129 | 38 (29.5) | .04 |
| Yes | 73 | 12 (16.4) | |
| Surgical procedure | |||
| Major hepatic resection | 99 | 21 (21.2) | .25 |
| Minor hepatic resection | 103 | 29 (28.2) | |
| RAS status | |||
| Wild type | 129 | 28 (21.7) | .18 |
| Mutant | 73 | 22 (30.1) | |
Abbreviations: ASA, American Society of Anesthesiologists physical status classification; CLMs, colorectal liver metastases; EGFR, epidermal growth factor receptor; NA, not applicable; NLR, neutrophil to lymphocyte ratio.
SI conversion factors: To convert aspartate aminotransferase and lactate dehydrogenase levels to microkatals per liter, multiply by 0.0167; carcinoembryonic antigen level to micrograms per liter, multiply by 1.0; platelet count to ×109/µL, multiply by 1.0; and potassium level to millimoles per liter, multiply by 1.0.
Thresholds of these variables were based on the median values.
Variables entered into the multinominal logistic regression analyses.
Table 3. Multivariable Analysis of Remnant Liver Ischemia (RLI) Grade 2 or Higher After Hepatic Resection.
| Variable | Odds Ratio (95% CI) | Multivariable P Value | ||
|---|---|---|---|---|
| Largest metastasis, cma | 2.74 (1.35-5.70) | .005 | ||
| No. of CLMsa | 2.51 (1.25-5.24) | .009 | ||
| Anatomic resectiona | 3.29 (1.52-7.63) | .002 | ||
Abbreviation: CLMs, colorectal liver metastases.
Variables entered into the multinominal logistic regression analyses.
RLI as an Independent Predictor of CSS
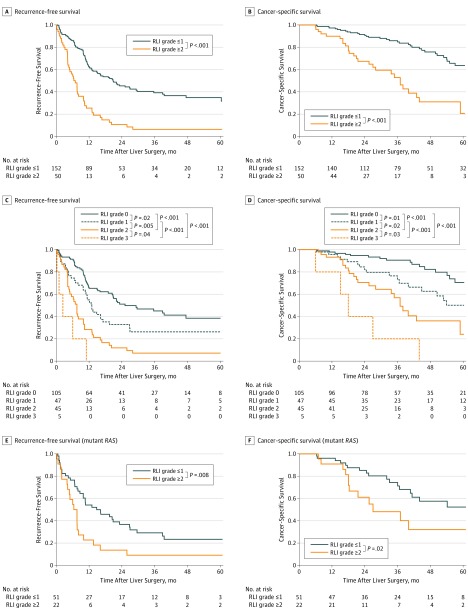
At a median follow-up of 37 months (range, 6.1-96 months), RFS and CSS after hepatic resection were significantly worse in patients with RLI grade 2 or higher (Figure 2). The 3-year RFS rate in patients with RLI grade 0 was 45.0% (47 of 105), which was superior to the rates in patients with RLI grade 1 (26.2% [12 of 47], P = .02), RLI grade 2 (7.1% [3 of 45], P < .001), and RLI grade 3 (0% [0 of 5], P < .001). The 5-year CSS rate in patients with RLI grade 0 was 70.6% (74 of 105), which was superior to the rates in patients with RLI grade 1 (50.1% [24 of 47], P = .02), RLI grade 2 (24.1% [11 of 45], P < .001), and RLI grade 3 (0% [0 of 5], P < .001).
Figure 2. Recurrence-Free Survival and Cancer-Specific Survival in Patients With Colorectal Liver Metastases Who Underwent Curative Resection.
Results are shown by severity of remnant liver ischemia (RLI) (grade ≤1 vs grade ≥2) (A and B), each grade of RLI (C and D), and severity of RLI in patients with mutant RAS (E and F).
In multivariable analysis of factors associated with RFS after hepatic resection, the following 3 factors were independent predictors of worse outcome: multiple CLMs, mutant RAS, and RLI grade 2 or higher (eTable 2 in the Supplement). Table 4 lists univariable analysis of CSS. In multivariable analysis of factors associated with CSS after hepatic resection, the following 3 factors were independent predictors of worse outcome: largest metastasis at least 3 cm, mutant RAS, and RLI grade 2 or higher (Table 5).
Table 4. Univariable Analysis of Cancer-Specific Survival.
| Variable | No. | Cancer-Specific Survival, No. (%)a | Univariable P Valueb | |
|---|---|---|---|---|
| 3 y | 5 y | |||
| All patients | 202 | 155 (76.9) | 109 (53.9) | NA |
| Sex | ||||
| Male | 118 | 87 (74.0) | 63 (53.5) | .38 |
| Female | 84 | 68 (80.8) | 46 (54.6) | |
| Age at hepatic resection, y | ||||
| ≥60 | 74 | 56 (75.3) | 34 (46.6) | .15 |
| <60 | 128 | 99 (77.6) | 76 (59.7) | |
| Primary Tumor | ||||
| Location | ||||
| Rectum | 41 | 32 (78.4) | 20 (49.4) | .74 |
| Colon | 161 | 123 (76.6) | 89 (55.1) | |
| T category | ||||
| T3/T4 | 167 | 127 (75.8) | 85 (50.9) | .31 |
| T1/T2 | 35 | 29 (82.4) | 24 (67.4) | |
| Lymph node metastasesc | ||||
| Yes | 125 | 89 (71.1) | 62 (49.9) | .048 |
| No | 77 | 67 (87.4) | 47 (61.4) | |
| Prehepatic resection chemotherapy | ||||
| Yes | 168 | 128 (76.0) | 88 (52.5) | .49 |
| No | 34 | 28 (82.8) | 22 (65.1) | |
| Hepatic Resection | ||||
| Operative time, min | ||||
| >180 | 125 | 100 (80.3) | 69 (55.4) | .34 |
| ≤180 | 77 | 55 (71.0) | 40 (52.2) | |
| Estimated blood loss, mL | ||||
| ≥500 | 37 | 30 (82.4) | 19 (51.6) | .96 |
| <500 | 165 | 124 (75.4) | 91 (55.3) | |
| Red blood cell transfusion | ||||
| Yes | 17 | 14 (82.4) | 9 (51.8) | .76 |
| No | 185 | 141 (76.1) | 101 (54.4) | |
| Surgical procedure | ||||
| Major hepatic resection | 99 | 76 (76.6) | 50 (51.0) | .27 |
| Minor hepatic resection | 103 | 79 (76.9) | 59 (57.3) | |
| Carcinoembryonic antigen at hepatic resection, ng/mL | ||||
| ≥5 | 84 | 62 (74.4) | 48 (57.2) | .93 |
| <5 | 118 | 93 (78.7) | 61 (52.1) | |
| Timing of diagnosis of primary tumor and CLMsc | ||||
| Synchronous | 148 | 113 (76.1) | 78 (52.5) | .89 |
| Metachronous | 54 | 43 (79.1) | 31 (58.2) | |
| No. of CLMsc | ||||
| Multiple | 113 | 79 (69.9) | 52 (46.1) | .005 |
| Solitary | 89 | 77 (86.7) | 58 (65.2) | |
| Largest metastasis, cmc | ||||
| ≥3 | 86 | 58 (67.7) | 43 (50.1) | .09 |
| <3 | 116 | 97 (84.0) | 66 (57.1) | |
| Residual tumor | ||||
| R1 | 17 | 12 (72.6) | 5 (30.3) | .19 |
| R0 | 185 | 143 (77.3) | 103 (55.8) | |
| RAS statusc | ||||
| Mutant | 73 | 47 (64.1) | 31 (42.8) | .003 |
| Wild type | 129 | 109 (84.2) | 79 (60.9) | |
| Major morbidity with Clavien-Dindo grade ≥IIIac | ||||
| Yes | 51 | 32 (63.7) | 15 (29.2) | .002 |
| No | 151 | 122 (81.1) | 92 (60.8) | |
| RLI after hepatic resectionc | ||||
| Grade ≥2 | 50 | 26 (52.7) | 10 (20.7) | <.001 |
| Grade ≤1 | 152 | 129 (84.8) | 97 (63.7) | |
| Posthepatic resection chemotherapy | ||||
| Yes | 184 | 140 (76.1) | 101 (54.7) | .85 |
| No | 18 | 16 (87.2) | 9 (47.7) | |
Abbreviations: CLMs, colorectal liver metastases; NA, not applicable; RLI, remnant liver ischemia.
SI conversion factor: To convert carcinoembryonic antigen level to micrograms per liter, multiply by 1.0.
By Kaplan-Meier method.
By log-rank test.
Variables entered into the Cox proportional hazards regression model.
Table 5. Multivariable Analysis of Cancer-Specific Survival.
| Variable | Hazard Ratio (95% CI) | Multivariable P Valuea |
|---|---|---|
| Primary Tumor | ||
| Lymph node metastasesb | 1.33 (0.75-2.45) | .34 |
| Hepatic Resection | ||
| No. of CLMsb | 1.64 (0.95-2.97) | .08 |
| Largest metastasis, cmb | 1.70 (1.01-2.88) | .045 |
| RAS statusb | 2.15 (1.27-3.64) | .005 |
| Major morbidity with Clavien-Dindo grade ≥IIIab | 1.70 (0.97-2.91) | .06 |
| RLI after hepatic resectionb | 2.90 (1.69-4.94) | <.001 |
Abbreviations: CLMs, colorectal liver metastases; NA, not applicable; RLI, remnant liver ischemia.
By Cox proportional hazards regression model.
Variables entered into the Cox proportional hazards regression model.
Among 168 patients treated with chemotherapy before hepatic resection, independent predictors of worse RFS were minor pathologic response, multiple CLMs, mutant RAS, and RLI grade 2 or higher. Independent predictors of worse CSS were minor pathologic response, a largest metastasis at least 3 cm, mutant RAS, and RLI grade 2 or higher (eTable 3 in the Supplement).
In the subgroup of 73 patients with mutant RAS, RFS and CSS after hepatic resection were significantly worse in patients with RLI grade 2 or higher (Figure 2). Subgroup analysis of patients with mutant RAS by RLI grade was not performed because of the small number of patients with mutant RAS and RLI grade 3 (n = 2).
Discussion
We have identified the severity of RLI as an independent predictor of RFS and CSS in patients undergoing hepatic resection of CLMs. Compared with patients having RLI grade 1 or lower, patients having RLI grade 2 or higher had worse RFS and CSS. Our results also suggest a dose-response relationship in which increases in RLI grade correlate with incremental decreases in survival. To our knowledge, this article is the first report to demonstrate the influence of the severity of postoperative RLI on CSS after resection of CLMs in the contemporary era of effective perioperative chemotherapy and tumor genotyping. Remnant liver ischemia may be the initial prognosticator under the control of the surgeon at the time of liver resection.
In the present study, RLI grade 2 or higher was independently correlated with larger size of metastasis, multiple CLMs, and performance of nonanatomic resection. Although recent investigations have shown parenchymal-sparing hepatic resection techniques (eg, multiple nonanatomic resections) to be associated with higher salvageability rates in case of recurrence in patients with CLMs, our data show that the use of such techniques can negatively influence patient outcome by unintentionally causing devascularization of liver tissue.
As a possible explanation for the association between RLI grade 2 or higher and poor prognosis, an experimental in vivo model of ALPPS may be applicable. After the first step of conventional ALPPS leading to an ischemic segment IV, mice had a significantly higher Ki-67 index on immunohistological examination, which has been shown to be a reliable and reproducible marker of tumor proliferation in colorectal cancer. Furthermore, a possible explanation is the subsequent occurrence of ischemia-reperfusion injury as shown in experimental models. Reports in the literature indicate that in hepatic ischemia-reperfusion injury hypoxia-induced matrix metallopeptidase 9 (associated with neutrophil recruitment), which has an important role in extracellular matrix remodeling and angiogenesis, facilitates cancer micrometastases. In addition, inhibition of matrix metallopeptidase 9 reduced progression of hepatic micrometastasis in colorectal tumors in an animal model of liver ischemia-reperfusion injury. Our results suggest an association between high postoperative NLR and severe RLI. This association might be attributable to increased matrix metallopeptidase 9 levels given that high levels of this protein have been shown to promote expansion of the neutrophil compartment.
We also found that patients with RLI grade 2 or higher had higher rates of major complications and lower rates of posthepatic resection chemotherapy, which may also contribute to the negative influence of RLI on prognosis. While we observed no influence of posthepatic resection chemotherapy on survival in the multivariable analysis, an absence of such chemotherapy may contribute to the difference in prognosis between patients with RLI grade 1 or lower and patients with RLI grade 2 or higher. Further evaluation may be needed regarding a negative prognostic influence of the delay or nonuse of adjuvant chemotherapy owing to postoperative complications.
Similar to previous reports, we found that not only traditional tumor factors (multiple CLMs and larger metastasis) but also RAS mutations were a strong and independent predictor of worse survival. Moreover, despite the known strong negative influence of RAS mutations on survival, even among patients with RAS mutations, RLI grade (≤1 vs ≥2) stratified patients into cohorts with significantly better vs worse, respectively, RFS and CSS. These data suggest that RLI can help to stratify patients with RAS mutations and, most important, indicate that the quality of surgery is a strong predictor of long-term survival. Unlike prognostic factors inherent to tumor biology (eg, number of lesions and RAS status), RLI is under the control of the operating surgeon. Remnant liver ischemia may be the only prognostic factor that can be positively influenced by the liver surgeon.
Limitations
The present study has some limitations. First, it is limited by its retrospective nature and associated biases, including selection bias. Furthermore, the cohort was restricted to patients in whom enhanced computed tomography was available within 30 days after hepatic resection (202 of 430 [47.0%]). Second, preoperative chemotherapy may render the future liver remnant more sensitive to an ischemic insult, which has been suggested in previous studies. Whether this limitation is a valid concern cannot be determined from our data because of the small number of patients who did not receive preoperative chemotherapy (50 of 252 [19.8%]). Nevertheless, we found no differences in terms of presence or absence of chemotherapy and type of regimen between patients with RLI grade 1 or lower and patients with RLI grade 2 or higher. Third, it remains possible that a small cohort of patients with presence of necrosis (RLI grade 4) but absence of gas pockets in the hypoperfused area on computed tomography may have been misclassified using the previously established definition of RLI by Gertsch et al. Similarly, while the distinction between grade 1 and grade 2 is subject to interpretation in some cases, low interobserver variability (<5%) verified the validity of the grading system. Fourth, RAS mutation status was assessed on either CLMs or the primary tumor, and mutation status may differ between these 2 sites. However, a growing body of evidence suggests a high rate of concordance (>90%) in somatic gene mutation status between a primary tumor and related metastases.
Conclusions
The present study demonstrates that greater extent of RLI after hepatic resection is a significant predictor of worse RFS and CSS in patients who undergo curative resection of CLMs. In the era of modern chemotherapy and increased knowledge regarding the influence of mutation status on patient outcome, meticulous surgery based on detailed anatomic knowledge of each patient and precise intraoperative ultrasonography to avoid RLI remains of critical importance. On an optimistic note, RLI may be the only prognostic factor today that can be positively influenced by liver surgeons caring for patients with CLMs.
eTable 1. Univariable and Multivariable Analyses of NLR>5 at 3 Months After Hepatic Resection
eTable 2. Univariable and Multivariable Analysis of Recurrence-Free Survival
eTable 3. Multivariable Cox Regression Models for RFS and CSS Among 168 Patients Undergoing Preoperative Chemotherapy and Resection of CLM
References
- 1.Kopetz S, Chang GJ, Overman MJ, et al. . Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27(22):3677-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonney GK, Coldham C, Adam R, et al. ; LiverMetSurvey International Registry Working Group . Role of neoadjuvant chemotherapy in resectable synchronous colorectal liver metastasis: an international multi-center data analysis using LiverMetSurvey. J Surg Oncol. 2015;111(6):716-724. [DOI] [PubMed] [Google Scholar]
- 3.Rubbia-Brandt L, Giostra E, Brezault C, et al. . Importance of histological tumor response assessment in predicting the outcome in patients with colorectal liver metastases treated with neo-adjuvant chemotherapy followed by liver surgery. Ann Oncol. 2007;18(2):299-304. [DOI] [PubMed] [Google Scholar]
- 4.Blazer DG III, Kishi Y, Maru DM, et al. . Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26(33):5344-5351. [DOI] [PubMed] [Google Scholar]
- 5.Vauthey JN, Zimmitti G, Kopetz SE, et al. . RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg. 2013;258(4):619-626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicoud IB, Jones CM, Pierce JM, et al. . Warm hepatic ischemia-reperfusion promotes growth of colorectal carcinoma micrometastases in mouse liver via matrix metalloproteinase-9 induction. Cancer Res. 2007;67(6):2720-2728. [DOI] [PubMed] [Google Scholar]
- 7.Khandoga A, Kessler JS, Hanschen M, et al. . Matrix metalloproteinase-9 promotes neutrophil and T cell recruitment and migration in the postischemic liver. J Leukoc Biol. 2006;79(6):1295-1305. [DOI] [PubMed] [Google Scholar]
- 8.Holbert BL, Baron RL, Dodd GD III. Hepatic infarction caused by arterial insufficiency: spectrum and evolution of CT findings. AJR Am J Roentgenol. 1996;166(4):815-820. [DOI] [PubMed] [Google Scholar]
- 9.Gertsch P, Vandoni RE, Pelloni A, Krpo A, Alerci M. Localized hepatic ischemia after liver resection: a prospective evaluation. Ann Surg. 2007;246(6):958-964. [DOI] [PubMed] [Google Scholar]
- 10.Cho JY, Han HS, Choi Y, et al. . Association of remnant liver ischemia with early recurrence and poor survival after liver resection in patients with hepatocellular carcinoma. JAMA Surg. 2017;152(4):386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 2016;263(1):146-152. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Kikuchi Y, Kawaguchi D, Murakami T, Hiroshima Y, Matsuo K. Modified ALPPS procedures avoiding division of portal pedicles. Ann Surg. 2017;265(2):e14-e20. [DOI] [PubMed] [Google Scholar]
- 13.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16(3):614-622. [DOI] [PubMed] [Google Scholar]
- 14.Pawlik TM, Scoggins CR, Zorzi D, et al. . Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik HZ, Prasad KR, Halazun KJ, et al. . Preoperative prognostic score for predicting survival after hepatic resection for colorectal liver metastases. Ann Surg. 2007;246(5):806-814. [DOI] [PubMed] [Google Scholar]
- 16.Kishi Y, Abdalla EK, Chun YS, et al. . Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250(4):540-548. [DOI] [PubMed] [Google Scholar]
- 17.Chun YS, Vauthey JN, Boonsirikamchai P, et al. . Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302(21):2338-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shindoh J, Makuuchi M, Matsuyama Y, et al. . Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol. 2016;64(3):594-600. [DOI] [PubMed] [Google Scholar]
- 19.Shindoh J, Tzeng CW, Aloia TA, et al. . Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg. 2014;18(1):45-51. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brudvik KW, Mise Y, Chung MH, et al. . RAS mutation predicts positive resection margins and narrower resection margins in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 2016;23(8):2635-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlegel A, Lesurtel M, Melloul E, et al. . ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg. 2014;260(5):839-846. [DOI] [PubMed] [Google Scholar]
- 23.Porschen R, Lohe B, Hengels KJ, Borchard F. Assessment of cell proliferation in colorectal carcinomas using the monoclonal antibody Ki-67: correlation with pathohistologic criteria and influence of irradiation. Cancer. 1989;64(12):2501-2505. [DOI] [PubMed] [Google Scholar]
- 24.Song J, Wu C, Zhang X, Sorokin LM. In vivo processing of CXCL5 (LIX) by matrix metalloproteinase (MMP)-2 and MMP-9 promotes early neutrophil recruitment in IL-1β–induced peritonitis. J Immunol. 2013;190(1):401-410. [DOI] [PubMed] [Google Scholar]
- 25.Tohme S, Yazdani HO, Al-Khafaji AB, et al. . Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76(6):1367-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aloia TA, Zimmitti G, Conrad C, Gottumukalla V, Kopetz S, Vauthey JN. Return to intended oncologic treatment (RIOT): a novel metric for evaluating the quality of oncosurgical therapy for malignancy. J Surg Oncol. 2014;110(2):107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102(10):1175-1183. [DOI] [PubMed] [Google Scholar]
- 28.Taieb J, Zaanan A, Le Malicot K, et al. . Prognostic effect of BRAF and KRAS mutations in patients with stage III colon cancer treated with leucovorin, fluorouracil, and oxaliplatin with or without cetuximab: a post hoc analysis of the PETACC-8 trial. JAMA Oncol. 2016;2(5):643-653. [DOI] [PubMed] [Google Scholar]
- 29.Denbo JW, Yamashita S, Passot G, et al. . RAS mutation is associated with decreased survival in patients undergoing repeat hepatectomy for colorectal liver metastases. J Gastrointest Surg. 2017;21(1):68-77. [DOI] [PubMed] [Google Scholar]
- 30.Weiss MJ, Ito H, Araujo RL, et al. . Hepatic pedicle clamping during hepatic resection for colorectal liver metastases: no impact on survival or hepatic recurrence. Ann Surg Oncol. 2013;20(1):285-294. [DOI] [PubMed] [Google Scholar]
- 31.Miyashita T, Nakanuma S, Ahmed AK, et al. . Ischemia reperfusion–facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation. Eur Surg. 2016;48:92-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takamoto T, Hashimoto T, Sano K, et al. . Recovery of liver function after the cessation of preoperative chemotherapy for colorectal liver metastasis. Ann Surg Oncol. 2010;17(10):2747-2755. [DOI] [PubMed] [Google Scholar]
- 33.Knijn N, Mekenkamp LJ, Klomp M, et al. . KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104(6):1020-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torzilli G, Makuuchi M. Tricks for ultrasound-guided resection of colorectal liver metastases. Hepatogastroenterology. 2003;50(49):1-3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Univariable and Multivariable Analyses of NLR>5 at 3 Months After Hepatic Resection
eTable 2. Univariable and Multivariable Analysis of Recurrence-Free Survival
eTable 3. Multivariable Cox Regression Models for RFS and CSS Among 168 Patients Undergoing Preoperative Chemotherapy and Resection of CLM