Abstract
Purpose
We advanced a multifactorial, dynamic account of the complex, nonlinear interactions of motor, linguistic, and emotional factors contributing to the development of stuttering. Our purpose here is to update our account as the multifactorial dynamic pathways theory.
Method
We review evidence related to how stuttering develops, including genetic/epigenetic factors; motor, linguistic, and emotional features; and advances in neuroimaging studies. We update evidence for our earlier claim: Although stuttering ultimately reflects impairment in speech sensorimotor processes, its course over the life span is strongly conditioned by linguistic and emotional factors.
Results
Our current account places primary emphasis on the dynamic developmental context in which stuttering emerges and follows its course during the preschool years. Rapid changes in many neurobehavioral systems are ongoing, and critical interactions among these systems likely play a major role in determining persistence of or recovery from stuttering.
Conclusion
Stuttering, or childhood onset fluency disorder (Diagnostic and Statistical Manual of Mental Disorders, 5th edition; American Psychiatric Association [APA], 2013), is a neurodevelopmental disorder that begins when neural networks supporting speech, language, and emotional functions are rapidly developing. The multifactorial dynamic pathways theory motivates experimental and clinical work to determine the specific factors that contribute to each child's pathway to the diagnosis of stuttering and those most likely to promote recovery.
We have advanced a multifactorial, dynamic account of the complex, nonlinear interactions of motor, linguistic, and emotional factors that contribute to the onset and development of stuttering (Smith, 1990, 1999; Smith & Kelly, 1997). Most researchers investigating stuttering now agree that stuttering cannot be explained by a single-factor theory although current accounts provide primary emphasis on varying factors, for example, the dual diathesis-stressor model of Walden et al. (2012). Our original perspective emphasized ongoing interactions among speech motor, linguistic, and emotional processes and drew from dynamic systems theory (e.g., Bassingthwaighte, Liebovitch, & West, 1994; Glass & Mackey, 1988) to better understand the idea of causality in the development of complex human behaviors such as stuttering. We offered what we believed to be a more integrated approach to understanding stuttering because the literature on stuttering in the 1990s was “fragmented and confusing” (Smith, 1999, p. 27). It was common practice for experimental and clinical papers on stuttering to be written on the basis of the assumption that the variable of interest, whether motor timing, various levels of linguistic processing, or auditory feedback loops, were candidate “causes” of stuttering. We argued that such theories could not adequately account for the full range of phenomena associated with stuttering in children and adults.
Another major obstacle we perceived for understanding stuttering at that time was a misguided focus on counts and detailed measurement of disfluencies as “the window through which all phenomena associated with the disorder must be observed” (Smith, 1999, p. 29). For example, studies of physiological processes related to fluent and stuttered speech were sometimes questioned because it was suggested that the temporal onsets and offsets of disfluencies were not adequately pinpointed (e.g., Ingham, Cordes, Ingham, & Gow, 1995). Therefore, any conclusions made on the basis of analyses of physiological or other continuous signals were suspect because it was not clear whether they occurred during fluent or disfluent speech. We argued that perceptible disfluencies were only convenient anchor points in time and that the underlying processes leading up to, during, and after perceptible disfluencies were continuous and should be studied as such. We suggested that “stuttering events” are not static; rather, the dynamic and continuous processes contributing to stuttering disfluencies “may be quite distant in time and space” (Smith, 1999, p. 30). As an analogy, we offered the study of volcanoes, which until the 1960s were subject to purely descriptive analyses on the basis of the shape of the volcanic landform and characteristics of the eruption. There was no integrative, coherent theory of volcanoes until the mapping of the Earth's seafloor provided convincing support for the theory of plate tectonics (Decker & Decker, 1991). It then became clear that the distribution of volcanoes on the Earth's surface was not random, but predictable by the location of adjacent tectonic plates. Our analogy to stuttering, then, was that disfluencies are the surface behaviors that an integrated, comprehensive theory of the underlying dynamic processes must explain.
Moving Beyond Either/Or Dynamic Systems and Mechanistic Approaches
Why did we invoke a dynamic, theoretical account rather than propose a more traditional mechanistic, causal approach to understand stuttering? Dynamic systems theories, which originated as mathematical models of physical phenomena, were widely adopted in the 1990s to explain complex human behaviors. Dynamic systems approaches (e.g., Bassingthwaighte et al., 1994) and the seminal work of Thelen and Smith (1994) on the application of dynamic systems theory to human motor development had a major impact on our work and, indeed, on many scientists studying human behavior and its development (e.g., Lewis, 2000). In brief, the difference between more traditional mechanistic or computational theories of human behavior and dynamic accounts is as follows: Mechanistic theories postulate that the system of interest is composed of component parts, each accomplishing specific function(s), and that when these parts are assembled and operating appropriately in relation to one another, the behavior of the system is predictable (Bechtel & Richardson, 1993). When one of the subcomponents is malfunctioning, errors in the output occur. Mechanistic theories of stuttering posit that there is a core deficit that is the cause of the disorder. The core deficit has been proposed to be in linguistic processing, for example, Wingate's (1988) fault-line hypothesis (syllable onset is produced, but the rime component encoding is delayed), the covert repair hypothesis (Postma & Kolk, 1993; deficit in an internal phoneme-monitoring loop), and Karniol's (1995) account of stuttering (a result of a suprasegmental sentence-planning deficit). Other examples target the motor system. Starkweather (1995) proposed the “proximal” cause of stuttering is the overactivation of the muscles involved in speech. Packman, Code, and Onslow (2007) more recently pinpointed the proximal cause of stuttering as a difficulty in initiating syllables as a result of disrupted function in the supplementary motor area's role in initiation and sequencing of syllable-based speech motor programs. In the EXPLAN theory of Howell (2004), linguistic planning is slowed so that motor execution processes repeat or stop on the current element, resulting in disfluencies. These theoretical accounts are often diagrammed showing directional flows of stepwise processing and indicating the site of the disrupted element in the system. In our view and in the view of the dynamic system theorists, a critical feature missing from these accounts is change over time.
Dynamic approaches, in contrast, critically focus on change over time, on the unfolding trajectory of a system's behavior and how it is affected by external and internal conditions and their nonlinear interactions (Beer, 2000; Thelen & Smith, 1994; van Gelder & Port, 1995). Dynamic system theories use mathematical equations to describe how the system's behavior evolves over time and emphasize emergent properties (properties that “emerge” from a complex system as a whole but that are not found in single subcomponents). This idea stands in contrast to the notion of a stepwise, linear, causal process that led to the current state of a system (see Beer, 2000, for example). Dynamic theories provide key insights into the nonlinear, interactive properties of complex systems and the sudden dynamic changes in output that may be driven by relatively small changes in underlying “control parameters.” The utility of dynamic systems theories in the experimental study of normal or disordered speech production, however, has been extremely limited because the formal tools of dynamics are difficult to apply in natural speaking situations. Formal dynamic models work well when there are repeating cycles in the behavior of the system and have had major impacts on the study of, for example, bimanual coordination and circadian rhythms (Bechtel & Abrahamsen, 2010). In fact, investigators studying speech production using dynamic models often limit speech samples to repetitive trains of syllables (e.g., van Lieshout, 2004). Thus, although dynamic system theory has had a very significant impact on our thinking about the development of stuttering as a complex human behavior that operates over many different time scales and many different levels, it has not provided a fruitful experimental “toolkit” with which to study the many phenomena related to speech production or stuttering.
A Synthesis: Dynamic Mechanistic Theories
Dynamic systems theorists often argue that dynamic theories are not compatible with and are preferred to mechanistic theories that invoke representational elements (e.g., Stepp, Chemero, & Turvey, 2011). We prefer the view of Bechtel and Abrahamsen (2010; see also Kaplan & Bechtel, 2011). They suggest that although dynamic systems models play an important role in cognitive science, they should not be viewed as an alternative to mechanistic explanations. Scientists ubiquitously attempt to explain phenomena by decomposing them into component operations located in various parts of the system. However, these systems do not operate in isolation. Bechtel and Abrahamsen (2010) suggest that the various mechanistically detailed subsystems must then be recomposed as a dynamic collective to understand the influence of higher order and/or external factors. They propose that this logical synthesis produces a “dynamic mechanistic explanation” (p. 321). We propose that a dynamic mechanistic approach is the optimal conceptual strategy for understanding stuttering. There are many ongoing attempts to explain how subsystems operate in stuttering—from auditory integration processes to feedback and feed-forward motor control models, central linguistic processing networks, and temperamental valences and autonomic arousal. We review these efforts in detail in the following sections.
The essential and central point we make here is that an integrative and complete theory of stuttering must arise from the reassembly of these subsystems (motor control, auditory integration, language processing, emotional aspects) and an understanding of their complex, nonlinear interactions over time, including developmental trajectories. In other words, experiments exploring mechanistic explanations will continue to produce valuable insights into the detailed operations of the many different systems involved in language production and how these operations are perturbed in stuttering. The next step is to use a dynamic mechanistic systems approach to view the critical participating and interacting elements as they perform in real-life speaking situations. This is obviously a great challenge for those of us who investigate stuttering, but in terms of understanding stuttering and being able to effectively communicate about the “cause” of stuttering, it is an essential step. At this point, claims that whatever subsystem one is studying in relation to stuttering will reveal the “core cause” of stuttering are, in our view, spurious.
Basic Tenets of a Multifactorial, Dynamic Pathways Theory
Recent research efforts from our own and many other laboratories have adapted experimental methods previously typically applied only in studies of older children and adults so that young children can be studied. From this work, knowledge of the early behavioral, physiological, and neuroanatomical bases of stuttering near its onset has expanded exponentially over the past decade. The purpose of the present article is to update our theoretical account by integrating recent experimental findings that provide critical new insights on the emergence of stuttering in the preschool years. We note our current views of the onset and development of stuttering are consistent with the three general principles suggested to provide “the essential groundwork” for future models of the development of stuttering in the final chapter of the textbook by Yairi and Ambrose (2005, p. 463).
It is now clear that stuttering belongs within the class of neurodevelopmental disorders that includes, for example, specific language impairment, dyslexia, and autism. We note that in the DSM-5 (APA, 2013) stuttering is now labeled “childhood-onset fluency disorder” (p. 45) and is classified as a neurodevelopmental disorder. These disorders arise during early childhood due to atypical development of the central nervous system (CNS). Although stuttering has received relatively little attention as a neurodevelopmental disorder, significant progress has been made in understanding the genetic, epigenetic, and neural growth patterns related to specific language impairment (e.g., Rice, 2012) and other neurodevelopmental disorders, such as autism (Muhle, Trentacoste, & Rapin, 2004). We review the evidence relevant to our current understanding of stuttering, including genetic and epigenetic factors; motor, linguistic, and emotional features of stuttering; and advances in neuroimaging studies revealing atypical neural connections supporting speech and language functions. In addition, we update the evidence supporting a central tenet of our earlier theoretical account: Although stuttering ultimately reflects impairment in sensorimotor processes involved in speech production, its emergence, persistence, and severity over the life span is strongly conditioned by linguistic and emotional factors.
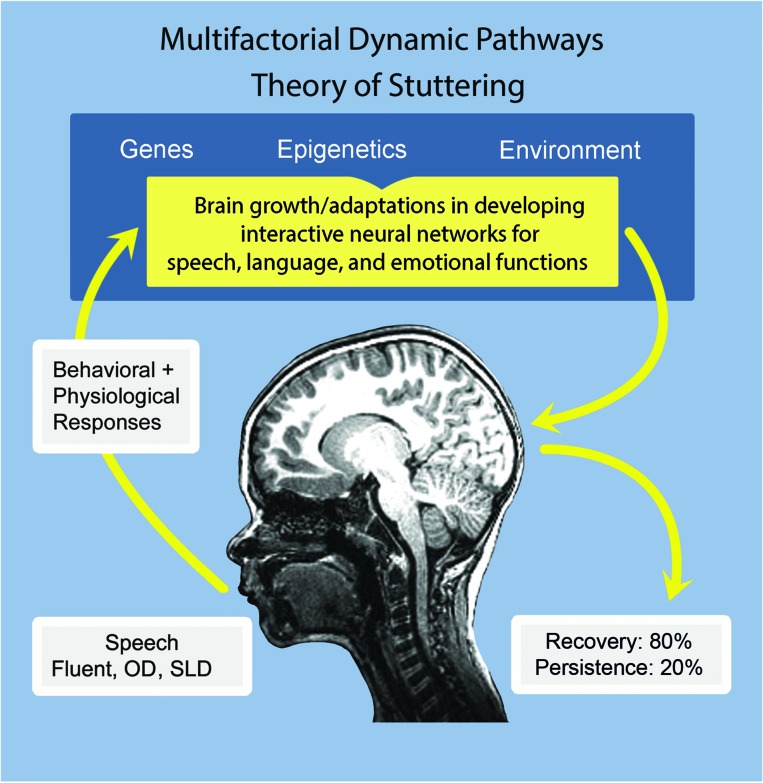
As illustrated in Figure 1, we call our current theory the multifactorial, dynamic pathways (MDP) account of stuttering, because a primary feature is an emphasis on the multilevel events ongoing in the time window in childhood when stuttering emerges and recovery or persistence occurs. We encourage clear delineation of the factors that contribute to the onset and persistence of stuttering, and as in our earlier work, we emphasize the key assertion that these factors have varying weights in different individuals who stutter (e.g., see the “snowmen figure” in Smith & Kelly, 1997). Thus, each child who experiences stuttering has a dynamic pathway into the diagnosis of stuttering, one that ultimately leads to recovery for most children, but it is critical to note that for some children the pathway leads to persistence. A central goal of the MDP account of stuttering is to motivate experimental and clinical work that improves our understanding of how best to aid children in finding the pathway to recovery.
Figure 1.
An illustration of the major features of the multifactorial dynamic pathways theory of stuttering. OD = other disfluencies; SLD = stuttering-like disfluencies.
What Is Stuttering?
Stuttering is a neurodevelopmental disorder whose primary symptoms are disfluencies, involuntary disruptions in the normal flow of speech. It has proven to be very useful to classify the observable symptoms of developmental stuttering as stuttering-like disfluencies (SLDs) and to distinguish them from other disfluencies (e.g., interjections such as um and ah…, phrase revisions; Ambrose & Yairi, 1999). SLDs include syllable and sound repetitions; disrhythmic phonations, such as blocks and prolongations; and broken words. Ambrose and Yairi (1999) were the first to complete a large-scale, detailed analysis of speech samples from preschool children (age 23 to 60 months), 90 characterized as stuttering by their parents and project clinicians and 50 who were developing typically with no concern about stuttering. They classified all of the disfluencies in large speech samples from each child into specific subtypes of SLDs and other disfluencies. Their results clearly demonstrated that the disfluent behaviors of children who were viewed as stuttering were distinct in both quantity and quality from the disfluencies of those who were not. To be specific, the children viewed as stuttering by parents and clinicians had significantly more disfluencies classified as SLDs than the nonstuttering children, and both groups of children had similar numbers of other disfluencies.
Ambrose and Yairi (1999) further demonstrated that a weighted SLD formula correctly classified all children thought to have a stuttering problem. The weighted formula produced not a single false classification of a nonstuttering participant. This was an important experimental demonstration because many early, influential theorists (e.g., Johnson et al., 1959; Bloodstein, 1995), strongly argued that near its onset, stuttering is characterized by disfluencies that are not distinguishable from those of typically developing children and that the disorder usually followed a course from very mild to more severe stuttering in later childhood. In fact, Yairi and Ambrose (2005) found that the onset of stuttering is often abrupt (41% of their cases) with severe stuttering present at onset. The work of the investigators on the Illinois Stuttering Project (summarized in Yairi & Ambrose, 2005) is seminal in many aspects, but critically important for both experimenters and clinicians is that it makes clear that the diagnosis of stuttering in early childhood is straightforward.
The typical age of stuttering onset is 30–48 months with a mean of 33 months; it is estimated that 5%–8% of preschool children experience stuttering and the male/female ratio is approximately 1.5:1 at that age (Bloodstein & Ratner, 2008; Yairi & Ambrose 2005, 2013). Approximately 80% of these children recover either with or without therapy (Yairi & Ambrose, 2005). The prevalence of stuttering in the teenage and adult populations is approximately 1% worldwide with boys and men outnumbering girls and women approximately 4:1. It is clear that the dramatic change in the male/female ratio in the school-age and adult years is indicative of the much higher recovery rate for girls who begin to stutter.
As noted, the symptoms of stuttering can be characterized by classification of the types of disfluencies that occur in speech. The question of what causes these atypical disruptions in speech production has been given a remarkably diverse set of answers depending on the theoretical perspective and scientific training of the author. Theories of the cause of stuttering range from early emotional trauma to parenting behavior, deficits in linguistic processing, and purely motor accounts (reviewed in Bloodstein & Ratner, 2008). Our theoretical account is novel in that we focus not only on the onset and course of the fluency disorder, but also on co-occurring dramatic changes in other behavioral and neural systems during early childhood. We argue that understanding the multidimensional developmental context during the time window when stuttering typically starts and ultimately resolves or persists is critical for any theory of stuttering. As the following review demonstrates, the factors that contribute to the onset, development, and persistence of stuttering lie both within speech sensorimotor systems and the neural systems specialized for a range of other functions, especially those involved in linguistic and emotional processing.
A Neurodevelopmental Perspective
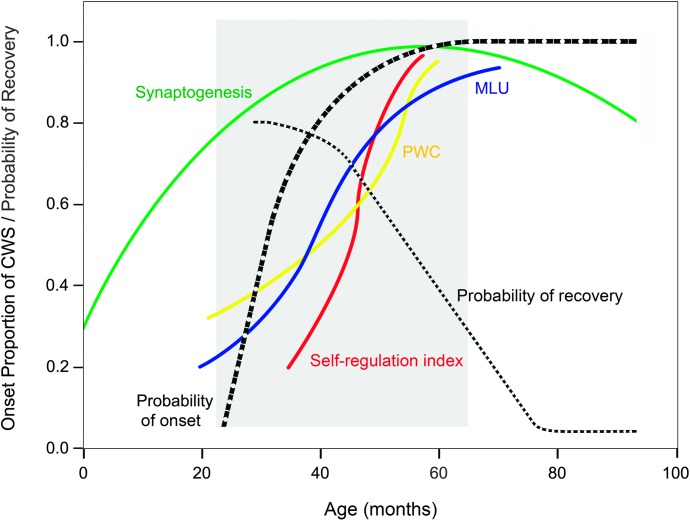
The structure of the brain at any point in time across the life span is a product of interactions among genetic, environmental, and epigenetic factors (Lenroot & Giedd, 2006). Genes are segments of DNA present at birth, and epigenetics encompasses the timing and intensity of gene expression throughout life. A useful analogy is to think of genes as the keys on a piano, and epigenetics is the “playing of the piano keys,” which can vary in timing and intensity (P. Miller, 2012, p. 62). Epigenetic processes are influenced by the environment, which includes the external environment and the internal physiological milieu. Thus, patterns of growth of the CNS and the ultimate phenotype (e.g., stuttering vs. not stuttering) are not fixed at birth, but arise over development through the interaction of genes, epigenetic processes, and experience. Highly significant for neurodevelopmental disorders in general are findings from longitudinal structural imaging studies of children's typically developing brains (Brown & Jernigan, 2012; Jernigan, Baaré, Stiles, & Skak Madsen, 2011). In their review of studies of brain development in the preschool years, Brown and Jernigan (2012) describe this as a period of “blossoming” (p. 313) because the brain shows some of its most dynamic and extensive anatomical and physiological transformations. Longitudinal MRI studies show that an individual child may exhibit a relatively rapid maturational growth curve for one fiber tract in the brain and a delayed course to maturation of other tracts. In addition to this regional specificity in growth, these investigations reveal remarkable individual differences in the growth patterns of specific fiber tracts (Jernigan et al., 2011).
These heterogeneous and asynchronous brain development profiles emerge in childhood as genes, the environment, and epigenesis interact to sculpt the child's brain structure and function. Petronis (2010) makes a strong case that epigenetic modifications of DNA will be crucial for understanding the molecular bases of complex phenotypes (for example, in the present case, stuttering) because “epigenetic factors are sometimes malleable and plastic enough to react to cues from the external and internal environments” (p. 721). He argues that older etiological accounts of complex non-Mendelian human disorders, such as schizophrenia, autism, and diabetes, attributed far too much weight to DNA variation. Rather, he suggests current accounts should give greatest emphasis to the role of epigenetic misregulation as the most significant underlying etiological factor in complex disorders. There is clear evidence to support such an account of stuttering. The most straightforward evidence related to genetic and epigenetic factors in stuttering comes from classic twin studies in which the concordance rates for monozygotic (MZ) and dizygotic (DZ) twin pairs are contrasted. For stuttering, the percentages are approximately 18% for DZ twins and 55% for MZ twins (Felsenfeld et al., 2000; Howie, 1981). For comparison, the respective concordance rates for specific language impairment are approximately 45% DZ and 85% MZ (Leonard, 2014). Although results of twin studies clearly suggest that one's DNA is an important factor in determining whether or not one will have chronic stuttering, it is also clear that specific language impairment has a greater heritability factor. Although the role of specific epigenetic processes in stuttering is unknown, the MZ twin discordance rates suggest that the emergence of stuttering and the path to persistence or recovery depends critically upon the timing and intensity of gene expression over development—that is, upon epigenesis. This understanding of the importance of experience in shaping the development of the brain in childhood leads to an increasing emphasis on understanding the specific factors that promote recovery from stuttering in the preschool years.
Motor Aspects of Stuttering
It has long been clear that stuttering involves problems in speech motor planning and execution with frank breakdowns in speech motor processes resulting in perceptible disfluencies varying in duration and form. Disfluencies arise when the motor commands to the muscles are disrupted, and normal patterns of muscle activity required for fluent speech are not generated (Smith, 1989). Although many older accounts of stuttering postulated that muscle activity was excessive during disfluencies, many electromyographic studies in adults who stutter (AWS) have revealed heterogeneity in muscle activation patterns underlying stuttering disfluencies (McClean, Goldsmith, & Cerf, 1984; Smith, Denny, Shaffer, Kelly, & Hirano, 1996; Smith et al., 1993) with each individual who stutters tending to show a consistent pattern across disfluent intervals. Thus, it is clear from studies of AWS that excessive muscle activation is not the cause of stuttering nor even a consistent symptom of stuttering. The only neurologically abnormal muscle activation pattern observed in AWS is tremor, involuntary rhythmic muscle contractions with a frequency in the 5- to 15-Hz range (Fibiger, 1971; McClean et al., 1984; Smith, 1989; Smith et al., 1996). These investigations report that tremor is observed during SLDs and is present in a subgroup of AWS, typically those with longer disfluencies and a more severe stuttering problem.
The speech motor systems of AWS are continuously affected by the underlying speech motor instabilities as even the perceptibly fluent speech of AWS shows signs of atypical patterns (e.g., Kleinow & Smith, 2000; Smith et al., 1996; Zimmermann, 1980a). It has been hypothesized that the underlying speech motor deficit in adults with persistent stuttering is a failure to form stable underlying motor programs for speech (feed-forward motor control processes) and that this underlying instability leads to overreliance on feedback systems (Max, Guenther, Gracco, Ghosh, & Wallace, 2004; Neilson & Neilson, 1987). This hypothesis is supported by many lines of evidence, for example, the instabilities observed during the fluent speech of AWS cited above. In addition, in a recent investigation of a novel nonword learning task (Smith, Sadagopan, Walsh, & Weber-Fox, 2010), we observed that normally fluent adults produced highly consistent interarticulator coordination patterns (quantified using a measure of the consistency of upper lip, lower lip, and jaw coordination on repeated productions). Even in the earliest trials, normally fluent adults performed at ceiling and did not improve with practice. AWS, on the other hand, showed significantly more variable articulatory coordination patterns in early nonword learning trials. They then adjusted motor commands as practice continued because the later trials of AWS were more consistently coordinated than the early trials (all nonword productions included in the learning analysis were perceptibly fluent). We concluded that the speech motor learning process observed in the AWS was an immature pattern because we had observed the same pattern of improved performance over the course of the experiment in typically fluent school-age children but not in adults (Walsh, Smith, & Weber-Fox, 2006). Kleinow and Smith (2000) investigated the fluent speech of AWS producing sentences of increasing length and linguistic complexity and found that the speech of AWS was increasingly unstable (again as indexed by an interarticulator coordination measure) in the face of increasing utterance demands. Their normally fluent peers did not show increased motor instability when producing longer and more complex sentences.
We have suggested that the less stable coordination patterns in fluent speech production in both the nonword learning and sentence production paradigms reflect the failure of the CNS to develop stable, basic motor programs and muscle synergies for speech in AWS. A recent transcranial magnetic stimulation study provides compelling evidence supporting this assertion. Transcranial magnetic stimulation was applied to left and right primary tongue motor cortical areas, and motor evoked potentials were recorded in tongue muscles while AWS and control participants performed a verb generation task (Neef, Hoang, Neef, Paulus, & Sommer, 2015). By varying the latency of the transcranial magnetic stimulation pulse relative to the onset of speech, these investigators could map the time course of any cortically generated increases in tongue motor neuron excitability prior to speech onset. Their results showed dramatic differences in the time course of cortical excitability during the speech motor planning and motor initiation phases in AWS compared with fluent speakers. Fluent speakers showed a left motor cortex facilitation of tongue motor neuron excitability during the 300-ms interval prior to speech onset. AWS did not show a left or a right facilitation of tongue muscle activation in the prespeech interval. These findings provide strong evidence that speech motor programming is typically left lateralized for fluent speakers but not for AWS. It is notable that the degree of reduction in primary motor cortex prespeech excitability was correlated with stuttering severity.
Such results are highly consistent with the neuroanatomical evidence of deficits in left speech premotor and motor areas and among tracts connecting motor, auditory, and language areas (reviewed in the section on CNS aspects below). Differences in the use of auditory feedback in AWS during speech production have been reported when the speaker's auditory feedback was perturbed during speech production (Cai et al., 2012). AWS and normally fluent adults both responded by adjusting articulatory patterns to compensate for the error, but AWS produced compensations that were approximately half the amplitude of the responses of the fluent controls. This suggests that the gain of the auditory feedback loop is lower during speech in AWS and, if this is the case, it would seem to make excessive reliance on feedback to adjust motor programs highly inefficient.
It is clear that persistent, childhood-onset stuttering is a sensorimotor problem, but it should also be noted that observations of characteristics of the phenotype of stuttering are not limited to speech production tasks. Evidence of atypical coordinative and motor timing processes can be observed in performance of some limb motor tasks by AWS, for example, in tapping complex finger sequences (Smits-Bandstra, De Nil, & Rochon, 2006) although the results of studies of nonspeech motor performance in AWS are mixed and appear to be task-dependent (Max, Caruso, & Gracco, 2003; Zelaznik, Smith, & Franz, 1994).
Most work on sensorimotor processes related to stuttering has been done in adults although some aspects of speech motor control in children who stutter (CWS) have been inferred from analyses of speech acoustic data (e.g., Hall, Amir, & Yairi, 1999). Our laboratory has provided perhaps the most extensive collection of work using direct assessment of speech and nonspeech motor dynamics in preschool and school-age CWS, and we therefore review this work in some detail. We have recruited CWS at age 4–5 years and followed them for up to 5 years to collect longitudinal data on articulatory motion tracking and a wide range of other protocols, including standardized tests, event-related brain potential (ERP) studies, and recording autonomic signals to examine autonomic nervous system function (Smith & Weber, 2016). During direct recording of articulatory movements of the lips and jaw, children have completed tasks including nonword learning and production of sentences of varying length and syntactic complexity. We also have recorded electromyographic signals during natural conversational speech in spontaneous play sessions. In addition, we have examined basic, nonspeech motor timing abilities in performance of a bimanual clapping task (Hilger, Zelaznik, & Smith, 2016; Olander, Smith, & Zelaznik, 2010).
Perhaps the most important outcome of these studies to date is the finding of early speech motor delays in CWS. CWS, by 4–5 years, especially boys, are already showing atypical speech motor development (MacPherson & Smith, 2013; Walsh, Mettel, & Smith, 2015). We document atypical development of coordinative processes in the same manner that we used with adult kinematic data and in large-scale studies of typical speech motor development—with a measure of oral interarticulator coordination (e.g., Smith & Zelaznik, 2004). On repeated production of an utterance, adults show highly stable, consistent interarticulator motions. We use a coordinative index to quantify the degree of consistency; the lower the value (i.e., lower variability) indicates higher consistency in the underlying coordinative pattern. The developmental time course to achieve remarkably stable coordinative patterns, reflecting highly consistent underlying muscle synergies and motor programs, is surprisingly protracted; coordinative consistency is not adult-like until after 16 years of age. We also have shown that before age 5 years, typically developing boys lag girls in speech motor development; then they “catch up” by age 7 years, and no further sex differences are seen during the school-age and adolescent years in the growth trajectories of oral motor coordination (Smith & Zelaznik, 2004; Walsh & Smith, 2002). Using the same methodology, we assessed speech motor coordination in a relatively large-scale study of 4- and 5-year-old children who were stuttering and matched controls. They produced simple, short sentences (e.g., “buy Bobby a puppy”). We found that male CWS (but not the girls) showed higher variability in articulatory coordination patterns and also differences in basic movement parameters, such as movement amplitude and velocity (Walsh et al., 2015).
Of course, at 4 and 5 years of age, these CWS have an estimated 50% to 60% likelihood of recovery (Yairi & Ambrose, 2005), so our sample of approximately 60 CWS was a mixed group in terms of future stuttering outcomes, yet we still found that the boys who were stuttering lagged boys who were not stuttering on speech motor development in the preschool years. It is interesting to note that the female preschool CWS were not different from their peers. In a similar manner, the neuroimaging data of Chang, Zhu, Choo, and Angstadt (2015) demonstrated sex differences in the relationships among the robustness of speech white matter tracts and stuttering severity (found primarily for boys). Thus, our kinematic findings provide additional evidence of the apparently “deeper” physiological imprint of deficits related to stuttering in preschool boys. This result also points to factors contributing to the well-documented lower probability for boys of recovery from stuttering.
Our studies of more complex sentence production and nonword learning in the preschool stuttering population also reveal atypical speech motor developmental pathways for CWS compared with their fluent peers (MacPherson & Smith, 2013; Smith, Goffman, Sasisekaran, & Weber-Fox, 2012). Mirroring the data for AWS, which reflect the outcome after many years of experience with stuttering, our findings suggest that even as early as age 4 to 5 years, the lagging development of speech motor systems in CWS is also characterized by reduced facility in managing the challenges of novel phonological sequence learning and in producing real utterances with increasing length and syntactic complexity.
To investigate the potential contribution of a general motor timing deficit in the onset of stuttering, we used a bimanual clapping task, in which children clapped to a beat for a sequence of beats, and then the beat was turned off. They continued to clap and tried to keep the beat for 20 claps; multiple trials were completed. We analyzed the interclap interval variability and interhand timing coordination during the unpaced, continuation clapping series. In our initial report on this protocol, we found that 60% of CWS (n = 17; age 4 to 5 years), performed more poorly than any of the 13 children who did not stutter (CWNS) tested (Olander et al., 2010). We found this to be a promising result, indicative of a general motor timing deficit in a large proportion of preschool CWS, and so we followed up with testing a much larger sample (n = 70) of CWS. When we expanded the sample size and completed the same experiment (Hilger et al., 2016), this distributional difference between CWS and their fluent peers was not found: The clapping ability of CWS overlapped those of their nonstuttering peers, and no group differences were observed. Nor was there a subgroup of CWS whose clapping ability was outside the typical range. Therefore, we concluded that there is no evidence, on the basis of assessment of clapping performance, that a basic motor timing deficit is present in a significant proportion of CWS. It could be argued that a different task, perhaps a more complex motor task, would reveal a deficit in manual motor timing. In response to this suggestion, we note that the repertoire of motor timing tasks that a 4-year-old can perform is limited. In fact, clapping to the beat and maintaining it was difficult for the children as evidenced by their poor timing accuracy and high variability on the interclap intervals. Our follow-up clapping study clearly showed that we had underestimated the range of typical performance in our earlier sample of typically developing children. Therefore, we argued that sampling error resulted in our earlier positive finding in the smaller n samples of CWNS and CWS. The results of our two clapping investigations are important to emphasize as they are an excellent cautionary note for research on stuttering. The majority of investigations of stuttering in adults and children use small numbers of participants, often fewer than the ns we used in our 2010 clapping study. Thus, in future work, it is essential to use larger samples of participants who do and do not stutter and to include reports of individual data to reveal the true range of performance of the participants who do and do not stutter.
Last, another of our large-scale, cross-sectional studies of preschoolers who stutter and their fluent peers addressed the old, widely held notion that excessive articulatory muscle activity is a feature of stuttering. We found no evidence of any differences in CWS and CWNS in perioral electromyographic amplitude during conversational speech or sentence production, no differences in the bilateral synchrony of activation, and no group differences in left/right amplitude ratios (Walsh & Smith, 2013). These data, considered with the kinematic data reviewed above, point to a deficit in speech motor processes in early childhood stuttering—that is, a speech motor programming and execution deficit and not a hyperactivation or overactivation of the speech production system.
In summary, for decades, we have known many of the atypical sensorimotor features related to stuttering in adults: poorer interarticulator coordination during fluent speech; documented disruptions in the spatiotemporal patterns of articulatory, laryngeal, and respiratory muscle activity during SLDs; atypical integration of sensory feedback; and tremor in the physiological range in some AWS. The question often raised in discussions of such results was if these atypical sensorimotor aspects of stuttering were a result of years of stuttering—that is, years of experience using an inefficient and unstable speech production system. Now, on the basis of the work reviewed above, it seems that the answer to this question is that atypical and/or lagging development of speech motor control processes are features of early stuttering. It seems likely that as the child grows and stuttering persists, the sensorimotor characteristics of that individual's stuttering change, and preliminary evidence suggests that tremor is a feature of stuttering that emerges later in the school-age years (Kelly, Smith, & Goffman, 1995). It will be important for future work to clarify if instabilities in speech motor processes observed near stuttering onset are predictive of persistent stuttering. In persistent stuttering, compensatory central neural processes are insufficient, and the child ultimately does not develop stable motor programs and functional synergies to enlist in aid of what most speakers experience daily: effortlessly fluent speech.
Language Aspects of Stuttering
Stuttering onset typically occurs when the child's linguistic abilities are developing very rapidly, such as rapid growth in mean length of utterance (MLU; e.g., J. F. Miller & Chapman, 1981) and phonological skills (e.g., Rvachew & Brosseau-Lapré, 2012), and researchers have long recognized the importance of examining potential relationships among the development of stuttering and various aspects of language proficiency of young CWS (e.g., Bauman, Hall, Wagovich, Weber-Fox, & Ratner, 2012; Bloodstein & Ratner, 2008; Wagovich, Hall, & Clifford, 2009; R. V. Watkins & Yairi, 1997; Yairi & Ambrose, 2005). Interactions between language demands and stuttering were revealed in several studies that found a link between exacerbated stuttering for productions of more syntactically complex utterances (e.g., Gaines, Runyan, & Meyers, 1991; Logan & Conture, 1997; N. B. Ratner & Sih, 1987; Weiss & Zebrowski, 1992; Yaruss, 1999; Zackheim & Conture, 2003). Further, as noted above, increased variability of speech oral motor coordination patterns in both CWS and AWS is associated with increased syntactic complexity of an utterance, indicating an impact of language demands on speech motor stability (MacPherson & Smith, 2013; Smith et al., 2012). A number of studies have found that the language abilities of some young CWS lag subtly on some measures of language and articulation performance compared with their typically developing peers (e.g., Anderson & Conture, 2000; Ntourou, Conture, & Lipsey, 2011; N. B. Ratner & Silverman, 2000) or show greater dissociations among specific abilities, for example, across receptive and expressive language abilities (Anderson, Pellowski, & Conture, 2005; Coulter, Anderson, & Conture, 2009). It has been suggested that a mismatch between the language proficiency of the child and the increasing demands of utterance length and complexity (e.g., MLU) contributes to the number of disfluencies the child displays (Zackheim & Conture, 2003). However, it is important to note that CWS as a group do not display frank language deficits (Nippold, 2002, 2012), and some studies have shown that specific measures of language abilities of CWS are similar to or above those of typically developing children (Kadi-Hanifi & Howell, 1992; Reilly et al., 2009; R. V. Watkins, Yairi, & Ambrose, 1999). Therefore, the “language” factor in stuttering is heterogeneous among different children. In one child, relatively robust language systems may be interacting with a lagging speech motor system, resulting in an increased probability of stuttering. Another CWS may experience either frank or subtle language and/or phonological delays that are interacting with immature speech motor networks that may result in a greater weighting of speech and language complexity, influencing fluency.
Investigators using a cognitive neuroscience approach to understand the relationships between language processing and stuttering have utilized electrophysiological measures of time-locked brain activity: ERPs. ERPs are sensitive to synchronous activity of pyramidal neuronal networks elicited by a specific stimulus or cognitive process (Luck, 2005) and have been used in numerous studies to better understand the underlying neural processes mediating semantic, syntactic, and phonological processing in typical development across the life span (e.g., Friederici, 2011; Gouvea, Phillips, Kazanina, & Poeppel, 2010; Holcomb, Coffey, & Neville, 1992; Kutas & Federmeier, 2011). ERPs are sensitive indicators of language proficiency in children and adults even when the range of participants' proficiencies is within normal limits (Hampton Wray & Weber-Fox, 2013; Pakulak & Neville, 2010). In a series of studies from our laboratory, we have used paradigms that elicit well-known language-related ERP components (e.g., N400, P600) to determine if and how neural activity patterns mediating semantic, syntactic, and phonological processing differ for AWS and CWS compared with their fluent peers (e.g., Cuadrado & Weber-Fox, 2003; Mohan & Weber, 2015; Usler & Weber-Fox, 2015; Weber-Fox, 2001; Weber-Fox & Hampton, 2008; Weber-Fox, Hampton Wray, & Arnold, 2013; Weber-Fox, Spencer, Spruill, & Smith, 2004; Weber-Fox, Spruill, Spencer, & Smith, 2008). The N400 component reflects the identification, retrieval, and integration of semantic meaning (Kutas & Federmeier, 2011), and the P600 is thought to index reanalysis and repair processes, often for violations of syntax or grammar or garden path sentences (Gouvea et al., 2010). ERP studies of AWS demonstrate that the neural activity mediating semantic and syntactic processing is atypical despite normal performance on standardized tests of language. Differences in ERPs have been observed for language stimuli presented in both visual and auditory modalities (Cuadrado & Weber-Fox, 2003; Weber-Fox, 2001; Weber-Fox & Hampton 2008), suggesting that the subtle differences in language-related ERPs of AWS are fundamentally related to the cognitive operations required for linguistic processing. In general, the findings from this series of studies suggest that neural patterns for semantic and syntactic processing are subtly different in AWS as a group. For example, in a semantic and syntactic processing study of naturally spoken simple sentences, unexpected verbs (reduced semantic expectation) elicited a classic N400 component in the typically fluent adults, and the violation in verb agreement (morphosyntactic error) elicited the well-known P600 component (Weber-Fox & Hampton, 2008). In contrast, the same stimuli elicited a biphasic N400–P600 waveform for both conditions in the AWS. It is interesting to note that this biphasic pattern can also be observed in typically fluent adults; however, it is typically elicited by more syntactically complex sentences (Friederici & Frisch, 2000). These findings suggest that the neural systems mediating semantic and syntactic processing in AWS are less fluid and may engage the semantic and syntactic processing streams less efficiently compared with those of their typically fluent peers.
Another aspect of language that has importance for understanding stuttering is phonological processing. Phonological disorders have a higher incidence among CWS compared with their nonstuttering peers (e.g., Paden, Yairi, & Ambrose, 1999; Yaruss, LaSalle, & Conture, 1998). Yairi, Ambrose, and Cox (1996) reported that although phonological development may be within normal limits for CWS whose stuttering persists, these children produce more errors compared with fluent peers and CWS who recover. Several studies have utilized nonword repetition tasks to investigate phonological processing in stuttering because these tasks rely on phonological awareness, representation, and memory as well as speech motor planning and execution (Anderson, Wagovich, & Hall, 2006; Hakim & Ratner, 2004; Seery, Watkins, Ambrose, & Throneburg, 2006; Smith et al., 2012; Spencer & Weber-Fox, 2014; Weber-Fox et al., 2008). With regard to behavioral measures of nonword repetition task performance, the results of these studies (Anderson et al., 2006; Hakim & Ratner, 2004; Smith et al., 2012; Weber-Fox et al., 2008) have been mixed—some finding poorer performance by CWS compared with typically developing peers and some reporting comparable performance if CWS are screened for co-occurring language or phonological disorders (Smith et al., 2012). Thus, differences in findings may be, in part, related to the inclusion criteria and matching on the basis of language abilities and socioeconomic status (Smith et al., 2012). Even with comparable behavioral performance on nonword repetition tasks, the studies in which speech kinematics were analyzed revealed differences in underlying motor performance in CWS and AWS compared with their normally fluent peers (Smith et al., 2012). And nonword repetition task accuracy and articulation proficiency (as indexed by the Bankson-Bernthal Test of Phonology–Consonant Inventory; Bankson & Bernthal, 1990) were found to be lower in 4- to 5-year-old CWS who would eventually persist in stuttering compared with the CWS who were eventually to recover (Spencer & Weber-Fox, 2014).
ERPs can also be used to examine the neural underpinnings of phonological processing in stuttering during the performance of rhyming tasks because rhyming requires phonological awareness (Weber-Fox et al., 2004). Investigations of ERPs elicited in rhyming tasks have been widely used in the study of typical development, and classic ERP measures for this task are well established. Nonrhyming targets elicit a greater negativity relative to rhyming targets, an ERP component that is in the family of the N400 (Rugg, 1984). In a rhyming task in which orthographic and phonological congruence were manipulated (e.g., target rhyme pairs, such as blown–own, cone–own, gown–own, chair–own), we found that ERPs to phonological processing for AWS were similar to those of fluent controls; however, they displayed a greater right hemisphere–dominant distribution of the N400 elicited by the rhyming/nonrhyming targets. These findings are consistent with the functional near-infrared spectroscopy findings by Sato et al. (2011) showing atypical right lateralization for processing phonemic distinctions in AWS as well as CWS. The behavioral accuracy and response times for the rhyme judgments were similar for AWS and controls on all conditions except for the most challenging condition in which the orthography of the prime and target words matched but did not rhyme (e.g., gown–own). For this condition, accuracy was lower and the response times longer for the AWS compared with fluent peers.
In an identical rhyming ERP experiment with school-age CWS (aged 9–12 years) and matched controls, we found qualitatively different results compared with those for AWS. First, the overall accuracy for rhyme judgments was reduced for the CWS regardless of condition, and response times were longer. Also, an ERP component elicited by the prime words, known as the contingent negative variation, was reduced in the CWS compared with peers, and the contingent negative variation was identical for the AWS and their control group. This suggests that the development of the neural processes related to phonological silent rehearsal (Rugg, 1984) or resource allocation (Coch, Grossi, Skendzel, & Neville, 2005; Grossi, Coch, Coffey-Corina, Holcomb, & Neville, 2001) lags in CWS but eventually matures—no longer being a distinguishing characteristic of stuttering in adulthood. The differences in findings for the CWS and AWS highlight the need for studies across development as neural functions related to language processing will be affected by brain developmental changes, growth in language proficiencies, and experience with stuttering.
In a recent ERP study, we examined language processing closer to the onset of stuttering, in preschool CWS, aged 4–5 years (Weber-Fox et al., 2013). In this study, semantic and syntactic violations were embedded in stories presented with natural speech and accompanied by child-directed cartoon videos. The children made no overt responses or judgments. They simply watched the videos and listened to the stories. All of the children had normal language skills, and there was no significant difference for CWS and CWNS on standard tests of language. The groups also had comparable socioeconomic status and nonverbal intelligence. We found that CWS had slightly longer latency N400s for processing semantic information and a more right-lateralized P600 for processing violations in syntactic phrase structure relative to canonical sentences. Thus, differences in the neural activity mediating language processing distinguish CWS as young as 4–5 years of age. Of course, it is important to note that in a group of 4- to 5-year-old CWS, a proportion of them (approximately 50%) are likely to recover from stuttering. So the results of this initial ERP study of preschool CWS may be confounded by differences in language processing that may be associated with eventual recovery or persistence of stuttering.
In additional ERP studies of slightly older CWS, we found that the children who recovered from stuttering (CWS-Rec) by ages 6–7 years displayed P600 ERPs similar to those of CWNS for processing of syntactic violations embedded in naturally spoken jabberwocky sentences (Usler & Weber-Fox, 2015). The CWS who persisted (CWS-Per) at this age, however, displayed an N400 ERP instead—in this case, a less mature pattern similar to responses of typically developing younger children (ages 3–5 years) in the same task (Usler & Weber-Fox, 2015). In the phonological domain, 7- to 8-year-old CWNS, CWS-Rec, and CWS-Per all showed a robust central–parietal N400 effect elicited by nonrhyming targets (Mohan & Weber, 2015). However, the more anterior ERP, in which the onset of the waveform elicited by the rhyming targets is earlier (presumably facilitated by the prime word), was bilateral for the CWNS but right-lateralized for the CWS-Rec. CWS-Per did not show the anterior ERP facilitation effect (Mohan & Weber, 2015). These results indicate that the development of some neural circuits mediating linguistic processes is related to the child's developmental course to recovery or persistence of stuttering. Further, for some aspects of language processing, ERP patterns distinguish CWNS, CWS-Rec, and CWS-Per despite the groups displaying similar behavioral accuracy and language proficiencies. Also, it is important to keep in mind that the language processing differences we have reviewed are based on group differences. There is considerable overlap in the ERPs of AWS and CWS with their typically developing peers. These findings support the notion that in the developmental dynamics of stuttering, language is a critical factor whose role varies among individuals who stutter.
In conclusion, it is essential to emphasize that the neural indices of delayed or atypical language processing in CWS are windows or snapshots taken at specific developmental time points. Differences in processing observed at a particular age for a specific aspect of language may not be observed at another developmental time point. The findings reviewed here highlight the dynamic nature of the neurodevelopmental course of interactions between specific aspects of language processing, speech motor development, and stuttering status. They also provide evidence that stuttering arises during dynamic phases of maturational lags or atypical development in multiple neural networks involved in language processing and speech production. The parallels in the language and motor domains for early stuttering are striking, showing evidence of atypical and/or delayed development of the neural networks supporting these functions. The language and motor systems must interact in complex ways during speech production, and stuttering often reflects anomalous early development in both domains. To be clear, we are not proposing that stuttering is a language disorder; rather, the experimental findings we present demonstrate that atypical developmental processes related to stuttering are not confined to neural networks supporting speech motor functions. Because speech motor and language networks are highly interactive, it is not surprising that the atypical development in one system often is accompanied by atypical development in the other. In future research, it will be important to determine if there are critical interactions between language and motor processes that are most likely to result in persistent stuttering. For example, are there specific combinations of multidimensional profiles, such as low speech motor coordination scores combined with delayed phonological development that are most likely to give rise to persistent stuttering?
Emotional Aspects of Stuttering
Various personality characteristics have been discussed as potentially important in stuttering; however, the role of anxiety has received the most attention in the literature, and many studies have supported the conclusion that there is a relatively high rate of social anxiety in AWS (Bloodstein, 1995; Iverach, Menzies, O'Brian, Packman, & Onslow, 2011; Iverach & Rappee, 2014). Iverach et al. (2011) note that stuttering often has negative consequences that adversely affect social interactions and overall quality of life and conclude that “as a result, anxiety in speaking-related or social situations can be considered a predictable outcome of the negative communication consequences experienced across the life span for people who stutter” (p. 221).
Most investigations of anxiety and stuttering have relied on standardized questionnaires—some designed specifically to assess stuttering-related anxiety. A few investigators have attempted to assess the physiological effects of speaking-related anxiety by measuring electrodermal responses and blood pulse volume—signals that reflect autonomic nervous system activity. Two studies revealed that speaking elicits high levels of autonomic arousal in both AWS and normally fluent adults (Peters & Hulstijn, 1984; Weber & Smith, 1990). For example, we observed that spontaneous speaking in response to neutral questions in both individuals who do and do not stutter produced high levels of autonomic arousal, higher than those observed in a strenuous breath-holding maneuver (Weber & Smith, 1990). On average, autonomic arousal was not higher during speech of AWS compared with fluent controls; however, when AWS were disfluent, they showed the largest autonomic responses recorded during speech. To be specific, in AWS, disfluency was associated with increased sympathetic arousal. More recent studies have examined autonomic arousal in AWS in response to hearing stuttered speech and to anticipation of their own stuttering. Results suggested that in AWS hearing and anticipating overt disfluencies produces increases in sympathetic arousal (Bowers, Saltuklaroglu, & Kalinowski, 2012; Guntupalli, Kalinowski, Nanjundeswaran, Saltuklaroglu, & Everhart, 2006).
In order to understand how emotional factors contribute to the early development of stuttering, an important step has been to adapt both the behavioral/clinical measures of communication anxiety and physiological indices to age-appropriate protocols for young CWS. In addition, researchers have used tools to assess dimensions of temperament and suggested that a child's temperament interacts with the development and acquisition of speech and language abilities (e.g., Dixon & Hull Smith, 2000; Garello, Viterbori, & Usai, 2012; Karrass, VanDeventer, & Braungart-Rieker, 2003; Slomkowski, Nelson, Dunn, & Plomin, 1992). There is evidence that greater negative affect imposes a greater cognitive load for emotional regulation, decreasing the availability of resources for language development (Rothbart, Sheese, Rueda, & Posner, 2011). Vanryckeghem and colleagues (Vanryckeghem & Brutten, 2006; Vanryckeghem, Brutten, & Hernandez, 2005) developed the KiddyCAT, a self-report measure used to assess attitudes toward speech in preschoolers. They found that the attitude toward speech of 45 preschool and kindergarten CWS was significantly more negative compared with CWNS. A subsequent study reported similar results (Clark, Conture, Frankel, & Walden, 2012). Ambrose and Yairi (1994) asked preschool children to discriminate between fluent and disfluent speech produced by puppets and to identify the puppet who “speaks like me.” They found some awareness of disfluency at age 3 years and that full awareness of disfluent speech was present by 5 years of age. They also reported that negative evaluation of disfluent speech was present by 4 years of age. These are important findings because they strongly contradict earlier notions that preschoolers are not aware of their stuttering and only develop negative attitudes toward their speech later in the school-age years (e.g., Bloodstein, 1995).
There is increasing attention to other aspects of temperament in the development of stuttering with accumulating evidence for differences in emotional development between CWS and CWNS (see R. Jones, Choi, Conture, & Walden, 2014, for review). Multidimensional measures have been obtained by parent report utilizing well-established metrics, such as the Children's Behavior Questionnaire (Rothbart, Ahadi, Hershey, & Fisher, 2001) or the Behavioral Style Questionnaire (McDevitt & Carey, 1978). Using these measures, investigators have reported that greater negative affect is associated with stuttering in children (Eggers, De Nil, & Van den Bergh, 2010; Embrechts, Ebben, Franke, & van de Poel, 2000; Ntourou, Conture, & Walden, 2013) along with less effective emotional regulation (Ntourou et al., 2013) and greater reactivity to changes in background stimuli (Schwenk, Conture, & Walden, 2007) and startle stimuli (Gregg & Scott, 2015) compared with CWNS. Several studies have reported a negative relationship between stuttering and inhibitory control (Anderson, Pellowski, Conture, & Kelly, 2003; Eggers et al., 2010; Embrechts et al., 2000; Walden et al., 2012); CWS are reported to be less able to inhibit inappropriate responses. CWS and CWNS recently were found to have similar behavioral inhibition skills; however, within the group of CWS, those with greater behavioral inhibition skills displayed greater stuttering severity (Choi, Conture, Walden, Lambert, & Tumanova, 2013). Findings are mixed with regard to attentional control with some studies reporting CWS to be higher in attentional control (Anderson et al., 2003) and others finding these children to be lower (Embrechts et al., 2000). It is important to note that, although some consistent differences in temperament have been observed between CWS and CWNS groups, the individual variability indicates that not all CWS display increased negative affect, increased emotional reactivity, or decreased emotional regulation. Therefore, as with language abilities, emotional factors contribute in varying ways to the developmental dynamics of stuttering in CWS.
There have been only two studies of physiological indices of autonomic nervous system activity in CWS during speech. Jones, Buhr, et al. (2014) measured respiratory sinus arrhythmia (an indicator of parasympathetic nervous system activity) and skin conductance (an indicator of sympathetic nervous system activity) in preschool children who watched a positive or negative video prior to a speaking task. Their results were difficult to interpret. Compared with CWNS, the CWS exhibited lower respiratory sinus arrhythmia at baseline and higher skin conductance during speaking following the positive (but not the negative) video (Jones, Buhr, et al., 2014). Zengin-Bolatkale, Conture, and Walden (2015) reported greater tonic skin conductance in 3-year-old CWS compared with their nonstuttering peers but not 4-year-old CWS. Again, these mixed findings likely point to real differences among some CWS on autonomic as well as other factors.
In summary, the role of emotional/temperamental factors in the development of stuttering is not well understood. An obvious obstacle to work in this area is the difficulty in developing behavioral or physiological protocols that provide reliable and valid measures of emotional factors in preschool children. In our laboratory, we use the KiddyCAT (Vanryckeghem & Brutten, 2006) to assess preschoolers' attitudes toward their speech combined with the information provided by the Children's Behavior Questionnaire as an index of their temperament on the basis of their parents' knowledge. We also have been working on protocols to record skin conductance and blood pulse volume signals in preschool children. It is extremely difficult to obtain meaningful measures of autonomic arousal in these youngsters. The recommended approach is to obtain baseline autonomic measures during well-controlled rest periods (e.g., Martin & Venables, 1981). These resting, baseline measures become the standard reference for subsequent tasks in which the subject becomes active. It is straightforward to subtract the resting autonomic levels to determine task-related increases in arousal, thus calibrating the task-related arousal relative to the individual's resting levels of autonomic activity. A well-controlled “rest” period analogous to one obtained in older children and adults (typical rest periods are several minutes as autonomic signals return to baseline very slowly) is not possible in preschoolers. One possible solution to the issue of a resting baseline measure is to use repeated-measures designs to compare task effects between groups of CWS and CWNS. As is the case in the motor and language domains, future studies presumably will continue to solve these problems, and the links between patterns of emotional development and the emergence of stuttering will be better understood.
In conclusion, motor and language factors, and their interaction, play a critical role in the development of stuttering. Emotional/temperamental factors clearly are significant in stuttering in older children and adults; their role is less well understood in relation to the onset and emergence of stuttering. However, recent work (Vanryckeghem et al., 2005; Zengin-Bolatkale et al., 2015) using both behavioral and physiological assays suggests that temperamental/emotional factors may be significant in the early years of stuttering, and we expect that this is an area that will receive much more experimental attention in the future.
Central Neural Aspects of Stuttering
Our understanding of the factors underlying chronic stuttering has been transformed by major advances over the past two decades in specifying the central neural correlates of stuttering in AWS (Neef, Anwander, & Friederici, 2015). Clear regions of interest for stuttering are left premotor and motor areas that are typically specialized for speech motor planning and production. Anatomical studies using diffusion tensor imaging in AWS have been used to measure the fractional anisotropy (FA, an index reflecting the fiber coherence or efficiency of conduction of white matter tracts) of the tracts connecting the left premotor area to primary motor areas involved in articulation. Results of these studies suggest deficits in connectivity between these critical areas for speech production and in perisylvian tracts important for auditory/motor integration in individuals who stutter (Chang, Horwitz, Ostuni, Reynolds, & Ludlow, 2011; Cykowski, Fox, Ingham, Ingham, & Robin, 2010; Sommer, Koch, Paulus, Weiller, & Büchel, 2002). A recent study examined the frontal aslant tract, which connects the inferior frontal gyrus with the supplementary motor area and the pre–supplementary motor area and found evidence of atypical structure of this important part of the motor pathway for speech bilaterally in AWS (Kronfeld-Duenias, Amir, Ezrati-Vinacour, Civier, & Ben-Shachar, 2016). Additional evidence has suggested reduced connectivity in AWS in the corpus callosum, the basal ganglia, corticospinal tracts, and the cerebellum (Connally, Ward, Howell, & Watkins, 2014; K. E. Watkins, Smith, Davis, & Howell, 2008), implicating widespread neural anomalies in stuttering.
Studies using functional imaging techniques consistently show that AWS have reduced activity in left hemisphere areas specialized for speech and that they overactivate homologous areas of the right hemisphere (Braun et al., 1997; Chang, Kenney, Loucks, & Ludlow, 2009; Fox et al., 1996). It has been hypothesized that the right hemisphere overactivation arises as an attempt to compensate for the structural and functional deficits in the left premotor and primary motor speech areas (Kell et al., 2009; Preibisch et al., 2003). Using magnetoencephalography to study the sequence of activation of multiple brain areas during single word reading, Salmelin, Schnitzler, Schmitz, and Freund (2000) found the expected sequence of brain activity in fluent controls; activation moved from posterior visual processing areas to left inferior frontal areas for motor programming and finally to the primary motor areas involved in speech production. This sequence was not observed in AWS, such that they activated primary motor areas during the earliest processing phase and before motor programming areas.
Atypical CNS functioning is not limited to speech production tasks in AWS. Chang et al. (2009) assessed if neural activity measured by the blood-oxygen-level dependent response of AWS differed from controls during planning and execution of speech and nonspeech oral gestures. They reported widespread brain activation differences in the two groups, including in left superior temporal gyrus and premotor areas and bilateral Heschl's gyrus, insula, putamen, and precentral motor regions. These atypical activation patterns were present in AWS for both speech and nonspeech oral tasks.
Differences in brain activity patterns between AWS and adults who do not stutter can also be observed in speech perception tasks with both auditory and visual linguistic stimuli (Biermann-Ruben, Salmelin, & Schnitzler, 2005; Cuadrado & Weber-Fox, 2003; Halag-Milo et al., 2016; Weber-Fox, 2001, Weber-Fox & Hampton, 2008; Weber-Fox et al., 2004). Using magnetoencephalography, Biermann-Ruben et al. (2005) observed differences in brain activation in the left inferior frontal and right Rolandic regions in AWS compared with fluent adults in a language perception task that required no speech production. A recent functional MRI study reported differences in the blood-oxygen-level dependent signals for speech perception for AWS compared with fluent adults in the right inferior frontrol gyrus and left Heschl's gyrus (Lu et al., 2016). In a series of studies utilizing measures of ERPs (computed from an electroencephalogram that is time locked to the onset of a stimulus), Weber-Fox and colleagues have reported differences in neural functions in AWS for specific aspects of semantic, syntactic, and phonological processing (Cuadrado & Weber-Fox, 2003; Weber-Fox, 2001; Weber-Fox & Hampton, 2008; Weber-Fox et al., 2004; reviewed above in section on language).
It is obvious that it is critical to map the developmental trajectories of atypical CNS structural and functional characteristics related to stuttering. This has been the motivation for increased experimental attention to stuttering in preschool and school-age children. There are few neuroimaging studies of CWS, and the results are in some cases consistent and in other cases inconsistent with earlier observations in AWS. Chang, Erickson, Ambrose, Hasegawa-Johnson, and Ludlow (2008) compared gray matter volume and used diffusion tensor imaging to assess the integrity of white matter tracts in groups of eight children with persistent stuttering, seven children who had recovered from stuttering, and seven who never stuttered (boys ages 9–12 years). Their results revealed deficits in the growth of white matter tracts in the oral motor regions on the left and reduced gray matter development in the left inferior frontal region (Broca's area) for both persistent and recovered CWS compared with typically developing controls. The reduced white matter integrity in tracts connecting left premotor and motor speech areas were also reported in AWS (e.g., Sommer et al., 2002), and the left reduced gray matter volume contrasts with earlier observations for adults (Jäncke, Hänggi, & Steinmetz, 2004). In a study of 11 CWS, ages 6–12 years, and 11 matched controls, Beal, Gracco, Brettschneider, Kroll, and De Nil (2013) reported widespread gray and white matter abnormalities in CNS areas subserving speech motor control, including less gray matter volume bilaterally in the inferior frontal gyri. In another study from this laboratory (Beal et al., 2015), this group reported that the posterior part of Broca's area, which is an integral component of speech motor programming, did not show typical effects of aging in a cross-section of CWS and AWS (ages 6–48 years).
Functional MRI studies of young children performing experimental tasks pose many methodological challenges, but various analytic procedures applied to resting-state fMRI data can yield detailed anatomical information as well as computations of probabilistic functional connectivities among brain regions. In two studies, Chang and colleagues recently reported analyses of resting-state fMRI data from relatively large samples of CWS and fluent controls ages 3 to 10 years (Chang & Zhu, 2013; Chang et al., 2015). Using epochs from the time-varying, resting blood-oxygen-level dependent signals, Chang and Zhu (2013) computed functional correlations for activity between specific brain areas implicated in stuttering in earlier studies of adults. They also used diffusion tensor imaging analyses to map fiber tracts to assess structural connectivity between regions of interest. For CWS compared with controls, they found reduced functional and structural connectivity in the basal ganglia-thalamo-cortical network, a network implicated in the control of self-paced movements. Also consistent with earlier studies of adults, Chang and Zhu found reduced connectivity among networks involved in auditory–motor interactions in CWS (areas included the left posterior superior temporal gyrus, insula, supplementary motor area, and inferior frontal gyrus). They reported preliminary evidence that deficits in the left long-range white matter tracts supporting auditory–motor integration were more pronounced in boys compared with the girls who stutter.
Chang et al. (2015) presented the most comprehensive study to date of white matter neuroanatomical differences in the brains of CWS and controls (ages 3–10 years). They completed whole brain analyses using tract-based spatial statistics to conduct group comparisons on FA (note that FA generally increases over the course of typical development and is decreased in pathological conditions; Chang et al., 2015). They found extensive differences in the CWS in long-range connectivity underlying sensorimotor integration for speech and in the corpus callosum, which supports interhemispheric communication. Given their relatively large sample sizes, Chang et al. (2015) computed correlations for FA values for specific tracts and age of the subjects. It is interesting to note that for the controls they found the expected positive correlations between FA and age for specific fiber tracts, such as those in the left inferior frontal gyrus, suggesting increased neural organization and efficiency with age. In contrast, for this tract in the CWS, there was no correlation between FA and age, suggesting a delayed or interrupted developmental trajectory for this important speech-related brain area. Another intriguing finding of this investigation was a significant negative relationship between FA for specific fiber tracts (e.g., left external capsule, left supramarginal gyrus) and stuttering severity as measured by the Stuttering Severity Instrument–Fourth Edition (Riley & Bakker, 2009), such that greater severity of stuttering was associated with the greatest reductions in FA. Further, correlations between FA and stuttering severity differed for boys and girls who stutter. Sex differences in the CWS in relationships between neural substrates and stuttering behavior is notable, as Chang et al. (2015) point out because girls are much more likely to recover from stuttering than boys.
In conclusion, the “imprint” of stuttering in the brain of an adult with persistent stuttering or of one who has recovered from stuttering is variable (Kell et al., 2009). However, across the many studies using a variety of experimental and analytic approaches, certain brain areas and neural networks are repeatedly implicated in stuttering in adults: left premotor and motor areas specialized for speech motor programming and execution, left perisylvian networks underlying auditory and language functions and their interactions with speech motor areas, the interhemispheric connections of the corpus callosum, and basal ganglia-thalamo-cortical loops. The most recent work in young children, while revealing some differences from earlier findings in adults, also generally implicates the left-dominant, widespread neural network important for the complex integration of neural functions necessary for fluent speech production as a significant factor in early stuttering.
Our Current Approach: The MDP Theory
Stuttering, or childhood onset fluency disorder (DSM-5, APA, 2013), is a neurodevelopmental disorder that begins during the preschool years when emerging neural networks critical for speech motor development produce unstable, aberrant control signals that give rise to SLDs. The occurrences of involuntary disruptions in speech, in turn, produce responses in the child's internal and external milieu at both behavioral and physiological levels. These processes then may have epigenetic influences on the expression of genes involved in the development of speech motor systems. Figure 1 illustrates the central features of our account. As indicated, approximately 80% of children who experience a period of stuttering will recover. In these children, brain adaptations occur that ultimately successfully compensate for the atypical neural activity underlying stuttering disfluencies. In other words, there are neural growth and connectivity changes that lead to more stable speech production networks most likely in areas adjacent to left hemisphere areas evolutionarily “domain relevant for speech” (Karmiloff-Smith, 2007, p. 85) but not yet speech-specific areas, and/or by shifting these functions to analogous right hemisphere regions (Kell et al., 2009). For approximately 20% of children who begin to stutter in the preschool years, brain adaptations are inadequate, and stuttering becomes a long-term, often lifelong problem. For these children, compensatory neural processes are not successful in developing neural connections that support stable, perturbation-resistant speech motor programs. Their speech motor systems remain vulnerable to breakdowns in the face of increasingly complex language demands and to psychosocial pressures in the environment. As the child matures and if stuttering persists, the complex sequences of central neural activity that drive the speech motor and other behaviors characteristic of that child's stuttering become overlearned patterns, interfering with fluent speech production into adolescence and adulthood.
Multifactorial Dynamic Developmental Context
Novel and central to our account of stuttering is primary emphasis on the dynamic developmental context in which stuttering emerges and in which the trajectory to persistence or recovery occurs. Onset and eventual recovery/persistence occur during a developmental time frame that is characterized by dramatically and dynamically changing growth trajectories of many diverse but interactive neural systems. In Figure 2, we show selected growth curves relevant to stuttering during the time when stuttering onset typically occurs. The thicker, black, dotted line indicates the typical age in months of the cumulative proportion of onsets of stuttering. For example, the earliest onset of stuttering is observed at around 24 months, and by 60 months or 5 years, it is rare for children to begin stuttering. The thinner, black, dotted line indicates the probability of recovery from stuttering as a function of age (these plots are estimated timelines on the basis of data from the Illinois Stuttering Project, summarized in Yairi & Ambrose, 2005). The highest rate of recovery occurs early, around 30 months of age, and decreases thereafter. Recovery from stuttering becomes highly unlikely if the child continues to stutter past 7 years of age. Other functions plotted in this graph were selected to illustrate the dramatically changing trajectories of neural connectivities, speech motor, language, and psychosocial development. The emergence of specialized neural networks supporting speech, language, and many other brain functions, including both short- and long-range connections, is influenced by experience during this period. Synapse formation is extremely rapid from birth to about 60 months, when synaptic pruning begins. As a result of extensive pruning, gray matter volume decreases from age 5 to 20 years, and as a general principle, primary motor and sensory areas mature earlier than regions involved in higher cognitive functions (Gogtay et al., 2004). As an example of the time course of developing neural networks, the green line is a plot of the process of synaptogenesis in the prefrontal cortex (adapted from Huttenlocher & Dabholkar, 1997). As this plot suggests, there is an extremely rapid growth of synapses in the frontal cortex ongoing during the period of stuttering onset, and pruning starts at about 5 years of age.
Figure 2.
These continuous line plots are interpolations on the basis of data available from the relevant literature. Gray area: the time window when stuttering onset and the pathways to persistence or recovery typically occur (on the basis of data from Yairi & Ambrose, 2005). Black dotted lines: Thicker line, the cumulative percentage of stuttering onsets as a function of age; thinner line, the probability of recovery as a function of age (Yairi & Ambrose, 2005). Other lines are selected to show developmental trajectories of neural and behavioral processes codeveloping during this window of time. Green curve: Growth of synapses and the beginning of synaptic pruning in the prefrontal cortex (units are synapses/100 μm3; range plotted is 10 to 60 synapses/100 μm3; adapted from Huttenlocher & Dabholkar, 1997). Blue curve: Growth of mean length of utterance (MLU) in morphemes (range one to five morphemes; J. F. Miller & Chapman, 1981; Rice et al., 2010). Yellow curve: Increase in percentage of whole words correct (PWC) a child produces, range approximately 30% to 88% (Rvachew & Brosseau-Lapré, 2012, pp. 240–245). Red curve: Index of the growth in the child's self-regulatory skills in a behavioral inhibition task. As the plot shows, at 36 months, children correctly inhibited their behavioral responses on just 22% of the trials, and children just 1 year older correctly inhibited with 91% accuracy (adapted from L. B. Jones et al., 2003). See text for more detailed explanation. CWS = children who stutter.
Reflecting language and speech growth characteristics during this period, the blue line is a plot of the growth of MLU in morphemes (J. F. Miller & Chapman, 1981; Rice et al., 2010). MLU rises rapidly from approximately one to five morphemes in the time window when stuttering emerges. The percentage of whole words correct a child produces (yellow line) also increases steeply around the time of stuttering onset from approximately 30% at age 25 months to about 88% at 60 months of age (Rvachew & Brosseau-Lapré, 2012, pp. 240–245). These plots indicate the dramatic increases in length and complexity of motor and language demands the child must manage in the period from 20 to 60 months. Last, in Figure 2, as an illustration of the rapid development of emotional/temperamental dimensions, we have plotted a curve indexing the growth in the child's self-regulatory skills (red line). Investigators studying the growth of self-regulatory processes have used a “Simon Says” task (children are instructed to respond to the commands of one toy animal but not to the commands of another) to measure children's ability to inhibit behavioral responses. As the plot shows, at 36 months, children correctly inhibited their behavioral responses on just 22% of the trials, and children just 1 year older correctly inhibited with 91% accuracy (adapted from L. B. Jones, Rothbart, & Posner, 2003).
A critical feature of the MDP account is an emphasis on the heterogeneity of the role of motor, language, and psychosocial factors in determining the course of this disorder in CWS. We have noted that different regions of the brains of individual children show highly asymmetric growth curves; some neural networks may be advanced, and others may be lagging the typical developmental growth curve (Jernigan et al., 2011). Within the current framework, we recognize that individual CWS will show variable growth curves in the domains relevant to stuttering. For example, the literature supports the assertion that CWS can be typical, precocious, or delayed in aspects of language development (N. Ratner, 1997). Our work on speech motor development has demonstrated that CWS, on average, have speech coordination skills lagging those of their peers; however, CWS show the full range of scores so that they also can demonstrate speech motor skills in the normal-to-high range (Walsh et al., 2015). On this point, it is important to note that young children's speech motor processes are inherently highly variable whether the child is stuttering or not (e.g., Smith & Zelaznik, 2004). In a similar manner, we would suggest that there is no single psychosocial profile that will characterize CWS. This is not to suggest that there are not consistent patterns characteristic of the trajectories to persistent stuttering versus recovery from stuttering. For example, our preliminary work suggests that relatively low speech motor coordination consistency is associated with the likelihood of persistence of stuttering (Usler, Smith, & Weber, 2017).
Discussions of heterogeneity in complex, multifactorial disorders naturally lead to the question of whether or not subtypes are present. The possibility of defining useful subtypes of stuttering has been discussed for decades (see review by Yairi, 2007). However, none of the proposed subtyping strategies have been viewed as successful, and none have been widely adopted by researchers or clinicians. Our research has had a primary focus on developing a weighted, multidimensional formula that accurately predicts the probability of persistence or recovery in preschool CWS. In a sense, this represents an effort to define two basic subtypes in early stuttering, eP (eventually persistent) and eR (eventually recovered). Precisely this conceptual approach was used by the Illinois team in a recent article titled “Relation of Motor, Linguistic and Temperament Factors in Epidemiologic Subtypes of Persistent and Recovered Stuttering: Initial Findings” (Ambrose, Yairi, Loucks, Seery, & Throneburg, 2015). Using longitudinal data with measures related to each of these factors (measures in some cases identical to ours, including the Children's Behavior Questionnaire and a measure of articulatory kinematic variability, the spatiotemporal index), they reported promising preliminary results that are consistent with ours (reported above) in the language and motor domains. Also promising is that in the domain of temperament, Ambrose et al. (2015) reported significant differences on negative affectivity scores between eP and eR. Whether one views preschool CWS who will persist versus recover as subtypes, as the Illinois group has, or conceives of them, as we do, as phenotypic outcomes that one wants to be able to predict with reasonable accuracy, a major goal of research now should be to find the factors associated with recovery and persistence.
A Dynamic Mechanistic Perspective on Stuttering
Returning to our call in the introduction for a “dynamic mechanistic” explanation of childhood-onset stuttering, MDP suggests clear experimental directions and hypotheses examining operation of the subsystems involved in developing speech production skills. First, we have argued that stuttering is fundamentally a disorder of sensorimotor processes involved in speech production. A criterion number of SLDs must be present in a child's speech for him or her to be diagnosed as stuttering (Ambrose & Yairi, 1999). Although, on the basis of perceptual evaluations, we can classify SLDs into distinct categories, such as syllable repetition or disrupted phonation, all SLDs have a common source. The signals controlling the spatiotemporal patterns of inhibition/excitation in the groups of motor neurons that control the muscles active for speech are “incorrect” so that the resulting speech output is aberrant.
Evidence we have reviewed in the sections above strongly suggests that these aberrant patterns in the neuromotor control signals to muscles arise in the primary cortical motor areas controlling respiratory, laryngeal, and articulatory muscles. Although our review focused primarily on sensorimotor aspects of the articulatory system in stuttering, many studies have documented atypical patterns of control and coordination in the respiratory and laryngeal systems during speech in AWS (e.g., Peters & Boves, 1988; Smith et al., 1993; Zocchi et al., 1990). Tightly coupled and controlled activity of all three systems is required for fluent speech. We propose that a central element of persistent stuttering is that the individual does not follow the typical path to developing well-organized, stable, and perturbation-resistant speech networks in the left premotor and primary motor areas. In individuals who stutter, the speech motor system may continuously show signs of instability even during fluent productions (e.g., Kleinow & Smith, 2000; Zimmermann, 1980a); however, SLDs occur when the behavior of the dynamic collective moves outside the fluent operating space. Some degree of variability in motor commands to muscles is always present and can be assessed relative to one's maturational stage. Preschool CWS, like CWNS, produce highly variable command signals, but in the case of SLDs, these signals deviate too far from the target spatiotemporal pattern of muscle activation, such that control and coordination parameters of speech output exceed the range that supports what we perceive to be normally fluent speech. In other words, we propose that there is an operating range that the speaker must stay within to continue to produce perceptibly fluent speech. When command signals to muscles deviate outside this range, speech is interrupted, and we perceive SLDs. These suprathreshold events that lead to SLDs can be within a system, for example, a breakdown in tongue–jaw coordination, or between systems, for example, too long a delay between oral opening and voice onset. Increasing linguistic demands produce increases in speech motor variability (Kleinow & Smith, 2000; MacPherson & Smith, 2013). In a similar manner, increases in autonomic arousal during speech lead to increased speech motor variability (Kleinow & Smith, 2006). Thus, SLDs are more likely to occur when linguistic and/or emotional/cognitive demands are higher.
It is interesting to note that our proposal shares features with that of Zimmermann's (1980c) early and influential article titled “Stuttering: A disorder of movement.” On the basis of analyses of articulatory kinematics of disfluent speech of AWS (Zimmermann, 1980b), he suggested that speech motor systems operate within a certain range of variability and that when these ranges are exceeded, stuttering disfluencies occur. In contrast to our hypotheses focused on developing cortical speech neural networks, he proposed (on the basis of general models of motor control dominant at that time) that the underlying mechanism driving the speech motor system outside the normal operating range was an imbalance in the gains of brainstem reflexes. This motivated subsequent work to investigate if AWS showed abnormal responses in brainstem-mediated reflexes arising from cutaneous and stretch receptors that would be activated during speaking (Smith & Luschei, 1983). We, however, found no evidence in support of the hypothesis that AWS had unusually higher or lower gains in oral motor reflexes; rather, our results clearly demonstrated that oral motor reflex responses were highly variable among individuals, both normally fluent adults and AWS.
A critical experimental need is to understand when and how these neural control systems develop atypically in CWS and the steps that aid compensation and recovery. We find very few studies of cortical organization assessed during speech production in young children. Hodgson, Hirst, and Hudson (2016) used functional transcranial Doppler imaging to obtain a general measure of right and left hemispheric blood flow during speech production in normally fluent children ages 3 to 10 years. From these measures, they computed a laterality index for hemispheric activation and reported that left speech lateralization is present by age 3 years. It is notable, however, that 13 of the 38 children tested did not show left lateralized speech-related activity. In the Purdue Stuttering Project, functional near-infrared spectroscopy is being used to assess regional blood flow in school-age CWS (Walsh, 2016). Preliminary results show that compared with controls, CWS have dramatic deactivations in left speech motor areas during speech production.
In addition to the critical need to discover the trajectory of development of left cortical motor and premotor speech areas, it will also be important to characterize peripheral speech motor development in CWS and CWNS. There is ample evidence that stuttering in older children and adults reflects a failure to develop stable, perturbation-resistant speech motor programs. Peripheral measures, such as kinematics and electromyography, can be used to derive indices of the stability of speech motor programs. Using measures of kinematics and electromyography, we can estimate the time course of the development of adult-like speech motor programs (Smith & Zelaznik, 2004; Walsh & Smith 2002; Wohlert & Smith, 2002). The general pattern of change in indices of articulatory coordination variability indicate that extremely rapid development of speech motor programs occurs from ages 4 to 7 years, after which the growth rate significantly slows (Smith & Zelaznik, 2004). We hypothesize that lagging speech motor developmental profiles are a significant risk factor for persistent stuttering. An experimental challenge in this area is to develop indices of speech motor program stability that can be applied to signals recorded during natural speech production and index coordination and consistency of relationships among respiratory, laryngeal, and articulatory systems.
Of course, speech motor systems are interacting with linguistic networks, and another hypothesis motivated by the MDP account is that periods of rapid change in linguistic development, for example, the growth in MLU from approximately 30 to 50 months (see Figure 2), present a significant destabilizing influence on the developing speech motor system. Using measures of speech motor program consistency, future investigations could explore if increasing MLU produces temporary halts or reversals in speech production stability and if children who are stuttering experience unusually destabilizing language/motor interactions during this period. In a similar manner, the extremely rapid rise in self-regulatory skills characteristic of typically developing children in the period from approximately 42 to 55 months (see Figure 2) suggests another logical hypothesis: CWS experience negative emotions when trying to regulate speech production behaviors that are susceptible to involuntary disruption. In turn, emotional arousal could further destabilize speech motor systems via autonomic nervous system activity (Kleinow & Smith, 2006). In general, the MDP encourages experimenters to consider a range of potentially important developmental trajectories across motor, linguistic, and temperamental domains to generate hypotheses about how these developing neural systems could interact in critical ways to either promote speech motor learning or to destabilize or interfere with speech motor learning. In taking these suggested experimental steps to explore interactions across domains, we begin to reassemble the dynamic collective of neural systems engaged in language production and to understand the conditions that lead to the generation of the disruptive neural control signals that we perceive as SLDs.
Clinical Implications of MDP
The MDP leads to clinical insights regarding the diagnosis and treatment of stuttering in young children. On the basis of the convergence of evidence for the roles of multiple factors (e.g., motor, language, emotion) in the onset and course of recovery or persistence in stuttering, a multidimensional assessment is most appropriate to determine the factors that may be contributing to instability in an individual child's speech motor system. For some children, support in the language or emotional areas could potentially facilitate greater fluency. On the basis of the research findings summarized above, a comprehensive assessment for a CWS would include indices of the child's speech motor development, language and phonological proficiencies, and temperamental profile. There are a number of tools for assessing language and temperamental profiles (some examples appear in the review above). However, sensitive assessments of speech motor coordination skill available for clinical testing are lacking. As noted above, there is a need to devise clinically applicable tests of speech motor coordination skills to index speech motor development similar to the kinematically derived spatiotemporal index used in our lab and others. Using behavioral measures, another fruitful avenue may be to test early nonword repetition skills and the predictive value of these scores for ultimate persistence/recovery (Spencer & Weber-Fox, 2014).
From the MDP perspective, treatment approaches for enhancing fluent speech and reducing occurrences of SLDs should begin as early as possible, particularly for children who have a higher risk of persistence. SLDs are motor behaviors that are involuntary and maladaptive, but like any other motor behavior, their repeated occurrence leads from brain adaptation, changes in the underlying neural networks, to formation of neural connections that are not optimized for generating stable speech motor programs necessary for fluent and ultimately effortless speech. Repeated occurrences of SLDs lead to the development of “stable” but maladaptive patterns of neural activity. “Neurons that fire together wire together” (Hebb's Law; Hebb, 1949), so that (as reviewed in sections above) older CWS and AWS show widespread atypical structure and function in neural networks involved in language processing and speech production. Once these atypical neural patterns are well established in later childhood and adolescence, the speech motor patterns underlying stuttering are notoriously difficult to alter or eliminate. The ultimate maladaptive end point of this process is the “locking” of the speech motor system into tremor oscillations observed in a significant portion of older CWS and AWS (Kelly et al., 1995; Smith, 1989). Therefore, a “wait and see” approach for the children who are at high risk for chronic stuttering is not optimal. As can be seen in Figure 2, the onset and greater likelihood of recovery from stuttering fall within a specific neurodevelopmental time window in which rapid and dramatic growth occurs in speech and language capabilities and emotional regulation. Therefore, a good strategy would be to take advantage of the high levels of neural plasticity present in this period to optimize connectivity in networks that support fluent speech, concurrently also minimizing SLDs.
One question related to the clinical relevance of MDP is how it relates to earlier, widely used models in the clinic and the classroom. The demands and capacities account (e.g., Starkweather & Gottwald, 1990) is one of the most widely used, especially in communicating with parents of CWS. We believe the MDP is consistent with the basic tenets of demands and capacities. The two accounts “explain” stuttering at very different levels. Depending on the audience and the goals of communicating what causes stuttering, demands and capacities and/or MDP may be useful.
Conclusion
In a nutshell, the MDP asserts that the mechanism that produces stuttering is a failure of the CNS to generate patterns of motor commands necessary for fluent speech to continue. Neural systems that interact with unstable speech motor networks place pressures on the collective system and push it outside the boundaries of fluent operation. The evidence suggests this occurs when there are increased linguistic and/or psychosocial demands. This is why treatments that incorporate an awareness of contributing factors that may help promote fluency are likely to be most effective when coupled with strategies for promoting speech motor coordination that result in fluent productions.
We offer the MDP theory to account for the onset and development of stuttering. This account grows from our earlier multifactorial, nonlinear approaches to stuttering (Smith, 1990, 1999; Smith & Kelly, 1997). Given major advances in available methodologies to study speech behaviors and their neural correlates in young children, we believe considerable progress will be made over the next decades in understanding stuttering and devising new treatment protocols for young children. Critical to the success of these efforts is the interpretation of experimental results arising from many different levels of observation within a coherent theory of the origins of stuttering as a neurodevelopmental disorder.
Acknowledgments
The work from the Purdue Stuttering Project summarized in this article was supported by the National Institute on Deafness and Other Communication Disorders Grant R01 DC00559 first funded in 1988. We express our gratitude to Gerald Zimmermann for his inspired approach to understanding the “causes” of stuttering. We thank David McFarland, Evan Usler, Bridget Walsh, and two anonymous reviewers for very helpful comments on earlier drafts of this article. Finally, our thanks to Soo-Eun Chang for providing the MRI image we used in Figure 1.
Funding Statement
The work from the Purdue Stuttering Project summarized in this article was supported by the National Institute on Deafness and Other Communication Disorders Grant R01 DC00559 first funded in 1988.
References
- Ambrose N., & Yairi E. (1994). The development of awareness of stuttering in preschool children. Journal of Fluency Disorders, 19, 229–245. [Google Scholar]
- Ambrose N. G., & Yairi E. (1999). Normative disfluency data for early childhood stuttering. Journal of Speech, Language, and Hearing Research, 42, 895–909. [DOI] [PubMed] [Google Scholar]
- Ambrose N. G., Yairi E., Loucks T. M., Seery C. H., & Throneburg R. (2015). Relation of motor, linguistic, and temperament factors in epidemiologic subtypes of persistent and recovered stuttering: Initial findings. Journal of Fluency Disorders, 45, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Anderson J. D., & Conture E. G. (2000). Language abilities of children who stutter: A preliminary study. Journal of Fluency Disorders, 25, 283–304. [Google Scholar]
- Anderson J. D., Pellowski M. W., & Conture E. G. (2005). Childhood stuttering and dissociations across linguistic domains. Journal of Fluency Disorders, 30, 219–253. [DOI] [PubMed] [Google Scholar]
- Anderson J. D., Pellowski M. W., Conture E. G., & Kelly E. M. (2003). Temperamental characteristics of young children who stutter. Journal of Speech, Language, and Hearing Research, 46, 1221–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D., Wagovich S. A., & Hall N. E. (2006). Nonword repetition skills in young children who do and do not stutter. Journal of Fluency Disorders, 31, 177–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankson N. W., & Bernthal J. E. (1990). Bankson-Bernthal Test of Phonology. Austin, TX: Pro-Ed. [Google Scholar]
- Bassingthwaighte J. B., Liebovitch L. S., & West B. J. (1994). Fractal physiology. New York, NY: Oxford University Press. [Google Scholar]
- Bauman J., Hall N. E., Wagovich S. A., Weber-Fox C. M., & Ratner N. B. (2012). Past tense marking in the spontaneous speech of preschool children who do and do not stutter. Journal of Fluency Disorders, 37, 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal D. S., Gracco V. L., Brettschneider J., Kroll R. M., & De Nil L. F. (2013). A voxel-based morphometry (VBM) analysis of regional grey and white matter volume abnormalities within the speech production network of children who stutter. Cortex, 49, 2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal D. S., Lerch J. P., Cameron B., Henderson R., Gracco V. L., & De Nil L. F. (2015). The trajectory of gray matter development in Broca's area is abnormal in people who stutter. Frontiers in Human Neuroscience, 9, 89 https://doi.org/10.3389/fnhum.2015.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel W., & Abrahamsen A. (2010). Dynamic mechanistic explanation: Computational modeling of circadian rhythms as an exemplar for cognitive science. Studies in History and Philosophy of Science Part A, 41, 321–333. [DOI] [PubMed] [Google Scholar]
- Bechtel W., & Richardson R. C. (1993). Discovering complexity: Decomposition and localization as strategies in scientific research. Princeton, NJ: Princeton University Press. [Google Scholar]
- Beer R. D. (2000). Dynamical approaches to cognitive science. Trends in Cognitive Science, 4, 91–99. [DOI] [PubMed] [Google Scholar]
- Biermann-Ruben K., Salmelin R., & Schnitzler A. (2005). Right rolandic activation during speech perception in stutters: A MEG study. NeuroImage, 25, 793–801. [DOI] [PubMed] [Google Scholar]
- Bloodstein O. (1995). A handbook on stuttering (5th ed.). San Diego, CA: Plural. [Google Scholar]
- Bloodstein O., & Ratner N. B. (2008). A handbook on stuttering (6th ed.). New York, NY: Thomson-Delmar. [Google Scholar]
- Bowers A., Saltuklaroglu T., & Kalinowski J. (2012). Autonomic arousal in adults who stutter prior to various reading tasks intended to elicit changes in stuttering frequency. International Journal of Psychophysiology, 83, 45–55. [DOI] [PubMed] [Google Scholar]
- Braun A. R., Varga M., Stager S., Schulz G., Selbie S., Maisog J. M., … Ludlow C. L. (1997). Altered patterns of cerebral activity during speech and language production in developmental stuttering: An H2 15O positron emission tomography study. Brain, 120, 761–784. [DOI] [PubMed] [Google Scholar]
- Brown T. T., & Jernigan J. L. (2012). Brain development during the preschool years. Neuropsychology Review, 22, 313–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S., Beal D. S., Ghosh S. S., Tiede M. K., Guenther F. H., & Perkell J. S. (2012). Weak responses to auditory feedback perturbation during articulation in persons who stutter: Evidence for abnormal auditory-motor transformation. PLoS One, 7, e41830 https://doi.org/10.1371/journal.pone.0041830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. E., Erickson K. I., Ambrose N. G., Hasegawa-Johnson M. A., & Ludlow C. L. (2008). Brain anatomy differences in childhood stuttering. NeuroImage, 39, 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. E., Horwitz B., Ostuni J., Reynolds R., & Ludlow C. L. (2011). Evidence of left inferior frontal-premotor structural and functional connectivity deficits in adults who stutter. Cerebral Cortex, 21, 2507–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. E., Kenney M. K., Loucks T. M., & Ludlow C. L. (2009). Brain activation abnormalities during speech and non-speech in stuttering speakers. NeuroImage, 46, 201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. E., & Zhu D. C. (2013). Neural network connectivity differences in children who stutter. Brain, 136, 3709–3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. E., Zhu D. C., Choo A. L., & Angstadt M. (2015). White matter neuroanatomical differences in young children who stutter. Brain, 138, 694–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D., Conture E. G., Walden T. A., Lambert W. E., & Tumanova V. (2013). Behavioral inhibition and childhood stuttering. Journal of Fluency Disorders, 38, 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. E., Conture E. G., Frankel C. B., & Walden T. A. (2012). Communicative and psychological dimensions of the KiddyCAT. Journal of Communication Disorders, 45, 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch D., Grossi G., Skendzel W., & Neville H. (2005). ERP nonword rhyming effects in children and adults. Journal of Cognitive Neuroscience, 17, 168–182. [DOI] [PubMed] [Google Scholar]
- Connally E. L., Ward D., Howell P., & Watkins K. E. (2014). Disrupted white matter in language and motor tracts in developmental stuttering. Brain and Language, 131, 25–35. [DOI] [PubMed] [Google Scholar]
- Coulter C. E., Anderson J. D., & Conture E. G. (2009). Childhood stuttering and dissociations across linguistic domains: A replication and extension. Journal of Fluency Disorders, 34, 257–278. https://doi.org/10.1016/j.jfludis.2009.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado E. M., & Weber-Fox C. M. (2003). Atypical syntactic processing in individuals who stutter: Evidence from event-related brain potentials and behavioral measures. Journal of Speech, Language, and Hearing Research, 46, 960–976. [DOI] [PubMed] [Google Scholar]
- Cykowski M. D., Fox P. T., Ingham R. J., Ingham J. C., & Robin D. A. (2010). A study of the reproducibility and etiology of diffusion anisotropy differences in developmental stuttering: A potential role for impaired myelination. NeuroImage, 52, 1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker R. W., & Decker B. B. (1991). Mountains of fire: The nature of volcanoes. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Dixon W. E. Jr., & Hull Smith P. (2000). Links between early temperament and language acquisition. Merrill-Palmer Quarterly, 46, 417–440. [Google Scholar]
- Eggers K., De Nil L. F., & Van den Bergh B. R. H. (2010). Temperament dimensions in stuttering and typically developing children. Journal of Fluency Disorders, 35, 355–372. [DOI] [PubMed] [Google Scholar]
- Embrechts M., Ebben H., Franke P., & van de Poel C. (2000). Temperament: A comparison between children who stutter and children who do not stutter. In Bosshardt H. G., Yaruss J. S., & Peters H. F. M. (Eds.), Proceedings of the third world congress on fluency disorders: Theory, research, treatment, and self-help (pp. 557–562). Nijmegen, the Netherlands: University of Nijmegen Press. [Google Scholar]
- Felsenfeld S., Kirk K. M., Zhu G., Statham D. J., Neale M. C., & Martin N. G. (2000). A study of the genetic and environmental etiology of stuttering in a selected twin sample. Behavior Genetics, 30, 359–366. [DOI] [PubMed] [Google Scholar]
- Fibiger S. (1971). Stuttering explained as a physiological tremor. Speech Transmission Lab Quarterly Progress and Status Report, 2(3), 1–24. [Google Scholar]
- Fox P. T., Ingham R. J., Ingham J. C., Hirsch T. B., Downs J. H., Martin C., … Lancaster J. L. (1996, July 11). A PET study of the neural systems of stuttering. Nature, 382, 158–161. [DOI] [PubMed] [Google Scholar]
- Friederici A. D. (2011). The brain basis of language processing: From structure to function. Physiological Reviews, 91, 1357–1392. [DOI] [PubMed] [Google Scholar]
- Friederici A. D., & Frisch S. (2000). Verb argument structure processing: The role of verb-specific and argument-specific information. Journal of Memory and Language, 43, 476–507. [Google Scholar]
- Gaines N. D., Runyan C. M., & Meyers S. C. (1991). A comparison of young stutterers' fluent versus stuttered utterances on measures of length and complexity. Journal of Speech and Hearing Research, 34, 37–42. [DOI] [PubMed] [Google Scholar]
- Garello V., Viterbori P., & Usai M. C. (2012). Temperamental profiles and language development: A replication and an extension. Infant Behavior & Development, 35, 71–82. [DOI] [PubMed] [Google Scholar]
- Glass L., & Mackey M. C. (1988). From clocks to chaos. Princeton, NJ: Princeton University Press. [Google Scholar]
- Gogtay N., Giedd J. N., Lusk L., Hayashi K. M., Greenstein D., Vaituzis A. C., … Thompson P. M. (2004). Dynamic mapping of human cortical development during childhood though early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea A. C., Phillips C., Kazanina N., & Poeppel D. (2010). The linguistic processes underlying the P600. Language and Cognitive Processes, 25, 149–188. [Google Scholar]
- Gregg B. A., & Scott M. (2015). Comparison of acoustic startle response in school-age children who stutter and their fluent peers. Procedia – Social and Behavioral Sciences, 193, 115–122. [Google Scholar]
- Grossi G., Coch D., Coffey-Corina S., Holcomb P. J., & Neville H. J. (2001). Phonological processing in visual rhyming: A developmental ERP study. Journal of Cognitive Neuroscience, 13, 610–625. [DOI] [PubMed] [Google Scholar]
- Guntupalli V. K., Kalinowski J., Nanjundeswaran C., Saltuklaroglu T., & Everhart D. E. (2006). Psychophysiological responses of adults who do not stutter while listening to stuttering. International Journal of Psychophysiology, 62, 1–8. [DOI] [PubMed] [Google Scholar]
- Hakim H. G., & Ratner N. B. (2004). Nonword repetition abilities of children who stutter: An exploratory study. Journal of Fluency Disorders, 29, 179–199. [DOI] [PubMed] [Google Scholar]
- Halag-Milo T., Stoppelman N., Kronfeld-Duenias V., Civier O., Amir O., Ezrati-Vinacour R., & Ben-Shachar M. (2016). Beyond production: Brain responses during speech perception in adults who stutter. NeuroImage: Clinical, 11, 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K. D., Amir O., & Yairi E. (1999). A longitudinal investigation of speaking rate in preschool children who stutter. Journal of Speech, Language, and Hearing Research, 42, 1367–1377. [DOI] [PubMed] [Google Scholar]
- Hampton Wray A., & Weber-Fox C. (2013). Specific aspects of cognitive and language proficiency account for variability in neural indices of semantic and syntactic processing in children. Developmental Cognitive Neuroscience, 5, 149–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D. O. (1949). The organization of behavior: A neuropsychological theory. New York, NY: Wiley. [Google Scholar]
- Hilger A., Zelaznik H. N., & Smith A. (2016). Evidence that a motor timing deficit is not a factor in the development of stuttering. Journal of Speech, Language, and Hearing Research, 59, 674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J. C., Hirst R. J., & Hudson J. M. (2016). Hemispheric speech lateralisation in the developing brain is related to motor praxis ability. Developmental Cognitive Neuroscience, 22, 9–17. https://doi.org/10.1016/j.dcn.2016.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcomb P. J., Coffey S. A., & Neville H. J. (1992). Visual and auditory sentence processing: A developmental analysis using event-related brain potentials. Developmental Neuropsychology, 8, 203–241. [Google Scholar]
- Howell P. (2004). Assessment of some contemporary theories of stuttering that apply to spontaneous speech. Contemporary Issues in Communication Science and Disorders, 31, 122–139. [PMC free article] [PubMed] [Google Scholar]
- Howie P. M. (1981). Concordance for stuttering in monozygotic and dizygotic twin pairs. Journal of Speech and Hearing Research, 24, 317–321. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. R., & Dabholkar A. S. (1997). Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology, 387, 167–178. [DOI] [PubMed] [Google Scholar]
- Ingham R. J., Cordes A. K., Ingham J. C., & Gow M. L. (1995). Identifying the onset and offset of stuttering events. Journal of Speech and Hearing Research, 38, 315–326. [DOI] [PubMed] [Google Scholar]
- Iverach L., Menzies R. G., O'Brian S., Packman A., & Onslow M. (2011). Anxiety and stuttering: Continuing to explore a complex relationship. American Journal of Speech-Language Pathology, 20, 221–232. [DOI] [PubMed] [Google Scholar]
- Iverach L., & Rapee R. M. (2014). Social anxiety disorder and stuttering: Current status and future directions. Journal of Fluency Disorders, 40, 69–82. [DOI] [PubMed] [Google Scholar]
- Jäncke L., Hänggi J., & Steinmetz H. (2004). Morphological brain differences between adult stutterers and non-stutterers. BMC Neurology, 4, 23 https://doi.org/10.1186/1471-2377/4/23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T. L., Baaré W. F. C., Stiles J., & Skak Madsen K. (2011). Postnatal brain development: Structural imaging of dynamic neurodevelopmental processes. In Braddick O., Atkinson J., & Innocenti G. M. (Eds.), Progress in Brain Research, Vol. 189 (pp. 77–92). Burlington, MA: Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W., Boehmler R., Dahlstrom W., Darley F., Goodstein L., Kools J., & Young M. (1959). The onset of stuttering. Minneapolis, MN: University of Minnesota Press. [Google Scholar]
- Jones L. B., Rothbart M. K., & Posner M. I. (2003). Development of executive attention in preschool children. Developmental Science, 6, 498–504. [Google Scholar]
- Jones R., Choi D., Conture E., & Walden T. (2014). Temperament, emotion and childhood stuttering. Seminars in Speech and Language, 35, 114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. M., Buhr A. P., Conture E. G., Tumanova V., Walden T. A., & Porges S. W. (2014). Autonomic nervous system activity of preschool-age children who stutter. Journal of Fluency Disorders, 41, 12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi-Hanifi K., & Howell P. (1992). Syntactic analysis of the spontaneous speech of normally fluent and stuttering children. Journal of Fluency Disorders, 17, 151–170. [Google Scholar]
- Kaplan D. M., & Bechtel W. (2011). Dynamical models: An alternative or complement to mechanistic explanations. Topics in Cognitive Science, 3, 438–444. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. (2007). Atypical epigenesist. Developmental Science, 10, 84–88. [DOI] [PubMed] [Google Scholar]
- Karniol R. (1995). Stuttering, language, and cognition: A review and a model of stuttering as suprasegmental sentence plan alignment (SPA). Psychological Bulletin, 117, 104–124. [DOI] [PubMed] [Google Scholar]
- Karrass J., VanDeventer M. C., & Braungart-Rieker J. M. (2003). Predicting shared parent-child book reading in infancy. Journal of Family Psychology, 17, 134–146. [PubMed] [Google Scholar]
- Kell C. A., Neumann K., von Kriegstein K., Posenenske C., von Gudenberg A. W., Euler H., & Giraud A. L. (2009). How the brain repairs stuttering. Brain, 132, 2747–2760. [DOI] [PubMed] [Google Scholar]
- Kelly E. M., Smith A., & Goffman L. (1995). Orofacial muscle activity of children who stutter: A preliminary study. Journal of Speech and Hearing Research, 38, 1025–1036. [DOI] [PubMed] [Google Scholar]
- Kleinow J., & Smith A. (2000). Influences of length and syntactic complexity on the speech motor stability of the fluent speech of adults who stutter. Journal of Speech, Language, and Hearing Research, 43, 548–559. [DOI] [PubMed] [Google Scholar]
- Kleinow J., & Smith A. (2006). Potential interactions among linguistic, autonomic, and motor factors in speech. Developmental Psychobiology, 48, 275–287. [DOI] [PubMed] [Google Scholar]
- Kronfeld-Duenias V., Amir O., Ezrati-Vinacour R., Civier O., & Ben-Shachar M. (2016). The frontal aslant tract underlies speech fluency in persistent developmental stuttering. Brain Structure and Function, 221, 365–381. [DOI] [PubMed] [Google Scholar]
- Kutas M., & Federmeier K. D. (2011). Thirty years and counting: Finding meaning in the N400 component of the event-related brain potential (ERP). Annual Review of Psychology, 62, 621–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R. K., & Giedd J. N. (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Behavioral Reviews, 30, 718–729. [DOI] [PubMed] [Google Scholar]
- Lewis M. D. (2000). The promise of dynamic systems approaches for an integrated account of human development. Child Development, 71, 36–43. [DOI] [PubMed] [Google Scholar]
- Leonard L. B. (2014). Children with specific language impairment. (2nd ed.) Cambridge, MA: MIT Press. [Google Scholar]
- Logan K. J., & Conture E. G. (1997). Selected temporal, grammatical, and phonological characteristics of conversational utterances produced by children who stutter. Journal of Speech, Language, and Hearing Research, 40, 107–120. [DOI] [PubMed] [Google Scholar]
- Lu C., Long Y., Zheng L., Shi G., Liu L., Ding G., & Howell P. (2016). Relationship between speech production and speech perception in people who stutter. Frontiers in Human Neuroscience, 10, 224 https://doi.org/10.3389/fnhum.2016.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck S. J. (2005). An introduction to the event-related potential technique. Cambridge, MA: MIT Press. [Google Scholar]
- MacPherson M. K., & Smith A. (2013). Influences of sentence length and syntactic complexity on the speech motor control of children who stutter. Journal of Speech, Language, and Hearing Research, 56, 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I., & Venables P. H. (Eds.). (1981). Techniques in psychophysiology. Chichester, United Kingdom: Wiley. [Google Scholar]
- Max L., Caruso A. J., & Gracco V. L. (2003). Kinematic analyses of speech, orofacial nonspeech, and finger movements in stuttering and nonstuttering adults. Journal of Speech, Language, and Hearing Research, 46, 215–232. [DOI] [PubMed] [Google Scholar]
- Max L., Guenther F. H., Gracco V. L., Ghosh S. S., & Wallace M. E. (2004). Unstable or insufficiently activated internal models and feedback-biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemporary Issues in Communication Science and Disorders, 31, 105–122. [Google Scholar]
- McClean M., Goldsmith H., & Cerf A. (1984). Lower-lip EMG and displacement during bilabial disfluencies in adult stutterers. Journal of Speech and Hearing Research, 27, 342–349. [DOI] [PubMed] [Google Scholar]
- McDevitt S. C., & Carey W. B. (1978). The measurement of temperament in 3–7 year old children. Journal of Child Psychology and Psychiatry and Allied Disciplines, 19, 245–253. [DOI] [PubMed] [Google Scholar]
- Miller J. F., & Chapman R. S. (1981). The relation between age and mean length of utterance in morphemes. Journal of Speech and Hearing Research, 24, 154–161. [DOI] [PubMed] [Google Scholar]
- Miller P. (2012, January). A thing or two about twins. National Geographic. Retrieved from http://ngm.nationalgeographic.com/print/2012/01/twins/miller-text
- Mohan R., & Weber C. (2015). Neural systems mediating processing of sound units of language distinguish recovery versus persistence in stuttering. Journal of Neurodevelopmental Disorders, 7, 28 https://doi.org/10.1186/s11689-015-9124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle R., Trentacoste S. V., & Rapin I. (2004). The genetics of autism. Pediatrics, 113, 472–486. [DOI] [PubMed] [Google Scholar]
- Neef N. E., Anwander A., & Friederici A. D. (2015). The neurological grounding of persistent stuttering: From structure to function. Current Neurology and Neuroscience Reports, 15, 63 https://doi.org/10.1007/s11910-015-0579-4 [DOI] [PubMed] [Google Scholar]
- Neef N. E., Hoang T. N., Neef A., Paulus W., & Sommer M. (2015). Speech dynamics are coded in the left motor cortex in fluent speakers but not in adults who stutter. Brain, 138, 712–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson M. D., & Neilson P. D. (1987). Speech motor control and stuttering: A computational model of adaptive sensory-motor processing. Speech Communication, 6, 325–333. [Google Scholar]
- Nippold M. A. (2002). Stuttering and phonology: Is there an interaction? American Journal of Speech-Language Pathology, 11, 99–110. https://doi.org/10.1044/1058-0360(2002/er01) [Google Scholar]
- Nippold M. A. (2012). Stuttering and language ability in children: Questioning the connection. American Journal of Speech-Language Pathology, 21,183–196. [DOI] [PubMed] [Google Scholar]
- Ntourou K., Conture E. G., & Lipsey M. W. (2011). Language abilities of children who stutter: A meta-analytical review. American Journal of Speech-Language Pathology, 20, 163–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntourou K., Conture E. G., & Walden T. A. (2013). Emotional reactivity and regulation in preschool-age children who stutter. Journal of Fluency Disorders, 38, 260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olander L., Smith A., & Zelaznik H. N. (2010). Evidence that a motor timing deficit is a factor in the development of stuttering. Journal of Speech, Language, and Hearing Research, 53, 876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packman A., Code C., & Onslow M. (2007). On the cause of stuttering: Integrating theory with brain and behavioral research. Journal of Neurolinguistics, 20, 353–362. [Google Scholar]
- Paden E. P., Yairi E., & Ambrose N. G. (1999). Early childhood stuttering II: Initial status of phonological abilities. Journal of Speech, Language, and Hearing Research, 42, 1113–1124. [DOI] [PubMed] [Google Scholar]
- Pakulak E., & Neville H. J. (2010). Proficiency differences in syntactic processing of monolingual native speakers indexed by event-related potentials. Journal of Cognitive Neuroscience, 22, 2728–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H. F. M., & Boves L. (1988). Coordination of aerodynamic and phonatory processes in fluent speech utterances of stutterers. Journal of Speech, Language, and Hearing Research, 31, 352–361. [DOI] [PubMed] [Google Scholar]
- Peters H. F. M., & Hulstijn W. (1984). Stuttering and anxiety: The difference between stutterers and nonstutterers in verbal apprehension and physiologic arousal during the anticipation of speech and non-speech tasks. Journal of Fluency Disorders, 9, 67–84. [Google Scholar]
- Petronis A. (2010, June 10). Epigenetics as a unifying principle in the aetiology of complex traits and diseases. Nature, 465, 721–727. [DOI] [PubMed] [Google Scholar]
- Postma A., & Kolk H. (1993). The covert repair hypothesis: Prearticulatory repair processes in normal and stuttered dysfluencies. Journal of Speech and Hearing Research, 36, 472–487. [PubMed] [Google Scholar]
- Preibisch C., Neumann K., Raab P., Euler H. A., von Gudenberg A. W., Lanfermann H., & Giraud A. L. (2003). Evidence for compensation for stuttering by the right frontal operculum. NeuroImage, 20, 1356–1364. [DOI] [PubMed] [Google Scholar]
- Ratner N. (1997). Stuttering: A psycholinguistic perspective. In Curlee R. & Siegel G. (Eds.), Nature and treatment of stuttering (pp. 99–127). Boston, MA: Allyn & Bacon. [Google Scholar]
- Ratner N. B., & Sih C. C. (1987). Effects of gradual increases in sentence length and complexity on children's dysfluency. Journal of Speech and Hearing Disorders, 52, 278–287. [DOI] [PubMed] [Google Scholar]
- Ratner N. B., & Silverman S. (2000). Parental perceptions of children's communicative development at stuttering onset. Journal of Speech, Language, and Hearing Research, 43, 1252–1263. [DOI] [PubMed] [Google Scholar]
- Reilly S., Onslow M., Packman A., Wake M., Bavin E. L., Prior M., … Ukoumunne O. C. (2009). Predicting stuttering onset by the age of 3 years: A prospective, community cohort study. Pediatrics, 123, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M. L. (2012). Toward epigenetic and gene regulation models of specific language impairment: Looking for links among growth, genes, and impairments. Journal of Neurodevelopmental Disorders, 4, 27 https://doi.org/10.1186/1866-1955-4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M. L., Smolik F., Perpich D., Thompson T., Rytting N., & Blossom M. (2010). Mean length of utterance levels in 6-month intervals for children 3 to 9 years with and without language impairments. Journal of Speech, Language, and Hearing Research, 53, 333–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley G. D., & Bakker K. (2009). Stuttering Severity Instrument–Fourth Edition. Austin, TX: Pro-Ed. [Google Scholar]
- Rothbart M. K., Ahadi S. A., Hershey K. L., & Fisher P. (2001). Investigations of temperament at three to seven years: The Children's Behavior Questionnaire. Child Development, 72, 1394–1408. [DOI] [PubMed] [Google Scholar]
- Rothbart M. K., Sheese B. E., Rueda M. R., & Posner M. I. (2011). Developing mechanisms of self-regulation in early life. Emotion Review, 3, 207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M. D. (1984). Event-related potentials in phonological matching tasks. Brain and Language, 23, 225–240. [DOI] [PubMed] [Google Scholar]
- Rvachew S., & Brosseau-Lapré F. (2012). Developmental phonological disorders: Foundations of clinical practice. San Diego, CA: Plural. [Google Scholar]
- Salmelin R., Schnitzler A., Schmitz F., & Freund H. J. (2000). Single word reading in developmental stutterers and fluent speakers. Brain, 123, 1184–1202. [DOI] [PubMed] [Google Scholar]
- Sato Y., Mori K., Koizumi T., Minagawa-Kawai Y., Tanaka A., Ozawa E., … Mazuka R. (2011). Functional lateralization of speech processing in adults and children who stutter. Frontiers in Psychology, 2, 70 https://doi.org/10.3389/fpsyg.2011.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk K. A., Conture E. G., & Walden T. A. (2007). Reaction to background stimulation of preschool children who do and do not stutter. Journal of Communication Disorders, 40, 129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery C. H., Watkins R. V., Ambrose N., & Throneburg R. (2006, November). Non-word repetition of school-age children who stutter. Paper presented at the American Speech-Language-Hearing Association Convention, Miami, FL. [Google Scholar]
- Slomkowski C. L., Nelson K., Dunn J., & Plomin R. (1992). Temperament and language: Relations from toddlerhood to middle childhood. Developmental Psychology, 28, 1090–1095. [Google Scholar]
- Smith A. (1989). Neural drive to muscles in stuttering. Journal of Speech and Hearing Research, 32, 252–264. [DOI] [PubMed] [Google Scholar]
- Smith A. (1990). Factors in the etiology of stuttering. In Cooper J. A. (Ed.), Research needs in stuttering: Roadblocks and future directions (pp. 39–47). Rockville, MD: American Speech-Language-Hearing Association. [Google Scholar]
- Smith A. (1999). Stuttering: A unified approach to a multifactorial, dynamic disorder. In Bernstein Ratner N. & Healey E. C. (Eds.), Stuttering research and practice: Bridging the gap (pp. 27–44). Hove, United Kingdom: Psychology Press. [Google Scholar]
- Smith A., Denny M., Shaffer L. A., Kelly E. M., & Hirano M. (1996). Activity of intrinsic laryngeal muscles in fluent and disfluent speech. Journal of Speech and Hearing Research, 39, 329–348. [DOI] [PubMed] [Google Scholar]
- Smith A., Goffman L., Sasisekaran J., & Weber-Fox C. (2012). Language and motor abilities of preschool children who stutter: Evidence from behavioral and kinematic indices of nonword repetition performance. Journal of Fluency Disorders, 37, 344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., & Kelly E. (1997). Stuttering: A dynamic, multifactorial model. In Curlee R. & Siegel G. (Eds.), Nature and treatment of stuttering: New directions (pp. 204–217). Boston, MA: Allyn & Bacon. [Google Scholar]
- Smith A., & Luschei E. (1983). Assessment of oral–motor reflexes in stutterers and normal speakers: Preliminary observations. Journal of Speech and Hearing Research, 26, 322–328. [DOI] [PubMed] [Google Scholar]
- Smith A., Luschei E., Denny M., Wood J., Hirano M., & Badylak S. (1993). Spectral analyses of activity of laryngeal and orofacial muscles in stutterers. Journal of Neurology, Neurosurgery, & Psychiatry, 56, 1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Sadagopan N., Walsh B., & Weber-Fox C. (2010). Increasing phonological complexity reveals heighted instability in inter-articulatory coordination in adults who stutter. Journal of Fluency Disorders, 35, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., & Weber C. (2016). Physiological windows onto language, motor, and emotional domains related to speech development in preschool children. In van Lieshout P., Maassen B., & Terband H. (Eds.), Speech motor control in normal and disordered speech: Future developments in theory and methodology. Retrieved from http://www.asha.org
- Smith A., & Zelaznik H. N. (2004). Development of functional synergies for speech motor coordination in childhood and adolescence. Developmental Psychobiology, 45, 22–33. [DOI] [PubMed] [Google Scholar]
- Smits-Bandstra S., De Nil L., & Rochon E. (2006). The transition to increased automaticity during finger sequence learning in adult males who stutter. Journal of Fluency Disorders, 31, 22–42. [DOI] [PubMed] [Google Scholar]
- Sommer M., Koch M. A., Paulus W., Weiller C., & Büchel C. (2002). Disconnection of speech-relevant brain areas in persistent developmental stuttering. The Lancet, 360, 380–383. [DOI] [PubMed] [Google Scholar]
- Spencer C., & Weber-Fox C. (2014). Preschool speech articulation and nonword repetition abilities may help predict eventual recovery or persistence of stuttering. Journal of Fluency Disorders, 41, 32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkweather C. W. (1995). A simple theory of stuttering. Journal of Fluency Disorders, 20, 91–116. [DOI] [PubMed] [Google Scholar]
- Starkweather C. W., & Gottwald S. R. (1990). The demands and capacities model II: Clinical applications. Journal of Fluency Disorders, 15, 143–157. [Google Scholar]
- Stepp N., Chemero A., & Turvey M. T. (2011). Philosophy for the rest of cognitive science. Topics in Cognitive Science, 3, 425–437. [DOI] [PubMed] [Google Scholar]
- Thelen E., & Smith L. B. (1994). A dynamic systems approach to the development of cognition and action. Cambridge, MA: MIT Press. [Google Scholar]
- Usler E., Smith A., & Weber C. (2017). A lag in speech motor coordination is associated with stuttering persistence in young children. Journal of Speech, Language, and Hearing Research, 60, 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usler E., & Weber-Fox C. (2015). Neurodevelopment for syntactic processing distinguishes childhood stuttering recovery versus persistence. Journal of Neurodevelopmental Disorders, 7, 4 https://doi.org/10.1186/1866-1955-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelder T., & Port R. (1995). It's about time: An overview of the dynamical approach to cognition. In Port R. & van Gelder T. (Eds.), Mind as motion: Explorations in the dynamics of cognition (pp. 1–43). Cambridge, MA: MIT Press. [Google Scholar]
- van Lieshout P. (2004). Dynamical systems theory and its application in speech. In Maassen B., Kent R., Peters H., van Lieshout P., & Hulstijn W. (Eds.), Speech motor control in normal and disordered speech (pp. 51–82). Oxford, United Kingdom: Oxford University Press. [Google Scholar]
- Vanryckeghem M., & Brutten G. (2006). KiddyCAT: Communication attitude test for preschool and kindergarten children who stutter. San Diego, CA: Plural. [Google Scholar]
- Vanryckeghem M., Brutten G., & Hernandez L. (2005). The KiddyCAT: A normative investigation of stuttering and nonstuttering preschoolers' speech-associated attitude. Journal of Fluency Disorders, 30, 307–318. [DOI] [PubMed] [Google Scholar]
- Wagovich S. A., Hall N. E., & Clifford B. A. (2009). Speech disruptions in relation to language growth in children who stutter: An exploratory study. Journal of Fluency Disorders, 34, 242–256. [DOI] [PubMed] [Google Scholar]
- Walden T. A., Frankel C. B., Buhr A. P., Johnson K. N., Conture E. G., & Karrass J. M. (2012). Dual diathesis-stressor model of emotional and linguistic contributions to developmental stuttering. Journal of Abnormal Child Psychology, 40, 633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B. (2016, November). Shedding light on stuttering: An fNIRS study of speech production. Paper presented at the annual convention of the American Speech-Language-Hearing Association, Philadelphia, PA. [Google Scholar]
- Walsh B., Mettel K. M., & Smith A. (2015). Speech motor planning and execution deficits in early childhood stuttering. Journal of Neurodevelopmental Disorders, 7, 27 https://doi.org/10.1186/s11689-015-9123-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B., & Smith A. (2002). Articulatory movements in adolescents: Evidence for protracted development of speech motor control processes. Journal of Speech, Language, and Hearing Research, 45, 1119–1133. [DOI] [PubMed] [Google Scholar]
- Walsh B., & Smith A. (2013). Oral electromyography activation patterns for speech are similar in preschoolers who do and do not stutter. Journal of Speech, Language, and Hearing Research, 56, 1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh B., Smith A., & Weber-Fox C. (2006). Short-term plasticity in children's speech motor systems. Developmental Psychobiology, 48, 660–674. [DOI] [PubMed] [Google Scholar]
- Watkins K. E., Smith S. M., Davis S., & Howell P. (2008). Structural and functional abnormalities of the motor system in developmental stuttering. Brain, 131, 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins R. V., & Yairi E. (1997). Language production abilities of children whose stuttering persisted or recovered. Journal of Speech, Language, and Hearing Research, 40, 385–399. [DOI] [PubMed] [Google Scholar]
- Watkins R. V., Yairi E., & Ambrose N. G. (1999). Early childhood stuttering III: Initial status of expressive language abilities. Journal of Speech, Language, and Hearing Research, 42, 1125–1135. [DOI] [PubMed] [Google Scholar]
- Weber C. M., & Smith A. (1990). Autonomic correlates of stuttering and speech assessed in a range of experimental tasks. Journal of Speech and Hearing Research, 33, 690–706. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C. (2001). Neural systems for sentence processing in stuttering. Journal of Speech, Language, and Hearing Research, 44, 814–825. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C., & Hampton A. (2008). Stuttering and natural speech processing of semantic and syntactic constraints on verbs. Journal of Speech, Language, and Hearing Research, 51, 1058–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C., Hampton Wray A., & Arnold H. (2013). Early childhood stuttering and electrophysiological indices of language processing. Journal of Fluency Disorders, 38, 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fox C., Spencer R. M. C., Spruill J. E. III, & Smith A. (2004). Phonologic processing in adults who stutter: Electrophysiological and behavioral evidence. Journal of Speech, Language, and Hearing Research, 47, 1244–1258. [DOI] [PubMed] [Google Scholar]
- Weber-Fox C., Spruill J. E. III, Spencer R., & Smith A. (2008). Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science, 11, 321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. L., & Zebrowski P. M. (1992). Disfluencies in the conversations of young children who stutter: Some answers about questions. Journal of Speech and Hearing Research, 35, 1230–1238. [DOI] [PubMed] [Google Scholar]
- Wingate M. E. (1988). The structure of stuttering: A psycholinguistic analysis. New York, NY: Springer Verlag. [Google Scholar]
- Wohlert A. B., & Smith A. (2002). Developmental change in variability of lip muscle activity during speech. Journal of Speech, Language, and Hearing Research, 45, 1077–1087. [DOI] [PubMed] [Google Scholar]
- Yairi E. (2007). Subtyping stuttering I: A review. Journal of Fluency Disorders, 32, 165–196. [DOI] [PubMed] [Google Scholar]
- Yairi E., & Ambrose N. G. (2005). Early childhood stuttering: For clinicians by clinicians. Austin, TX: Pro-Ed. [Google Scholar]
- Yairi E., & Ambrose N. (2013). Epidemiology of stuttering: 21st century advances. Journal of Fluency Disorders, 38, 66–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yairi E., Ambrose N., & Cox N. (1996). Genetics of stuttering: A critical review. Journal of Speech and Hearing Research, 39, 771–784. [DOI] [PubMed] [Google Scholar]
- Yaruss J. S. (1999). Utterance length, syntactic complexity, and childhood stuttering. Journal of Speech, Language, and Hearing Research, 42, 329–344. [DOI] [PubMed] [Google Scholar]
- Yaruss J. S., LaSalle L. R., & Conture E. G. (1998). Evaluating stuttering in young children: Diagnostic data. American Journal of Speech-Language Pathology, 7(4), 62–76. [Google Scholar]
- Zackheim C. T., & Conture E. G. (2003). Childhood stuttering and speech disfluencies in relation to children's mean length of utterance: A preliminary study. Journal of Fluency Disorders, 28, 115–141. [DOI] [PubMed] [Google Scholar]
- Zelaznik H. N., Smith A., & Franz E. A. (1994). Motor-performance of stutterers and nonstutterers on timing and force control tasks. Journal of Motor Behavior, 26, 340–347. [DOI] [PubMed] [Google Scholar]
- Zengin-Bolatkale H., Conture E. G., & Walden T. A. (2015). Sympathetic arousal of young children who stutter during a stressful picture naming task. Journal of Fluency Disorders, 46, 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann G. (1980a). Articulatory behaviors associated with stuttering: A cineofluorographic analysis. Journal of Speech and Hearing Research, 23, 108–121. [DOI] [PubMed] [Google Scholar]
- Zimmermann G. (1980b). Articulatory dynamics of fluent utterances of stutterers and nonstutterers. Journal of Speech and Hearing Research, 23, 95–107. [DOI] [PubMed] [Google Scholar]
- Zimmermann G. (1980c). Stuttering: A disorder of movement. Journal of Speech and Hearing Research, 23, 122–136. [PubMed] [Google Scholar]
- Zocchi L., Estenne M., Johnston S., Del Ferro L., Ward M. E., & Macklem P. T. (1990). Respiratory muscle incoordination in stuttering speech. The American Review of Respiratory Disease, 141, 1510–1515. [DOI] [PubMed] [Google Scholar]