Introduction
A longer duration of delirium among patients with critical illness is predictive of mortality, increased hospital length of stay, greater healthcare costs, long-term cognitive impairment, and disability in activities of daily living.1–8 While delirium affects 2 out of 3 patients with critical illness, these patients can also demonstrate acute brain dysfunction that does not meet the formal criteria for delirium using a validated delirium screening tool. This intermediate state of brain dysfunction is known as subsyndromal delirium (SSD).9–22
Previous studies in patients with acute and critical illness have found the presence of SSD to be associated with worse clinical outcomes.10,12–14,18,23 These studies, however, categorized patients into mutually exclusive groups according to their worst mental status (ie, coma, delirium, SSD, or normal) thereby ignoring fluctuations in mental status that are characteristic of delirium. Moreover, these studies have not assessed the effect of the duration of SSD on clinical outcomes while also considering the effect of delirium. Therefore, because patients may suffer both delirium and SSD during critical illness, these limitations may underestimate the true prevalence of SSD and do not provide data on the outcomes independently associated with the duration of SSD.
To address these gaps in knowledge, we characterized the prevalence and duration of SSD in patients who were hospitalized for critical illness and evaluated the independent association between the duration of SSD and institutionalization. We hypothesized that SSD would have a high prevalence and that a longer duration of SSD would predict discharge to an institution.
To test these hypotheses, we performed this investigation that was conceived a priori as part of the multicenter BRAIN-ICU prospective cohort study.5
Materials and Methods
Study Design and Participants
Complete inclusion and exclusion criteria for the BRAIN-ICU study are reported elsewhere.5 Briefly, we enrolled patients treated for respiratory failure or shock in the medical or surgical ICUs at Vanderbilt University Medical Center and Saint Thomas Hospital in Nashville. We excluded those with severe dementia, recent ICU exposure, those in whom delirium could not be assessed, and those in whom follow-up would be difficult due to active substance abuse or residence greater than 200 miles from Nashville. Patients or their proxies provided informed consent. The institutional review boards of the participating centers approved conduct of the investigation. All data were prospectively collected.
Defining Subsyndromal Delirium
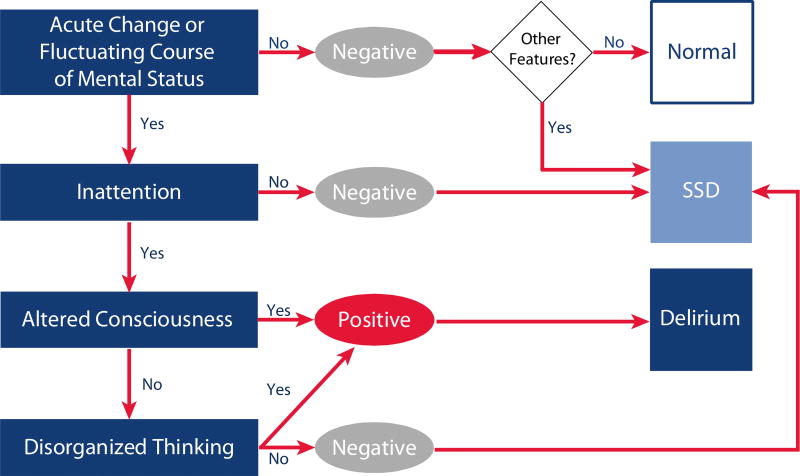
We used the CAM-ICU24,25 to assess all non-comatose patients for delirium twice each day while in the ICU and once per day thereafter until hospital discharge (or for up to 30 days). Delirium was considered present during each assessment that the CAM-ICU was positive. SSD was considered present if the CAM-ICU was negative but the patient demonstrated at least one CAM-ICU feature (Figure 1).9–11,16,20,26
Figure 1. Assessing Subsyndromal Delirium Using the Confusion Assessment Method for the ICU (CAM-ICU) framework.
We determined the presence of subsyndromal delirium (SSD) using the Confusion Assessment Method for the ICU (CAM-ICU) assessed twice each day while patients were in the ICU and once per day thereafter. At each assessment each of the 4 CAM-ICU features were assessed. According to the CAM-ICU framework, if a patient demonstrated an acute change in mental status or a fluctuating course of mental status (Feature 1) and inattention (Feature 2) plus either Altered Consciousness (Feature 3) or Disorganized Thinking (Feature 4), the CAM-ICU was considered positive and delirium was present (dark blue box). If the CAM-ICU was negative, but the patient exhibited any of the CAM-ICU features, subsyndromal delirium was considered present (light blue box). If no CAM-ICU features were present, was the patient considered to have normal mental status (white box).
The primary exposure variable was the duration of SSD, defined as the number of days with SSD. To determine the presence of SSD and delirium, we considered all assessments with abnormal CAM-ICU features, regardless of the potential underlying etiology (e.g., sedative-associated delirium). We chose this conservative and inclusive approach for three reasons: first, data do not exist to suggest that any specific underlying etiology of delirium features is unimportant for our outcome of interest. Second, it avoids any attempt to claim outright knowledge of the etiology of a particular patient’s delirium features. Finally, if sedative-associated delirium features are not associated with institutionalization then their inclusion will result in an underestimation of the true association between the duration of SSD and institutionalization.
Outcomes and Covariates
We defined institutionalization as discharge to a location other than the patient’s home, which included institutions such as rehabilitation hospitals, long-term acute care hospitals (LTACHs), nursing home, hospice care, or other locations.
We selected all covariates a priori. Detailed descriptions are available in the Supplement. We included age, years of education, pre-illness cognitive status via the Informant Questionnaire on Cognitive Decline in the Elderly,27 pre-illness disability status via the Katz Index of Activities of Daily Living,28 medical comorbidities via the Charlson comorbidity index,29 severity of illness via the mean daily modified Sequential Organ Failure Assessment score,30,31 delirium duration defined as the number of days where the CAM-ICU was positive, duration of coma defined as the number of days the Richmond Agitation Sedation Scale32,33 was −4 or −5, duration of severe sepsis, mean daily doses of benzodiazepines in midazolam equivalents, mean daily dose of propofol and mean daily doses of opioids in fentanyl equivalents. We also hypothesized that delirium duration may modify the potential association between SSD and institutionalization. Therefore, we included an interaction term between SSD duration and delirium duration in our statistical models.
Missing Data
To reduce potential bias due to missing or incomplete data and to allow us to calculate the duration of SSD without assuming normal status in cases of missing delirium features,34 we used single imputation for any missing CAM-ICU features (using data from the assessments closest to the incomplete assessment).
Statistical Analysis
We used multiple logistic regression to determine the relationship between duration of SSD and institutionalization. Continuous covariates were allowed to be non-linear using restricted cubic splines. Non-linear and interaction terms were excluded from the models if they were non-significant (ie, p >0.20). We used R (version 3.1.0) for all analyses. P-values <0.05 were considered statistically significant.
Results
We included 821 patients who were a median age of 61 (Interquartile Range [IQR] 51–71) years old with a high severity of illness (Table 1). Of the 636 patients who survived the index hospitalization, 281 (44%) were discharged to an institution with 135 (21%) discharged to a rehabilitation facility, 57 (9%) to an LTACH, 56 (9%) to a nursing home, 25 (4%) to hospice care, 6 (1%) to another hospital and 2 (1%) to other locations.
Table 1.
Characteristics of In-hospital cohort and hospital survivors
| Characteristic | In-hospital cohort (n=821) |
Hospital survivors (n=636) |
|---|---|---|
| Age (years) | 61 (51–71) | 61 (50–70) |
| Male Sex, n (%) | 420 (51) | 325 (51) |
| Medical ICU, n (%) | 559 (68) | 413 (65) |
| Years of education | 12 (12–14) | 12 (12–14) |
| Charlson scorea | 2 (1–4) | 2 (1–4) |
| APACHE II at enrollment | 25 (19–31) | 25 (19–30) |
| SOFA at enrollment | 9 (7–12) | 9 (7–12) |
| Cognitive impairment at enrollmentb | 51 (6) | 39 (6) |
| Disability in ADLs at enrollmentc | 229 (29) | 183 (29) |
| Ever severely septic, n (%)d | 572 (70) | 424 (67) |
| Duration, (days)e | 5 (2–9) | 5 (2–9) |
| Ever delirious, n (%)f | 596 (73) | 478 (75) |
| Duration, (days)e | 4 (2–7) | 4 (2–7) |
| Ever in shock, n (%)g | 457 (56) | 313 (49) |
| Duration, (days)e | 2 (1–5) | 2 (1–5) |
| Ever mechanically ventilated, n (%) | 746 (91) | 571 (90) |
| Duration, (days)e | 2 (1–5) | 2 (1–5) |
| ICU length of stay | 5 (2–10) | 5 (2–10) |
| Hospital length of stay | 10 (6–17) | 10 (6–16) |
| Discharge location, n (%) | ||
| Home | 355 (56) | |
| Rehabilitation hospital | 135 (21) | |
| LTACH | 57 (9) | |
| Nursing home | 56 (9) | |
| Hospice | 25 (4) | |
| Other | 8 (1) |
ADL, activities of daily living; APACHE II, Acute Physiologic and Chronic Health Evaluation, version II; Charlson score, Charlson comorbidity index; ICU, Intensive Care Unit; LTACH, Long-term acute care hospital; SOFA, Sequential Organ Failure Assessment
Charlson comorbidity index score ranges from 0 to 33, where higher scores indicate a greater burden of illness
Defined as an IQCODE score ≥ 3.6, where higher scores indicate worse cognition.
Defined as a Katz ADL score ≥1, where higher scores indicate greater disability.
Defined as suspected infection in the setting of two or more systemic inflammatory response syndrome (SIRS) criteria plus any signs of organ failure (e.g., mechanical ventilation, cardiovascular or renal Sequential Organ Failure Assessment (SOFA) score ≥2, delirium or coma.
Among those who patients who developed severe sepsis, delirium, coma, shock or required mechanical ventilation, respectively
Defined as a positive Confusion Assessment Method for the ICU (CAM-ICU) assessment.
Defined as a cardiovascular (CV) SOFA score ≥1.
Prevalence and characteristics of subsyndromal delirium
At some point during the study, 702 (86%) patients had SSD lasting a median of 3 days (IQR 2–5 days). Of these 702 patients, 531 (76%) were also delirious during the study. There were 72 patients (9%) who had delirium but did not have SSD during the study.
Of the 19,995 mental status assessments performed during the study, SSD was present in 29% (Table 2), delirium was present in 26%, coma was present in 20%, and normal mental status (i.e., no SSD, delirium or coma) was present in 26%. The individual delirium features present during CAM-ICU assessments are presented in Table 2. Single imputation was used in 4,331 (28%) of all individual mental status assessments but did not result in a substantial reclassification of mental status assessments (see Table S1 in the Supplement).
Table 2.
Classifications of mental status assessments
| Mental status classificationa | Assessments among in-hospital cohort (n= 19,995)b,c |
Assessments among hospital survivors (n= 16,738)b,c |
|---|---|---|
| Normal, n (%) | 5164 (26) | 4847 (29) |
| Subsyndromal delirium, n (%) | 5728 (29) | 5025 (30) |
| Feature 1 only | 2877 (50) | 2549 (51) |
| Features 1 and 4 | 1243 (22) | 1053 (21) |
| Features 1 and 3 | 397 (7) | 349 (7) |
| Features 1, 3 and 4 | 321 (6) | 256 (5) |
| Features 1 and 2 | 283 (5) | 250 (5) |
| Feature 4 only | 225 (4) | 218 (4) |
| Feature 3 only | 166 (3) | 152 (3) |
| Feature 2 only | 125 (2) | 117 (2) |
| Other combinationsd | 91 (2) | 81 (2) |
| Delirium, n (%) | 5165 (26) | 4272 (26) |
| Features 1, 2, 3 and 4 | 3454 (67) | 2815 (66) |
| Features 1, 2 and 3 | 1057 (20) | 921 (22) |
| Features 1, 2 and 4 | 391 (8) | 320 (7) |
| Missing feature, CAM-ICU positivee | 263 (5) | 216 (5) |
| Coma, n (%) | 3938 (20) | 2594 (15) |
CAM-ICU, Confusion Assessment Method for the Intensive Care Unit
The four features of the CAM-ICU are: Acute change or fluctuating course of mental status (Feature 1), Inattention (Feature 2), Altered level of consciousness (Feature 3), and Disorganized thinking (Feature 4). The CAM-ICU is positive (and delirium present) if Features 1, 2 and either 3 or 4 are present. We considered subsyndromal delirium to be present if any delirium features were present in the setting of a negative CAM-ICU. See Figure 1. Patients were considered to be comatose if the Richmond Agitation-Sedation Scale was rated as −4 or −5. Non-comatose patients were considered to have ‘normal’ mental status if none of the CAM-ICU features were present.
Percentages may not sum to 100% due to rounding
Overall n reflects mental status assessments using single imputation.
“Other” combinations of subsyndromal delirium each occurred in <1% of assessments and included: Features 2 and 3; Features 2 and 4; Features 3 and 4; and Features 2, 3 and 4.
CAM-ICU raters entered data on both individual features, as well as an overall CAM-ICU score. The CAM-ICU feature data were used to determine the CAM-ICU rating according to conventional CAM-ICU scoring. If a CAM-ICU feature was missing, we used the overall score to determine CAM-ICU rating. For example if a patient was positive for features 1 and 4 and feature 2 was missing but the overall CAM-ICU score was entered as “positive”, that assessment was considered as positive for delirium. Alternatively, for the same scenario, if the CAM-ICU score was “negative”, then that assessment was considered negative for delirium, but positive for subsyndromal delirium.
The most common pattern of SSD was an acute change from baseline or fluctuation in mental status alone (CAM-ICU Feature 1) which was present in 50% of assessments (Table 2). Inattention (CAM-ICU Feature 2), a cardinal feature of the full delirium syndrome,16 was present in 473 assessments (8%).
Duration of subsyndromal delirium and institutionalization
After adjusting for the potential confounders listed above, the number of days of SSD was an independent predictor of increased odds of institutionalization (p=0.007). A sensitivity analysis, that included mental status assessments with complete data as the exposure variable, also found the duration of SSD to have an independent association with increased odds of discharge to an institution (P=0.004).
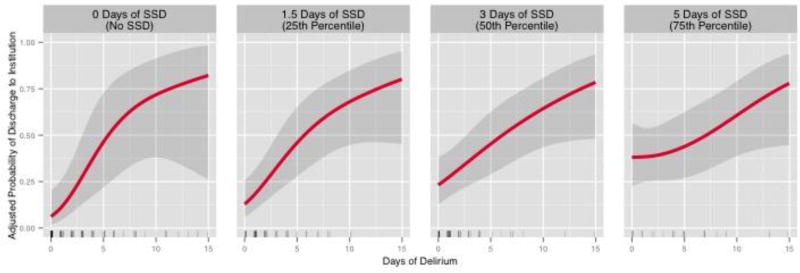
Our model showed a significant interaction between the duration of SSD and duration of delirium (p for interaction=0.01), indicating that how long a patient suffered delirium affected the strength and/or the direction of the association between the duration of SSD and institutionalization. Therefore, we present the associations between the duration of SSD and institutionalization for patients who experienced 4 different durations of delirium (Figure 2). The strength of the independent association between the duration of SSD and institutionalization was greatest among those with the fewest days of delirium.
Figure 2. The relationship between the duration of subsyndromal delirium and odds of institutionalization stratified by the duration of delirium.
The overall association between duration of subsyndromal delirium (SSD) and adjusted probability of institutionalization was significant (p=0.007). We hypothesized a priori that this association would be modified by the delirium duration and found this interaction to be significant (p=0.01), indicating a greater independent risk of disability from SSD in patients with shorter durations of overt delirium. Therefore, we present the association stratified by delirium duration. The panels display the step-wise reduction in the strength of the association among patients with 0 days of delirium (no delirium), 1 day of delirium (25th percentile), 2 days of delirium (50th percentile) and 6 days of delirium (75th percentile), respectively, with all other covariates adjusted to their respective median or mode. Black lines represent the independent association and shading represents the 95% confidence intervals. The rug plots illustrate the distribution of patients with a given duration of SSD.
To place the independent effect of the duration of SSD on the odds of institutionalization into clinical context and to understand better the meaning of the statistical interaction term, we contrast the effect of the duration of SSD on institutionalization in patients who never became delirious during the study with the effect of the duration of SSD in patients who also suffered 6 days of delirium (the upper IQR for delirium duration). Comparing two typical patients who never developed delirium and were alike in all ways (i.e., with all other covariate values adjusted to the median or mode), the patient who had SSD for 5 days was 4.2 times more likely to be discharged to an institution than one who had SSD for 1.5 days (Table 3). Conversely, a similar comparison among typical patients who both suffered 6 days of delirium, the odds of institutionalization did not differ, regardless of the duration SSD (Table 3).
Table 3.
Duration of subsyndromal delirium and the odds of institutionalization (stratified by days of delirium)
| Duration of SSDa | ||
|---|---|---|
| 1.5 daysb | 5 daysc | |
| 0 days of deliriumd | Reference | 4.2 (1.8 to 9.7)e |
| 1 day of deliriumd | Reference | 2.8 (1.5 to 5.1) |
| 2 days of deliriumd | Reference | 1.9 (1.1 to 3.2) |
| 6 days of deliriumd | Reference | 0.8 (0.4 to 1.7) |
The overall association between the duration of subsyndromal delirium (SSD) using the upper and lower IQR (interquartile ranges as boundaries by which to view this relationship) and the adjusted odds of institutionalization (ie, survival and discharge to rehabilitation hospital, LTACH, nursing home, or hospice care) was significant (P=0.007). This association was modified by the delirium duration (P for interaction=0.01); therefore we present odds ratios for the association between the duration of SSD and institutionalization for different delirium durations. The associations between the duration of SSD and the adjusted odds of institutionalization, at various durations of delirium, are presented in Figure 2.
25th percentile IQR (interquartile range) of duration of SSD.
75th percentile IQR (interquartile range) of duration of SSD
Durations of delirium represent the IQRs for delirium duration: no delirium (0 days), the 25th percentile of duration of delirium (1 day), the 50th percentile of delirium duration (2 days), and the 75th percentile of delirium duration (6 days).
Adjusted Odds Ratios (95% CI) comparing patients with a duration of SSD at the 75th percentile to those at the 25th percentile (Reference), controlling for age, education, pre-illness cognitive function and disability status, comorbid medical conditions, severity of illness, the durations of delirium, coma, and severe sepsis, as well as mean daily doses of sedatives and opiates. For example, among patients who never had delirium, the odds of institutionalization among those with 5 days of SSD were 4.2 times greater than those who never developed delirium but had 1.5 days of SSD, with all other covariates held constant.
Discussion
This large, multicenter cohort investigation conducted in a diverse population of patients with critical illness, found that of 20,000 patient evaluations in over 800 patients, SSD (i.e., the presence of delirium features that did not fulfill the diagnostic criteria for delirium) was present in over 80% at some point throughout their ICU stay. Though there was a high prevalence of SSD, the degree to which patients were affected varied as demonstrated by wide-ranging durations of SSD. One out of four patients developed SSD without ever developing delirium. Conversely, among patients who had delirium nine out of ten also had at least 1 day of SSD.
We found that the duration of SSD had an independent association with institutionalization even after adjusting for a number of a priori selected confounders including the number of days a patient was delirious. This association was strongest among patients with the fewest days of delirium (see Figure 2). Because, unlike the full syndrome of delirium, routine screening for SSD is not performed in many ICUs, these data indicate greater odds of institutionalization in the type of patient in whom acute brain dysfunction is overlooked. These findings are of particular importance to bedside ICU nurses, as the clinicians who assess for delirium with greatest frequency, since they suggest that patients with critical illness should be reported as having SSD when some CAM-ICU features are positive even though the CAM-ICU is “negative”. Thus, nurses would report that a patient who is not normal is in SSD, delirium, or coma – three different tiers of acute brain dysfunction. Additionally, future trials aimed at reducing delirium in patients with critical illness should assess effects on SSD.
We found SSD to be present in one-third of all mental status assessments and that the majority of SSD-positive assessments were characterized by an acute change or fluctuating course of mental status (i.e., CAM-ICU Feature 1). This finding has important implications for the conduct of routine delirium screening. The CAM-ICU training manual refers to assessing those features “needed to get your answer”. For example, if Feature 1 is absent, it is not necessary to assess Features 2, 3, or 4 because the patient can not have delirium.35 Our data indicate, however, that stopping the CAM-ICU assessment at Feature 1 will fail to detect 10% of SSD. Thus, consideration should be given to screening for all 4 CAM-ICU features. Moreover, our findings that the CAM-ICU Feature 1 was present in almost all SSD-positive assessments and the duration of SSD is of prognostic importance suggest that efforts to maintain baseline mental status through strategies such as those designed to minimize exposure to psychoactive medications such as targeting light levels of sedation,36,37 spontaneous awakening trials,38,39 or use of protocols of “no” sedation40 may be a means by which to improve outcomes.
Our approach to detect SSD with the CAM-ICU used previous definitions9–11,14,19–22,26,41,42 including the DSM-5 classification for attenuated delirium syndrome (a synonym for SSD)16 to classify those patients with delirium symptoms who did not meet the full delirium diagnostic criteria as having SSD. We then considered the effect of the overall number of days a patient had SSD on outcomes, while also considering the number of days a patient had coma and delirium. By considering mental status each day, on a fluctuating basis, compared with a single ever/never status, our analysis both better resembles the real-life clinical scenarios of patients with delirium and avoids potential bias resulting from classifying patients according to a single mental status for all study days, regardless of how many days it was present. Our inclusive approach yielded a prevalence of SSD of 86%, greater than the previous study of SSD in patients with critical illness which observed 33% of patients had SSD14 and greater than previous studies in patients without critical illness where 16% to 51% of patients were found to have SSD.10–14,18,21–23,26,43–45 Applying a more restrictive definition of SSD that classified patients according to a single mental status (i.e., considering those who ever developed delirium as delirious, regardless of if they also had SSD) to the current cohort resulted in a prevalence of SSD of 21%. Nevertheless, we found that 9 out of 10 patients who would be classified as “delirious” using the restrictive approach also suffered at least one day of SSD. These data suggest that the overall duration of delirium and SSD during critical illness may be underestimated using the time-fixed, more restrictive classification of mental status. Because the duration of SSD was an important independent predictor of institutionalization in the majority of patients with delirium, our findings advance the understanding of the prognostic significance of SSD.
In addition to considering all days of acute brain dysfunction as having potential importance, our study provides other methodological advancements in the study of SSD during critical illness: 1) While previous investigators have used a variety of dimensional (ie, Delirium Rating Scale Revised-98 [DRS-R98],11,21,22 Neelon and Champagne [NEECHAM] Confusion Scale,26 Memorial Delirium Assessment Scale [MDAS]9 and the Intensive Care Delirium Screening Checklist [ICDSC]14) or categorical delirium screening tools (ie, Confusion Assessment Method9,10,12,13,15,20,23,26,45) to detect SSD, our study is the first to use the CAM-ICU, and 2) In a manner complementing the previous studies of SSD patients with critical illness,14,43 we considered the presence of delirium symptoms to indicate either delirium (if the CAM-ICU was positive) or SSD (if the CAM-ICU was negative) even in the setting of sedation, since such medications may have important implications in the majority of patients.46,47
This is the first study of patients with critical illness to our knowledge to address use multivariable analysis to show that the duration of SSD is an independent predictor of institutionalization. These findings build on those of Ouimet et al., who observed a similar relationship in patients with SSD during critical illness but did not consider the duration of SSD or adjust for potential confounders14 and those of Levkoff18 and Bourdel-Marchasson12 who found an independent association between the presence of SSD and institutionalization following hospitalization for acute, but non-critical, illness. Thus, all of these studies taken together have important clinical implications about how we as a critical care community should start to consider SSD, a mental status overlooked in routine clinical practice.
Strengths of this investigation include enrollment of a large number of patients with a wide range of critical illness diagnoses from the medical and surgical ICUs at two tertiary referral centers. In addition, we collected detailed physiologic and pharmacologic data including twice-daily delirium assessments conducted by experienced research personnel using a well-validated delirium assessment tool, the CAM-ICU. We also considered the spectrum of acute brain dysfunction in our models by including durations of coma, delirium and SSD. Next, those who conducted the follow-up assessments were masked to the details of each patient’s ICU course, and clinicians who determined discharge disposition were unaware of formal delirium assessments. Finally, we used a conservative approach to determine the exposure to SSD and delirium. We considered each delirium feature to have the potential for harm, regardless of potential underlying etiology (e.g., sedation-associated delirium). A small, but important, subset (i.e., 12%) of patients with delirium while on sedation may resolve their symptoms following interruption of sedation.46,47 The inclusion of all patients regardless of whether or not they were receiving sedation is a strength of this investigation since the positive findings mean that, if anything, we may have underestimated the prognostic value of SSD.
This investigation has some limitations. First, while the CAM-ICU detects four key features of delirium, it has not been used as a formal severity scoring system in the ICU—though in non-ICU patients the Confusion Assessment Method has been applied as a severity score.48 Studies by Trzepacz21 and Meagher22 that assessed for a wide array of delirium symptoms, report a similar symptom profiles between delirium and SSD.21,22 Second, although our definition of SSD is in keeping with those from both prior studies and the DSM-5, there is no standard definition of SSD. Studies are needed to determine the specific delirium symptoms that may drive worse outcomes for patients and would be important in the development of a standardized SSD definition. Fourth, we did not collect data on residence in an institution prior to developing critical illness, which may bias our results. Nevertheless, we enrolled a cohort of young patients with few pre-illness comorbid medical conditions and disabilities and excluded those with known or probable dementia. Finally, like all studies of patients with emergent illnesses, we were unable to assess the trajectory of pre-illness cognition and disability status given the unplanned nature of critical illness49. Nevertheless, we used well-validated and robust proxy-based tools to determine pre-illness cognition and disability status and included these important predictors of post-ICU functioning in our multivariable regression models5,27,28.
Conclusions
Subsyndromal delirium was pervasive among patients with critical illness and is a strong independent predictor of institutionalization (ie, discharge to a location other than home). The duration of this intermediate form of acute brain dysfunction was more predictive of institutionalization in patients who had fewer days of delirium. Building on earlier studies, these data add to the literature. Because ICU nurses are the clinicians who screen for delirium with greatest frequency, standardized nursing assessment and reporting of SSD is indicated. Given the prognostic implications of SSD, the presence of delirium symptoms, even when they do not meet full diagnostic criteria, should prompt all ICU clinicians to evaluate and address modifiable delirium risk factors.
Supplementary Material
Acknowledgments
Funding/Support:
National Institutes of Heath under awards (R01AG027472, KL2TR000446, R03AG040549, R01AG035117, and R01HL111111), the Vanderbilt Clinical and Translational Scholars Program and the Department of Veterans Affairs Tennessee Valley Healthcare System Geriatric Research, Education and Clinical Center (GRECC).
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The contents of this paper are solely the responsibility of the authors and do not necessarily represent those of the National Institutes of Health, the Department of Veterans Affairs or Vanderbilt University.
Dr. Girard has received honoraria for CME activities from Hospira unrelated to the current manuscript. Dr. Pandharipande has received a research grant from Hospira unrelated to the current manuscript and honoraria for CME activities from Hospira unrelated to the current manuscript. Dr. Ely has received research grants and/or honoraria from Abbott, Hospira, Orion, and Pfizer unrelated to the current manuscript.
Footnotes
Institutions at which work was performed:
Vanderbilt University Medical Center, Nashville, TN, USA
Saint Thomas Hospital, Nashville, TN, USA
Author contributions:
Drs. Brummel and Ely had full access to the all study data, take responsibility for the integrity of the data and the accuracy of the data analysis.
Design and conduct of the study: NEB, TDG, PPP, EWE
Data acquisition, analysis and interpretation of the data: All authors
Statistical Analysis: JLT, RC
Drafting of the manuscript: NEB, LMB, TDG, EWE
Critical revision of the article for important intellectual content: All authors
Final approval of the article: All authors
Obtaining Funding: NEB, PPP, GRB, RSD, EWE
Conflicts of interest:
The remaining authors report no financial conflicts of interest.
References
- 1.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 2.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180(11):1092–1097. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shehabi Y, Riker RR, Bokesch PM, et al. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 4.Girard TD, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Ely EW. Duration of Delirium as a Predictor of Long-Term Cognitive Impairment in Survivors of Critical Illness. American Journal of Respiratory and Critical Care Medicine. 2009;179:A5477. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brummel NE, Jackson JC, Pandharipande PP, et al. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation*. Crit Care Med. 2014;42(2):369–377. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milbrandt EB, Deppen S, Harrison PL, et al. Costs associated with delirium in mechanically ventilated patients. Crit Care Med. 2004;32(4):955–962. doi: 10.1097/01.ccm.0000119429.16055.92. [DOI] [PubMed] [Google Scholar]
- 9.Marcantonio ER, Ta T, Duthrie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50:850–857. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 10.Cole M, McCusker J, Dendukuri N, Han L. The prognostic significance of subsyndromal delirium in elderly medical inpatients. J Am Geriatr Soc. 2003;51(6):754–760. doi: 10.1046/j.1365-2389.2003.51255.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiely DK, Bergmann MA, Murphy KM, Jones RN, Orav EJ, Marcantonio ER. Delirium among newly admitted postacute facility patients: Prevalence, symptoms, and severity. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2003;58(5):441–445. doi: 10.1093/gerona/58.5.m441. [DOI] [PubMed] [Google Scholar]
- 12.Bourdel-Marchasson I, Vincent S, Germain C, et al. Delirium symptoms and low dietary intake in older inpatients are independent predictors of institutionalization: a 1-year prospective population-based study. J Gerontol A Biol Sci Med Sci. 2004;59(4):350–354. doi: 10.1093/gerona/59.4.m350. [DOI] [PubMed] [Google Scholar]
- 13.Dosa D, Intrator O, McNicoll L, Cang Y, Teno J. Preliminary derivation of a Nursing Home Confusion Assessment Method based on data from the Minimum Data Set. J Am Geriatr sSoc. 2007;55(7):1099–1105. doi: 10.1111/j.1532-5415.2007.01239.x. [DOI] [PubMed] [Google Scholar]
- 14.Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: evidence for a disease spectrum. Intensive Care Med. 2007;33(6):1007–1013. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 15.Cole MG, McCusker J, Ciampi A, Belzile E. The 6- and 12-month outcomes of older medical inpatients who recover from subsyndromal delirium. J Am Geriatr Soc. 2008;56(11):2093–2099. doi: 10.1111/j.1532-5415.2008.01963.x. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5. Washington, D.C.: American Psychiatric Association; 2013. American Psychiatric Association. DSM-5 Task Force. [Google Scholar]
- 17.Lipowski ZJ. Transient cognitive disorders (delirium, acute confusional states) in the elderly. Am J Psychiatry. 1983;140(11):1426–1436. doi: 10.1176/ajp.140.11.1426. [DOI] [PubMed] [Google Scholar]
- 18.Levkoff SE, Liptzin B, Cleary PD, et al. Subsyndromal delirium. American Journal of Geriatric Psychiatry. 1996;4(4):320–329. doi: 10.1097/00019442-199622440-00006. [DOI] [PubMed] [Google Scholar]
- 19.Meagher D, Trzepacz PT. Phenomenological distinctions needed in DSM-V: delirium, subsyndromal delirium, and dementias. J Neuropsychiatry Clin Neurosci. 2007;19(4):468–470. doi: 10.1176/jnp.2007.19.4.468. [DOI] [PubMed] [Google Scholar]
- 20.Voyer P, Richard S, Doucet L, Carmichael PH. Detecting delirium and subsyndromal delirium using different diagnostic criteria among demented long-term care residents. JAmMedDirAssoc. 2009;10(3):181–188. doi: 10.1016/j.jamda.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Trzepacz PT, Franco JG, Meagher DJ, et al. Phenotype of subsyndromal delirium using pooled multicultural Delirium Rating Scale--Revised-98 data. J Psychosom Res. 2012;73(1):10–17. doi: 10.1016/j.jpsychores.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Meagher D, Adamis D, Trzepacz P, Leonard M. Features of subsyndromal and persistent delirium. Br J Psychiatry. 2012;200(1):37–44. doi: 10.1192/bjp.bp.111.095273. [DOI] [PubMed] [Google Scholar]
- 23.McCusker J, Cole MG, Voyer P, et al. Six-month outcomes of co-occurring delirium, depression, and dementia in long-term care. J Am Geriatr Soc. 2014;62(12):2296–2302. doi: 10.1111/jgs.13159. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 26.DeCrane SK, Culp KR, Wakefield B. Twelve-month mortality among delirium subtypes. Clin Nurs Res. 2011;20(4):404–421. doi: 10.1177/1054773811419497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 28.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychological function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 31.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 32.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 33.Ely EW, Truman B, Shintani A, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003;289(22):2983–2991. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 34.Little RJ, D'Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ely EW, Pun BT. The Confusion Assessment Method for the ICU (CAM-ICU) training manual. 2014 http://www.icudelirium.org/docs/CAM_ICU_training.pdf.
- 36.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–731. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 37.Shehabi Y, Chan L, Kadiman S, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med. 2013;39(5):910–918. doi: 10.1007/s00134-013-2830-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kress JP, Pohlman AS, O'Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 39.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 40.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375(9713):475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 41.Neto AS, Nassar AP, Jr, Cardoso SO, et al. Delirium screening in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2012;40(6):1946–1951. doi: 10.1097/CCM.0b013e31824e16c9. [DOI] [PubMed] [Google Scholar]
- 42.Gusmao-Flores D, Salluh JI, Chalhub RA, Quarantini LC. The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) and Intensive Care Delirium Screening Checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16(4):R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skrobik Y, Ahern S, Leblanc M, Marquis F, Awissi DK, Kavanagh BP. Protocolized Intensive Care Unit Management of Analgesia, Sedation, and Delirium Improves Analgesia and Subsyndromal Delirium Rates. AnesthAnalg. 2010;111(2):451–463. doi: 10.1213/ANE.0b013e3181d7e1b8. [DOI] [PubMed] [Google Scholar]
- 44.Levkoff SE, Evans DA, Liptzin B, et al. Delirium - the Occurrence and Persistence of Symptoms among Elderly Hospitalized-Patients. Archives of Internal Medicine. 1992;152(2):334–340. doi: 10.1001/archinte.152.2.334. [DOI] [PubMed] [Google Scholar]
- 45.Marcantonio ER, Kiely DK, Simon SE, et al. Outcomes of older people admitted to postacute facilities with delirium. J Am Geriatr Soc. 2005;53(6):963–969. doi: 10.1111/j.1532-5415.2005.53305.x. [DOI] [PubMed] [Google Scholar]
- 46.Patel SB, Poston JT, Pohlman A, Hall JB, Kress JP. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189(6):658–665. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 47.Haenggi M, Blum S, Brechbuehl R, Brunello A, Jakob SM, Takala J. Effect of sedation level on the prevalence of delirium when assessed with CAM-ICU and ICDSC. Intensive Care Med. 2013;39(12):2171–2179. doi: 10.1007/s00134-013-3034-5. [DOI] [PubMed] [Google Scholar]
- 48.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: Development and Validation of a New Scoring System for Delirium Severity in 2 Cohorts. Ann Intern Med. 2014;160(8):526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwashyna TJ, Netzer G, Langa KM, Cigolle C. Spurious inferences about long-term outcomes: the case of severe sepsis and geriatric conditions. Am J Respir Crit Care Med. 2012;185(8):835–841. doi: 10.1164/rccm.201109-1660OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.