Abstract
Objective
In women with postmenopausal osteoporosis, Vitamin K2 appears to decrease the incidence of hip, vertebral and non-vertebral fractures. Women with post-menopausal osteoporosis have more circulating activated T cells compared to healthy post-menopausal and pre-menopausal women, but the effects of Vitamin K2 on T-cells has not been studied. In this study, we have looked at T-cell suppression by Vitamin K2.
Materials and methods
Peripheral blood mononuclear cells (PBMCs) from three healthy donors were used. The PBMCs were stimulated with the mitogens phytohemagglutinin and concanavalin A, and T-cell proliferation was analyzed using flow cytometry based on carboxyfluorescein succinimidyl ester (CSFE) dye dilution.
Results
Vitamin K2 (60 and 100 µM) inhibited T-cell proliferation. Vitamin K1 at the same concentrations did not inhibit T-cell proliferation.
Conclusion
Vitamin K2 has immunomodulatory activities.
Keywords: Vitamin K2, Immunomodulation, T-cells, Osteoporosis
Introduction
Vitamin K is a fat-soluble vitamin. Two vitamin K species are known: vitamin K1 (phylloquinone), vitamin K2 (menaquinones). Vitamin K1 activates blood clotting factors. Vitamin K2, acts on extra-hepatic tissues (bone, brain, vasculature, testis, pancreas, kidneys and lungs) to activate K2 dependent proteins such as osteocalcin and matrix gla protein. The most common forms of vitamin K2 in the human diet are MK-4 and MK-7, short- and long-chained molecules, respectively (Beulens et al., 2013; Booth, 1997, 2012; Myneni and Mezey, 2016; Shearer and Newman, 2008). The effect of vitamin K2, mainly MK-4, on the bone health of women with post-menopausal osteoporosis has been studied by a number of clinical investigators (Inoue et al., 2009; Iwamoto et al., 2003; Knapen et al., 2007; Tanaka and Oshima, 2007; Ushiroyama et al., 2002; Yonemura et al., 2004; Yonemura et al., 2000). A meta-analysis of studies in which MK-4 was used at a dose of 45 mg/day revealed that vitamin K2 reduced hip, vertebral and non-vertebral fractures (Cockayne et al., 2006). Women with post-menopausal osteoporosis have more circulating activated T cells compared to healthy post-menopausal and pre-menopausal women (Adeel et al., 2013; D'Amelio et al., 2008). T-cells are known to play a critical role in promoting bone loss in postmenopausal osteoporosis as well as bone cancers (Zhang et al., 2011), rheumatoid arthritis (Kong et al., 1999), and periodontitis (Teng et al., 2000). Given the protective effect of vitamin K2 on bone health and the role of activated T-cells in osteoporosis, we asked if vitamin K2 could suppress T-cell proliferation. This could contribute to the activities described above. We report that vitamin K2 indeed can suppress T-cell proliferation. This activity is specific to vitamin K2, and not shown by vitamin K1.
Materials and Methods
Reagents
All reagents used in this study were obtained from Sigma-Aldrich (St Louis, MO) unless mentioned. CFSE, phytohemagglutinin and Concanavalin A were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CD3 APC antibody and 7-AAD were purchased from eBioscience.
PBMC staining and culture
Human PBMCs from healthy adult volunteers were obtained from the NIDCR core facility. Healthy volunteer blood was collected in accordance with the Declaration of Helsinki received from the NIH blood Bank. PBMCs were cultured in RPMI-1640 media with 10% Heat inactivated FBS, 2 mM glutamine, 10 mM HEPES, 1 mM sodium pyruvate, 1× nonessential amino acids and 1% penicillin-streptomycin. PBMCs were stained with 2.5 µM CFSE for 15 min at room temperature in the dark at a cell density of 10 ×106 PBMC/ml. The reaction was stopped by adding an equal amount of cold RPMI-1640 media with 10% Heat inactivated FBS and incubated on ice for 5 min and centrifuged at 400×g for 5 min. The cells are washed twice with cold media and resuspended at a density of 1×106 PBMCs/ml. The CFSE labelled PBMCs were used for the T-cell proliferation assay.
T-cell proliferation assay
The CFSE-labeled PBMCs were cultured in triplicate at a density of 5 ×104 cells/ml in a round bottom 96-well plate. T-cell proliferation was induced with phytohemagglutinin (PHA-5 µg/ml) or concanavalin A (ConA-5 µg/ml) for 96 hr with or without vitamin K2 (MK-4) or vitamin K1 in a total volume of 200 µl. Cells were cultured in a humidified incubator at 37°C and 5% CO2. T-cell proliferation was determined by CFSE dye dilution of CD3 positive cells using AccuriC6 flow cytometer. The gating strategy is shown in Supplemental Fig 1. The Flow cytometry data were analyzed using Flow Jo software.
Statistical analysis
All values are expressed as standard error of the mean (SEM) of three donors. Statistical significance was assessed by unpaired ANOVA followed by Tukey’s post hoc testing using Prism 7. P values are as follows: ***p>0.001, ****p>0.0001.
Results and Discussion
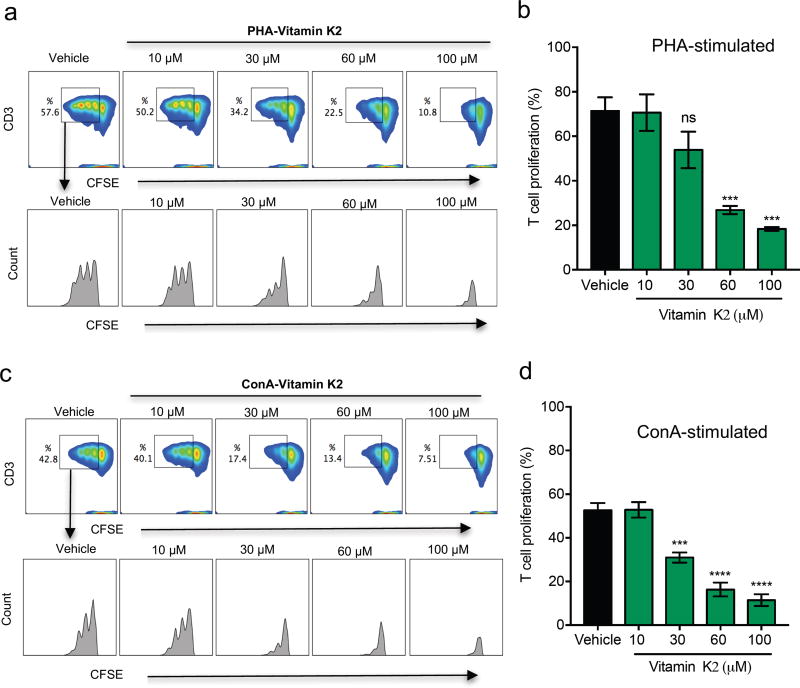
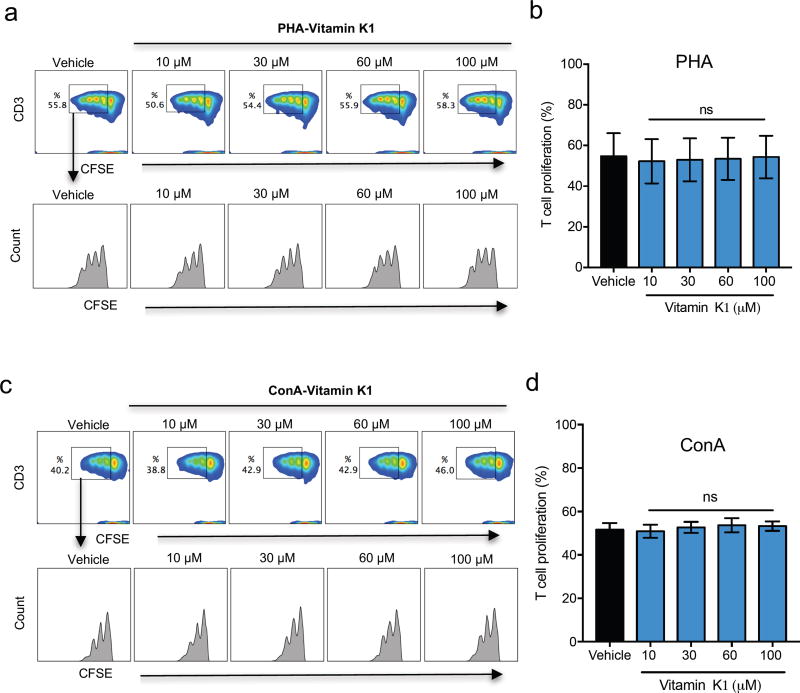
In an initial series of experiments, we determined optimal concentrations of the mitogens PHA and ConA to promote T-cell activation using three donors to balance individual immune response variability (Supplemental Fig.2). A concentration of 5 µg/ml was chosen for both PHA and ConA, and was used for the rest of the study. We initially tested the effect of vitamin K2 (MK-4) on T-cell proliferation at concentrations ranging from 0 µM to 10 µM, concentrations most commonly reported in literature to affect the function of cells such as osteoblasts (Ichikawa et al., 2007), osteoclasts (Koshihara et al., 2003), hematopoietic stem cells (Miyazawa and Aizawa, 2004), and erythroid and myeloid cells (Sada et al., 2010). Vitamin K2 at these concentrations did not inhibit T-cell proliferation (Supplemental Fig.3). Vitamin K2 concentrations greater than 10 µM were shown to induce testosterone production (Ito et al., 2011). For the next set of experiments we used vitamin K2 concentrations as high as 100 µM. Vitamin K2 significantly inhibited T-cell proliferation at 60 and 100 µM in both PHA (Fig.1. a, b) and ConA (Fig.1. c, d) stimulated PBMCs. 30 µM of K2 significantly inhibited ConA-stimulated, but not PHA-stimulated PBMCs (Fig.1. b, d). We next asked whether vitamin K1 also inhibits T-cell proliferation. As shown in Fig.2. a, b, c, d, vitamin K1 did not inhibit T-cell proliferation at any of the concentrations used. These results suggest that inhibition of T-cell proliferation is specific to Vitamin K2.
Fig. 1. Vitamin K2 inhibits T-cell proliferation.
(A) Flow cytometry density plots show expression of PHA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of proliferating T-cells following PHA stimulation with or without vitamin K2 for 96 hr of culture. The histogram plots of the gated regions show the suppression of vitamin K2 on PHA stimulated CD3 T-cells, each peak in the histogram represents one generation of proliferating cells. (B) Percentage of T-cell proliferation in presence of vitamin K2 at the indicated doses or vehicle control with PHA stimulation. (C) Flow cytometry density plots show expression of ConA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of proliferating T-cells with ConA stimulation with or without vitamin K2 after 96 hr of culture. The histogram plots of the gated regions show the suppression of vitamin K2 on ConA stimulated CD3 T-cells. (D) Percentage of T-cell proliferation in presence of vitamin K2 at the indicated doses or vehicle control with ConA stimulation. n=3 donors; Error bars represent standard error of mean (SEM); ns-not significant ;***p<0.001; ****p<0.0001.
Fig. 2. Vitamin K1 has no inhibitory effect on T-cell proliferation.
(A) Flow cytometry density plots show expression of PHA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of proliferating T-cells following PHA stimulation with or without vitamin K1 after 96 hr of culture. The histogram plots of the gated regions show the lack of suppression by vitamin K1 on PHA stimulated CD3 T-cells, each peak in the histogram represents a generation of proliferating cells. (B) Percentage of T-cell proliferation in presence of vitamin K1 at the indicated doses or vehicle control following PHA stimulation. (C) Flow cytometry density plots show expression of ConA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of proliferating T-cells with ConA stimulation with or without vitamin K1 after 96 hr of culture. The histogram plots of the gated regions show the lack of suppression by vitamin K1 on ConA stimulated CD3 T-cells. (D) Percentage of T-cell proliferation in presence of vitamin K1 at the indicated doses or vehicle control with ConA stimulation. n=3 donors; Error bars represent standard error of mean (SEM); ns-not significant.
It has been reported that vitamin K2, but not vitamin K1, promotes osteoblasts differentiation from mesenchymal stem cells, preventing osteoclast formation. This is similar to the effect on T-cell proliferation that we observed. Our results also suggest that immunomodulation might contribute to the overall anabolic effect of vitamin K2 on bone. In clinical trials with vitamin K2, investigators used a dose of 45 mg/day. This is the minimal effective dose to improve bone health, and there were no side effects reported when it was given continuously for three years (Inoue et al., 2009; Iwamoto, 2014). In principal, this dose of K2 taken orally could achieve a maximum blood concentration of 40 to 50 µM, but this needs to be further investigated. This would have clinical impact in that by modulating the dosage of vitamin K2 T-cell function can be modulated without affecting its functions on bone cells and other cell types. In this study, we have not looked at T-cells subsets, however. Doing this will provide a better understanding of the specific cell(s) that contribute to the function of vitamin K2 in promoting immunomodulatory activities. Further studies will be needed to determine the mechanism of immune regulatory functions of vitamin K2, and to examine the function of other vitamin K2 isoforms on immune regulation.
Supplementary Material
Supplemental Figure 1: Gating strategy for proliferation analysis. (a) Unstimulated PBMCs stained with CFSE and CD3, gated on live cells using 7AAD. Histogram representing a single peak denotes the original population. (b) ConA stimulated PBMCs stained with CFSE and CD3, gated on live cells using 7AAD. (i) Histogram representing total population with the highest peak denotes the original population, and each samller peak represents one generation of proliferating cells. (ii) Histogram showing generations of proliferating cells. (iii) Histogram showing original generation/non proliferating cells.
Supplemental Figure 2: Optimal concentration of mitogens. (A) Flow cytometry density plots show PHA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of T-cells proliferating with various concentrations of PHA stimulation after 96 hr of culture. (B) Flow cytometry density plots show ConA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of T-cells proliferating with various concentrations of ConA stimulation after 96 hr of culture.
Data shown is from one of the three donor PBMCs used. (n=3 donors).
Supplemental Figure 3: Reported Vitamin K2 concentrations do not inhibit T-cell proliferation.
(A) Flow cytometry density plots show expression of PHA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of proliferating T-cells on PHA stimulation with or without vitamin K2 for 96 hr of culture. (B) Flow cytometry density plots show expression of ConA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of proliferating T-cells with ConA stimulation with or without vitamin K2 for 96 hr of culture. Data shown are from one of the three donor PBMCs used. (n=3 donors).
Acknowledgments
The authors would like to thank Dr. Michael J. Brownstein for critical reading and editing this manuscript. The help of the Combined Technical Research Core (ZIC DE000729-09) is greatly appreciated.
This research was supported by the Intramural Research Program of the NIH, NIDCR.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Author contributions
VM designed the study, analyzed the data and wrote the manuscript. EM reviewed the data and edited the manuscript.
References
- Adeel S, Singh K, Vydareny KH, Kumari M, Shah E, Weitzmann MN, Tangpricha V. Bone loss in surgically ovariectomized premenopausal women is associated with T lymphocyte activation and thymic hypertrophy. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2013;61:1178–1183. doi: 10.231/JIM.0000000000000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beulens JW, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K(2)) in human health. The British journal of nutrition. 2013;110:1357–1368. doi: 10.1017/S0007114513001013. [DOI] [PubMed] [Google Scholar]
- Booth SL. Skeletal functions of vitamin K-dependent proteins: not just for clotting anymore. Nutrition reviews. 1997;55:282–284. doi: 10.1111/j.1753-4887.1997.tb01619.x. [DOI] [PubMed] [Google Scholar]
- Booth SL. Vitamin K: food composition and dietary intakes. Food & nutrition research. 2012;56 doi: 10.3402/fnr.v56i0.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Archives of internal medicine. 2006;166:1256–1261. doi: 10.1001/archinte.166.12.1256. [DOI] [PubMed] [Google Scholar]
- D'Amelio P, Grimaldi A, Di Bella S, Brianza SZ, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43:92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Horie-Inoue K, Ikeda K, Blumberg B, Inoue S. Vitamin K2 induces phosphorylation of protein kinase A and expression of novel target genes in osteoblastic cells. Journal of molecular endocrinology. 2007;39:239–247. doi: 10.1677/JME-07-0048. [DOI] [PubMed] [Google Scholar]
- Inoue T, Fujita T, Kishimoto H, Makino T, Nakamura T, Nakamura T, Sato T, Yamazaki K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase IV clinical study of 15-mg menatetrenone capsules. Journal of bone and mineral metabolism. 2009;27:66–75. doi: 10.1007/s00774-008-0008-8. [DOI] [PubMed] [Google Scholar]
- Ito A, Shirakawa H, Takumi N, Minegishi Y, Ohashi A, Howlader ZH, Ohsaki Y, Sato T, Goto T, Komai M. Menaquinone-4 enhances testosterone production in rats and testis-derived tumor cells. Lipids in health and disease. 2011;10:158. doi: 10.1186/1476-511X-10-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto J. Vitamin K(2) therapy for postmenopausal osteoporosis. Nutrients. 2014;6:1971–1980. doi: 10.3390/nu6051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto J, Takeda T, Ichimura S. Treatment with vitamin D3 and/or vitamin K2 for postmenopausal osteoporosis. The Keio journal of medicine. 2003;52:147–150. doi: 10.2302/kjm.52.147. [DOI] [PubMed] [Google Scholar]
- Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2007;18:963–972. doi: 10.1007/s00198-007-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, Capparelli C, Li J, Elliott R, McCabe S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–309. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- Koshihara Y, Hoshi K, Okawara R, Ishibashi H, Yamamoto S. Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. The Journal of endocrinology. 2003;176:339–348. doi: 10.1677/joe.0.1760339. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Aizawa S. Vitamin K2 improves the hematopoietic supportive functions of bone marrow stromal cells in vitro: a possible mechanism of improvement of cytopenia for refractory anemia in response to vitamin K2 therapy. Stem cells and development. 2004;13:449–451. doi: 10.1089/scd.2004.13.449. [DOI] [PubMed] [Google Scholar]
- Myneni VD, Mezey E. Regulation of bone remodeling by vitamin K2. 2016 doi: 10.1111/odi.12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sada E, Abe Y, Ohba R, Tachikawa Y, Nagasawa E, Shiratsuchi M, Takayanagi R. Vitamin K2 modulates differentiation and apoptosis of both myeloid and erythroid lineages. European journal of haematology. 2010;85:538–548. doi: 10.1111/j.1600-0609.2010.01530.x. [DOI] [PubMed] [Google Scholar]
- Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thrombosis and haemostasis. 2008;100:530–547. [PubMed] [Google Scholar]
- Tanaka I, Oshima H. Vitamin K2 as a potential therapeutic agent for glucocorticoid-induced osteoporosis. Clinical calcium. 2007;17:1738–1744. [PubMed] [Google Scholar]
- Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. The Journal of clinical investigation. 2000;106:R59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiroyama T, Ikeda A, Ueki M. Effect of continuous combined therapy with vitamin K(2) and vitamin D(3) on bone mineral density and coagulofibrinolysis function in postmenopausal women. Maturitas. 2002;41:211–221. doi: 10.1016/s0378-5122(01)00275-4. [DOI] [PubMed] [Google Scholar]
- Yonemura K, Fukasawa H, Fujigaki Y, Hishida A. Protective effect of vitamins K2 and D3 on prednisolone-induced loss of bone mineral density in the lumbar spine. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004;43:53–60. doi: 10.1053/j.ajkd.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Yonemura K, Kimura M, Miyaji T, Hishida A. Short-term effect of vitamin K administration on prednisolone-induced loss of bone mineral density in patients with chronic glomerulonephritis. Calcified tissue international. 2000;66:123–128. doi: 10.1007/pl00005832. [DOI] [PubMed] [Google Scholar]
- Zhang K, Kim S, Cremasco V, Hirbe AC, Collins L, Piwnica-Worms D, Novack DV, Weilbaecher K, Faccio R. CD8+ T cells regulate bone tumor burden independent of osteoclast resorption. Cancer research. 2011;71:4799–4808. doi: 10.1158/0008-5472.CAN-10-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Gating strategy for proliferation analysis. (a) Unstimulated PBMCs stained with CFSE and CD3, gated on live cells using 7AAD. Histogram representing a single peak denotes the original population. (b) ConA stimulated PBMCs stained with CFSE and CD3, gated on live cells using 7AAD. (i) Histogram representing total population with the highest peak denotes the original population, and each samller peak represents one generation of proliferating cells. (ii) Histogram showing generations of proliferating cells. (iii) Histogram showing original generation/non proliferating cells.
Supplemental Figure 2: Optimal concentration of mitogens. (A) Flow cytometry density plots show PHA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of T-cells proliferating with various concentrations of PHA stimulation after 96 hr of culture. (B) Flow cytometry density plots show ConA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of T-cells proliferating with various concentrations of ConA stimulation after 96 hr of culture.
Data shown is from one of the three donor PBMCs used. (n=3 donors).
Supplemental Figure 3: Reported Vitamin K2 concentrations do not inhibit T-cell proliferation.
(A) Flow cytometry density plots show expression of PHA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of proliferating T-cells on PHA stimulation with or without vitamin K2 for 96 hr of culture. (B) Flow cytometry density plots show expression of ConA stimulated viable CSFE and CD3 positive T-cells. The gated region shows the percentage of proliferating T-cells with ConA stimulation with or without vitamin K2 for 96 hr of culture. Data shown are from one of the three donor PBMCs used. (n=3 donors).