Abstract
Purpose
Low tidal volume ventilation (LTVV) reduces mortality in acute respiratory distress syndrome (ARDS) patients. Understanding local barriers to LTVV use at a former ARDS Network hospital may provide new insight to improve LTVV implementation.
Methods
A cohort of 214 randomly selected adults met the Berlin definition of ARDS at Harborview Medical Center between 2008 and 2012. The primary outcome was the receipt of LTVV (tidal volume of ≤6.5 mL/kg predicted body weight) within 48 hours of ARDS onset. We constructed a multivariable logistic regression model to identify factors associated with the outcome.
Results
Only 27% of patients received tidal volumes of ≤6.5 mL/kg PBW within 48 hours of ARDS onset. Increasing plateau pressure (OR 1.11; 95% CI 1.03 to 1.19; p-value <0.01) was positively associated with LTVV use while increasing PaO2:FIO2 ratio was negatively associated (OR 0.75, 95% CI 0.57 to 0.98; p-value 0.03). Physicians documented an ARDS diagnosis in only 21% of the cohort. Neither patient height nor gender were associated with LTVV use.
Conclusions
Most ARDS patients did not receive LTVV despite significant institutional efforts to improve utilization, which suggests that ARDS remains under-recognized and untreated.
Keywords: ARDS, Low-tidal volume ventilation, quality of care
INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a common and devastating form of respiratory failure that affects 190,000 patients annually in the United States and has a mortality rate of 39%[1]. Despite 30 years of clinical trials and drug research, only a handful of interventions reduce mortality in ARDS patients[2, 3]. In 2000, the ARDS Network published the results of the landmark randomized controlled trial that demonstrated a 9% absolute reduction in mortality utilizing a low tidal volume ventilation (LTVV) strategy, defined as ≤6 milliliters (mL) per kilogram (kg) of predicted body weight (PBW) with a goal plateau pressure (Pplat) ≤30 cm H2O[4]. This ventilation strategy is now termed “lung protective ventilation” and has become the standard of care for patients with ARDS[5]. Many studies have confirmed the benefits of LTVV, and have suggested a time-dependent mortality benefit from early utilization of LTVV[5-10]. New evidence also suggests that LTVV may prevent incident ARDS in critically ill patients at risk[11, 12].
The adoption of lung protective ventilation strategies in clinical practice has been slow despite convincing evidence of the benefits of LTVV[13-19]. Studies performed in the initial five years of the ARDS Network trial demonstrated only 40% of ARDS patients received appropriate LTVV therapy[20, 21]. Low rates of compliance with LTVV have prompted further examination of barriers to utilization. Several barriers to LTVV use have already been described and include mismeasurement or missing documentation of patient height used to calculate predicted body weight, concern for patient discomfort or perceived need for greater doses of sedating medications, and physician failure to recognize ARDS[13, 20, 22, 23]. Studies of sedation practices in the LTVV era have not found an association between LTTV and increased sedation use in either dose or duration[24, 25]. Factors associated with higher utilization of LTVV in prior studies include a written protocol for delivery of appropriate ventilator settings and a closed ICU staffing model[20, 26].
This analysis was conducted at Harborview Medical Center, an academic hospital with a level one trauma designation and a former ARDS Network contributing site. Our hospital is a unique location as a contributing site in the original ARDS Network trial and we have already adopted several mechanisms to increase utilization of LTVV. Our institution has implemented written ventilator protocols and uses a ventilator order set for LTVV[27, 28]. We also operate in a “closed” ICU staffing model, which has been associated with delivery of lower tidal volumes[26]. Our primary aim was to describe patient and physician factors associated with the use of LTVV in patients meeting the Berlin Definition of ARDS[29], over ten years since the original trial. We hypothesized that the proportion of ARDS patients who receive LTVV remains low at our academic center, despite significant institutional efforts to increase utilization.
METHODS
The University of Washington institutional review board approved this study with waiver of informed consent.
Study Population: ARDS Cohort
We conducted a retrospective cohort study using a pre-existing registry of mechanically ventilated patients admitted to a Harborview Medical Center (HMC) intensive care unit (ICU) between January 1, 2008 and December 31, 2012. We identified patients who received mechanical ventilation via an endotracheal tube for at least 48 hours and included patients meeting the Berlin Definition of ARDS[29]. We required a PaO2:FIO2 ratio ≤ 300 mmHg on two consecutive arterial blood gas (ABG) measurements to meet criteria for hypoxemia in our study. We then selected a random sample of the hypoxemic patients and evaluated chest radiographs obtained within 24 hours of the qualifying ABG. Study authors blinded to ventilator settings analyzed each radiograph, and ten percent of the total radiographs were reviewed by two readers (LJS and CLH). A qualifying chest radiograph met Berlin criteria with demonstration of bilateral infiltrates. ARDS onset was defined as the latter time of either the second qualifying ABG or qualifying chest radiograph.
Definition of LTVV
We defined our primary outcome of LTVV as ventilation with tidal volumes (VT) ≤6.5 mL/kg of predicted body weight (PBW) from values charted by respiratory therapists in our electronic medical record. A VT of 6.5 mL/kg PBW is consistent with the cutoff chosen by the ARDS Network when evaluating LTVV adherence, and permits for slight deviations from the goal of 6.0 mL/kg PBW that can happen due to miscalculations or rounding in practical use[20]. LTVV use was not dependent on ventilator mode in our study and thus patients receiving ventilation via a pressure controlled mode met criteria for LTVV if the delivered tidal volume was ≤6.5 mL/kg PBW. We also collected plateau pressures from values charted by respiratory therapists. We measured the number of cases that received a low tidal volume at the time of the first qualifying ABG, and then again at 24 hours and 48 hours after ARDS onset. Patients met criteria for our primary outcome if they received LTVV at any of those three time points.
Collection of Covariates
We abstracted electronic medical record charts to obtain demographic data, physiologic variables, lab values, ventilator data and ICU type. We reviewed admission and daily notes in the first 48 hours after ARDS onset to identify the underlying ARDS risk factor(s). We also reviewed physician notes to assess for documentation of concurrent acute brain injury as a potential contraindication to the use of LTVV or acute cardiac events that could call an ARDS diagnosis into question. We did not exclude patients with chart documentation of acute cardiac events. We also reviewed progress notes for interpretation of ABG and/or chest radiographs, documentation of respiratory failure and/or of an ARDS diagnosis by physicians and trainees. Documentation of Acute Lung Injury and the more general, “lung injury,” were also considered equivalent to an ARDS diagnosis as this cohort existed prior to the current Berlin definition[29].
Statistical Analysis
We computed descriptive statistics for all study variables including binomial confidence intervals for proportions. Difference testing between groups was performed using two-tailed t-tests for means, Mann-Whitney nonparametric tests for medians, and chi-square tests for proportions, as appropriate. A p-value of less than 0.05 was considered significant. We used SAS statistical software for all analyses (SAS version 9.4; SAS Institute, Inc., Cary, NC).
We built a multivariable logistic regression model using five factors selected a priori (age, type of ICU, sepsis/pneumonia; PaO2: FIO2 ratio; Pplat) to examine associations with the receipt of LTVV at any time within 48 hours of ARDS onset. There are potential contraindications to the use LTVV per the ARDS Network trial protocol including a pH <7.15, PaO2<55 mmHg, RR >40 breaths per minute, and/or co-morbid acute brain injury. We examined the subset of patients without these potential contraindications, collected at ARDS onset, to assess for the possible effect of these contraindications on the use of LTVV at our center.
RESULTS
From 31,722 patients admitted to an ICU at Harborview Medical Center between 2008 and 2012, we randomly selected 700 adult patients (≥18 years of age) for evaluation (Figure 1). Of those, 255 patients (36%) were intubated for at least 48 hours and had two consecutive ABG measurements with a PaO2:FIO2 ratio ≤300 mmHg, meeting our criteria for possible ARDS. We excluded fourteen patients with chronic respiratory failure, three with brain death on arrival, four with missing radiographs, and 20 with radiographs inconsistent with ARDS. The final study cohort included 214 patients.
Figure 1.
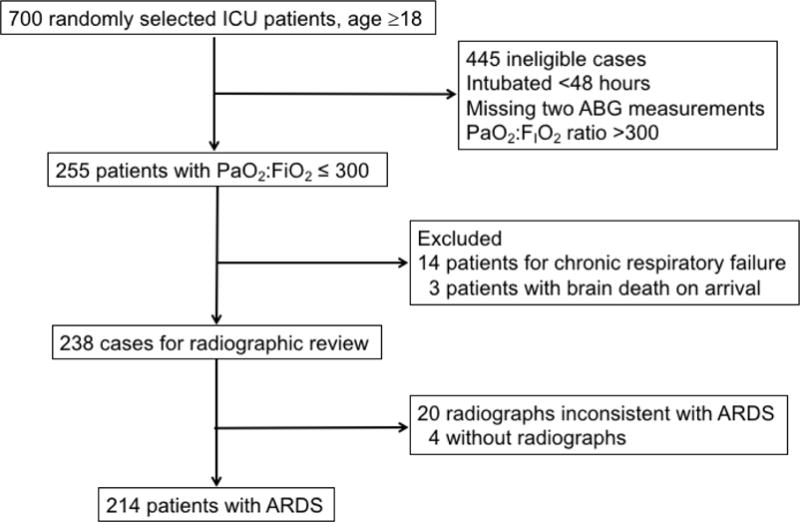
Flow chart demonstrating eligibility for cases included in the cohort.
The cohort had a mean age of 55 ± 16 years, was mostly male (71%) and predominately white race (83%) as shown in Table 1. We identified at least one ARDS risk factor in 95% of patients. At ARDS onset, 211 patients (99%) were on assist control, volume-cycled ventilation. Patients had a mean PaO2:FIO2 of 199 ± 68, a mean Pplat of 22.7± 5 mmHg, and received a mean tidal volume of 8.0 ± 0.96 mL/kg PBW. At ARDS onset, only 16 patients (7.5%) received a tidal volume of ≤6.5 mL/kg PBW, while 24 patients (11.2%) received tidal volumes >9 mL/kg PBW (Figure 2). At 24 hours, LTVV use increased to 30 of 200 cases with tidal volumes reported (15.0%). At 48 hours, LTVV use again increased to 45 of 214 patients (21.0%). Overall, only 58 patients (27.1%) ever received LTVV within 48 hours after ARDS onset. Only 28 (13%) patients had a potential contraindication to LTVV use (pH <7.15, PaO2<55, RR >40, acute brain injury). Among the remaining 186 patients without a potential contraindication, 38 (20%) received LTVV within 48 hours of ARDS onset.
Table 1.
Demographic and physiologic characteristics of ARDS patients with and without receipt of LTVV.
| All Patients (n = 214) |
No LTVV (n = 169) |
+LTVV at 48h (n = 45) |
P value | |
|---|---|---|---|---|
| Age, years | 55 ± 16 | 57 ± 16 | 50 ± 15 | 0.02 |
| Male sex, N (%) | 152 (71%) | 121 (72%) | 31 (69%) | 0.72 |
| Height, cm | 173 ± 12 | 173 ± 12 | 174 ± 11 | 0.69 |
| BMI | 31 ± 9 | 31 ± 9 | 31 ± 9 | 0.64 |
| Caucasian, N (%) | 178 (83%) | 144 (85%) | 34 (76%) | 0.23 |
| Type of ICU, N (%) | <0.01 | |||
| Medicine | 56 (26%) | 36 (21%) | 20 (44%) | |
| CCU | 13 (6%) | 13 (8%) | 0 | |
| Neurology, Neurosurgery | 49 (23%) | 43 (25%) | 6 (13%) | |
| Surgery/Trauma | 92 (43%) | 74 (44%) | 18 (40%) | |
| Burn/Plastics | 4 (2%) | 3 (2%) | 1 (2%) | |
| APACHE II score | 26 ± 8 | 25 ± 8 | 27 ± 9 | 0.13 |
| Tidal Volume, mL/kg PBW | 8.0 ± 0.96 | 8.2 ± 0.7 | 7.3 ± 1.3 | <0.01 |
| Plateau airway pressure, cm H2O | 22.7 ± 5 | 22 ± 5 | 25.3 ± 6 | <0.01 |
| PEEP, cm H2O | 6.5 ± 2.8 | 6.1 ± 2.1 | 8.2 ± 4.0 | <0.01 |
| Minute Ventilation, L/min | 10 ± 3 | 10 ± 3 | 11 ± 3 | 0.47 |
| Respiratory Rate, breaths/min | 20 ± 6 | 19 ± 6 | 22 ± 7 | <0.01 |
| FIO2, % | 70 ± 29 | 67 ± 29 | 81 ± 26 | <0.01 |
| ARDS Severity by PaO2:FIO2 | 199 ± 68 | 209 ± 66 | 172 ± 66 | <0.01 |
| Mild (PaO2:FIO2 200 – 300), N (%) | 121 (57%) | 103 (61%) | 18 (40%) | 0.03 |
| Moderate (PaO2:FIO2 100 – 200), N (%) | 62 (29%) | 46 (27%) | 16 (36%) | |
| Severe (PaO2:FIO2 <100), N (%) | 31 (14%) | 20 (12%) | 11 (24%) | |
| ARDS Risk Factor, N (%)* | ||||
| Aspiration | 56 (26%) | 41 (24%) | 15 (33%) | 0.30 |
| Pneumonia | 68 (32%) | 49 (29%) | 19 (42%) | 0.13 |
| Sepsis | 56 (26%) | 33 (20%) | 23 (51%) | <0.01 |
| Trauma | 92 (43%) | 78 (46%) | 14 (31%) | 0.12 |
| Other | 17 (8%) | 12 (7%) | 5 (11%) | 0.47 |
| None | 11 (5%) | 10 (6%) | 1 (2%) | 0.04 |
| Hospital Length of Stay, days (median, IQR) | 19, 11–33 | 18, 11–33 | 23, 9–32 | 0.80 |
| Duration of Mechanical Ventilation, days (median, IQR) | 6, 3–11 | 5, 3–10 | 7, 4–12 | 0.07 |
| Died, N (%) | 48 (22%) | 38 (22%) | 10 (22%) | 0.87 |
All values taken at ARDS onset. Continuous variables are shown as mean ± standard deviation.
Risk factors are not mutually exclusive
Figure 2.
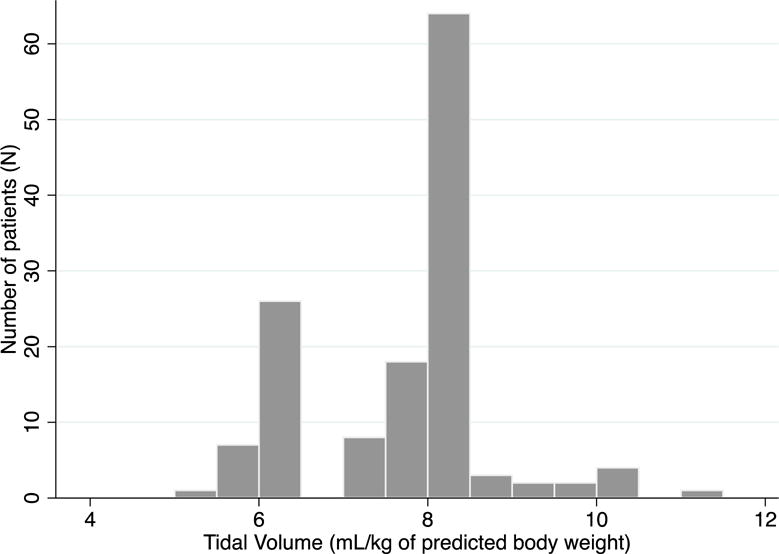
Most patients receive TV ≥ 8 mL/kg predicted body weight within 48 hours after ARDS onset.
The ARDS Network lung protective ventilation strategy included a goal Pplat <30 cm H2O in addition to a low tidal volume. Of the 58 patients who received a tidal volume ≤ 6.5 mL/kg PBW in this study, 30% (N=18) had Pplat above the goal of 30 cmH2O. Per the lung protective protocol, additional decreases in tidal volume should be undertaken to reach a goal Pplat <30cm H2O in ARDS patients. Only 6 (33%) of the 18 patients had the recommended decrease in tidal volume to meet the goal plateau pressure.
Patient demographic and clinical characteristics significantly differed by outcome at ARDS onset in unadjusted analyses are shown in Table 1. Patients who did receive LTVV were an average of seven years younger, were more than twice as likely to be admitted to a medical ICU, and were twice as likely to have severe ARDS with a PaO2:FIO2 <100 mmHg. ARDS patients who received LTVV also had higher mean positive end expiratory pressure (PEEP). Neither patient height or gender was differed with the use of LTVV in our study.
When examining physician documentation, three variables differed by LTVV use in univariate analysis. Patients who received LTVV were more likely to have an interpretation of a chest radiograph documented in the admission or daily note (84% vs. 52%, p <0.01), more likely to have documentation of the ABG (64% vs. 24%, p <0.01), and more likely to have physician documentation of an ARDS diagnosis (67% vs. 4%, p <0.01). Twenty-one percent (N = 46) of the entire cohort had a physician document a diagnosis of ARDS. Amongst patients with a physician diagnosis of ARDS, 81% (N = 37) received LTVV within 48 hours of ARDS onset and nine (19%) patients did not. Fifty-two (24%) of ARDS patients lacked even the simplest documentation of respiratory failure in the electronic chart. Forty-nine patients (23%) had documentation of pulmonary edema, but the proportion did not differ between patients who received LTVV and those who did not (24% vs. 22%, p=0.94). Thirty-five patients (16%) had documentation of heart failure or myocardial infarction (MI) in the electronic chart either as a diagnosis or in consideration. Again, the proportion of heart failure and MI did not differ between the groups of ARDS patients that received LTVV and those that did not (17% vs. 16%, p=0.99).
In multivariable analysis, the presence of sepsis and/or pneumonia was independently associated with LTVV use (Table 2). Milder cases of ARDS were associated with reduced LTVV use; for every 50 mmHg increase in the PaO2:FIO2 ratio, the likelihood of receiving LTVV fell by 25%. Higher Pplat was also positively associated with LTVV use, with the odds of receiving LTVV increasing by 11 % for every 1 cm H2O increase in Pplat.
Table 2.
Association between patient demographic and clinical characteristics and receipt of low tidal volume ventilation within 48 hours of ARDS onset, as estimated using multivariable logistic regression.
| Variable | Adjusted OR (95% CI) | p-value |
|---|---|---|
| Age (years) | 0.98 (0.96 – 1) | 0.07 |
| Sepsis and/or Pneumonia | 2.76 (1.11 – 6.83) | 0.03 |
| Medical Intensive Care Unit* | 1.56 (0.68 – 3.57) | 0.34 |
| PaO2:FIO2 (per 50 mmHg) | 0.75 (0.57 – 0.98) | 0.03 |
| Plateau Pressure (per cm H2O) | 1.11 (1.03 – 1.19) | <0.01 |
denotes binary variable (Medical ICU vs. Other ICU)
DISCUSSION
We found that LTVV was infrequently used and often delayed in the 48 hours after ARDS onset in our hospital, which is consistent with prior studies. We purport that while the utilization of LTVV is low at our institution, it may represent an overestimate to what happens in daily ICU practice in hospital settings outside of the ARDS Network. A Hawthorne effect is likely present at our center as a participating site in the former ARDS Network clinical trials with increased awareness of ARDS and the evidence supporting the use of LTVV. We also found that 100% of our cohort had a patient height measurement documented in the electronic medical record, and that neither patient height or gender was associated with LTVV use in our study. Despite all of these efforts, still only 23% of ARDS patients ever received guideline recommended LTVV therapy in our hospital.
The reason why LTVV is not utilized remains unclear. Previous literature has suggested that physicians may not pursue low tidal volumes in patients with ARDS when plateau pressures are not high[30]. Some investigators have suggested that a Pplat less than 30 cmH2O is safe, and does not require the use of low tidal volumes[31, 32]. However, subsequent studies have shown that managing ARDS patients solely by a pressure-limited strategy does not convey the same mortality benefit as the dual volume and pressure limited strategy utilized in the ARDS Network trial[33]. A recent prospective study found that even one mL/kg PBW increase in initial tidal volume was associated with a 23% increased risk of ICU mortality, independent of Pplat [9]. In our study, each one cm H2O increase in Pplat was associated with an 11% increase in the odds of LTVV use, suggesting that physicians are targeting pressure-limitations when managing the ventilator in ARDS. The concept of ventilator driving pressure described by Amato et al. combines the two metrics of tidal volume and Pplat with PEEP and may provide a bridge to this physician knowledge gap for the future[34].
ARDS may have been under-recognized by physicians in our study, with even less recognition amongst mild cases of ARDS. Physician under-recognition, and that severe cases of ARDS are likely to be more recognizable to physicians, have been described as barriers to LTVV use in prior study, [13, 30, 35, 36]. LTVV use was associated with increased ARDS severity and with physician documentation of an ARDS diagnosis in our hospital. However, the LUNG SAFE study demonstrated that even when physicians correctly diagnose ARDS, high-quality therapeutics such as LTVV are not used[36]. Continued efforts to bridge the physician knowledge gap and increase ARDS recognition are still needed. Additionally, utilizing empirically lower tidal volumes in mechanically ventilated patients would also increase compliance with the LTVV protocol[19].
The independent association between sepsis and LTVV use in our study is a newer finding and recently demonstrated by Weiss et al[37]. Several subphenotypes of ARDS have been described with sepsis-associated ARDS being the most common. This sepsis subphenotype may be easier for physicans to identify radiographically, leading to improved diagnosis and greater LTVV use [38]. However, the association with LTVV use may also be due to the Surviving Sepsis campaign recommendation that all severe sepsis cases requiring mechanical ventilatory support utilize a low tidal volume strategy, and is further reinforced via clinical protocols[39].
Our study has several potential limitations. First, we used the Berlin criteria to define ARDS in our study. The Berlin definition lacks the cardiac limitations from the American European Consensus Conference definition and could lead to misclassification bias. However, we examined chart documentation by physicians for relevant terms such as pulmonary edema, acute heart failure, etc. Documentation of these diagnosis occurred in only a small minority of the cohort, and did not change the proportional use of LTVV. Additionally, physician documentation is an imperfect surrogate for physician recognition of ARDS. However, the majority of patients with a documented ARDS diagnosis did go on to receive LTVV and we believe it was important to reflect whether practicing physicians were identifying their patients that were eligible for LTVV. This study was conducted at a single site in a patient population that included a large proportion of patients who were white, male, and/or victims of trauma; thus, our findings may be less generalizable to other settings in the United States. We required two consecutive ABG measurements for entry into the cohort. This was done to ensure the patient truly suffered from hypoxemic respiratory failure and likely ARDS, and not under-resuscitation or under-treatment. However, requiring two ABG measurements may bias towards a more proactive ventilator management strategy given the frequency of ABGs drawn. If true, this bias would likely shift in favor of the outcome and may mean that LTVV use is overestimated in our sample relative to the population as a whole.
CONCLUSION
We found the majority of ARDS patients at a single, academic center with targeted interest in ARDS research do not receive lung protective ventilation in the first 48 hours after ARDS onset despite institutional efforts to increase use. Our findings support the conclusion that ARDS is under-recognized and often not managed according to standard of care practices. Empiric low tidal volume ventilation in cases of hypoxemic respiratory failure would benefit ARDS patients and solve the issue of adherence to LTVV. However, an empiric tidal volume strategy does not fully address physician recognition of ARDS which is crucial to the appropriate use of additional, advanced therapies such as proning or neuromuscular blockade. The results of this study suggest that future efforts aimed at increasing use of therapies that improve ARDS outcomes should consider the empiric use of LTVV in all ventilated patients while simultaneously targeting improved physician and care team recognition of ARDS.
Highlights.
Rate of use of Low Tidal Volume Ventilation (LTVV) remains low a decade after the ARDS Network trial demonstrating the benefit of this therapy.
Rate of LTVV use was low at our center despite multiple efforts to increase use, including written protocols, ventilator order bundles, with closed ICU staffing models.
Physician diagnosis of ARDS was associated with use of LTVV in our study and supports that physicians may under recognize ARDS in clinical practice.
Acknowledgments
Funding: This work was supported by the National Institutes of Health (NIH/NHLBI T32 HL007287, University of Washington and NIH T32 DK007467, University of Washington).
Financial Disclosure: I certify that no party having direct interest in the results of the research supporting this article has or will confer a benefit on me.
Abbreviations
- ARDS
Acute Respiratory Distress Syndrome
- APACHE
Acute Physiology, Age and Chronic Health Evaluation
- FIO2
fraction of inspired oxygen
- ICU
Intensive Care Unit
- LTVV
Low Tidal Volume Ventilation
- PaO2
partial pressure of arterial oxygen
- Pplat
plateau pressure
- PEEP
positive end-expiratory pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors whose names are listed certify that they have no affiliations with or involvement in any organization or entity with an financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
BIBLIOGRAPHY
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Critical care medicine. 2006;34(5):1311–8. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 3.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–68. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 4.Network TA. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Petrucci N, De Feo C. Lung protective ventilation strategy for the acute respiratory distress syndrome. The Cochrane database of systematic reviews. 2013;2:CD003844. doi: 10.1002/14651858.CD003844.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan E, Needham DM, Stewart TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA : the journal of the American Medical Association. 2005;294(22):2889–96. doi: 10.1001/jama.294.22.2889. [DOI] [PubMed] [Google Scholar]
- 7.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Annals of internal medicine. 2009;151(8):566–76. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 8.Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Critical care. 2010;14(1):R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Needham DM, Yang T, Dinglas VD, Mendez-Tellez PA, Shanholtz C, Sevransky JE, et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. American journal of respiratory and critical care medicine. 2015;191(2):177–85. doi: 10.1164/rccm.201409-1598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–54. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 11.Fuller BM, Mohr NM, Drewry AM, Carpenter CR. Lower tidal volume at initiation of mechanical ventilation may reduce progression to acute respiratory distress syndrome: a systematic review. Critical care. 2013;17(1):R11. doi: 10.1186/cc11936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonis FD, Binnekade JM, Braber A, Gelissen HP, Heidt J, Horn J, et al. PReVENT–protective ventilation in patients without ARDS at start of ventilation: study protocol for a randomized controlled trial. Trials. 2015;16:226. doi: 10.1186/s13063-015-0759-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Critical care medicine. 2004;32(6):1289–93. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 14.Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, et al. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Critical care medicine. 2006;34(2):300–6. doi: 10.1097/01.ccm.0000198328.83571.4a. [DOI] [PubMed] [Google Scholar]
- 15.Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. American journal of respiratory and critical care medicine. 2003;167(10):1304–9. doi: 10.1164/rccm.200205-478OC. [DOI] [PubMed] [Google Scholar]
- 16.Schultz MJ, Wolthuis EK, Moeniralam HS, Levi M. Struggle for implementation of new strategies in intensive care medicine: anticoagulation, insulin, and lower tidal volumes. Journal of critical care. 2005;20(3):199–204. doi: 10.1016/j.jcrc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133(5):1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Malinchoc M, Cartin-Ceba R, Venkata CV, Kor DJ, Peters SG, et al. Eight-year trend of acute respiratory distress syndrome: a population-based study in Olmsted County, Minnesota. American journal of respiratory and critical care medicine. 183(1):59–66. doi: 10.1164/rccm.201003-0436OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serpa Neto A, Cardoso SO, Manetta JA, Pereira VG, Esposito DC, Pasqualucci Mde O, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a metaanalysis. JAMA : the journal of the American Medical Association. 2012;308(16):1651–9. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 20.Umoh NJ, Fan E, Mendez-Tellez PA, Sevransky JE, Dennison CR, Shanholtz C, et al. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Critical care medicine. 2008;36(5):1463–8. doi: 10.1097/CCM.0b013e31816fc3d0. [DOI] [PubMed] [Google Scholar]
- 21.Checkley W, Brower R, Korpak A, Thompson BT, Acute Respiratory Distress Syndrome Network I Effects of a clinical trial on mechanical ventilation practices in patients with acute lung injury. American journal of respiratory and critical care medicine. 2008;177(11):1215–22. doi: 10.1164/rccm.200709-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herasevich V, Yilmaz M, Khan H, Hubmayr RD, Gajic O. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35(6):1018–23. doi: 10.1007/s00134-009-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walkey AJ, Wiener RS. Risk factors for underuse of lung-protective ventilation in acute lung injury. Journal of critical care. 2012;27(3):323, e1–9. doi: 10.1016/j.jcrc.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kahn JM, Andersson L, Karir V, Polissar NL, Neff MJ, Rubenfeld GD. Low tidal volume ventilation does not increase sedation use in patients with acute lung injury. Critical care medicine. 2005;33(4):766–71. doi: 10.1097/01.ccm.0000157786.41506.24. [DOI] [PubMed] [Google Scholar]
- 25.Wolthuis EK, Veelo DP, Choi G, Determann RM, Korevaar JC, Spronk PE, et al. Mechanical ventilation with lower tidal volumes does not influence the prescription of opioids or sedatives. Critical care. 2007;11(4):R77. doi: 10.1186/cc5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooke CR, Watkins TR, Kahn JM, Treggiari MM, Caldwell E, Hudson LD, et al. The effect of an intensive care unit staffing model on tidal volume in patients with acute lung injury. Critical care. 2008;12(6):R134. doi: 10.1186/cc7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kallet RH, Corral W, Silverman HJ, Luce JM. Implementation of a low tidal volume ventilation protocol for patients with acute lung injury or acute respiratory distress syndrome. Respiratory care. 2001;46(10):1024–37. [PubMed] [Google Scholar]
- 28.Fessler HE, Brower RG. Protocols for lung protective ventilation. Critical care medicine. 2005;33(3 Suppl):S223–7. doi: 10.1097/01.ccm.0000155919.53727.d5. [DOI] [PubMed] [Google Scholar]
- 29.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA : the journal of the American Medical Association. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 30.Mikkelsen ME, Dedhiya PM, Kalhan R, Gallop RJ, Lanken PN, Fuchs BD. Potential reasons why physicians underuse lung-protective ventilation: a retrospective cohort study using physician documentation. Respiratory care. 2008;53(4):455–61. [PubMed] [Google Scholar]
- 31.Eichacker PQ, Gerstenberger EP, Banks SM, Cui X, Natanson C. Meta-analysis of acute lung injury and acute respiratory distress syndrome trials testing low tidal volumes. American journal of respiratory and critical care medicine. 2002;166(11):1510–4. doi: 10.1164/rccm.200208-956OC. [DOI] [PubMed] [Google Scholar]
- 32.Ricard JD. Are we really reducing tidal volume–and should we? American journal of respiratory and critical care medicine. 2003;167(10):1297–8. doi: 10.1164/rccm.2303003. [DOI] [PubMed] [Google Scholar]
- 33.Hager DN, Krishnan JA, Hayden DL, Brower RG, Network ACT Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. American journal of respiratory and critical care medicine. 2005;172(10):1241–5. doi: 10.1164/rccm.200501-048CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–55. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 35.Rubenfeld GD, Caldwell E, Granton J, Hudson LD, Matthay MA. Interobserver variability in applying a radiographic definition for ARDS. Chest. 1999;116(5):1347–53. doi: 10.1378/chest.116.5.1347. [DOI] [PubMed] [Google Scholar]
- 36.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA : the journal of the American Medical Association. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 37.Weiss CH, Baker DW, Weiner S, Bechel M, Ragland M, Rademaker A, et al. Low Tidal Volume Ventilation Use in Acute Respiratory Distress Syndrome. Critical care medicine. 2016;44(8):1515–22. doi: 10.1097/CCM.0000000000001710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6):1539–48. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Critical care medicine. 2004;32(3):858–73. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]


