Abstract
Affective neuroscience research has revealed that reward contains separable components of ‘liking’, ‘wanting’, and learning. Here we focus on current ‘liking’ and ‘wanting’ findings and applications to clinical disorders. ‘Liking’ is the hedonic impact derived from a pleasant experience, and is amplified by opioid and related signals in discrete sites located in limbic-related brain areas. ‘Wanting’ refers to incentive salience, a motivation process for reward, and is mediated by larger systems involving mesocorticolimbic dopamine. Deficits in incentive salience may contribute to avolitional features of depression and related disorders, whereas deficits in hedonic impact may produce true anhedonia. Excesses in incentive salience, on the other hand, can lead to addiction, especially when narrowly focused on a particular target. Finally, a fearful form of motivational salience may even contribute to some paranoia symptoms of schizophrenia and related disorders.
INTRODUCTION
A fundamental question in affective neuroscience is how reward is generated in the brain. Answers may provide valuable insight not only into normal reward experiences, but also into how dysfunction in reward mechanisms contributes to neuropsychological disorders such as drug and behavioral addictions, major depressive disorder (MDD), Parkinson’s disease (PD), and schizophrenia. Reward contains major components of ‘liking’ (hedonic pleasure), ‘wanting’ (incentive salience or motivation), and learning, and we will focus here especially on relations between ‘liking’ and ‘wanting. Research has indicated these two components are dissociable, and mediated by separable neural substrates. Here, we consider these components, and their application to reward dysfunctions.
‘LIKING’ AND ‘WANTING’ AS SEPARATE ASPECTS OF REWARD
In ordinary experience, ‘liking’ and ‘wanting’ seem conjoined. For example, we eat cake because we enjoy it, and often eat more than intended if the cake tastes good. However, brain processes underlying liking and wanting can sometimes cause these components to diverge in certain circumstances.
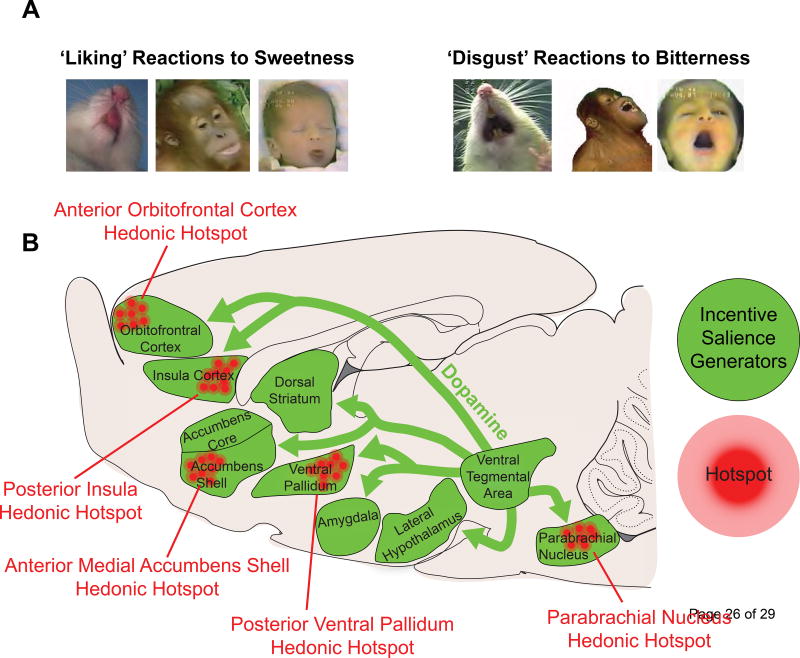
‘Liking’ (in quote marks) refers to the hedonic impact derived from a stimulus (which can be conscious or unconscious) [1,2]. Consciously, it corresponds to subjectively experienced pleasure commonly denoted by liking (without quote marks). An objective form of ‘liking’ reaction has been measured in affective neuroscience studies of rodents using the taste reactivity (TR) test based on hedonic facial expressions to taste, first developed in human infants by Steiner [3] and later adapted for rats by Grill and Norgren [4]. The taste-elicited orofacial reactions are homologous across many species, including rodents, apes, and humans [3,5]. For example, sweetness causes positive, appetitive orofacial reactions (e.g. tongue protrusions), which represent a ‘liked’ tastant. Alternatively, bitterness elicits aversive, negative orofacial reactions (e.g. gapes) and reflects ‘disgust’ (Figure 1A). Importantly, ‘liking’ can be distinguished from the sensory properties of a stimulus, such as sweetness. For example, when a sweet food that was once ‘liked’ is now disliked after being paired with visceral illness- a phenomenon known as conditioned taste aversion (CTA) [6–9]. Similarly, physiological states may shift hedonic tone, called alliesthesia [10], in which food may become more ‘liked’ when one is hungry [10] or even tasty chocolate can become less ‘liked’ when satiated [11].
Figure 1.
Reward systems in the brain. (A) Examples of positive hedonic orofacial reactions (‘liking’) in response to the taste of sweet sucrose (left). Negative affective reactions (‘disgust’) in response to the taste of bitter quinine solution (right). These affective orofacial reactions to tastes are conserved across species in rodents, non-human primates, and humans infants. (B) Sagittal view of a rat brain depicting brain reward systems. Discrete sites, known as hedonic hotspots (clusters of red circles), can enhance ‘liking’ through the actions of opioids, endocannabinoids and orexins, but not dopamine. ‘Wanting’ is derived from dopamine signaling (green arrows), originating from the ventral tegmental area, acting in brain areas that generate incentive salience (purple structures). Hyperactivity in this ‘wanting’ circuit underlies many conditions characterized by excessive motivation, such as addiction, whereas hypoactivity (depicted by the smaller, darker arrows) may produce avolition seen in depression. Finally, aversive dysfunction of this ‘wanting’ circuit, particularly signalling onto the nucleus accumbens shell, may promote fearful salience to otherwise neutral stimuli- a trait commonly observed in patients with paranoid psychosis.
‘Wanting’, or incentive salience, refers to attention-grabbing and motivational features of rewards and their learned cues [12]. Reward cues have the ability to often trigger bursts of reward-seeking motivation [13,14], and the cue itself becomes attractive as a ‘motivational magnet’. Cue ‘wanting’ can be experimentally tested using the Pavlovian autoshaping or sign-tracking test [15,16], or in conditioned reinforcement tests where instrumentally working can earn cue presentations [17], and cue-triggered spurts of reward seeking are often experimentally tested using the Pavlovian Instrumental Transfer (PIT) test [13,18].
SEPARATE NEURAL SUBSTRATES MEDIATE ‘LIKING’ AND ‘WANTING’
Liking in the brain
Research in our laboratory has identified a network of discrete sites, called ‘hedonic hotspots’, within limbic-related brain structures in which pleasure amplification mechanisms are localized (Figure 1B) [5]. For example, one hedonic hotspot can be found in the anterior-dorsal portion of the medial shell of the nucleus accumbens (NAc). Within hedonic hotspots, microinjection of opioid-stimulating drugs or a few other agents can enhance positive ‘liking’ reactions to sucrose taste [19–23]. Other subcortical hedonic hotspots have been found in the posterior portion of the ventral pallidum (VP) [24,25], and near the brainstem parabrachial nucleus of the pons (PBN) [26]. Recent findings have also identified two cortical hotspots: one in the anterior orbitofrontal cortex (OFC) and another in posterior insula [27], consistent with human neuroimaging findings that implicate these cortical regions in pleasure and/or disgust [11,28,29].
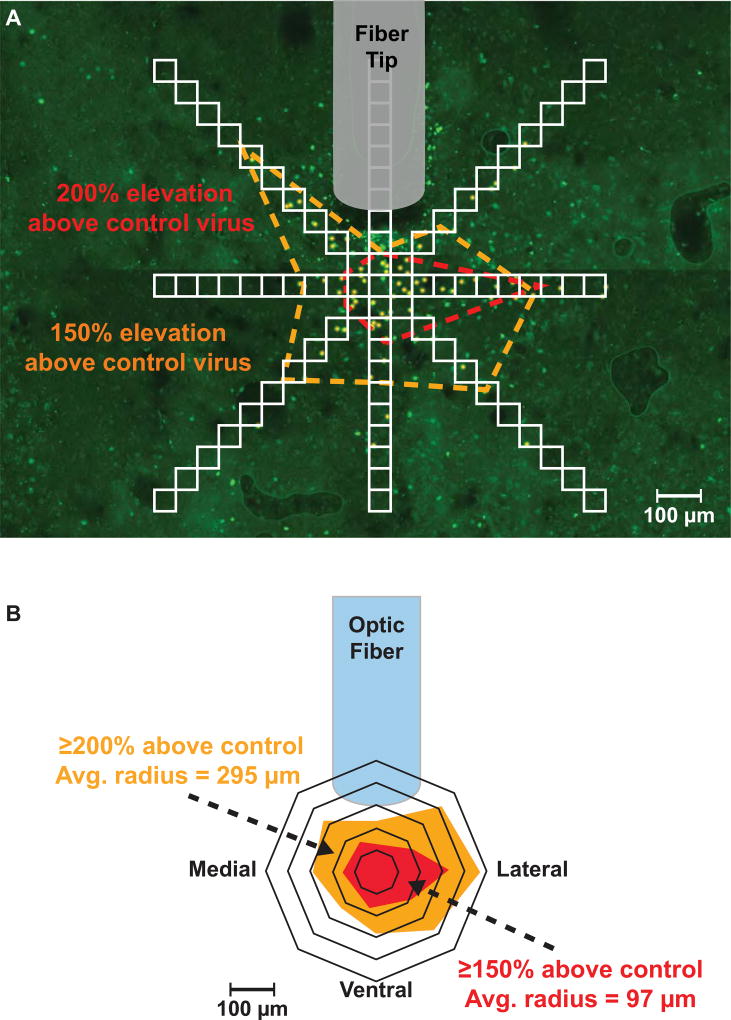
Notably, enhancement of hedonic reactions is restricted to these hotspots- the boundaries of which are defined by measuring the extent of neuronal activation surrounding the microinjector or optic fiber tip (known as ‘Fos plumes’; Figure 2). Opioid microinjections beyond hotspot boundaries do not enhance ‘liking’ reactions, even in the same structure and even if they still amplify ‘wanting’ to eat the reward. In some oppositely-valenced hedonic coldspot sites, opioid stimulation may instead produce the opposite suppression of ‘liking’ reactions to sweetness, even while still amplifying ‘wanting’ to eat [19,24,27].
Figure 2.
Example of a ‘Fos plume’ produced by laser stimulation of local brain tissue containing channelrhodopsin (ChR2; excitatory optogenetic construct). Laser illumination of ChR2 stimulates expression of the immediate early gene, Fos (a marker of neuronal activity), in neurons immediately surrounding the optic fiber tip. (A) To measure Fos plumes, a radial grid of contiguous 50 µm squares is placed over a photomicrograph containing the area of the brain surrounding the tip of the optic fiber. Expression of Fos is quantified by counting the number of Fos immunoreactive neurons found within each square of the grid (yellow points). Comparing the degree of Fos expression in treatment animals relative to that of control animals, which receive laser illumination but lack the optogenetic ChR2 construct, provides a means to measure the percentage in ChR2 laser-induced enhancement of neuronal activity relative to baseline (red dotted line = 200% control; orange dotted line = 150% control). (B) An average Fos plume is created by mapping the mean diameter of Fos elevation over baseline in each direction around the center. These Fos plumes provide highly objective and neuroanatomically precise information of where a laser stimulation or drug microinjection acts on the brain to enhance ‘liking’ and/or ‘wanting.’
Among hedonic hotspots, the posterior VP is the only known one where neuronal damage induced by lesions causes a loss-of-function elimination of positively valenced ‘liking’ reactions to sweetness and replacement with negatively valenced ‘disgust’ reactions [30–32]. This unique feature suggests that the VP hotspot not only amplifies ‘liking’, but is necessary for the expression of normal levels of ‘liking’ reaction.
Additionally, several hedonic hotspots appear to be functionally linked together, so that stimulation of one hotspot leads to activation of the others, indicating that the entire network acts as an integrated whole circuit to enhance ‘liking’ [27,33,34]. Moreover, unanimous activation of hotspots appears to be required for enhancing ‘liking’, as blocking opioid signaling in one hotspot prevents the opioid stimulation-induced enhancement of ‘liking’ reactions by another hotspot [33]. Together, these findings indicate that these hotspots form a larger functional circuit that underlies hedonic reactions.
‘Wanting’ in the brain
Incentive salience or ‘wanting’ is powered by a much larger mesocorticolimbic network of brain structures, and often involves a hyperactive mesolimbic dopamine system (Figure 1B) [35]. Despite its early label as a pleasure neurotransmitter, dopamine has never enhanced ‘liking’ in our studies, even in hedonic hotspots, and is no longer so widely thought to mediate pleasure. Instead, it is well known that presentation of previously learned reward cues activates midbrain DA neurons, causing release to a large network of structures, spurring the individual to action, and invigorating reward-seeking [35,36]. Structures involved in incentive salience include the ventral tegmentum containing dopamine neurons, the entire NAc, much of the dorsal neostriatum, the central amygdala (CeA), lateral hypothalamus (LH), OFC and other parts of prefrontal cortex, and VP, among others [37–39].
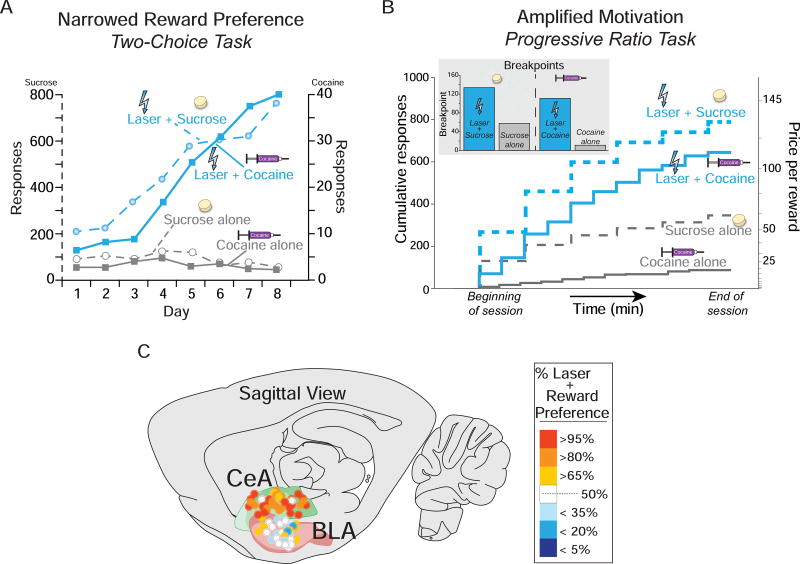
Beyond generating ‘wanting’, this circuitry also focuses motivation on particular targets. This allows one to adaptively ‘want’ different rewards at different times, but can be biased towards one particular target in addiction. Focusing of incentive salience may involve mesocorticolimbic interactions with amygdala. For example, our lab has found that pairing a particular sugar or cocaine reward with optogenetic laser-excitation of the central nucleus of amygdala narrowly focuses excessive ‘wanting’ on just that laser-paired reward (Figure 3) [38,40]. We think something like an equivalent neural change may happen without laser in the brain of addicts to focus ‘wanting’ on a particular addictive target.
Figure 3.
Central Amygdala optogenetic ChR2-pairing narrows and amplifies reward choice. (A) When given the choice between earning a sucrose reward alone (grey circles and dashed lines) or earning a sucrose reward paired with optogenetic central amygdala (CeA) ChR2 stimulation (blue circles and dashed lines), rats intensely prefer the CeA-paired sucrose reward above and beyond the sucrose reward alone. When given the choice between earning a cocaine reward alone (0.3mg/kg/infusion; grey squares and solid lines) or earning a cocaine reward paired with optogenetic CeA stimulation (blue squares and solid lines), rats prefer the CeA-paired cocaine above and beyond cocaine alone. (B) In a breakpoint test of motivation intensity, rats are willing to work harder in a progressive ratio task, reaching higher breakpoints when laser is paired with rewards. (C) Notably, narrowing of motivation produced by optogenetic laser stimulation was observed in the CeA, but not the basolateral amygdala (BLA). Figure modified from [38] and [40]. CeA, central amygdala; BLA, basolateral amygdala.
Normally, focusing of ‘wanting’ is sensitive to current neurobiological and physiological states, allowing for appetites, satiety and other factors to direct craving [37,41]. In human addicts, states of intense stress, or drug exposure may also magnify cue-elicited ‘wanting’ to generate even more intense desire in particular encounters [42–44]. Related to stress, findings from our lab suggest corticotrophin-releasing factor, a brain stress-related neurotransmitter, can amplify cue-triggered ‘wanting’ in some brain structures similarly to dopamine stimulation [45].
BLUNTED INCENTIVE SALIENCE AND THE ANHEDONIA PARADOX
Anhedonia (also termed consummatory anhedonia), or the inability to experience pleasures, has long been considered a defining symptom of some neuropsychological disorders, such as major depression (MDD), Parkinson’s disease (PD), and the negative symptoms of schizophrenia [46–52]. [53–55]. However, a number of clinical investigators have recently suggested that these patients have signs of avolition (sometimes called anticipatory anhedonia in clinical settings), or a lack of motivational ‘wanting’, instead of an actual lack of ‘liking’ or pleasure capacity [56–60]. For example, recent evidence has emerged to suggest that when liking is measured specifically, patients with these conditions exhibit no deficits in rating sensory pleasures such as ice cream [56,61–68], which suggests intact ‘liking’. Patients with these disorders do, however, fail to value those and other rewards in life. Accordingly, a hypoactive dopamine system of incentive salience has been implicated with each of these disorders [49,51,69]. Together, these interpretations are consistent with our understanding of the role of dopamine in ‘wanting’ but not ‘liking.’
Why have such deficits been characterized for so long in medical textbooks as anhedonia, rather than as avolition? One possibility is that traditional clinical measures of anhedonia do not adequately parse ‘liking’ from ‘wanting’, but rather blend them together. Indeed, the presence of anhedonia is often determined by patient self-report in that they expect rewards to have little value [70–72]. Patients’ ability to separate self-reports of ‘liking’ versus ‘wanting’ may be limited without careful questioning [73]. Even the newest generation of the DSM, for example, may not adequately differentiate between ‘liking’ and ‘wanting’ as it defines anhedonia as “lack of enjoyment from, [or] engagement in…life’s experiences…” [74] - with enjoyment resembling ‘liking’ and engagement reflecting ‘wanting.’ Together, these oversights may contribute to inconsistencies in the literature [67,75,76].
We applaud those researchers of depression, Parkinson’s disease, and schizophrenia who have addressed the potential dissociation between ‘liking’ and ‘wanting’ by adopting separate terms for each, such as anhedonia and avolition [59,77–80]. More specific definitions may assist clinicians in better differentiating between these symptoms, which, in turn, could lead to more accurate diagnoses and eventually allow more targeted and efficacious treatments.
HEIGHTENED INCENTIVE SALIENCE
The original clinical application of ‘wanting’ and ‘liking’ dissociation was to drug addiction, based on evidence that drugs of abuse can sensitize mesolimbic dopamine systems of incentive salience in vulnerable individuals [81]. According to that Incentive Sensitization Theory, drug addiction is characterized by a hyper-reactive mesolimbic dopaminergic system in response to drug reward cues, causing excessive cue-triggered ‘wanting’ to take more drugs [35]. Genetic and environmental susceptibilities to dopamine sensitization, coupled with repeated bingeing of drugs, can sensitize the mesocorticolimbic system of particular individuals to create addiction [42,82]. In an extension of the incentive sensitization theory beyond drugs to behavioral addictions, recent neuroimaging studies have shown that a sensitization-like dopamine hyper-reactivity brain signature may also arise in some individuals without need of drugs, resulting in compulsive pursuit of other incentives such as gambling, sex, shopping, etc. [83–87].
For example, applied to gambling addiction, a recent fMRI study looking at cue reactivity to a food or gambling cue revealed that individuals diagnosed with gambling disorder demonstrated a greater change in activity of reward-regions in response to gambling cues than food cues, unlike in moderate gamblers who are not ‘addicted’ and less activated by the gambling cue [88]. Similarly, applied to sex, brain mesolimbic activations elicited by cues that predict a pornographic image elicited stronger mesolimbic brain activations and quicker reaction times in individuals with problematic pornography use (PPU) that rises to arguably compulsive levels than in non-compulsive users, which was interpreted by the investigators as consistent with incentive-sensitization [89].
Some of the most striking evidence that dopamine stimulation can produce behavioral addictions has recently come from dopamine dysregulation syndrome in medicated Parkinson’s patients. For example, approximately 17% of patients with PD receiving medication that stimulates dopaminergic systems, especially high doses of direct agonist drugs that directly stimulate dopamine D2/D3 receptors, report the emergence of a new behavioral addiction, such as compulsive levels of gambling, shopping, sex, eating, hobbies, etc. [90]. Behavioral addictions may be magnified in early onset PD, in which upwards of 50% may develop an impulse control disorder involving behavioral addictions [91]. Further, some PD patients with this disorder also addictively over-consume their dopamine-stimulating medication, despite such medications not being reported as producing much pleasure [91–93]. Human imaging studies have pointed to excessive incentive salience via heightened mesocorticolimbic reactivity important for impulse control disorder development and addictive-like disorders [94,95].
CAN INCENTIVE SALIENCE FLIP TO FEARFUL SALIENCE IN PARANOIA?
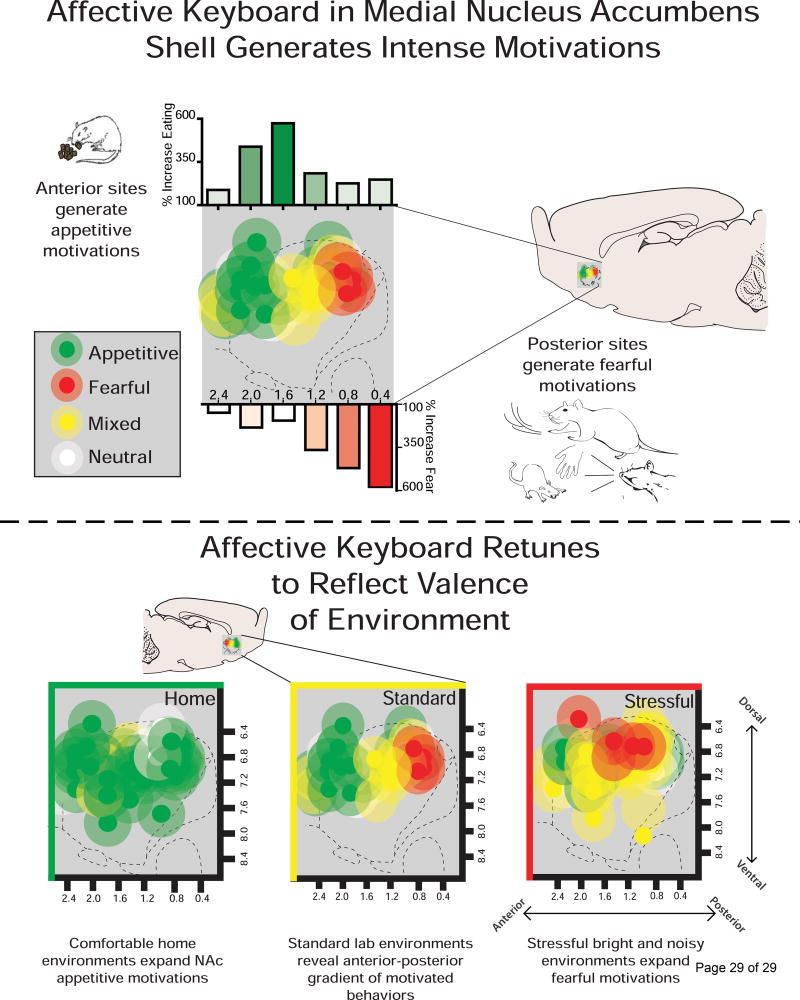
Finally, a very different side of incentive salience, flipped to negative valence as an active-coping form of fearful salience may be manifested in motivational features of psychostimulant-induced psychosis and schizophrenic psychosis. Our laboratory has shown that excessive incentive salience generated by dopamine-glutamate interaction in the NAc can be flipped to a negative form of fearful salience [96–99]. Fear-biased generation caused by drug microinjections (e.g., AMPA antagonist, DNQX) that alter local glutamate-dopamine interactions occurs especially in posterior sites of NAc medial shell. However, most of the rest of NAc shell can also be recruited for fear generation in stressful situations, retuning the NAc’s normal keyboard gradient of motivational valence (whereas comfortable environments bias the NAc manipulations towards generating positive incentive salience; Figure 4) [100,101]. Dopamine in NAc shell is crucial for generation of both incentive salience and fearful salience generated by these microinjections [102]. In particular, fearful salience especially needs D2/D3 dopamine receptor stimulation in NAc, whereas D1 receptor stimulation participates in both motivations [103].
Figure 4.
Desire and dread in the nucleus accumbens. (A) Disrupting glutamatergic and/or dopaminergic activation in the medial nucleus accumbens shell reveals a anterior to posterior gradient of intense motivations. Microinjections of glutamate and/or dopamine antagonists in more anterior sites generate appetitive motivations (depicted in green) such as increased food intake. The same microinjections targeted posteriorly reveal fearful motivations (depicted in red) such as increased escape attempts, defensive treading, and vocalizations or bites toward experimenter. (B) The valence of the environment the rat is tested in reveals ‘retuning’ of the affective keyboard. When the same microinjections are given in a comfortable home environment, appetitive motivations span caudally and replace fearful motivations with appetitive motivations. On the contrary, stressful environments equipped with bright lights and loud music reveals an affective keyboard with more fearful/mixed motivations replacing appetitive motivations.
In a major hypothesis of human schizophrenia, Kapur and colleagues have suggested that elevated dopamine signaling in striatum and NAc may underlie excessive motivational salience assigned to innocuous stimuli, resulting in their gaining excessive meaningfulness and even taking on a threatening aspect to the patient [104–107]. Indeed, there is strong evidence to suggest that hyperdopaminergic activity in striatum underlies these positive symptoms seen in schizophrenia, making them more motivationally compelling, even if the hallucinations or delusions themselves arise from other neural causes [104]. For example, higher dopamine metabolite levels in striatum is associated with greater positive symptoms [108], and levels of released striatal dopamine are almost doubled in schizophrenia compared to controls [109,110]. Elevating dopamine levels even in healthy humans, such as taking high doses of amphetamine or other psychostimulants, can sometimes lead to drug-induced psychosis and paranoia symptoms similar to schizophrenia [111]. Conversely, antipsychotic medications that generally block D2 receptors (though some also block serotonin receptors) may reduce paranoia symptoms in schizophrenia [92]. As Kapur and colleagues have suggested, it is conceivable that dopamine dysregulation in striatum resulting in abnormal dopamine release may lead to an aberrant assignment of salience to neutral stimuli [105,106]. Thus, psychosis may appear as a result of dopamine dysregulation in combination with other factors relevant to cognitive and sociocultural context.
Aberrant salience for some authors may mean simply exaggeration in attentional, physical sensory, or novelty/surprise features of stimulus perception, without any distorted motivational component. Such attentional salience has sometimes been posited to be mediated by phasic (rather than tonic) dopamine signals [112], or by early stages of a phasic dopamine signal, and that the later stage of the phasic signal encodes reward prediction error [113]. Other views have suggested that only some midbrain dopamine neurons encode reward prediction error value while different dopamine neurons encode motivational salience for both reward-related or aversive-related stimuli [114]. We note that views of aberrant salience as purely sensory/attentional, or of dopamine as prediction error teaching signals or hedonic values of reward, are distinct from our hypothesis that dopamine can mediate incentive/fearful salience independently from sensory features, prediction error learning, or hedonic reward values of stimuli [115], and different from motivational interpretations of aberrant salience in psychosis.
However, an additional motivational component of aberrant salience in schizophrenia has been posited by Kapur, Howes and their colleagues, and especially linked to striatal dopamine hyper-reactivity [104–107]. For example, as Howes and Nour [107] describe in a recent review, “the aberrant salience hypothesis of schizophrenia proposed that disordered mesostriatal dopamine release results from over-attribution of meaning and motivational value (incentive salience) to irrelevant environmental events” (p. 3). Elevated limbic reactivity to reward-related stimuli related to incentive salience may even precede full-blown psychosis. For example, Winton-Brown and colleagues [115] report “During reward anticipation, ultra-high risk [for psychosis] subjects showed greater activation than controls in the VP bilaterally” (p. 1) (but not during aversive anticipation or to neutral stimuli). Dopamine antagonist medications for psychosis are suggested by this group specifically to reduce excessive motivational salience: as Bolstad and colleagues [116] put it, the “…increased activity of the dopaminergic mesolimbic motivational system results in an aberrant assignment of salience in patients with schizophrenia, and antipsychotic drugs are thought to relieve positive symptoms by dampening this aberrant salience through diminishing the dopaminergic hyperactivity” (p. 2259). All these views of aberrant salience and dopamine in psychosis expressed by Kapur, Howes and colleagues seem quite consistent with our view described above of the role of mesolimbic dopamine in incentive salience and fearful salience.
CONCLUSION
As a modular process, reward is best understood when ‘liking’ and ‘wanting’ components can be examined independently. In the affective neuroscience laboratory, experimental tools have disentangled these components in animal studies in order to probe their underlying neurobiological mechanisms. In human clinical populations, these findings seem to be having increasing applications to better understand reward dysfunction in depression, addiction, schizophrenia and other conditions. A greater understanding of reward components and their mechanisms thus may continue to facilitate strategies to understand and treat these complex human psychopathologies.
HIGHLIGHTS.
Reward is a modular process that can be broken down into smaller components, including ‘wanting’ (incentive salience) and ‘liking’ (hedonic impact).
Dopamine is necessary for ‘wanting’ but does not contribute to ‘liking.’
Hypoactivity in dopamine-related systems may produce avolition (i.e. blunted ‘wanting’), a symptom common among patients with depression.
Hyper-reactivity in sensitized dopamine-related systems of addicts may intensify ‘wanting.’
Dysregulated dopamine signaling in the nucleus accumbens may assign fearful salience to otherwise neutral stimuli observed in patients with paranoid psychosis.
Acknowledgments
This work was supported by National Institutes of Health grants DA015188 (KCB) MH063649 (KCB), DA007268 (JJO), DC00011 (SMW), and DA007281 (EEN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Winkielman P, Berridge KC, Wilbarger JL. Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers Soc Psychol Bull. 2005;31:121–135. doi: 10.1177/0146167204271309. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 3.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 4.Grill HJ, Norgren R. The taste reactivity test. II. Mimetic responses to gustatory stimuli in chronic thalamic and chronic decerebrate rats. Brain Res. 1978;143:281–297. doi: 10.1016/0006-8993(78)90569-3. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA. A general theory of aversion learning. Ann N Y Acad Sci. 1985;443:8–21. doi: 10.1111/j.1749-6632.1985.tb27060.x. [DOI] [PubMed] [Google Scholar]
- 7.Rozin P, Haidt J, McCauley CR. Disgust. In: Lewis M, Haviland M, editors. Handbook of Emotions. 1993. [no volume] [Google Scholar]
- 8.Reilly S, Schachtman TR. Conditioned taste aversion: neural and behavioral processes. Oxford University Press; 2008. [Google Scholar]
- 9.Spector AC, Breslin P, Grill HJ. Taste reactivity as a dependent measure of the rapid formation of conditioned taste aversion: a tool for the neural analysis of taste-visceral associations. Behav Neurosci. 1988;102:942–952. doi: 10.1037//0735-7044.102.6.942. [DOI] [PubMed] [Google Scholar]
- 10.Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- 11.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–1733. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 12.Bindra D. How adaptive behavior is produced: a perceptual-motivational alternative to response reinforcements. Behav. Brain Sci. 1978;1:41. [Google Scholar]
- 13.Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered “wanting” for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37:1529–1540. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Robinson MJF, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, Ferrario CR. Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity. Neuropsychopharmacology. 2015;40:2113–2123. doi: 10.1038/npp.2015.71. This study assessed changes in ‘wanting’ and ‘liking’ after exposure to a high-fat diet in rats. Rats who gained weight with exposure to the high fat diet showed enhanced ‘wanting’, i.e. conditioned approach or autoshaping, amphetamine-induced locomotion, and conditioned reinforcement to a sucrose cue, without showing enhancement in ‘liking’, i.e. positive orofacial reactions to sucrose solution. Not only do these data support the notion that 'liking' and 'wanting' are separate, dissociable aspects of reward, they also demonstrate that rewarding stimuli beyond simply drugs of abuse (in this case a high-fat diet) may trigger incentive sensitization and produce excessive 'wanting.'. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hearst E, Jenkins HM. Sign-tracking: The stimulus-reinforcer relation and directed action. Sign-tracking: The stimulus-reinforcer relation and directed action. 1974 [no volume] [Google Scholar]
- 16.Boakes RA, Poli M, Lockwood MJ, Goodall G. A study of misbehavior: token reinforcement in the rat. J Exp Anal Behav. 1978;29:115–134. doi: 10.1901/jeab.1978.29-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robbins TW, Watson BA, Gaskin M, Ennis C. Contrasting interactions of pipradrol, d-amphetamine, cocaine, cocaine analogues, apomorphine and other drugs with conditioned reinforcement. Psychopharmacology (Berl) 1983;80:113–119. doi: 10.1007/BF00427952. [DOI] [PubMed] [Google Scholar]
- 18.Mahler SV, Berridge KC. What and when to “want”? Amygdala-based focusing of incentive salience upon sugar and sex. Psychopharmacology (Berl) 2012;221:407–426. doi: 10.1007/s00213-011-2588-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peciña S, Berridge KC. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci. 2014;34:4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro DC, Terry RA, Berridge KC. Orexin in rostral hotspot of nucleus accumbens enhances sucrose “liking” and intake but scopolamine in caudal shell shifts “liking” toward “disgust” and “fear”. Neuropsychopharmacology. 2016;41:2101–2111. doi: 10.1038/npp.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances “liking” of a sweet reward. Neuropsychopharmacology. 2007;32:2267–2278. doi: 10.1038/sj.npp.1301376. [DOI] [PubMed] [Google Scholar]
- 23.Faure A, Richard JM, Berridge KC. Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLoS ONE. 2010;5:e11223. doi: 10.1371/journal.pone.0011223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho C-Y, Berridge KC. An orexin hotspot in ventral pallidum amplifies hedonic “liking” for sweetness. Neuropsychopharmacology. 2013;38:1655–1664. doi: 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Söderpalm AH, Berridge KC. The hedonic impact and intake of food are increased by midazolam microinjection in the parabrachial nucleus. Brain Res. 2000;877:288–297. doi: 10.1016/s0006-8993(00)02691-3. [DOI] [PubMed] [Google Scholar]
- 27••.Castro DC, Berridge KC. Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc Natl Acad Sci U S A. 2017;114:E9125–E9134. doi: 10.1073/pnas.1705753114. The existence of cortical hedonic hotspots was mostly speculative until they were recently discovered and characterized by Castro and Berridge. These hotspots, found within the orbitofrontal cortex and insula, enhance ‘liking’ reactions to sucrose following opioid or orexin stimulation. Moreover, activation of these hotspots leads to increased activity in other subcortical hotspots suggesting that these novel sites form functional connections with existing hedonic hotspots. Together, these findings extend our understanding of how hedonic pleasure is manifested in the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 29.Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39:701–711. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- 30.Cromwell HC, Berridge KC. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 1993;624:1–10. doi: 10.1016/0006-8993(93)90053-p. [DOI] [PubMed] [Google Scholar]
- 31.Ho C-Y, Berridge KC. Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. Eur J Neurosci. 2014;40:3556–3572. doi: 10.1111/ejn.12720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimura T, Imaoka H, Yamamoto T. Neurochemical modulation of ingestive behavior in the ventral pallidum. Eur J Neurosci. 2006;23:1596–1604. doi: 10.1111/j.1460-9568.2006.04689.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith KS, Berridge KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci U S A. 2011;108:E255–64. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71:670–679. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD. Mesolimbic dopamine signals the value of work. Nat Neurosci. 2016;19:117–126. doi: 10.1038/nn.4173. Dopamine neurotransmission was recorded via microdialysis and fast-scan cyclic voltammetry, and manipulated using optogenetic methods in rats performing a novel reward task. Authors conclude that dopamine signals motivational excitement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90. doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warlow SM, Robinson MJF, Berridge KC. Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine. J Neurosci. 2017;37:8330–8348. doi: 10.1523/JNEUROSCI.3141-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiFeliceantonio AG, Berridge KC. Dorsolateral neostriatum contribution to incentive salience: opioid or dopamine stimulation makes one reward cue more motivationally attractive than another. Eur J Neurosci. 2016;43:1203–1218. doi: 10.1111/ejn.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson MJF, Warlow SM, Berridge KC. Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J Neurosci. 2014;34:16567–16580. doi: 10.1523/JNEUROSCI.2013-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson MJF, Berridge KC. Instant transformation of learned repulsion into motivational “wanting”. Curr Biol. 2013;23:282–289. doi: 10.1016/j.cub.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prisciandaro JJ, Joseph JE, Myrick H, McRae-Clark AL, Henderson S, Pfeifer J, Brady KT. The relationship between years of cocaine use and brain activation to cocaine and response inhibition cues. Addiction. 2014;109:2062–2070. doi: 10.1111/add.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang KS, Smith DV, Delgado MR. Using fMRI to study reward processing in humans: past, present, and future. J Neurophysiol. 2016;115:1664–1678. doi: 10.1152/jn.00333.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dias NR, Schmitz JM, Rathnayaka N, Red SD, Sereno AB, Moeller FG, Lane SD. Anti-saccade error rates as a measure of attentional bias in cocaine dependent subjects. Behav Brain Res. 2015;292:493–499. doi: 10.1016/j.bbr.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peciña S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger PA, Faull KF, Kilkowski J, Anderson PJ, Kraemer H, Davis KL, Barchas JD. CSF monoamine metabolites in depression and schizophrenia. Am J Psychiatry. 1980;137:174–180. doi: 10.1176/ajp.137.2.174. [DOI] [PubMed] [Google Scholar]
- 47.Lambert G, Johansson M, Ågren H, Friberg P. Reduced Brain Norepinephrine and Dopamine Release in Treatment-Refractory Depressive Illness. Arch Gen Psychiatry. 2000;57:787. doi: 10.1001/archpsyc.57.8.787. [DOI] [PubMed] [Google Scholar]
- 48.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 49.Ruhé HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007;12:331–359. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 50.Bremner JD, Vythilingam M, Ng CK, Vermetten E, Nazeer A, Oren DA, Berman RM, Charney DS. Regional brain metabolic correlates of alpha-methylparatyrosine-induced depressive symptoms: implications for the neural circuitry of depression. JAMA. 2003;289:3125–3134. doi: 10.1001/jama.289.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14:518–531. doi: 10.1016/S1474-4422(15)00019-8. [DOI] [PubMed] [Google Scholar]
- 52.Gold JM, Waltz JA, Prentice KJ, Morris SE, Heerey EA. Reward processing in schizophrenia: a deficit in the representation of value. Schizophr Bull. 2008;34:835–847. doi: 10.1093/schbul/sbn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kraepelin E. Dementia praecox and paraphrenia. Dementia praecox and paraphrenia. 1971 [no volume] [Google Scholar]
- 54.Bleuler E. Dementia Praecox or the group of Schizophrenias. 1950 [no volume] [PubMed] [Google Scholar]
- 55.Meehl PE. Hedonic capacity: some conjectures. Bull Menninger Clin. 1975;39:295–307. [PubMed] [Google Scholar]
- 56.Schmidt L, d Arc BF, Lafargue G, Galanaud D, Czernecki V, Grabli D, Schüpbach M, Hartmann A, Lévy R, Dubois B, et al. Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain. 2008;131:1303–1310. doi: 10.1093/brain/awn045. [DOI] [PubMed] [Google Scholar]
- 57.Hartmann MN, Hager OM, Reimann AV, Chumbley JR, Kirschner M, Seifritz E, Tobler PN, Kaiser S. Apathy but not diminished expression in schizophrenia is associated with discounting of monetary rewards by physical effort. Schizophr Bull. 2015;41:503–512. doi: 10.1093/schbul/sbu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fervaha G, Graff-Guerrero A, Zakzanis KK, Foussias G, Agid O, Remington G. Incentive motivation deficits in schizophrenia reflect effort computation impairments during cost-benefit decision-making. J Psychiatr Res. 2013;47:1590–1596. doi: 10.1016/j.jpsychires.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 59••.Rømer Thomsen K, Whybrow PC, Kringelbach ML. Reconceptualizing anhedonia: novel perspectives on balancing the pleasure networks in the human brain. Front Behav Neurosci. 2015;9:49. doi: 10.3389/fnbeh.2015.00049. These authors present an excellent account of the different components of reward (i.e. liking, wanting, and learning) and discuss how deficits in each may manifest across a range of neuropsychological disorders. Even across a large number of studies, the evidence for true deficits in ‘liking’ is sparse. Accordingly, these authors advocate the clinical concept of anhedonia be revised. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung YS, Barch DM. Frontal-striatum dysfunction during reward processing: Relationships to amotivation in schizophrenia. J Abnorm Psychol. 2016;125:453–469. doi: 10.1037/abn0000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kazes M, Danion JM, Grangé D, Pradignac A, Simon C, Burrus-Mehl F, Schlienger JL, Singer L. Eating behaviour and depression before and after antidepressant treatment: a prospective, naturalistic study. J Affect Disord. 1994;30:193–207. doi: 10.1016/0165-0327(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 62.Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression. Biol Psychiatry. 1987;22:1481–1485. doi: 10.1016/0006-3223(87)90108-9. [DOI] [PubMed] [Google Scholar]
- 63.Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–309. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 64.Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27:859–863. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Mucci A, Dima D, Soricelli A, Volpe U, Bucci P, Frangou S, Prinster A, Salvatore M, Galderisi S, Maj M. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol Med. 2015;45:1765–1778. doi: 10.1017/S0033291714002943. These researchers found that patients with schizophrenia exhibited much lower motivation to work for a monetary reward relative to healthy controls. Notably, these groups showed statistically equivalent levels of consummatory pleasure (i.e. anhedonia). What is more, functional imaging revealed that these patients displayed reduced activity within the dorsal caudate, a mesolimbic structure involved in reward processing, and hypoactivity within this region was correlated with lower motivation, but not anhedonia. This study provides compelling evidence that avolition, not anhedonia, is a key feature of the negative symptoms of schizophrenia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohen AS, Minor KS. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr Bull. 2010;36:143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dumais KM, Franklin TR, Jagannathan K, Hager N, Gawrysiak M, Betts J, Farmer S, Guthier E, Pater H, Janes AC, et al. Multi-site exploration of sex differences in brain reactivity to smoking cues: Consensus across sites and methodologies. Drug Alcohol Depend. 2017;178:469–476. doi: 10.1016/j.drugalcdep.2017.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. In this review, Whitton and colleagues discuss evidence of the hypoactive and dysfunctional nature of the dopamine system in major depressive disorder and schizophrenia, respectively. These authors also make special note that deficits in dopamine signaling associated with depression may lead to blunted incentive salience and that this symptom is an underappreciated feature of the disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McFarland BR, Klein DN. Emotional reactivity in depression: diminished responsiveness to anticipated reward but not to anticipated punishment or to nonreward or avoidance. Depress Anxiety. 2009;26:117–122. doi: 10.1002/da.20513. [DOI] [PubMed] [Google Scholar]
- 71.Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clin Psychol Rev. 2010;30:839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. Am J Psychiatry. 2012;169:364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kringelbach ML. Limbic forebrain: the functional neuroanatomy of emotion and hedonic processing. In: Pfaff DW, editor. Neuroscience in the 21st century. Springer; New York: 2013. pp. 1335–1363. [Google Scholar]
- 74.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013. [Google Scholar]
- 75.Forbes EE. Where’s the fun in that? Broadening the focus on reward function in depression. Biol Psychiatry. 2009;66:199–200. doi: 10.1016/j.biopsych.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kring AM, Moran EK. Emotional response deficits in schizophrenia: insights from affective science. Schizophr Bull. 2008;34:819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bucci P, Galderisi S. Categorizing and assessing negative symptoms. Curr Opin Psychiatry. 2017;30:201–208. doi: 10.1097/YCO.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 78.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Treadway MT, Zald DH. Parsing Anhedonia: Translational Models of Reward-Processing Deficits in Psychopathology. Curr Dir Psychol Sci. 2013;22:244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zald DH, Treadway MT. Reward processing, neuroeconomics, and psychopathology. Annu Rev Clin Psychol. 2017;13:471–495. doi: 10.1146/annurev-clinpsy-032816-044957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 82.Robinson MJF, Anselme P, Suchomel K, Berridge KC. Amphetamine-induced sensitization and reward uncertainty similarly enhance incentive salience for conditioned cues. Behav Neurosci. 2015;129:502–511. doi: 10.1037/bne0000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mayo LM, de Wit H. Acquisition of responses to a methamphetamine-associated cue in healthy humans: self-report, behavioral, and psychophysiological measures. Neuropsychopharmacology. 2015;40:1734–1741. doi: 10.1038/npp.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kessler RM, Hutson PH, Herman BK, Potenza MN. The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev. 2016;63:223–238. doi: 10.1016/j.neubiorev.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 85•.Joyner MA, Kim S, Gearhardt AN. Investigating an Incentive-Sensitization Model of Eating Behavior: Impact of a Simulated Fast-Food Laboratory. Clinical Psychological Science. 2017 doi: 10.1177/2167702617718828. These authors present a superb example of taking principals of incentive-sensitization theory and translating it to uncontrollable eating behavior. In comparison to a neutral environment, participants in a cue-rich environment worked harder for food rewards and had higher self-reported ‘wanting.’. [DOI] [Google Scholar]
- 86.Simon JJ, Wetzel A, Sinno MH, Skunde M, Bendszus M, Preissl H, Enck P, Herzog W, Friederich H-C. Integration of homeostatic signaling and food reward processing in the human brain. JCI Insight. 2017 doi: 10.1172/jci.insight.92970. [no volume] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Holst RJ, Sescousse G, Janssen LK, Janssen M, Berry AS, Jagust WJ, Cools R. Increased striatal dopamine synthesis capacity in gambling addiction. Biol Psychiatry. 2017 doi: 10.1016/j.biopsych.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Limbrick-Oldfield EH, Mick I, Cocks RE, McGonigle J, Sharman SP, Goldstone AP, Stokes PRA, Waldman A, Erritzoe D, Bowden-Jones H, et al. Neural substrates of cue reactivity and craving in gambling disorder. Translational psychiatry. 2017;7:e992. doi: 10.1038/tp.2016.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89•.Gola M, Wordecha M, Sescousse G, Lew-Starowicz M, Kossowski B, Wypych M, Makeig S, Potenza MN, Marchewka A. Can Pornography be Addictive? An fMRI Study of Men Seeking Treatment for Problematic Pornography Use. Neuropsychopharmacology. 2017;42:2021–2031. doi: 10.1038/npp.2017.78. Men with a history of problematic pornography use (PPU) and control men without a history of use, underwent an incentive delay task. Men with PPU elicited greater ventral striatum reactivity to cues predicting erotic images compared to controls and in relation to cues predicting monetary rewards. This study provides another example of how incentive sensitization theory, which was originally developed to explain drug addiction, may be applied behavioral addictions as well. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 91•.Vela L, Martínez Castrillo JC, García Ruiz P, Gasca-Salas C, Macías Macías Y, Pérez Fernández E, Ybot I, Lopez Valdés E, Kurtis MM, Posada Rodriguez IJ, et al. The high prevalence of impulse control behaviors in patients with earlyonset Parkinson’s disease: A cross-sectional multicenter study. J Neurol Sci. 2016;368:150–154. doi: 10.1016/j.jns.2016.07.003. Men with early-onset Parkinson’s disease displayed significantly more impulse control behaviors compared to healthy, age-matched controls. Importantly, dopamine medications were found to significantly increase this risk. This supports a role for dopamine sensitizing reward regions and increasing addictive behaviors in susceptible individuals. [DOI] [PubMed] [Google Scholar]
- 92.Heiden P, Heinz A, Romanczuk-Seiferth N. Pathological gambling in Parkinson’s disease: what are the risk factors and what is the role of impulsivity? Eur J Neurosci. 2017;45:67–72. doi: 10.1111/ejn.13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weintraub D, David AS, Evans AH, Grant JE, Stacy M. Clinical spectrum of impulse control disorders in Parkinson’s disease. Mov Disord. 2015;30:121–127. doi: 10.1002/mds.26016. [DOI] [PubMed] [Google Scholar]
- 94.Tessitore A, Santangelo G, De Micco R, Giordano A, Raimo S, Amboni M, Esposito F, Barone P, Tedeschi G, Vitale C. Resting-state brain networks in patients with Parkinson’s disease and impulse control disorders. Cortex. 2017;94:63–72. doi: 10.1016/j.cortex.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Ishii T, Sawamoto N, Tabu H, Kawashima H, Okada T, Togashi K, Takahashi R, Fukuyama H. Altered striatal circuits underlie characteristic personality traits in Parkinson’s disease. J Neurol. 2016;263:1828–1839. doi: 10.1007/s00415-016-8206-0. [DOI] [PubMed] [Google Scholar]
- 96.Reynolds SM, Berridge KC. Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J Neurosci. 2001;21:3261–3270. doi: 10.1523/JNEUROSCI.21-09-03261.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reynolds SM, Berridge KC. Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/“disliking” reactions, place preference/avoidance, and fear. J Neurosci. 2002;22:7308–7320. doi: 10.1523/JNEUROSCI.22-16-07308.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- 99.Richard JM, Berridge KC. Metabotropic glutamate receptor blockade in nucleus accumbens shell shifts affective valence towards fear and disgust. Eur J Neurosci. 2011;33:736–747. doi: 10.1111/j.1460-9568.2010.07553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Richard JM, Plawecki AM, Berridge KC. Nucleus accumbens GABAergic inhibition generates intense eating and fear that resists environmental retuning and needs no local dopamine. Eur J Neurosci. 2013;37:1789–1802. doi: 10.1111/ejn.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J Neurosci. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Richard JM, Berridge KC. Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D(1) alone for appetitive eating but D(1) and D(2) together for fear. J Neurosci. 2011;31:12866–12879. doi: 10.1523/JNEUROSCI.1339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kapur S, Mizrahi R, Li M. From dopamine to salience to psychosis--linking biology, pharmacology and phenomenology of psychosis. Schizophr Res. 2005;79:59–68. doi: 10.1016/j.schres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- 107.Howes OD, Nour MM. Dopamine and the aberrant salience hypothesis of schizophrenia. World Psychiatry. 2016;15:3–4. doi: 10.1002/wps.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 109.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- 110.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Howes OD, Montgomery AJ, Asselin M-C, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20. doi: 10.1001/archgenpsychiatry.2008.514. [DOI] [PubMed] [Google Scholar]
- 112.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat Rev Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schultz W. Dopamine reward prediction-error signalling: a two-component response. Nat Rev Neurosci. 2016;17:183–195. doi: 10.1038/nrn.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Winton-Brown T, Schmidt A, Roiser JP, Howes OD, Egerton A, Fusar-Poli P, Bunzeck N, Grace AA, Duzel E, Kapur S, et al. Altered activation and connectivity in a hippocampal-basal ganglia-midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl Psychiatry. 2017;7:e1245. doi: 10.1038/tp.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bolstad I, Andreassen OA, Groote I, Server A, Sjaastad I, Kapur S, Jensen J. Effects of haloperidol and aripiprazole on the human mesolimbic motivational system: A pharmacological fMRI study. Eur Neuropsychopharmacol. 2015;25:2252–2261. doi: 10.1016/j.euroneuro.2015.09.016. [DOI] [PubMed] [Google Scholar]