Abstract
Background
Cardiovascular disease (CVD) is a complex disease with multifactorial etiology. The presence of endothelial dysfunction constitutes an early risk factor for CVD in children. Circulating microRNAs (miRNAs) are small noncoding RNAs that regulate gene expression and represent a novel class of biomarkers and therapeutic targets; therefore, we examined whether the presence of endothelial dysfunction is associated with differential expression of plasma miRNAs in otherwise healthy children.
Methods
A total of 70 children (aged 5-10 years) were recruited and classified into two groups (normal endothelial function [NEF] and endothelial dysfunction). Time to peak postocclusive reperfusion (Tmax) was considered as the indicator of either normal endothelial function (NEF; Tmax < 45 s) or endothelial dysfunction (Tmax ≥ 45 s). Lipid profiles, high-sensitivity C-reactive protein, fasting glucose, and insulin were assayed using enzyme-linked immunosorbent assay. miRNAs isolated from plasma were assayed with a custom human CVD array, followed by quantitative polymerase chain reaction verification of candidates. In addition, bioinformatics approaches including combinatorial target prediction algorithms and gene ontology were applied.
Results
Three miRNAs that have been previously linked to cardiomyopathy, hsa-miR-125a-5p, hsa-miR-342-3p, and hsa-miR-365b-3p, were identified as potential biomarkers of children with endothelial dysfunction. The miRNA predicted gene targets revealed 31 common targets among all three putative candidate biomarker miRNAs and encompass three biologic pathways, including transforming growth factor-β signaling, cytokine-cytokine receptor interactions, and activin receptor-like kinase in cardiac myocytes.
Conclusions
Plasma miRNAs may be useful as potential screening tools for the presence of endothelial dysfunction in children and may reveal endothelial dysfunction-relevant target genes.
Key Words: endothelium, microRNA, obesity
Abbreviations: CVD, cardiovascular disease; GO, gene ontology; HOMA, homeostasis model assessment; hsCRP, high-sensitivity C-reactive protein; miRNA, microRNA; NEF, normal endothelial function; qRT-PCR, quantitative real-time polymerase chain reaction; TGF-β, transforming growth factor-β; Tmax, time to peak postocclusive reperfusion
Obesity is one of the world’s greatest public health challenges in both developed and developing countries, affecting both adults and children. The presence of obesity has now been conclusively linked to heightened morbidity and mortality through increased risk for a multitude of chronic diseases, including type 2 diabetes mellitus, hypertension, dyslipidemia, and coronary artery disease.1, 2, 3, 4, 5 The pathogenesis of obesity is multifactorial, incorporating both genetics and lifestyle factors. In children, obesity is associated with increased risk for multifaceted derangements in metabolic and cardiovascular function, including endothelial dysfunction.6, 7, 8, 9 Overweight children are more likely to prematurely develop endothelial dysfunction, hypertension, and type 2 diabetes mellitus, an array of conditions that would be expected in older obese adults,4 a finding that has prompted the recommendation to screen for such morbidities at even earlier ages.10
Obesity in children is associated with an increased risk for the development of endothelial dysfunction prior to the onset of hypertension.8 However, not every obese child will develop endothelial dysfunction, suggesting that both genetic and environmental factors may play a role. Conversely, a small subset of otherwise healthy children who are not obese may manifest abnormal endothelial function, and such functional phenotype may be determined by genetic variance in endothelial function-related genes.11 Endothelial dysfunction, an early risk marker of cardiovascular disease, refers to a loss of normal homeostatic function in the blood vessels and is characterized by altered vasodilatory and vasoconstrictive functions and inflammatory activity.12 Endothelial dysfunction is involved in the development of vascular complications related to dyslipidemia and cardiovascular disease (CVD) such as hypertension, coronary artery disease, and chronic heart failure.13, 14 Thus, extraordinary efforts have been directed to determine the molecular and pathologic characteristics of the diseased heart and vasculature and to develop novel diagnostic and therapeutic strategies.
One of the emerging and promising diagnostic tools is the use of circulating microRNAs (miRNAs). miRNAs are small noncoding RNAs (approximately 22 nucleotides) that posttranscriptionally regulate gene expression by blocking translation or inducing degradation of the targeted mRNA.15 The presence of circulating miRNAs in plasma suggests that miRNAs may fulfill important biologic functions outside their corresponding cell sources in both physiologic and pathologic conditions and serve as potential biomarkers for disease states.16 Circulating miRNAs are protected from RNase-dependent degradation by several mechanisms, including their inclusion in microvesicles, exosomes, and apoptotic bodies as well as through formation of protein-miRNA complexes that are resistant to degradation.17 Of note, although the expression of miRNAs in plasma and serum are believed to reflect the extrusion of miRNAs from relevant remote tissues or organs or disease processes,18 it is likely that peripheral blood miRNAs do not reflect miRNAs expressed in remote tissues. miRNAs are involved in the regulation of several key cellular processes, including cellular development, differentiation, proliferation, cell death, and metabolism.19 miRNAs have been implicated in almost every cardiovascular disorder in which they have been examined, including heart failure, cardiac hypertrophy, remodeling after myocardial infarction, arrhythmias, atherosclerosis, atrial fibrillation, and peripheral artery disease.20, 21, 22, 23, 24 Since miRNAs can be readily detected in various body fluids,25, 26 they have been advanced as useful biomarkers for the diagnosis and characterization of systemic diseases,27 including type 2 diabetes mellitus,28 hypertension,29 obesity,30 and cardiovascular disease.31 Peripheral blood miRNAs, including miRNAs expressed by peripheral blood mononuclear cells, as well as extracellular/circulating miRNAs may, thus, provide an easy and rapid screening approach in clinical populations32 and add to the accuracy of current CVD risk stratification criteria.
In this study, we hypothesized that circulating miRNAs may have predictive value for identification of children at increased risk for endothelial dysfunction. To examine this issue, plasma samples of children with and without endothelial dysfunction were examined using pathway-fused cardiovascular miRNA arrays, followed by bioinformatic assessments of functional information of the differentially expressed specific miRNAs and their related regulatory networks, as well as putative identification of their target genes as predicted by function and pathway enrichment analysis of at least three public datasets (TargetScan, PicTar, and miRanda).
Materials and Methods
Subjects
The study was approved by the University of Louisville Human Research Committee (Protocol 474.99), and informed consent was obtained from the legal caregiver of each participant. Consecutive healthy overweight and obese prepubertal children (aged 5-10 years) were recruited from the community to investigate endothelial function. Children were excluded if they had any chronic medical condition, were receiving medications, or had any genetic or craniofacial syndromes. All children who were hypertensive or using antihypertensive therapies were excluded. Also excluded from the study were children with a known episode of infection in the 8 weeks preceding the study or children with asthma or allergies receiving specific therapy (desensitization, leukotriene inhibitors, topical or systemic corticosteroids). Height and weight were obtained, systemic BP was measured,33 and BMI z score was calculated (e-Appendix 1).
Endothelial Function
Endothelial function was assessed by using a modified hyperemic test after cuff-induced occlusion of the radial and ulnar arteries by placing the cuff over the wrist, as previously reported.8, 34, 35, 36, 37 We defined endothelial dysfunction as a time to peak postocclusive reperfusion (Tmax) cutoff value of ≥ 45 s, whereas values < 45 s were considered as normal endothelial function (NEF)36 (see e-Appendix 1).
Biochemical Assays
Fasting blood samples were drawn from the subjects in the morning and immediately centrifuged at 2,000g for 20 min at 4°C, and aliquoted plasma samples were frozen at −80°C until assayed. Plasma high-sensitivity C-reactive protein (hsCRP), lipids, insulin, and glucose were measured (e-Appendix 1), and insulin resistance was assessed using the homeostasis model assessment (HOMA) equation (fasting insulin × fasting glucose/22.5).38
Circulating miRNA Isolation, Quality, and Integrity
Total RNA including miRNA was isolated from plasma using miRNeasy Mini Kit column-based system following the manufacturer’s instructions (Qiagen). RNA quantity was evaluated by spectrophotometry using NanoDrop ND-1000 (Thermo Fisher Scientific Inc). The RNA quality and integrity were determined using the Eukaryote Total RNA Nano 6000 LabChip assay (Agilent Technologies) on the Agilent 2100 Bioanalyzer. The quality of miRNA was determined using Agilent Small RNA Kit according to the manufacturer’s protocol. All the purified samples were stored at −80°C until further analyses (e-Appendix 1).
miRNA Polymerase Chain Reaction Array for CVD
Pathway-specific for human CVD miRNA arrays (84 miRNAs) were used in age-, sex-, ethnicity-, and BMI z score-matched children with either normal endothelial function (n = 8) or endothelial dysfunction (n = 8) (Qiagen) (Fig 1). Each of the arrays contains a specific set of selected miRNAs with CVD significance based on published studies. A set of 12 miRNA controls preset on this array (96-well plates) enables data analysis using the ΔΔCT method of relative quantification, assessment of reverse transcription performance, and assessment of polymerase chain reaction (PCR) performance using SYBR Green real-time PCR. For target verification purposes, quantitative real-time PCR (qRT-PCR) analyses were performed using ABI 7500 (Thermo Fisher Scientific Inc) using validated housekeeping genes39, 40 (e-Appendix 1).
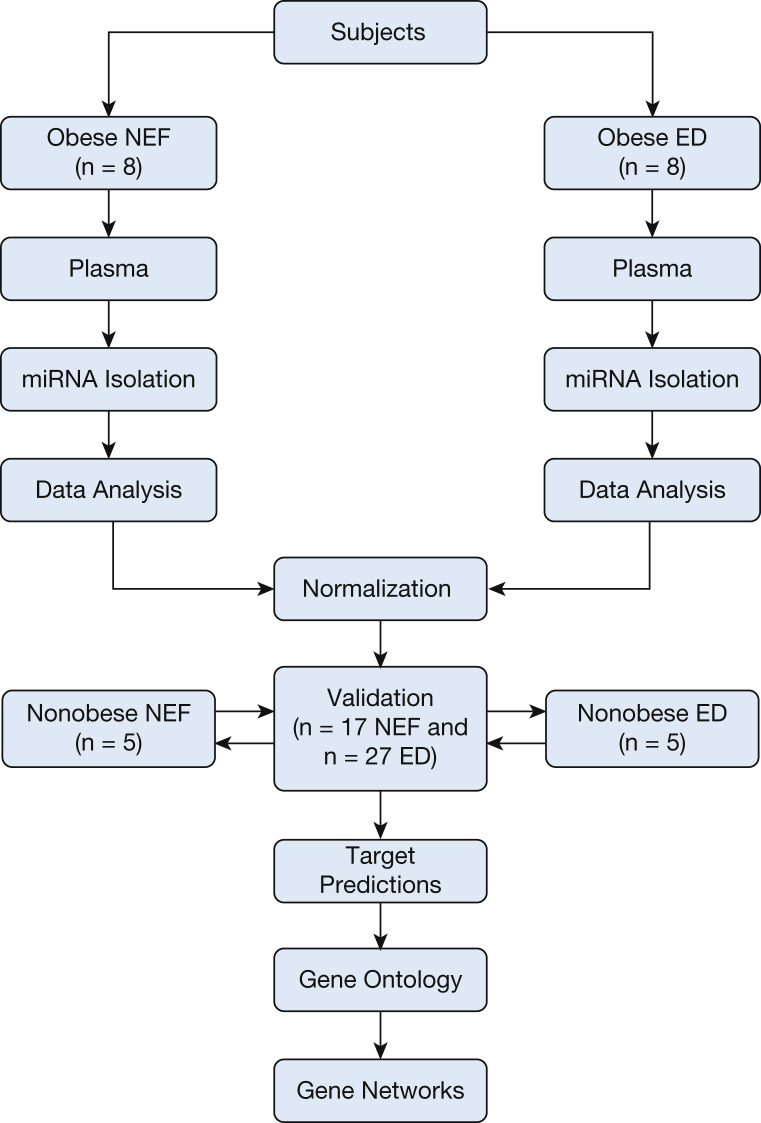
Figure 1.
Schema illustrating subject recruitment and data analysis. The cohort was matched for age, sex, ethnicity, and BMI z score and differed only in their time to peak postocclusive reperfusion characteristics (ie, endothelial dysfunction or NEF). Plasma miRNAs were isolated from each subject and equal concentrations of each sample were converted into cDNA. Each individual sample was applied into Qiagen arrays (84 miRNAs). The data were normalized with housekeeping miRNA, and the averages of each miRNA were compared between NEF vs ED. Target predictions for each statistically significant miRNA were performed by at least four different software programs. The common target predictions genes as derived from Venn diagram were then used to build gene networks. The number of subjects tested in each step is indicated (eight subjects in ED and NEF groups served as the initial exploratory phase, 17 NEF and 27 ED subjects served as post hoc verification phase, and additional nonobese control subjects (n = 5 in endothelial dysfunction and NEF groups). ED = endothelial dysfunction; miRNA = microRNA; NEF = normal endothelial function.
Target Predictions
Gene targets for differentially expressed miRNAs were computationally predicted using established miRNA target-prediction programs: MicroInspector, miRanda, PicTar, RNA22, RNAhybrid, and TargetScan (Fig 1). To improve the reliability of the miRNA targets, only target genes predicted by at least three of the programs were selected for extraction. The predicted genes of individual miRNA were uploaded to the online DAVID (Database for Annotation, Visualization, and Integrated Discovery) program (http://david.abcc.ncifcrf.gov/) for their functional annotation and clustering analysis. The predominant biologic pathways for the selected miRNAs were identified.
Gene Ontology and Functional Annotation
Analysis of gene ontology (GO) annotation was performed by applying DAVID 6.7 functional annotation tool. This DAVID software (http://david. abcc.ncifcrf.gov/) is able to identify the most relevant (overrepresented) biologic terms associated with a given gene list.41 The DAVID functional annotation cluster tool groups genes based on their associated GO annotations, and the related terms are clustered into groups with enrichment scores calculated from their EASE Score, the modified Fisher exact P value.42 Moreover, the web server hosts an updated version of the KEGG database providing a relevant search module based on KEGG pathway descriptions.43
Pathway Analysis Enrichment
The web-based computational tool DIANA-mirPath v2.144 was used to predict the target genes and altered pathways of the differentially expressed miRNAs. The software performs an enrichment analysis of multiple miRNA target genes by comparing each set of miRNA targets to all known KEGG pathways (Kyoto Encyclopedia of Genes and Genomes). The pathways exhibiting a false discovery rate adjusted P value of < .05 were considered significantly enriched between the compared classes.
Statistical Analysis
All data were expressed as mean ± SD. Analysis of variance tests or Student t tests were used for statistical analyses. We performed analysis of variance and/or paired t tests to study differences on quantitative variables between endothelial dysfunction and NEF groups. All analyses were conducted using SPSS software (version 21.0; IBM Corporation), and data are presented as mean ± SD. A P value < .05 was considered statistically significant for all analyses.
Results
Cohort Phenotype
The demographic and clinical parameters of the 60 children initially recruited to the study (25 in the NEF and 35 in the endothelial dysfunction groups) are summarized in Table 1, and illustrate their similar age, sex, ethnicity, and BMI z scores. The Tmax value for NEF children was 26.8 ± 11.4 s as compared with 62.4 ± 15.9 s for children with endothelial dysfunction (P value < .0001). However, there were no differences in either systolic or diastolic blood pressures between endothelial dysfunction and NEF groups.
Table 1.
Demographic Characteristics and Metabolic Data in Children With and Without Endothelial Dysfunction
| Variables | NEF (n = 25) | Endothelial Dysfunction (n = 35) | P Value |
|---|---|---|---|
| Age, y | 7.59 ± 1.26 | 8.41 ± 1.20 | .14 |
| BMI z-score | 1.69 ± 0.62 | 2.00 ± 0.68 | .43 |
| Sex, % male | 70.4 | 63.8 | .35 |
| Ethnicity, % | |||
| White | 77.3 | 74.1 | .21 |
| African American | 22.4 | 26.2 | .26 |
| SBP, mm Hg | 107.55 ± 10.35 | 107.32 ± 10.68 | .47 |
| DBP, mm Hg | 62.28 ± 8.15 | 63.56 ± 7.16 | .32 |
| Tmax, s | 26.84 ± 11.35 | 62.42 ± 15.88 | .0001 |
| TG, mg/dL | 67.30 ± 30.71 | 89.0.06 ± 53.64 | .04 |
| TC, mg/dL | 160.34 ± 27.41 | 164.90 ± 26.98 | .27 |
| HDL-C, mg/dL | 52.78 ± 7.44 | 49.01 ± 7.60 | .03 |
| LDL-C, mg/dL | 94.04 ± 22.49 | 98.06 ± 23.87 | .27 |
| Glucose, mg/dL | 76.23 ± 9.90 | 81.45 ± 13.43 | .15 |
| Insulin, mg/dL | 5.88 ± 2.98 | 9.11 ± 6.53 | .02 |
| HOMA-IR | 0.98 ± 0.79 | 1.66 ± 1.58 | .04 |
| hsCRP, mg/dL | 0.43 ± 0.31 | 1.51 ± 1.18 | .007 |
Data presented as mean ± SD unless otherwise noted. DBP = diastolic BP; HDL-C = high-density lipoprotein cholesterol; HOMA-IR = homeostatic model assessment-insulin resistance; hsCRP = high-sensitivity C-reactive protein; LDL-C = low-density lipoprotein cholesterol; NEF = normal endothelial function; SBP = systolic BP; TC = total cholesterol; TG = triglyceride; Tmax = time of maximum perfusion after occlusion release.
The metabolic data for the two groups are shown in Table 1. Serum triglyceride levels were significantly higher in children with endothelial dysfunction (P value ≤ .04), whereas high-density lipoprotein levels were significantly lower (P value < .03). Although fasting glucose levels were similar, plasma insulin concentrations were higher in children with endothelial dysfunction (P value < .05), and hsCRP levels also differed among the groups (P value = 0.007) (Table 1).
miRNA Profiling and qRT-PCR Validation
Microarray-based analyses revealed that three miRNAs were differentially expressed in children with endothelial dysfunction (Fig 1, Table 2). Out of 84 miRNAs listed on the PCR array, 76 miRNAs were detected. We further confirmed these findings in 17 additional subjects with NEF and 27 subjects with endothelial dysfunction. Notably, qRT-PCR results on differentially expressed miRNAs (ie, hsa-miR-365b-3p, hsa-miR-125a-5p, and hsa-miR-342-3p) were consistent with the expression patterns observed in miRNA microarray profiling (Table 2). To ascertain that BMI z-score status was not biasing our findings and that the miRNA findings were specifically ascribable to endothelial dysfunction, we further compared additional nonobese children with otherwise normal endothelial function (n = 5) and additional nonobese children with endothelial dysfunction (n = 5). MiRNA expression patterns in these 10 nonobese children further confirmed the increased levels of hsa-miR-365b-3p in endothelial dysfunction and the reductions in circulating hsa-miR-125a-5p and hsa-miR-342-3p miRNAs in endothelial dysfunction. Thus, the number of subjects participating in this study were 60 obese children, either NEF or endothelial dysfunction, and 10 nonobese children (NEF or endothelial dysfunction), thereby totaling 70 subjects.
Table 2.
List of Differentially Expressed miRNAs in Children With Endothelial Dysfunction and Validations of the Same miRNAs Using qRT-PCR Analysis
| List of miRNAs | Reference Sequence | Chromosome Location | Transcript ID | Cardiovascular Arrays |
Validation (qRT-PCR) |
||
|---|---|---|---|---|---|---|---|
| Fold Changes | P Value | Fold Changes | P Value | ||||
| hsa-miR-365b-3p | NR_029856 | 17q11.2 | ENSG00000199187 | 1.41 ± 0.14 | .004 | 1.52 ± 0.23 | .001 |
| hsa-miR-125a-5p | NR_029693 | 19q13.41 | ENST00000385273 | −1.33 ± 0.11 | .02 | −1.27 ± 0.12 | .01 |
| hsa-miR-342-3p | NR_029888.1 | 14q32.2 | ENST00000362212 | −1.41 ± 0.08 | .03 | −1.22 ± 0.06 | .02 |
miRNA = microRNA; qRT-PCR = quantitative real-time polymerase chain reaction.
Prediction of Potential Targets of Differentially Expressed miRNAs
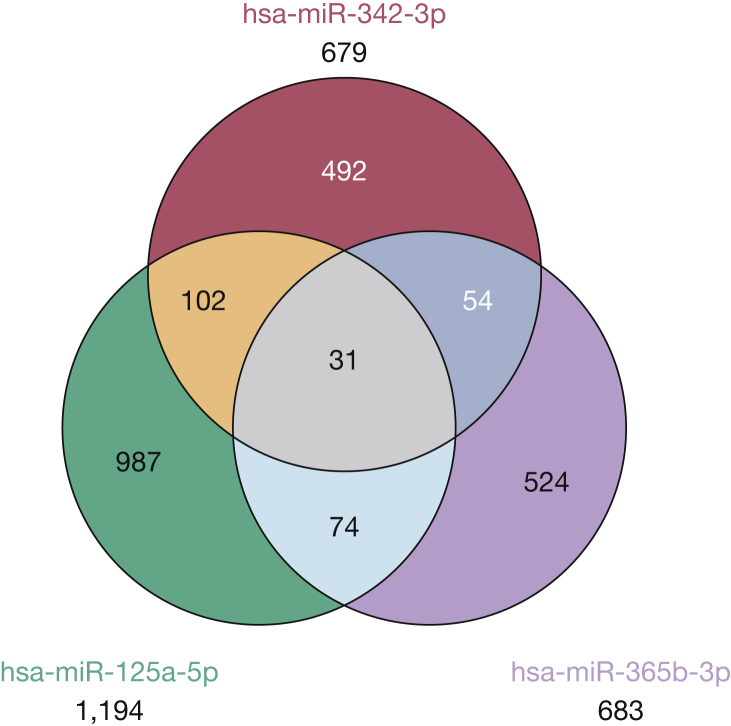
A Venn diagram in Figure 2 shows the intersections among the three target genes list, and the list of these 31 putative target genes is provided in Table 3. hsa-miR-125a-5p had the most gene target hit rates with 1,194, whereas hsa-miR-365-3p had 683 and hsa-miR-342-3p had 679 target genes, respectively (Fig 3). To evaluate the potential biologic roles of the three differentially expressed miRNAs, we assessed the GO of their potential gene targets using several established computational algorithms, and the annotated genes were classified according to the GO categories: cellular component (n = 3), biologic process (n = 50), and molecular function (n = 13) based on P value < .05. GO analyses further revealed that a broad range of biologic processes categories were enriched among the target genes list (Table 4), including metabolic process, anterior/posterior pattern formation, tube development, and respiratory tube development. The list of significant molecular functions associated with endothelial dysfunction is shown in Table 5, and the most common of these biologic processes were transmembrane receptor protein serine/threonine kinase activity and TGF-β receptor activity. Also, the lists of significant cellular components included cell surface, plasma membrane, and Srb-mediator complex (Table 6). Next, we found that three biologic pathways were associated with the gene target predictions, including transforming growth factor (TGF)-β signaling, cytokine-cytokine receptor interactions, and activin receptor-like kinase in cardiac myocytes.
Figure 2.
Venn diagram showing the intersections of the putative target gene predictions from the three differentially expressed microRNAs (miRNAs) in children with endothelial dysfunction. The final common list of target predictions genes among all three differentially expressed miRNAs were identified from the Venn diagram and used to define the putative common candidate target genes.
Table 3.
List of Predicted Target Genes Derived From Shared Venn Diagram of the Three Differentially Expressed miRNAs
| Gene Name | Ensembl Gene | UniGene | RefseqRNA | Description | Chromosome | Start, bp | End, bp |
|---|---|---|---|---|---|---|---|
| ACVR2B | ENSG00000114739 | Hs.174273 | NM_001106 | Activin A receptor, type IIB | 3 | 38470794 | 38509637 |
| APC | ENSG00000134982 | Hs.158932 | NM_000038 | Adenomatosis polyposis coli | … | 32236465 | 32246349 |
| B4GALT1 | ENSG00000071626 | Hs.651277 | NM_018959 | UDP-Gal:betaGlcNAc β 1,4-galactosyltransferase, polypeptide 1 | 19 | 1358584 | 1386683 |
| BMPR2 | ENSG00000204217 | Hs.471119 | NM_001204 | Bone morphogenetic protein receptor, type 2 (serine/threonine kinase) | 2 | 202949916 | 203140719 |
| C1orf21 | ENSG00000116667 | Hs.497159 | NM_030806 | Chromosome 1 open reading frame 21 | 1 | 182622773 | 182864778 |
| CACNA1C | ENSG00000151067 | Hs.118262 | NM_000719.5 | Calcium channel, voltage-dependent, L type, α 1C subunit | 12 | 2032725 | 2677376 |
| CBFB | ENSG00000067955 | Hs.460988 | NM_001755 | Core-binding factor, β subunit | 16 | 65620551 | 65692457 |
| CREB5 | ENSG00000146592 | Hs.437075 | NM_001011666.1 | CAMP responsive element binding protein 5 | 7 | 28305465 | 28832036 |
| DOCK3 | ENSG00000129596 | Hs.476284 | NM_001801 | Dedicator of cytokinesis 3 | 5 | 115168333 | 115180304 |
| FBXO33 | ENSG00000165355 | Hs.324342 | NM_203301 | F-box protein 33 | 14 | 38936718 | 38971371 |
| FRAS1 | ENSG00000138759 | Hs.369448 | NM_025074.4 | Fraser syndrome 1 | 4 | 79198120 | 79684447 |
| GABRB2 | ENSG00000121966 | Hs.303527 | NM_001008540 | γ-aminobutyric acid (GABA) A receptor, β 2 | 5 | 160648014 | 160907708 |
| GPR158 | ENSG00000151025 | Hs.499108 | NM_020752 | G protein-coupled receptor 158 | 10 | 25504296 | 25931164 |
| GRB10 | ENSG00000106070 | Hs.164060 | NM_001001549.1 | Growth factor receptor-bound protein 10 | 7 | 50625260 | 50828652 |
| HIATL1 | ENSG00000148110 | Hs.555996 | NM_032558 | Hippocampus abundant transcript-like 1 | 9 | 96176654 | 96263022 |
| HIPK1 | ENSG00000163349 | Hs.532363 | NM_152696 | Homeodomain interacting protein kinase 1 | 1 | 114203267 | 114321949 |
| KLHL24 | ENSG00000114796 | Hs.407709 | NM_017644 | Kelch-like 24 (Drosophila) | 3 | 184836105 | 184884996 |
| LMO3 | ENSG00000048540 | Hs.504908 | NM_001001395 | LIM domain only 3 (rhombotin-like 2) | 12 | 16592574 | 16652291 |
| MED13 | ENSG00000108510 | Hs.282678 | NM_005121.2 | Mediator complex subunit 13 | 17 | 57374749 | 57497425 |
| MED14 | ENSG00000180182 | Hs.407604 | NM_004229 | Mediator complex subunit 14 | 2 | 28828118 | 28879300 |
| MTF1 | ENSG00000188786 | Hs.471991 | NM_005955 | Metal-regulatory transcription factor 1 | 1 | 38047827 | 38097879 |
| NFIB | ENSG00000147862 | Hs.644095 | NM_005596 | Nuclear factor I/B | 9 | 14071847 | 14388630 |
| PCDH7 | ENSG00000169851 | Hs.479439 | NM_002589 | Protocadherin 7 | 4 | 30331135 | 30753569 |
| PHF15 | ENSG00000043143 | Hs.483419 | NM_015288 | PHD finger protein 15 | 5 | 133889246 | 133946817 |
| PLAG1 | ENSG00000181690 | Hs.14968 | NM_002655 | Pleomorphic adenoma gene 1 | 8 | 57236037 | 57286392 |
| PURB | ENSG00000146676 | Hs.349150 | NM_033224 | Purine-rich element binding protein B | 7 | 44889059 | 44891485 |
| RICTOR | ENSG00000164327 | Hs.407926 | NM_152756 | Rapamycin-insensitive companion of mTOR | 5 | 38973779 | 39110260 |
| SETD7 | ENSG00000145391 | Hs.480792 | NM_030648 | SET domain containing (lysine methyltransferase) 7 | 4 | 140646642 | 140697027 |
| SLC44A1 | ENSG00000070214 | Hs.573495 | NM_080546 | Solute carrier family 44, member 1 | 9 | 107046724 | 107241273 |
| SP7 | ENSG00000170374 | Hs.209402 | NM_152860.1 | Sp7 transcription factor | 12 | 52008197 | 52009489 |
| TGFBR1 | ENSG00000128268 | Hs.494622 | NM_001098270.1 | Transforming growth factor, β receptor I (activin A receptor type 2-like kinase, 53kDa) | 22 | 38183271 | 38218143 |
Figure 3.
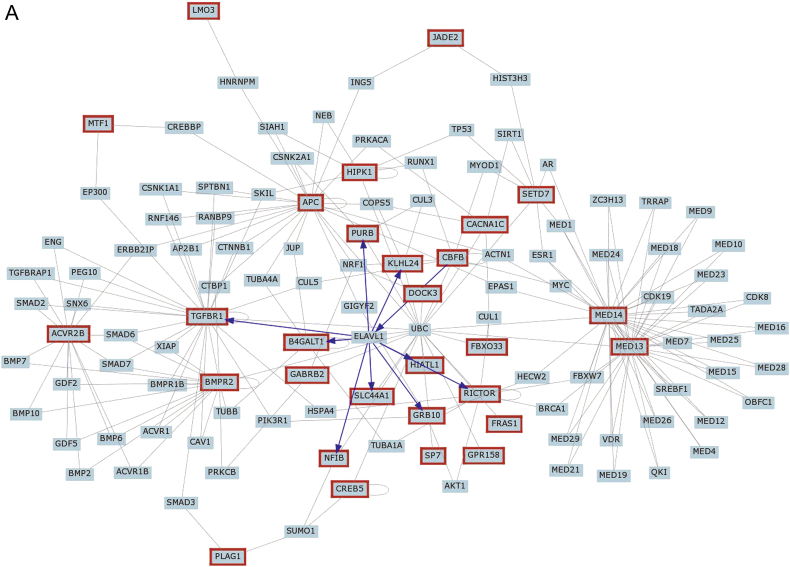
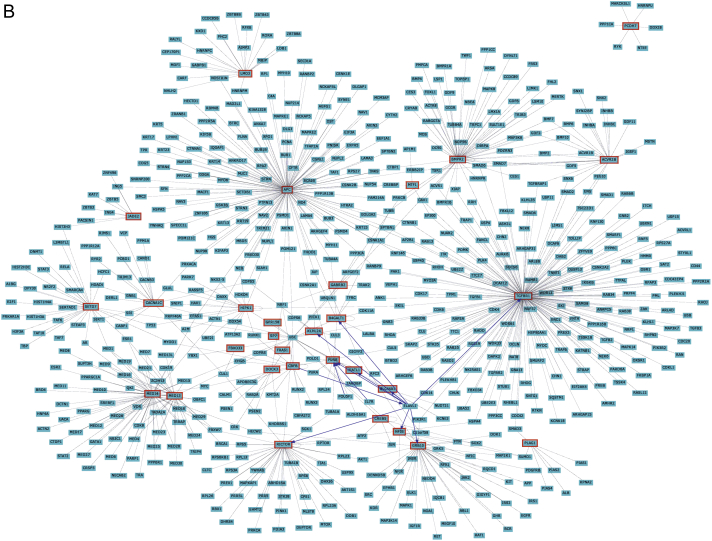
Gene network representation of interactions between common gene target predictions as identified from the Venn diagram in Figure 2. A, Interactions of the target genes with at least two list members of the query. B, Interactions of the target genes with all known interactions of gene products. Note that these densely connected nodes capture a significant proportion of the overall network connectivity.
Table 4.
List of the Most Significant Gene Ontology Biologic Processes As Derived From Interrogation of Predicted mRNA Target Genes
| GO ID | GO Term | Gene Count | Percentage | P Value | Genes |
|---|---|---|---|---|---|
| GO:0010604 | Positive regulation of macromolecule metabolic process | 10 | 32.26 | .000026 | MTF1, TGFBR1, BMPR2, CREB5, MED14, MED13, RICTOR, CBFB, NFIB, APC |
| GO:0009952 | Anterior/posterior pattern formation | 5 | 16.13 | .00016 | ACVR2B, HIPK1, TGFBR1, BMPR2, APC |
| GO:0006350 | Transcription | 13 | 41.94 | .00034 | PLAG1, LMO3, BMPR2, MED14, CREB5, MED13, CBFB, PURB, HIPK1, MTF1, SETD7, SP7, NFIB |
| GO:0003002 | Regionalization | 5 | 16.13 | .00058 | ACVR2B, HIPK1, TGFBR1, BMPR2, APC |
| GO:0045449 | Regulation of transcription | 14 | 45.16 | .00063 | PLAG1, LMO3, TGFBR1, MED14, CREB5, MED13, CBFB, PURB, ACVR2B, HIPK1, MTF1, SETD7, SP7, NFIB |
| GO:0045941 | Positive regulation of transcription | 7 | 22.58 | .00071 | MTF1, TGFBR1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0010628 | Positive regulation of gene expression | 7 | 22.58 | .00083 | MTF1, TGFBR1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0035295 | Tube development | 5 | 16.13 | .00089 | B4GALT1, ACVR2B, TGFBR1, BMPR2, NFIB |
| GO:0030324 | Lung development | 4 | 12.90 | .00098 | ACVR2B, TGFBR1, BMPR2, NFIB |
| GO:0030323 | Respiratory tube development | 4 | 12.90 | .001067 | ACVR2B, TGFBR1, BMPR2, NFIB |
| GO:0006366 | Transcription from RNA polymerase II promoter | 5 | 16.13 | .00112 | BMPR2, CREB5, MED14, MED13, CBFB |
| GO:0045935 | Positive regulation of nucleic acid metabolic process | 7 | 22.58 | .001212 | MTF1, TGFBR1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0060541 | Respiratory system development | 4 | 12.90 | .001259 | ACVR2B, TGFBR1, BMPR2, NFIB |
| GO:0051173 | Positive regulation of nitrogen compound metabolic process | 7 | 22.58 | .001427 | MTF1, TGFBR1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0010557 | Positive regulation of macromolecule biosynthetic process | 7 | 22.58 | .001544 | MTF1, TGFBR1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0007389 | Pattern specification process | 5 | 16.13 | .001821 | ACVR2B, HIPK1, TGFBR1, BMPR2, APC |
| GO:0031328 | Positive regulation of cellular biosynthetic process | 7 | 22.58 | .001957 | MTF1, TGFBR1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0009891 | Positive regulation of biosynthetic process | 7 | 22.58 | .002106 | MTF1, TGFBR1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0045893 | Positive regulation of transcription, DNA-dependent | 6 | 19.35 | .002265 | MTF1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0051254 | Positive regulation of RNA metabolic process | 6 | 19.35 | .002349 | MTF1, CREB5, MED14, MED13, CBFB, NFIB |
| GO:0006351 | Transcription, DNA-dependent | 5 | 16.13 | .002522 | BMPR2, CREB5, MED14, MED13, CBFB |
| GO:0032774 | RNA biosynthetic process | 5 | 16.13 | .002649 | BMPR2, CREB5, MED14, MED13, CBFB |
| GO:0045667 | Regulation of osteoblast differentiation | 3 | 9.68 | .003294 | ACVR2B, BMPR2, APC |
| GO:0045944 | Positive regulation of transcription from RNA polymerase II promoter | 5 | 16.13 | .005928 | MTF1, MED14, MED13, CBFB, NFIB |
| GO:0030278 | Regulation of ossification | 3 | 9.68 | .010493 | ACVR2B, BMPR2, APC |
| GO:0051247 | Positive regulation of protein metabolic process | 4 | 12.90 | .012182 | TGFBR1, BMPR2, RICTOR, APC |
| GO:0042325 | Regulation of phosphorylation | 5 | 16.13 | .01301 | ACVR2B, TGFBR1, BMPR2, RICTOR, APC |
| GO:0006357 | Regulation of transcription from RNA polymerase II promoter | 6 | 19.35 | .013315 | MTF1, SP7, MED14, MED13, CBFB, NFIB |
| GO:0001934 | Positive regulation of protein amino acid phosphorylation | 3 | 9.68 | .013501 | TGFBR1, BMPR2, RICTOR |
| GO:0051174 | Regulation of phosphorus metabolic process | 5 | 16.13 | .014883 | ACVR2B, TGFBR1, BMPR2, RICTOR, APC |
| GO:0042327 | Positive regulation of phosphorylation | 3 | 9.68 | .015896 | TGFBR1, BMPR2, RICTOR |
| GO:0010562 | Positive regulation of phosphorus metabolic process | 3 | 9.68 | .016838 | TGFBR1, BMPR2, RICTOR |
| GO:0051094 | Positive regulation of developmental process | 4 | 12.90 | .017444 | B4GALT1, ACVR2B, BMPR2, APC |
| GO:0007178 | Transmembrane receptor protein serine/threonine kinase signaling pathway | 3 | 9.68 | .017803 | ACVR2B, TGFBR1, BMPR2 |
| GO:0042127 | Regulation of cell proliferation | 6 | 19.35 | .018247 | B4GALT1, HIPK1, TGFBR1, BMPR2, NFIB, APC |
| GO:0006355 | Regulation of transcription, DNA-dependent | 9 | 29.03 | .019046 | MTF1, SETD7, CREB5, SP7, MED14, MED13, PURB, CBFB, NFIB |
| GO:0001655 | Urogenital system development | 3 | 9.68 | .020145 | ACVR2B, TGFBR1, APC |
| GO:0051252 | Regulation of RNA metabolic process | 9 | 29.03 | .02157 | MTF1, SETD7, CREB5, SP7, MED14, MED13, PURB, CBFB, NFIB |
| GO:0007167 | Enzyme linked receptor protein signaling pathway | 4 | 12.90 | .029914 | ACVR2B, GRB10, TGFBR1, BMPR2 |
| GO:0044087 | Regulation of cellular component biogenesis | 3 | 9.68 | .032355 | TGFBR1, RICTOR, APC |
| GO:0051896 | Regulation of protein kinase B signaling cascade | 2 | 6.45 | .033413 | TGFBR1, RICTOR |
| GO:0060491 | Regulation of cell projection assembly | 2 | 6.45 | .035344 | TGFBR1, APC |
| GO:0030501 | Positive regulation of bone mineralization | 2 | 6.45 | .035344 | ACVR2B, BMPR2 |
| GO:0070169 | Positive regulation of biomineral formation | 2 | 6.45 | .037272 | ACVR2B, BMPR2 |
| GO:0001932 | Regulation of protein amino acid phosphorylation | 3 | 9.68 | .046311 | TGFBR1, BMPR2, RICTOR |
| GO:0045778 | Positive regulation of ossification | 2 | 6.45 | .046856 | ACVR2B, BMPR2 |
| GO:0032273 | Positive regulation of protein polymerization | 2 | 6.45 | .048762 | RICTOR, APC |
| GO:0045669 | Positive regulation of osteoblast differentiation | 2 | 6.45 | .048762 | ACVR2B, BMPR2 |
| GO:0015850 | Organic alcohol transport | 2 | 6.45 | .048762 | SLC44A1, HIATL1 |
GO = gene ontology.
Table 5.
List of the Most Significant Molecular Function Biologic Processes as Derived From Interrogation of Predicted mRNA Target Genes
| GO ID | GO Term | Gene Count | Percentage | P Value | Genes |
|---|---|---|---|---|---|
| GO:0004675 | Transmembrane receptor protein serine/threonine kinase activity | 3 | 9.68 | .00058 | ACVR2B, TGFBR1, BMPR2 |
| GO:0005024 | Transforming growth factor β receptor activity | 3 | 9.68 | .00058 | ACVR2B, TGFBR1, BMPR2 |
| GO:0030145 | Manganese ion binding | 4 | 12.90 | .00348327 | B4GALT1, ACVR2B, TGFBR1, BMPR2 |
| GO:0030528 | Transcription regulator activity | 9 | 29.03 | .00754371 | PLAG1, MTF1, CREB5, SP7, MED14, MED13, PURB, CBFB, NFIB |
| GO:0016563 | Transcription activator activity | 5 | 16.13 | .0084461 | MTF1, MED14, MED13, CBFB, NFIB |
| GO:0003713 | Transcription coactivator activity | 4 | 12.90 | .00868169 | MTF1, MED14, MED13, CBFB |
| GO:0008134 | Transcription factor binding | 5 | 16.13 | .01805295 | MTF1, MED14, MED13, PURB, CBFB |
| GO:0019838 | Growth factor binding | 3 | 9.68 | .0185566 | ACVR2B, TGFBR1, BMPR2 |
| GO:0003712 | Transcription cofactor activity | 4 | 12.90 | .03502498 | MTF1, MED14, MED13, CBFB |
| GO:0003700 | Transcription factor activity | 6 | 19.35 | .04146813 | PLAG1, MTF1, CREB5, PURB, CBFB, NFIB |
| GO:0042809 | Vitamin D receptor binding | 2 | 6.45 | .04509705 | MED14, MED13 |
| GO:0043167 | Ion binding | 14 | 45.16 | .04984618 | PLAG1, B4GALT1, FRAS1, GABRB2, LMO3, TGFBR1, BMPR2, CREB5, PCDH7, ACVR2B, MTF1, PHF15, SP7, CACNA1C |
See Table 4 legend for expansion of abbreviation.
Table 6.
List of the Most Significant Cellular Component Biologic Processes as Derived From Interrogation of Predicted mRNA Target Genes
| GO ID | GO Term | Gene Count | Percentage | P Value | Genes |
|---|---|---|---|---|---|
| GO:0009986 | Cell surface | 4 | 12.90 | .020994 | FRAS1, B4GALT1, ACVR2B, BMPR2 |
| GO:0044459 | Plasma membrane part | 9 | 29.03 | .025195 | FRAS1, B4GALT1, ACVR2B, GABRB2, TGFBR1, BMPR2, PCDH7, CACNA1C, APC |
| GO:0016592 | Srb-mediator complex | 2 | 6.45 | .0487818 | MED14, MED13 |
See Table 4 legend for expansion of abbreviation.
Gene Networks
Individual miRNAs are involved in a variety of biologic responses, and certain categories are also enriched in their own target genes. We therefore constructed gene networks for all target predicted genes (Fig 3, e-Figs 1A, 1B). We also determined gene networks for the putative predicted target genes for each of the three differentially expressed miRNAs (e-Figs 1-3, e-Tables 1-3).
Discussion
The present study demonstrates that endothelial dysfunction in otherwise healthy obese and nonobese children is associated with a distinctive circulating miRNA signature. Many diseases are characterized by an abnormal miRNA expression pattern, and identification of the miRNA signature for specific diseases should prove useful for early screening and diagnosis of asymptomatic diseases such as endothelial dysfunction. Here, we identified three unique miRNAs in the circulating plasma of children with endothelial dysfunction and further examined putative gene target prediction genes for those miRNAs through implementation of several previously validated and established algorithms. Venn diagrams of the intersections between predicted target genes showed that 31 genes are common gene targets among all three differentially expressed miRNAs linked to endothelial dysfunction and also revealed some of the putative biologic functions and signaling pathways that may be pathophysiologically relevant in the context of abnormal endothelial function in children.45
In the present study, we identified three miRNAs in plasma, namely hsa-miR-125a-5p, hsa-miR-342-3p, and hsa-miR-365b-3p, whose concentrations were significantly altered in the plasma of eight otherwise asymptomatic children with endothelial dysfunction when compared with eight matched children in whom normal endothelial function was present. Further confirmatory efforts revealed the specificity of these miRNAs, as the miRNA differential expression persisted in a post hoc cohort of 60 children (including the original 16 subjects), even when obese NEF children (n = 5) were compared with nonobese children with endothelial dysfunction (n = 5). It has been suggested that hsa-mir-125a-5p is involved in microarray expression profiling in human myocardial infarction and remodeling process during the progression of heart disease.46 This mir-125a has been suggested to coordinate the suppression of ERBB2 and ERBB3 by enforced expression of miR-125a.47 Plasma microRNAs can serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure and have included hsa-mir-342-3p.48, 49 For hsa-mir-365b, it has been reported to be involved in advanced heart failure and also following support with a left ventricular assist device.50
Increasing evidence indicates that miRNAs play critical roles in many key biologic processes, such as cell growth, tissue differentiation, embryonic development, cell proliferation, and apoptosis.51 As such, dysregulation of miRNAs and their effects on downstream gene targets may result in disease states that include the cardiovascular system.52, 53 Recently, several studies have shown that plasma miRNA levels are significantly altered in specific CVDs and that some of these miRNAs have the potential to serve as novel biomarkers for early diagnosis.54 Since a single miRNA can target several genes, and multiple miRNAs may share common targets, miRNAs are particularly suited for regulating processes and pathways at the “network” level.55 The current challenge is to accurately identify targets that are specifically regulated by miRNAs.
To further understand the function of the three differentially expressed plasma miRNAs in children with endothelial dysfunction, we proceeded to identify their putative targets using multiple bioinformatics tools. Although miRNAs traditionally influence the expression of their target genes in a negative manner via mRNA degradation and translational repression, there is also evidence supporting that they may also enhance the expression of genes.56 Prediction of miRNA target genes is challenging in that most bioinformatics approaches, when used alone, usually yield an excessive number of genes. Thus, we applied a strict curation of such lists through combinatorial bioinformatics analyses. In this study, we found some potential gene targets involved in metabolic processing of macromolecules such as those included in the GO terms MED13 and MED14 (Table 4, Table 5, Table 6). Mediators (MED) are multi-subunit complexes, which act as a bridge between DNA-bound transcription factors and RNA polymerase II, which joins transcription factors bound at the upstream regulatory elements, such as nuclear receptors and the transcription machinery at the promoter region.57, 58, 59, 60 A study demonstrated that MED13 functions in the heart to control metabolic homeostasis and energy expenditure,58 whereas MED14 directly interacts with the N-terminus of PPARγ in a ligand-independent manner and is required for the transcription activity of peroxisome proliferator-activated receptor γ.61 Activin A receptor, type IIB (ACVR2B) activins are dimeric growth and differentiation factors that belong to the TGF-β superfamily of structurally related signaling proteins. Activin A has been recognized as a multifunctional cytokine expressed in a wide range of tissues and cells with roles in regulation of wound repair, cell differentiation, apoptosis, embryogenesis, and inflammation.62 Activin A is induced by angiotensin II in smooth muscle cells and may be induced by inflammatory cytokines such as tumor necrosis factor-α and IL-1.63 Thus, there is clearly biologic plausibility to the miRNA-RNA networks identified in children with endothelial dysfunction that could potentially be used in future multicenter studies aimed at validating the miRNA signature discovered herein.
Some of the strengths and limitations of the study should be mentioned. First, our recruitment approaches using a laser Doppler-based technique for assessment of endothelial function in children enabled inclusion of otherwise healthy children from the community. Furthermore, implementation of a previously validated 60-s occlusion duration rather than the more extensively used 5 min in adults prevents frequent motion-induced artifacts in children without detracting from the physiologic significance of the measurement.36, 64 In addition, all vascular assessments were conducted in the fasting state at the same time of the day to minimize potential confounders introduced by differences in meal content and timing relative to the testing and also to ensure that circadian variation in endothelial function would not play a role. Particular precautions were implemented to avoid hemolysis during plasma isolation, since it can affect circulating miRNA levels. However, we did not assess miR-15b and/or miR-16 as hemolysis indicators. In addition, exploratory in silico analyses (eg, Tarbase query) did not reveal any known relevant vascular function for two of the identified three miRNAs. Since these databases are continuously being updated, prospective re-evaluation of such miRNAs is justified. Finally, since the initial screening cohort was relatively small, the possibility exists that additional differentially expressed miRNAs may have not been identified, and as such additional biomarkers may be found in future larger studies.
Endothelial dysfunction induces impairment of regional blood flow in various organs and tissues. It is now evident from many studies that childhood obesity is correlated with adult excess weight status and the development of risk factors for cardiovascular diseases in adulthood, including hypertension, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome.65 Findings about obese children include not only the alteration characteristic of metabolic syndrome but also systemic low-grade inflammation and disorders indicative of endothelial dysfunction.66, 67, 68 In this study we found that some of the metabolic parameters are significantly higher, such as triglyceride, insulin, and HOMA-insulin resistance, as well as hsCRP as shown in Table 1. The magnitude and characteristics of such metabolic and inflammatory parameters would ultimately underlie the probability of end-organ vascular injury with its attendant morbid consequences. However, the specificity of the three putative miRNAs as being indicative of endothelial dysfunction, rather than reflecting obesity-related or metabolic-related abnormalities, was confirmed by the differentially expressed miRNAs in five nonobese children with NEF and five nonobese children with endothelial dysfunction in whom there was no evidence of either dyslipidemia or insulin resistance. In the next a few years, we likely will see further breakthroughs in cardiovascular miRNA research, such as initial phase 1 and II projects using miRNA therapeutics in cardiovascular medicine that may pave the way for large-scale mechanism-orientated miRNA-based therapeutic trials in pediatric cardiovascular risk management approaches.
Conclusions
This study provides initial evidence that a subset of circulating miRNAs are deregulated in children with endothelial dysfunction. Biomarkers for early detection of children in general, and more particularly overweight and obese children, at risk for developing vascular abnormalities would greatly improve delineation of targeted interventions in this high-risk population. We further show that systems biology-based approaches provide new avenues for biologic interpretation of miRNA profiling and generation of experimentally testable hypotheses regarding collective regulatory functions of miRNAs in cardiovascular disease.
Acknowledgments
Author contributions: D. G. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. A. K. contributed to performing miRNA experiments, performed data analyses, and drafted the initial version of the manuscript; L. K.-G. contributed to performing endothelial function studies, and performed data analyses; R. B. contributed to recruiting experimental subjects and assisted with endothelial function studies; A. A. K. contributed to performing bioinformatics analyses; D. G. contributed to conceptualizing the project, provided the funding for the project, critically reviewed data, and contributed to all phases of manuscript drafting; D. G., A. K., L. K.-G., R. B., and A. A. K. contributed to reviewing and approving the final version of the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work is supported by the National Institutes of Health [Grant HL-65270 to D. G. and L. K.-G.], the Herbert T. Abelson Endowed Chair in Pediatrics, and the American Heart Association [Grant 13SDG14780079 to R. B.].
Supplementary Data
References
- 1.Kelishadi R. Childhood overweight, obesity, and the metabolic syndrome in developing countries. Epidemiol Rev. 2007;29:62–76. doi: 10.1093/epirev/mxm003. [DOI] [PubMed] [Google Scholar]
- 2.Haslam D.W., James W.P. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Mi J., Shan X.Y., Wang Q.J., Ge K.Y. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes. 2007;31(1):177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 4.Reilly J.J., Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011;35(7):891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 5.Landsberg L., Aronne L.J., Beilin L.J. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment–a position paper of the Obesity Society and The American Society of Hypertension. Obesity (Silver Spring) 2013;21(1):8–24. doi: 10.1002/oby.20181. [DOI] [PubMed] [Google Scholar]
- 6.Ribeiro M.M., Silva A.G., Santos N.S. Diet and exercise training restore blood pressure and vasodilatory responses during physiological maneuvers in obese children. Circulation. 2005;111(15):1915–1923. doi: 10.1161/01.CIR.0000161959.04675.5A. [DOI] [PubMed] [Google Scholar]
- 7.Aggoun Y., Farpour-Lambert N.J., Marchand L.M., Golay E., Maggio A.B., Beghetti M. Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J. 2008;29(6):792–799. doi: 10.1093/eurheartj/ehm633. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee R., Alotaibi W.H., Kheirandish-Gozal L., Capdevila O.S., Gozal D. Endothelial dysfunction in obese non-hypertensive children without evidence of sleep disordered breathing. BMC Pediatr. 2010;10:8. doi: 10.1186/1471-2431-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gozal D., Kheirandish-Gozal L. Cardiovascular morbidity in obstructive sleep apnea: oxidative stress, inflammation, and much more. Am J Respir Crit Care Med. 2008;177(4):369–375. doi: 10.1164/rccm.200608-1190PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shashaj B., Bedogni G., Graziani M.P. Origin of cardiovascular risk in overweight preschool children: a cohort study of cardiometabolic risk factors at the onset of obesity. JAMA Pediatr. 2014;168(10):917–924. doi: 10.1001/jamapediatrics.2014.900. [DOI] [PubMed] [Google Scholar]
- 11.Chatsuriyawong S., Gozal D., Kheirandish-Gozal L. Genetic variance in nitric oxide synthase and endothelin genes among children with and without endothelial dysfunction. J Transl Med. 2013;11:227. doi: 10.1186/1479-5876-11-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davignon J., Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 suppl 1):III27–III32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- 13.Hirata Y., Nagata D., Suzuki E., Nishimatsu H., Suzuki J., Nagai R. Diagnosis and treatment of endothelial dysfunction in cardiovascular disease. Int Heart J. 2010;51(1):1–6. doi: 10.1536/ihj.51.1. [DOI] [PubMed] [Google Scholar]
- 14.Versari D., Daghini E., Virdis A., Ghiadoni L., Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(suppl 2):S314–S321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartel D.P. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilad S., Meiri E., Yogev Y. Serum microRNAs are promising novel biomarkers. PLoS One. 2008;3(9):e3148. doi: 10.1371/journal.pone.0003148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khalyfa A., Gozal D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J Transl Med. 2014;12:162. doi: 10.1186/1479-5876-12-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell P.S., Parkin R.K., Kroh E.M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krützfeldt J., Stoffel M. MicroRNAs: a new class of regulatory genes affecting metabolism. Cell Metab. 2006;4(1):9–12. doi: 10.1016/j.cmet.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Bonauer A., Boon R.A., Dimmeler S. Vascular microRNAs. Curr Drug Targets. 2010;11(8):943–949. doi: 10.2174/138945010791591313. [DOI] [PubMed] [Google Scholar]
- 21.Thai T.H., Christiansen P.A., Tsokos G.C. Is there a link between dysregulated miRNA expression and disease? Discov Med. 2010;10(52):184–194. [PubMed] [Google Scholar]
- 22.Kawashima T., Shioi T. MicroRNA, emerging role as a biomarker of heart failure. Circ J. 2011;75(2):268–269. doi: 10.1253/circj.cj-10-1254. [DOI] [PubMed] [Google Scholar]
- 23.Latronico M.V., Condorelli G. MicroRNAs and cardiac pathology. Nat Rev Cardiol. 2009;6(6):419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 24.Adachi T., Nakanishi M., Otsuka Y. Plasma microRNA 499 as a biomarker of acute myocardial infarction. Clin Chem. 2010;56(7):1183–1185. doi: 10.1373/clinchem.2010.144121. [DOI] [PubMed] [Google Scholar]
- 25.Weber J.A., Baxter D.H., Zhang S. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markham D.W., Hill J.A. MicroRNAs and heart failure diagnosis: MiR-acle or MiR-age? Circ Res. 2010;106(6):1011–1013. doi: 10.1161/CIRCRESAHA.110.219030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zampetaki A., Willeit P., Drozdov I., Kiechl S., Mayr M. Profiling of circulating microRNAs: from single biomarkers to re-wired networks. Cardiovasc Res. 2012;93(4):555–562. doi: 10.1093/cvr/cvr266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zampetaki A., Kiechl S., Drozdov I. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107(6):810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 29.Contu R., Latronico M.V., Condorelli G. Circulating microRNAs as potential biomarkers of coronary artery disease: A promise to be fulfilled? Circ Res. 2010;107(5):573–574. doi: 10.1161/CIRCRESAHA.110.227983. [DOI] [PubMed] [Google Scholar]
- 30.Ortega F.J., Mercader J.M., Catalán V. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59(5):781–792. doi: 10.1373/clinchem.2012.195776. [DOI] [PubMed] [Google Scholar]
- 31.D’Alessandra Y., Devanna P., Limana F. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur Heart J. 2010;31(22):2765–2773. doi: 10.1093/eurheartj/ehq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Stefano V., Zaccagnini G., Capogrossi M.C., Martelli F. microRNAs as peripheral blood biomarkers of cardiovascular disease. Vascul Pharmacol. 2011;55(4):111–118. doi: 10.1016/j.vph.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 33.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents Synopsis book: the fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(suppl 2):555–576. [PubMed] [Google Scholar]
- 34.Gozal D., Kheirandish-Gozal L., Serpero L.D., Sans Capdevila O., Dayyat E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: effect of adenotonsillectomy. Circulation. 2007;116(20):2307–2314. doi: 10.1161/CIRCULATIONAHA.107.696823. [DOI] [PubMed] [Google Scholar]
- 35.Kheirandish-Gozal L., Bhattacharjee R., Kim J., Clair H.B., Gozal D. Endothelial progenitor cells and vascular dysfunction in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2010;182(1):92–97. doi: 10.1164/rccm.200912-1845OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharjee R., Kim J., Alotaibi W.H., Kheirandish-Gozal L., Capdevila O.S., Gozal D. Endothelial dysfunction in children without hypertension: potential contributions of obesity and obstructive sleep apnea. Chest. 2012;141(3):682–691. doi: 10.1378/chest.11-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wahlberg E., Olofsson P., Swendenborg J., Fagrell B. Changes in postocclusive reactive hyperaemic values as measured with laser Doppler fluxmetry after infrainguinal arterial reconstructions. Eur J Vasc Endovasc Surg. 1995;9(2):197–203. doi: 10.1016/s1078-5884(05)80090-5. [DOI] [PubMed] [Google Scholar]
- 38.Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 39.Mestdagh P., Hartmann N., Baeriswyl L. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (miRQC) study. Nat Methods. 2014;11(8):809–815. doi: 10.1038/nmeth.3014. [DOI] [PubMed] [Google Scholar]
- 40.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Huang W., Sherman B.T., Lempicki R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 42.Dennis G., Jr., Sherman B.T., Hosack D.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4(5):3. [PubMed] [Google Scholar]
- 43.Kanehisa M., Goto S., Sato Y., Furumichi M., Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40(database issue):D109–D114. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlachos I.S., Kostoulas N., Vergoulis T. DIANA miRPath v.2.0: investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012;40(Web Server issue):W498–W504. doi: 10.1093/nar/gks494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu F., Guo N.J., Tian H. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2011;17(1):241–250. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Small E.M., Olson E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011;469(7330):336–342. doi: 10.1038/nature09783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ikeda S., Kong S.W., Lu J. Altered microRNA expression in human heart disease. Physiol Genomics. 2007;31(3):367–373. doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
- 48.Dickinson B.A., Semus H.M., Montgomery R.L. Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure. Eur J Heart Fail. 2013;15(6):650–659. doi: 10.1093/eurjhf/hft018. [DOI] [PubMed] [Google Scholar]
- 49.Bronze-da-Rocha E. MicroRNAs expression profiles in cardiovascular diseases. Biomed Res Int. 2014;2014:985408. doi: 10.1155/2014/985408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang K.C., Yamada K.A., Patel A.Y. Deep RNA sequencing reveals dynamic regulation of myocardial noncoding RNAs in failing human heart and remodeling with mechanical circulatory support. Circulation. 2014;129(9):1009–1021. doi: 10.1161/CIRCULATIONAHA.113.003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esquela-Kerscher A., Slack F.J. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 52.Latronico M.V., Catalucci D., Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circ Res. 2007;101(12):1225–1236. doi: 10.1161/CIRCRESAHA.107.163147. [DOI] [PubMed] [Google Scholar]
- 53.Liu N., Olson E.N. MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010;18(4):510–525. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sayed A.S., Xia K., Salma U., Yang T., Peng J. Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ. 2014;23(6):503–510. doi: 10.1016/j.hlc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Inukai S., Slack F. MicroRNAs and the genetic network in aging. J Mol Biol. 2013;425(19):3601–3608. doi: 10.1016/j.jmb.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huntzinger E., Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet. 2011;12(2):99–110. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 57.Liu X., Bushnell D.A., Kornberg R.D. RNA polymerase II transcription: structure and mechanism. Biochim Biophys Acta. 2013;1829(1):2–8. doi: 10.1016/j.bbagrm.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grueter C.E., van Rooij E., Johnson B.A. A cardiac microRNA governs systemic energy homeostasis by regulation of MED13. Cell. 2012;149(3):671–683. doi: 10.1016/j.cell.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schiano C., Casamassimi A., Vietri M.T., Rienzo M., Napoli C. The roles of mediator complex in cardiovascular diseases. Biochim Biophys Acta. 2014;1839(6):444–451. doi: 10.1016/j.bbagrm.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 60.Taatjes D.J. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem Sci. 2010;35(6):315–322. doi: 10.1016/j.tibs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grøntved L., Madsen M.S., Boergesen M., Roeder R.G., Mandrup S. MED14 tethers mediator to the N-terminal domain of peroxisome proliferator-activated receptor gamma and is required for full transcriptional activity and adipogenesis. Mol Cell Biol. 2010;30(9):2155–2169. doi: 10.1128/MCB.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen Y.G., Lui H.M., Lin S.L., Lee J.M., Ying S.Y. Regulation of cell proliferation, apoptosis, and carcinogenesis by activin. Exp Biol Med (Maywood) 2002;227(2):75–87. doi: 10.1177/153537020222700201. [DOI] [PubMed] [Google Scholar]
- 63.Shao L.E., Frigon N.L., Jr., Yu A., Palyash J., Yu J. Contrasting effects of inflammatory cytokines and glucocorticoids on the production of activin A in human marrow stromal cells and their implications. Cytokine. 1998;10(3):227–235. doi: 10.1006/cyto.1997.0282. [DOI] [PubMed] [Google Scholar]
- 64.Kheirandish-Gozal L., Wang Y., Duggan R.C. Nitric oxide production by monocytes in children with OSA and endothelial dysfunction. Clin Sci (Lond) 2014;127(5):323–330. doi: 10.1042/CS20130679. [DOI] [PubMed] [Google Scholar]
- 65.Herouvi D., Karanasios E., Karayianni C., Karavanaki K. Cardiovascular disease in childhood: the role of obesity. Eur J Pediatr. 2013;172(6):721–732. doi: 10.1007/s00431-013-1932-8. [DOI] [PubMed] [Google Scholar]
- 66.Valle M., Gascón F., Martos R. Metabolic cardiovascular syndrome in obese prepubertal children: the role of high fasting insulin levels. Metabolism. 2002;51(4):423–428. doi: 10.1053/meta.2002.31319. [DOI] [PubMed] [Google Scholar]
- 67.Csábi G., Török K., Jeges S., Molnár D. Presence of metabolic cardiovascular syndrome in obese children. Eur J Pediatr. 2000;159(1-2):91–94. doi: 10.1007/pl00013812. [DOI] [PubMed] [Google Scholar]
- 68.Süheyl Ezgü F., Hasanoğlu A., Tümer L., Ozbay F., Aybay C., Gündüz M. Endothelial activation and inflammation in prepubertal obese Turkish children. Metabolism. 2005;54(10):1384–1389. doi: 10.1016/j.metabol.2005.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.