Abstract
Traumatic brain injury (TBI) affects 5.3 million people in the United States, and there are 12,500 new cases of spinal cord injury (SCI) every year. There is yet a significant need for in vitro models of TBI and SCI in order to understand the biological mechanisms underlying central nervous system (CNS) injury and to identify and test therapeutics to aid in recovery from neuronal injuries. While TBI or SCI studies have been aided with traditional in vivo and in vitro models, the innate limitations in specificity of injury, isolation of neuronal regions, and reproducibility of these models can decrease their usefulness in examining the neurobiology of injury. Microfluidic devices provide several advantages over traditional methods by allowing researchers to 1) examine the effect of injury on specific neural components, 2) fluidically isolate neuronal regions to examine specific effects on subcellular components, and 3) reproducibly create a variety of injuries to model TBI and SCI. These microfluidic devices are adaptable for modeling a wide range of injuries, and in this review, we will examine different methodologies and models recently utilized to examine neuronal injury. Specifically, we will examine vacuum-assisted axotomy, physical injury, chemical injury, and laser-based axotomy. Finally, we will discuss the benefits and downsides to each type of injury model and discuss how researchers can use these parameters to pick a particular microfluidic device to model CNS injury.
Keywords: Microfluidics, neuronal culture, chemical neuronal injury, physical neuronal injury, vacuum assisted neuronal injury, laser based neuronal injury, traumatic brain injury, axotomy, spinal cord injury
Introduction
Traumatic brain injury (TBI) affects 5.3 million people in the United States, and there are 12,500 new cases of spinal cord injury (SCI) every year (Center 2015; Langlois and Sattin 2005). During and after TBI and SCI, neurons and neuronal tissue undergo broad structural and biochemical changes. These changes can range from axonal degeneration such as Wallerian, retrograde, or anterograde degeneration, to biochemical changes such as mitochondrial dysfunction, excitotoxicity, and others (Cheriyan et al. 2014; Morrison et al. 2011; Rishal and Fainzilber 2014). Understanding these structural and biochemical responses is critical to the development of new therapeutics. Therefore, there is a tremendous need to 1) model TBI and SCI to understand the biological mechanisms underlying central nervous system (CNS) injury and 2) find and test therapeutics for these neuronal injuries. Various in vitro and in vivo models have been used to mimic these injuries to both investigate the biological response to injury and to examine potential treatments for these conditions (Cheriyan et al. 2014; Xiong et al. 2013). While in vivo models such as weight drop, fluid percussion, or blast injury allow for more accurate simulations of either TBI or SCI (Cheriyan et al. 2014; Xiong et al. 2013), in vitro models such as glutamate excitotoxicity, allow researchers to examine how secondary injury resulting from TBI or SCI can affect individual neurons and other neural cell types (Benam et al. 2015). In addition, in vitro models of TBI were found by Morrison et. Al. to be predictive of 88% of results in vivo highlighting the importance of in vitro injury models (Morrison et al. 2011).
Although TBI or SCI can be mimicked by these in vivo and in vitro models, innate limitations can decrease their usefulness in examining the neurobiology of injury. For example, in vivo models can be resource intensive and are more variable in the extent of injury while traditional in vitro models are limited by the types of injury that can be applied. In addition, in both of these models, it is difficult to specifically examine the effects of injury localized to specific subcellular regions, such as dendrites and axons. Furthermore, there are significant differences in protein expression in neuronal soma versus axon and these differences may impact injury or disease (Rishal and Fainzilber 2014). Therefore, incorporating the ability to segregate neuritic subcellular components (i.e. axons from soma) in either in vivo or in vitro models of injury has long been sought after for many decades to understand the biological mechanisms that underlie neuronal injury or to discover potential treatments.
Microfluidics is an adaptive tool and explored beyond the patterning of neurons in studies of neuroscience (Shrirao et al. 2014; Shrirao et al. 2017). One of the first models to successfully separate the axon from the soma in vitro was created by Campenot in 1977 (Campenot 1977). These were simple devices consisting of a Teflon ring coated with silicone grease placed on top of a scratched cell culture surface. The scratches allowed neurites to burrow through the grease layer and to extend into the outer region free of somal contamination (Figure 1). Jeon and colleagues subsequently improved upon this initial design by incorporating microfluidic channels allowing for more precise control in the separation of neurites and soma (Taylor et al. 2005; Taylor et al. 2003).
Figure 1.

Microfluidic Neuronal culture devices. (A) Original Campenot chamber design. Neurites grow through scratches into adjacent chambers (Campenot 1977). (B) Improvement to Campenot device using microfluidics (Taylor et al. 2005). Precise microfluidic channels allow for consistent and reproducible neurite isolation and fluidic isolation of the separate chambers.
The development of these microfluidic devices allowed for specific isolation of subcellular components (e.g. neuronal soma, proximal and distal axons) to investigate neuronal injury, and enabled the examination of specific sites of CNS or PNS injury with higher specificity and ease compared to previous methods (Campenot 1977; Taylor et al. 2005). However, microfluidic device creation requires close collaboration between biologists and engineers. For example, neurobiologists must carefully communicate their needs to microfabrication engineers in order to fabricate microfluidic devices that enhance modeling of neuronal injury. These devices must accommodate an appropriate method of injuring neurons, for example chemical injuries, stretch strain, axotomy, or other forms of injury, either in vitro or in vivo.
When considering in vitro studies to examine injury to the CNS or PNS, it is imperative to choose an injury method that is pertinent to the biological phenomena being studied. In this review, we will examine different methodologies and models utilizing microfluidic devices which were recently developed to examine neuronal injury or diseases. The review focuses four microfluidic models of neuronal injury: vacuum-assisted axotomy, physical injury, chemical injury, and laser-based axotomy. In each section, we will describe the basic mechanisms of the injury method, the motivation behind choosing a particular type of injury method, and review findings resulting from the use of these microfluidic devices/injury models discussed in Table 1, 2 and 3. Microfluidic models and approaches cited in this review emphasized the relevance of their findings with respect to conventional in vivo models in their publications (Table 1). Finally, we will highlight the benefits and downsides of each type of microfluidic injury model and discuss how these parameters can be used for the selection of a particular microfluidic device to model neuronal injury.
Table 1.
Microfluidic multi-compartment platforms used to apply axonal injury and treatment. Neurons are cultured in somal compartments. Axons of neurons culture in the somal compartment grow through interconnected microchannel and enter neighboring axonal compartment. Interconnected microchannel cross sections are smaller than the diameter of neuronal somas to prevent cell bodies from entering the microchannel yet still large enough to allow axonal growth. This design also ensures fluidic isolation between somal and axonal microchannel compartment. Length of microchannels varies from 100 μm to 2 mm. Side compartments are over-pressurized with medium to prevent the reagents from leaking into microchannels. Dolle et al. used open top two compartments. Hosmane et al. 2012 used axonal compartment to co-culture glial cells as a treatment. Magdesian et al. removed PDMS device to allow axons to make neighboring synaptic connections. Deleglise et al. used asymmetric microchannels to connect central and axonal compartment in order to prevent axon growth in the direction of axonal to central compartment.
| Microfluidic device | Injury Mechanism | References |
|---|---|---|
| Two microchannel compartments (somal and axonal/somal/co-culture) interconnected with microchannels. Figure 2A | Vacuum aspiration, chemical injury, and physical strain/stress Injury. | (Charier et al. 2015; Dolle et al. 2013; Hosmane et al. 2012; Magdesian et al. 2012; Park et al. 2006; Sauer et al. 2013; Taylor et al. 2005; Yap et al. 2014) |
| Three microchannel compartments (somal, axonal, and axonal (Figure 2) or somal, central/injury, and axonal (Figure 4)) interconnected with microchannels. | (Deleglise et al. 2013; Fournier et al. 2014; Holland et al. 2016; Hosmane et al. 2011; Kilinc et al. 2011) | |
| Four microchannel compartments (glial, axonal, somal and glial) interconnected with microchannels. Pneumatic microvalves used to control the injection of reagents in and between the compartments. (Figure 6) | Chemical injury. | (Li et al. 2012) |
| Five microchannel compartments (each act as somal and axonal) interconnected with microchannels. Neurons were cultured in each (somal/axonal) compartment. Spreads of chemical injury introduced in central compartment through neuronal transmission is evaluated. Figure 5A | (Samson et al. 2016). | |
| Three layer device with seven microchannel compartments (somal in the center and six axonal surrounding or three on each side with inlet and outlet). (Figure 5B) | (Kim et al. 2016; Park et al. 2014) |
Table 2.
List of studies that used microfluidic vacuum assisted and physical strain/stress injury. Vacuum aspiration in axonal compartmentindicates studies using vacuum aspiration and other studies utilizes physical strain/stress injury.
| Aim of study (Neuronal cells used) | Injury Approach | References |
|---|---|---|
| Develop compartmentalize neuronal culture device to isolate soma and axons in modeling CNS injury and neurodegeneration. (Embryonic hippocampal and cortical neurons of rat) | Vacuum aspiration in axonal compartment. | (Park et al. 2006) |
| Isolation of axons without somata or dendrites to analyze pure axonal fractions and apply local physical and chemical treatments to axons or somata. (Oligodendrocytes of postnatal rat) | Vacuum aspiration in axonal compartment. | (Taylor et al. 2005) |
| Determine the importance of palmitoyl-DLK for retrograde injury signaling. (Dorsal root ganglia embryonic sensory neurons of rat) | Vacuum aspiration in axonal compartment. | (Holland et al. 2016) |
| Examine the effect of mechanical loads on cytoskeletal degeneration, neurofilament and microtubule structure of axons. (Hippocampal embryonic neurons of rat) | Pneumatic deflection of a PDMS injury pad in the central compartment to apply compressive injury onto axons. | (Fournier et al. 2014; Hosmane et al. 2011) |
| Investigate the effect of a very mild to mild stretch injury on distal axonal degeneration is studied. (Embryonic cortical neurons of rat) | Pneumatic deflection of a cavity beneath the axonal microchannels | (Yap et al. 2014) |
| Study the role of axon diameter and mitochondrial membrane potential changes in response to axonal strain injuries. (Organotypic hippocampal brain slices of rat) | Pneumatic deflection of a cavity beneath the axonal microchannels | (Dolle et al. 2013) |
| Investigate the force required to uncouple axonal transport without impairing axonal survival and compromise axonal survival. (Hippocampal and DRG embryonic neurons of rat) | Force applied using tip of atomic force microscope to repairable and irreparable axonal injury. | (Magdesian et al. 2012) |
Table 3.
List of studies that used microfluidic devices to apply a chemical injury and drug/biomolecule or co-culture treatment after injury.
| Aim of study (Neuronal cells used) | Injury Approach | References |
|---|---|---|
| Examination of the potential protective effects of memantine + vitamin D against lysed or clotted blood in cortical neuronal cultures. (Embryonic cerebral cortices neurons of mouse) | Somal compartment treated with media cultured with blood clot, memantine and vitamin D | (Charier et al. 2015). |
| Investigation of microglial-axon interactions and role of TRIF in mediating microglial clearance of degenerating axons. (Embryonic hippocampal rat neurons and cortices of rat or mice) | Somal compartment treated with diethylamineNONOate (DEA NONOate) | (Hosmane et al. 2012) |
| Direct axonal injury using antigen-specific CD8+ lymphocytes through a MHC-I- and GzB-dependent mechanism. (Embryonic cortical (gang enriched) neurons of mouse) | Somal compartment treated with Interferon gamma (IFNγ), SIINFEKL, and RAHYNIVTF peptides. | (Sauer et al. 2013). |
| The role of axotomy and treatment of cortical fibers in triggering a rapid presynaptic disconnection from striatal dendrites. (Embryonic cortices and striatal neurons of mice) | Detergent injected in central and drug treatment applied on neurons in left and right compartment. | (Deleglise et al. 2013) |
| Investigated effect of localized extracellular matrix components and brain-derived neurotropic factor treatments on axon growth. (Embryonic CNS neurons of rat forebrains) | CSPG injected in axonal compartment inlet for injury. | (Kim et al. 2016; Park et al. 2014) |
| Investigated neurodegeneration molecular pathways using axotomy and independent treatment of distal and proximal axons. (Embryonic cortices of Mouse) | Detergent (0.1% saponin) injected in central and treatment applied in somal and axonal compartment. | (Kilinc et al. 2011). |
| Study of neuronal degeneration and regeneration after acrylamide stimulation and monosialoganglioside or cocultured treatment. (Hippocampal neurons, astrocytes, and Schwann cells of rat) | Acrylamide injected in somal/axonal and astrocytes and Schwann cells co-culture in glial compartment. | (Li et al. 2012) |
| Replicate secondary spreading toxicity and study the role of GluN2A and GluN2B receptors in its spread. (Hippocampal neurons of rat) | Excitotoxic and sub-lethal concentrations of glutamate were injected in the central compartment. | (Samson et al. 2016). |
Vacuum-assisted neuronal injury
The microfluidic vacuum aspiration induced axonal injury technique is by far the most widely used microfluidic injury model due to 1) its simplicity and compatibility with biological analytical techniques and 2) the availability of a variety of microfluidic devices with different channel lengths, tunnel widths, and devices with reversible bonding. This compatibility allows for the utilization of vacuum aspiration in conjunction with techniques such as immunofluorescent staining (Taylor et al. 2003), transcriptional analysis (Taylor et al. 2009) and electrophysiology (Nakatomi et al. 2002).
Jeon and colleagues (Park et al. 2006; Taylor et al. 2005) used their microfluidic device (Table 1) and laboratory vacuum to injure axons (Figure 2). Once axonal growth reached the adjacent compartment, vacuum aspiration with a pasteur pipette was briefly applied to the inlet of the second chamber. This vacuum created an air bubble which sheared only the axons in the second compartment. As such, this device allows injury of axons without affecting somas, which is impossible with traditional non-microfluidic methods.
Figure 2.

Microfluidic device for axonal injury by vacuum aspiration. (A) Left: Typical protocol for vacuum-assisted axotomy (Kim et al. 2012). Top Right: Phase contrast images of neurons grown in microfluidic device before and after application of vacuum-assisted axotomy. Neuronal soma are visibly undisturbed after injury (Park et al. 2006). (B) Modified device to apply vacuum injury with neurons expressing GFP after appropriate lentiviral infection with cell bodies in the center and axonal growth guided through microgrooves in side compartments. (C) Vacuum aspiration injury in the right chamber triggered retrograde signals that induced robust c-Jun phosphorylation. Microfluidic cultures were immunostained to detect phospho-cJun (pcJun) and Neurofilament. The retrograde signaling is tracked from uninjured and injured axon chambers on either side to the central cell body chamber(Holland et al. 2016).
Following injury, real-time polymerase chain reaction of the axonal compartment did not have detectable somal contamination (Park et al. 2006). This injury approach has subsequently been utilized to screen potential palliatives for axonal regeneration (Kim et al. 2012; Lezana et al. 2016; Taylor et al. 2005). Treatment of vacuum-assisted axotomized neurons with brain-derived neurotrophic factor and neurotrophin-3 dramatically increased axonal branching, growth, and growth cone formation, suggesting the potential utility of neurotrophins in neuronal regeneration (Taylor et al. 2005). Conversely, the peptide NOGO-66 inhibited axonal regeneration (Kim et al. 2012). Recently, vacuum-assisted axotomy was used to investigate the role of peroxisome proliferator-activated receptor gamma in axonal regeneration, and the results suggest that activating this pathway increases axonal regeneration after injury (Lezana et al. 2016). Microfluidics simplified the experiment and analysis of neuronal regeneration compared to tradition non-microfluidic approaches.
Using a similar two chambered microfluidic system, Taylor et. Al performed microarray analysis specifically on cortical axons without somal contamination. The analysis revealed an upregulation of transcripts for cytoskeletal and intracellular transport following vacuum-assisted axotomy further demonstrating isolation of axonal mRNA or other biomolecules following injury with microfluidic devices. (Taylor et al. 2009). Microfluidic and vacuum-based injury mechanisms can also be used to model and characterize acute axonal degeneration (AAD). Using this method, Zhang and colleagues identified calpain and its target, collapsin response mediator protein-2 (CRMP2), as a regulator of AAD in vitro (Zhang et al. 2016). Furthermore, the spatial organization of axons inside the microfluidic channels facilitated the examination of mitochondrial transport (using an AAV.mito-RFP) in axons. Calpain inhibition resulted in an increase in CRMP2 expression, a delay in bulb formation at the site of axotomy, and rescue of mitochondrial transport after AAD.
The versatility of microfluidic based vacuum-assisted axotomy allows the modelling of intrinsic synaptic remodeling following long range axotomy (Nagendran et al. 2016). Using microgrooves 900 μm in length, Nakatomi et. Al examined the effect of axotomy on synaptic remodeling similar to in vivo conditions where long projection neurons are affected far from the site of CNS injury (Nakatomi et al. 2002). By first applying the injury and then subsequently removing the polydimethylsiloxane (PDMS) microfluidic device, Nakatomi et Al. electrophysiologically probed neurons following a controlled axotomy. These microfluidic devices allowed fluidic isolation of components and access to neurons following injury expanding the number of potentially compatible methods and tools to investigate neuronal injury.
Recently, a microfluidic vacuum aspiration injury model was used to examine the pathway responsible for the decline in regrowth of mature axons observed following injury (Zhou et al. 2016). In mature axons, syntaphilin (SNPH) mediated mitochondrial anchoring obstructs transport of mitochondria creating an energy deficit at the site of injury. Enhancing mitochondrial transport by deletion of the SNPH gene facilitated axon regrowth after injury by increasing mitochondrial transport and maintaining the ATP supply to injured axons (Sheng and Cai 2012). Thus, vacuum aspiration injury characterized mitochondrial trafficking and energy supply in injured axons and enabled the development new axon regeneration strategies.
The role of palmitoylation of dual leucine-zipper kinase (DLK) in retrograde injury signaling has also been investigated (Table 2) using the vacuum aspiration injury model (Holland et al. 2016). Palmitoylation is a multifunctional lipid modification that controls the localization, protein interaction, and activity of signaling kinases involved in neuronal communication over long distances and times. The neuronal cultures in microfluidic devices were infected with lentiviruses expressing either wtDLK-GFP or DLK-CS-GFP and the morphology marker mCherry in order to track DLK along length of axons. The microfluidic platform has enabled precise neuronal injury to trigger retrograde signals that induce robust c-Jun phosphorylation and shed light on how palmitoylation can control DLK dynamics following injury (Figure 2C).
Despite the benefits of these studies, several limitations to vacuum aspiration may limit its effectiveness in studying neuronal injury. Vacuum aspiration requires high fluidic resistance between the interconnected compartments to limit injury to specific neuronal regions. This resistance is generally provided by microgrooves in the microfluidic device, however, the duration and intensity of the vacuum step must be carefully tuned to decrease damage to unwanted regions. In addition, vacuum mediated axotomy rarely occurs in physical injuries to the CNS, and thus may not be appropriate to study aspects of neuronal injury.
Physical (stretch/strain) injury
During a TBI or SCI, a sudden physical force or motion, such as blast injuries, sports concussions and automobile accidents, generates and exerts strain and shear forces on the neuronal architecture leading to diffuse axonal injuries and either stretching or rupture of the long axons and/or damage to the cell body, resulting in axon degeneration. While several models exist to reproduce this stretch/strain injury in vitro or in organotypic slices (Morrison Iii et al. 2006; Pfister et al. 2003), these models suffer from the reproducibility of applied strain and are often unable to specifically isolate the stretch injury to axons alone.
Microfluidic platforms can precisely and efficiently deliver stress/strain-based physical injury to single axons, diffuse portions of axons, or over a varied range of axonal lengths and facilitate the examination of post-injury axonal degeneration. Microfluidic devices can be designed to generate either stress/strain-induced injury using pneumatic-based deformation of a PDMS membrane or compression-induced injury via pads that apply direct compression to the axons. Axons directionally grown in elastic microchannels can be uniaxially stretched by pressurizing a pneumatic cavity beneath the axonal compartment. Microfluidic devices can precisely and consistently control the location of injury and the extent of injury via pneumatic pressure. Conversely, in non-microfluidic platform-based in vitro or on organotypic models, injuries are difficult to reproduce due to unpredictable distribution of applied stress or strain.
Dollé and colleagues designed a (Dolle et al. 2013) microfluidic device (Figure 3) to apply a pneumatic strain-based injury specifically to the axons grown within the microchannels (Table 2). This design facilitated subsequent microscopy of the injured axons to examine the dynamics of axonal degeneration and regeneration. To simulate subthreshold to severe TBI strain thresholds, the group applied various degrees of strain injuries to axons inside of the microchannels from 10% to 45% and observed axonal beading and the breakdown of microtubules post-injury (Dolle et al. 2013). This injury model was subsequently used to characterize the biochemical changes in mitochondria induced by diffuse axonal injury (Dolle et al. 2014) and found a dramatic increase in mitochondrial membrane potential, using the lipophilic cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′- tetraethylbenzimi-dazolylcarbocyanine iodide (JC-1), after mild strain (10%), but a dramatic decrease after drastic strain (45%) (Dolle et al. 2014; Mathur et al. 2000; Troyan et al. 1997). These dramatic oscillations in mitochondrial membrane potential were ameliorated using a sodium-hydrogen exchange inhibitor, ethylisopropyl amiloride, and the mitochondrial permeability transition pore inhibitor, cyclosporine A, which resulted in a decrease in axonal degradation in the 25% and 45% strain conditions (Xu et al. 2001). These results demonstrated axonal degeneration is dependent on the axon bundle diameter and that a threshold strain injury is required for delayed hyperpolarization and immediate depolarization of mitochondria. This device demonstrated the the biochemical consequences of diffuse axonal injury and could be utilized to screen potential therapeutic agents (Dolle et al. 2014).
Figure 3.

Uniaxial Strain Microfluidic Platform for Organotypic Slice Culture. (A) Platform schematic depicting PDMS deflection after the application of pressure into pneumatic valve. (B) Top view of microfluidic device. (C) Time progression of delayed elastic effect on a single axon after application of 42% strain, i) before injury, ii) after injury t=0 min, iii) 20 mins, iv) 50 mins, v) 75 mins, vi) 210 mins, vii) 24hrs. Arrows show individual “waves/undulations” and arrow heads show beading(Dolle et al. 2013).
In a similar approach, Yap and colleagues designed a microfluidic device (Table 2) to induce strain injury to dissociated rat cortical axons (Yap et al. 2014). Unlike Dollé’s strain injury model (Dolle et al. 2013; Dolle et al. 2014) which applied injury to axons over 2 mm, this device applies a localized stretch injury to axons over 90 μm. A very mild stretch, or a mild stretch, consisting of 0.5% strain or 5% strain, respectively, was applied, and it was observed that a very mild stretch did not have significant axonal degeneration 1 day following injury, but did have significant degeneration 3 days post injury. In addition, ‘dying back’ axon degeneration was reported to occur after the application of very mild stretch injury, as evidenced by the presence of dendritic beading in the somatic compartment 1 day post injury. Conversely, the mild stretch injury led to significant axonal degeneration at both 1 and 3 days following injury (Yap et al. 2014). Recently, this in vitro model of axonal stretch injury was used to investigate axonal responses to single and repetitive stretch injuries in a fluidically isolated microenvironment (Yap et al. 2017). The study compared the morphology and cytoskeletal profile of growth cones on the tips of the axons following mild (5% strain), very mild (0.5% strain), and repetitive very mild (2×0.5% strain) axonal stretch injury. Following the very mild and mild axonal stretch injury, growth cones at the tips of axons exhibited abnormal colocalization of actin and microtubules as well as the formation of abnormal retraction bulbs on the tips of distal retractions. Furthermore, these abnormalities in cytoskeletal structure could be decreased through the application of the potentially clinically therapeutic microtubule stabilizing agent, Epothilone D (Brunden et al. 2012). While these results exemplify the utility of their device for studying neuronal responses to discrete axonal stretch injury, their device lacked optical transparency for high resolution live time-lapse analysis and the ability to investigate synaptically-connected axons from two different neuronal populations (Yap et al. 2017).
Hosmane and colleagues (Hosmane et al. 2011) developed an axonal injury micro-compression (AIM) platform for the application of compressive load onto axons (Table 2), also through the use of a pneumatic injury pad (Figure 4). This platform allows for the characterization of axonal deformation during and promptly following the application of a compression injury onto single axons. Single axons were compressed at varying compressive loads, and then monitored using phase contrast microscopy for up to 12 hours. It was found that the majority of axons continued to grow when exposed to mild injury conditions (<55 kPa compression), while there was a significant reduction in the number of axons that continued to grow under moderate compression (55-95 kPa). The highest level of compression (>95 kPa) led to the transection of the majority of axons, half of which regenerated during the subsequent 12 hour period without the addition of any exogenous molecules. Fournier and colleagues recently redesigned a microcompression axonal injury device to perform a compression injury (Fournier et al. 2014). The injury pad layer was reversibly sealed to the glass coverslip to allow access to the injured neurons for subsequent transmission electron microscopy (TEM) analysis (Table 2). This microfluidic platform allows for the examination of the molecular processes that take place in neurons during mechanical loading. Pneumatic pressure is applied to press the injury pad onto the culture glass substrate containing neuronal axons to model mechanical loading. The group used confocal microscopy and TEM to examine changes to neurofilaments and microtubules following the application of the mechanical load onto the axons. During the first five-minute period after axonal compression, there was a significant decrease in neurofilament levels, while there was no change in the microtubule concentration. These findings suggest that local neurofilament changes may act as a trigger for impending secondary damage. Using the same microfluidic platform in combination with TEM and immunolabeling to examine changes in the ultrastructural composition of neurofilaments and microtubules following axonal focal compression, the same group found significant decreases in neurofilament and microtubule number, density, and spacing after the application of the compressive load (Fournier et al. 2015). These results suggest that axon caliber dependency for microtubule and neurofilament number and density exists for axons undergoing compressive load.
Figure 4.

Microfluidic device to perform injury using compression pad. Microfluidic device controls for neuronal growth through microchannels into injury region. Application of pressure causes a downward deflection of the injury pad and compression injury of axon growing underneath. (B) Wallerian degeneration (nodal swellings) observed when a tau-labeled (microtubule marker) axon was subjected to severe (235 kPa) compression injury. After injury, the transected axon tip (white arrows) first retracted (30 mins), and then began to reform a growth cone (1 h 30 mins). Dotted lines demarcate the injury pad region. Scale bar 25 mm (Hosmane et al. 2011).
Additionally, microfluidic isolation of axons allows for integration of specialized tools to study distinct phenomena in neuronal injury. For example, Magdesian and colleagues applied microfluidics in tandem with Atomic Force Microscopy (AFM) to examine the pressure threshold for axonal degeneration post-injury (Magdesian et al. 2012). Injury was induced using an AFM to apply force to the axons with the use of a beaded or tipless cantilever (Table 2). Using this setup, Magdesian et. al. found that axons of rat hippocampal neurons were unable to recover axonal transport after compressed with pressures above 65 ± 10 Pa. On the other hand, axons of dorsal root ganglion neurons withstood significantly greater compression pressures of 540 ± 220 Pa (Magdesian et al. 2012). Additionally, the group discovered that the elastic modulus of dorsal root ganglion axons is 20 % less than that of the axons of hippocampal neurons. The use of this system demonstrated the ability of microfluidic platforms to aid in the guidance of axonal connections, which could be further investigated with the use of advanced physical tools such as atomic force microscopy to elucidate the mechanical characteristics of axons, such as their ability to withstand the application of specific forces in relation to axonal transport.
Despite the precise control of injuries afforded by the described devices, careful consideration is needed as utilizing this technique often requires pneumatic equipment for the actuation necessary to create a stress, strain, or compression injury. Creating an air tight interface between the pneumatic equipment and the miniaturized microfluidic platforms is often challenging and requires custom made couplers compatible with the microchannel dimensions and materials. In addition, each microfluidic device requires careful calibration of the injury mechanism as the amount of injury can vary greatly depending upon device design. Finally, creating microfluidic devices for these physical injuries can be difficult as they often require multilayer devices with precise dimensions for creating accurate injuries to the axonal or somal compartments. Often neurobiologists or biological laboratories working with axonal injury have limited microfabrication skills and resources in terms of microfabrication equipment. Although, the pneumatic layer devices can be fabricated using simple techniques such as the adhesive tape method (Shrirao and Perez-Castillejos 2010; Shrirao et al. 2012), fabrication of these complex multilayer microfluidic devices may be challenging without access to the technical expertise and the equipment necessary for fabrication.
Chemical Injury
Microfluidic platforms can also mimic biochemical processes seen in vivo. Following the primary insult after TBI or SCI, secondary damage, such as glutamate excitotoxicity, can greatly affect neuronal survival and activity (Neukomm and Freeman 2014; Sharif-Alhoseini et al. 2017). Microfluidic platforms can ascertain the effects of biochemical injury mechanisms on precise subcellular regions by precisely controlling the spatio-temporal distribution of pharmacological agents (Li et al. 2012; Taylor and Jeon 2010; Velve-Casquillas et al. 2010). Typically, a detergent or neurotransmitter is used to damage neurons or simulate excitotoxicity (Lee et al. 2013) but other molecules such as hemolytic compounds and immunogenic compounds have been used to study mechanisms of neuronal damage (Kilinc et al. 2011; Li et al. 2012). However, one should note a clear distinction between models employing chemical-mediated axotomy, i.e. detergent application, with models that utilize glutamate or toxins to injure neuronal and neuronal network function.
Detergents can be used to simulate axotomy by disrupting axonal structure. The use of detergents allows for disruption of the plasma membrane without the application of mechanical force, which can lead to the disruption of axonal adherence to the substrate and induce degeneration (George et al. 1988; Lee et al. 2013). Deleglise and colleagues examined the synapto-protective properties of pharmacological agents (Table 3) on axonal injury in neuronal networks through the use of a microfluidic platform and detergent-based injury (Deleglise et al. 2013). By filling the central microchannel of a microfluidic device with medium containing the detergent 0.1% Triton X-100, Deleglise found that pre-synaptic loss in axotomized cortical neurons occurred prior to axonal fragmentation and post-synaptic spine changes. Additionally, pre-treatment of the cultures with resveratrol and a caspase inhibitor carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone (z-VAD-fmk) showed no significant synapto-protection. However, pre-treatment with Rho Kinase inhibitor (Y27632) and a caspase inhibitor nicotinamide adenine dinucleotide (NAD+) were significantly synapto-protective and NAD+ led to retention of 65% of synapses post-axotomy compared to 25% retention in control cultures. Similarly, Kilinc and colleagues developed a microfluidic chemical injury device (Table 3) which simulated distal Wallerian degeneration but without undergoing somatic cell death (Kilinc et al. 2011). Additionally, the group reported that pre-treatment with NAD+ delayed fragmentation of the axonal cytoskeleton, while pre-treatment with the calpain inhibitors N-Acetyl-Leu-Leu-Norleu-al (ALLN) and z-VAD, had no effect on axonal fragmentation.
In contrast to chemical-mediated axotomy, application of excess excitatory neurotransmitters to microfluidic systems is also used to model glutamate excitotoxicity which is a component of secondary injury that accompanies a primary physical insult (Guerriero et al. 2015). Samson and colleagues developed a five compartment microfluidic platform interconnected with microchannels (Figure 5A) to examine the effects of neuronal networks on neuroprotection (Table 3) from excitotoxic and sub-lethal concentrations of glutamate injury (Samson et al. 2016). Hippocampal neurons were seeded in all five chambers to establish synaptic connectivity through interconnecting microchannels. These neurons were then subjected to excitotoxic insult induced by injecting glutamate in the central chamber (labeled 0 in Figure 5A). The role of synaptic neurotransmission in abolishing spreading toxicity and importance of preconditioning stimulus to protect against subsequent direct glutamate excitotoxic insult were tested by introducing blocker (glutamate transporters, NMDA receptors) and/or preconditioning stimulus in chambers (−1 and −2) on one side of injury chamber (0) and compared with other side chambers (1 and 2). Samson et. al. observed that excitotoxic injury spread to fluidically isolated, non-injured neurons in adjacent compartments via a GluN2B type N-methyl-D-aspartate (NMDA) receptor mediated mechanism (Samson et al. 2016). Additionally, neuroprotective signaling following excitotoxic injury provided resistance to the ensuing excitotoxic insult.
Figure 5.
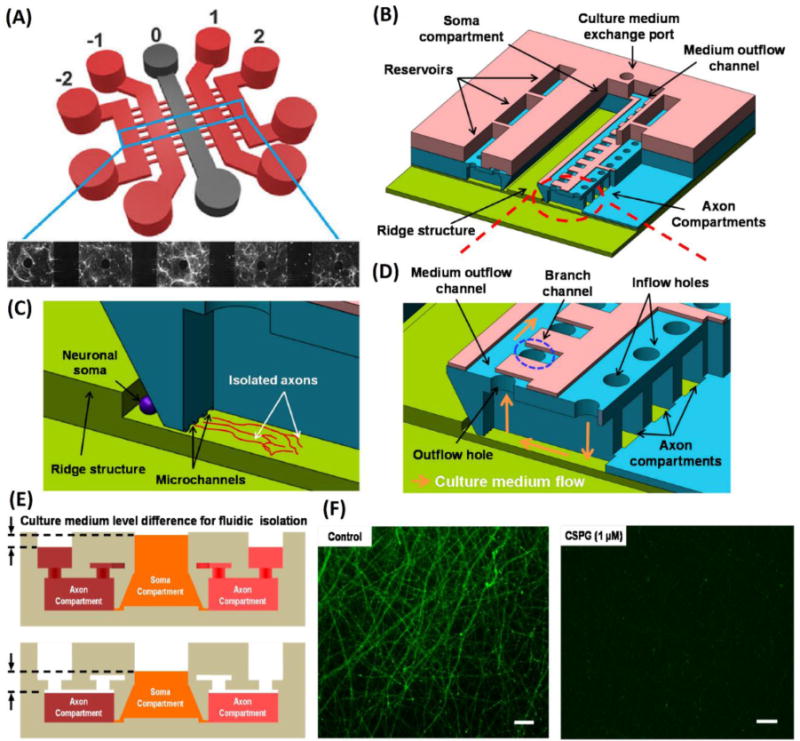
Microfluidic platforms to performchemical injury. (A) Schematic of five chamber microfluidic device. Fluidic isolation between chambers allows for investigation of effects of neural networks after application of chemical injury to one of the chambers (Samson et al. 2016). (B) Schematic of highly complex three layer compartmentalized microfluidic platform. (C) Isolation of cell body and axonal growth. (D) Axonal chamber with inlet for introducing treatments and outlet for media changes. (E) Differential fluid levels ensure fluidic isolation between somal and axonal chamber during treatment or media changes. (F) Axonal degeneration induced by injecting CSPG treatment in axonal chamber. Cells were stained with Calcein-AM(Kim et al. 2016).
Blood has also been used to induce neurotoxicity in microfluidic models of axonal damage. Charier and colleagues explored the neurotoxic and calcium stress effects of blood on neurons which occurs during intracranial hemorrhage (Charier et al. 2015) using microfluidic devices combined with lysed blood or medium cultured with a blood clot (Table 3). While exposure to blood clot medium did not lead to axonal fragmentation, exposure to lysed blood led to a ‘dying back’ fragmentation pattern in the axons. Treatment with the combination of the NMDA receptor antagonist memantine and vitamin D significantly reduced axonal fragmentation 24 hours after injury, while no significant reduction in axonal fragmentation occurred when the two treatments were performed separately thus demonstrating a potential palliative for intracranial hemorrhage.
Microfluidic platforms also enable the examination of axonal response to a variety of biomolecules, allowing investigators to explore specific biological mechanisms following injury. Park and colleagues (Kim et al. 2016; Park et al. 2014) used microfluidic chemical injury (Figure 5B-E) to demonstrated that the treatment of isolated axons with chondroitin sulfate proteoglycans (CSPG) (5 μg/ml) leads to significant fragmentation and degeneration (Figure 5F) of neuronal axons (Table 3). The devices used by Park et. Al. were composed of a single central soma chamber and connected 6 axonal chambers. The central soma chamber contains a ridge in the center of the chamber which encourages neurons to adhere and grow near the axonal chamber inlets. Each axonal chamber was connected to the central chamber by 4 inlets which prevented somal contamination in these chambers. A single outlet allowed researchers to efficiently aspirate all 6 axonal chambers and the central soma chamber simultaneously. This design allowed Park et. Al. to run 6 different studies (one per axonal chamber), specifically treat the axonal portions of neurons, and simultaneously ensure fluidic isolation between the chambers using differences in fluidic height between the axonal and somal chambers (Figure 5E).
In addition, integrated microfluidic systems may aid investigators in examining the dynamics between neuronal injury and regeneration, localization of injury to neuronal soma or axons, the influence of glial, and or pharmacological agents. Li and colleagues (Li et al. 2012) examined neuronal regeneration (Table 3) after chemical injury in an integrated microfluidic platform (Figure 6). Once the axonal growth of neurons seeded in the somal chamber reaches the axonal chamber, chemical injury can be applied to axonal and/or somal chambers via injection of injury reagents or potential treatments into either the axonal or somal chamber. In addition, the microfluidic device was designed to enable co-culture of glial cells in the two glial cell chambers. These glial chambers could be selectively exposed to or shut out from the connected axonal and/or somal compartments via integrated valves. Neuronal axons and cell bodies showed different responses to chemically induced injury, with axons displaying greater resistance to damage compared to somata. Additionally, the application of the neurotrophic agent monosialotetrahexosylganglioside (GM1) and co-culture with astrocytes and Schwann cells promoted the regeneration of retracted axons. In contrast, GM1 application did not avert degeneration following damage to neuronal somata. These types of microfluidic devices may further elucidate the interactions between neurons and glial cells before and after injury and how these interactions influence neuronal degeneration and regeneration.
Figure 6.

Integrated microfluidic system developed to examine the effects of glial co-culture and pharmacological intervention on axonal degeneration and regeneration after chemical injury. (A) Image of dye-loaded device. B. Schematic displaying microvalve mechanism. (C) Various modes of intervention performed utilizing the integrated microfluidic system (Li et al. 2012).
Other types of chemical modulators, such as nitric oxide (NO), can be used in microfluidic platforms to specifically induce microglial mediated axonal degeneration in a controlled fashion. Hosmane and colleagues (Hosmane et al. 2012) developed a microfluidic platform to examine microglial phagocytosis of axonal debris after injury (Table 3). This platform allowed for investigation into the mechanisms behind clearance of axonal debris by microglia, without directly affecting the microglia during the chemical injury. Hosmane et. al. found that the clearance of axonal debris by microglia is mediated by the TIR-domain-containing adapter-inducing interferon-β (TRIF) and by p38 mitogen-activated protein kinase (MAPK) downstream of TRIF thus furthering our understanding of how microglia respond to injury (Hosmane et al. 2012). Microfluidic platforms also allow for the elucidation of mechanisms in neurological disease in addition to neuronal injury. Sauer and colleagues investigated the relationship between CD8+ lymphocytes (CTLs) and axonal injury (Table 3) in inflammatory lesions found in multiple sclerosis patients (Sauer et al. 2013). Application of the stimulatory Interferon gamma (IFNγ) peptides led to the upregulated expression of axonal major histocompatibility complex class I (MHC I) molecules, which present antigens procured from endogenous proteins to CD8+ lymphocytes. Sauer et. al. exposed neuronal axons which were pretreated with the SIINFEKL peptide and IFNγ to CD8+ lymphocytes and found that CD8+ lymphocytes developed cytotoxic immune synapses on the axons expressing MHC I molecules, leading to the degradation of axons but not neuronal apoptosis (Sauer et al. 2013).
Microfluidic devices provide a platform for examination of how the chemical microenvironment of neuronal tissues can affect survival, growth, and regeneration. By taking advantage of the ability of microfluidic devices to fluidically isolate specific neuronal regions, a wide variety of secondary injuries, such as glutamate-induced excitotoxicity (Samson et al. 2016), radical oxygen species generation (Hosmane et al. 2011; Hosmane et al. 2012), and the immunogenic response to various inflammatory mechanisms (Sauer et al. 2013) can be studied. Despite this wide variety of potential chemical injuries, fluidic isolation requires precise management of fluid levels in the microfluidic compartments, making the system prone to human error in fluid loading. This can create a level of uncertainty in the degree of fluidic isolation in these microfluidic systems. More complex microfluidic devices may allow for more precise control of fluidic isolation within the device but significantly raises the complexity of the microfluidic device. Finally, chemical injuries may not fully recapitulate the physical force induced aspects of injury to the CNS such as effect of deformation, detachment, stress and strain on axons, but potentially be used to study in vivo biological phenomena such as inflammation or excitotoxicity responses.
Laser-based injury for microfluidic devices
Microfluidics and laser-based methods can work synergistically to enhance the specificity of neuronal injury models. Organization, isolation, and stabilization of the target axon allows for injury of single axons either in vitro or in vivo. In 2004, Yanik and colleagues demonstrated the first use of lasers for axotomy of single axons in C. elegans (Yanik et al. 2004). This technique was subsequently combined with microfluidic technology to enhance the utility of laser-based axotomy by Zeng and colleagues in 2008 (Zeng et al. 2008) and later adapted for use both in cultured mammalian neurons (Kim et al. 2009) and in Drosophila larvae (Ghannad-Rezaie et al. 2012). Microfluidics allows for the isolation, stabilization, and high throughput screening of these in vitro and in vivo models in conjunction with precision laser-based axotomy. This recent work aided in the discovery of key aspects of neuronal injury and regeneration as discussed below. The exact mechanism by which laser-based axotomy functions depends greatly upon the type of femto-second pulsed laser utilized. These mechanisms can be broadly divided into three categories, with the lowest power (~0.26 × 1012 W/cm2) creating low levels of reactive oxygen species in the area and causing damage to subcellular organelle (Tsai et al. 2009), and the highest powers (~6.54 × 1012 W/cm2) resulting in the formation of plasma cavitation bubbles, injuring tissue via large bubble formation (Ben-Yakar and Bourgeois 2009). The work discussed here used the middle category of power (5.1 × 1012 W/cm2), which results in the formation of micron sized bubbles, which serve to axotomize the target neurite either in vitro or in vivo. In general, these laser-based methods take advantage of precision optics in microscope objectives to specifically target single axons. Lasers focused in this manner will generally leave the surrounding tissue intact (Ben-Yakar and Bourgeois 2009; Guo et al. 2008), allowing for precise damage to tissues and cells. Further discussion of the mechanisms behind optically-induced damage to axons or other biological tissues are covered in other reviews (Ben-Yakar and Bourgeois 2009; Tsai et al. 2009).
Microfluidic devices serve to enhance the precision of laser-based axotomy in several ways (Figure 7). In vitro, the microfluidic devices serve to organize single axons into a specified area, allowing for simple targeting of the axon (Kim et al. 2009). With Drosophila and C. elegans model systems using laser-induced axotomy, microfluidic devices allow for stabilization and entrapment of the organism to precisely injure the animal (Ghannad-Rezaie et al. 2012; Guo et al. 2008) and to avoid the use of anesthetics, which may affect the injury and regeneration of neurons (Tsai et al. 2009). The precise dissection of an axon allows for controlled examination of both the proximal and distal portions of the cut axon. In 2010, Hellman and colleagues combined a microfluidic device with laser-induced axotomy to accurately break a single axon in vitro and examine its degenerative and regenerative processes (Hellman et al. 2010). In this study, they reported that following axotomy, degeneration is followed by neuronal “dieback” involving retraction of the axon and subsequent regeneration of the axon (Figure 7E-F). By utilizing a calcium chelator EGTA, they demonstrated that the processes of both degeneration and regeneration are calcium-dependent (Hellman et al. 2010). In 2012, Ghannad-Rezaie and colleagues reported a new system using immobilized Drosophila larvae with neurons expressing the calcium sensor gCaMP 3.0. Laser-based axotomy of these neurons showed an immediate calcium rise and decay following injury. Synaptic core vesicles were also imaged using the Gal4/UAS system, and a significant increase in anterograde transportation in the proximal portion of axotomized axons after injury was observed. These discoveries were facilitated by the use of microfluidics, which allowed mechanical immobilization and precise control of the microenvironment of the Drosophila larvae (Ghannad-Rezaie et al. 2012).
Figure 7.
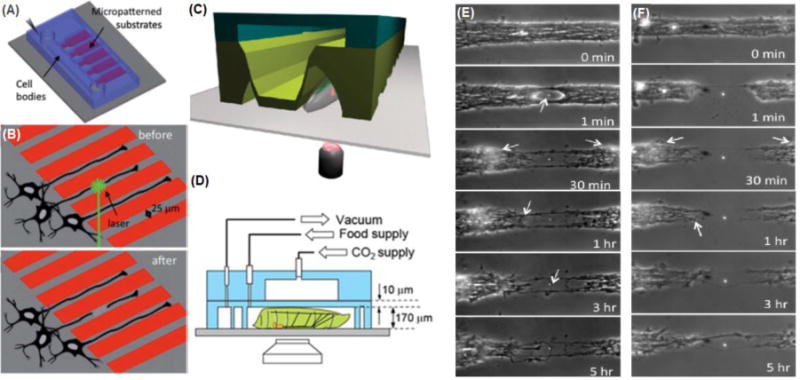
Microfluidic devices to perform laser assisted neuronal injury. (A) Microfluidic device to perform laser-based axotomy. (B) Schematic showing axotomy of single axons grown through microfluidic channels with an integrated laser. (C) Microfluidic device for axotomy of single axons in vivo (Samara et al. 2010). (D) Drosophila or C. elegans are held in place with microfluidic channels, and single axons are visualized and axotomized via integrated optical components (Ghannad-Rezaie et al. 2012). (E-F) Axonal regeneration after (E) a partial defect created using single 400 nJ pulse and (F) a complete transection created using single 800 nJ pulse in a 25 μm-wide axon bundle. Arrows pointing initial dieback and growth cones (Hellman et al. 2010).
Microfluidics combined with laser-based axotomy can enable high-throughput screening. In 2010, Yanik and colleagues utilized microfluidic isolation of C. elegans combined with a femtosecond laser to injure individual neurons and screen the effect of compounds on regeneration of mechanosensory GABAergic, AWP and CEP neurons. Several inhibitors of protein kinase C (PKC) reduced regeneration and a PKC activator increased regeneration. Microfluidics allowed for fast high-throughput screening of these compounds with each worm taking only ~30 seconds to treat, axotomize, and subsequently unload (Samara et al. 2010). In 2012, Driscoll and colleagues used a laser axotomy and microfluidic entrapment system to reveal the roles of CED-3 and CED-4 in neuronal regeneration in C. elegans. CED-3 and CED-4 are caspases that traditionally function in cell death as part of the apoptotic pathway. However, by specifically cutting a single mechanosensory axon in these worms, regeneration was monitored in worms lacking CED-3 and CED-4. Microfluidics allowed them to perform these experiments by immobilizing worms without the use of anesthetics, thus mimicking normal physiological regeneration process (Pinan-Lucarre et al. 2012).
Microfluidic devices utilized in conjunction with laser-based methods of axotomizing single axons allows for precise control of the extent and localization of injury in both in vitro and in vivo systems. By taking advantage of the ability of microfluidic platforms to isolate single axons in vitro, single axons can be cut and their regrowth and regeneration examined. In addition, microfluidic devices in conjunction with laser-based single axon axotomy allow for immobilization of C. elegans and Drosophila larvae for high throughput screening. Studies can be performed without the use of anesthetics, which may affect the growth and regeneration of neurons post-injury. While the extreme precision of this technique allows for very detailed analysis of regeneration, the use of a laser-based method requires complicated and expensive optical components integrated into inverted microscope systems. These components require a high level of technical expertise for setup, maintenance, and calibration, which may deter use of this method.
Finally, single neuron axotomy using microfluidic injury models may not be suitable for examining physical CNS injuries as injuries to the CNS are rarely limited to single axons. However, reducing injury to a well-defined isolated region with these microfluidic approaches can drastically simplify studies.
Summary
Microfluidic platforms used to study CNS injury provide many advantages over traditional in vitro or in vivo models. However, each of these injury platforms has various advantages and disadvantages that need to be carefully considered before deciding on which platform to use for a particular study. In particular, researchers need to carefully consider whether the microfluidic device can simulate their injury of interest. In Table 4, we highlight the pros and cons of each of the injury models described in this review. The list of analytical techniques supported by these models is provided in Table 5.
Table 4.
Microfluidic injury models: applications, advantages, and limitations
| Injury Model | Applications and Examples | Advantages | Limitations |
|---|---|---|---|
| Vacuum-assisted Injury | Simple method of applying axotomy or neuronal injury in vitro. |
|
|
| Chemical Injury | Mimic variety of biochemical microenvironments in injury or diseases:
|
|
|
| Physical Injury | Simulate variety of physical aspects of injury to specific cellular regions:
|
|
|
| Laser-based injury | Precise axotomy of individual axons in a variety of model systems:
|
|
|
Table 5.
Compatible analytical techniques supported by microfluidic injury models previously reported and discussed above.
| Analytical technique | Vacuum-assisted Injury | Chemical Injury | Physical Injury | Laser-based injury |
|---|---|---|---|---|
| Immunofluorescence | ☑ | ☑ | ☑ | ☑ |
| Biochemical Assays | ☑ | ☑ | ☑ | ☑ |
| Protein Analysis | ☑ | ☑ | ☑ | ☑ |
| mRNA Analysis | ☑ | ☑ | ☑ | ☑ |
| Morphological analysis | ☑ | ☑ | ☑ | ☑ |
| High throughput screening | ☑ | ☑ | ☑ | |
| In vivo functionality | ☑ |
Note: The list of compatible analytical techniques is not all inclusive and microfluidic devices can potentially be modified to include others.
Vacuum-induced injury with microfluidic platforms is a reliable, efficient, and simple method of simulating axotomy in vitro. Its simplicity allows it to be utilized with a variety of biological techniques for in depth analysis of neuronal response to injury. However, because axotomy is a relatively rare neuronal injury, biological responses discovered with vacuum-induced injury may not be representative of all neuronal injuries. Microfluidic platforms can be modified to study physical stress/strain/compression injury and produce models to reliably physically injure specific subcellular regions of neurons in vitro. However, actuating these injuries often requires the use of difficult to interface pneumatic equipment, the fabrication of more complex multilayered microfluidic devices and extensive calibration of these devices with specific equipment that may not be available in most biological laboratories. Microfluidic platforms used to simulate chemical injury take advantage of the ability to fluidically isolate subcellular regions of the neuron, such as the soma or axon, to allow for the controlled spatial and temporal application of agents to the neuron. This ability allows microfluidic platforms to mimic phenomena, such as glutamate-induced excitotoxicity, hemolysis-induced damage, and immunogenic-mediated damage to axons. Chemical injury, however, does not capture the complete injury response to physical trauma. Furthermore, microfluidic channels developed to create regions of fluidic isolation can be simple and constructed with commercially available microfluidic devices (Taylor et al. 2005), or they can be extremely complex and difficult to fabricate in most biological laboratories. Laser-based injury coupled with microfluidic devices allows for extremely precise injury for both in vitro and in vivo models of neuronal injury. In vitro, laser-based injury allows single axotomy and examination of regeneration. In vivo, microfluidics allows for the performance of high throughput screening of regenerative compounds in Drosophila and C. elegans model systems without the use of anesthetics. However, laser-based injury requires complex optical setups, which may be difficult to establish in most laboratories.
In summary, each of these methods has advantages and disadvantages for the study of neuronal injury. Careful consideration is warranted when choosing an injury model suitable for particular applications or research interests. That being said, the precise control in the application of injury can enhance the reliability, reproducibility, and efficiency of microfluidic devices. This allows researchers to gather innovative insights into the biological mechanisms that mediate neuronal injury or disease and the potential discovery of new avenues for preserving or regenerating the CNS.
Acknowledgments
Funding for this work was provided by NSF CBET 1512170, New Jersey Commission Brain Injury Research #CBIR14IRG019, New Jersey Commission on Spinal Cord Research #CSCR14IRG005, National Institutes of Health under the Ruth L. Kirschstein National Research Service Award T32 GM8339 from the NIGMS.
Footnotes
Conflicts of interest
There are no conflicts of interest to declare.
Contributor Information
Anil B. Shrirao, Department of Biomedical Engineering, Rutgers University, 599, Taylor Road, Piscataway, NJ 08854.
Frank H. Kung, Department of Cell Biology & Neuroscience, Rutgers University, 604 Allison Road, Nelson Labs D-411, Piscataway, NJ 08854.
Anton Omelchenko, Department of Cell Biology & Neuroscience, Rutgers University, 604 Allison Road, Nelson Labs D-411, Piscataway, NJ 08854.
Rene S. Schloss, Department of Biomedical Engineering, Rutgers University, 599, Taylor Road, Piscataway, NJ 08854
Nada N. Boustany, Department of Biomedical Engineering, Rutgers University, 599, Taylor Road, Piscataway, NJ 08854
Jeffrey D. Zahn, Department of Biomedical Engineering, Rutgers University, 599, Taylor Road, Piscataway, NJ 08854
Martin L. Yarmush, Department of Biomedical Engineering, Rutgers University, 599, Taylor Road, Piscataway, NJ 08854
Bonnie L. Firestein, Department of Cell Biology & Neuroscience, Rutgers University, 604 Allison Road, Nelson Labs D-411, Piscataway, NJ 08854
References
- Ben-Yakar A, Bourgeois F. Ultrafast laser nanosurgery in microfluidics for genome-wide screenings. Current opinion in biotechnology. 2009;20(1):100–105. doi: 10.1016/j.copbio.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benam KH, Dauth S, Hassell B, Herland A, Jain A, Jang K-J, Karalis K, Kim HJ, MacQueen L, Mahmoodian R. Engineered in vitro disease models. Annual Review of Pathology: Mechanisms of Disease. 2015;10:195–262. doi: 10.1146/annurev-pathol-012414-040418. [DOI] [PubMed] [Google Scholar]
- Brunden KR, Ballatore C, Lee VM, Smith AB, 3rd, Trojanowski JQ. Brain-penetrant microtubule-stabilizing compounds as potential therapeutic agents for tauopathies. Biochem Soc Trans. 2012;40(4):661–6. doi: 10.1042/BST20120010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. Local control of neurite development by nerve growth factor. Proceedings of the National Academy of Sciences. 1977;74(10):4516–4519. doi: 10.1073/pnas.74.10.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center NSCIS. Facts and Figures at a Glance. Birmingham, AL: University of Alabama at Birmingham; 2015. pp. 1–2. [Google Scholar]
- Charier D, Beauchet O, Bell M, Brugg B, Bartha R, Annweiler C. Memantine plus vitamin D prevents axonal degeneration caused by lysed blood. ACS Chem Neurosci. 2015;6(3):393–7. doi: 10.1021/cn500303k. [DOI] [PubMed] [Google Scholar]
- Cheriyan T, Ryan D, Weinreb J, Cheriyan J, Paul J, Lafage V, Kirsch T, Errico T. Spinal cord injury models: a review. Spinal Cord. 2014;52(8):588–595. doi: 10.1038/sc.2014.91. [DOI] [PubMed] [Google Scholar]
- Deleglise B, Lassus B, Soubeyre V, Alleaume-Butaux A, Hjorth JJ, Vignes M, Schneider B, Brugg B, Viovy J-L, Peyrin J-M. Synapto-Protective Drugs Evaluation in Reconstructed Neuronal Network. PLOS ONE. 2013;8(8):e71103. doi: 10.1371/journal.pone.0071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle J-P, Morrison B, Iii, Schloss RS, Yarmush ML. An organotypic uniaxial strain model using microfluidics. Lab on a Chip. 2013;13(3):432–442. doi: 10.1039/c2lc41063j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle JP, Morrison B, 3rd, Schloss RS, Yarmush ML. Brain-on-a-chip microsystem for investigating traumatic brain injury: Axon diameter and mitochondrial membrane changes play a significant role in axonal response to strain injuries. Technology (Singap World Sci) 2014;2(2):106. doi: 10.1142/S2339547814500095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AJ, Hogan JD, Rajbhandari L, Shrestha S, Venkatesan A, Ramesh KT. Changes in Neurofilament and Microtubule Distribution following Focal Axon Compression. PLoS One. 2015;10(6):e0131617. doi: 10.1371/journal.pone.0131617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier AJ, Rajbhandari L, Shrestha S, Venkatesan A, Ramesh KT. In vitro and in situ visualization of cytoskeletal deformation under load: traumatic axonal injury. Faseb j. 2014;28(12):5277–87. doi: 10.1096/fj.14-251942. [DOI] [PubMed] [Google Scholar]
- George EB, Schneider BF, Lasek RJ, Katz MJ. Axonal shortening and the mechanisms of axonal motility. Cell Motil Cytoskeleton. 1988;9(1):48–59. doi: 10.1002/cm.970090106. [DOI] [PubMed] [Google Scholar]
- Ghannad-Rezaie M, Wang X, Mishra B, Collins C, Chronis N. Microfluidic chips for in vivo imaging of cellular responses to neural injury in Drosophila larvae. PloS one. 2012;7(1):e29869. doi: 10.1371/journal.pone.0029869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero RM, Giza CC, Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Current neurology and neuroscience reports. 2015;15(5):27–27. doi: 10.1007/s11910-015-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SX, Bourgeois F, Chokshi T, Durr NJ, Hilliard MA, Chronis N, Ben-Yakar A. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nature methods. 2008;5(6):531–533. doi: 10.1038/nmeth.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman AN, Vahidi B, Kim HJ, Mismar W, Steward O, Jeon NL, Venugopalan V. Examination of axonal injury and regeneration in micropatterned neuronal culture using pulsed laser microbeam dissection. Lab on a chip. 2010;10(16):2083–2092. doi: 10.1039/b927153h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SM, Collura KM, Ketschek A, Noma K, Ferguson TA, Jin Y, Gallo G, Thomas GM. Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc Natl Acad Sci U S A. 2016;113(3):763–8. doi: 10.1073/pnas.1514123113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmane S, Fournier A, Wright R, Rajbhandari L, Siddique R, Yang IH, Ramesh KT, Venkatesan A, Thakor N. Valve-based microfluidic compression platform: single axon injury and regrowth. Lab on a Chip. 2011;11(22):3888–3895. doi: 10.1039/c1lc20549h. [DOI] [PubMed] [Google Scholar]
- Hosmane S, Tegenge MA, Rajbhandari L, Uapinyoying P, Kumar NG, Thakor N, Venkatesan A. TRIF mediates microglial phagocytosis of degenerating axons. The Journal of Neuroscience. 2012;32(22):7745–7757. doi: 10.1523/JNEUROSCI.0203-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilinc D, Peyrin JM, Soubeyre V, Magnifico S, Saias L, Viovy JL, Brugg B. Wallerian-like degeneration of central neurons after synchronized and geometrically registered mass axotomy in a three-compartmental microfluidic chip. Neurotox Res. 2011;19(1):149–61. doi: 10.1007/s12640-010-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Jeong S, Koo C, Han A, Park J. A Microchip for High-Throughput Axon Growth Drug Screening. Micromachines. 2016;7(7):114. doi: 10.3390/mi7070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Park JW, Byun JH, Vahidi B, Rhee SW, Jeon NL. Integrated microfluidics platforms for investigating injury and regeneration of CNS axons. Ann Biomed Eng. 2012;40(6):1268–76. doi: 10.1007/s10439-012-0515-6. [DOI] [PubMed] [Google Scholar]
- Kim Y-t, Karthikeyan K, Chirvi S, Davé DP. Neuro-optical microfluidic platform to study injury and regeneration of single axons. Lab on a Chip. 2009;9(17):2576–2581. doi: 10.1039/b903720a. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Sattin RW. Traumatic brain injury in the United States: research and programs of the Centers for Disease Control and Prevention (CDC) J Head Trauma Rehabil. 2005;20(3):187–8. doi: 10.1097/00001199-200505000-00001. [DOI] [PubMed] [Google Scholar]
- Lee CY, Romanova EV, Sweedler JV. Laminar stream of detergents for subcellular neurite damage in a microfluidic device: a simple tool for the study of neuroregeneration. Journal of neural engineering. 2013;10(3):036020–036020. doi: 10.1088/1741-2560/10/3/036020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezana JP, Dagan SY, Robinson A, Goldstein RS, Fainzilber M, Bronfman FC, Bronfman M. Axonal PPARγ promotes neuronal regeneration after injury. Developmental Neurobiology. 2016;76(6):688–701. doi: 10.1002/dneu.22353. [DOI] [PubMed] [Google Scholar]
- Li L, Ren L, Liu W, Wang JC, Wang Y, Tu Q, Xu J, Liu R, Zhang Y, Yuan MS, et al. Spatiotemporally controlled and multifactor involved assay of neuronal compartment regeneration after chemical injury in an integrated microfluidics. Anal Chem. 2012;84(15):6444–53. doi: 10.1021/ac3013708. [DOI] [PubMed] [Google Scholar]
- Magdesian MH, Sanchez FS, Lopez M, Thostrup P, Durisic N, Belkaid W, Liazoghli D, Grütter P, Colman DR. Atomic force microscopy reveals important differences in axonal resistance to injury. Biophysical journal. 2012;103(3):405–414. doi: 10.1016/j.bpj.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur A, Hong Y, Kemp BK, Barrientos AA, Erusalimsky JD. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovascular Research. 2000;46(1):126–138. doi: 10.1016/s0008-6363(00)00002-x. [DOI] [PubMed] [Google Scholar]
- Morrison B, 3rd, Elkin BS, Dolle JP, Yarmush ML. In vitro models of traumatic brain injury. Annu Rev Biomed Eng. 2011;13:91–126. doi: 10.1146/annurev-bioeng-071910-124706. [DOI] [PubMed] [Google Scholar]
- Morrison B, Iii, Cater HL, Benham CD, Sundstrom LE. An in vitro model of traumatic brain injury utilising two-dimensional stretch of organotypic hippocampal slice cultures. Journal of Neuroscience Methods. 2006;150(2):192–201. doi: 10.1016/j.jneumeth.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Nagendran T, Larsen R, Bigler R, Frost S, Philpot B, Nudo RJ, Taylor AM. Distal axotomy enhances retrograde presynaptic excitability onto injured pyramidal neurons via trans-synaptic signaling. bioRxiv. 2016 doi: 10.1038/s41467-017-00652-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110(4):429–41. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Neukomm LJ, Freeman MR. Diverse cellular and molecular modes of axon degeneration. Trends in cell biology. 2014;24(9):515–523. doi: 10.1016/j.tcb.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim S, Park SI, Choe Y, Li J, Han A. A microchip for quantitative analysis of CNS axon growth under localized biomolecular treatments. J Neurosci Methods. 2014;221:166–74. doi: 10.1016/j.jneumeth.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Microfluidic culture platform for neuroscience research. Nat Protocols. 2006;1(4):2128–2136. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- Pfister BJ, Weihs TP, Betenbaugh M, Bao G. An In Vitro Uniaxial Stretch Model for Axonal Injury. Annals of Biomedical Engineering. 2003;31(5):589–598. doi: 10.1114/1.1566445. [DOI] [PubMed] [Google Scholar]
- Pinan-Lucarre B, Gabel CV, Reina CP, Hulme SE, Shevkoplyas SS, Slone RD, Xue J, Qiao Y, Weisberg S, Roodhouse K. The core apoptotic executioner proteins CED-3 and CED-4 promote initiation of neuronal regeneration in Caenorhabditis elegans. PLoS Biol. 2012;10(5):e1001331. doi: 10.1371/journal.pbio.1001331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishal I, Fainzilber M. Axon-soma communication in neuronal injury. Nature Reviews Neuroscience. 2014;15(1):32–42. doi: 10.1038/nrn3609. [DOI] [PubMed] [Google Scholar]
- Samara C, Rohde CB, Gilleland CL, Norton S, Haggarty SJ, Yanik MF. Large-scale in vivo femtosecond laser neurosurgery screen reveals small-molecule enhancer of regeneration. Proceedings of the National Academy of Sciences. 2010;107(43):18342–18347. doi: 10.1073/pnas.1005372107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson AJ, Robertson G, Zagnoni M, Connolly CN. Neuronal networks provide rapid neuroprotection against spreading toxicity. Scientific Reports. 2016;6:33746. doi: 10.1038/srep33746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer BM, Schmalstieg WF, Howe CL. Axons are injured by antigen-specific CD8(+) T cells through a MHC class I- and granzyme B-dependent mechanism. Neurobiol Dis. 2013;59:194–205. doi: 10.1016/j.nbd.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Alhoseini M, Khormali M, Rezaei M, Safdarian M, Hajighadery A, Khalatbari MM, Safdarian M, Meknatkhah S, Rezvan M, Chalangari M, et al. Animal models of spinal cord injury: a systematic review. Spinal Cord. 2017 doi: 10.1038/sc.2016.187. [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Cai Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13(2):77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrirao A, Perez-Castillejos R. Simple fabrication of microfluidic devices by replicating Scotch-tape masters. Chips Tips 2010 [Google Scholar]
- Shrirao AB, Hussain A, Cho CH, Perez-Castillejos R. Adhesive-tape soft lithography for patterning mammalian cells: application to wound-healing assays. Biotechniques. 2012;53(5):315–18. doi: 10.2144/000113928. [DOI] [PubMed] [Google Scholar]
- Shrirao AB, Kung FH, Yip D, Cho CH, Townes-Anderson E. Vacuum-assisted fluid flow in microchannels to pattern substrates and cells. Biofabrication. 2014;6(3):035016. doi: 10.1088/1758-5082/6/3/035016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrirao AB, Kung FH, Yip D, Firestein BL, Cho CH, Townes-Anderson E. A Versatile Method of Patterning Proteins and Cells. J Vis Exp. 2017;120 doi: 10.3791/55513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Berchtold NC, Perreau VM, Tu CH, Li Jeon N, Cotman CW. Axonal mRNA in uninjured and regenerating cortical mammalian axons. J Neurosci. 2009;29(15):4697–707. doi: 10.1523/JNEUROSCI.6130-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat Methods. 2005;2(8):599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Jeon NL. Micro-scale and microfluidic devices for neurobiology. Curr Opin Neurobiol. 2010;20(5):640–7. doi: 10.1016/j.conb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Rhee SW, Tu CH, Cribbs DH, Cotman CW, Jeon NL. Microfluidic multicompartment device for neuroscience research. Langmuir. 2003;19(5):1551–1556. doi: 10.1021/la026417v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyan MB, Gilman VR, Gay CV. Mitochondrial membrane potential changes in osteoblasts treated with parathyroid hormone and estradiol. Exp Cell Res. 1997;233(2):274–80. doi: 10.1006/excr.1997.3570. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Blinder P, Migliori BJ, Neev J, Jin Y, Squier JA, Kleinfeld D. Plasma-mediated ablation: an optical tool for submicrometer surgery on neuronal and vascular systems. Current opinion in biotechnology. 2009;20(1):90–99. doi: 10.1016/j.copbio.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velve-Casquillas G, Le Berre M, Piel M, Tran PT. Microfluidic tools for cell biological research. Nano today. 2010;5(1):28–47. doi: 10.1016/j.nantod.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Mahmood A, Chopp M. Animal models of traumatic brain injury. Nature Reviews Neuroscience. 2013;14(2):128–142. doi: 10.1038/nrn3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wang Y, Ayub A, Ashraf M. Mitochondrial K(ATP) channel activation reduces anoxic injury by restoring mitochondrial membrane potential. Am J Physiol Heart Circ Physiol. 2001;281(3):H1295–303. doi: 10.1152/ajpheart.2001.281.3.H1295. [DOI] [PubMed] [Google Scholar]
- Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432(7019):822–822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- Yap YC, Dickson TC, King AE, Breadmore MC, Guijt RM. Microfluidic culture platform for studying neuronal response to mild to very mild axonal stretch injury. Biomicrofluidics. 2014;8(4):044110. doi: 10.1063/1.4891098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap YC, King AE, Guijt RM, Jiang T, Blizzard CA, Breadmore MC, Dickson TC. Mild and repetitive very mild axonal stretch injury triggers cystoskeletal mislocalization and growth cone collapse. PLOS ONE. 2017;12(5):e0176997. doi: 10.1371/journal.pone.0176997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Rohde CB, Yanik MF. Sub-cellular precision on-chip small-animal immobilization, multi-photon imaging and femtosecond-laser manipulation. Lab on a Chip. 2008;8(5):653–656. doi: 10.1039/b804808h. [DOI] [PubMed] [Google Scholar]
- Zhang J-N, Michel U, Lenz C, Friedel CC, Köster S, d’Hedouville Z, Tönges L, Urlaub H, Bähr M, Lingor P, et al. Calpain-mediated cleavage of collapsin response mediator protein-2 drives acute axonal degeneration. Scientific Reports. 2016;6:37050. doi: 10.1038/srep37050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Yu P, Lin M-Y, Sun T, Chen Y, Sheng Z-H. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. The Journal of Cell Biology. 2016;214(1):103–119. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]


