Abstract
A new method for cysteine-lysine crosslinking in peptides and proteins using palladium oxidative addition complexes is presented. First, a biarylphosphine-supported palladium reagent is used to transfer an aryl group bearing an O-phenyl carbamate substituent to a cysteine residue. Next, this carbamate undergoes chemoselective acyl substitution by a proximal lysine to form a crosslink. The linkage so formed is stable towards acid, base, oxygen and external thiol nucleophiles. This method was applied to crosslink cysteine with nearby lysines in sortase A*. Furthermore, we used this method for the intermolecular crosslinking between a peptide and a protein based on the p53-MDM2 interaction. These studies demonstrate the potential for palladium-mediated methods to serve as a platform for the development of future crosslinking techniques for peptides and proteins with natural amino acid residues.
Graphical Abstract
INTRODUCTION
Intra- and intermolecular crosslinking of peptides and proteins have been recognized as an important strategy to control protein conformations, enhance protein stability, study protein-protein interactions, and improve pharmacological properties. For example, disulfide bonds formed between two cysteine side chains serve as a part of natural protein’s secondary or tertiary structures, providing the essential framework for protein function and/or stability.1 However, these naturally occurring crosslinking processes are significantly limited. As a consequence, chemists have developed numerous synthetic crosslinking methodologies2 that can link specific side chains in peptides3–7 and proteins8,9 for engineering their structures and properties.
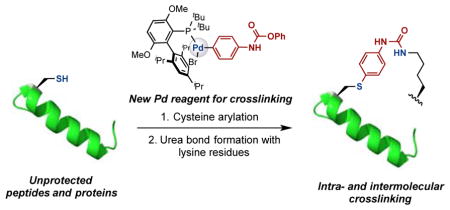
Our groups have been interested in applying transition-metal-mediated cross-coupling strategies towards the modification of complex biomolecules.7e,7f,10 Recently, we reported that aryldialkylphosphine-based palladium oxidative addition complexes are able to mediate cysteine- or lysine-selective arylations.7f,10a Furthermore, bis-palladium complexes have been applied to prepare stapled peptides through the construction of intramolecular cysteine-cysteine and lysine-lysine linkages.7e,7f In order to expand the generality of transition-metal-mediated intra- and intermolecular protein crosslinking technology, we envisioned the use of a palladium oxidative addition complex bearing an appropriate electrophilic functional group. This bifunctional reagent could react with unprotected peptides or proteins through palladium-mediated cysteine-arylation,10a after which the bound electrophilic group could react with a proximal nucleophilic residue to furnish the desired crosslink. The main challenges associated with this strategy include avoiding hydrolysis of the bioconjugation handle prior to the crosslinking step and achieving chemo- and site-selectivity. Therefore, we anticipated that judicious choice of the electrophilic functional group would be required to achieve the desired transformation with a high level of selectivity.
O-aryl carbamates are mild electrophiles, which react with amines under basic conditions to form stable urea adducts. Recently, Schultz and co-workers reported an elegant new protein crosslinking method involving incorporation of noncanonical amino acids with side chains bearing an aryl carbamate.9 They demonstrated the efficient crosslinking formation between the aryl carbamate and the proximal lysine residues within proteins. Despite its utility, a limitation of this approach is that it requires the incorporation of an unnatural amino acid into each individual protein of interest.11 To compliment the Schultz technique, we aimed to develop a direct method to incorporate the O-aryl carbamate functional group at specific natural amino acid sites in unprotected peptides and proteins to form protein crosslinked connections. Specifically, we envisioned using a palladium complex bearing a phenyl carbamate group as a cysteine-lysine crosslinking method at natural amino acid residues to form a linker with stable S-aryl and urea attachments (Figure 1). In analogy to our previous work,10a we expected that the SH group of a cysteine would displace an appropriate leaving group (X) on Pd. The ensuing reductive elimination would provide the S-arylated intermediate. Subsequent nucleophilic attack by a proximal lysine residue would provide the crosslinked peptide or protein (Figure 1a). In addition, the same strategy could be used for intermolecular crosslinking between two biomolecules (Figure 1b).
Figure 1.
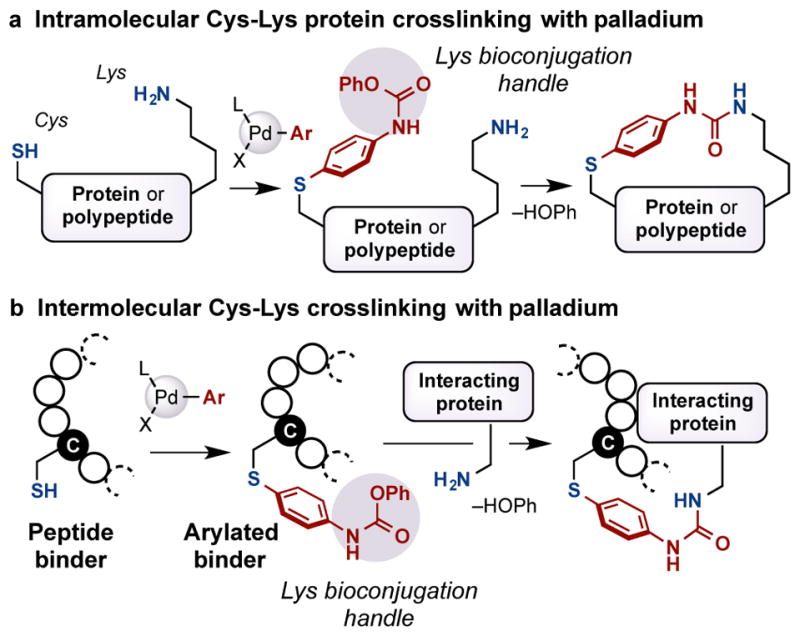
Strategy for palladium-mediated peptide and protein crosslinking.
RESULTS AND DISCUSSION
The palladium oxidative addition complex 1 bearing a phenyl carbamate can be prepared from phenyl N-(4-bromophenyl)carbamate in high yield, using our previously reported protocol with tBuBrettPhos as the ligand (Scheme 1).10a,12 This phenyl carbamate transfer reagent is a solid that is stable in the air, and may be stored at 4°C for at least 5 months without detectable decomposition.13
Scheme 1.
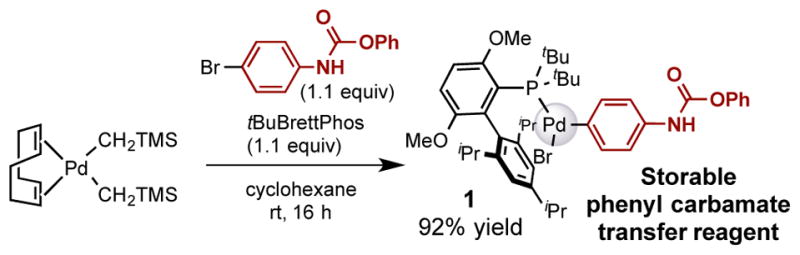
Synthesis of the Palladium Oxidative Addition Complex 1, which Bears a Phenyl Carbamate.
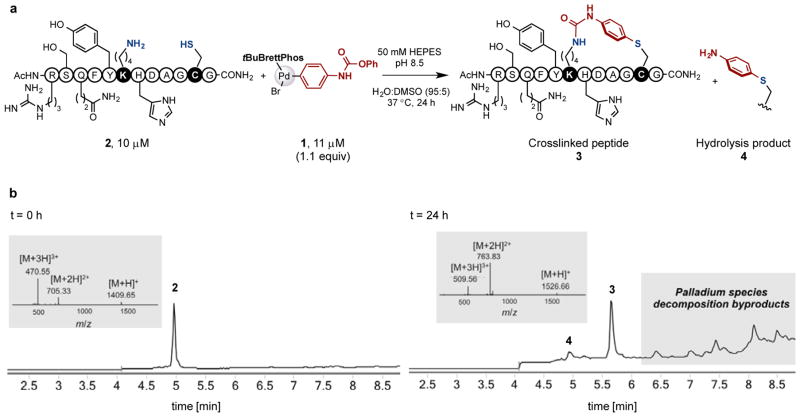
We first examined the use of 1 for the intramolecular cysteine-lysine crosslinking in a model peptide 2, which also contained a variety of other nucleophilic amino acid residues such as tyrosine, serine, arginine, histidine and glutamine which possess nucleophilic groups on their side chains (Figure 2). In the presence of 1 (11 μM, 1.1 equiv) in 50 mM pH 8.5 HEPES buffer in water/DMSO (95:5) solvent at 37° C for 24 hours,14 the substrate 2 (10 μM) was converted to the desired intramolecular crosslinked product 3. The reaction outcome was characterized by liquid chromatography-ESI-QTOF mass spectrometry (LC-MS). We observed the complete disappearance of 2, accompanied by the appearance of a new major peak corresponding to the loss of phenol and formation of a urea crosslinked product 3 (Figure 2). A small amount of the in-situ hydrolysis cysteine-arylation product 4 was detected.
Figure 2.
Selective intramolecular peptide crosslinking using the palladium oxidative addition complex 1. (a) Palladium complex 1-mediated intramolecular crosslinking of model peptide 2. (b) LC-MS analysis of starting material and the reaction mixture. The mass spectra for peptide 2 and 3 are shown as insets.
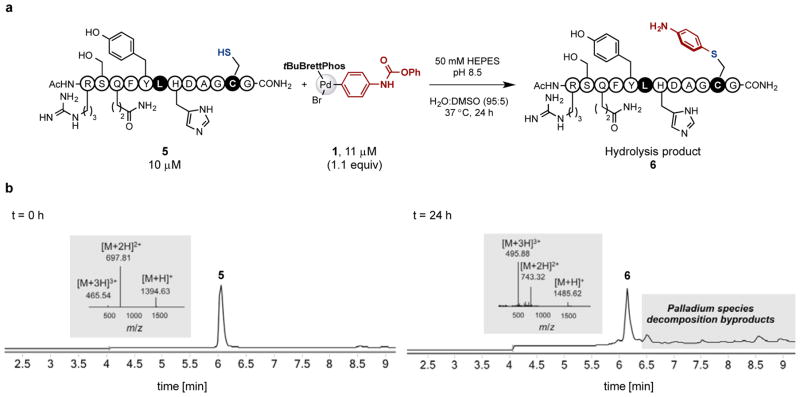
As confirmation of the chemoselectivity of this reaction, we mutated the lysine in peptide 2 to leucine (Figure 3). Under identical conditions as above, crosslinking was not observed. Instead, despite the presence of numerous nucleophilic residues, only hydrolysis of the carbamate derived from cysteine S-arylation was observed by LC-MS analysis. This suggests crosslinking occurs selectively between cysteine and lysine residues in peptide 2 using 1.
Figure 3.
Chemoselectivity of palladium complex 1-mediated peptide crosslinking is confirmed by mutation study. (a) Even in the presence of nucleophilic residues, no crosslink was formed in lysine-t0-leucine mutated model peptide 5 using the palladium complex 1, indicating crosslinking occurs between cysteine and lysine residues. (b) LC-MS analysis of starting material and the reaction mixture. The mass spectra for peptide 5 and 6 are shown as insets.
We next varied the distance between the lysine and cysteine residues to adjust the size of the newly formed macrocycle. Analogs of peptide 2, in which the relative position of cysteine and lysine residues ranged from i,i+1 to i,i+11, were prepared. When treated with the palladium complex 1 under the standard conditions, good-excellent yields of product were observed in all cases (Figure 4).
Figure 4.
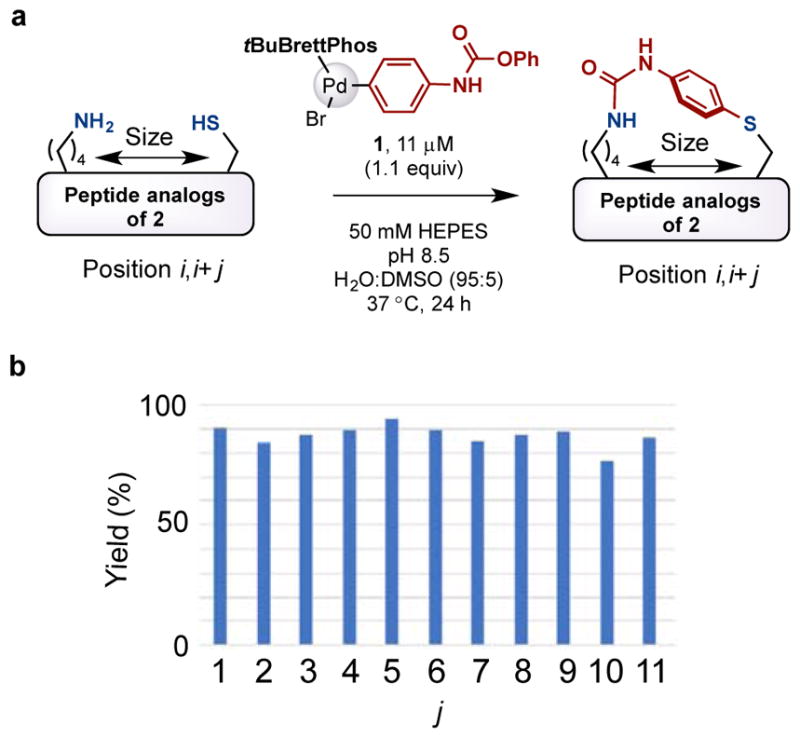
Palladium oxidative addition complex 1 enables highly efficient cysteine-lysine crosslinking to form macrocycles with different sizes. (a) Tuning the size of the macrocycles. (b) Yield summary for the macrocyclization scan. Yield of the desired products were determined by LC-MS. See the Supporting Information for the details.
A high degree of stability of the linker and the macrocyclic peptide is key for any practical crosslinking methodology. Thus, we investigated the stability of the cysteine-lysine crosslinked peptide 3 under a number of conditions (Figure 5). When conjugate 3 (100 μM) was subjected to basic (500 μM K2CO3)15, acidic (10 mM HCl) or oxidative (open air) conditions, LC-MS analysis showed that no degradation had occurred after 24 hours. Additionally, no reaction of 3 was observed in the presence of the external nucleophile glutathione (500 μM) in Tris buffer (100 mM) at pH 7.5 over the same period of time.
Figure 5.
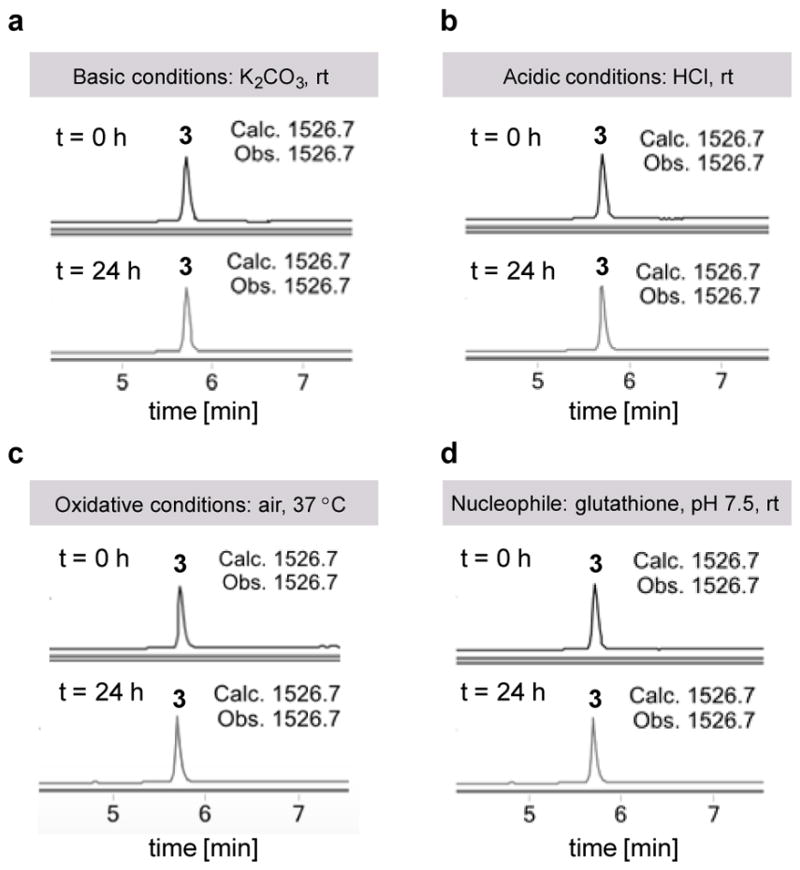
Chemical stability of the intramolecular crosslinked peptide 3 (100 μM) under (a) basic (500 μM K2CO3) condition; (b) acidic (10 mM HCl) condition; (c) oxidative conditions (air) in Tris buffer (100 mM, pH 7.5) and (d) in the presence of glutathione (500 μM) in Tris buffer (100 mM, pH 7.5). LC-MS analysis indicated that little or no decomposition had occurred after 24 hours.
This palladium-mediated methodology was next applied to the intramolecular crosslinking within a protein (Figure 6). Engineered sortase A (SrtA*, 7) containing one cysteine and 18 lysine residues was chosen,16 in order to investigate the site-selectivity of the crosslinking. The X-ray crystal structure (PDB ID 2KID) of wild type SrtA shows that Cys(184) is close to Lys(134), Lys(162) and Lys(198) (Figure S1). Thus, we would expect that crosslinking between cysteine and these residues would be most probable. The reaction of SrtA* (7) with 1 under the standard reaction conditions was conducted and the resulting reaction mixture was analysed by LC-MS. As expected, the disappearance of the peak corresponding to the SrtA* (7) with a mass of 19,214.5 Da was observed. This was accompanied by the appearance of a new peak corresponding to a mass of 19,331.7 Da, consistent with the formation of an S-aryl and urea linkage to produce the desired product 8. To confirm the crosslinking site, the reaction mixture was subjected to trypsin and the resultant mixture was analysed by the LC-MS/MS (See the Supporting Information for details). The MS/MS fragmentation of the products (Figure S2) indicated that crosslinks had occurred between Cys(184) and either Lys(162) or Lys(198), in approximately equal proportion.
Figure 6.
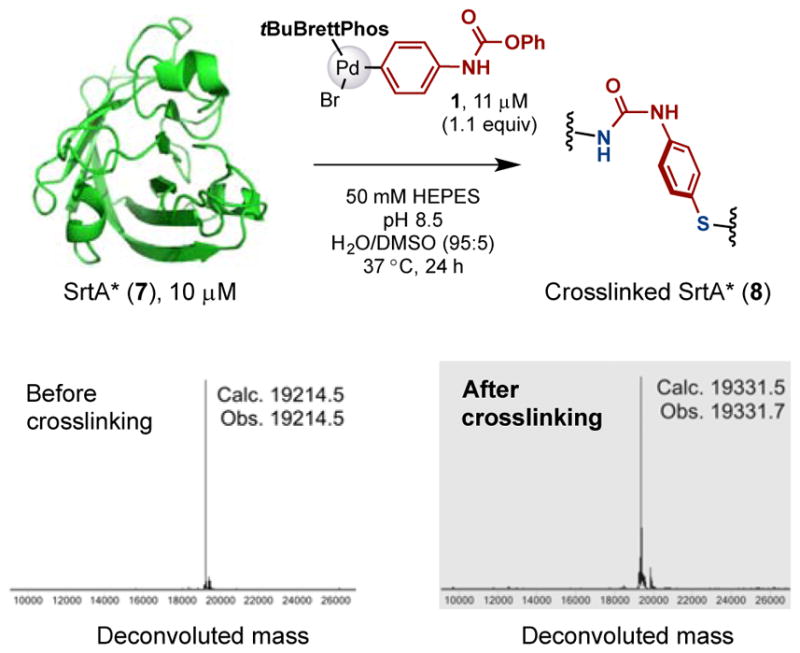
SrtA* (7) was crosslinked intramolecularly by the palladium complex 1. The deconvoluted mass spectra for the whole protein peak from LC-MS analysis of the starting material and crude reaction mixture are shown.
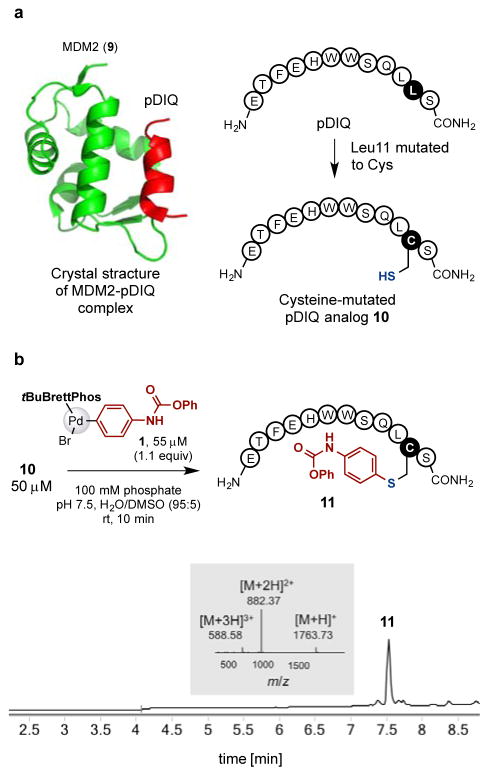
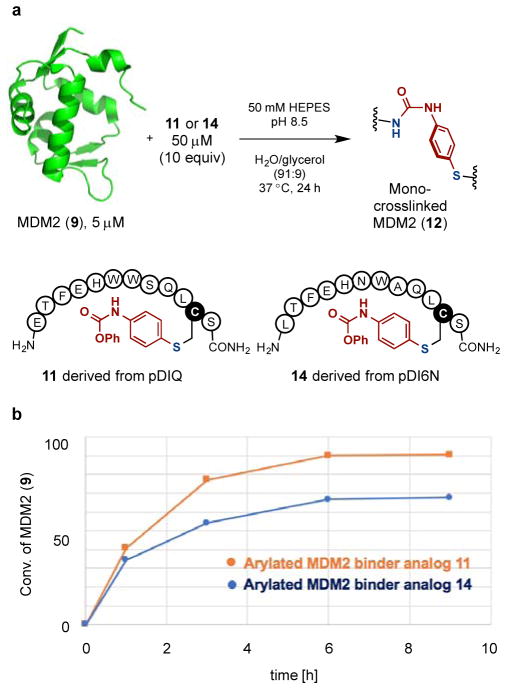
Our method was also used to form an intermolecular crosslinking between a peptide and an interacting protein. MDM2 protein (9) is known to bind to the peptide pDIQ with high affinity (Figure 7a).17 We carried out the crosslinking process in two separate steps. An advantage of this approach is that the product of the first step can be stored and used over a period of time. First, pDIQ analog 10 was prepared with the leucine residue mutated to cysteine (Figure 7a). To incorporate a phenyl carbamate group into the peptide substrate 10, we carried out the arylation reaction of 10 (50 μM) with the palladium oxidative addition complex 1 (55 μM, 1.1 equivalents, relative to 10) with 100 mM pH 7.5 phosphate buffer in water/DMSO (95:5) co-solvent at room temperature for 10 minutes. This yielded the desired arylation product 11 with complete conversion of 10 (Figure 7b). The product 11 was purified by a reverse phase HPLC to provide the pure product. Notably, even though it contains a free N-terminal amine, 11 does not undergo macrocyclization in acidic media during HPLC purification and can be stored in water at 500 μM in the freezer for at least three months.
Figure 7.
Preparation of MDM2 binding peptide analog 11 bearing a phenyl carbamate. (a) X-ray crystal structure of MDM2-pDIQ complex (PDB ID: 3JZS) and design of pDIQ analog 10 for cysteine arylation. (b) The palladium-mediated cysteine arylation of 10. LC-MS analysis showed full conversion of 10 to form the desired product 11.
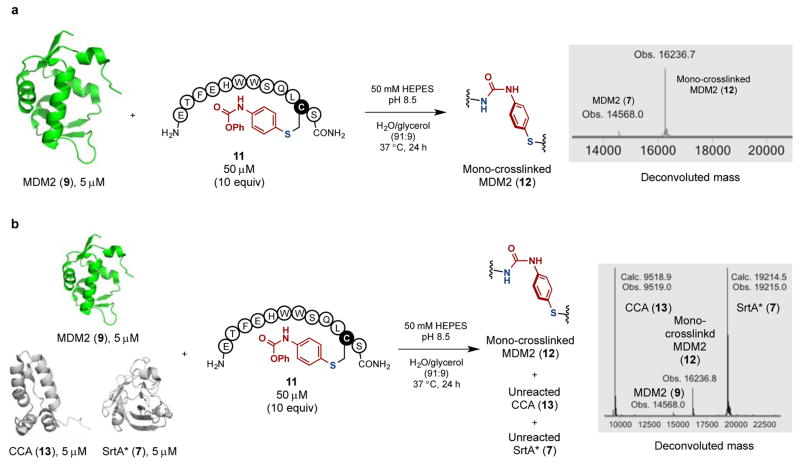
The crosslinking reaction between the conjugate 11 and MDM2 (9) was carried out. MDM2 (9) contains one cysteine and 9 lysine residues (Figure 8). The reaction of 9 (5 μM) and 10 equivalents of 11 (50 μM) in pH 8.5 HEPES buffer produced the mono-crosslinked product 12 (Figure 8a). In the deconvoluted mass spectra from LC-MS analysis, the original mass peak corresponding to 9 at 14,568.0 Da entirely disappeared and a single new peak at 16,236.7 Da emerged, consistent with the loss of phenol and formation of a urea crosslink in the desired product 12. Notably, multiply crosslinked products were not observed. The LC-MS analysis also showed that the conjugate 11 underwent intramolecular cyclization between the phenyl carbamate and its terminal amine to form the corresponding macrocyclic peptide. We note that although we only see product from the addition of one equivalent of 11 to 9, we cannot say whether only one crosslinked species is formed. To demonstrate the observed selectivity in the crosslinking reaction of MDM2 (9) is not derived from the competing cyclization of 11, we added an additional 10 equivalents of 11 to the reaction mixture and waited for another 24 hours. Only a trace amount of the double-crosslinked product was observed (Figure S3), highlighting the crosslinking reaction is primarily mediated by the protein-peptide interaction.
Figure 8.
Protein-selective intermolecular crosslinking between a peptide and an interacting protein. (a) Intermolecular crosslinking of MDM2 (9) with the arylated pDIQ binder analog 11. The deconvoluted mass spectra for the whole protein peak from LC-MS analysis of the crude reaction mixture showed no multiple crosslinked products. (b) Protein-selective intermolecular crosslinking of MDM2 (9) in a protein mixture. Only MDM2 (9) was modified as indicated by the LC-MS analysis.
Given the efficient crosslinking reaction between 9 and 11, we wondered whether the reaction was MDM2 (9) selective if carried out in a mixture of proteins (Figure 8b). To this end, 10 equivalents of the arylated pDIQ analog 11 (50 μM) and a mixture of three proteins, MDM2 (9, 5 μM), the C-terminal domain of HIV-1 capsid assembly polyprotein (CCA, 13, 5 μM), which contains 6 lysines, and SrtA* (7, 5 μM), which contains 18 lysines, were incubated. Analysis by LC-MS indicated that the mono-crosslinked MDM2 (12) had been formed (94% conversion determined by LC-MS) leaving CCA (13) and SrtA* (7) unmodified (Figure 8b).
In order to probe the relationship between binding affinity of the substrate peptide and the rate of intermolecular crosslinking with MDM2 (9), a second arylated MDM2 binder analog 14 was prepared (See the Supporting Information for the details) and its reaction with MDM2 (9) was examined (Figure 9). Peptides 11 and 14 were derived from pDIQ (IC50 = 8 nM) and pDI6N (IC50 = 400 nM) respectively, which have different binding affinities toward 9.17 Indeed, the more tightly binding pDIQ analog 11 provided a higher conversion at every time point (1, 3, 6 and 9 hours) compared to that of the arylated pDI6N analog 14 (Figure 9).
Figure 9.
The efficiency of intermolecular crosslinking of MDM2 is mediated by peptide binding affinity. (a) MDM2 (9) was reacted with two analogs 11 and 14 derived from two MDM2 binding peptides of different affinities. (b) Summary of MDM2 conversion at different time points.
CONCLUSION
In summary, we have developed a new protocol for peptide and protein crosslinking at natural amino acid residues using palladium oxidative addition complexes. This method involves the palladium-mediated arylation of cysteine residues to incorporate an O-phenyl carbamate group into peptides or proteins, followed by acyl substitution by proximal lysine residues. Compared to other cysteine-lysine crosslinking reagents such as N-hydroxysuccinimide(NHS)-maleimide, NHS-pyridyldithiol or NHS-haloacetyl crosslinkers, the palladium oxidative complex 1 shows potential advantages in high linker stability and minimal hydrolysis side reactions. The method was successfully applied to intramolecular crosslinking of SrtA* (7) and intermolecular crosslinking between interacting MDM2 (9) and pDIQ analog 11 with excellent protein-selectivity. These studies demonstrate the potential for palladium oxidative addition complexes to serve as new reagents for protein crosslinking.
Supplementary Material
Acknowledgments
Financial support for this work was provided by the National Institutes of Health (R01GM46059 and R01GM110535). MIT has patents on some of the ligands and precatalysts used in this work from which S.L.B. and former co-workers receive royalty payments. We gratefully acknowledge Sigma-Aldrich for the gift of RuPhos and BrettPhos. K.K. gratefully acknowledge support from the Japan Society for the Promotion of Science. We thank Dr. Ekaterina Vinogradova for performing related initial experiments. We thank Dr. Mette Ishoey and Dr. Faycal Touti for assistance in protein expression. We thank Richard Liu, Dr. Nicholas White, and Dr. Andy Thomas (MIT) for assistance on the preparation of this manuscript.
Footnotes
Experimental procedures, data and computational details. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Fass D. Annu Rev Biophys. 2012;41:63–79. doi: 10.1146/annurev-biophys-050511-102321. [DOI] [PubMed] [Google Scholar]
- 2.The selected reviews on synthetic polypeptide crosslinking strategies, see: Koniev O, Wagner A. Chem Soc Rev. 2015;44:5495–5551. doi: 10.1039/c5cs00048c.Kluger R, Alagic A. Bioorg Chem. 2004;32:451–472. doi: 10.1016/j.bioorg.2004.08.002.
- 3.For the selected examples of peptide crosslinking by olefin metathesis, see: Wang D, Chen K, Kulp JL, Arora PS. J Am Chem Soc. 2006;128:9248–9256. doi: 10.1021/ja062710w.Speltz TE, Fanning SW, Mayne CG, Fowler C, Tajkhorshid E, Greene GL, Moore TW. Angew Chem Int Ed. 2016;55:4252–4255. doi: 10.1002/anie.201510557.
- 4.For the selected examples of peptide crosslinking by alkylation, see: Muppidi A, Doi K, Edwardraja S, Drake E, Gulick A, Wang H, Lin Q. J Am Chem Soc. 2012;134:14734–14737. doi: 10.1021/ja306864v.Jo H, Meinhardt N, Wu Y, Kulkarni S, Hu Z, Low KE, Davies PL, DeGrado WF, Greenbaum DC. J Am Chem Soc. 2012;134:17704–17713. doi: 10.1021/ja307599z.Wang Y, Chou D. Angew Chem Int Ed. 2015;54:10931–10934. doi: 10.1002/anie.201503975.Zambaldo C, Luo X, Mehta AP, Schultz PG. J Am Chem Soc. 2017;139:11646–11649. doi: 10.1021/jacs.7b04159.
- 5.For the selected examples of peptide crosslinking by cycloaddition, see: Scrima M, Le Chevalier-Isaad A, Rovero P, Papini AM, Chorev M, D’Ursi AM. Eur J Org Chem. 2010;2010:446–457.Lau Y, de Andrade P, Quah S-T, Rossmann M, Laraia L, Skold N, Sum T, Rowling P, Joseph T, Verma C, Hyvonen M, Itzhaki L, Venkitaraman A, Brown C, Lane D, Spring D. Chem Sci. 2014;5:1804–1809.
- 6.For the selected examples of peptide crosslinking by lactamization, see: Taylor JW. Biopolymers. 2002;66:49–75. doi: 10.1002/bip.10203.de Araujo AD, Hoang HN, Kok WN, Diness F, Gupta P, Hill TA, Driver RW, Prince DA, Liras S, Fairlie DP. Angew Chem Int Ed. 2014;53:6965–6969. doi: 10.1002/anie.201310245.
- 7.For peptide crosslinking by arylation, see: Spokoyny AM, Zou Y, Ling JJ, Yu H, Lin YS, Pentelute BL. J Am Chem Soc. 2013;135:5946–5949. doi: 10.1021/ja400119t.Brown S, Smith A. J Am Chem Soc. 2015;137:4034–4037. doi: 10.1021/ja512880g.Kalhor-Monfared S, Jafari MR, Patterson JT, Kitov PI, Dwyer JJ, Nuss JM, Derda R. Chem Sci. 2016;7:3785–3790. doi: 10.1039/c5sc03856a.Lautrette G, Touti F, Geun Lee H, Dai P, Pentelute BL. J Am Chem Soc. 2016;138:8340–8343. doi: 10.1021/jacs.6b03757.Rojas AJ, Zhang C, Vinogradova EV, Buchwald N, Reilly J, Pentelute BL, Buchwald SL. Chem Sci. 2017;8:4157–4263. doi: 10.1039/c6sc05454d.Geun Lee H, Lautrette G, Pentelute BL, Buchwald SL. Angew Chem Int Ed. 2017;56:3177–3181. doi: 10.1002/anie.201611202.
- 8.The selected examples of protein crosslinking, see: Xiang Z, Ren H, Hu YS, Coin I, Wei J, Cang H, Wang L. Nat Methods. 2013;10:885–888. doi: 10.1038/nmeth.2595.Furman JL, Kang M, Choi S, Cao Y, Wold ED, Sun SB, Smider VV, Schultz PG, Kim CH. J Am Chem Soc. 2014;136:8411–8417. doi: 10.1021/ja502851h.Chen X-H, Xiang Z, Hu YS, Lacey VK, Cang H, Wang L. ACS Chem Biol. 2014;9:1956–1961. doi: 10.1021/cb500453a.Hoppmann C, Lacey VK, Louie GV, Wei J, Noel JP, Wang L. Angew Chem Int Ed. 2014;53:3932–3936. doi: 10.1002/anie.201400001.
- 9.Xuan W, Shao S, Schultz PG. Angew Chem Int Ed. 2017;56:5096–5100. doi: 10.1002/anie.201611841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Vinogradova EV, Zhang C, Spokoyny AM, Pentelute BL, Buchwald SL. Nature. 2015;526:687–691. doi: 10.1038/nature15739. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhao W, Geun Lee H, Buchwald SL, Hooker JM. J Am Chem Soc. 2017;139:7152–7155. doi: 10.1021/jacs.7b02761. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rojas AJ, Pentelute BL, Buchwald SL. Org Lett. 2017;19:4263–4266. doi: 10.1021/acs.orglett.7b01911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Xiao H, Schultz PG. Cold Spring Harbor Perspect Biol. 2016;8:a023945. doi: 10.1101/cshperspect.a023945. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu CC, Schultz PG. Annu Rev Biochem. 2010;79:413–444. doi: 10.1146/annurev.biochem.052308.105824. [DOI] [PubMed] [Google Scholar]
- 12.McAtee JR, Martin SES, Ahneman DT, Johnson KA, Watson DA. Angew Chem Int Ed. 2012;51:3663–3667. doi: 10.1002/anie.201200060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.After aging the palladium reagent 1 for 5 months at 4°C in a freezer, we performed the crosslinking of peptide 2 using this batch and quantitatively obtained the desired product 3.
- 14.The full optimization data is described in the Supporting Information.
- 15.In contrast, a significant amount of degradation of maleimide-cysteine conjugate was observed under basic conditions in our previous study (ref. 10a).
- 16.The SrtA* used here contains P94S/D160N/K196T triple mutation, which promotes enhanced catalytic efficiency, see: Chen I, Dorr BM, Liu DR. Proc Natl Acad Sci U S A. 2011;108:11399–11404. doi: 10.1073/pnas.1101046108.
- 17.Jason P, Zhenyu L, Agnieszka K, Baozong L, Said S, Wayne G, Ernst S. J Biol Chem. 2010;285:2174–2183. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.