Abstract
Purpose
We aimed to estimate the independent effect of pre-hospital frailty (PHF) on hospital mortality and prolonged hospital length of stay (pLOS) while adjusting for other patient level factors.
Methods
This is a cohort study of hospitalized adults with acute respiratory failure (ARF) who required invasive mechanical ventilation for ≥ 24 hours in 2013. We used inpatient/outpatient claims from a list of diagnoses from the year before index hospital admission to define PHF. Differences in characteristics/outcomes by PHF were explored using descriptive statistics; multivariable logistic regression was used to estimate association between PHF and hospital outcomes.
Results
Among 1157 patients (mean age (standard deviation) 67.1 [16.4]), 53.2 % had PHF. PHF was independently associated with higher hospital mortality (44.2% in PHF patients vs. 34.6% in those without, adjusted Odds Ratio (aOR) (95% Confidence Interval [CI] 1.56 (1.19 -2.05), p <0.001). PHF was also significantly associated with pLOS in hospital survivors (55.5% PHF patients had pLOS versus 34.2% in those without, aOR (95% CI) 2.61 (1.87-3.65), p<0.001).
Conclusions
PHF, identified by frailty diagnoses from before index hospitalization, may be a useful approach for identifying adults with ARF at increased risk of hospital mortality and pLOS.
Keywords: frailty, acute respiratory failure, hospital mortality, hospital length-of-stay
Introduction
Acute respiratory failure requiring mechanical ventilation (ARF) is the most common acute organ dysfunction in US hospitals, associated with high hospital mortality and about 12% of all hospital costs [1, 2]. ARF survivors are less likely to be discharged home compared to hospital survivors without ARF and the legacy of ARF survival continues after hospital discharge, with high rates of cognitive and mobility impairments that persist in the months and years that follow [3, 4].
With the aging of the population and the increase in ARF survivors with physical and cognitive impairments, there is an increased recognition that the heterogeneity in patient's pre-hospital health status is helpful in predicting outcomes and characterizing patients' needs [5-8]. Pre-hospital frailty (PHF), a medical syndrome of age or disease-related decline in physical and cognitive reserve, has become an increasingly relevant approach to describing the pre-hospital health status of critically ill adults [9]. Recent research has validated several approaches for screening or identifying adults with PHF prospectively in the Intensive Care Unit (ICU) setting [10-14]. These studies, taken together, suggest that critically ill adults who present acutely ill with evidence of PHF are at increased risk of short-term mortality and morbidity [10, 11, 13, 14].
Previous research suggests that diagnosis codes prior to the index hospitalization can be used to classify patients' pre-hospital health status [8, 15, 16]. Moreover, PHF, identified using diagnosis codes from the year prior to index admission, was associated with higher hospital and 3-year mortality in elderly adults [8]. Whether this approach can be useful in risk-stratifying critically ill adults of all ages has not been explored but is timely and relevant in light of the emerging research highlighting the importance of frailty in younger critically ill adults [12, 17].
Therefore, we aimed to estimate the association between PHF and hospital mortality and prolonged hospital length of stay (pLOS) while adjusting for patient-level factors such as severity of illness and co-morbidities.
Methods
Study Design and Study Setting
This retrospective cohort study included consecutive adult patients admitted to the hospital requiring invasive mechanical ventilation for ≥ 24hrs at two academic hospitals within the Montefiore Health System between January 1st and December 31st, 2013. Montefiore Health System is an academic health system with hospitals and outpatient centers located in Bronx and Westchester, New York. The date and time of invasive mechanical ventilation was abstracted from the hospital data warehouse which serves as a repository for the electronic health records and hospital administrative data.
Defining Pre-Hospital Frailty
Conceptually, we aimed to diagnose PHF as a binary variable using a list of diagnoses that could be extracted from administrative health data. We used Montefiore's healthcare surveillance software Clinical Looking Glass (CLG™ Emerging Health Information Technology, Yonkers, New York) to extract all inpatient and outpatient diagnoses associated with each patient from the year before their index hospital admission with ARF using the International Classification (ICD-9-CM) diagnosis codes [8, 16]. Patients were classified as having PHF if they had at least one claim in the year prior to index hospitalization associated with any of the following diagnoses: dementias or dementia from Alzheimer's disease or senility; sub-acute delirium; cerebrovascular diseases; Parkinson's disease; pathologic fracture; functional urinary and fecal incontinence; dehydration; debility; pressure ulcer; other unspecified protein-calorie malnutrition, kwashiorkor, nutritional marasmus, severe protein calorie malnutrition or abnormal loss of weight or adult failure to thrive; accidental falls or abnormality of gait or lack of coordination (see e-Table 1) [8,16].
Hospital Processes and Outcomes
Data including laboratory (such as total bilirubin, creatinine, platelet count), clinical findings (such as a level of sedation or consciousness measured by the Richmond Agitation and Sedation Scale (RASS) [18], demographic data, interventions and hospital processes and outcomes such as date of admission, date of discharge, discharge status including hospital mortality and discharge to home with/without services were abstracted from the clinical data warehouse and directed to an identity-based encryption server. Additional data on palliative care consultation during the index admission, nursing home residence prior to admission, major comorbid conditions (including Charlson Comorbidity Score) were also extracted from CLG™. The severity of illness on day 1 of invasive mechanical ventilation was quantified using the total Sequential Organ Failure Assessment (SOFA) score which is calculated using physiological and laboratory parameters from six organ systems, each scored from 0 (no organ dysfunction) to 4 (severe organ dysfunction) and then summed for a total score between 0 and 24;[19] RASS was substituted for Glasgow Coma Scale Score[20] and SpO2/FiO2 for PaO2/FiO2 ratios when an arterial blood gas was unavailable [21]. This study was reviewed by the Institutional Review Board of Albert Einstein College of Medicine–Montefiore Medical Center and informed consent was waived.
Statistical Methods
Descriptive statistics including means, frequencies and proportions were used to examine the characteristics, hospital processes and outcomes of the patient sample. The exposure variable of interest was PHF expressed as a binary variable. Hospital mortality and pLOS were the primary outcomes of interest. A priori, we aimed to create a binary variable capturing pLOS that would be a clinically relevant outcome for patients with ARF [22]. After reviewing the distribution of the hospital length of stay in the whole cohort, we defined pLOS as those patients with hospital length-of stay (LOS) within the highest tertile of the whole cohort which was ≥ 20 days [22]. Other secondary outcomes explored included: 1) palliative care specialty consultation during the index hospitalization and 2) being discharged to home with or without services from the index hospitalization for patients admitted to the hospital from home. Multivariable logistic regression was used to determine the independent association between PHF and hospital outcomes after adjusting for other covariates. For our multivariable regression models, certain variables were selected a priori based on prior studies; additional risk factors identified to be associated with the outcome on bivariate analyses (P < 0.05) that were scientifically plausible were also included in the multivariate model. In developing the final models for PHF and hospital outcomes, we used the lowess command to confirm the linear relationship between continuous predictors and the log odds of the dependent variable. Logistic regression model fit was assessed using the Hosmer-Lemeshow test and the discrimination and calibration of the final models were evaluated using sensitivity, specificity and area under the receiver operating characteristic curve [23].
Although we had conceptualized PHF as a binary variable, in sensitivity analyses we explored frailty as a continuous variable using the number of unique frailty diagnoses to create a post-hoc frailty index by assigning increasing scores of 0, 1, 2, 3 based on the number of unique frailty diagnosis claims 0, 1-2, 3-4, ≥5 respectively. In addition, we explored the redundancy and robustness of the frailty classification by exploring the association between type of frailty diagnoses (evidence of pre-hospital cognitive impairment or stroke and evidence of pre-hospital mobility or nutritional impairment) and our outcomes of interest.
All statistical analyses were performed using Stata (Statacorp, College Station, Texas) and a two-sided p-value < 0.05 was used as the threshold for statistical significance.
Results
Of the 1411 patients who had acute respiratory failure requiring invasive mechanical ventilation, 254 were intubated for 1 day, leaving 1157 total patients in the analysis cohort (see table 1). 616 (53.2%) patients were classified as having PHF based on a total of 1157 diagnoses from the year prior to the index hospitalization. 80.5% (n=931) of the PHF claims came from inpatient admissions; 19.5% (n=226) were from the outpatient, ambulatory surgery or emergency department visits. The study population had a mean age (standard deviation) of 67.1 (16.4), with 57.6% older than 65 years old. Patients with PHF were older (mean (standard deviation SD) age 70.8 (14.7) versus 62.9 (17.1), p-value <0.001) although frailty was not limited to older adults (with 40.1% of patients ≤ 60 years old classified as having PHF compared with 65.9% in patients older than 75 years, p< 0.001, see Figure 1). Patients with PHF presented to the hospital with lower average BMI (mean (SD) 27.8 (9.1) versus 29.1 (9.9), p=0.034), were more likely to be admitted from a nursing home (14.8% versus 4.2%, p<0.001). Patients with PHF were also more likely to have higher number of co-morbidities despite similar median and interquartile ranges in their Charlson Co-morbidity scores (73.2% of patients with PHF had ≥ 3 on their Charlson Co-morbidity Score versus 65.1%, p=0.003).
Table 1. showing the baseline characteristics of adult patients with acute respiratory failure requiring more than 1 day of invasive mechanical ventilation.
| Baseline Characteristics | Total N=1157 | Not Frail N=541 | Frail N=616 | P value |
| Age, mean (SD) | 67.1 (16.4) | 62.9 (17.1) | 70.8 (14.7) | <0.001 |
| Male Gender, n (%) | 520 (44.9) | 243 (44.9) | 277 (45.0) | 0.986 |
| Race, n (%) | 0.091 | |||
| White | 292 (25.2) | 135 (25.0) | 157 (25.5) | |
| Black/African American | 376 (32.5) | 160 (29.6) | 216 (35.1) | |
| Alaska/Pacific/Asian | 29 (2.5) | 14 (2.6) | 15 (2.4) | |
| Other/Unknown | 460 (39.8) | 232 (42.9) | 228 (37.0) | |
| Hispanic Ethnicity, n (%) | 421 (36.4) | 212 (39.2) | 209 (33.9) | 0.147 |
| BMI, mean (SD) | 28.4 (9.3) | 29.1 (9.9) | 27.8 (9.1) | 0.034 |
| Admitted from nursing home, n (%) | 114 (9.9) | 23 (4.3) | 91 (14.8) | <0.001 |
| SOFA Score, median (IQR) | 8 (6-12) | 9 (7-12) | 8 (5-11) | <0.001 |
| Charlson Co-Morbidity Score, median (IQR) | 4 (2-6) | 4 (2-6) | 4 (2-6) | <0.001 |
Abbreviations: SD – Standard Deviation; SOFA – Sequential Organ Failure Assessment; IQR – Interquartile Range.
Figure 1.
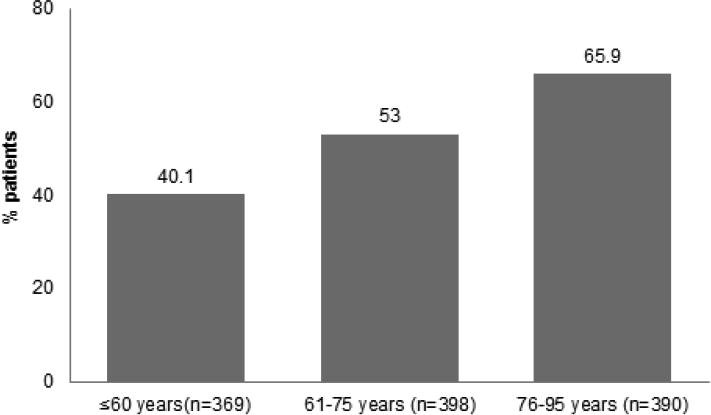
Shows the percent of the study population classified as frail within each age tertile.
Pre-Hospital Frailty and Hospital Outcomes
Hospital Mortality
459/1157 (39.7%) patients died before discharge from the hospital. Patients with PHF were more likely to die (44.2% mortality in those classified as frail versus 34.6% in those without frailty, p=0.001, see Table 2). After adjusting for other predictors of hospital mortality, PHF was independently associated with an increased risk of dying in the hospital (adjusted Odds Ratio (aOR) (95% Confidence Interval (95% CI), 1.56 (1.19-2.05), p<0.001 see Table 3). Other predictors of increased hospital mortality in the study sample included older age, higher day 1 SOFA scores, higher Charlson co-morbidity scores and a BMI < 18.5 kg/m2 (see table 3).
Table 2. Clinical Processes and Outcomes by Pre-Hospital Frailty Status.
| Total (N=1157) | Not Frail (N=541) | Frail (N=616) | p-value | |
|---|---|---|---|---|
| Days on mechanical ventilator, median (IQR) | 5 (2-13) | 4 (2-9) | 7.5 (3-18) | <0.001 |
| Days on mechanical ventilator for hospital survivors†, median (IQR) | 5 (2-14) | 3 (2-8) | 10 (3-23.5) | <0.001 |
| Days on mechanical ventilator for hospital deaths§, median (IQR) | 6 (2-13) | 6 (2-10) | 6 (2-15) | 0.113 |
| Hospital LOS, median days (IQR) | 15 (9-26) | 13 (8-23) | 17 (10-30) | <0.001 |
| Hospital LOS in hospital survivors, median days (IQR)† | 17 (11-30) | 14 (9-24) | 21.5 (14-35) | <0.001 |
| Hospital LOS in hospital deaths, median days (IQR)§ | 12 (6-22) | 11 (7-19) | 12 (6-23) | 0.349 |
| Prolonged Hospital LOS, n (%) | 468 (33.2) | 180 (24.7) | 288 (42.2) | <0.001 |
| Palliative Care Consultation, n (%) | 390 (35.2) | 124 (24.2) | 266 (44.6) | <0.001 |
| Discharge Status | ||||
| Discharge to Home w/without services, n (%) | 257 (22.2) | 198 (38.2) | 58 (11.1) | <0.001 |
| Discharge to Hospice, n (%) | 29 (2.5) | 13 (2.4) | 16 (2.6) | 0.833 |
| Died in Hospital, n (%) | 459 (39.7) | 187 (34.6) | 272 (44.2) | 0.001 |
Abbreviations: IQR - Interquartile Range; LOS - Length of Stay;
from a total N=698 hospital survivors;
from a total N=459 hospital deaths.
Table 3. Multivariable predictor models showing the independent predictors of hospital mortality.
| Predictors | Adjusted OR (95% CI) | P-value |
|---|---|---|
| Pre-Hospital Frailty | 1.56 (1.19–2.05) | 0.001 |
| Age, per 1 year increase | 1.04 (1.03-1.05) | <0.001 |
| SOFA Score, per unit increase | 1.15 (1.11-1.19) | <0.001 |
| Charlson Co-morbidity Score, per unit | 1.08 (1.04-1.13) | <0.001 |
| increase | ||
| Body Mass Index < 18kg/m2 | 2.33 (1.35 – 4.01) | 0.002 |
| Admitted from a nursing home | 0.14 (0.07- 0.28) | <0.001 |
Abbreviations: OR – Odds Ratio; 95% CI – 95% Confidence Interval; SOFA – Sequential Organ Failure Assessment
Prolonged Hospital Length-of-Stay
Patients with PHF spent more days in the hospital compared with those without frailty (median (IQR) days in hospital 17 (10-30) for frail patients versus 13 (8-23) for those without frailty, p <0.001) and were more likely to have a prolonged LOS (42.2% of frail patients had prolonged LOS versus 24.7% of patients without frailty p<0.001 see Table 2). As shown in table 4, PHF was independently associated with prolonged LOS in hospital survivors (aOR (95% CI) 2.61 (1.87 – 3.65) p<0.001) as well as in those who died in the hospital (aOR (95% CI) 1.82 1.17-2.95), p=0.009).
Table 4. Logistic regression models showing the independent predictors of prolonged hospital length of stay.
| Hospital Survivors (n=698) | Hospital Deaths (n=459) | |||
|---|---|---|---|---|
| Predictors | Adjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value |
| Pre-Hospital Frailty | 2.61 (1.87–3.65) | <0.001 | 1.82(1.17-2.85) | 0.009 |
| Age, per 1 year increase | 1.00(1.00-1.02) | 0.321 | 0.98(0.97-1.00) | 0.016 |
| SOFA score, per unit increase | 1.11 (1.06-1.17) | <0.001 | 1.04(0.99–1.09) | 0.139 |
| Charlson Co-morbidity Score | 1.05(0.99-1.11) | 0.132 | 1.06(1.00-1.13) | 0.068 |
Abbreviations: OR – Odds Ratio; 95% CI – 95 percent Confidence Interval; SOFA – Sequential Organ Failure.
Other Secondary Outcomes
Patients with PHF also spent more days on mechanical ventilation than those without PHF (median (IQR) 7.5 (3-18) in frail patients versus 4 (2-9) in those without frailty, p<0.001) and were more likely to have a palliative care consultation during the index hospital admission (44.6% in frail patients versus 24.2%, p<0.001). 256/1043 (24.5%) of the patients who were admitted from home were discharged to home with or without services. Patients admitted from home with PHF were less likely to be discharged home from the hospital (11.1% versus 38.2% in those not classified as frail, p<0.001). After adjusting for other independent predictors of being discharged to home, PHF was independently associated with a lower odds of being discharged home after the index admission in the patients who were admitted from home (aOR (95% CI) 0.23 (0.16-0.33), p<0.001).
Sensitivity Analyses
Patients with PHF had a median (IQR) of 2 (1-3) unique frailty diagnoses in the year prior to index admission. Our post-hoc frailty severity index was independently associated with hospital mortality (aOR (95% CI) 1.18 (1.00-1.38), p=0.046) and with pLOS (aOR (95% CI) 1.65 (1.37-2.06) in hospital survivors, p<0.001) (see e-Table 2). When we tested the relationship between two frailty sub-categories of diagnoses and our outcomes, we found that the frailty diagnoses related to cognitive or neurologic impairments were significantly associated with increased hospital mortality (aOR 1.62 (1.22-2.15), p=0.001) while both sub-categories of frailty diagnoses were significantly associated with pLOS (see e-Table 2).
Discussion
Summary of Key Findings
In an urban population of adults with ARF, although PHF was more common in older adults, 40% of adults 60 years or younger were classified as frail using diagnosis codes from the year prior to hospital admission. Importantly, we show that PHF was associated with 1.5x the odds of dying in the hospital and more than 2.5x the odds of surviving after a pLOS and that these effects were independent to other patient-level confounders such as age, prior comorbidities and severity of illness. The effect estimates we have found between PHF and hospital outcomes are consistent with previous research in acutely ill adults [10-14] and our results suggest that health systems may be able to use a diagnosis-based approach to risk stratify acutely ill patients, many of whom will be unable to be identified as having PHF using other performance or judgment-based approaches.
Relationship to Previous Findings
Although previous studies have used diagnosis codes to clarify the population-level association between frailty and adverse outcomes in large administrative datasets, this approach often precludes careful adjustment for patient level factors such as co-morbidity and severity of illness scores [8, 15, 16, 24]. We previously used a similar approach to diagnose preICU frailty in an elderly Medicare patient population but could not fully adjust for co-morbidity and severity of illness scores [8]. McIsaac et al. used a similar list of frailty defining diagnostic codes to identify frailty in a cohort of older adults admitted for elective surgery and found that frailty was significantly associated with an increase risk of 1-year mortality [24]. In their post-hoc analyses, the frailty effect was attenuated after adjusting for all co-morbidities but remained statistically significant [24]. This current study builds and extends the research on the use of frailty diagnosis codes to identify PHF by showing that this objective approach has potential utility and validity at the level of the health system for identifying patients with ARF who are at high risk of hospital mortality and prolonged LOS.
The strong association found between PHF and adverse hospital outcomes in this cohort is consistent with the emerging literature from multiple studies showing that PHF is relevant to short-term outcomes in elderly and non-elderly patients with critical illness [9]. Bagshaw et al., in their landmark prospective cohort study of critically ill adults > 50 years old, found a similar effect estimate for frailty and hospital mortality that we have shown in this population using a judgment based approach to identifying frailty [10]. In a follow-up study by Bagshaw et al. that assessed the effect of frailty in a cohort of younger critically ill adults (age between 50-64.5 years), frailty was not significantly associated with hospital mortality but was significantly associated with 1-year mortality [12]. Similarly, Brummel et al. in a cohort of critically ill adults (median (IQR) age of 62 (53-72) found that frailty was not significantly associated with hospital mortality but was significantly associated with 3- and 12- month mortality [17]. Our cohort is different from previous prospective cohort studies in that we included all adults ≥ 18 years old who needed invasive mechanical ventilation for at least one day in the hospital and although we could not assess long-term mortality in these analyses, the hospital mortality in our cohort was higher than in other cohort studies that included non-elderly adults which increased our ability to show an effect between frailty and hospital mortality.
Implications of Study Findings
How to integrate frailty identification into the care of patients with critical illness is still an open question. In particular, approaches such as the one used in this study has the advantage of being objective, easy to obtain from the medical record and can be useful for health care administrators planning for healthcare of large groups of patients. If replicated in future studies, identifying PHF using diagnosis codes might be a useful screening approach in health systems with electronic health records where the majority of the critically ill adults will have had the majority of their outpatient/inpatient care within the same health system. Frailty identification may facilitate 1) modifications in communication or values facilitation and 2) interventions such as mobility and/or cognitive rehabilitation efforts to improve long-term outcomes in frail survivors. If, as previous studies suggest, survivors with pLOS are indeed at higher risk of long-term complications, frail survivors after pLOS may be a useful sub-group of adult patients in which to focus on innovative approaches to improve long-term outcomes. These results may also suggest a need for health systems to develop approaches to help support clinicians taking care of frail patients with prolonged LOS in order to decrease the impact of moral distress and nihilism on patient outcomes [25].
Strengths and Limitations
The study has several strengths and limitations. We used administrative data and were able to capture mortality and LOS data completely in our cohort. We defined PHF using a diagnosis-based approach that has been used previously though this approach may not be generalizable to all health systems. Although we used data from a single health system, our sample size is large and we were able to adjust for patient-level factors. Our approach to frailty identification is different from the phenotypic approach in which frailty is identified by the presence of a critical mass of biologic markers of frailty. We used a binary definition of frailty that did not account for the impact of the increasing levels of frailty although, in sensitivity analyses, we did explore frailty as a continuous variable. In addition, our results cannot distinguish the biologic effect of frailty from the effects of ageism and other heuristic biases already present in the clinical care of critically ill adults.
Conclusion
Pre-hospital frailty, identified using a list of frailty diagnoses from the year prior to index admission, is strongly associated with an increased risk of hospital mortality and prolonged hospital length of stay in adults with acute respiratory failure requiring invasive mechanical ventilation. Such a diagnosis-based approach to identifying pre-hospital frailty potentially allows health systems to use their administrative data to improve the risk stratification and care of a broader population of acutely ill patients than is feasible with other approaches to frailty identification.
Supplementary Material
Highlights.
Acute respiratory failure requiring mechanical ventilation is the most common acute organ dysfunction in US hospitals, associated with a high risk of morbidity and mortality.
Pre-hospital frailty, a medical syndrome of age or disease-related decline in physical and cognitive reserve, has been shown to be an important predictor of short-term outcomes in acutely ill elderly adults but has not been explored in adults with acute respiratory failure.
In a cohort of 1157 adults with acute respiratory failure, we found that about 53% had diagnoses in the year prior to the index hospitalization consistent with pre-hospital frailty.
Pre-hospital frailty was associated with 1.5x the odds of dying in the hospital and more than 2.5x the odds of surviving after a prolonged hospital length-of-stay; these effects were independent to other patient-level confounders such as age, prior co-morbidities and severity of illness.
Our findings suggest that health systems may be able to use a diagnosis-based approach to systematically risk stratify acutely ill patients.
Acknowledgments
Role of the Sponsors: none
Abbreviations List
- PHF
Pre-Hospital Frailty
- pLOS
Prolonged hospital length-of-stay
- aOR
adjusted odds ratio
- ARF
acute respiratory failure
- ICU
Intensive Care Unit
- SOFA
Sequential Organ Failure Assessment
Footnotes
Conflicts of Interest statements: none of the authors have any conflicts to report
Funding Information: National Institute of Aging: R03 AG050927 (Hope); National Heart, Lung and Blood Institute: U01 HL122998 and UH3 HL125119 (MNG);
Author Contributions: The study was conceived and designed by AAH and MNG; MNG, AAH and EHC performed the data collection; all authors were involved in the data analysis; AAH and OA prepared the first draft of the manuscript under the guidance of MNG. All authors assisted with data interpretation and preparation of subsequent versions of the manuscript. AAH was the Principal Investigator of the study and is responsible for the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 2.Cartin-Ceba R, Kojicic M, Li G, Kor DJ, Poulose J, Herasevich V, Kashyap R, Trillo-Alvarez C, Cabello-Garza J, Hubmayr R, et al. Epidemiology of critical care syndromes, organ failures, and life-support interventions in a suburban US community. Chest. 2011;140:1447–1455. doi: 10.1378/chest.11-1197. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, Orme JF., Jr Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171:340–347. doi: 10.1164/rccm.200406-763OC. [DOI] [PubMed] [Google Scholar]
- 4.Barnato AE, Albert SM, Angus DC, Lave JR, Degenholtz HB. Disability among elderly survivors of mechanical ventilation. Am J Respir Crit Care Med. 2011;183:1037–1042. doi: 10.1164/rccm.201002-0301OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185:835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors Associated with Functional Recovery Among Older ICU Survivors. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hope AA, Gong MN, Guerra C, Wunsch H. Frailty Before Critical Illness and Mortality for Elderly Medicare Beneficiaries. J Am Geriatr Soc. 2015;63:1121–1128. doi: 10.1111/jgs.13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, Sibley S, Rockwood K. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017 doi: 10.1007/s00134-017-4867-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, Artiuch B, Ibrahim Q, Stollery DE, Rokosh E, Majumdar SR. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E95–102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagshaw SM, Stelfox HT, Johnson JA, McDermid RC, Rolfson DB, Tsuyuki RT, Ibrahim Q, Majumdar SR. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med. 2015;43:973–982. doi: 10.1097/CCM.0000000000000860. [DOI] [PubMed] [Google Scholar]
- 12.Bagshaw M, Majumdar SR, Rolfson DB, Ibrahim Q, McDermid RC, Stelfox HT. A prospective multicenter cohort study of frailty in younger critically ill patients. Crit Care. 2016;20:175. doi: 10.1186/s13054-016-1338-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hope AA, Hsieh SJ, Petti A, Hurtado-Sbordoni M, Verghese J, Gong MN. Assessing the Utility and Validity of Frailty Markers in Critically Ill Adults. Ann Am Thorac Soc. 2017;14:952–959. doi: 10.1513/AnnalsATS.201607-538OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Maguet P, Roquilly A, Lasocki S, Asehnoune K, Carise E, Saint Martin M, Mimoz O, Le Gac G, Somme D, Cattenoz C, et al. Prevalence and impact of frailty on mortality in elderly ICU patients: a prospective, multicenter, observational study. Intensive Care Med. 2014;40:674–682. doi: 10.1007/s00134-014-3253-4. [DOI] [PubMed] [Google Scholar]
- 15.Lunney JR, Lynn J, Foley DJ, Lipson S, Guralnik JM. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–2392. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 16.Lunney JR, Lynn J, Hogan C. Profiles of older medicare decedents. J Am Geriatr Soc. 2002;50:1108–1112. doi: 10.1046/j.1532-5415.2002.50268.x. [DOI] [PubMed] [Google Scholar]
- 17.Brummel NE, Bell SP, Girard TD, Pandharipande PP, Jackson JC, Morandi A, Thompson JL, Chandrasekhar R, Bernard GR, Dittus RS, et al. Frailty and Subsequent Disability and Mortality Among Patients With Critical Illness. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ely EW, Truman B, Shintani A, Thomason JW, Wheeler AP, Gordon S, Francis J, Speroff T, Gautam S, Margolin R, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289:2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Vasilevskis EE, Pandharipande PP, Graves AJ, Shintani A, Tsuruta R, Ely EW, Girard TD. Validity of a Modified Sequential Organ Failure Assessment Score Using the Richmond Agitation-Sedation Scale. Crit Care Med. 2016;44:138–146. doi: 10.1097/CCM.0000000000001375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crit Care Med 2006. Vol. 34. Orlando, Florida, USA: Feb 17-21, 2007. Abstracts of the 36th Critical Care Congress; pp. A1–176. [PubMed] [Google Scholar]
- 22.Nguyen TN, Cumming RG, Hilmer SN. The Impact of Frailty on Mortality, Length of Stay and Re-hospitalisation in Older Patients with Atrial Fibrillation. Heart Lung Circ. 2016;25:551–557. doi: 10.1016/j.hlc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Hosmer D, Lemeshow S. Applied Logistic Regression. New York, U.S.A: John Wiley & Sons, Inc.; 2000. [Google Scholar]
- 24.McIsaac DI, Bryson GL, van Walraven C. Association of Frailty and 1-Year Postoperative Mortality Following Major Elective Noncardiac Surgery: A Population-Based Cohort Study. JAMA Surg. 2016;151:538–545. doi: 10.1001/jamasurg.2015.5085. [DOI] [PubMed] [Google Scholar]
- 25.Henrich NJ, Dodek PM, Gladstone E, Alden L, Keenan SP, Reynolds S, Rodney P. Consequences of Moral Distress in the Intensive Care Unit: A Qualitative Study. Am J Crit Care. 2017;26:e48–e57. doi: 10.4037/ajcc2017786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


