Abstract
Background
Italian pediatric antimicrobial prescription rates are among the highest in Europe. As a first step in an Antimicrobial Stewardship Program, we implemented a Clinical Pathway (CP) for Community Acquired Pneumonia with the aim of decreasing overall prescription of antibiotics, especially broad-spectrum.
Materials and methods
The CP was implemented on 10/01/2015. We collected antibiotic prescribing and outcomes data from children aged 3 months-15 years diagnosed with CAP from 10/15/2014 to 04/15/2015 (pre-intervention period) and from 10/15/2015 to 04/15/2016 (post-intervention period). We assessed antibiotic prescription differences pre- and post-CP, including rates, breadth of spectrum, and duration of therapy. We also compared length of hospital stay for inpatients and treatment failure for inpatients and outpatients. Chi-square and Fisher’s exact test were used to compare categorical variables and Wilcoxon rank sum test was used to compare quantitative outcomes.
Results
120 pre- and 86 post-intervention clinic visits were identified with a diagnosis of CAP. In outpatients, we observed a decrease in broad-spectrum regimens (50% pre-CP vs. 26.8% post-CP, p = 0.02), in particular macrolides, and an increase in narrow-spectrum (amoxicillin) post-CP. Post-CP children received fewer antibiotic courses (median DOT from 10 pre-CP to 8 post-CP, p<0.0001) for fewer days (median LOT from 10 pre-CP to 8 post-CP, p<0.0001) than their pre-CP counterparts. Physicians prescribed narrow-spectrum monotherapy more frequently than broad-spectrum combination therapy (DOT/LOT ratio 1.157 pre-CP vs. 1.065 post-CP). No difference in treatment failure was reported before and after implementation (2.3% pre-CP vs. 11.8% post-CP, p = 0.29). Among inpatients we also noted a decrease in broad-spectrum regimens (100% pre-CP vs. 66.7% post-CP, p = 0.02) and the introduction of narrow-spectrum regimens (0% pre-CP vs. 33.3% post-CP, p = 0.02) post-CP. Hospitalized patients received fewer antibiotic courses post-CP (median DOT from 18.5 pre-CP to 10 post-CP, p = 0.004), while there was no statistical difference in length of therapy (median LOT from 11 pre-CP to 10 post-CP, p = 0.06). Days of broad spectrum therapy were notably lower post-CP (median bsDOT from 17 pre-CP to 4.5 post-CP, p <0.0001). No difference in treatment failure was reported before and after CP implementation (16.7% pre-CP vs. 15.4% post-CP, p = 1).
Conclusions
Introduction of a CP for CAP in a Pediatric Emergency Department led to reduction of broad-spectrum antibiotic prescriptions, of combination therapy and of duration of treatment both for outpatients and inpatients.
Background
Pneumonia is the single greatest cause of death in children worldwide: 1–4% of the pediatric population is treated every year for community acquired pneumonia (CAP) and 0.1–2% of those children are hospitalized [1–3].
Inpatient healthcare costs associated with CAP are estimated to be more than one billion dollars per year [4].
Inappropriate antibiotic prescribing for CAP has been frequently reported, as many patients receive antibiotics for viral pneumonia or broad-spectrum antibiotics for uncomplicated bacterial pneumonia [5]. The Italian antimicrobial prescription rate is one of highest in the EU (52%) [6], and antibiotic resistance has become a serious health threat with high social costs and severe consequences including prolonged illness, increased length of hospitalization and mortality [6]. Increasing penicillin and macrolide resistance of Streptococcus pneumoniae strains pose an important threat to effective treatment [7]. There is also widespread β-lactamase production in Haemophilus influenzae and macrolide resistance in Streptococcus pyogenes [8].
Thus, it is imperative to reduce improper use of these drugs. Clinical Pathways (CPs) along with educational programs have shown to be a reasonable and feasible first step for Antimicrobial Stewardship Program (ASP) implementation by reducing antibiotic prescriptions in both community and in-hospital settings [9–13].
To date, ASPs have primarily targeted the inpatient setting, and there is a paucity of literature regarding antimicrobial stewardship strategies in the Pediatric Emergency Department (PED), despite the substantial proportion of antibiotics prescribed to children in this setting [14–17]. Since PEDs are uniquely positioned at the interface of inpatient and outpatient settings, PED physicians could have a consistent impact on prescribing trends in both locations.
In the PED setting, challenges include high turnover rates for both patients and practitioners, the need for rapid decision-making, and diagnostic uncertainty in empiric prescription [18].
Since CPs have been effective in reducing antibiotic prescriptions in primary care and in hospital settings, we hypothesized that their implementation in the PED could decrease overall prescription of antibiotics, especially broad-spectrum, for common infectious diseases such as CAP [9–13].
The primary aim of this study was to assess changes in antibiotic prescription before and after CP implementation for CAP in a large Italian PED. Secondary aims were to compare treatment failures before and after CP implementation.
Materials and methods
Study design
The study was set at the PED of the Department for Women and Children Health at Padua University Hospital. Our Children’s Hospital provides primary and secondary care for a metropolitan area of 350,000 people (45,000 younger than 15 years) and tertiary care for a regional and extra-regional population, with approximately 26,000 PED visits per year and an overall hospital admission rate from PED of around 7 out of 100 visits.
From the PED, children with moderate-severe CAP (criteria listed in the CP S1 Fig) are usually admitted to the Pediatric Acute Care Unit (PACU), an acute care unit near the emergency department, which shares the same medical staff.
This is a pre-post quasi-experimental study that assesses the changes in antibiotic prescribing for CAP during a 6-month period preceding CP implementation (pre-intervention, from 15 October 2014 to 15 April 2015) and during the six months after CP implementation (post intervention, from 15 October 2015 to 15 April 2016). The decision to analyse the same period in different years was made in order to limit the effects of seasonality.
Intervention
On 1 October 2015 CPs for the management of CAP were implemented.
The CP is a one-page decision support algorithm designed to assist providers in determining whether an antibiotic should be prescribed, and if so, the optimal agent and duration of therapy.
The CP summarizes international guidelines [1,8] for the diagnosis and treatment of the clinical condition and was developed by the Division of Pediatric Infectious Diseases and Pediatric Emergency Department of Padua in collaboration with the Division of Pediatric Infectious Diseases of the Children’s Hospital of Philadelphia.
Three CP training sessions (two during the first weeks of October and one during the first week of November) were presented to PED and Pediatric Acute Care Unit (PACU) physicians and residents along with an overview of the guidelines, the rationale behind the treatment.
Study population
All patients aged between 3 months and 15 years with International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 485 and 486 at discharge diagnosis or descriptive diagnosis of CAP were included.
Exclusion criteria were: cystic fibrosis or other chronic pulmonary diseases (except for asthma), immunodeficiency or immunosuppressive therapy, sickle cell disease, tracheostomy, patients at risk for aspiration pneumonia, hospitalization during previous 30 days, concomitant infections, ongoing antibiotic therapy.
Participating patients were divided in two groups:
Outpatients: patients evaluated at the PED and discharged;
Inpatients: patients admitted to the PACU.
Data source
All patients with a clinical diagnosis of pneumonia (medical progress notes) or documentation of a chest infiltrate (radiology notes) were included. All clinical, demographic, diagnostic and antimicrobial data were manually collected from electronic medical records, using a password protected REDCap® data collection form and stored in the secure server at the University of Padua.
We considered treatment based on amoxicillin or ampicillin alone narrow-spectrum. Broad-spectrum antimicrobials were defined as: β-lactam and β-lactamase inhibitor combinations, second- and third-generation cephalosporins, clindamycin, glycopeptides, fluoroquinolones and macrolides. Therapeutic regimens including at least one broad-spectrum prescription, despite the association with amoxicillin, were considered broad-spectrum. In line with expert consensus CAP guidelines [1], our CP suggested a dosage of amoxicillin of 90 mg/kg/day divided every 8 hours.
Amoxicillin per os rather than penicillin G is recommended due to its better gastrointestinal absorption and higher levels in blood and lung parenchyma [1,18].
Privacy was guaranteed in two ways: a unique, study specific survey number was assigned to each patient and no personally identifying data were collected.
To evaluate the effectiveness and safety of the intervention, follow up phone calls to the family were made within 30 days to assess for treatment failure, defined as new admission, prescription of a new antibiotic (instead of or in addition to the previous one) for persistence or relapse of symptoms or for drug side effects (eg. rash, diarrhea) within 30 days after discharge.
Admissions for CAP in the same patient occurring greater than 30 days apart were analysed as separate events.
This study was approved by the Institutional Review Board of Department for Woman and Child Health at the University of Padua.
Determination of outcomes
Primary outcome
The following aspects of antibiotic prescriptions for CAP were assessed every month over the six months before and the six months after CP implementation:
Narrow-spectrum prescription rate;
Duration of therapy expressed in Days of therapy (DOT) and Length of Therapy (LOT) [19–21], DOT/LOT ratio, median DOTs of broad-spectrum antibiotics (bsDOTs) and bsDOT/DOT ratio.
Dosage of the most frequently prescribed antibiotics, expressed in mg/kg/day;
Length of hospital stay (LOS) for inpatients.
Secondary outcome
Thirty-day treatment failures investigated through a phone call, defined as: changes in antibiotic prescription for persistence or worsening of symptoms; treatment changes for antibiotic side effects or new antibiotic prescriptions within 30 days from discharge date for relapse of symptoms and mortality.
Data analysis
Results are summarized as frequencies and proportions for categorical variables and as median and range for quantitative variables.
Comparisons of categorical and quantitative variables were conducted with chi-square or Fisher’s exact test and Wilcoxon rank sum test respectively, since the data were not normally distributed (Shapiro-Wilk test). Statistical significance was declared for p ≤0.05. Statistical analysis was conducted with SAS 9.2 (SAS Institute, Inc., Cary, NC) for Windows.
Results
Over the 6-month pre-intervention period, 13,262 children were evaluated in the PED and 12,335 children were seen during the 6-month post-intervention period.
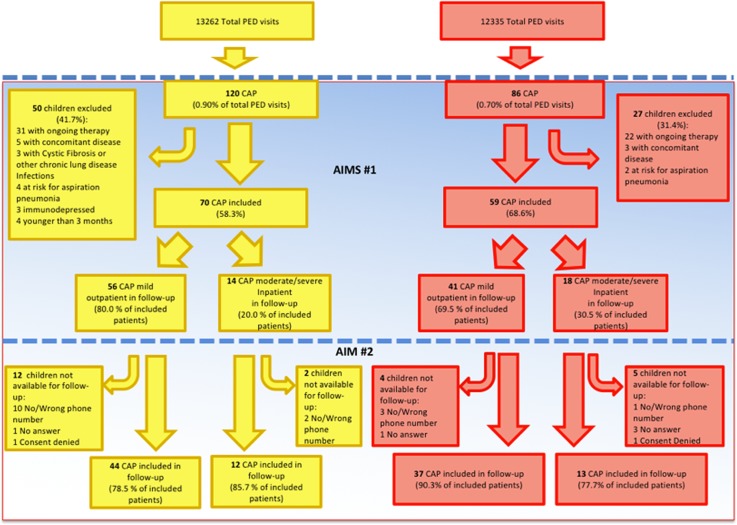
During the pre-intervention period, 120 patients were diagnosed with CAP, accounting for 0.90% (120/13,262) of total PED visits. In the post-intervention period 86/12,335 (0.70%) children were evaluated for CAP. Of these, 70/120 (58.3%) children and 59/86 (68.6%) met the inclusion criteria in the two analysed periods of time (Fig 1).
Fig 1. Flowchart of children enrolled during the pre and post-intervention period.
Characteristics of the studied population
Variables including sex, age and severity were assessed in pre- and post-CP populations. The two groups were similar with respect to sex (p = 0.76): 50.0% (35/70) females pre-intervention and 54.2% (28/59) post-intervention.
Age was stratified into two age ranges: 3 months-5 years and 5 years-15 years. In both groups, the highest prevalence of CAP was reported in the 3 months-5 years years group with 76.8% (43/56) pre and 92.7% (38/41) post-intervention respectively (p = 0.07). The same analysis was performed also for excluded patients with similar results.
For both the inpatient and outpatient groups, there was no statistically significant difference in frequency or proportion of patients diagnosed with Mycoplasma pneumoniae by serology. Proportion of inpatients presenting with hypoxemia and with pleural effusion were similar in the pre- and post-CP groups (Tables 1 and 2).
Table 1. Characteristics of outpatients population.
| Pre-Intervention Period | Post-Intervention Period | p value | ||||
|---|---|---|---|---|---|---|
| Included 0utpatients | 56 (80.0% of included patients) | 41 (69.5% of included patients) | ||||
| N | % | N | % | |||
| Sex | m | 24 | 42.9 | 19 | 46.3 | 0.73 |
| f | 32 | 57.1 | 22 | 53.7 | ||
| Age | 3 mo—5 yr | 43 | 76.8 | 38 | 92.7 | 0.07 |
| 5 yr—15 yr | 13 | 23.2 | 3 | 7.3 | ||
| Mycoplasma Pneumoniae IgM test | performed | 1 | 1.8 | 3 | 7.3 | 0.31 |
| not performed | 55 | 98.2 | 38 | 92.7 | ||
| Number of M. Pneumoniae IgM positive test/number of test performed | 1 | 100 | 0 | 0 | 0.40 | |
Table 2. Characteristics of inpatient population.
| Pre-Intervention Period | Post-Intervention Period | p value | ||||
|---|---|---|---|---|---|---|
| Included Inpatients | 14 (20.0% of included patients) | 18 (30.5% of included patients) | ||||
| N | % | N | % | |||
| Sex | m | 11 | 78.6 | 8 | 44.4 | 0.11 |
| f | 3 | 21.4 | 10 | 55.6 | ||
| Age | 3 mo—5 yr | 12 | 85.8 | 14 | 77.8 | 0.9 |
| 5 yr—15 yr | 2 | 14.2 | 4 | 22.2 | ||
| Mycoplasma Pneumoniae IgM test | performed | 8 | 57.1 | 13 | 72.2 | 0.47 |
| not performed | 6 | 42.9 | 5 | 27.8 | ||
| Number of M. pneumoniae IgM positive test/number of test performed | 3 | 37.5 | 2 | 15.4 | 0.33 | |
| Hypoxia | 8 | 57.1 | 11 | 72.2 | 1 | |
| Pleural effusion | 4 | 28.6 | 3 | 16.7 | 0.67 | |
| Chest Drainage | 1 | 7.1 | 0 | 0 | 0.44 | |
Antibiotic prescription in outpatients
Changes in prevalence of antibiotic prescriptions for CAP
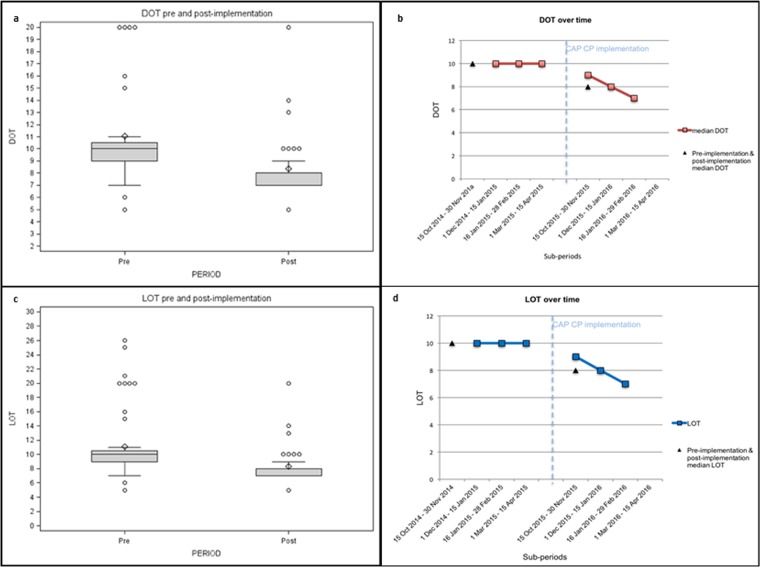
Before implementation 50% of children (28/56) received exclusively amoxicillin, compared with 73.2% (30/41) after CP release. Due to the high prevalence of combination therapy, further analysis on antibiotic prescriptions were performed using Days of Therapy (DOTs) for each patient. The median DOT for the pre-intervention period was 10 (range, 5–26), the median DOT for the post-intervention period was 8 (range, 5–20) (p<0.0001).
The median DOT was calculated for every sub-period of observation (Fig 2A and 2B). DOTs analysis for each antimicrobial reflected the prescriptions prevalence. Statistically significant increase in use of amoxicillin (54.5% pre-CP vs. 71.1% post-CP, p <0.0001) and decrease in use of macrolides (21.3% pre-CP vs. 6.4% post-CP, p <0.0001) was observed. Cephalosporins and amoxicillin-clavulanate use decreased as well (9.7% and 14.5% pre-CP vs. 8.5% and 14.0% post-CP), but the difference was not statistically significant.
Fig 2.
a–b. Median DOT pre and post-implementation for outpatients and DOT over time for outpatients. c. Median LOT pre and post-implementation for outpatients. d. LOT over time for outpatients.
Changes in prevalence of broad-spectrum antibiotic prescriptions for CAP
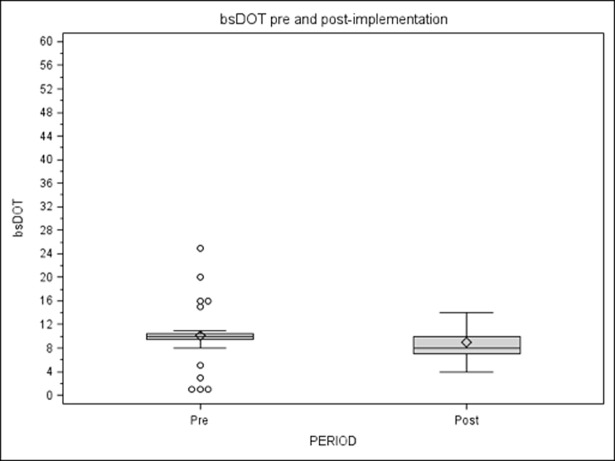
In the pre-intervention period the median bsDOT was 10 (range 1–25) and in the post-intervention period 8 (range 4–14), with a significant and stable difference in prescribing between pre- and post-intervention groups reported for each sub-period in the time series (Fig 3). As a result, pre-intervention bsDOT/DOT was 0.45, while in post-intervention it was 0.29.
Fig 3. bsDOT/DOT for outpatients.
Changes in duration of therapy
For treating mild CAP our clinical pathway recommends a 7-day antibiotic therapy.
Pre-intervention median LOT was 10 (range 3–15), while post-intervention median LOT was 8 (range 5–10) (p<0.0001) as recommended in the CP, with a decreasing trend over all sub-periods after implementation.
DOT/LOT ratio indicates use of combination therapy and the length of therapy: pre-intervention DOT/LOT was 1.16, post-intervention 1.07. Specifically, during the related sub-periods, pre-CP DOT/LOT was included between 1.25 and 1.08, post-CP ratio, instead, ranged from 1.19 to 1.04 (Fig 2C and 2D).
Changes in dosage for the most commonly prescribed antibiotic for CAP
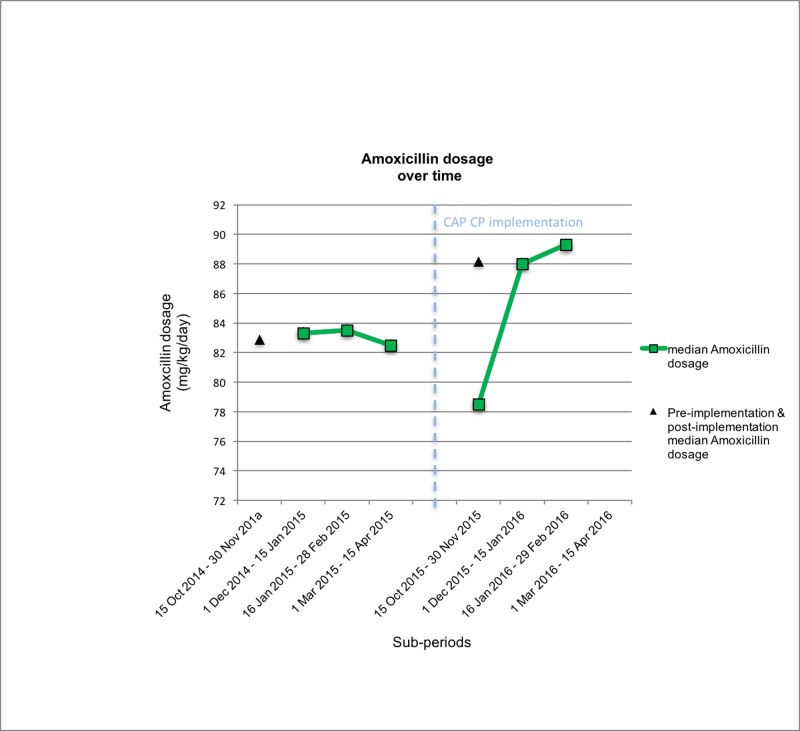
The most commonly prescribed antibiotic for outpatients with CAP was amoxicillin. Pre-intervention median dosage corresponds to 82.9mg/kg/day (range 28.6–102). Post-intervention median dosage was 88.15mg/kg/day (range 64–95.5) (p = 0.03) as recommended in the CP (Fig 4).
Fig 4. Median amoxicillin dosage over time.
Antibiotic prescription in inpatients
Changes in prevalence of antibiotic prescriptions for CAP in inpatients
We observed an increase in narrow-spectrum regimens prescribed in the post-CP period: 6/18 children (33.3%) after CP implementation received exclusively narrow-spectrum antibiotics in contrast with 0% in the pre-intervention period (p = 0.02). Broad-spectrum antibiotics also showed a decreasing trend (14/14 pre-CP, 12/18 post-CP).
Median DOT for the pre-intervention period was 18.5 days (range 11–32), for the post-intervention it was 10 (range 3–26) (p = 0.004).
We reported a statistically significant increase in use of ampicillin and amoxicillin and a concomitant decrease in use of cephalosporins and macrolides. Furthermore, use of amoxicillin-clavulanate, ampicillin-sulbactam, carbapenems and glycopeptides was abandoned. Clindamycin was prescribed only in the post-CP period.
Changes in prevalence of broad-spectrum antibiotic prescriptions for CAP
Broad-spectrum antibiotic use was assessed through median bsDOTs: pre-intervention median bsDOT was 17 (range 11–24) and it decreased to 4.5 (range 1–23) in the post-intervention period (p<0.0001).
Broad-spectrum in relation to overall antibiotic use evaluated through bsDOT/DOT ratio decreased from 0.83 in the pre-CP to 0.41 in the post-CP period (p<0.0001).
Changes in duration of therapy and length of stay
Pre-CP median LOT was 11 days (range 5–17), while post-CP LOT was 10 days (range 3–15) (p = 0.06). Pre-intervention ratio of DOT/LOT, which measures the quantity of antibiotics prescribed per day was 1.70, while post-intervention DOT/LOT was 1.26.
Median LOS in the pre-CP period was 5 days (range 3–16) and in the post-CP period, 4 days (range 2–14) (p = 0.23).
Changes in dosage for the most commonly prescribed antibiotics for CAP
The CAP CP developed for this study recommends ampicillin dosage of 200–300 mg/kg/day. In the pre-CP period, there were no ampicillin prescriptions. In the post-intervention period, 6/18 (16.7%) patients received ampicillin with median dosage of 200 mg/kg/day (range 200–307.7).
The recommended dosage of ceftriaxone in our CAP CP is 50–100 mg/kg/day. Ceftriaxone was prescribed in 8/14 (57.1%) pre-CP patients and 8/18 (44.4%) post-CP. The median dosage was 75 mg/kg/day both pre-intervention (range 38.5–100) and post-intervention (range 75–100).
Treatment failure in outpatients
As a balancing measure, we assessed treatment failure: 44/56 (78.5%) children were available for CAP follow-up in the baseline period, in comparison with 37/41 (90.3%) in post-intervention period.
The two groups were compared for prevalence of prescriptions: amoxicillin and broad spectrum antibiotic prescriptions did not show significant differences before and after CP implementation, though trends for all antibiotics indicated improvement post-intervention (Table 3).
Table 3. Antibiotics prescriptions for outpatients follow-up.
| Pre-intervention period | Post-intervention period | p value | |||
| Number of outpatients available for follow-up | 44 | 34 | |||
| Number of prescriptions | 56 | 39 | |||
| Prescriptions/patients ratio | 1.3 | 1.1 | |||
| N | % | N | % | ||
| Amoxicillin | 28 | 50 | 25 | 64.1 | 0.17 |
| Cephalosporins | 9 | 16.1 | 4 | 10.3 | 0.42 |
| Macrolides | 10 | 17.8 | 3 | 7.7 | 0.16 |
| Amoxicillin-clavulanate | 9 | 16.1 | 7 | 17.9 | 0.97 |
In the pre-CP period, treatment failure occurred in 2.3% (1/44) of cases, while 11.8% (4/34) failed treatment in the post-CP period (p = 0.29). All these cases consist of change of antibiotic for persistence or worsening of symptoms.
Treatment failure in inpatients
Twelve out of 14 (85.7%) parents were available for a follow-up call in the pre-intervention period and 13/18 (72.2%) in the post-intervention period (p = 0.36). In the pre-CP period 83.3% (10/12) of prescribed therapy was effective, with 2/12 cases of antibiotic change during hospitalization for persistence or worsening of symptoms. There was no significant change in the post-CP period, where 84.6% (11/13) of prescribed therapies was effective and for 2/13 patients’ antibiotics were changed for persistence of symptoms.
Discussion
In accordance with the literature, the highest prevalence of CAP was observed in children <5 years of age in our population [22].
Current recommendations, reflected in our CAP CP, indicate that children with a clinical diagnosis of pneumonia should receive antibiotics, as bacterial and viral pneumonia cannot be reliably distinguished from each other [8]. Narrow-spectrum monotherapy (amoxicillin) is the first option for mild CAP in fully immunized children, as S. pneumoniae accounts for 21–44% of disease [23–28].
Use of macrolides is only appropriate if atypical bacterial ethology is suspected, as the use of azithromycin has been associated with the selection of resistant organisms because of its prolonged serum elimination half–life [1,8].
According to other authors, our study showed relevant changes in physicians’ prescribing behaviours for both outpatients and inpatients [16,17]. In contrast to other settings, where significant effects were achieved only during the second year of the intervention, in this case changes took place immediately after CP implementation [11].
During the post-intervention period more narrow-spectrum regimens were prescribed for fewer days.
In our population, macrolide over-prescription in the pre-CP period may have resulted from the perception that two antimicrobials were more reliable, as only one patient was actually infected with Mycoplasma pneumoniae in this period. Indeed, the pre and post populations were similar in terms of number of tests performed to detect M. pneumoniae infection and positive tests (only one in the pre-CP period).
All inpatients were admitted to the PACU for an episode of moderate CAP.
No statistical difference was reported between the two groups before and after implementation with regard to sex, age, symptoms, M. pneumoniae IgM serology positivity and presence of complications (hypoxia, pleural effusion, necrotizing pneumonia).
After CAP CP implementation, prescription of narrow-spectrum regimens (ampicillin or amoxicillin) among inpatients decreased significantly, with a concomitant decrease of broad-spectrum antibiotics. This is in line with most recent recommendations, which suggest high doses of narrow-spectrum β-lactams as the first-line parenteral therapy for moderate CAP if the child is fully immunized or is not admitted for a previous amoxicillin treatment failure. Alternatively, parenteral third generation cephalosporins are recommended [1] when these criteria are not met.
Median DOTs showed a substantial decrease from 18.5 to 10 days in pre- versus post-CP periods, indicating a decrease in the prevalence of antibiotic prescriptions. As reported by Smith et al [17], we saw a significant increase in use of ampicillin and amoxicillin and decrease in cephalosporins and macrolides. This is an important achievement for inpatients: before the CP implementation physicians didn’t even consider ampicillin for moderate CAP treatment in our centre.
Furthermore, use of broad-spectrum antibiotics like amoxicillin-clavulanate, ampicillin-sulbactam, carbapenems and glycopeptides were abandoned after CP implementation in our centre, while clindamycin was prescribed in the post-CP period only in case of complicated pneumonia (parapneumonic effusion, necrotizing pneumonia).
Carbapenems are one of the β-lactams with the broadest antibacterial spectrum currently available, with a relatively low rate of adverse effects. They are recommended as “last-line agents” for severe infections or resistant bacteria, since carbapenems are not destroyed by most β-lactamases [29]. From this study, it emerged that their empiric use was not exclusively for severe nosocomial infections in critically ill patients, but was prescribed as drug of choice for moderate CAP during pre-intervention period, as also reported by other authors [30]. The wide use of these lifesaving drugs is problematic due to the emergence of carbapenem-resistant bacteria which cause severe infections [31–35].
The dramatic change in antimicrobial choices is attributable to the shift in suggested first-line therapy for CAP and, since the starting therapy is established for both outpatients and inpatients by the PED, improvements in PED prescriptions determine improvements in PACU prescriptions, even because the two wards share the same medical staff.
Indeed, starting with penicillin (amoxicillin/ampicillin) gives physicians the possibility to observe children for 48–96 hours and, in case of persistence of symptoms, to switch to a third-generation cephalosporin. On the other hand, physicians starting with ceftriaxone are more prone to change to a broader spectrum antibiotic such as carbapenem.
Furthermore, the introduction of clindamycin recommendations for complicated moderate CAP gave the opportunity to avoid glycopeptides, hence reducing their use.
DOT, LOT and DOT/LOT ratio analysis was performed to describe inpatient prescriptions. Our intervention resulted in a statistically significant decrease in overall median DOT (narrow and broad-spectrum) from 18.5 to 10, as well as bsDOT from 17 to 4.5. LOT median did not significant decrease, despite CAP guidelines recommending 7 days of therapy in case of uncomplicated CAP and 14 days if complicated (parapneumonic effusion, necrotizing pneumonia). This suggests pediatricians are more inclined to change their attitude towards the choice of antibiotic prescribed rather than the duration of therapy.
The increased use of ampicillin/amoxicillin also resulted in a decrease of the median LOS after CP implementation. Indeed, a rapid and uneventful improvement during the first 24–48 hours after ampicillin administration has a favourable impact on switch to oral antibiotic and early discharge [36].
For both outpatient and inpatient populations, no differences in treatment failure were reported despite a remarkable decrease in broad-spectrum antibiotic prescription. During the post-CP period, an increase in treatment failure was reported in outpatients but it was not statistically significant. This may be due to relatively low sample sizes and to the very low occurrence of treatment failure overall, even in the pre-CP period. Continuing surveillance is needed to confirm this trend, as such a substantial reduction in treatment failure after CP would be clinically significant.
This study has strengths and limitations. It is the first study that evaluates the effectiveness of ASP through CPs in an Italian hospital. This intervention was designed to be feasible and was developed by a multidisciplinary team to guarantee a high quality and level of coordination, with cooperation between the Infectious Diseases and PED teams. Furthermore, following CP presentation a prominent educational campaign included lectures and distribution of handy pocket cards and posters.
This is the first study with a phone call follow-up to assess antimicrobial stewardship in the PED context, allowing evaluation of antibiotic changes for persistence of symptoms or side effects.
Limitations include the retrospective nature of the analysis, its single-centre setting, the short period of observation,the inability to assess appropriateness of single antimicrobial prescriptions and the small amount of inpatients.
Thus, a longer term follow up study evaluating the longevity of observed changes in antimicrobial prescription is warranted to analyse further improvements, as well as expanding to include other Italian PED for validation of this tools.
Conclusions
This study provides evidence that clinical pathway implementation in an Italian PED setting is an effective tool for antimicrobial stewardship, appearing to be associated with the kind of treatment children receive.
An evidence-based CP supplemented by educational and explanatory lectures was associated with significant changes in prescribing habits of physicians at our centre, decreasing the use of broad-spectrum antibiotics in favour of narrow-spectrum, and reducing the length of therapy without increasing treatment failure both for outpatients and inpatients.
Supporting information
(PDF)
Abbreviations
- ASPs
Antimicrobial stewardship programs
- bsDOT
Broad-spectrum days of therapy
- CP
Clinical Pathway
- CAP
Community-acquired pneumonia
- DOT
Days of therapy
- LOS
Length of hospital stay
- LOT
Length of therapy
- PACU
Pediatric Acute Care Unit
- PED
Pediatric Emergency Department
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
the authors received no specific funding for this work.
References
- 1.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America: The Management of Community-Acquired Pneumonia in Infants and Children Older Than 3 Months of Age: Clinical Practice Guidelines by the Pediatric Infectious Diseases Society of America. Clin Infect Dis 2011; 53(7): e25–76 doi: 10.1093/cid/cir531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardlaw T, Salama P, Johansson EW, Mason E. Pneumonia: the leading killer of children. Lancet 2006; 368: 1048–1050 doi: 10.1016/S0140-6736(06)69334-3 [DOI] [PubMed] [Google Scholar]
- 3.Don M, Canciani M, Korppi M. Community-acquired pneumonia in children: what’s old? What’s new? Acta Paediatrica 2010; 99: 1602–1608 doi: 10.1111/j.1651-2227.2010.01924.x [DOI] [PubMed] [Google Scholar]
- 4.Leyenaar JK, Lagu T, Shieh MS, Pekow PS, Lindenauer PK: Variation in resource utilization for the management of uncomplicated community-acquired pneumonia across community and children’s hospitals. J Pediatr 2014; 165(3): 585–591 doi: 10.1016/j.jpeds.2014.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein RT, Marostica PJ. Community-acquired pneumonia: a review and recent advances. Pediatr Pulmonol 2007; 42: 1095–1103 doi: 10.1002/ppul.20652 [DOI] [PubMed] [Google Scholar]
- 6.Clavenna A, Bonati M. Differences in antibiotic prescribing in paediatric outpatients. Arch Dis Child 2011; 96: 590–595 doi: 10.1136/adc.2010.183541 [DOI] [PubMed] [Google Scholar]
- 7.Hyde TB, Gay K, Stephens DS, Vugia DJ, Pass M, Johnson S, et al. Macrolide resistance among invasive streptococcus pneumoniae isolates. JAMA 2001; 286(15): 1857–62 [DOI] [PubMed] [Google Scholar]
- 8.Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society Standards of Care Committee. Thorax 2011; 66(2): 1–23 [DOI] [PubMed] [Google Scholar]
- 9.Jenkins TC, Irwin A, Coombs L, Dealleaume L, Ross SE, Rozwadowski J, et al. Effects of Clinical Pathways for Common Outpatient Infections on Antibiotic Prescribing. Am J Med 2013; 126(4): 327–335 doi: 10.1016/j.amjmed.2012.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frei CR, Bell AM, Traugott KA, Jaso TC, Daniels KR, Mortensen EM, et al. A clinical pathway for community-acquired pneumonia: an observational cohort study. BMC Infect Dis 2011; 11: 188 doi: 10.1186/1471-2334-11-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samore MH, Bateman K, Alder SC, Hannah E, Donnelly S, Stoddard GJ, et al. Clinical Decision Support and Appropriateness of Antimicrobial Prescribing. JAMA 2005; 294(18): 2305–2314 doi: 10.1001/jama.294.18.2305 [DOI] [PubMed] [Google Scholar]
- 12.South M, Royle J, Starr M. A simple intervention to improve hospital antibiotic prescribing. Med J Aust 2003; 178(5): 207–209 [DOI] [PubMed] [Google Scholar]
- 13.Marrie TJ, Lau CY, Wheeler SL, Wong CJ, Vandervoort MK, Feagan BG. A Controlled Trial of a Critical Pathway for Treatment of Community-Acquired Pneumonia. JAMA 2000; 283(6): 749–755 [DOI] [PubMed] [Google Scholar]
- 14.Center for Disease Control and Prevention website (CDC), Ambulatory Health Care Data; 2015. Available at: http://www.cdc.gov/nchs/ahcd.htm
- 15.Launay E, Levieux K, Levy C, Dubos F, Martinot A, Vrignaud B, et al. GPIP. Compliance with the current recommendations for prescribing antibiotics for paediatric community-acquired pneumonia is improving: data from a prospective study in a French network. BMC Pediatr. 2016. August 12;16(1):126 doi: 10.1186/s12887-016-0661-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambroggio L, Thomson J, Murtagh Kurowski E, Courter J, Statile A, Graham C,et al. Quality improvement methods increase appropriate antibiotic prescribing for childhood pneumonia. Pediatrics 2013. May;131(5):e1623–31 doi: 10.1542/peds.2012-2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MJ, Kong M, Cambon A, Woods CR. Effectiveness of antimicrobial guidelines for community-acquired pneumonia in children. Pediatrics. 2012. May;129(5):e1326–33 doi: 10.1542/peds.2011-2412 [DOI] [PubMed] [Google Scholar]
- 18.Bradley JS, Garonzik SM, Forrest A, Bhavnani SM. Pharmacokinetics, pharmacodynamics, and Monte Carlo simulation: selecting the best antimicrobial dose to treat an infection. Pediatr Infect Dis J 2010; 29: 1043–1046 doi: 10.1097/INF.0b013e3181f42a53 [DOI] [PubMed] [Google Scholar]
- 19.Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168(20): 2254–2260 doi: 10.1001/archinte.168.20.2254 [DOI] [PubMed] [Google Scholar]
- 20.Polk RE, Fox C, Mahoney A, Letcavage J, MacDougall C. Measurement of adult antibacterial drug use in 130 US hospitals: comparison of defined daily dose and days of therapy. Clin Infect Dis 2007; 44(5): 664–670 doi: 10.1086/511640 [DOI] [PubMed] [Google Scholar]
- 21.Polk RE, Hohmann SF, Medvedev S, Ibrahim O. Benchmarking risk-adjusted adult antibacterial drug use in 70 US academic medical center hospitals. Clin Infect Dis 2011; 53(11): 1100–1110 doi: 10.1093/cid/cir672 [DOI] [PubMed] [Google Scholar]
- 22.Rudan I, Tomaskovic L, Boschi-Pinto C, Campbell H. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull World Health Organ 2004; 82(12):895–903 doi: /S0042-96862004001200005 [PMC free article] [PubMed] [Google Scholar]
- 23.Michelow IC, Olsen K, Lozano J, Rollins NK, Duffy LB, Ziegler T, et al. Epidemiology and Clinical Characteristics of Community-Acquired Pneumonia in Hospitalized Children. Pediatrics 2004. 113(4): 701–707 [DOI] [PubMed] [Google Scholar]
- 24.Nascimento-Carvalho CM, Ribeiro CT, Cardoso MR, Barral A, Araújo-Neto CA, Oliveira JR, et al. The role of respiratory viral infections among children hospitalized for community-acquired pneumonia in a developing country. Pediatr Infect Dis J 2008; 27(10):939–41 doi: 10.1097/INF.0b013e3181723751 [DOI] [PubMed] [Google Scholar]
- 25.Juvén T, Mertsola J, Waris M, Leinonen M, Meurman O, Roivainen M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J 2000; 19(4):293–8 [DOI] [PubMed] [Google Scholar]
- 26.Hasegawa J, Mori M, Showa S, Matsushima A, Ohnishi H, Tsufawa T, et al. Pneumococcal vaccination reduced the risk of acute otitis media: Cohort study. Pediatr Int 2015; 57(4): 582–585 doi: 10.1111/ped.12587 [DOI] [PubMed] [Google Scholar]
- 27.Gagliotti C, Buttazzi R, Moro ML, Di Mario S. Uso di antibiotici e resistenze antimicrobiche in età pediatrica. Rapporto Emilia-Romagna 2013. Agenzia sanitaria e sociale dell’Emilia-Romagna 2014. Available at: http://assr.regione.emilia-romagna.it/it/servizi/pubblicazioni/rapporti-documenti/uso-antibiotici-resistenze-eta-pediatrica-2013 [Google Scholar]
- 28.Rosenblüt A, Santolaya ME, Gonzalez P, Borel C, Cofré J. Penicillin resistance is not extrapolable to amoxicillin resistance in Streptococcus pneumonia isolated from middle ear fluid in children with acute otitis media. Ann Otol Rhinol Laryngol 2006; 115(3): 186–190 doi: 10.1177/000348940611500305 [DOI] [PubMed] [Google Scholar]
- 29.De Luca M, Donà D, Montagnani C, Lo Vecchio A, Romanengo M, Tagliabue C, et al. Antibiotic Prescriptions and Prophylaxis in Italian Children. Is It Time to Change? Data from the ARPEC Project. PLoS ONE 2016; 1(5): e0154662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. Participating National Healthcare Safety Network Facilities: NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008; 29(11): 996–1011 doi: 10.1086/591861 [DOI] [PubMed] [Google Scholar]
- 31.Bratu S, Mooty M, Nichani S, Landman D, Gullans C, Pettinato B, et al. Emergence of KPC-possessing Klebsiella Pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob Agents Chemother 2005; 49(7): 3018–3020 doi: 10.1128/AAC.49.7.3018-3020.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29(12): 1099–1106 doi: 10.1086/592412 [DOI] [PubMed] [Google Scholar]
- 33.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 2008; 52(3): 1028–1033 doi: 10.1128/AAC.01020-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe M, Iyobe S, Inoue M, Mitsuhashi S. Transferable imipenem resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 1991; 35(1): 147–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, Steward CD, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45(4): 1151–1161 doi: 10.1128/AAC.45.4.1151-1161.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simbalista R, Araújo M, Nascimento‐Carvalho CM. Outcome of children hospitalized with community‐acquired pneumonia treated with aqueous penicillin G. Clinics 2011; 66(1):95–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.