Abstract
Mesenchymal stem cell derived extracellular matrix (MSC-ECM) is a natural biomaterial with robust bioactivity and good biocompatibility, and has been studied as a scaffold for tissue engineering. In this investigation, we tested the applicability of using decellularized human bone marrow derived MSC-ECM (hBMSC-ECM) as a culture substrate for chondrocyte expansion in vitro, as well as a scaffold for chondrocyte-based cartilage repair. hBMSC-ECM deposited by hBMSCs cultured on tissue culture plastic (TCP) was harvested, and then subjected to a decellularization process to remove hBMSCs. Compared with chondrocytes grown on TCP, chondrocytes seeded onto hBMSC-ECM exhibited significantly increased proliferation rate, and maintained better chondrocytic phenotype than TCP group. After being expanded to the same cell number and placed in high-density micromass cultures, chondrocytes from the ECM group showed better chondrogenic differentiation profile than those from the TCP group. To test cartilage formation ability, composites of hBMSC-ECM impregnated with chondrocytes were subjected to brief trypsin treatment to allow cell-mediated contraction, and folded to form 3-dimensional chondrocyte-impregnated hBMSC-ECM (Cell/ECM constructs). Upon culture in vitro in chondrogenic medium for 21 days, robust cartilage formation was observed in the Cell/ECM constructs. Similarly prepared Cell/ECM constructs were tested in vivo by subcutaneous implantation into SCID mice. Prominent cartilage formation was observed in the implanted Cell/ECM constructs 14 days post-implantation, with higher sGAG deposition compared to controls consisting of chondrocyte cell sheets. Taken together, these findings demonstrate that hBMSC-ECM is a superior culture substrate for chondrocyte expansion and a bioactive matrix potentially applicable for cartilage regeneration in vivo.
Keywords: Extracellular matrix, Bone marrow mesenchymal stem cells, Chondrocyte expansion, Redifferentiation, Chondrogenesis, Micromass, In vivo cartilage formation
Graphical abstract
1. Introduction
Focal cartilage defect, a common knee problem with a very high prevalence (20%) among people of all ages [1–3], represents a challenge in orthopedics, as the avascular articular cartilage has limited intrinsic self-healing ability. If left untreated, these cartilage defects frequently lead to the onset of osteoarthritis (OA), which causes pain, stiffness and limited mobility, with total joint replacement as the final solution [4]. A number of reparative and regenerative approaches have been developed, including microfracture, mosaicplasty, autologous chondrocyte implantation (ACI) or matrix-induced autologous chondrocyte implantation (MACI). Although some treatments have shown benefits in short term follow-ups, such as reduced pain and enhanced mobility [5], complete re-surfacing of articular cartilage has not been achieved. Among these, ACI or MACI, surgical procedures for cartilage repair through the implantation of autologous chondrocytes, represent promising regenerative treatments over other methods [6], but still have many drawbacks that limit their applicability and efficacy. For ACI, the limitations include chondrocyte dedifferentiation during in vitro expansion, the extended time to achieve sufficient cell number needed for implantation, cell leaching from the implantation site, and lack of physical and chondrogenic support for neo-cartilage formation [6–8]. In comparison, MACI also faces the chondrocyte dedifferentiation challenge during expansion, but provides partial solution for some of the problems in ACI by the use of a regeneration biomatrix template. However, the materials used in MACI are often xenogeneic, such as collagen-based scaffolds derived from animals, with the potential of immune reactions and disease transmission [9]. In addition, whether such scaffolds actually support hyaline cartilage formation remains unsettled.
To overcome the dedifferentiation of chondrocytes and the insufficiency of current biomaterials for MACI, various strategies have been utilized for in vitro chondrocyte expansion such as the inclusion of growth factors or coating cell culture substrates with different proteins, as well as developing different scaffolds to assist cartilage regeneration [10–12]. In recent years, the use of extracellular matrices (ECM) derived from native tissues or from in vitro cultured cells, has drawn increasing attention owing to their availability and intrinsic bioactivities. For example, human dermal fibroblast derived ECM has been shown to improve stem cell proliferation and chondrogenic potential compared to TCP [13]. In particular, mesenchymal stem cell (MSC) derived extracellular matrix (MSC-ECM), a natural material with good biocompatibility and bioactivity, has been utilized as a culture substrate to rejuvenate aged mouse stem cells and enhance their lineage differentiation capacity [14]. In our previous study, we have extracted the urea-soluble fraction from human MSC-ECM (U-MECM), and found that U-MECM coated TCP promoted stem cells proliferation, migration and multi-lineage differentiation potential, which was significantly superior to substrates coated with collagen, suggesting that the non-collagenous proteins are likely the bioactive components in U-MECM [15]. Interestingly, porcine synovium-derived stem cells deposited ECM has been shown to be able to improve porcine chondrocyte proliferation and delay chondrocyte dedifferentiation compared with TCP [16]. However, given that both the stem cells and chondrocytes in this study were from 3-month old, juvenile pigs, more investigation involving human adult cells are needed to further demonstrate the utility of MSC-ECM in terms of potential clinical relevance. In addition to being used as a cell culture substrate, ECM has also been tested as a carrier to deliver cells into the defect sites [17]. For example, MSC or chondrocyte derived ECM scaffolds as well as decellularized stem cell deposited ECM have been applied to repair cartilage defects [18–21]. Lu et al. found that MSC or chondrocyte derived ECM scaffolds could promote MSC adhesion, proliferation, chondrogenic potential, as well as cartilage matrix deposition compared to MSCs in pellet culture [20]. Cai et al. also showed that ECM scaffolds mimicking the early stage of chondrogenesis could support better chondrogenesis of MSCs whereas scaffolds mimicking the late stage of chondrogenesis suppressed it [21]. In addition, decellularized chondrocyte-derived ECM has been shown to have a positive effect on the chondrogenesis of human placenta-derived MSCs [22]. It is noteworthy that ECM-generating cells may not have to be removed if they have the potential to become tissue specific cells. Thus, the cell sheet technique, which utilizes both live cells and their deposited ECM, represents an exogenous scaffold-free approach for tissue regeneration. Chondrocyte cell sheets have been shown to be capable of inducing new cartilage formation in vivo as a way to implant chondrocytes [23,24]. An important of this ECM strategy for tissue repair is the potential of generating autologous tissues by using the patient’s own (autologous) cells, thus avoiding the shortcomings of allogenic scaffolds. However, as extended in vitro culturing is required to produce the large number of chondrocytes for deposition of sufficient ECM for transplantation, cell phenotype is likely to be compromised. Also, the mechanical property of stacked cell sheets before and after transplantation requires further optimization.
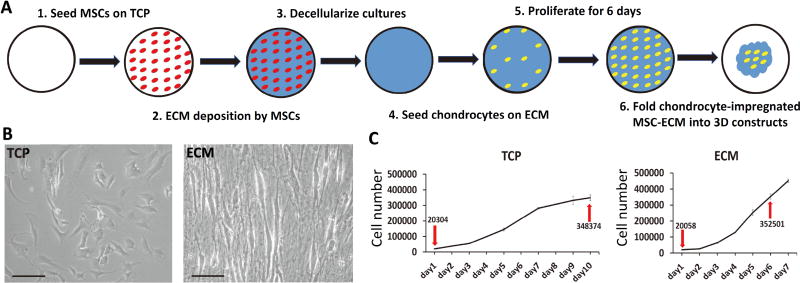
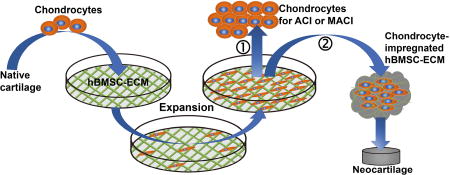
In this study, we tested the applicability of using decellularized human bone marrow derived MSC-ECM (hBMSC-ECM) as a culture substrate for chondrocyte expansion in vitro, as well as a cell-delivery scaffold for chondrocyte-based cartilage repair. As depicted in Figure 1A, chondrocytes were seeded on decellularized hBMSC-ECM at 2,000 cells/cm2 and allowed to expand in vitro following standard chondrocyte culture procedure. Proliferation rate of chondrocytes, chondrogenic potential of expanded cells and redifferentiation ability after expansion were analyzed and compared to cells growing on TCP as control. After 6 days of culture when cells reached 80–90% confluence, the chondrocyte-impregnated hBMSC-ECM cultures were folded into constructs. The cartilage formation capacity of the chondrocyte-impregnated hBMSC-ECM constructs (Cell/ECM constructs), without cell detachment after expansion, was assessed in vitro and in vivo. We hypothesized that hBMSC-ECM would significantly enhance chondrocyte proliferation, maintain chondrocyte phenotype, and promote robust cartilage tissue formation in vitro and in vivo.
Figure 1.
(A) Schematic representation of preparation of chondrocyte-impregnated hBMSC-ECM. (B) Bright field images of chondrocytes growing on TCP and ECM for 5 days or 2 days. (C) chondrocyte growth curve on TCP or ECM. Chondrocytes were seeded initially at the same density (2,000 cells/cm2, shown by down arrows). After proliferation on TCP for 10 days, chondrocytes reached a cell density similar to those growing on ECM for 6 days (shown by up arrows). Bar = 100 µm in B.
2. Materials and Methods
2.1 Isolation of human bone marrow derived mesenchymal stem cells (hBMSCs)
Human bone marrow samples were collected from surgical wastes after total hip arthroplasty with Institutional Review Board approval (University of Pittsburgh and University of Washington). Briefly, trabecular bone was cored out using curette or rongeur and sieved through 40 µm mesh screens to remove remaining debris, and cells were pelleted by centrifugation (300g, 5 min). After rinsing, cells were re-suspended in MSC growth medium (GM, α-MEM containing 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA, USA), 1% antibiotics-antimycotics (Life Technologies, Carlsbad, CA, USA), and 1.5 ng/ml FGF-2 (RayBiotech, Norcross, GA, USA)), and then plated into 150 cm2 tissue culture flasks. On day 4, cells were washed with phosphate-buffered saline (PBS) and fresh GM was added. Medium was changed every 3 to 4 days. Once 70 to 80% confluence was reached, cells were detached with 0.25% trypsin-EDTA (Invitrogen) and passaged. MSC populations isolated from individual patients were routinely validated as capable of osteogenic, adipogenic and chondrogenic differentiation (data not shown). All experiments were performed with passage 4 (P4) MSCs. MSCs isolated and pooled from 3 patients (44 years old male, 69 years old female and 62 years old male) were used in this study.
2.2 Isolation of human articular chondrocytes
Human knee articular chondrocytes were isolated from surgical wastes of patients undergone total knee replacement with IRB approval (University of Pittsburgh and University of Washington). Cartilage pieces from non-fibrillated areas were cut away from the underlying bone and then minced into 1–2 mm morsels with scalpel blades. The minced cartilage was then washed several times with rinsing medium (α-MEM, 2% antibiotics-antimycotics), and weighed. Afterwards, cartilage morsels were digested, with gentle agitation, with collagenase type II (1 mg/ml, 10 ml/gram cartilage) (Worthington Biochemical corporation, Lakewood, NJ, USA) at 37°C for 16 h. The dissociated cells were collected by filtering through a 70 µm mesh. Afterwards, cells were re-suspended in fresh chondrocyte growth medium (DMEM containing 10% fetal bovine serum (FBS, Invitrogen), 1% antibiotics-antimycotics) and seeded into 150 cm2 tissue culture flasks. Medium was changed every 3 to 4 days. Once 70 to 80% confluence was reached, cells were detached with 0.25% trypsin-EDTA (Invitrogen) and ready to use. Chondrocytes isolated from 3 patients (49 years old female, 78 years old male and 48 years old male) were used in this study.
2.3 Preparation of decellularized hBMSC-ECM
hBMSC-ECM was prepared according to a previously reported protocol [15]. Briefly, P4 hBMSCs were seeded into 6-well tissue culture plates (Corning Incorporated, Corning, NY, USA) at a density of 1 × 104 cells/cm2 and cultured in MSC growth medium. After cells reached 100% confluence, 50 µg/mL L-ascorbic acid phosphate (Sigma-Aldrich, St. Louis, MO, USA) was added into GM and cultured for 10 days. The medium was changed every 2 days. The hBMSC-ECM was harvested by treating the cultures with PBS containing 0.5% Triton X-100 (Sigma-Aldrich) and 20 mM ammonium hydroxide at 37 for 5 min to lyse and remove live hBMSCs, followed by washing with PBS for 5 times.
After washing, decellularized hMSC-ECM was collected from 3 different wells and its dsDNA content determined using the Quant-iT PicoGreen Reagent (Invitrogen) according to the manufacturer’s instructions. The dimension of each decellularized hMSC-ECM sample was calculated by dividing its volume by its area, which is 950 mm2 for each well of a 6-well culture plate (n = 6; 3 individual plates; total n = 18).
2.4 In Vitro Chondrocyte expansion on hBMSC-ECM or TCP
Chondrocytes were seeded in 6-well culture plates (TCP), some of which were coated with hBMSC-ECM, at a density of 2,000 cells/cm2, and cultured with fresh chondrocyte growth medium for up to 10 days. Medium was changed every 2–3 days. Cell morphology at different time points on different substrates was captured using an Olympus CKX 41 microscope equipped with a Leica camera, using LAS V4.9 software. The groups were denoted as ECM and TCP, respectively. Cell proliferation rate of chondrocytes growing on different substrates was analyzed with MTS assay (Promega, Madison, WI, USA) over a period of 10 days, as cells growing on ECM reach confluence after 7 days and those on TCP after 10 days. Briefly, at day 1, 2, 3, 4, 5, 6, 7 for ECM group and day 1, 3, 5, 7, 9, 10 for TCP group, 3 ml of diluted MTS solution was added to each of the three wells of cultures of ECM and TCP groups. After incubation in 37°C for 2h, a 200 µl aliquot of the MTS solution was aspirated from each well and transferred to a 96-well plate for measurement of absorbance at 492 using a plate reader (Biotek Synergy HT, Winooski, VT, USA). Corresponding cell numbers were calculated based on a standard curve calibrated using a set of cultures of known cell numbers.
2.5 Quantitative real-time RT-PCR
Total RNA of the cells growing on different substrates were extracted at different time points with TRIZOL reagent (Invitrogen) and purified using RNeasy® Plus Mini Kit (Qiagen, Germantown, MD, USA). Reverse transcription was achieved using SuperScript® VILO™ cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s protocol. Real-time PCR was performed using the SYBR Green Reaction Mix (Applied Biosystems, Foster City, CA, USA) with a StepOne-Plus thermocycler (Applied Biosystems). Gene expression levels of collagen type II (i.e., the alpha 1 chain of collagen type II, COL2A1), aggrecan, collagen type I (i.e., the alpha 2 chain of collagen type I, COL1A2), collagen type X, alkaline phosphatase (ALP) and matrix metalloproteinase 13 (MMP13) were analyzed. Sequence information of primers for the tested genes is provided in Table 1. All gene expression levels were determined using the 2-ΔΔCt method, and normalized to that of human 18S rRNA as a housekeeping gene standard.
Table 1.
Primer sequences for real-time PCR
| Genes | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| Collagen type I (COL1A2) | GGG CTC TAA TGA TGT TGA ACT TGT | ATG ATT GTC TTT CCC CAT TCA TTT |
| Aggrecan | AGT CAC ACC TGA GCA GCA TC | AGT TCT CAA ATT GCA TGG GGT GTC |
| Collagen type II (COL2A1) | GGA TGG CTG CAC GAA ACA TAC CGG | CAA GAA GCA GAC CGG CCC TAT G |
| Collagen type X | GTG TTT TAC GCT GAA CGA TAC CAA | ACC TGG TTT CCC TAC AGC TGA TG |
| Matrix metalloproteinase 13 | ATG CAG TCT TTC TTC GGC TTA G | ATG CCA TCG TGA AGT CTG GT |
| Alkaline phosphatase | ATC TTT GGT CTG GCC CCC ATG | AGT CCA CCA TGG AGA CAT TCT CTC |
| 18S rRNA | GTA ACC CGT TGA ACC CCA TT | CCA TCC AAT CGG TAG TAG CG |
2.6 Re-differentiation of expanded chondrocytes in micromass culture
P1 chondrocytes were seeded at 2,000 cells/cm2 and grown on ECM for 6 days and on TCP for 10 days, respectively, at which point they had reached the same cell number (see Results), and were then trypsinized and detached from the substrates and suspended at 2 × 107 cells/ml with chondrogenic medium (DMEM, 40 µg/mL proline (Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), 1% antibiotics-antimycotics, 50 µg/mL L-ascorbic acid (Sigma-Aldrich), and 1×Insulin-Transferrin-Selenium (Life Technologies)) with the supplementation of 10 ng/mL of transforming growth factor-β3 (TGFβ3) (Peprotech, Rocky Hill, NJ, USA). Then 5 µl of the suspension was pipetted into the center of each well of a 48-well tissue culture plate, and allowed to attach for 30 minutes before another 300 µl fresh chondrogenic medium was applied. The micromasses were cultured with chondrogenic medium for 14 days. Micromass cultures derived from chondrocytes that were expanded on TCP for 10 days or on hBMSC-ECM for 6 days were designated as TCP or ECM group.
2.7 Western blot
The chondrogenic response of expanded chondrocytes in response to TGFβ3 stimulation was analyzed by assessing Smad2/3 phosphorylation. Chondrocytes harvested from cultures grown on hBMSC-ECM for 6 days, or on TCP for 10 days were exposed to 10 ng/ml TGFβ3 for 40 min, and protein extracts prepared using RIPA buffer (Sigma-Aldrich). After reducing SDS-PAGE, protein blots were performed using low fluorescence background polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA), blocked in 3% milk in TBS-T (0.25% Tween-20 in TBS) for 1 hour, and probed overnight at 4°C with various antibodies (GAPDH, Smad2/3 and phospo-Smad2/Smad3 (Cell Signaling Technology, Danvers, MA, USA)) in 1% milk/TBS-T. Immunodetection was performed with HRP-conjugated secondary antibodies (Thermo Scientific, Waltham, MA, USA), followed by chemiluminescent HRP substrate (Thermo Scientific), and imaged with a Fotodyne/Analyst FX CCD camera system (FOTODYNE Incorporated, Hartland, WI, USA).
2.8 Histology
Micromass cultures were fixed in 4% paraformaldehyde at 4°C overnight, dehydrated with a gradient ethanol series, followed by clearing with xylene and embedding in paraffin. Histological sections (7 µm thickness) were stained with Safranin O solution to detect sGAG with Fast Green as a counterstain.
2.9 Biochemical analyses
Cartilage ECM deposition was quantified by measuring sGAG as a function of dsDNA content. Constructs were homogenized and then digested for 18 h in a papain solution (125 µg/ml papain, 50mM sodium phosphate buffer, 2 mM N-acetyl cysteine (Sigma-Aldrich), pH 6.5) at 500 µl/construct. An aliquot of the digest was assayed for sGAG content using the dimethylmethylene blue dye binding assay (Blyscan, Biocolor, United Kingdom) according to the manufacturer’s instruction. dsDNA was quantified with another aliquot of the digest using PicoGreen based assay. All assays were performed in triplicate.
2.10 Assessment of in vitro cartilage formation by chondrocyte-impregnated hBMSC-ECM constructs
Chondrocyte-impregnated hBMSC-ECM constructs detached from culture substrates displayed a very loose architecture (Supplementary Figure 1). We hypothesized that a brief trypsin treatment could improve cell-mediated contraction to result in a more compact structure. In this study, chondrocytes seeded at 2,000 cells/cm2 on hBMSC-ECM were expanded for 6 days, and then the cultures were treated with 0.25% trypsin-EDTA for 2.5 minutes until the cells were seen to adopt a round morphology but remain attached to the ECM as observed under the microscope. Afterwards, trypsin treatment was terminated with addition of chondrocyte growth medium. Cultures with or without trypsin pretreatment were detached by gentle agitation, dislodging the entire structure, which was then picked up with a small tweezer and placed individually into each well of a 48-well culture plate. The detached chondrocyte-impregnated hBMSC-ECM would slowly contract and form smaller individual constructs by themselves overnight. The folded constructs were then cultured in full chondrogenic medium for 21 days. The folded constructs were denoted as Cell/ECM constructs, and groups with or without trypsin pretreatment were denoted as Trypsin+ group and Trypsin- group, respectively.
2.11 Chondrocyte cell sheet formation
Chondrocyte cell sheets were prepared using the procedure of Kaneshiro et al.[25] Chondrocytes were seeded at 10,000 cells/cm2 on TCP and cultured in cell sheet growth medium (DMEM containing 20% FBS, 1% antibiotics-antimycotics, 50 µg/mL L-ascorbic acid) for 10 days. After the cell sheets formed, they detached with a cell scraper and rounded up to smaller constructs. The folded constructs were then put in 48-well plate and cultured in full chondrogenic medium for 10 days. In the present study, chondrocyte cell sheet constructs are denoted as Cell Sheet constructs.
2.12 In vivo cartilage formation
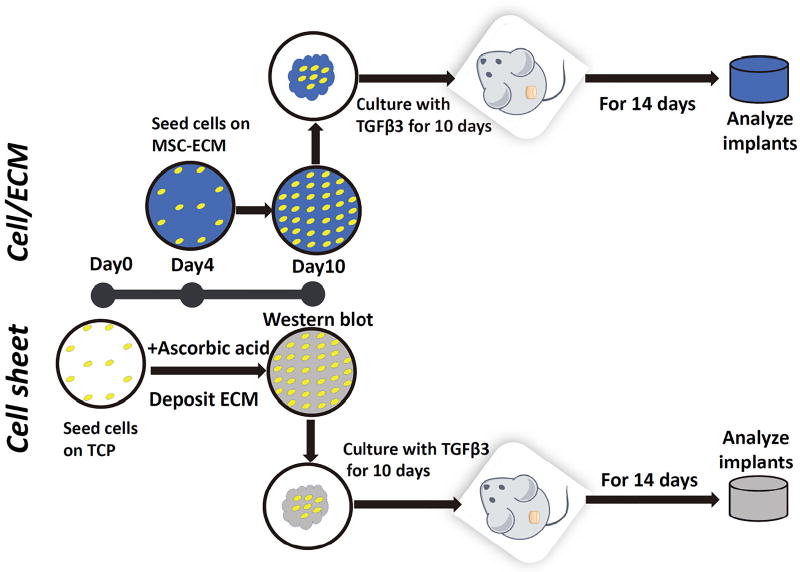
Female Severe Combined Immunodeficiency (CB17/Icr-Prkdcscid/IcrIcoCrl SCID®) mice (8–12 weeks old; Charles River Laboratories; Wilmington, MA) were used to assess in vivo cartilage formation by chondrocyte cell sheets (Cell Sheet group) or chondrocyte-impregnated hBMSC-ECM (Cell/ECM group), prepared with the procedures described above, using a protocol by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) and performed in accordance with relevant guidelines and regulations. The experimental timeline is summarized in Figure 7. Cell/ECM constructs and Cell sheet constructs were formed at culture day 10, and then maintained in chondrogenic medium with TGFβ3 for additional 10 days in vitro and then implanted subcutaneously into SCID mice. After 2 weeks, the constructs were harvested. Gene expression levels of chondrogenesis markers (collagen type II, aggrecan), sGAG content, and histological analysis were performed as described above.
Figure 7.
Schematic of production of cell sheet constructs and Cell/ECM constructs and in vivo cartilage formation testing via subcutaneous implantation in SCID mice. Chondrocytes were thawed and expanded in tissue culture flasks for 6 days and seeded at 10,000 cells/cm2 on TCP and cultured in cell sheet growth medium at day 0. Same number of chondrocytes were expanded for 6 days and seeded at a density of 2,000 cells/cm2 on hBMSC-ECM at day 4. At day 10 of total culture period, folded chondrocytes-impregnated hBMSC-ECM and chondrocytes cell sheets were treated with full chondrogenic medium for an additional 10 days of in vitro culture, and then subcutaneously implanted in SCID mice. At 2 weeks post-implantation, the mice were sacrificed and the implants were excised and processed for different analyses of cartilage formation in vivo.
2.13 Statistical analysis
All data from control and experimental groups were analyzed using the unpaired Student's t-test or two-way ANOVA, with statistical difference set as p < 0.05. All values were presented as mean ± standard deviation.
3. Results
3.1 Culturing on hBMSC-ECM promotes proliferation of chondrocytes
After decellularization, the hMSC-ECM used, with dimension of 950 mm2 in area and 31.11 ± 2 µm in thickness, showed little residual dsDNA remaining (24 ng per piece of hMSC-ECM). P1 chondrocytes seeded at 2,000 cells/cm2 onto hBMSC-ECM were compared to cells similarly seeded on TCP in terms of proliferation and morphology. The MTS assay results (Figure 1C) showed that cells seeded on hBMSC-ECM proliferated much faster than those on TCP. Cell counting data showed that after 6 days of culture expansion, chondrocytes on hBMSC-ECM reached confluence with a 17.5-folder increase in cell number, which was equal to those after 10 days of expansion on TCP. Cells from these 2 culture conditions were denoted as day 6 ECM and day 10 TCP, which had undergone similar population doublings. Compared to a flat and polygon morphology seen in the day 10 TCP group, cells of the day 6 ECM group were smaller, more refractile, and displayed a spindle shape (Figure 1B).
3.2 Culturing on hBMSC-ECM enhances chondrocytic phenotype
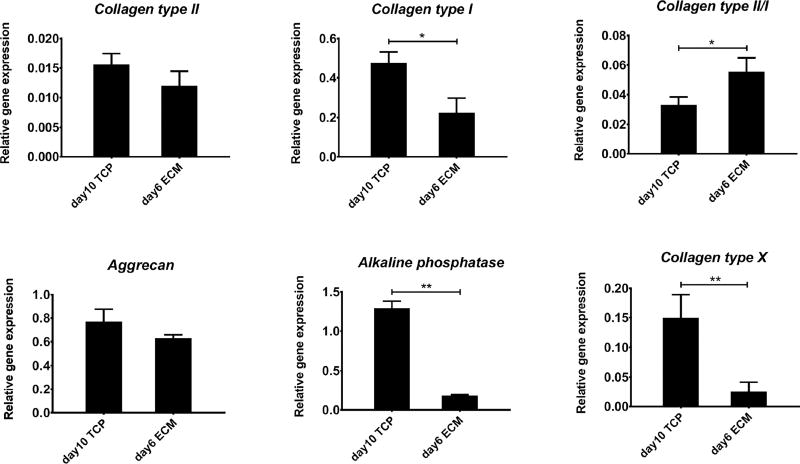
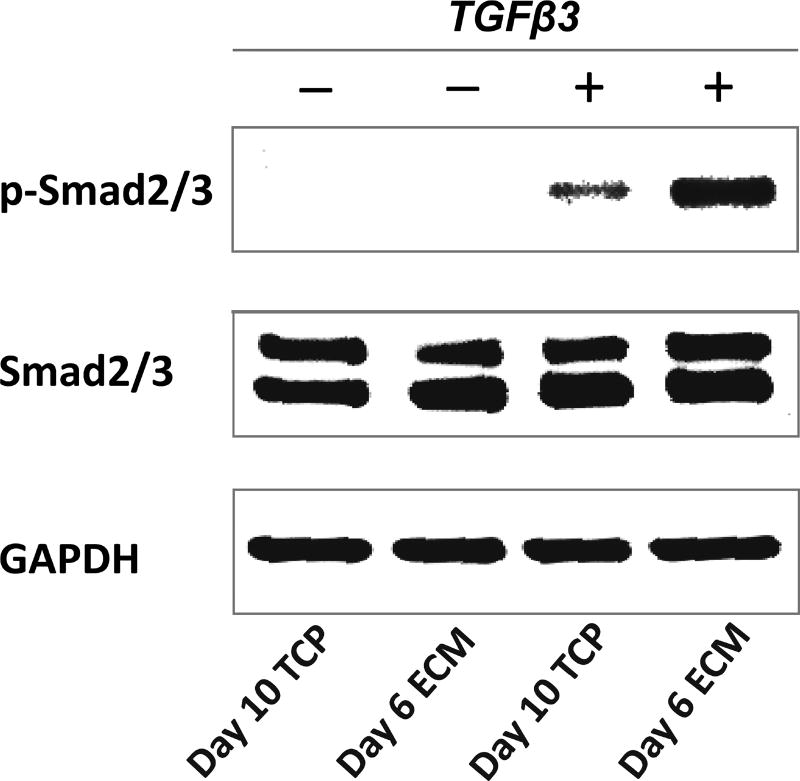
At similar population doubling, cells grown on hBMSC-ECM maintained better chondrocytic phenotype indicated by higher ratio collagen type II/collagen type I gene expression, and significantly lower expression of hypertrophy genes, such as collagen type X and ALP, compared to those expanded on TCP (Figure 2), suggesting the advantage of hBMSC-ECM on the maintenance of chondrocyte phenotype. Specifically, we evaluated the signaling response of the chondrocytes to the chondrogenesis-inducing factor, TGFβ3. As shown in Figure 3, Western blot analysis showed that higher level of p-Smad2/3 after 40 min TGFβ3 exposure in day 6 ECM chondrocytes compared to day 10 TCP chondrocytes, suggesting a more robust chondrogenic potential in the former.
Figure 2.
Real time RT-PCR analysis of expression levels of chondrogenic and hypertrophic genes in chondrocytes growing on TCP for 10 days (day 10 TCP), or on ECM for 6 days (day 6 ECM), both of which have undergone similar rounds of proliferation or population doubling. Collagen type II stands for COL2A1, and collagen type I stands for COL1A2. Results are normalized to those in cells prior to seeding, and shown as mean ± SD (*, p<0.05; **, p<0.01).
Figure 3.
Representative Western blot analysis of phosphorylated Smad2/3 (p-Smad2/3) and total Smad2/3 (Smad2/3) in chondrocytes after being expanded on TCP for 10 days (day 10 TCP), or on ECM for 6 days (day 6 ECM), with or without TGFβ3 stimulation. Western blots of GAPDH indicate relatively even protein loading among different lanes. The results showed that higher p-Smad2/3 level in day 6 ECM group compared to day 10 TCP group upon chondro-induction.
3.3 hBMSC-ECM-expanded chondrocytes exhibited higher chondrogenic capacity in micromass culture in vitro
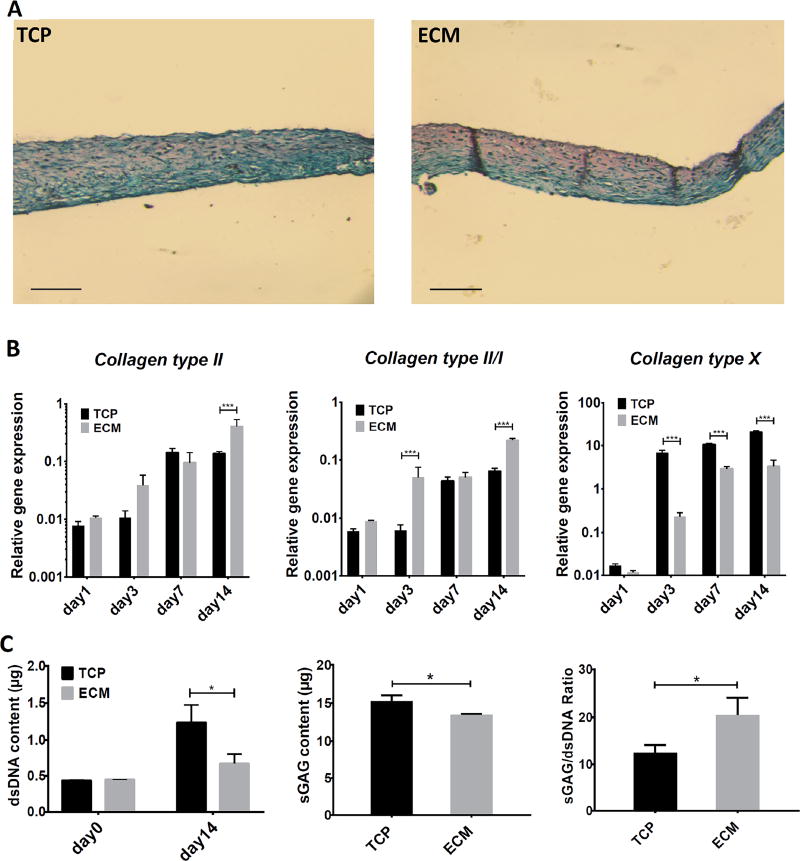
The redifferentiation ability of chondrocytes after expansion on different substrates (day 6 ECM and day 10 TCP) were tested using high-density micromass cultures in TGFβ3-containing, full chondrogenic medium. At culture day 14, chondrogenic phenotype was assayed using a number of assays, including quantitative RT-PCR on two sets of marker genes (chondrogenesis and hypertrophy), sGAG content, and histology. The RT-PCR data revealed that micromasses formed by cells from the ECM group showed higher collagen type II and collagen type II/collagen type I gene expression and significantly lower collagen type X expression (Figure 4B), although expression of aggrecan and MMP-13 showed no significant difference (data not shown). Histological analysis showed that micromass cultures from ECM group had more intense Safranin O staining than their counterparts derived from TCP group (Figure 4A). Biochemical analysis showed that although ECM micromasses had individually slightly lower total sGAG content, but their dsDNA content (or cell number) was considerably less, similar to the value at day 0, suggesting limited cell proliferation. The significantly higher sGAG/dsDNA in the ECM group thus suggested their preference for chondrogenesis instead of proliferation.
Figure 4.
Assessment of redifferentiation ability of chondrocytes expanded on TCP for 10 days (TCP), or on ECM for 6 days (ECM). Cells were cultured as high-density micromass for 14 days with the treatment of full chondrogenic medium. Chondrogenesis was estimated by histology, real time PCR and biochemical analysis. (A) sGAG of TCP (left) and ECM (right) micromass cultures was detected with Safranin O staining. ECM cultures showed deeper stained sGAG. (B) Real-time PCR analysis of relative mRNA expression levels of chondrogenic (collagen type II and collagen type II/collagen type I) and hypertrophic (collagen type X) genes at different time points. Results are normalized to mRNA level in chondrocytes without culture expansion. ECM micromasses showed higher collagen type II, collagen type II/collagen type I gene expression and considerably lower collagen type X expression. Collagen type II refers to COL2A1, and collagen type I refers to COL1A2. (C) Dimethylmethylene blue dye binding assay and picogreen assay were used to detect sGAG and dsDNA, respectively, and sGAG/dsDNA was calculated. The sGAG/dsDNA ratio of ECM cultures was significantly higher than that of TCP cultures. Data are shown as average ± SD for n = 3. *, p<0.05; ***, p<0.001. Bar=150µm.
3.4 Trypsin pre-treatment enhanced cartilage formation by Cell/ECM constructs in vitro
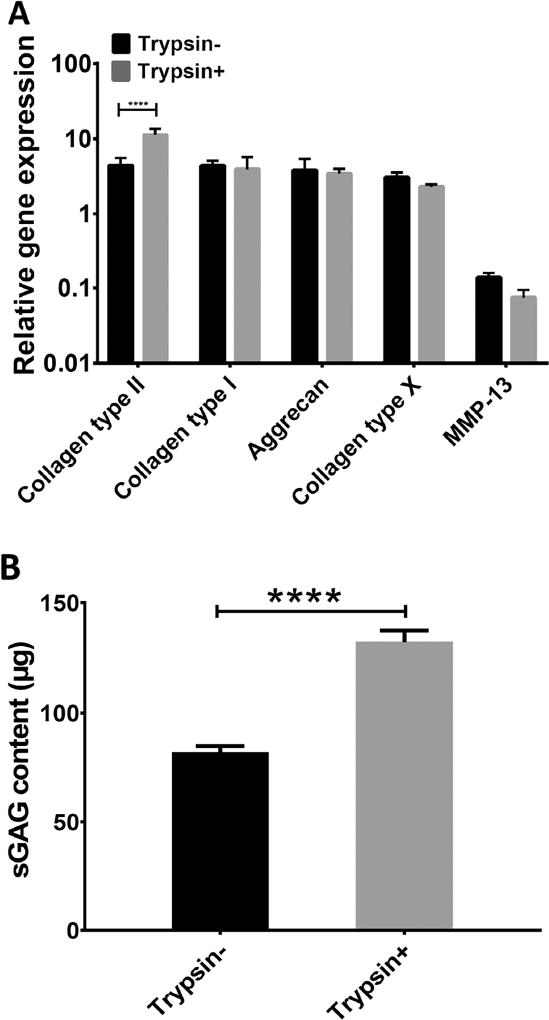
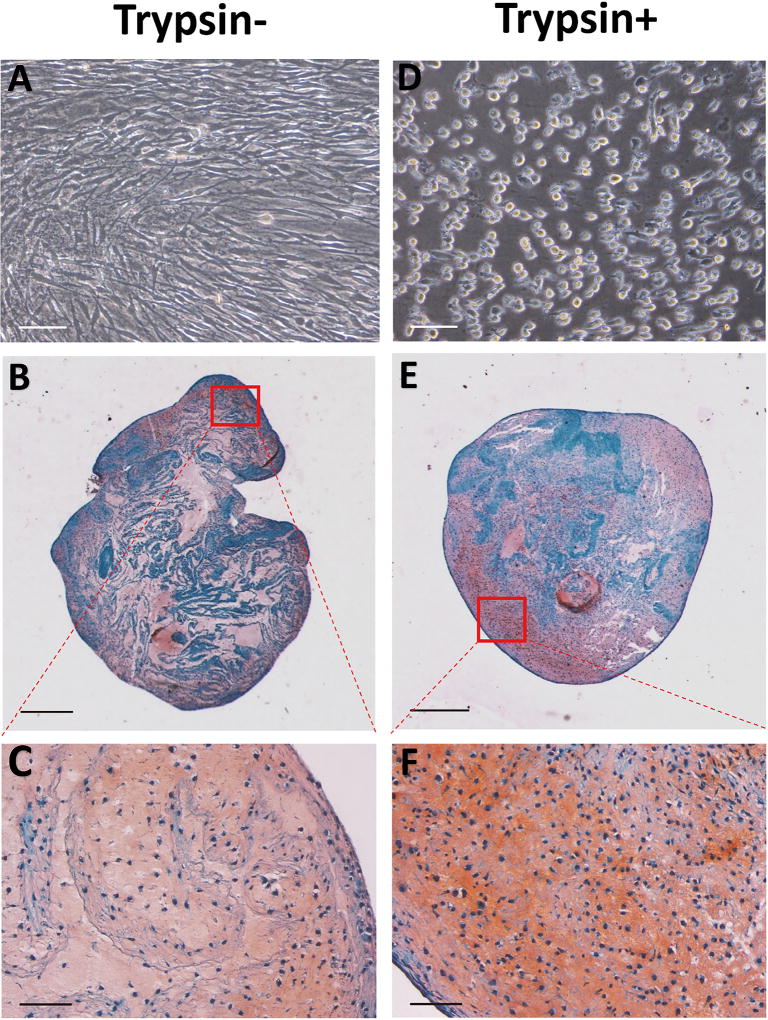
As Cell/ECM constructs consisted of a loose structure when left without further treatment (Supplementary Figure 1), we tested whether a brief trypsin treatment would allow improved cell-mediated contraction so that the constructs would become more compact. We observed that after being exposed to brief trypsin treatment, followed by washing, Cell/ECM constructs readily formed more compact structures. To examine the cartilage formation ability of such constructs, trypsin treated (Trypsin+) and untreated constructs (Trypsin-) were then placed in culture with TGFβ3-containing, full chondrogenic medium. After 21 days, the Trypsin+ group showed significantly higher collagen type II expression compared with Trypsin- group, although no significant difference was seen with regard to the rest of the genes between the two groups (Figure 6A). Histological analysis showed that the Trypsin+ group formed a more condensed structure. Image J-based area measurement revealed that the average dimension of the Trypsin+ constructs was 22.4 less than that of the Trypsin- group, suggesting a contracting process occurring during the 21-day culture). The Trypsin+ group also displayed more uniform distribution of Safranin O-positive sGAG compared to Trypsin- group (Figure 5B, C, E, F). Quantitation by methylmethylene blue dye binding assay showed significantly higher total sGAG content in the Trypsin+ group (Figure 6B). Taken together, a brief trypsin-EDTA treatment of the Cell/ECM cultures resulted in uniform and compact construct formation and significantly improved the level of cartilage formation by the seeded chondrocytes.
Figure 6.
Assessment of in vitro chondrogenic capacity of chondrocyte-impregnated hMSC-ECM: Gene expression and sGAG analysis. After being expanded on ECM for 6 days, chondrocytes were subjected to 2.5 minutes trypsin-EDTA treatment (Trypsin+), with no treatment as control (Trypsin-). Afterwards, these chondrocytes-impregnated hBMSC-ECM constructs (Cell/ECM constructs) were detached from culture plate and cultured for 21 days of culture in full chondrogenic medium. (A) Real time RT-PCR analysis of mRNA expression levels of chondrogenesis (collagen type II, aggrecan and collagen type I) and hypertrophy (collagen type X, MMP-13) genes at various culture time points, normalized to those of non-culture expanded cells. Collagen type II expression level was higher in the Trypsin+ group, while other genes showed similar expression levels in the Trypsin+ and Trypsin- groups. Collagen type II refers to COL2A1, and collagen type I refers to COL1A2. (B) Dimethylmethylene blue dye binding assay showed significantly higher sGAG content in the Trypsin+ group. ****, p<0.0001.
Figure 5.
Assessment of in vitro chondrogenic capacity of chondrocyte-impregnated hMSC-ECM. Chondrocytes cultured on hMSC-ECM were briefly trypsinized, detached together with the ECM, and cultured for additional 21 days in full chondrogenic medium. The extent of chondrogenesis was estimated histologically. (A, D) Microscopic images of on-ECM chondrocytes with (D) or without (A) trypsin-EDTA treatment. Cells displayed round shapes after the treatment. (B, C, E, F) Safranin O staining of Cell/ECM constructs with (E, F) or without (B, C) trypsin-EDTA treatment after chondro-induction for 21 days. Trypsin+ group showed more compact structure and more uniformly distributed Safranin O staining of sulfated proteoglycan. Bar = 500 µm (B, E) or 150 µm (A, D, C, F).
3.5 In vivo cartilage formation by cultured Cell/ECM constructs and Cell sheet constructs in SCID mice
The ability of the Cell/ECM constructs to form neo-cartilage in vivo was next tested by subcutaneous implantation in SCID mice, with standard chondrocyte cell sheet constructs as control. The experimental timeline is summarized in Figure 7. Day 10 chondrocyte-impregnated hBMSC-ECM (Cell/ECM group) and chondrocyte cell sheets (Cell sheet group) were folded into 3D constructs, cultured in TGFβ3-containing, full chondrogenic medium for another 10 days, and then implanted subcutaneously in SCID mice. At 2 weeks post-implantation, the implanted constructs were harvested for different analyses.
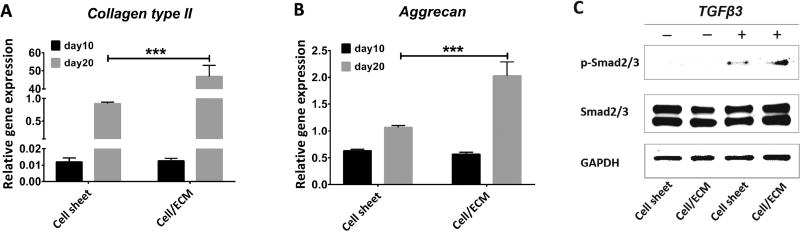
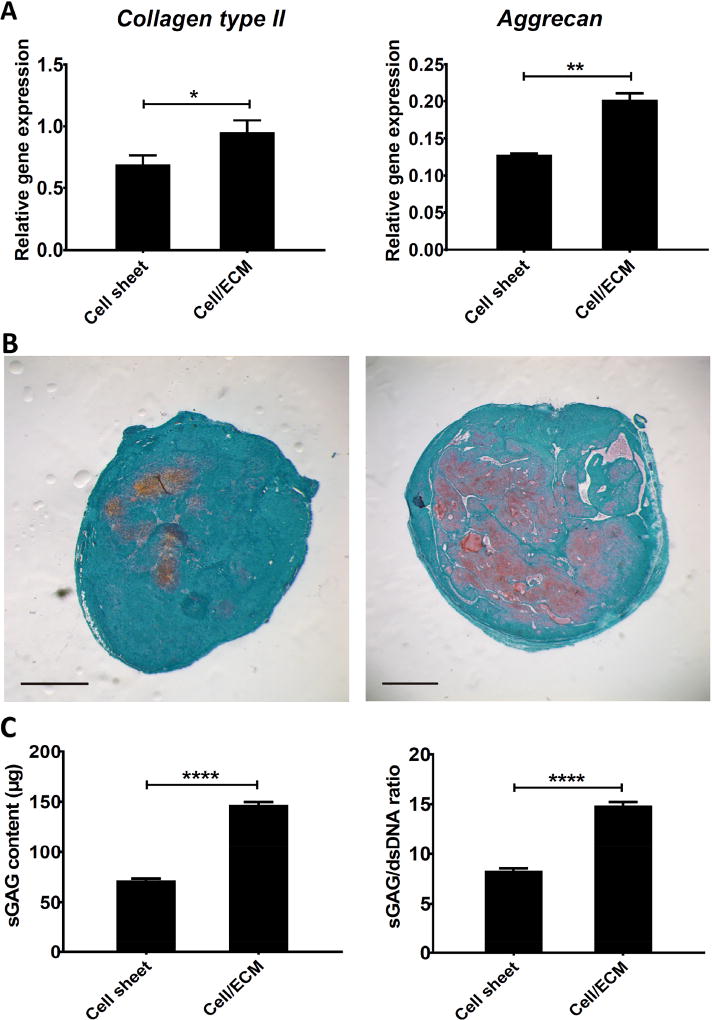
At day 10, before chondrogenic stimulation, cells from both groups showed statistically similar expression levels of chondrogenesis marker genes (collagen type II, aggrecan) (Figure 8A, B). However, it should be noted that, due to enhanced cell proliferation on the hBMSC-ECM, the starting cell number needed for the Cell/ECM group was 5 times less than for the Cell sheet group. Western blot was performed to assess the responsiveness to TGFβ3 of chondrocytes in the Cell/ECM group and Cell sheet group. The results showed that after a 40-minute treatment with full chondrogenic medium, higher p-Smad2/3 level was in the Cell/ECM group compared to the Cell sheet group (Figure 8C), suggesting enhanced chondrogenic potential in the former. After an additional 10-day chondro-inductive treatment, significantly higher gene expression levels of chondrogenesis markers (collagen type II, aggrecan) were also seen in the Cell/ECM constructs (Figure 8A, B). When harvested at 2 weeks post-implantation, implants of Cell/ECM constructs were uniformly stiffer to the touch than those from Cell sheet constructs, suggesting substantial matrix deposition. However, the irregular shapes of these constructs preclude rigorous measurement of mechanical properties, such as compressive modulus. As shown in Figure 9A, the Cell/ECM implants expressed higher levels of chondrogenesis markers (collagen type II and aggrecan). Histological observation showed that the larger-size Cell/ECM implants had substantially more intense Safranin O staining of sGAG than Cell sheet constructs (Figure 9B). This was further confirmed by the biochemical analysis results, showing significantly higher total sGAG content and sGAG/dsDNA in implants of Cell/ECM constructs versus those in implants of Cell sheet constructs (Figure 9C).
Figure 8.
Characterization of chondrocyte-impregnated hBMSC-ECM constructs used for subcutaneous implantation. Chondrocytes were seeded to form chondrocyte cell sheets (Cell sheet group) or chondrocyte-impregnated hBMSC-ECM (Cell/ECM group) in culture. After 10 days (day 10), RT-PCR and western blotting were performed. Both groups were then folded into 3D constructs. After 10 additional days of in vitro chondro-induction (day 20), RT-PCR assay was performed prior to the constructs being used for subcutaneously implantation. (A) Collagen type II (COL2A1) expression; and (B) aggrecan expression. RT-PCR analysis showed similar levels in the cell sheet and Cell/ECM groups on day 10, but significantly higher collagen type II and aggrecan expression in the Cell/ECM group at day 20. (C) Representative western blot analysis of Smad2/3 activation in cell sheet and Cell/ECM groups upon TGF-β3 treatment. The results showed that the Cell/ECM group displayed higher TGF-β3 dependent p-Smad2/3 level, consistent with higher chondro-induction, compared with the Cell sheet group. Values shown are mean ± SD (n = 3; *, p<0.05; **, p<0.01).
Figure 9.
Assessment of in vivo cartilage forming potential of chondrocyte-impregnated hBMSC-ECM constructs. Chondrocytes were cultured on hBMSC-ECM for 6 days to generate chondrocyte-impregnated hBMSC-ECM constructs (Cell/ECM), or for 10 days on TCP to generate chondrocyte cell sheets. After 10 additional days of stimulation with chondrogenic medium, the constructs were subcutaneously implanted, and cartilage formation capacity was examined 2 weeks post-implantation. (A) Collagen type II (COL2A1) and aggrecan gene expression. RT-PCR results, normalized to those prior to culture expansion, showed that Cell/ECM group had significantly higher level of both genes compared with Cell sheet group. (B) Histological analysis of cell sheet (left) and Cell/ECM (right) implants, based on Safranin O staining, showed more abundant and evenly distributed sulfated proteoglycan distribution in the Cell/ECM group compared with the Cell sheet group. Bar = 1 mm. (C) Results from the Dimethylmethylene blue and picogreen based assays showed that the Cell/ECM group had significantly higher sGAG content and sGAG/dsDNA ratio compared with the Cell sheet group. *, p<0.05; **, p<0.01; ****, p<0.0001.
4. Discussion
The goal of this investigation was to address a key challenge in the practice of ACI and MACI, i.e., loss of chondrocytic phenotype during in vitro expansion of chondrocytes [23]. The results presented here showed that hBMSC-ECM, as a culture substrate, promoted cell proliferation, delayed dedifferentiation and improved chondrogenic potential for expanded human chondrocytes, compared to TCP. Harvested cells that have been expanded on hBMSC-ECM also showed better redifferentiation ability when subsequently placed in a micromass culture system, compared with cells expanded on TCP. Moreover, chondrocyte-impregnated hBMSC-ECM manifested good chondrogenesis in both in vitro and in vivo experiments, suggesting applicability of the use of hBMSC-ECM as a means to improve clinical outcomes of ACI and MACI procedures.
Decellularized ECM has emerged in recent years as a type of natural, bioactive biomaterial potentially applicable in tissue engineering, to provide physical, chemical and mechanical cues for seeded and neighboring cells. In addition to tissue-derived ECM preparations, cell-derived ECM represents a safer, alternative ECM source, as autologous cells may be used to minimize the potential risk of immune responses and pathogen transmission [26]. A number of approaches are currently being used to expose cultured cells to ECM to simulate natural cell-ECM interactions. One approach is to directly coat the culture substrate with ECM protein. It has been shown that decellularized ECM from human MSC cultures could drastically promote MSC proliferation compared with TCP.[27] In fact, our previous findings showed that coating of an urea-extracted fraction of human MSC-ECM (U-MECM) was able to improve MSC proliferation more than coatings consisting of single proteins, such as collagen type I, suggesting the involvement of multiple proteins in the ECM [15]. In addition, ECM deposited by porcine synovium-derived stem cells has been shown to improve proliferation and delay dedifferentiation of chondrocytes, compared to TCP [16]. Our results shown in Figure 1C agree with these findings. Although the underlying mechanism responsible for the proliferation supportive activity of the ECM derived from stem cells is not known, the following possibility may be considered. First, it should be noted that collagen type I, a major component of the ECM, has known mitogenic effects on cells such as hBMSCs [28]. However, this is unlikely in the case of cultured chondrocytes, since collagen type I is not a natural component of cartilage matrix, and it has been shown that collagen type I coating alone did not significantly affect chondrocyte proliferation rate [11]. In fact, in our previous study, non-collagenous components were shown to account for the enhanced proliferation of hBMSCs [15]. A more likely possibility is that as the ECM acts as a natural depot of growth factors, which are introduced into the chondrocyte cultures to stimulate cell proliferation. For example, we have recently identified more than 10 growth factors, including high level of the mitogen factor, FGF-2, in urea-solubilized ECM derived from articular cartilage [29]. We therefore postulate that hBMSC-ECM proteins and sequestered growth factors together contribute to the mitogenic effect on chondrocytes [30], although the exact underlying mechanism requires further study in the future.
Aside from promotion of proliferation, cell-derived ECM also exhibits other effects on cells. For example, it has been shown that decellularized MSC-derived ECM enhanced maintenance of MSC stemness after extensive proliferation [31]. Pei et al. demonstrated that porcine chondrocytes retained a better collagen type II mRNA level after expansion on synovium derived stem cell ECM, compared to cells expanded on TCP (16). In the present study, we also found that comparing chondrocytes growing on different substrates that have reached the same cell number, cells on ECM retained better chondrogenic gene expression in terms of higher collagen type II /collagen type I ratio. Interestingly, previous studies have shown that chondrocytes cultured on a collagen type I-coated substrates displayed a suppressed dedifferentiation phenotype [11], while exposure to fibronectin, a component of MSC-ECM [27] enhanced the response of chondrocytes to insulin-like growth factor-1 (IGF-1), and maintained better chondrocytic phenotype [32]. In this study, as shown in Figure 2, we observed that expansion on hBMSC-ECM resulted in significantly inhibited expression of chondrocyte hypertrophy associated genes, including collagen type X and ALP, which is critical for hyaline cartilage formation. Similar results have not been reported for chondrocytes cultured on other ECM preparations or individual ECM components. While the exact mechanism for this effect remains to be elucidated, chondrocytes cultured on MSC-ECM generally appeared smaller in size than those cultured on TCP, consistent with a less hypertrophic state. Our findings, taken together with those of others, strongly suggest that chondrocytes are highly responsive to their ECM environment, which may be manipulated for their optimal application in cartilage repair and regeneration.
The production of neo-cartilage by implanted chondrocytes, which are generally used after culture expansion, is critically dependent on their redifferentiation ability when introduced into a 3D environment. Supplementation of culture medium with TGFβ3 (e.g., 10 ng/mL) is commonly used to stimulate the redifferentiation of chondrocytes [33]. In our study, we used TGFβ3 supplemented medium for high density micromass cultures of expanded chondrocytes to examine their chondrogenic redifferentiation activity. After 14 days of culture, dsDNA quantitation showed only a low level of cell proliferation in micromass cultures of chondrocytes exposed to hBMSC-ECM, while TCP-expanded chondrocytes maintained in similar micromass cultures more than doubled in cell number (Figure 4C). In addition, RT-PCR analysis showed that the former exhibited better chondrogenic marker gene expression (Figure 4B). Preconditioning of the chondrocytes with different substrates likely contributed to this difference. Specifically, we observed that chondrocytes expanded on hBMSC-ECM displayed higher level of p-Smad2/3 level upon TGFβ3 induced chondrogenesis (Figure 3), consistent with the known signaling pathway of TGFβ3 action on mesenchymal chondrogenesis [34,35]. While TGFβ1 has also been reported to promote chondrocyte proliferation, also via p-Smad2/3 signaling [36], we observed here that higher p-Smad2/3 is associated with decreased cell proliferation. Such difference could be related to the difference in culture conditions, i.e., monolayer culture versus micromass culture, and TGFβ1 versus TGFβ3). It is also possible that the longer trypsin treatment period required to dissociate chondrocytes from the hBMSC-ECM could have altered cell surface receptors to result in a different cellular response to TGFβ3. In addition to Smad2/3, Smad1/5/8 may also be involved in the control of cell proliferation. For example, inhibition of p- Smad1/5/8 with SB505124 did not affect the level of p-Smad2/3, but significantly reduce chondrocyte proliferation.[36] Future studies should aim to elucidate the interplay between p- Smad2/3 an p-Smad1/5/8 signaling in controlling chondrocyte phenotype.
Another aim of our study is to investigate the utility of hBMSC-ECM as a vehicle to encapsulate and deliver cells ex vivo. After allowing chondrocytes to expand on hBMSC-ECM, we folded the chondrocytes-impregnated hBMSC-ECM into 3D Cell/ECM constructs. In our in vitro study, Cell/ECM constructs were cultured in full chondrogenic medium for 21 days and their ability to form cartilage examined. In initial experiments, we observed that while the Cell/ECM constructs did display Safranin O positive sGAG staining, the staining was not uniform and the constructs were structurally weak and loose (Supplementary Figure 1). As it has been previously suggested that cell-mediated contraction of scaffolds could lead to better structures and chondrogenesis [37], we developed a simple protocol to prepare a more optimal Cell/ECM construct by first enhancing cell rounding, followed by cell spreading and associated cell-mediated contraction, similar to what has been observed in collagen-based experiments [38]. The protocol involved: (1) brief trypsinization of the chondrocyte-impregnated hBMSC-ECM for 2.5 minutes using 0.25% trypsin-EDTA to make cells adopt a round shape, but remain attached to hBMSC-ECM which was observed under microscope; and (2) termination of the trypsin treatment using chondrocyte growth medium; (3) detachment and collection of the entire 3D construct off the culture plates using a cell scraper (Figure. 5A, D). As expected, the trypsinized Cell/ECM constructs showed a much more compact structure and more uniform distribution of Safranin O stained sGAG (Figure. 5E, F) with much higher sGAG/dsDNA ratio (Figure. 6B). Similar results have also been reported previously that MSC/collagen constructs with low crosslink densities of collagen experienced MSC-mediated contraction, a greater degree of chondrogenesis, and a denser internal structure, compared to more highly crosslinked scaffolds that resisted cellular contraction [37]. In addition, the higher expression of collagen type II and the compact structure of the Cell/ECM constructs likely result in greater sGAG retention, simulating ECM interaction in native cartilage [39]. These features could explain the higher content of sGAG in the Trypsin+ group compared to Trypsin- group, although they have similar aggrecan gene expression level.
To further assess the utility of hBMSC-ECM for cartilage formation in vivo, chondrocyte-impregnated hBMSC-ECM constructs were subcutaneously implanted into SCID mouse. As control, chondrocyte cell sheets were used. In previous studies [40], chondrocytes cultured on temperature responsive dishes were detached with their ECM to form sheets which were then stacked to form a 3D structure, and used as a scaffold free strategy to generate neo-cartilage both in vitro and in vivo. In one study, the formed multilayered cell sheets were used to repair lesions in a partial-thickness defect rabbit model. The results showed that the 3-layered chondrocyte cell sheet was able to prevent further deterioration after the defect was made, compared with groups with no covering after 4 weeks; however, histological analysis revealed minimal to no Safranin O stained cartilage [41]. In another study using stacked chondrocytes cell sheets to repair lesions in a minipig model of full-thickness defects, the part filled with cell sheets showed good cartilage formation indicated by Safranin O staining [24]. Thus, the utility of chondrocyte cell sheets for consistent and sufficient hyaline cartilage formation in vivo remains unsettled. In this study, we directly compared the cartilage formation ability of chondrocyte cell sheets (initial seeding density of 10,000 cells/cm2 at day 0) and chondrocyte-impregnated hBMSC-ECM (initial seeding density of 2,000 cells/cm2 at day 4) first in terms of chondrogenesis in vitro, and next for cartilage formation in a SCID mouse subcutaneous implantation model in vivo (Figure 7). At day 10, both groups exhibited similar gene expression of collagen type II and aggrecan (Figure 8A, B), and were then folded into constructs. It is noteworthy that the hBMSC-ECM method requires considerably less initial cell number and shorter culture period. For the in vivo test, constructs were placed in the subcutaneous portion of the back of the SCID mice, a common method used to estimate potential cartilage formation ability in a joint [42,43]. After 10 days of in vitro culture, compared with Cell sheet constructs, Cell/ECM constructs showed considerably higher gene expression of collagen type II and aggrecan (Figure 8A, B). In parallel, western blot results showed that treatment with full chondrogenic medium for 40 minutes showed higher phospho-Smad2/Smad3 level in the Cell/ECM group compared to the Cell sheet group, before being rounded up into constructs (Figure 8C), consistent with the more robust chondrogenic response in the former. In vivo, the higher cartilage formation ability of the Cell/ECM constructs continued, displaying both higher chondrogenic gene expression, higher sGAG content, and more prominent Safranin O staining at 2 weeks after implantation (Figure 9). It should be pointed out that as the Cell/ECM constructs were produced here by simple detachment from the culture dish, instead of being lifted off from temperature responsive culture substrate as in the case of the chondrocyte cell sheets (38, 39).
A number of limitations to the hBMSC-ECM based technology still remain. First, the preparation of hBMSC-ECM requires the isolation and culture of hBMSCs, with additional time and cost. Given the low immunogenicity and stability of MSC-ECM [19], the use of an MSC cell line or allogeneic MSC source may represent an “off-the-shelf” solution. Second, the exact mechanism of hBMSC-ECM enhancing chondrocyte proliferation and differentiation is not fully understood. Future research should focus on analyzing the activation of intracellular signaling pathways, as well as identifying key extracellular bioactive factors in the MSC-ECM. In particular, chondrocyte pericellular matrix related molecules will be included in these mechanistic studies. Third, we did not test the ability of the Cell/ECM constructs to repair cartilage defects in a clinically relevant cartilage defect animal model. Thus, the potential of Cell/ECM constructs to resurface an articular cartilage defect in vivo and integrate with surrounding cartilage remains unexplored. Ongoing work will address these issues. For example, we are planning additional in vivo studies in a clinically relevant animal model, namely the repair of full-thickness defect in goat knee articular cartilage. However, despite these limitations, our results strongly suggest a novel approach to improve clinical outcome of articular cartilage repair using current MACI practice.
5. Conclusion
We report there that hBMSC-ECM functions as a robust substrate for chondrocyte proliferation, maintenance of chondrocytic phenotype, and promotion of chondrocyte redifferentiation after expansion, and in particular may serve as a good vehicle for chondrocyte implantation. Compared to the chondrocyte cell sheet technology, the hBMSC-ECM based approach not only requires significantly less cell number, but also leads to a more robust cartilage formation both in vitro and in vivo. Additional studies are needed to further elucidate the mechanism of how hBMSC-ECM affects chondrocyte behavior, and to examine the reparative potential of chondrocyte-impregnated hBMSC-ECM in a clinically relevant animal model.
Supplementary Material
Statement of significance.
Current cell-based treatments for focal cartilage defects face challenges, including chondrocyte dedifferentiation, need for xenogenic scaffolds, and suboptimal cartilage formation. We present here a novel technique that utilizes adult stem cell-derived extracellular matrix, as a culture substrate and/or encapsulation scaffold for human adult chondrocytes, for the repair of cartilage defects. Chondrocytes cultured in stem cell-derived matrix showed higher proliferation, better chondrocytic phenotype, and improved redifferentiation ability upon in vitro culture expansion. Most importantly, 3-dimensional constructs formed from chondrocytes folded within stem cell matrix manifested excellent cartilage formation both in vitro and in vivo. These findings demonstrate the suitability of stem cell-derived extracellular matrix as a culture substrate for chondrocyte expansion as well as a candidate bioactive matrix for cartilage regeneration.
Acknowledgments
The authors gratefully thank Dr. Paul Manner (University of Washington) for generously providing human tissue, Dr. Jian Tan (University of Pittsburgh) for isolating hBMSCs, Dr. Guang Yang (Department of Cardiothoracic Surgery, Stanford University) and Dr. Yangzi Jiang (University of Pittsburgh) for instructions on experimental assays, and Zixuan Lin and Yuhao Deng for measuring the thickness of ECM and quantitating the dsDNA content after decellularization. This work is supported in part by the National Institutes of Health (R01 EB019430) and the U.S. Department of Defense (W81XWH-14-1-0217). Dr. He Shen thanks the National Natural Science Foundation of China (81501074) and China Postdoctoral Science Foundation (2015M571836). Yuanheng Yang thanks the Third Xiangya Hospital of Central South University for research fellowship support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–182. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Arøen A, Løken S, Heir S, Alvik E, Ekeland A, Granlund OG, Engebretsen L. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211–215. doi: 10.1177/0363546503259345. [DOI] [PubMed] [Google Scholar]
- 3.Heir S, Nerhus TK, Røtterud JH, Løken S, Ekeland A, Engebretsen L, Arøen A. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231–237. doi: 10.1177/0363546509352157. [DOI] [PubMed] [Google Scholar]
- 4.Makris EA, Gomoll AH, Malizos KN, Hu JC, Athanasiou KA. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gracitelli GC, Moraes VY, Franciozi CE, Luzo MV, Belloti JC. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst Rev. 2016;9:CD010675. doi: 10.1002/14651858.CD010675.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch T, Mandelbaum B, Tom M. Autologous chondrocyte implantation: past, present, and future. Sports Med Arthrosc. 2016;24:85–91. doi: 10.1097/JSA.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 7.Kon E, Filardo G, Di Martino A, Marcacci M. ACI and MACI. J Knee Surg. 2012;25:17–22. doi: 10.1055/s-0031-1299651. [DOI] [PubMed] [Google Scholar]
- 8.Deng Z, Jin J, Zhao J, Xu H. Cartilage Defect Treatments: With or without cells? Mesenchymal stem cells or chondrocytes? Traditional or matrix-assisted? A systematic review and meta-analyses. Stem Cells Int. 2016;2016:9201492. doi: 10.1155/2016/9201492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phull A-R, Eo S-H, Abbas Q, Ahmed M, Kim SJ. Applications of chondrocyte-based cartilage engineering: an overview. Biomed Res Int. 2016;2016:1879837. doi: 10.1155/2016/1879837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiménez G, López-Ruiz E, Kwiatkowski W, Montañez E, Arrebola F, Carrillo E, Gray PC, Izpisua Belmonte JC, Choe S, Perán M, Marchal JA. Activin A/BMP2 chimera AB235 drives efficient redifferentiation of long term cultured autologous chondrocytes. Sci Rep. 2015;5:16400. doi: 10.1038/srep16400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kino-Oka M, Yashiki S, Ota Y, Mushiaki Y, Sugawara K, Yamamoto T, Takezawa T, Taya M. Subculture of chondrocytes on a collagen type I-coated substrate with suppressed cellular dedifferentiation. Tissue Eng. 2005;11:597–608. doi: 10.1089/ten.2005.11.597. [DOI] [PubMed] [Google Scholar]
- 12.Chua KH, Aminuddin BS, Fuzina NH, Ruszymah BHI. Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur Cell Mater. 2005;9:58–67. doi: 10.22203/ecm.v009a08. discussion 67. [DOI] [PubMed] [Google Scholar]
- 13.Zhou Y, Zimber M, Yuan H, Naughton GK, Fernan R, Li W-J. Effects of human fibroblast-derived extracellular matrix on mesenchymal stem cells. Stem Cell Rev. 2016;12:560–572. doi: 10.1007/s12015-016-9671-7. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Hansen KC, Zhang Y, Dong C, Dinu CZ, Dzieciatkowska M, Pei M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials. 2014;35:642–653. doi: 10.1016/j.biomaterials.2013.09.099. [DOI] [PubMed] [Google Scholar]
- 15.Lin H, Yang G, Tan J, Tuan RS. Influence of decellularized matrix derived from human mesenchymal stem cells on their proliferation, migration and multi-lineage differentiation potential. Biomaterials. 2012;33:4480–4489. doi: 10.1016/j.biomaterials.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Pei M, He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol. 2012;227:2163–2174. doi: 10.1002/jcp.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravindran S, Kotecha M, Huang C-C, Ye A, Pothirajan P, Yin Z, Magin R, George A. Biological and MRI characterization of biomimetic ECM scaffolds for cartilage tissue regeneration. Biomaterials. 2015;71:58–70. doi: 10.1016/j.biomaterials.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y-C, Chen R-N, Jhan H-J, Liu D-Z, Ho H-O, Mao Y, Kohn J, Sheu MT. Development and characterization of acellular extracellular matrix scaffolds from porcine menisci for use in cartilage tissue engineering. Tissue Eng Part C Methods. 2015;21:971–986. doi: 10.1089/ten.tec.2015.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Zhu Y, Li J, Guo Q, Peng J, Liu S, Yang J, Wang Y. Cell-derived extracellular matrix: basic characteristics and current applications in orthopedic tissue engineering. Tissue Eng Part B Rev. 2016;22:193–207. doi: 10.1089/ten.TEB.2015.0290. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Hoshiba T, Kawazoe N, Koda I, Song M, Chen G. Cultured cell-derived extracellular matrix scaffolds for tissue engineering. Biomaterials. 2011;32:9658–9666. doi: 10.1016/j.biomaterials.2011.08.091. [DOI] [PubMed] [Google Scholar]
- 21.Cai R, Nakamoto T, Kawazoe N, Chen G. Influence of stepwise chondrogenesis-mimicking 3D extracellular matrix on chondrogenic differentiation of mesenchymal stem cells. Biomaterials. 2015;52:199–207. doi: 10.1016/j.biomaterials.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 22.Park Y-B, Seo S, Kim J-A, Heo J-C, Lim Y-C, Ha C-W. Effect of chondrocyte-derived early extracellular matrix on chondrogenesis of placenta-derived mesenchymal stem cells. Biomed Mater. 2015;10:035014. doi: 10.1088/1748-6041/10/3/035014. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura A, Akahane M, Shigematsu H, Tadokoro M, Morita Y, Ohgushi H, Dohi Y, Imamura T, Tanaka Y. Cell sheet transplantation of cultured mesenchymal stem cells enhances bone formation in a rat nonunion model. Bone. 2010;46:418–424. doi: 10.1016/j.bone.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 24.Ebihara G, Sato M, Yamato M, Mitani G, Kutsuna T, Nagai T, Ito S, Ukai T, Kobayashi M, Kokubo M, Okano T, Mochida J. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials. 2012;33:3846–3851. doi: 10.1016/j.biomaterials.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 25.Kaneshiro N, Sato M, Ishihara M, Mitani G, Sakai H, Kikuchi T, Mochida J. Cultured articular chondrocytes sheets for partial thickness cartilage defects utilizing temperature-responsive culture dishes. Eur Cell Mater. 2007;13:87–92. doi: 10.22203/ecm.v013a09. [DOI] [PubMed] [Google Scholar]
- 26.Shakouri-Motlagh A, O’Connor AJ, Brennecke SP, Kalionis B, Heath DE. Native and solubilized decellularized extracellular matrix: a critical assessment of their potential for improving the expansion of mesenchymal stem cells. Acta Biomater. 2017;55:1–12. doi: 10.1016/j.actbio.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 27.Lai Y, Sun Y, Skinner CM, Son EL, Lu Z, Tuan RS, Jilka RL, Ling J, Chen XD. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev. 2010;19:1095–1107. doi: 10.1089/scd.2009.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Somaiah C, Kumar A, Mawrie D, Sharma A, Patil SD, Bhattacharyya J, Swaminathan R, Jaganathan BG. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE. 2015;10:e0145068. doi: 10.1371/journal.pone.0145068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rothrauff BB, Yang G, Tuan RS. Tissue-specific bioactivity of soluble tendon-derived and cartilage-derived extracellular matrices on adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8:133. doi: 10.1186/s13287-017-0580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pei M. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials. 2016;117:10–23. doi: 10.1016/j.biomaterials.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao Pattabhi S, Martinez JS, Keller TCS. Decellularized ECM effects on human mesenchymal stem cell stemness and differentiation. Differentiation. 2014;88:131–143. doi: 10.1016/j.diff.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin JA, Buckwalter JA. Effects of fibronectin on articular cartilage chondrocyte proteoglycan synthesis and response to insulin-like growth factor-I. J Orthop Res. 1998;16:752–757. doi: 10.1002/jor.1100160618. [DOI] [PubMed] [Google Scholar]
- 33.Zeng L, Chen X, Zhang Q, Yu F, Li Y, Yao Y. Redifferentiation of dedifferentiated chondrocytes in a novel three-dimensional microcavitary hydrogel. J Biomed Mater Res A. 2015;103:1693–1702. doi: 10.1002/jbm.a.35309. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Song B, Anbarchian T, Shirazyan A, Sadik JE, Lyons KM. Smad2 and smad3 regulate chondrocyte proliferation and differentiation in the growth plate. PLoS Genet. 2016;12:e1006352. doi: 10.1371/journal.pgen.1006352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van der Kraan PM, Blaney Davidson EN, Blom A, van den Berg WB. TGF-beta signaling in chondrocyte terminal differentiation and osteoarthritis: modulation and integration of signaling pathways through receptor-Smads. Osteoarthr Cartil. 2009;17:1539–1545. doi: 10.1016/j.joca.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Narcisi R, Signorile L, Verhaar JAN, Giannoni P, van Osch GJVM. TGFβ inhibition during expansion phase increases the chondrogenic re-differentiation capacity of human articular chondrocytes. Osteoarthr Cartil. 2012;20:1152–1160. doi: 10.1016/j.joca.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Vickers SM, Gotterbarm T, Spector M. Cross-linking affects cellular condensation and chondrogenesis in type II collagen-GAG scaffolds seeded with bone marrow-derived mesenchymal stem cells. J Orthop Res. 2010;28:1184–1192. doi: 10.1002/jor.21113. [DOI] [PubMed] [Google Scholar]
- 38.Nirmalanandhan VS, Levy MS, Huth AJ, Butler DL. Effects of cell seeding density and collagen concentration on contraction kinetics of mesenchymal stem cell?seeded collagen constructs. Tissue Eng. 2006;12:1865–1872. doi: 10.1089/ten.2006.12.1865. [DOI] [PubMed] [Google Scholar]
- 39.Rojas FP, Batista MA, Lindburg CA, Dean D, Grodzinsky AJ, Ortiz C, Han L. Molecular adhesion between cartilage extracellular matrix macromolecules. Biomacromolecules. 2014;15:772–780. doi: 10.1021/bm401611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owaki T, Shimizu T, Yamato M, Okano T. Cell sheet engineering for regenerative medicine: current challenges and strategies. Biotechnol J. 2014;9:904–914. doi: 10.1002/biot.201300432. [DOI] [PubMed] [Google Scholar]
- 41.Kaneshiro N, Sato M, Ishihara M, Mitani G, Sakai H, Mochida J. Bioengineered chondrocyte sheets may be potentially useful for the treatment of partial thickness defects of articular cartilage. Biochem Biophys Res Commun. 2006;349:723–731. doi: 10.1016/j.bbrc.2006.08.096. [DOI] [PubMed] [Google Scholar]
- 42.Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54:3254–3266. doi: 10.1002/art.22136. [DOI] [PubMed] [Google Scholar]
- 43.Wang C-C, Yang K-C, Lin K-H, Liu Y-L, Liu H-C, Lin F-H. Cartilage regeneration in SCID mice using a highly organized three-dimensional alginate scaffold. Biomaterials. 2012;33:120–127. doi: 10.1016/j.biomaterials.2011.09.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.