Abstract
Purpose of review
Metabolic surgery is recommended for the treatment of type 2 diabetes for its potent ability to improve glycemic control. However, the mechanisms underlying the beneficial effects of metabolic surgery are still under investigation. We provide an updated review of recent studies into the molecular underpinnings of metabolic surgery, focusing in on what is known about the role of gut microbiota. Over the last seven years several reports have been published on the topic, however the field is expanding rapidly.
Recent findings
Studies have now linked the regulation of glucose and lipid metabolism, neuronal and intestinal adaptations, and hormonal and nutrient signaling pathways to gut microbiota. Given that the composition of gut microbiota is altered by metabolic surgery, investigating the potential mechanism and outcomes of this change are now a priority to the field.
Summary
As evidence for a role for microbiota builds, we expect future patients may receive microbe-based therapeutics to improve surgical outcomes and perhaps one day preclude the need for surgical therapies all together. In this review and perspective, we evaluate the current state of the field and its future.
Keywords: Bariatric Surgery, metabolic surgery, microbiota
Introduction
In the hours and days immediately following restructuring of the gastrointestinal tract through bariatric surgery, patients experience a shift toward improved glucose regulation. The shift occurs independent of weight loss or dietary improvement, though both are commonly touted benefits of the surgery. In recent years, these same surgical procedures have been shown to improve outcomes of other obesity-related diseases including type 2 diabetes (T2D), cardiovascular disease, and reverse some end-organ damage[1,2]. Moreover, the effects of surgery on weight loss, T2D, and cardiovascular disease risks factors are maintained even decades after surgery[3,4]. As such, the delegates of the 2nd Diabetes Surgery Summit (DSS-II) now suggest surgery as a treatment option for certain patients with T2D, including those with body-mass indices (BMIs) < 35, the traditional exclusion metric for patients[5]. It is increasingly clear that ‘metabolic surgery’ proves a more apt term for the procedures. Despite this clinical step forward, experimentally the molecular underpinnings behind many of the beneficial effects of metabolic surgery remain a mystery. Several lines of evidence, both new and old, suggest changes in secretion of incretin hormones, bile acid levels, lipid homeostasis, neuronal and intestinal adaptation may contribute to the beneficial effects (Fig. 1). Recently, research has delved in to investigating the role of the gut microbiome, largely because microbiota are linked to the regulation of these same factors.
Figure 1.
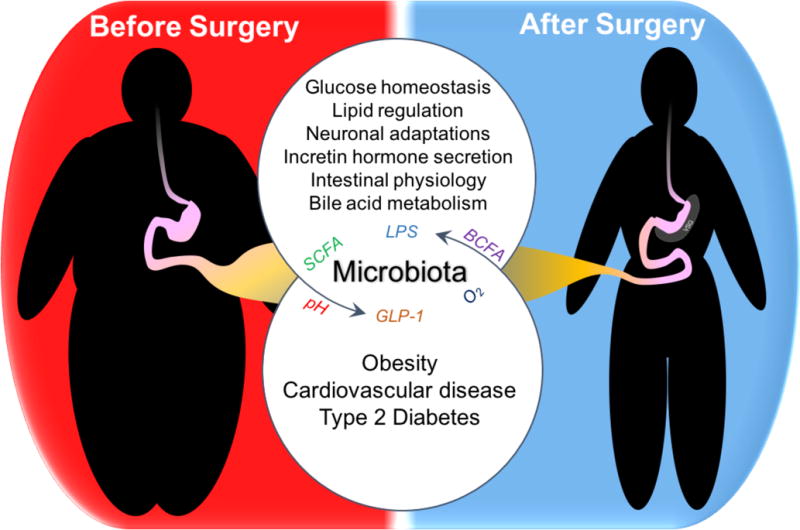
What is the role for microbiota in the beneficial outcomes of metabolic surgery?
Vertical Sleeve Gastrectomy (VSG) results in improved metabolic function. It is just a single example of the many metabolic surgeries available for patients with obesity, cardiovascular diseases, and type 2 diabetes. The systemic mechanisms behind the vast post-operative improvement are still under investigation, and microbiota may contribute. However, how surgery alters microbiota, and the specific mechanisms microbes employ to regulate metabolic function are still under investigation
By far the most widely appreciated benefit of metabolic surgery is the robust and sustained weight loss seen in patients in the weeks and months post-operation. According to the World Health Organization 39% of adults are overweight, 13% of which are classified as obese[6]. Yet, despite decades of research, few pharmaceutical treatments have entered the market with more than a modest effect on weight loss, and many of these drugs are accompanied by significant adverse effects for patients[7]. Glucose metabolism also rapidly improves following metabolic surgery, with surgery recipients demonstrating superior control relative to those making lifestyle changes alone[5]. In the most recent update of the STAMPEDE clinical trial, 5-year outcomes of T2D patients who were randomly assigned to undergo intensive medical therapy with or without metabolic surgery, showed that patients that received metabolic surgery achieved better glycemic control. Moreover, between 25% and 45% of patients, dependent on surgical procedure, were able to do so without taking additional medications[4]. Therefore, understanding how weight loss and improved glucose regulation is achieved following metabolic surgery, is critical not only to improve surgery outcomes, but also to someday preclude the need for such invasive therapies. Over the past five years, several strides have been made toward understanding the role of gut microbiota in regulating weight loss post-surgery. The microbiota may play a mechanistic role in mediating outcomes, though the current state of the field has numerous inconsistencies, which may reflect inherent variability in microbiomes based on age, ethnicity, and diet.
For historical reference and to our knowledge, the earliest study looking into changes in gut microbes following metabolic surgery was published in 2009[8]. Using just three patient samples taken 8-15 months post-surgery, Zhang et al. observed altered fecal bacteria composition compared to obese and normal weight patients[8]. A series of papers followed, with larger cohorts spanning humans and rodents, which further associated microbial species and metabolites with various post-surgical outcomes[9–12].
Compositional changes in the gut microbiome following metabolic surgery
Most studies to date have investigated whether certain phyla or species of bacteria from fecal samples can be linked to surgical outcomes[11–16]. These studies vary by surgical procedure (e.g. Roux-en-Y gastric bypass, vertical sleeve gastrectomy, duodenal-jejunal bypass) and study models, making cross-study comparisons difficult. Nevertheless, Guo et al. (2017) and Magouliotis et al. (2017) recently conducted meta-analyses of the published microbiome studies post-metabolic surgery[17,18]. These meta-analyses are important contributions to the recent literature, especially given the variability in microbiome studies, because they cohesively examine the changes in microbiota that are reliable across cohorts, surgical models, and species. They found that while diversity and richness of microbiota varied across studies post-operatively, several taxa had strong evidence for significant and consistent post-operative change (e.g. increased Proteobacteria, specifically genus Escherichia). Certain bacterial groups were strongly associated with patient parameters across studies, for example Bifidobacteria and BMI[17]. Recent evidence points to an association between gut microbiota, particularly Escherichia coli, and T2D remission and occasional recurrence following metabolic surgery[19,20]. This observation warrants mechanistic follow-up, as it may provide future avenues for therapeutic improvement of surgical outcomes. Interestingly, a study by Ilhan et al. (2017) suggest that restrictive procedures, like laproscopic adjustable gastric banding, which does not improve glucose regulation in excess of weight loss alone[5], does not have as profound an effect on microbial composition[21], which has implications for understanding how surgery alters overall microbial composition.
The microbiome itself varies regionally (e.g. duodenum versus colon) as well as locally (e.g. inter-fold versus digesta) within the gut[22,23]. While fecal samples are the most common and easiest to collect, they largely represent the microbial environment of just the distal colon. Less is known about the microbial changes that occur in the upper gastrointestinal tract following surgery, which is surprising given that recent studies have strongly implicated this region as mechanistically important in metabolic surgery (i.e. the foregut hypothesis)[24]. Following Roux-en-Y gastric bypass, gut microbiota is altered differently in each region of the intestine[12]. While it is likely that this is true of other procedures, very little is currently known about the mechanistic impact of these regional changes.
Microbial mechanisms regulating beneficial outcomes of metabolic surgery
The gold standard to determine whether microbiota contribute to a phenotype are fecal transplant studies, typically using germ-free mice, and more recently in humans[25]. Such studies have been conducted following bariatric surgery by transferring microbiota from both rodents[15,26] and humans[27] following metabolic surgery into germ-free mice. The earliest study of this type, published in 2013 by Liou and colleagues, found that cecal transplant from a mouse model of metabolic surgery induced reduced adiposity without reductions in food intake of the recipient germ-free mice[15]. Similar results were found by the Bäckhed laboratory using fecal samples from patients following metabolic surgery[27]. However, a recent study from the same laboratory found using a rat model of metabolic surgery that these effects show regional specificity, with mice colonized with ileal microbiota from rats receiving metabolic surgery unexpectedly having an impaired glucose tolerance, while cecal contents resulted in improved sensitivity[26]. Despite the high density of microbes in the colon, these results further emphasize the need to consider the effects of microbes throughout the gastrointestinal tract, including the small intestine.
Toward better mechanistic understanding of gut microbiota, a recent population study of Han Chinese individuals following metabolic surgery described post-operative shifts in fecal microbial populations with marked increases in Bacteroides thetaiotamicron abundance[28]. Interestingly, gavaging mice with B. thetaiotamicron resulted in reduced adiposity and resistance to weight gain on a high-fat diet. The authors hypothesized that the relationship between this specific bacterial population and body weight may be associated with circulating levels of amino acid and neurotransmitter, glutamate[28]. While this observation is correlational, the study provides some of the early mechanistic evidence for specific microbial species in regulating adiposity and the beneficial effects of metabolic surgery.
One of the conceptual and experimental challenges of transplant studies is evaluating the ability of transplanted microbes to replicate the donor state, including regional localization and density. Most transplant studies, such as the ones mentioned above, take a top-down approach by orally administering microbiota. Microbes must then survive the acidic stomach and relatively oxygen-rich small intestine before colonizing the comparable colon (in the case of fecal samples) in the recipient animal. An alternative strategy to evaluate the role of microbiota is via depletion, such as by administering antibiotics to reduce the density of microbiota[29]. These types of studies have their own limitations, ranging from the effect of antibiotics on the host to incomplete elimination of gut microbiota. Other recent studies have approached the role for microbiota using probiotics, though no significant beneficial or protective effect was found after 6 months administration following metabolic surgery[30]. However, in a recent study evaluating the effects of fecal transplant in humans in relation to metabolic disease, Kootte et al. showed that fecal transplant from lean donors into patients with metabolic disease improved peripheral insulin sensitivity, but the transient effect lasted only weeks[25]. Overall, we think it is important for future studies of these types to consider not only regional effects of microbes, but also temporal.
On a related note of microbial mechanisms, lipopolysacharides (LPS) have been hypothesized to contribute to improved metabolic health post-surgery. LPS, a gram-negative bacterial cell wall component and biomarker of chronic inflammation, when elevated in blood plasma can drive adipose precursor proliferation and macrophage infiltration contributing to metabolic disease[31]. While obese patients have elevated plasma LPS levels, there is evidence that metabolic surgery decreases these levels [32,33], though preoperative levels and post-operative effect sizes are variable across studies. How metabolic surgery acts to decrease these levels is under active investigation, and could be the result of improved barrier function and or altered microbial load or composition at the mucosal surface[34]. Much research over the last decade into the role of gut microbiota has focused on short-chain fatty acids (SCFAs), which are produced primarily by gut bacterial fermentation of fiber, and which are compositionally altered by metabolic surgery. Increased levels of certain SCFAs can improve intestinal barrier function[35], which may contribute to improved insulin signaling[36]. However, future research will need to be conducted to determine the direct and indirect roles of gut microbiota in regulating host metabolism and the beneficial effects of metabolic surgery.
How does surgery change microbial composition?
How surgical intervention alters gut microbiota is of considerable interest to the field. There are several hypotheses, while it is possible and very likely that multiple factors contribute to the overall compositional changes, very few have been experimentally tested. It is possible that the surgical interventions that alter the gastric pouch cause changes to pH or oxygen levels in the upper gastrointestinal tract thereby changing the environmental suitability for some bacteria, and likely also contributing the high rate of bacterial overgrowth following surgery[8,37]. The pH of fecal samples after bilio-intestinal bypass, are much lower than pre-operative controls, which may support this hypothesis[38]. Alternatively, it has been hypothesized that changes in intestinal length or transit time could favor colonization of faster growing species[8]. Nevertheless, it is also possible that changes in microbial composition are secondary to other effects of surgery. For example, farnesoid-x receptor (FXR) signaling and bile acid metabolism have been shown to modulate microbial composition and metabolic surgery outcome[39–41]. More recently, increased focus has been placed on the role of branched-chain amino acids and their microbially-derived by product branched-chain fatty acids[21]. Branched-chain fatty acids were observed to be increased after surgery[27], though others suggest that changes in plasma non-esterified fatty acids following surgery are more representative of the remodeling and improved lipid handling that occurs following surgery[42]. In the meta-analysis by Guo et al (2017), the authors identified six studies which evaluated KEGG enrichment of microbial gene categories, which showed a general enrichment in gene expression related to the phosphotransferase system (PTS), which is a bacterial system for the uptake of carbohydrates for energy using the substrate phosphoenolpyruvate. Interestingly, in a recent study investigating the connection between host genotype, microbiota, and metabolic pathways, sensitivity to a high-fat diet and increased insulin secretion was associated with microbiota with more PTS gene expression[43].
Conclusion
While the current state of the field indicates that the microbial landscape undergoes a significant and sustained alteration following metabolic surgery, we are still investigating the mechanistic impact of these changes. Several recent studies have touched on this knowledge deficit, and we expect many more such studies in the coming years. Research into the role of commensal microbiota in metabolic surgery outcomes is overall relatively new. While much can be gleaned from the broader studies of host-microbe interactions, communication, and metabolism, we are still awaiting the contextual studies to evaluate the impact and effect size following surgery. The future will likely see microbe-based therapeutics either as a mono- or combination-treatment option with metabolic surgery. However, these therapeutics will not progress without a firmer understanding of how surgery influences microbiota, and how they in turn mediate key beneficial effects of metabolic surgery.
Key points.
The beneficial effects of metabolic surgery on weight loss, glycemic control, and type 2 diabetes are not the result of mechanical restriction and reduced nutrient absorption.
Endocrine, neuronal, and metabolic improvements occur following metabolic surgery.
Gut microbiota are compositionally changed by metabolic surgery, and may contribute to the changes seen in the beneficial outcomes or surgery
Investigation into the microbial mechanisms mediating the beneficial effects of metabolic surgery are in their infancy, but the field is progressing rapidly.
Acknowledgments
None
Financial support and sponsorship
This work was supported by the National Institutes of Health, including the National Institute of Digestive and Kidney Diseases (RJS, R01DK107652; BCEP, 5T32DK101357).
Funding for this work generously provided by: The National Institutes of Health and the National Institute of Digestive and Kidney Diseases.
Footnotes
Conflicts of interest
RJS serves as a paid consultant for and receives research support from Ethicon Endo-Surgery (a subsidiary of Johnson & Johnson), Novo Nordisk, Janssen (a subsidary of Johnson & Johnson), and Kallyope. He serves as a paid consultant for Daiichi Sankyo, Novartis, Orexigen, and Scohia. RJF receives research support from Zafgen, Sanofi, and MedImmune. Additionally, RJS is a paid-consultant and expert witness for Paul Hastings Law Firm. BCEP has declared no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, (18 months/ 2016-2017) have been highlighted as being:
• of special interest
•• of outstanding interest
- 1*.Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. OBES SURG. 2017;27:2724–2732. doi: 10.1007/s11695-017-2866-4. This is a strong meta-analysis and review of the various diseases and complications that are improved following metabolic surgery, but that are not necessarily widely appreciated by the scientific and medical community. [DOI] [PubMed] [Google Scholar]
- 2*.Benotti PN, Wood GC, Carey DJ, et al. Gastric Bypass Surgery Produces a Durable Reduction in Cardiovascular Disease Risk Factors and Reduces the Long-Term Risks of Congestive Heart Failure. J Am Heart Assoc. 2017;6:e005126–12. doi: 10.1161/JAHA.116.005126. This massive long-term case-control study of metabolic surgery patients focusing on risk of cardiovascular diseases. Many studies have focused on type 2 diabetes risk and remission, but risk for other metabolic diseases are also decreased by surgery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3**.Adams TD, Davidson LE, Litwin SE, et al. Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med. 2017;377:1143–1155. doi: 10.1056/NEJMoa1700459. This 12-year follow-up report of a large observational study of patients who received gastric bypass demonstrates the long-term efficacy of metabolic surgery for weight loss and diabetes remission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4*.Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes — 5-year outcomes. N Engl J Med. 2017;376:641–651. doi: 10.1056/NEJMoa1600869. One of the important aspects of this study is the long-term follow-up, combined with the study design comparing intensive medical therapy with or without metabolic surgery. It definitively demonstrates that metabolic surgery has improved outcomes compared to medical therapies resulting in weight loss alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39:861–877. doi: 10.2337/dc16-0236. This is the recent statement by the delegates of the DSS-II suggesting a new algorithm for determining patient suitability for metabolic surgery. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Obesity and overweight: Fact sheet [Internet] 2016 [Google Scholar]
- 7*.Saltiel AR. New therapeutic approaches for the treatment of obesity. Science Translational Medicine. 2016;8:323r. doi: 10.1126/scitranslmed.aad1811. v2–323rv2. This is an excllentreview of the current pharmacological treatment options for patients with obesity. [DOI] [PubMed] [Google Scholar]
- 8.Zhang H, DiBaise JK, Zuccolo A, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furet J-P, Kong L-C, Tap J, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. [Internet] Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graessler J, Qin Y, Zhong H, et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J. 2013;13:514–522. doi: 10.1038/tpj.2012.43. [DOI] [PubMed] [Google Scholar]
- 11.Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut. 2011;60:1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osto M, Abegg K, Bueter M, et al. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav. 2013;119:92–96. doi: 10.1016/j.physbeh.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Shao Y, Ding R, Xu B, et al. Alterations of gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in Sprague-Dawley rats. OBES SURG. 2017;27:295–302. doi: 10.1007/s11695-016-2297-7. [DOI] [PubMed] [Google Scholar]
- 14.Guo Y, Liu C-Q, Shan C-X, et al. Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: Increased diversity and associations of discriminant genera with metabolic changes. Diabetes/Metabolism Research and Reviews. 2016;33:e2857. doi: 10.1002/dmrr.2857. [DOI] [PubMed] [Google Scholar]
- 15.Liou AP, Paziuk M, Luevano J-M, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Science Translational Medicine. 2013;5:178ra41:1–11. doi: 10.1126/scitranslmed.3005687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jahansouz C, Staley C, Bernlohr DA, et al. Sleeve gastrectomy drives persistent shifts in the gut microbiome. Surg Obes Relat Dis. 2017;13:916–924. doi: 10.1016/j.soard.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 17**.Guo Y, Huang Z-P, Liu C-Q, et al. Modulation of the gut microbiome: a systematic review of the effect of bariatric surgery. Eur J Endocrinol. 2017 doi: 10.1530/EJE-17-0403. This is an importantmeta-analysis looking at changes in microbial composition changes and their associations with patient outcome metrics. [DOI] [PubMed] [Google Scholar]
- 18*.Magouliotis DE, Tasiopoulou VS, Sioka E, et al. Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. OBES SURG. 2017;27:1345–1357. doi: 10.1007/s11695-017-2595-8. This meta-review came out a few months before Guo et al (2017), and evaluates not only the shifted bacterial communities, but also associated metabolites and microbial byproducts. [DOI] [PubMed] [Google Scholar]
- 19*.Zhong M-W, Liu S-Z, Zhang G-Y, et al. Alterations in gut microbiota during remission and recurrence of diabetes after duodenal-jejunal bypass in rats. World J Gastroenterol. 2016;22:6706–6715. doi: 10.3748/wjg.v22.i29.6706. This article evaluates progresses the field by looking at changes in colonic microbiota that can be associated with diabetes remission and recurrence using an animal model of metabolic surgery. It pairs well with Murphy et al. (2017) which takes a similar approach using fecal samples from human patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Murphy R, Tsai P, Jüllig M, et al. Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. OBES SURG. 2017;27:917–925. doi: 10.1007/s11695-016-2399-2. See comment on Zhong et al (2016). [DOI] [PubMed] [Google Scholar]
- 21*.Ilhan Z-E, DiBaise JK, Isern NG, et al. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017;11:2047–2058. doi: 10.1038/ismej.2017.71. This multidimensional study looks and both microbiome and metabolomic changes in a patient cohort that received a purely restrictive procedure versus permanent restructuring of the gastrointestinal tract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aidy El S, van Baarlen P, Derrien M, et al. Temporal and spatial interplay of microbiota and intestinal mucosa drive establishment of immune homeostasis in conventionalized mice. Mucosal Immunol. 2012;5:567–579. doi: 10.1038/mi.2012.32. [DOI] [PubMed] [Google Scholar]
- 23*.Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Micro. 2016;14:20–32. doi: 10.1038/nrmicro3552. This is a review of the mechanisms and factors driving microbial community biogeography within the gut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24**.Zhu J, Gupta R, Safwa M. The mechanism of metabolic surgery: Gastric center hypothesis. OBES SURG. 2016;26:1639–1641. doi: 10.1007/s11695-016-2175-3. This is a great review article, which furthers the discussion by laying out the various theories on mechanisms of action following metabolic surgery and giving an overview of the related evidence for each of them. [DOI] [PubMed] [Google Scholar]
- 25**.Kootte RS, Levin E, Salojärvi J, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metabolism. 2017;26:611–619.e6. doi: 10.1016/j.cmet.2017.09.008. This study performs fecal transplantation from lean donors directly into the duodenum of patients with metabolic diseases. They performed several metabolic follow-up studies over the course of 18 months to evaluate the long-term benefits and effects of gut microbiota in humans. [DOI] [PubMed] [Google Scholar]
- 26**.Arora T, Seyfried F, Docherty NG, et al. Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J. 2017;258:628. doi: 10.1038/ismej.2017.70. This study compared the effects of microbial shifts in two different intestinal regions following metabolic surgery in an animal model. Their results encourage future studies to evaluate microbial shifts within the small intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. [Internet] Cell Metabolism. 2015;22:228–238. doi: 10.1016/j.cmet.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Liu R, Hong J, Xu X, et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med. 2017 doi: 10.1038/nm.4358. This is one of the first studies to investigate the contribution of a single microbial species in driving metabolic outcomes following metabolic surgery. [DOI] [PubMed] [Google Scholar]
- 29*.Reijnders D, Goossens GH, Hermes GDA, et al. Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: A randomized double-blind placebo-controlled trial. Cell Metabolism. 2016;24:63–74. doi: 10.1016/j.cmet.2016.06.016. As evidence against microbiota having a role in metabolism, the authors give obese patients a 7-day course of antibiotics. They did not find any significant differences in patient metabolism following the treatment. [DOI] [PubMed] [Google Scholar]
- 30*.Sherf-Dagan S, Zelber-Sagi S, Zilberman-Schapira G, et al. Probiotics administration following sleeve gastrectomy surgery: A randomized double-blind trial. Int J Obes. 2017 doi: 10.1038/ijo.2017.210. Supporting negative findings of Reijnders et al. (2016) the authors did not find a significant change in difference between patients receiving probiotics or not, following metabolic surgery. [DOI] [PubMed] [Google Scholar]
- 31.Luche E, Cousin B, Garidou L, et al. Metabolic endotoxemia directly increases the proliferation of adipocyte precursors at the onset of metabolic diseases through a CD14-dependent mechanism. Molecular Metabolism. 2013;2:281–291. doi: 10.1016/j.molmet.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basso N, Soricelli E, Castagneto-Gissey L, et al. Insulin resistance, microbiota, and fat distribution changes by a new model of vertical sleeve gastrectomy in obese rats. Diabetes. 2016;65:2990–3001. doi: 10.2337/db16-0039. [DOI] [PubMed] [Google Scholar]
- 33.Clemente-Postigo M, del Mar Roca-Rodriguez M, Camargo A, et al. Lipopolysaccharide and lipopolysaccharide-binding protein levels and their relationship to early metabolic improvement after bariatric surgery. Surgery for Obesity and Related Diseases. 2015;11:933–939. doi: 10.1016/j.soard.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 34*.Yang P-J, Yang W-S, Nien H-C, et al. Duodenojejunal bypass leads to altered gut microbiota and strengthened epithelial barriers in rats. OBES SURG. 2016;26:1576–1583. doi: 10.1007/s11695-015-1968-0. Many studies have seen variability in plasma LPS levels following metabolic surgery. This paper provides a possible explanation by evaluating the health of the intestinal barrier following surgery. [DOI] [PubMed] [Google Scholar]
- 35.Kelly CJ, Zheng L, Campbell EL, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host & Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saad MJA, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 2016;31:283–293. doi: 10.1152/physiol.00041.2015. [DOI] [PubMed] [Google Scholar]
- 37.Beasley DE, Koltz AM, Lambert JE, et al. The evolution of stomach acidity and its relevance to the human microbiome. PLoS ONE. 2015;10:e0134116. doi: 10.1371/journal.pone.0134116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patrone V, Vajana E, Minuti A, et al. Postoperative changes in fecal bacterial communities and fermentation products in obese patients undergoing bilio-intestinal bypass. Front Microbiol. 2016;7:15718–13. doi: 10.3389/fmicb.2016.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryan KK, Tremaroli V, Clemmensen C, et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Zhang X, Wang Y, Zhong M, et al. Duodenal-jejunal bypass preferentially elevates serum taurine-conjugated bile acids and alters gut microbiota in a diabetic rat model. OBES SURG. 2016;26:1890–1899. doi: 10.1007/s11695-015-2031-x. This study is interesting in that it evaluates microbiota, and its association with luminal, fecal and serum bile acids at two different time points following metabolic surgery. It is also a nice follow-up to Ryan et al. (2014) and provides further evidence for FXR signaling and gut microbiota in mediating the effects of metabolic surgery. [DOI] [PubMed] [Google Scholar]
- 41*.Baud G, Daoudi M, Hubert T, et al. Bile diversion in Roux-en-Y gastric bypass modulates sodium-dependent glucose intestinal uptake. Cell Metabolism. 2016;23:547–553. doi: 10.1016/j.cmet.2016.01.018. In this study, the authors evaluate the effect of increased sodium in the distal small intestine, resulting from bile diversion, on glucose uptake. While not widely applicable across metabolic surgeries that do not re-route bile from the proximal to distal small intestine, the study is elegant and encourages future study into the role of other macronutrients and micronutrients in mediating the beneficial effects of metabolic surgery. [DOI] [PubMed] [Google Scholar]
- 42*.Grenier-Larouche T, Carreau A-M, Geloën A, et al. Fatty acid metabolic remodelling during type 2 diabetes remission after bariatric surgery. Diabetes. 2017 doi: 10.2337/db17-0414. This detailed study was aimed at understanding mobilization and effect of lipids during the weight loss associated with metabolic surgery. it presents some negative data important to the field, but also leads to more questions as to the role of adipose in T2D remission following metabolic surgery. [DOI] [PubMed] [Google Scholar]
- 43*.Kreznar JH, Keller MP, Traeger LL, et al. Host genotype and gut microbiome modulate insulin secretion and diet-induced metabolic phenotypes. Cell Rep. 2017;18:1739–1750. doi: 10.1016/j.celrep.2017.01.062. This is an interesting study evaluating the interconnected nature of genotype, microbiota and metabolic phenotypes using a mouse population genomics cohort. There results could encourage use of genotyping tools to evaluate metabolic surgery patient genetic stratification or encourage future association-based studies in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]


