Abstract
Background
Previous studies demonstrate that infant and childhood mortality differ among children with birth defects by maternal race/ethnicity, but limited mortality information is published for Hispanic ethnic subgroups.
Methods
We performed a retrospective cohort study using data for children with birth defects born to Hispanic mothers during 1999–2007 from 12 population-based state birth defects surveillance programs. Deaths were ascertained through multiple sources. Survival probabilities were estimated by the Kaplan-Meier method. Cox proportional hazards regression was used to examine the effect of clinical and demographic factors on mortality risk.
Results
Among 28,497 Hispanic infants and children with major birth defects, 1-year survival was highest for infants born to Cuban mothers at 94.6% (95% confidence intervals [CI] 92.7–96.0) and the lowest for Mexicans at 90.2% (95% CI 89.7– 90.6; p < .0001). For children aged up to 8 years, survival remained highest for Cuban Americans at 94.1% (95% CI 91.8–95.7) and lowest for Mexican Americans at 89.2% (95% CI 88.7–89.7; p= .0002). In the multivariable analysis using non-Hispanic White as the reference group, only infants and children born to Mexican mothers were noted to have a higher risk of mortality for cardiovascular defects.
Conclusions
This analysis provides a better understanding of survival and mortality for Hispanic infants and children with selected birth defects. The differences found in survival, particularly the highest survival rates for Cuban American children and lowest for Mexican American children with birth defects, underscores the importance of assessing Hispanic ethnic subgroups, as differences among subgroups appear to exist.
Keywords: birth defects, congenital heart disease, health disparities, Hispanic, public health, survival
1. Introduction
Birth defects affect approximately 1 in 33 newborns and are a leading cause of infant mortality in the United States (Centers for Disease Control and Prevention, 2008; Heron et al., 2009). Birth defects are also a major contributor to pediatric hospitalizations, chronic childhood illness, and developmental disabilities (Utah Department of Health, n.d.). Given the heterogeneity of the U.S. population, it is important to understand how birth defects are affecting different racial/ethnic communities. Health disparities in both prevalence and survival are well described in non-Hispanic (NH) Black as well as Hispanic children with birth defects compared with NH-White children (Broussard et al., 2012; Centers for Disease Control and Prevention, 2010; Nembhard, Pathak, & Schocken, 2008; Nembhard et al., 2011; Reddy et al., 2011; Yang et al., 2006). Previous analyses of survival differences using population-based data have largely been limited to large racial groups (e.g., Whites and Blacks) or combined minority groups and have rarely examined racial/ethnic survival differences across more than one type of birth defect. The few studies that have assessed multiple birth defects for maternal racial/ethnic groups, noted that researchers should conduct further studies of variations among Hispanic ethnic subgroups, as previous studies have suggested potential variations in outcomes among these subgroups (Kucik et al., 2014; Reichman & Kenney, 1998).
Hispanics are the fastest growing minority population in the United States, constituting 17% of the population (Brown, n.d.; State and County Quickfacts, n.d.), but are a very heterogeneous group with the largest proportions comprised of Mexicans, Puerto Ricans, and Cubans (Motel & Patten, n.d.). Overall, pediatric studies stratified by these Hispanic ethnic subgroups are limited. One statewide study assessing low birth weight and preterm delivery in Hispanic subgroups demonstrated that the risk of preterm delivery and low birth weight differed among these groups (Reichman & Kenney, 1998). In one of the few studies assessing the prevalence of an array of major birth defects prevalence among broader Hispanic subgroups, the study noted that Hispanic mothers born in Mexico/Central America were more likely to deliver babies with spina bifida and anotia or microtia than their U.S.-born counterparts (Ramadhani et al., 2009). Prevalence (but not survival) of birth defects among Hispanic ethnic subgroups was noted in one study, demonstrating that Mexican American children had a higher prevalence of anotia and microtia and Puerto Rican children had a higher prevalence of anencephaly (Canfield et al., 2014).
Obtaining information about Hispanic ethnic subgroups has been problematic in studies investigating survival among infants and children with birth defects that have relied on hospital discharge data, single institution medical records, or population-based surveillance data from a single state registry. These data sources typically do not have adequate sample sizes or sufficient subgroup variation to adequately evaluate this information among Hispanics. Also, while smaller studies have assessed birth defect prevalence and survival for Hispanic ethnic subgroups (Cohen, Friedman, Mahan, Lederman, & Munoz, 1993; Reichman & Kenney, 1998), there is a need for a large population-based study to explore survival of infants with birth defects among Hispanic ethnic subgroups.
The objectives of this study were to (a) evaluate survival of infants and children of Hispanic ethnic subgroups with selected major birth defects, and (b) identify potential demographic and clinical factors associated with survival.
2. Methods
2.1. Population studied
A retrospective, population-based cohort study was conducted on all live births (singletons and multiples) between 1999 and 2007 with selected major birth defects from 12 population-based state birth defects surveillance programs that were pooled for larger collaborative studies of survival among all children with birth defects (Meyer et al., 2016; Wang et al., 2015). The population-based systems used either active (four state birth defects surveillance programs) or passive (eight state birth defects surveillance programs) case finding methodologies to identify all potential cases within a defined catchment area. For active case finding methodology, birth defects cases are actively identified by individuals reviewing birth defects data; for passive case finding methodology, cases are reported by providers/facilities or administrative/other data sets. Data for birth cohorts were included from 12 states (Appendix A). Our data include 1-year survival from all years for all states. In the current analysis, we included infants born to Hispanic mothers, who were subcategorized into Mexican, Puerto Rican, Cuban, and Other (Central and South American and other and unknown Hispanic), and infants born to NH-White mothers. For the purposes of this article, infants and children born to Mexican, Puerto Rican, and Cuban mothers will be referred to as Mexican, Puerto Rican, and Cuban, including Hispanic Blacks, if any. The study protocol was approved by each state's Institutional Review Board.
2.2. Determining birth defects
The International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) and British Paediatric Association (BPA) codes (Appendix B) were used to classify birth defects by each state program in our study. We selected birth defect categories based on the likelihood that these defects were consistently diagnosed during early infancy and ascertained by state surveillance programs, given that these programs were either actively surveilled or cases were reported by providers/facilities or administrative/other data sets (Canfield et al., 2014). If a child had any of these selected birth defects, information on any additional birth defects of the child were also included in the data set.
2.3. Vital statistics
Each state surveillance program linked their birth defect case information to state death certificate data files (follow-up ranging from 1 to 8 years) to obtain the vital status of the study cohort. For deceased children, programs provided the date of death, duration of life in days, and underlying cause of death. Eight states only used state death certificates to determine child and infant death. Other states used additional data sources to obtain vital status information included hospital discharge files, medical records, and the National Death Index. Two state programs also collected information on deaths among their residents from neighboring states' vital records.
2.4. Demographic data
Surveillance programs matched cases to state birth certificate records to obtain demographic and clinical characteristics. These included infant sex (male, female), birth weight (<2,500 and ≥2,500 g), gestational age (<37 and ≥37 weeks), plurality (singleton, multiple births), method of delivery (vaginal, caesarean), maternal age (<34 and ≥35 years), maternal education (≤high school, > high school), maternal nativity (U.S.-born, foreign-born), marital status (married, single), prenatal care (yes, no), maternal prenatal cigarette smoking (yes, no), maternal diabetes of any type (yes, no), maternal residence at delivery (metropolitan, non-metropolitan [based on county of maternal residence], and state of residence), and maternal race/ethnicity (NH White, Hispanic [Mexican, Cuban, Puerto Rican, and Hispanic other]). The maternal race/ethnicity were obtained from birth certificates of the infants/children through matching of the birth defect cases of the participating state birth defect surveillance programs to state vital records (birth certificates). The maternal race/ethnicity information in the birth certificate records are self-reported by mothers/fathers. All 12 programs had complete data on Hispanic subgroups for all years of the study and all ethnic subgroups listed above.
2.5. Statistical analysis
Using the Kaplan-Meier Product Limit method, survival probabilities for infant (< 1 year of age) and early childhood (1–8 years of age) were calculated for individual defects by maternal race/ethnicity. Greenwood's method was used to calculate 95% confidence intervals (CI) for estimates of survival probabilities. Data from all 12 birth defect programs were used to calculate infant survival estimates. For the analyses of survival beyond infancy, 10 programs' data were analyzed using data for those born during 1999–2005; Illinois and Nebraska data were excluded because of unavailability of vital status beyond infancy. Additionally, the birth cohort for New Jersey was only through 2005, making this the last birth year to be included for all 10 programs in the analysis. Thus, the longest period of follow-up was 8 years.
An initial bivariate analysis of all the selected variables was performed to determine possible explanatory variables for inclusion in multivariable models. Multivariable analysis using Cox proportional hazards regression models was conducted to explore associations between the explanatory variables and survival, controlling for infant sex, gestational age, birth weight, maternal age, maternal metropolitan or nonmetropolitan county of residence at delivery, maternal nativity, maternal race/ethnicity, surveillance methodology (active or passive case ascertainment), maternal diabetes, plurality, prenatal care, method of delivery, and birth year. The proportionality assumption was examined in the Cox model. Children with more than one defect are represented in more than one defect category.
SAS Version 9.2 (SAS Institute, Cary, NC) was used for all statistical analyses.
3. Results
During the study period, the 12 programs ascertained cases among approximately 14 million live births (about 39% of all live births in the United States), with 98,833 infants identified as having at least one of the 21 selected birth defects. Of these, we identified 28,497 Hispanic births (28.8% of all births with birth defects), with all cases having vital status data for the infancy period, thus all being included in the first year survival analysis. A total of 18,342 cases who survived beyond infancy (≥1 year of age) were followed up to 8 years. Of all infants, we had 69.8% Mexican (n = 19,881), 7.1% Puerto Rican (n = 2,024), 2.7% Cuban (n = 773), and 20.4% Hispanic other (n = 5,819) (e.g., mothers from Central and South American countries) (Table 1). NH Whites were the referent group. A total of 2,688 Hispanic infants (9.4%) died in infancy with a survival probability of 90.6% (95% CI: 90.2–90.9); 4,165 (7.6%) of NH-White infants died in infancy, with a survival probability of 92.4% (95% CI: 92.1–92.6). There were 202 Hispanic children (1.1%) who died during childhood with the survival probability of 98.9% (95% CI: 98.799.0); 327 NH-White children (0.9%) died during childhood, with the survival probability of 99.1% (95% CI: 99.0–99.2). The highest infant survival probability was in Cubans at 94.6% (95% CI 92.7–96.0), followed by NH Whites at 92.4% (95% CI 92.1–92.6). The lowest infant survival probability was in Mexicans at 90.2% (95% CI 89.7–90.6). Analysis outcome were not statistically different from combined surveillance systems when assessed individually by type of registry (active vs. passive).
Table 1. Overall survival for children with selected birth defects born to Hispanic women by demographic and clinical characteristics in the United States, 1999–2007.
| Infant (<1 year)a | Childhood (1–8 years)b | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | No. of birth | No. of death (%) | Survival probability (95% CI) | No. of birth | No. of death (%) | Survival probability (95% CI) |
| Maternal ethnicity | p<.0001 | p=.0797 | ||||
| Mexican | 19,881 | 1,955 (9.8) | 90.2 (89.7–90.6) | 12,829 | 154 (1.2) | 98.8 (98.6–99.0) |
| Puerto Rican | 2,024 | 163 (8.1) | 91.9 (90.7–93.1) | 1,369 | 11 (0.8) | 99.2 (98.6–99.6) |
| Cuban | 773 | 42 (5.4) | 94.6 (92.7–96.0) | 541 | 1 (0.2) | 99.8 (98.7–100.0) |
| Other Hispanic | 5,819 | 528 (9.1) | 90.9 (90.2–91.6) | 3,603 | 36 (1.0) | 99.0 (98.6–99.3) |
| NH Whitec | 54,519 | 4,165 (7.6) | 92.4 (92.1–92.6) | 35,954 | 327 (0.9) | 99.1 (99.0–99.2) |
|
| ||||||
| Infant sex | p<.0001 | p=.0567 | ||||
| Male | 17,334 | 1,461 (8.4) | 91.6 (91.1–92.0) | 11,355 | 112 (1.0) | 99.0 (98.8–99.2) |
| Female | 11,152 | 1,218 (10.9) | 89.1 (88.5–89.6) | 6,987 | 90 (1.3) | 98.7 (98.4–99.0) |
|
| ||||||
| Gestation/birth weight | p<.0001 | p<.0001 | ||||
| <37 week/<2,500 g | 3,892 | 1,028 (26.4) | 73.6 (72.2–74.9) | 1,964 | 47 (2.4) | 97.6 (96.8–98.2) |
| <37 week/>2,500 g | 2,210 | 247 (11.2) | 88.8 (87.4–90.1) | 1,356 | 13 (1.0) | 99.0 (98.4–99.4) |
| >37 week/<2,500 g | 1,877 | 423 (22.5) | 77.5 (75.5–79.3) | 1,058 | 26 (2.5) | 97.5 (96.4–98.3) |
| >37 week/>2,500 g | 20,518 | 990 (4.8) | 95.2 (94.9–95.5) | 13,964 | 116 (0.8) | 99.2 (99.0–99.3) |
|
| ||||||
| Plurality | p<.0001 | p=.3992 | ||||
| Single | 27,706 | 2,554 (9.2) | 90.8 (90.4–91.1) | 17,885 | 199 (1.1) | 98.9 (98.7–99.0) |
| Multiple | 755 | 130 (17.2) | 82.8 (79.9–85.3) | 437 | 3 (0.7) | 99.3 (97.9–99.8) |
|
| ||||||
| Maternal age | p=.0059 | p=.0257 | ||||
| <19 years | 4,796 | 428 (8.9) | 91.1 (90.2–91.8) | 3,106 | 50 (1.6) | 98.4 (97.9–98.8) |
| 20–24 years | 7,836 | 700 (8.9) | 91.1 (90.4–91.7) | 5,142 | 54 (1.1) | 98.9 (98.6–99.2) |
| 25–29 years | 6,744 | 680 (10.1) | 89.9 (89.2–90.6) | 4,305 | 45 (1.0) | 99.0 (98.6–99.2) |
| 30–34 years | 4,845 | 431 (8.9) | 91.1 (90.3–91.9) | 3,163 | 34 (1.1) | 98.9 (98.5–99.2) |
| >35 years | 4,275 | 449 (10.5) | 89.5 (88.5–90.4) | 2,625 | 19 (0.7) | 99.3 (98.9–99.5) |
|
| ||||||
| Maternal education | p=.1180 | p=.1655 | ||||
| <High school | 13,537 | 1,290 (9.5) | 90.5 (90.0–91.0) | 8,767 | 108 (1.2) | 98.8 (98.5–99.0) |
| High school | 8,360 | 785 (9.4) | 90.6 (90.0–91.2) | 5,428 | 52 (1.0) | 99.0 (98.7–99.3) |
| >High school | 6,115 | 526 (8.6) | 91.4 (90.7–92.1) | 3,821 | 35 (0.9) | 99.1 (98.7–99.3) |
|
| ||||||
| Maternal diabetes | p=.0012 | p=.9170 | ||||
| Yes | 1,468 | 177 (12.1) | 87.9 (86.2–89.5) | 856 | 10 (1.2) | 98.8 (97.8–99.4) |
| No | 25,014 | 2,372 (9.5) | 90.5 (90.1–90.9) | 15,672 | 177 (1.1) | 98.9 (98.7–99.0) |
|
| ||||||
| Maternal nativity | p=.1644 | p=.1217 | ||||
| U.S. born | 12,349 | 1,122 (9.1) | 90.9 (90.4–91.4) | 8,083 | 100 (1.2) | 98.8 (98.5–99.0) |
| Non-U.S. born | 15,933 | 1,524 (9.6) | 90.4 (90.0–90.9) | 10,144 | 101 (1.0) | 99.0 (98.8–99.2) |
|
| ||||||
| Geographic area | p=.0212 | p=.1210 | ||||
| Metro | 26,106 | 2,432 (9.3) | 90.7 (90.3–91.0) | 16,796 | 179 (1.1) | 98.9 (98.8–99.1) |
| Nonmetro | 2,380 | 255 (10.7) | 89.3 (88.0–90.5) | 1,537 | 23 (1.5) | 98.5 (97.8–99.0) |
|
| ||||||
| Method of delivery | p<.0001 | p=.0504 | ||||
| Vaginal | 17,069 | 1,341 (7.9) | 92.1 (91.7–92.5) | 11,415 | 113 (1.0) | 99.0 (98.8–99.2) |
| Caesarean | 11,275 | 1,338 (11.9) | 88.1 (87.5–88.7) | 6,830 | 89 (1.3) | 98.7 (98.4–98.9) |
|
| ||||||
| Prenatal care | p<.0001 | p=.1398 | ||||
| Yes | 27,099 | 2,474 (9.1) | 90.9 (90.5–91.2) | 17,566 | 194 (1.1) | 98.9 (98.7–99.0) |
| No | 854 | 123 (14.4) | 85.6 (83.1–87.8) | 494 | 2 (0.4) | 99.6 (98.4–99.9) |
|
| ||||||
| Birth period | p=.0254 | p=.0034 | ||||
| 1999–2002 | 11,134 | 1,104 (9.9) | 90.1 (89.5–90.6) | 9,372 | 124 (1.3) | 98.7 (98.4–98.9) |
| 2003–2007 (2005) | 17,363 | 1,584 (9.1) | 90.9 (90.4–91.3) | 8,970 | 78 (0.9) | 99.1 (98.9–99.3) |
|
| ||||||
| Total | 28,497 | 2,688 (9.4) | 90.6 (90.2–90.9) | 18,342 | 202 (1.1) | 98.9 (98.7–99.0) |
Children born in 1999–2007 from all 12 states, n=28,497.
Children born in 1999–2005 from 10 states; data from IL and NE were excluded because no vital status data beyond infancy were available, n=18,342.
For comparison, data for NH Whites were added.
As shown in Table 1, survival probability was higher for infants who were male, of gestational age >37 weeks, an only child, born to a nondiabetic mother, lived in a metropolitan area, were delivered vaginally, had prenatal care, and were born after 2002. For Hispanic children who survived beyond infancy, childhood (1–8 years old) survival probability was higher for children who were of gestational age >37 weeks, had a vaginal delivery, and were born after 2002.
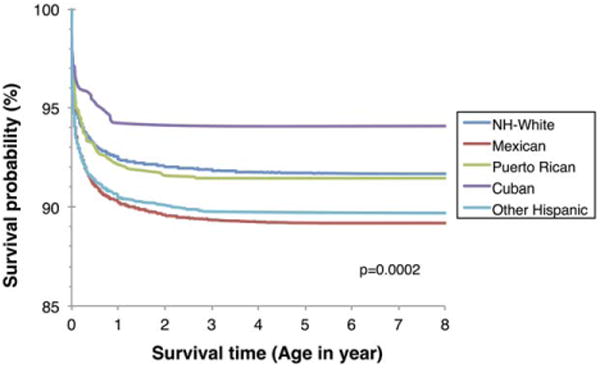
Figure 1 shows Kaplan-Meier survival curves for infants born between 1999 and 2005 from 10 surveillance programs stratified by Hispanic ethnic groups and NH Whites. When compared to NH Whites, the 8-year survival probability was the highest for Cubans (94.1% [95% CI 91.8–95.7]), similar for NH Whites and Puerto Ricans, and significantly lower for Mexicans (89.2% [95% CI 88.7–89.7; p = .0002]).
Figure 1. Survival curves of children born with birth defects to Hispanic women in the United States, by Hispanic subgroups, 1999–2005s.
Results from survival analysis of Hispanic children by birth defect category and Hispanic subgroup are shown in Table 2. For comparison, data for NH Whites were added. The lowest overall survival probability occurred for Mexican and Puerto Rican children (0–8 years old) with hypoplastic left heart syndrome (HLHS), 44.9% (95% CI 39.4–50.3) and 45.9% (95% CI 29.6–60.9), respectively. Mexican and Puerto Rican infants with HLHS also had the lowest survival probability. In addition, less than 70% survival was found among Mexican infants with encephalocele, atrioventricular septal defect, and omphalocele; Puerto Rican infants with an atrio-ventricular septal defect; and Cuban infants with an encephalocele and an atrioventricular septal defect.
Table 2. Survival estimates by type of birth defect for children born to Hispanic women in the United States and by Hispanic subgroup, 1999-2007.
| Infancy (<1 year) survival probabilitya (95% CI) | Childhood (≤8 years) survival probabilityb (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| NH Whitec | Mexican | Puerto Rican | Cuban | NH Whitec | Mexican | Puerto Rican | Cuban | |
| Central nervous system | ||||||||
| Spina bifida without anencephalus | 92.0 (90.7–93.1) | 92.4 (90.6–93.9) | 95.5 (88.3–98.3) | 91.3 (69.5–97.8) | 90.5 (88.8–91.9) | 90.7 (88.2–92.6) | 96.3 (86.0–99.1) | 91.3 (69.5–97.8) |
| Encephalocele | 73.6 (68.9–77.7) | 66.0 (59.0–72.1) | 84.2 (58.7–94.6) | 66.7 (5.4–94.5) | 72.8 (67.0–77.8) | 62.3 (53.9–69.6) | 100, no death | 66.7 (5.4–94.5) |
|
| ||||||||
| Cardiovascular and respiratory system | ||||||||
| Common truncus | 80.3 (76.7–83.5) | 64.7 (57.0–71.4) | 81.8 (58.5–92.8) | 100, no death | 76.4 (71.7–80.4) | 59.5 (50.4–67.5) | 85.7 (53.9–96.2) | 100, no death |
| Transposition of great arteries | 86.0 (84.6–87.4) | 82.8 (79.9–85.2) | 84.4 (74.2–90.8) | 86.0 (72.9–93.1) | 84.2 (82.4–85.8) | 80.6 (77.2–83.6) | 78.7 (66.1–87.0) | 87.2 (71.9–94.5) |
| Tetralogy of Fallot | 89.5 (88.3–90.6) | 83.2 (80.4–85.6) | 85.1 (77.1–90.5) | 96.3 (86.0–99.1) | 87.8 (86.3–89.1) | 80.0 (76.5–83.1) | 81.8 (72.0–88.4) | 95.2 (82.3–98.8) |
| Atrioventricular septal defect | 84.1 (82.7–85.5) | 72.6 (69.3–75.7) | 78.3 (67.8–85.7) | 78.6 (58.4–89.8) | 81.6 (79.8–83.2) | 67.9 (63.9–71.6) | 72.1 (59.1–81.7) | 81.0 (56.9–92.4) |
| Atrioventricular septal defect(occurring w/o DS) | 75.6 (73.1–77.9) | 64.6 (60.0–68.8) | 63.4 (46.8–76.1) | 68.4 (42.8–84.4) | 73.1 (70.1–75.8) | 58.8 (53.3–63.9) | 62.1 (42.1–76.9) | 69.2 (37.3–87.2) |
| Aortic valve stenosis | 85.5 (83.7–87.1) | 78.0 (74.2–81.4) | 97.2 (81.9–99.6) | 100, no death | 83.9 (81.7–85.8) | 75.7 (71.3–79.6) | 95.8 (73.9–99.4) | 100, no death |
| Hypoplastic left heart syndrome | 57.8 (55.4–60.2) | 50.1 (45.6–54.5) | 53.3 (37.9–66.6) | 72.7 (49.1–86.7) | 54.0 (51.1–56.8) | 44.9 (39.4–50.3) | 45.9 (29.6–60.9) | 71.4 (40.6–88.2) |
| Coarctation of aorta | 86.3 (85.1–87.3) | 83.4 (81.1–85.4) | 87.9 (80.5–92.7) | 86.2 (74.3–92.9) | 84.0 (82.5–85.3) | 80.1 (77.2–82.7) | 82.7 (72.6–89.4) | 85.7 (69.0–93.8) |
|
| ||||||||
| Oral clefts | ||||||||
| Cleft palate without cleft lip | 93.0 (92.2–93.7) | 86.6 (84.6–88.3) | 89.5 (83.4–93.4) | 87.0 (73.2–93.9) | 92.3 (91.3–93.2) | 86.2 (83.8–88.2) | 89.7 (82.5–94.0) | 86.1 (69.8–94.0) |
| Cleft lip with and without cleft palate | 93.5 (92.9–94.1) | 89.7 (88.4–90.8) | 90.3 (85.2–93.7) | 94.5 (84.0–98.2) | 92.8 (92.0–93.5) | 88.9 (87.3–90.2) | 88.3 (81.8–92.5) | 92.9 (79.5–97.6) |
|
| ||||||||
| Gastrointestinal | ||||||||
| Esophageal atresia/tracheoespphageal Fistula | 88.2 (86.7–89.6) | 79.5 (75.6–82.8) | 82.0 (69.8–89.6) | 90.6 (73.7–96.9) | 86.7 (84.8–88.5) | 79.3 (74.6–83.2) | 88.9 (75.3–95.2) | 91.7 (70.6v97.8) |
| Pyloric stenosis | 99.5 (99.4–99.6) | 99.4 (99.1–99.6) | 99.3 (98.1–99.7) | 99.6 (97.1–99.9) | 99.3 (99.2–99.5) | 99.1 (98.7–99.4) | 99.3 (97.7–99.8) | 99.4 (96.0–99.9) |
| Rectal and large intestinal atresia/stenosis | 90.3 (89.2–91.4) | 81.7 (79.2–83.8) | 85.2 (77.0–90.6) | 85.7 (70.9–93.3) | 90.1 (88.7–91.3) | 80.7 (77.8–83.3) | 84.7 (75.1–90.8) | 82.8 (63.4–2.4) |
|
| ||||||||
| Musculoskeletal | ||||||||
| Upper limb deficiencies | 91.4 (90.1–92.6) | 87.4 (84.8–89.5) | 87.5 (77.4–93.3) | 91.3 (69.5–97.8) | 89.9 (88.1–91.4) | 86.2 (83.1–88.7) | 91.2 (80.2–96.3) | 89.5 (64.1–97.3) |
| Lower limb deficiencies | 90.7 (88.7–92.3) | 81.5 (77.0–85.3) | 100, no death | 100, no death | 91.0 (88.6–92.9) | 80.8 (75.4–85.1) | 100, no death | 100, no death |
| Diaphragmatic hernia | 70.6 (68.3–72.7) | 70.6 (67.0–74.0) | 72.9 (60.8–81.7) | 62.5 (40.3–78.4) | 70.2 (67.4–72.8) | 69.1 (64.6–73.1) | 74.5 (59.4–84.6) | 55.6 (30.5–74.8) |
| Gastroschisis | 93.0 (91.8–94.1) | 93.2 (91.5–94.5) | 94.0 (86.3–97.5) | 100, no death | 92.5 (90.8–93.9) | 92.7 (90.5–94.4) | 90.9 (79.5–96.1) | 100, no death |
| Omphalocele | 73.9 (70.3–77.1) | 65.2 (59.3–70.4) | 81.3 (52.5–93.5) | no Case | 73.2 (68.6–77.2) | 64.3 (57.2–70.5) | 90.9 (50.8–98.7) | no Case |
|
| ||||||||
| Chromosomal | ||||||||
| Trisomy 21 (Down syndrome) | 94.5 (94.0–95.0) | 94.7 (93.9–95.4) | 94.3 (90.9–96.4) | 99.0 (93.4–99.9) | 93.2 (92.5–93.8) | 93.7 (92.6–94.6) | 92.9 (88.5–95.7) | 98.6 (90.7–99.8) |
Children born in 1999-2007 from all 12 states, n = 28,497.
Children born in 1999-2005 from 10 states; data from IL and NE were excluded because no vital status data beyond infancy were available, n = 18,342.
For comparison, data for NH Whites were added.
In the multivariable analysis using NH White as the reference group (Table 3), only Mexican infants and children were noted to have a higher risk of mortality for cardiovascular defects: the hazard ratio (HR) of infant death was 1.8 for common truncus, 1.3 for transposition of great arteries, 1.6 for tetralogy of Fallot and aortic valve stenosis, and 1.4 for atrioventricular septal defect; the HR of childhood mortality (1–8 years old) was 2.2 for hypoplastic left heart syndrome. Cuban infants had a statistically significant higher risk of mortality for cleft palate without cleft lip (HR: 2.7). Puerto Rican infants and children have no significantly different HR in the listed defects except for gastroschisis with a HR of 9.5 and a very broad range of 95% CI, which is possibly due to small numbers.
Table 3. Adjusted risk of death (HR) of children with selected birth defects born for Hispanic mothers compared with NH mothers in the United States, by Hispanic subgroups, Unites States, 1999–20071.
| Infancy (<1 year) HRa (95% CI) | Childhood (1–8 years) HRb,c (95% CI) | ||||
|---|---|---|---|---|---|
|
|
|
||||
| Characteristic | Mexican | Puerto Rican | Cuban | Mexican | Puerto Rican |
| Central Nervous system | |||||
| Spina bifida without anencephalus | 1.1 (0.7, 1.6) | 0.6 (0.2, 1.7) | 2.2 (0.5, 9.2) | 0.9 (0.2, 3.7) | No death |
| Encephalocele | 1.2 (0.7, 1.8) | 0.5 (0.2, 1.6) | 0.7 (0.1, 5.2) | 0.3 (0.0, 10.5) | No death |
|
| |||||
| Cardiovascular and respiratory system | |||||
| Common truncus | 1.8 (1.2, 2.8) | 1.1 (0.4, 3.2) | No death | 0.7 (0.1, 8.8) | No death |
| Transposition of great arteries | 1.3 (1.0, 1.7) | 1.0 (0.6, 1.8) | 1.0 (0.5, 2.1) | 1.5 (0.6, 3.6) | 1.7 (0.4, 7.8) |
| Tetralogy of Fallot | 1.6 (1.2, 2.0) | 1.2 (0.7, 2.1) | 0.5 (0.1, 1.9) | 1.3 (0.6, 2.6) | 0.7 (0.2, 3.1) |
| Atrioventricular septal defect | 1.4 (1.1, 1.7) | 1.3 (0.8, 2.2) | 1.3 (0.6, 3.0) | 1.6 (0.8, 2.9) | 2.5 (0.9, 7.0) |
| Atrioventricular septal defect(occurring w/o DS) | 1.1 (0.9, 1.5) | 1.5 (0.9, 2.6) | 1.2 (0.5, 2.7) | 1.9 (0.9, 4.2) | 1.6 (0.2, 12.5) |
| Aortic valve stenosis | 1.6 (1.2, 2.2) | 0.2 (0.0, 1.3) | 0.0 (0.0, 3E168) | 0.9 (0.3, 2.5) | No death |
| Hypoplastic left heart syndrome | 1.1 (0.9, 1.3) | 1.0 (0.6, 1.6) | 0.5 (0.2, 1.2) | 2.2 (1.1, 4.7) | 0.9 (0.1, 6.5) |
| Coarctation of aorta | 1.0 (0.8, 1.3) | 0.8 (0.5, 1.4) | 0.9 (0.5, 1.9) | 1.8 (0.9, 3.4) | 1.8 (0.4, 7.8) |
|
| |||||
| Oral Clefts | |||||
| Cleft palate without cleft lip | 1.4 (1.1, 1.8) | 1.2 (0.7, 2.0) | 2.7 (1.2, 6.2) | 1.6 (0.8, 3.2) | 0.6 (0.1, 4.5) |
| Cleft lip with and without cleft palate | 1.2 (1.0, 1.4) | 1.1 (0.7, 1.8) | 1.0 (0.3, 3.2) | 1.1 (0.5, 2.3) | No death |
|
| |||||
| Gastrointestinal | |||||
| Esophageal atresia/tracheoesophageal Fistula | 1.7 (1.3, 2.4) | 1.6 (0.8, 2.9) | 0.7 (0.2, 2.2) | 1.1 (0.3, 3.6) | No death |
| Pyloric stenosis | 1.1 (0.6, 2.1) | 1.3 (0.4, 3.6) | 0.8 (0.1, 5.9) | 0.7 (0.2, 2.5) | No death |
| Rectal and large intestinal atresia/stenosis | 1.5 (1.2, 1.9) | 1.5 (0.9, 2.5) | 2.1 (0.9, 4.8) | 1.6 (0.6, 4.6) | No death |
|
| |||||
| Musculoskeletal | |||||
| Upper limb deficiencies | 1.0 (0.7, 1.4) | 1.2 (0.6, 2.4) | 1.1 (0.3, 4.7v | 1.2 (0.4, 3.9) | No death |
| Lower limb deficiencies | 1.4 (0.9, 2.3) | No death | No death | 0.6 (0.0, 12.1) | No death |
| Diaphragmatic hernia | 1.0 (0.8, 1.2) | 0.9 (0.6, 1.5) | 1.6 (0.8, 3.1) | 0.4 (0.1, 3.2) | No death |
| Gastroschisis | 0.9 (0.6, 1.2) | 0.7 (0.3, 1.7) | No death | 1.6 (0.4, 6.9) | 9.5 (0.9, 104.0) |
| Omphalocele | 1.3 (0.9, 1.8) | 0.5 (0.1, 1.5) | No Case | 1.6 (0.2, 12.4) | No death |
|
| |||||
| Chromosomal | |||||
| Trisomy 21 (Down syndrome) | 1.0 (0.8, 1.3) | 1.0 (0.6, 1.6) | 0.2 (0.0, 1.6) | 0.7 (0.4, 1.4) | 1.1 (0.3, 3.6) |
Notes. Adjusted covariates includes infant gender, birth weight/gestational age, maternal nativity, plurality, maternal age, maternal diabetes, geographic area, surveillance methodology, method of delivery, prenatal care, and birth period. NH Whites were the referent/comparison group. Bold entries are noted to have statistical significant HR when compared to NH Whites. CI that are bolded and include 1 are because those numbers were rounded for the purposes of fitting the table.
Children born in 1999–2007 from all 12 states.
Children born in 1999–2005 from 10 states; data from IL and NE were excluded because no vital status data beyond infancy were available.
Data for Cuban subgroup is not show due to small numbers.
4. Discussion
Our study used multistate pooled data for 21 selected major birth defects to determine associated survival estimates and mortality risk for infants and children born to mothers of three major Hispanic ethnic groups. We identified 28,497 Hispanics (28.8%) with birth defects, providing a sufficiently large sample size to identify factors associated with Hispanic infant and child survival for a broad range of major birth defects. We found that demographic and clinical factors associated with survival for Hispanic infants included infant sex, gestational age/birth weight, plurality, maternal age, maternal diabetes, geographic area, method of delivery, prenatal care, and birth period.
The majority of studies use broad racial/ethnic groups (e.g., Blacks and Hispanics) when assessing survival outcomes. Using an analytic category of “Hispanic” makes it impossible to detect any disparities across the heterogeneous Hispanic community. We investigated infant and child survival within Hispanic ethnicities after adjustment for regional variations and other covariates. We observed differences in mortality and survival risk analysis between Hispanic ethnic groups and NH Whites for selected congenital heart defects, gastrointestinal defects, oral clefts, and central nervous system defects. A recent study using similar data from the 12 state birth defects programs used in our study demonstrated elevated prevalence in birth defects not found to be of higher mortality in our study (Canfield et al., 2014). This includes a 50% or higher prevalence of anotia or microtia for Mexicans and a higher prevalence of anencephaly for Puerto Ricans. Cubans had a lower or similar prevalence for all birth defects studied (Canfield et al., 2014). Another study examining congenital heart disease in the United States noted that no racial/ethnic difference was observed in the overall congenital heart disease prevalence in Hispanics except for atrial septal defects (not assessed in our study) (Egbe et al., 2014). Our study demonstrated specific increases in mortality risk for Mexican infants and children with common truncus, atrioventricular septal defects, and aortic valve stenosis, a finding not seen in Puerto Ricans or Cubans. Other studies have conflicting findings regarding increased gastroschisis prevalence in Hispanics (Kim, Wang, Kirby, & Druschel, 2013; Kirby et al., 2013). We found that rectal and large intestinal atresia/stenosis were associated with a higher risk of infant mortality for the three Hispanic ethnic groups, which has not been described in the literature previously. In general, it is difficult to compare our findings to those previously studies, given the lack of information available on the mortality or survival for Hispanic ethnic groups.
Perhaps most interesting is our finding of overall survival for children with birth defects from infancy through childhood. While literature reporting overall survival for several birth defects often demonstrates lower rates for Hispanics compared with NH Whites (Canfield et al., 2006; Yang et al., 2006), when separated into Hispanic ethnic groups, Cubans had a statistically significant higher survival than all groups, including NH Whites. Further, Puerto Ricans and NH Whites had similar survival rates. Thus, given that the Mexican ethnic group had the lowest rate of survival, it is possible that previous studies demonstrating lower survival in Hispanics were reflecting this specific community, and caution must be taken when generalizing survival rates to “Hispanics” (not accounting for ethnic differences).
This study has several important strengths. First, the large sample size supports relatively stable survival estimates for most phenotypes and risk factors examined. Also, the data were collected from population-based birth defect programs linked to vital records, which enhances the reliability and representativeness of the study population. Our study extends the findings of a previous study (Kucik et al., 2014) using a larger population to provide more detailed survival estimates and to compare the survival and mortality experience of infants across an array of phenotypes and risk factors, throughout infancy and early childhood for Hispanic ethnic subgroups.
Limitations of our study include incomplete ascertainment of deaths. While the majority of deaths were identified by matching the state birth to death certificate records, some deaths were ascertained from medical records at birth hospitals. Any child for whom there was no record of death was assumed to be alive. Although some children might have moved and died in another state, many states have agreements in place with neighboring states to report deaths back to the state of birth. Additionally, three of 12 programs utilized the National Death Index, thus deaths that occurred outside the state of maternal residence at delivery could be potentially captured. Missed deaths could result in an overestimation of the survival probabilities. Finally, there is some degree of uncertainty given the wide CI in risk of death for the Cuban population due to their smaller sample size, thus findings should be carefully interpreted.
Also, of seven surveillance programs using passive case ascertainment methodologies, only three validate the accuracy of their birth defect case identification. The misclassification could introduce a bias in estimating the survival probabilities by assigning noncases as cases. However, we minimized this potential bias by selecting phenotypes that were, for the most part, consistently diagnosed. Moreover, the mortality risk estimates reported in the current study were adjusted for case ascertainment methods as well as other risk factors using multivariable regression.
Another potential limitation includes the possible lack of accuracy of coding race/ethnicity on birth certificates across programs, defects, and time periods, which could lead to a misclassification of Hispanic subgroups and impact findings from our study. We attempted to ameliorate this by using several sources that identify the patient race/ethnicity, such as birth and death certificates, and maternal self-report.
Another potential limitation is that our finding of differences in survival among infants with specific birth defects such as atrioventricular septal defects, cleft lip/palate, and omphalocele could be due, in part, to differences in the prevalence of trisomies (e.g., trisomy 13 or 18) or other chromosomal anomalies among the different Hispanic subpopulations.
Finally, we were unable to adjust for other important factors, such as timing and age at initial diagnosis, clinical severity, socioeconomic status, maternal education, and whether the child had an isolated or nonisolated malformation. Unfortunately, these variables were not consistently available from all surveillance systems, and thus these may serve as confounders for our data. We limited our findings to three Hispanic subgroups, as the remaining data consisted of heterogeneous countries from Central and South America whose limited information made it challenge to be accurately analyzed. Many other rapidly growing populations of Hispanics in the United States, such as those from Central and South American countries (Zong & Batalova, 2015) were not captured in our analyses.
In conclusion, our study provides important estimates of survival probability and mortality risk for infants and children up to 8 years with selected birth defects using population-based birth defects programs. Differences in survival probabilities and mortality risks for Hispanic subgroups were found for selected congenital heart defects, gastrointestinal defects, oral clefts, and central nervous system defects. Additionally, we found a survival advantage for Cuban infants and children with birth defects, a finding requiring further investigation into other potential confounders, including socioeconomic status of the different Hispanic subgroups, as well as with NH Whites. Future studies should be performed to further ascertain differences in other Hispanic subgroups, and potential contribution of other relevant factors to infant and childhood survival among all Hispanic subgroups.
Acknowledgments
The authors would like to thank the 12 population-based state birth defects surveillance programs: Arizona, Colorado, Florida, Georgia/CDC, Illinois, Massachusetts, Michigan, North Carolina, Nebraska, New Jersey, New York, and Texas for their collaboration and preparation of data sets for the study. The authors have no financial relationships relevant to this article to disclose. The findings and conclusions in this report are those of the authors and do not represent the official position of the Centers for Disease Control and Prevention.
Appendix A
State Birth Cohort.
| State | Birth years | Death yearsa | Live births with birth defects | Surveillance methodologyb | Data source(s) used for ascertaining deaths | |
|---|---|---|---|---|---|---|
| AZ | 1999–2007 | 1999–2010 | 2,413 | Active | Death certificate, Medical records, hospital discharge files | |
| CO | 1999–2006 | Not providedc | 1,496 | Passive | Death certificate | |
| FL | 1999–2007 | 1999–2010 | 3,952 | Passive | Death certificate | |
| GA | 1999–2007 | 1999–2009 | 571 | Active | Death certificate, NDI | |
| IL | 1999–2006 | 1999–2007 | 1,562 | Passive | Death certificate | |
| MA | 2000–2007 | 2000–2008 | 374 | Active | Death certificate | |
| MI | 1999–2006 | 1999–2009 | 529 | Passive | Death certificate, NDI | |
| NC | 2003–2007 | 2003–2010 | 831 | Passive | Death certificate | |
| NE | 1999–2006d | 1999–2007 | 201 | Passive | Death certificate | |
| NJ | 1999–2005d | 1999–2006 | 1,253 | Passive | Death certificate | |
| NY | 1999–2007 | 1999–2008 | 1,142 | Passive | Death certificate | |
| TX | 1999–2007 | 1999–2009 | 14,173 | Active | Death certificate, Medical records, hospital discharge files | |
For records with death information beyond the cut-off death years, we treated them as alive.
Active case finding methodology: birth defects cases are actively identified by individuals reviewing birth defects data. Passive case finding methodology: cases are reported by providers/facilities or administrative/other data sets.
CO provided with age at death instead of death year.
NE: 2007 births are excluded because they were not followed for a full year after birth; NJ: 2006–2007 births are excluded because they were not followed for a full year after birth.
Appendix B
Selected Major Birth Defects, ICD-9-CM codes, and CDC/BPA codes.
| Birth defects | ICD-9-CM codes | CDC/BPA codes |
|---|---|---|
| Central nervous system defects | ||
| Spina bifida without anencephalus | 741.0–741.9 w/o 740.0–740.1 | 741.00–741.99 w/o 740.00–740.10 |
| Encephalocele | 742.0 | 742.00–742.09 |
|
| ||
| Congenital heart defects | ||
| Common truncus | 745.0 | 745.00–45.01 |
| Transposition of the great arteries | 745.10, .11, .12, .19 | 745.10, .11, .12, .19 |
| Tetralogy of Fallot | 745.2 | 745.20–745.21, 746.84, 747.31 |
| Atrioventricular septal defect | 745.60, .61, .69 w 758.0 | 745.60–745.69 w 758.00–758.09 |
| Atrioventricular septal defect (occurring without Down | syndrome) 745.60, .61, .69 wo758.0 | 745.60–745.69 wo758.00–758.09 |
| Aortic valve stenosis | 746.3 | 746.30 |
| Hypoplastic left heart syndrome | 746.7 | 746.70 |
| Coarctation of aorta | 747.10 | 747.10–747.19 |
|
| ||
| Oral clefts | ||
| Cleft palate without cleft lip | 749.0 | 749.00–749.09 |
| Cleft lip with or without cleft palate | 749.1, 749.2 | 749.10–749.29 |
|
| ||
| Gastrointestinal defects | ||
| Esophageal atresia/tracheoesophageal fistula | 750.3 | 750.30–750.35 |
| Pyloric stenosis | 750.5 | 750.51 |
| Rectal and large intestinal atresia/stenosis | 751.2 | 751.20–751.24 |
|
| ||
| Musculoskeletal defects | ||
| Upper limb deficiencies | 755.20–755.29 | 755.20–755.29 |
| Lower limb deficiencies | 755.30–755.39 | 755.30–755.39 |
| Diaphragmatic hernia | 756.6 | 756.610–756.617 |
| Gastroschisis | 756.79 | 756.71 |
| Omphalocele | 756.79 | 756.70 |
|
| ||
| Chromosomal defects | ||
| Trisomy 21 (Down syndrome) | 758.0 | 758.00–758.09 |
ICD-9-CM 5 International Classification of Diseases, 9th Revision, Clinical Modifications; CDC/BPA 5 Centers for Disease Control and Prevention/British Pediatric Association Classification.
Footnotes
Clinical Trial Registration: N/A
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Author Contribution: Drs. Lopez and Nembhard conceptualized and designed the study, drafted the initial manuscript, revised the manuscript, and approved the final manuscript as submitted. Mr. Liu carried out the initial statistical analyses and approved the final manuscript as submitted. Drs. Wang, Copeland, Kirby, and Canfield supervised data collection at their respective state departments (where applicable), critically reviewed and revised the manuscript, and approved the final manuscript as submitted. Drs. Kucik and Gilboa critically reviewed and revised the manuscript, and approved the final manuscript as submitted.
References
- Broussard CS, Gilboa SM, Lee KA, Oster M, Petrini JR, Honein MA. Racial/ethnic differences in infant mortality attributable to birth defects by gestational age. Pediatrics. 2012;130(3):e518–e527. doi: 10.1542/peds.2011-3475. https://doi.org/10.1542/peds.2011-3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, editor. U.S Hispanic and Asian populations growing, but for different reasons. Fact Tank - Pew Research Center. n.d. Retrieved from http://www.pewresearch.org/fact-tank/2014/06/26/u-s-hispanic-and-asian-populations-growing-but-for-different-reasons.
- Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, et al. Kirby RS, et al. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999-2001. Birth Defects Research Part A: Clinical and Molecular Teratology. 2006;76(11):747–756. doi: 10.1002/bdra.20294. https://doi.org/10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- Canfield MA, Mai CT, Wang Y, O'halloran A, Marengo LK, Olney RS, et al. National Birth Defects Prevention Network. The association between race/ethnicity and major birth defects in the United States, 1999-2007. American Journal of Public Health. 2014;104(9):e14–e23. doi: 10.2105/AJPH.2014.302098. https://doi.org/10.2105/AJPH.2014.302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects-Atlanta, Georgia, 1978-2005. Morbidity and Mortality Weekly Report. 2008;57(1):1–5. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Racial differences by gestational age in neonatal deaths attributable to congenital heart defects—United States, 2003-2006. Morbidity and Mortality Weekly Report. 2010;59(37):1208–1211. [PubMed] [Google Scholar]
- Cohen BB, Friedman DJ, Mahan CM, Lederman R, Munoz D. Ethnicity, maternal risk, and birth weight among Hispanics in Massachusetts, 1987-89. Public Health Reports (Washington, DC: 1974) 1993;108(3):363–371. [PMC free article] [PubMed] [Google Scholar]
- Egbe A, Uppu S, Stroustrup A, Lee S, Ho D, Srivastava S. Incidences and sociodemographics of specific congenital heart diseases in the United States of America: An evaluation of hospital discharge diagnoses. Pediatric Cardiology. 2014;35(6):975–982. doi: 10.1007/s00246-014-0884-8. https://doi.org/10.1007/s00246-014-0884-8. [DOI] [PubMed] [Google Scholar]
- Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: Final data for 2006. National Vital Statistics Reports. 2009;57(14):1–134. [PubMed] [Google Scholar]
- Kim K, Wang Y, Kirby RS, Druschel CM. Prevalence and trends of selected congenital malformations in New York State, 1983 to 2007. Birth Defects Research Part A: Clinical and Molecular Teratology. 2013;97(10):619–627. doi: 10.1002/bdra.23160. https://doi.org/10.1002/bdra.23160. [DOI] [PubMed] [Google Scholar]
- Kirby RS, Marshall J, Tanner JP, Salemi JL, Feldkamp ML, Marengo L, et al. National Birth Defects Prevention Network. Prevalence and correlates of gastroschisis in 15 states, 1995 to 2005. Obstetrics & Gynecology. 2013;122(2, PART 1):275–281. doi: 10.1097/AOG.0b013e31829cbbb4. https://doi.org/10.1097/AOG.0b013e31829cbbb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucik JE, Cassell CH, Alverson CJ, Donohue P, Tanner JP, Minkovitz CS, et al. Kirby RS, et al. Role of health insurance on the survival of infants with congenital heart defects. American Journal of Public Health. 2014;104(9):e62–e70. doi: 10.2105/AJPH.2014.301969. https://doi.org/10.2105/AJPH.2014.301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RE, Liu G, Gilboa SM, Ethen MK, Aylsworth AS, Powell CM, et al. National Birth Defects Prevention Network. Survival of children with trisomy 13 and trisomy 18: A multi-state population-based study. American Journal of Medical Genetics Part A. 2016;170A(4):825–837. doi: 10.1002/ajmg.a.37495. https://doi.org/10.1002/ajmg.a.37495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motel S, Patten E, editors. The 10 largest Hispanic origin groups: Characteristics, rankings, top counties. Fact Tank -Pew Research Center. n.d. Retrieved from http://www.pewhispanic.org/2012/06/27/the-10-largest-hispanic-origin-groups-characteristics-rankings-top-counties.
- Nembhard WN, Pathak EB, Schocken DD. Racial/ethnic disparities in mortality related to congenital heart defects among children and adults in the United States. Ethnicity & Disease. 2008;18(4):442–449. [PubMed] [Google Scholar]
- Nembhard WN, Salemi JL, Ethen MK, Fixler DE, DiMaggio A, Canfield MA. Racial/ethnic disparities in risk of early childhood mortality among children with congenital heart defects. Pediatrics. 2011;127(5):e1128–e1138. doi: 10.1542/peds.2010-2702. https://doi.org/10.1542/peds.2010-2702. [DOI] [PubMed] [Google Scholar]
- Ramadhani T, Short V, Canfield MA, Waller DK, Correa A, Royle M, et al. National Birth Defects Prevention Study (NBDPS) Are birth defects among Hispanics related to maternal nativity or number of years lived in the United States? Birth Defects Research Part A: Clinical and Molecular Teratology. 2009;85(9):755–763. doi: 10.1002/bdra.20584. https://doi.org/10.1002/bdra.20584. [DOI] [PubMed] [Google Scholar]
- Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko CW, Willinger M. Term Pregnancy. Obstetrics & Gynecology. 2011;117(6):1279–1287. doi: 10.1097/AOG.0b013e3182179e28. https://doi.org/10.1097/AOG.0b013e3182179e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichman NE, Kenney GM. Prenatal care, birth outcomes and newborn hospitalization costs: Patterns among Hispanics in New Jersey. Family Planning Perspectives. 1998;30(4):182–187. 200. [PubMed] [Google Scholar]
- State and County Quickfacts. n.d. Retrieved from http://quickfacts.census.gov/qfd/states/00000.html.
- Utah Department of Health. n.d. Retrieved from http://health.utah.gov/ubdn/defect.html.
- Wang Y, Liu G, Canfield MA, Mai CT, Gilboa SM, Meyer RE, et al. National Birth Defects Prevention Network. Racial/ethnic differences in survival of United States children with birth defects: A population-based study. The Journal of Pediatrics. 2015;166(4):819–826. e1–e2. doi: 10.1016/j.jpeds.2014.12.025. https://doi.org/10.1016/j.jpeds.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Chen H, Correa A, Devine O, Mathews TJ, Honein MA. Racial differences in infant mortality attributable to birth defects in the United States, 1989-2002. Birth Defects Research Part A: Clinical and Molecular Teratology. 2006;76(10):706–713. doi: 10.1002/bdra.20308. https://doi.org/10.1002/bdra.20308. [DOI] [PubMed] [Google Scholar]
- Zong J, Batalova J. Central American immigrants in the United States. Migration Information Source. 2015 Sep; Retrieved from http://scholar.google.com/scholar?q=related:Hy61pwsu1vQJ:scholar.google.com/&hl=en&num=20&as_sdt=0,5.


