Summary
Background
Exenatide, a glucagon-like peptide-1 (GLP-1) receptor agonist, has neuroprotective effects in preclinical models of Parkinson’s disease. We investigated whether these effects would be apparent in a clinical trial.
Methods
In this single-centre, randomised, double-blind, placebo-controlled trial, patients with moderate Parkinson’s disease were randomly assigned (1:1) to receive subcutaneous injections of exenatide 2 mg or placebo once weekly for 48 weeks in addition to their regular medication, followed by a 12-week washout period. Eligible patients were aged 25–75 years, had idiopathic Parkinson’s disease as measured by Queen Square Brain Bank criteria, were on dopaminergic treatment with wearing-off effects, and were at Hoehn and Yahr stage 2·5 or less when on treatment. Randomisation was by web-based randomisation with a two strata block design according to disease severity. Patients and investigators were masked to treatment allocation. The primary outcome was the adjusted difference in the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) motor subscale (part 3) in the practically defined off-medication state at 60 weeks. All efficacy analyses were based on a modified intention-to-treat principle, which included all patients who completed any post-randomisation follow-up assessments. The study is registered at ClinicalTrials.gov (NCT01971242) and is completed.
Findings
Between June 18, 2014, and March 13, 2015, 62 patients were enrolled and randomly assigned, 32 to exenatide and 30 to placebo. Our primary analysis included 31 patients in the exenatide group and 29 patients in the placebo group. At 60 weeks, off-medication scores on part 3 of the MDS-UPDRS had improved by 1·0 points (95% CI −2·6 to 0·7) in the exenatide group and worsened by 2·1 points (−0·6 to 4·8) in the placebo group, an adjusted mean difference of −3·5 points (−6·7 to −0·3; p=0·0318). Injection site reactions and gastrointestinal symptoms were common adverse events in both groups. Six serious adverse events occurred in the exenatide group and two in the placebo group, although none in either group were judged to be related to the study interventions.
Interpretation
Exenatide had positive effects on practically defined off-medication motor scores in Parkinson’s disease, which were sustained beyond the period of exposure. Whether exenatide affects the underlying disease pathophysiology or simply induces long-lasting symptomatic effects is uncertain. Exenatide represents a major new avenue for investigation in Parkinson’s disease, and effects on everyday symptoms should be examined in longer-term trials.
Funding
Michael J Fox Foundation for Parkinson’s Research.
Introduction
Perhaps the most important unmet need in Parkinson’s disease is the development of a neuroprotective or disease-modifying therapy that can slow or halt disease progression. None of the compounds that had potential neuroprotective properties in in-vitro or animal models have shown any effects on disease progression in clinical trials.1
Glucagon-like peptide-1 (GLP-1) agonists are licensed for the treatment of type 2 diabetes. These drugs activate GLP-1 receptors to promote glucose-level-dependent insulin secretion, inhibit glucagon secretion, and slow gastric emptying.2 Exenatide is a synthetic version of exendin-4, a naturally occurring analogue of human GLP-1 that was originally discovered in the saliva of the Gila monster (Heloderma suspectum) and is resistant to the normal metabolic processes that degrade endogenous human GLP-1.3 In addition to effects on glucose homoeostasis, evidence from studies in toxin-based rodent models of Parkinson’s disease show that exenatide crosses the blood–brain barrier and exerts neuroprotective and neurorestorative effects via GLP-1 receptors at doses similar to those used in type 2 diabetes, resulting in improvements in motor performance, behaviour, learning, and memory.4–8
We previously did a small, proof-of-concept, open-label trial9 of exenatide in patients with Parkinson’s disease of moderate severity. 12 months’ exposure to exenatide led to improvements in motor and cognitive assessments in the intervention group compared with the control group, which persisted 12 months after drug withdrawal.10 On the basis of these encouraging findings, we aimed to do a randomised, placebo-controlled trial (NCT01971242) to assess further the potential disease-modifying effects of 48 weeks’ exposure to exenatide, followed by a 12-week washout, on the motor severity of Parkinson’s disease.
Methods
Study design and participants
We did a randomised, double-blind, placebo-controlled, parallel-group, single-centre trial of exenatide once weekly in Parkinson’s disease of moderate severity. The trial was done at the Leonard Wolfson Experimental Neuroscience Centre (London, UK), a dedicated clinical trial research facility and part of the University College London (UCL) Institute of Neurology and the National Hospital for Neurology & Neurosurgery. The study was coordinated by the UCL Comprehensive Clinical Trials Unit (London, UK). Clinical oversight was provided by a trial steering committee, and an independent data and safety monitoring board. Trial operations were supported by the Leonard Wolfson Experimental Neuroscience Centre and the National Institute for Health Research Biomedical Research Centre at the UCL Institute of Neurology and the National Hospital for Neurology and Neurosurgery (London, UK).
Eligible men and women were aged 25–75 years, had idiopathic Parkinson’s disease as measured by Queen Square Brain Bank criteria,11 were on dopaminergic treatment with wearing-off effects, were judged able to administer the trial drug, and were at Hoehn and Yahr stage 2·5 or less when on treatment. We pre-screened patients over the phone against these criteria before formal in-person screening. Key exclusion criteria (see trial protocol for full list) included concurrent dementia (defined as a score <120 points on the Mattis-Dementia Rating scale), body-mass index (BMI) of less than 18·5, and diabetes (glycated haemoglobin [HbA1c] ≥48 mmol/mol at screening). This trial was approved by the Brent NHS Research Ethics Committee, London. All patients provided written informed consent.
Randomisation and masking
Patients were recruited from the pool of patients attending the National Hospital for Neurology & Neurosurgery, or approached us as a result of hearing about the trial on ClinicalTrials.gov or Fox Trial Finder. We used SealedEnvelope, an independent, commerical, internet-based randomisation service that generated the online randomisation list on the basis of guidance from the trial IT manager (SH) and trial statistician (SSS). After extensive testing to ensure that the service worked perfectly, the trial recruiting team used it for randomisation, with a block design of two strata according to disease severity (Hoehn and Yahr stage 1·0–2·0 vs stage 2·5). Patients were randomly assigned (1:1) to subcutaneous exenatide once weekly or matched placebo injections, in addition to their regular drugs. The trial statistician (SSS) generated and uploaded unique three-digit identifiers for every active and placebo drug kit to the randomisation service to allow allocation of masked study drug kits (sufficient for 12 weeks) at randomisation and follow-up visits by assessing clinicians. The randomisation service then provided the relevant kit numbers that were to be dispensed to the patient from the hospital pharmacy. Patients and investigators were masked to treatment allocation throughout the study. The study drug kit codes were known only to the qualified person at the Royal Free Pharmacy (London, UK), who ensured that exenatide and placebo kits were labelled with the appropriate codes, and to SealedEnvelope. Data were unblinded after data base lock, at which point the statistican analysing the data was unblinded.
Procedures
Our trial had a washout design, comprising a 48-week exposure period, in which patients self-administered exenatide 2 mg or placebo via subcutaneous injection once weekly, followed by study drug withdrawal and a final assessment 12 weeks later. At screening for trial entry, each patient underwent a physical and neurological examination, assessments of mood and cognition, and blood sampling for clinical laboratory tests; women of childbearing potential also had a pregnancy test. Electrocardiographic and [123I]FP-CIT single photon emission CT (DaTscan) imaging were also done. After confirmation of patient eligibility, subsequent visits were held at baseline (week 0) and weeks 12, 24, 36, 48, and 60. Immediately after completion of all baseline assessments, patients were instructed on how to assemble and self-administer the injections and were witnessed administering their first injection. At each visit, patients were supplied with study drug kits.
Patients were asked to attend each visit in an off-medication state, which was defined as a period of withdrawal of levodopa for at least 8 h (ie, overnight) or 36 h in the case of long-acting drugs such as ropinirole, pramipexole, rasagiline, and rotigotine. All assessments were done early in the morning. A dedicated trial team used the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) and timed motor tests (10 m timed walk, timed keyboard taps in 30 s with a novel internet-based program) to assess patients. Patients then underwent repeat motor assessments roughly 1 h after taking their regular Parkinson’s disease medications (to allow uniformity across patients), alongside assessments of cognition (Mattis Dementia Rating Scale), dyskinesia (Unified Dyskinesia Rating Scale), quality of life (EuroQol Five Dimensions Questionnaire, Parkinson’s Disease Questionnaire 39), mood (Montgomery-Asberg Depression Rating Scale), and non-motor symptoms (Non-Motor Symptoms Severity Scale). Empty drug vials and questionnaires were collected at each visit to assess compliance. Patients also kept Hauser diaries.
After 48 weeks, study drugs were withdrawn. We did a final clinical assessment and repeat DaTscan imaging at 60 weeks. Blood and urine were collected at each visit, and cerebrospinal fluid (CSF) was collected at weeks 12 and 48, for pharmacokinetic measurements. Assessment of serum and CSF concentrations of exenatide was done in a blinded manner in duplicate across all samples by fluorescent ELISA immunoassay (FEK-070-94; Phoenix Pharmaceuticals, Burlingame, CA, USA) at the National Institute on Aging (Baltimore, MD, USA). Levodopa equivalent dose (LED) was calculated at each visit.12 Changes in concurrent medication were allowed throughout the trial to minimise drop out. To prevent the possibility of adverse events compromising rater blinding, all adverse events, biochemical results, blood pressure, heart rate, and weight were recorded separately, by clinicians who were also masked to treatment allocation.
Outcomes
The primary outcome was change in MDS-UPDRS part 3 scores in the practically defined off-medication state at 60 weeks (ie, after any possible symptomatic effects of exenatide should have washed out). Predefined secondary outcomes were differences between exenatide and placebo in each subsection of the MDS-UPDRS in the on-medication state and the Mattis Dementia Rating Scale at weeks 48 and 60. Additional secondary measures included frequency of adverse events, changes in vital signs, weight, and clinical laboratory values. Exploratory outcomes included between-group differences in dopamine transporter availability as measured by DaTscan,13 timed motor tests in both off-medication and on-medication states, LED, 3-day Hauser diary of Parkinson’s disease state, and scores on the Unified Dyskinesia Rating Scale, Montgomery and Asberg Depression Rating Scale, Non-Motor Symptoms Severity Scale, Parkinson’s Disease Questionnaire 39, and EuroQol Five Dimensions Questionnaire.
Statistical analysis
All study analyses were done according to a predefined statistical analysis plan. To analyse the effect of treatment allocation on the primary outcome, we used a regression analysis of covariance (ANCOVA) approach to adjust for stratification factors (Hoehn and Yahr stage) and baseline raw MDS-UPDRS part 3 values. On the basis of previously collected pilot data9 and with a two-sided 5% significance level, we estimated that a sample size of 60 patients would be required to detect a difference of 5·8 MDS-UPDRS points between the two groups. These calculations were based on a common SD of 13, 90% power, and an overall type 1 error rate of 5%. Additionally, we assumed a correlation of 0·85 between baseline and follow-up MDS-UPDRS measurements. All efficacy analyses were based on a modified intention-to-treat principle, which included all patients who completed any post-randomisation follow-up assessments.
Differences in continuous motor and non-motor outcome measures in the on-medication state were estimated with the same regression approach, which was adjusted for stratification factors, baseline scores, and any change from baseline in LED to account for the possible confounding effect of changes in Parkinson’s disease medications during the trial. Comparison of gastrointestinal adverse events between treatment groups was done with χ2 tests. We used Pearson’s correlation to investigate a possible relation between noted treatment effects and potential confounding factors, such as weight loss and change in LED. A post-hoc exploratory analysis of the primary outcome additionally adjusted for change from baseline in LED was also subsequently done to address the possibility that differential increases in LED could have confounded motor assessments even in the off-medication state.
We used statistical parametric mapping to quantitatively analyse DaTscan data. Baseline and delayed images for each participant were smoothed and coregistered before spatial normalisation into Montreal Neurological Institute space via a DaTscan template. We used a fully flexible model after image scaling to assess between-group differences in loss of DaTscan uptake between baseline and 60-week scans by ANCOVA, adjusting for baseline differences in DaTscan signal, Hoehn and Yahr stage, and change in LED at 60 weeks. Further analysis was also done to assess the differences in the changes between the two allocations. The resulting statistical parametric maps were masked to restrict differences to bilateral caudate and putamen regions at a height threshold of p less than 0·01, uncorrected for multiple comparisons, and an extent threshold of ten voxels.
We did a planned interim analysis after 60 participants completed 24 weeks’ follow-up. The change in MDS-UPDRS part 3 score between baseline and 24 weeks was compared between groups and analysed by the trial statistician at UCL’s Comprehensive Clinical Trials Unit, who ensured that the trial team remained blinded to treatment allocations. The results of the interim analysis were communicated to the independent data-monitoring committee only, and recommendations to continue the trial, based on recruitment and adverse event profiles only, were communicated to both the trial steering committee and the study funders. We used STATA/MP and SPSS for all analyses.
Role of the funding source
The study funder had roles in study design and data interpretation, but no role in data collection or analysis, or writing of the Article. After the planned interim analysis, recommendations to continue the trial based on recruitment, and details about adverse event profiles were communicated to the funder, who remained blinded to individual treatment allocation. All authors had full access to all study data, and the corresponding author had responsibility for the final decision to submit the Article for publication.
Results
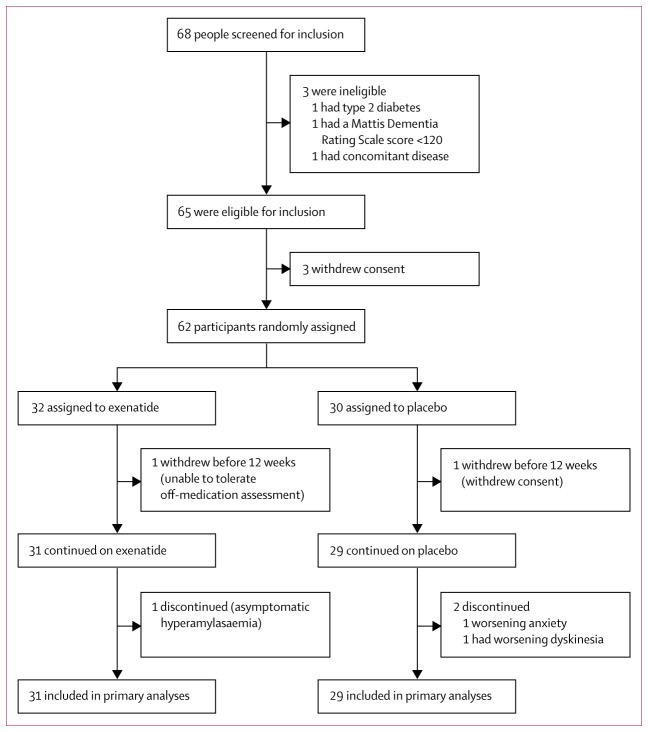
Between June 18, 2014, and March 13, 2015, 68 patients were screened for eligibility, 62 of whom were randomly assigned to either exenatide or placebo (figure 1). Patients randomly allocated to exenatide were slightly older, had higher baseline MDS-UPDRS part 3 scores, and had lower LED than did those assigned to placebo (table 1). Questionnaire responses and collection of empty drug vials at each visit suggested that compliance with study drugs was very high: 58 patients reported not missing a single dose (data not shown).
Figure 1. Trial profile.
Individuals who withdrew before 12 weeks could not contribute data to the primary outcome, and so were replaced per protocol. All 60 patients who completed at least the initial 12-week follow-up were included in the primary analysis.
Table 1.
Patient characteristics at baseline
| Exenatide (n=31) | Placebo (n=29) | |
|---|---|---|
| Age, years | 61·6 (8·2) | 57·8 (8·0) |
|
| ||
| Sex | ||
| Female | 9 (29%) | 7 (24%) |
| Male | 22 (71%) | 22 (76%) |
|
| ||
| Age at diagnosis, years | 55·9 (7·9) | 52·2 (7·7) |
|
| ||
| Duration of diagnosis at baseline, years | 6·4 (3·3) | 6·4 (3·3) |
|
| ||
| Hoehn and Yahr stage | ||
| 1·0–2·0 | 29 (94%) | 29 (100%) |
| 2·5 | 2 (6%) | 0 (0%) |
|
| ||
| MDS-UPDRS part 3 off medication | 32·8 (9·7) | 27·1 (10·3) |
|
| ||
| Levodopa equivalent dose, mg | 773·9 (260·9) | 825·7 (215·0) |
Data are mean (SD) or n (%). This table excludes two patients who were recruited but did not complete any follow-up visits. MDS-UPDRS=Movement Disorders Society Unified Parkinson’s Disease Rating Scale.
At 60 weeks, off-medication scores in MDS-UPDRS part 3 had worsened by 2·1 points (95% CI −0·6 to 4·8) in the placebo group and improved by 1·0 points (−2·6 to 0·7) in the exenatide group, a significant adjusted difference of −3·5 points (−6·7 to −0·3) favouring exenatide (p=0·0318; figure 2; table 2). At 48 weeks, scores in the placebo group had deteriorated by 1·7 points (−0·6 to 4·0) and those in the exenatide group had improved by 2·3 points (−4·1 to −0·7) points (table 2), resulting in a significant adjusted between-group difference of −4·3 points (−7·1 to −1·6; p=0·0026). On-medication scores on MDS-UPDRS parts 1–4 did not differ significantly between groups at 48 or 60 weeks (table 2). Data were missing for only one participant at 60 weeks, so we did not do sensitivity analyses for the primary outcome.
Figure 2. MDS-UPDRS part 3 scores (A) and changes in MDS-UPDRS part 3 scores (B), by study visit Data are means for the off-medication state. Error bars represent standard error of the mean.
MDS-UPDRS=Movement Disorders Society Unified Parkinson’s Disease Rating Scale.
Table 2.
MDS-UPDRS scores between baseline and week 60
| Baseline | 12 weeks | 24 weeks | 36 weeks | 48 weeks | Change (0–48 weeks) | Adjusted difference (0–48 weeks) | p value | 60 weeks | Change (0–60 weeks) | Adjusted difference (0–60 weeks) | p value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDS-UPDRS part 3 (off medication) | ||||||||||||
|
| ||||||||||||
| Exenatide | 32·8 (9·7) | 30·3 (10·9) | 30·6 (10·8) | 31·2 (11·3) | 30·2 (11·1) | −2·3 (−4·1 to −0·7) | −4·3 (−7·1 to −1·6) | 0·0026 | 31·9 (12·0) | −1·0 (−2·6 to 0·7) | −3·5 (−6·7 to −0·3) | 0·0318 |
| Placebo | 27·1 (10·3) | 27·6 (11·8) | 28·5 (11·0) | 28·6 (9·5) | 28·8 (10·8) | 1·7 (−0·6 to 4·0) | ·· | ·· | 29·2 (12·0) | 2·1 (−0·6 to 4·8) | ·· | ·· |
|
| ||||||||||||
| MDS-UPDRS part 1 (on medication) | ||||||||||||
|
| ||||||||||||
| Exenatide | 9·8 (4·8) | 8·6 (4·2) | 8·3 (3·6) | 8·0 (4·2) | 8·8 (4·4) | −1·0 (−2·4 to 0·4) | −1·3 (−3·4 to 0·8) | 0·21 | 9·3 (4·0) | −0·5 (−2·0 to 1·1) | −1·2 (−3·2 to 0·8) | 0·22 |
| Placebo | 9·2 (3·8) | 8·7 (5·0) | 8·9 (4·4) | 9·3 (4·6) | 9·7 (5·6) | 0·5 (−1·2 to 2·2) | ·· | ·· | 10·1 (5·3) | 0·7 (−0·8 to 2·3) | ·· | ·· |
|
| ||||||||||||
| MDS-UPDRS part 2 (on medication) | ||||||||||||
|
| ||||||||||||
| Exenatide | 12·5 (6·7) | 10·9 (7·0) | 11·2 (7·4) | 11·7 (7·8) | 11·7 (6·3) | −0·7 (−2·1 to 0·7) | −0·6 (−2·7 to 1·5) | 0·58 | 11·6 (6·6) | −0·8 (−2·2 to 0·6) | −0·6 (−2·7 to 1·5) | 0·55 |
| Placebo | 10·7 (5·3) | 10·2 (5·6) | 11·1 (6·0) | 10·1 (6·1) | 10·8 (5·6) | 0·1 (−1·6 to 1·9) | ·· | ·· | 11·0 (6·7) | 0·2 (−1·4 to 1·8) | ·· | ·· |
|
| ||||||||||||
| MDS-UPDRS part 3 (on medication) | ||||||||||||
|
| ||||||||||||
| Exenatide | 19·4 (8·4) | 19·3 (9·1) | 20·4 (9·7) | 19·6 (8·8) | 20·5 (9·5) | 1·1 (−0·8 to 3·0) | −0·002 (−2·4 to 2·4) | 0·99 | 19·9 (10·3) | 0·5 (−1·9 to 3·0) | 0·7 (−2·1 to 3·6) | 0·61 |
| Placebo | 14·4 (8·2) | 15·4 (8·3) | 16·0 (7·1) | 16·7 (7·7) | 15·7 (7·1) | 1·3 (−0·4 to 3·0) | ·· | ·· | 14·5 (7·1) | −0·02 (−1·8 to 1·8) | ·· | ·· |
|
| ||||||||||||
| MDS-UPDRS part 4 (on medication) | ||||||||||||
|
| ||||||||||||
| Exenatide | 4·7 (3·1) | 4·1 (3·4) | 4·2 (2·0) | 4·6 (2·5) | 4·9 (2·5) | 0·3 (−0·9 to 1·4) | −0·5 (−1·8 to 0·9) | 0·48 | 5·2 (2·3) | 0·5 (−0·5 to 1·6) | −0·6 (−2·1 to 0·9) | 0·42 |
| Placebo | 5·3 (3·0) | 5·8 (2·7) | 5·2 (3·2) | 5·3 (3·4) | 5·6 (3·0) | 0·3 (−0·9 to 1·5) | ·· | ·· | 6·1 (3·7) | 0·7 (−0·7 to 2·1) | ·· | ·· |
Data are mean (SD) or mean (95% CI). MDS-UPDRS=Movement Disorders Society Unified Parkinson’s Disease Rating Scale.
We noted no significant differences between the exenatide and placebo groups in scores on the Mattis Dementia Rating Scale, Montgomery-Asberg Depression Rating Scale, Unified Dyskinesia Rating Scale, Non-Motor Symptoms Severity Scale, Parkinson’s Disease Questionnaire summary index, or EuroQol Five Dimensions Questionnaire, nor in results on timed motor tests or Hauser diaries (table 3). Although no significant difference was noted in total LED at 60 weeks between the groups, the mean increase in LED was 19·6 mg higher in the exenatide than in the placebo group (table 3). In our post-hoc exploratory analysis adjusted for differences in LED from baseline, off-medication scores in part 3 of the MDS-UPDRS were 3·6 points (95% CI −6·8 to −0·4; p=0·0294) lower at 60 weeks and 4·4 points (−7·2 to −1·6; 0·0023) lower at 48 weeks in the exenatide group than in the placebo group. We noted no significant correlation between change in LED and change in the primary outcome (ρ=0·17; p=0·3588).
Table 3.
Changes in cognition, motor and non-motor symptoms, mood, quality of life, vital signs, and medications between baseline and week 60
| Baseline | 12 weeks | 24 weeks | 36 weeks | 48 weeks | Change (0–48 weeks) |
Adjusted difference (0–48 weeks) |
p value | 60 weeks | Change (0–60 weeks) |
Adjusted difference (0–60 weeks) |
p value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mattis Dementia Rating Scale* | ||||||||||||
|
| ||||||||||||
| Exenatide | 138·0 (5·0) | 139·0 (6·1) | 139·5 (4·2) | 140·3 (3·7) | 139·7 (4·1) | 1·7 (0·4 to 2·9) | 0·4 (−1·0 to 1·9) | 0·57 | 139·9 (3·6) | 1·9 (0·6 to 3·1) | 0·8 (−0·9 to 2·5) | 0·32 |
| Placebo | 139·8 (3·7) | 140·3 (3·1) | 139·7 (5·8) | 140·3 (4·1) | 140·2 (3·9) | 0·4 (−0·6 to 1·5) | ·· | ·· | 140·2 (4·6) | 0·4 (−1·1 to 1·8) | ·· | ·· |
|
| ||||||||||||
| Unified Dyskinesia Rating Scale | ||||||||||||
|
| ||||||||||||
| Exenatide | 5·4 (7·9) | 5·4 (8·0) | 4·4 (6·5) | 5·6 (7·9) | 5·1 (7·1) | −0·3 (−2·3 to 1·8) | −0·8 (−3·6 to 1·9) | 0·53 | 6·2 (7·2) | 0·8 (−1·7 to 3·3) | −1·6 (−5·1 to 1·8) | 0·35 |
| Placebo | 7·3 (9·4) | 6·8 (9·7) | 6·9 (9·8) | 6·8 (9·9) | 7·4 (10·7) | 0·1 (−1·7 to 1·8) | ·· | ·· | 9·0 (12·4) | 1·7 (−0·8 to 4·2) | ·· | ·· |
|
| ||||||||||||
| MADRS | ||||||||||||
|
| ||||||||||||
| Exenatide | 4·1 (3·7) | 3·4 (3·5) | 2·2 (1·8) | 2·7 (3·1) | 2·5 (2·7) | −1·6 (−3·4 to 0·07) | −1·4 (−3·2 to 0·5) | 0·15 | 2·1 (2·6) | −1·6 (−2·7 to −0·4) | −0·9 (−2·2 to 0·3) | 0·15 |
| Placebo | 3·7 (3·0) | 2·9 (3·8) | 3·5 (4·4) | 3·9 (4·4) | 3·8 (4·2) | 0·2 (−1·8 to 2·2) | ·· | ·· | 2·8 (2·6) | −0·9 (−2·3 to 0·5) | ·· | ·· |
|
| ||||||||||||
| NMSS | ||||||||||||
|
| ||||||||||||
| Exenatide | 24·6 (19·8) | 17·7 (15·4) | 16·4 (12·4) | 16·5 (10·3) | 19·7 (12·4) | −4·9 (−11·6 to 1·8) | −4·0 (−11·8 to 3·8) | 0·30 | 22·3 (14·2) | −2·3 (−9·6 to 5·1) | −3·3 (−11·7 to 5·1) | 0·43 |
| Placebo | 28·3 (24·7) | 22·0 (22·4) | 22·1 (20·2) | 23·1 (21·6) | 25·8 (22·8) | −2·5 (−9·5 to 4·6) | ·· | ·· | 27·6 (23·3) | −1·5 (−9·0 to 6·0) | ·· | ·· |
|
| ||||||||||||
| PDQ-39 summary index | ||||||||||||
|
| ||||||||||||
| Exenatide | 19·9 (13·7) | 17·1 (10·7) | 16·8 (10·6) | 17·2 (11·4) | 18·7 (12·7) | −1·2 (−4·7 to 2·3) | −1·7 (−5·6 to 2·1) | 0·38 | 18·4 (11·1) | −1·5 (−5·4 to 2·4) | −3·3 (−8·0 to 1·5) | 0·17 |
| Placebo | 21·1 (13·0) | 17·8 (10·9) | 18·6 (14·2) | 20·5 (15·6) | 20·1 (12·8) | −1·1 (−4·2 to 2·1) | ·· | ·· | 22·2 (14·8) | 0·3 (−3·4 to 4·0) | ·· | ·· |
|
| ||||||||||||
| EQ5D index* | ||||||||||||
|
| ||||||||||||
| Exenatide | 0·71 (0·20) | 0·72 (0·17) | 0·76 (0·14) | 0·81 (0·14) | 0·74 (0·23) | 0·03 (−0·07 to 0·12) | 0·06 (−0·03 to 0·15) | 0·21 | 0·72 (0·18) | 0·005 (−0·08 to 0·09) | −0·003 (−0·09 to 0·09) | 0·95 |
| Placebo | 0·79 (0·16) | 0·72 (0·19) | 0·77 (0·14) | 0·75 (0·16) | 0·74 (0·14) | −0·05 (−0·10 to 0·00) | ·· | ·· | 0·75 (0·14) | −0·06 (−0·12 to 0·01) | ·· | ·· |
|
| ||||||||||||
| EQ5D VAS (%)* | ||||||||||||
|
| ||||||||||||
| Exenatide | 73·6 (14·5) | 72·3 (13·7) | 71·5 (15·6) | 71·4 (16·6) | 70·1 (15·6) | −3·2 (−8·9 to 2·5) | 6·9 (−1·0 to 14·8) | 0·08 | 68·1 (14·4) | −5·6 (−12·2 to 1·1) | 5·3 (−3·0 to 13·5) | 0·21 |
| Placebo | 74·5 (16·0) | 68·6 (13·2) | 68·5 (18·7) | 69·0 (19·7) | 64·7 (20·5) | −9·3 (−15·4 to −3·1) | ·· | ·· | 65·1 (20·2) | −10·6 (−16·4 to −4·8) | ·· | ·· |
|
| ||||||||||||
| Right hand taps in 30 s* | ||||||||||||
|
| ||||||||||||
| Exenatide (off medication) | 46·5 (9·9) | 48·3 (10·7) | 48·5 (13·8) | 46·9 (12·4) | 47·9 (11·2) | 1·1 (−2·5 to 4·8) | −1·1 (−5·8 to 3·6) | 0·69 | 46·6 (12·1) | 0·4 (−3·0 to 3·8) | 1·1 (−4·3 to 6·4) | 0·64 |
| Placebo (off medication) | 53·9 (13·1) | 54·0 (13·0) | 52·2 (12·2) | 52·9 (11·4) | 50·5 (11·0) | −3·1 (−7·8 to 1·7) | ·· | ·· | 52·7 (9·8) | −1·0 (−4·5 to 2·5) | ·· | ·· |
| Exenatide (on medication) | 52·8 (11·7) | 53·0 (12·0) | 51·5 (11·9) | 51·5 (12·9) | 51·3 (12·9) | −1·5 (−7·0 to 3·9) | −3·2 (−8·4 to 2·1) | 0·28 | 52·6 (11·4) | −0·7 (−5·7 to 4·3) | −3·4 (−9·6 to 2·8) | 0·23 |
| Placebo (on medication) | 59·1 (14·5) | 59·3 (11·6) | 59·3 (12·4) | 59·7 (10·0) | 57·6 (10·3) | −1·3 (−5·9 to 3·4) | ·· | ·· | 58·7 (11·5) | 0·4 (−2·9 to 3·7) | ·· | ·· |
|
| ||||||||||||
| Left hand taps in 30 s* | ||||||||||||
|
| ||||||||||||
| Exenatide (off medication) | 47·8 (9·3) | 48·8 (9·4) | 49·0 (10·5) | 48·3 (8·7) | 47·7 (9·8) | 0·3 (−2·7 to 3·3) | −0·9 (−4·8 to 2·9) | 0·69 | 47·2 (9·7) | −0·6 (−3·8 to 2·6) | 0·2 (−4·0 to 4·4) | 0·62 |
| Placebo (off medication) | 50·6 (11·5) | 52·6 (11·8) | 50·2 (11·0) | 49·9 (10·5) | 49·7 (10·2) | −0·9 (−4·6 to 2·8) | ·· | ·· | 49·5 (9·7) | −0·3 (−3·0 to 2·3) | ·· | ·· |
| Exenatide (on medication) | 52·9 (10·0) | 49·8 (12·7) | 50·6 (10·1) | 50·5 (10·8) | 49·0 (10·2) | −4·1 (−7·4 to −0·9) | −1·2 (−5·2 to 2·8) | 0·18 | 50·9 (12·0) | −2·1 (−5·3 to 1·2) | −2·9 (−7·1 to 1·4) | 0·54 |
| Placebo (on medication) | 56·6 (13·0) | 56·3 (12·1) | 55·8 (10·3) | 56·3 (10·0) | 54·6 (11·9) | −2·2 (−5·6 to 1·2) | ·· | ·· | 54·1 (10·8) | −1·1 (−3·4 to 1·2) | ·· | ·· |
|
| ||||||||||||
| 10 m timed walk (s) | ||||||||||||
|
| ||||||||||||
| Exenatide (off medication) | 17·2 (4·5) | 16·2 (7·8) | 17·3 (9·6) | 16·7 (8·1) | 17·4 (11·1) | 0·2 (−3·1 to 3·4) | 0·8 (−4·6 to 6·1) | 0·69 | 19·5 (16·5) | 2·5 (−2·5 to 7·5) | −0·7 (−4·2 to 2·8) | 0·78 |
| Placebo (off medication) | 17·1 (6·3) | 16·2 (5·4) | 16·4 (7·1) | 14·8 (4·8) | 16·6 (8·8) | −0·5 (−2·9 to 1·9) | ·· | ·· | 19·1 (16·0) | 1·8 (−2·5 to 6·1) | ·· | ·· |
| Exenatide (on medication) | 15·2 (2·7) | 14·9 (3·4) | 14·7 (3·3) | 14·4 (3·3) | 15·1 (5·5) | −0·03 (−1·5 to 1·4) | −1·5 (−4·6 to 1·6) | 0·61 | 15·0 (5·8) | −0·1 (−1·6 to 1·4) | 0·3 (−0·9 to 1·6) | 0·35 |
| Placebo (on medication) | 14·7 (3·1) | 14·3 (3·2) | 14·4 (3·7) | 14·2 (3·3) | 13·6 (3·0) | −1·1 (−1·8 to −0·4) | ·· | ·· | 15·3 (7·5) | 0·6 (−2·4 to 3·7) | ·· | ·· |
|
| ||||||||||||
| Hauser diary: asleep (%) | ||||||||||||
|
| ||||||||||||
| Exenatide | 30 | 29 | 30 | 31 | 30 | ·· | ·· | 28 | ·· | ·· | ·· | |
| Placebo | 26 | 26 | 27 | 27 | 27 | ·· | ·· | 25 | ·· | ·· | ·· | |
|
| ||||||||||||
| Hauser diary (off; %) | ||||||||||||
|
| ||||||||||||
| Exenatide | 17 | 14 | 15 | 12 | 16 | ·· | ·· | 18 | ·· | ·· | ·· | |
| Placebo | 20 | 20 | 17 | 19 | 20 | ·· | ·· | 22 | ·· | ·· | ·· | |
|
| ||||||||||||
| Hauser diary: on without dyskinesia (%) | ||||||||||||
|
| ||||||||||||
| Exenatide | 49 | 53 | 48 | 52 | 49 | ·· | ·· | 50 | ·· | ·· | ·· | |
| Placebo | 49 | 50 | 50 | 48 | 47 | ·· | ·· | 47 | ·· | ·· | ·· | |
|
| ||||||||||||
| Hauser diary: on with non-troublesome dyskinesia (%) | ||||||||||||
|
| ||||||||||||
| Exenatide | 3 | 3 | 5 | 4 | 5 | ·· | ·· | 5 | ·· | ·· | ·· | |
| Placebo | 3 | 2 | 5 | 3 | 4 | ·· | ·· | 4 | ·· | ·· | ·· | |
|
| ||||||||||||
| Hauser diary: on with troublesome dyskinesia (%) | ||||||||||||
|
| ||||||||||||
| Exenatide | 1 | 4 | 2 | 1 | 1 | ·· | ·· | ·· | 5 | ·· | ·· | ·· |
| Placebo | 1 | 2 | 1 | 3 | 2 | ·· | ·· | ·· | 3 | ·· | ·· | ·· |
|
| ||||||||||||
| Mean arterial blood pressure (mm Hg) | ||||||||||||
|
| ||||||||||||
| Exenatide | 95·4 (15·8) | 95·8 (12·1) | 96·2 (11·5) | 93·8 (12·2) | 96·8 (11·0) | 1·4 (−2·7 to 5·6) | ·· | ·· | 95·8 (13·6) | 0·4 (−4·2 to 4·9) | ·· | ·· |
| Placebo | 94·2 (7·9) | 93·1 (11·2) | 93·8 (9·1) | 93·8 (9·5) | 95·0 (9·6) | 0·8 (−2·2 to 3·7) | ·· | ·· | 95·2 (7·6) | 1·3 (−2·1 to 4·7) | ·· | ·· |
|
| ||||||||||||
| Weight (kg) | ||||||||||||
|
| ||||||||||||
| Exenatide | 81·8 (16·6) | 80·0 (16·3) | 79·3 (16·5) | 78·1 (15·9) | 79·2 (16·1) | −2·6 (−4·0 to −1·2) | ·· | ·· | 80·9 (16·6) | −0·9 (−2·6 to 0·7) | ·· | ·· |
| Placebo | 80·8 (12·9) | 80·1 (14·3) | 80·2 (14·0) | 79·5 (13·6) | 80·2 (13·3) | −0·6 (−1·9 to 0·8) | ·· | ·· | 80·5 (14·3) | −0·09 (−1·5 to 1·3) | ·· | ·· |
|
| ||||||||||||
| Levodopa equivalent dose (mg) | ||||||||||||
|
| ||||||||||||
| Exenatide | 773·9 (260·9) | 804·5 (288·3) | 851·7 (336·5) | 849·3 (368·6) | 895·6 (337·7) | 121·8 (47·7 to 195·8) | ·· | ·· | 906·1 (328·8) | 132·2 (61·5 to 203·0) | ·· | ·· |
| Mean change per visit | ·· | 30·6 | 47·2 | −2·4 | 46·3 | ·· | ·· | ·· | 10·5 | ·· | ·· | ·· |
| Placebo | 825·7 (215·0) | 828·8 (225·4) | 897·5 (225·0) | 883·3 (218·9) | 913·0 (243·4) | 87·3 (−2·4 to 177·1) | ·· | ·· | 942·7 (235·2) | 112·6 (40·7 to 184·4) | ·· | ·· |
| Mean change per visit | ·· | 3·1 | 68·7 | −14·2 | 29·7 | ·· | ·· | ·· | 29·7 | ·· | ·· | ·· |
|
| ||||||||||||
| Parkinson’s drugs (n) | ||||||||||||
|
| ||||||||||||
| Exenatide | ||||||||||||
| Levodopa | 31 | 31 | 31 | 31 | 31 | ·· | ·· | 31 | ·· | ·· | ·· | |
| Dopamine agonists | 24 | 25 | 24 | 24 | 24 | ·· | ·· | 24 | ·· | ·· | ·· | |
| Monoamine oxidase B inhibitors | 17 | 17 | 17 | 17 | 17 | ·· | ·· | 17 | ·· | ·· | ·· | |
| Placebo | ||||||||||||
| Levodopa | 29 | 29 | 29 | 29 | 29 | ·· | ·· | 29 | ·· | ·· | ·· | |
| Dopamine agonists | 23 | 23 | 25 | 25 | 25 | ·· | ·· | 25 | ·· | ·· | ·· | |
| Monoamine oxidase B inhibitors | 13 | 13 | 14 | 14 | 15 | ·· | ·· | 15 | ·· | ·· | ·· | |
Data are mean (SD) or mean (95% CI), except for data for Parkinson’s drugs, which are n. All scores are for the on-medication state, unless otherwise specified. MADRS=Montgomery-Asberg Depression Rating
Scale. NMSS=Non-Motor Symptoms Scale. PDQ-39=Parkinson’s Disease Questionnaire 39. EQ5D=EuroQol Five Dimensions Questionnaire. VAS=visual analogue scale.
Higher scores show improved status.
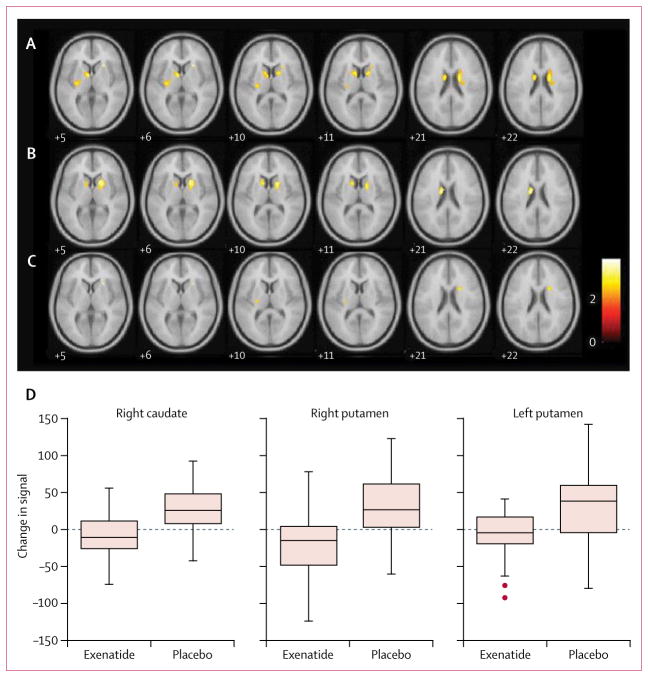
Statistical parametric mapping analysis contrasted to show regions with decreased DaTscan binding between the first and the second scan showed declines in both groups (figure 3). The contrasts suggested a reduced rate of decline of DaTscan binding in the exenatide group compared with the placebo group in the right putamen (x 22, y 8, z 22; T=2·98, 24 voxels; uncorrected p=0·0018), left putamen (−26, −18, 10; 2·76, 12 voxels; 0·0034), and right caudate (26, 20, 6; 3·83, 10 voxels; 0·0001).
Figure 3. ANCOVA comparing decline in DaTscan binding between the placebo and exenatide groups.
(A) Placebo group: reduced DaTscan binding in the left caudate, right caudate, and left putamen. (B) Exenatide group: reduced DaTscan binding in the left caudate and right caudate. (C) Significant clusters derived from the first level of analysis used to do an ANCOVA between placebo and exenatide groups showing a reduced rate of decline in the right caudate, left putamen, and right putamen. (D) Boxplots of mean change in DaTscan binding ratio for the relevant volume of interest. Montreal Neurological Institute of standardised space are shown in each slice; the error bars represent 1·5 times the IQR. ANCOVA=analysis of covariance. DaTscan= [123I]FP-CIT single photon emission CT.
Median peak serum exenatide concentrations were 543·3 pg/mL in the exenatide group. Median CSF concentrations were 11·4 pg/mL at 12 weeks and 11·7 pg/mL at 48 weeks. The frequency of adverse effects did not differ significantly between groups (table 4). Weight change occurred in both groups, but was more common in the exenatide than in the placebo group. At 48 weeks, patients in the exenatide group had lost a mean of 2·6 kg (95% CI −4·0 to −1·2), whereas those in the control group had lost 0·6 kg (−1·9 to 0·8). We noted no significant correlation between the degree of weight loss and change in the primary outcome (ρ=0·30; p=0·0986). Other gastrointestinal symptoms associated with exenatide occurred in both groups, and the presence or absence of weight loss, nausea, loss of appetite, or abdominal pain was not significantly associated with treatment allocation (χ2=0·388; p=0·5330). Eight serious adverse events were recorded, six in the exenatide group and two in the placebo group; none were judged to be related to the study interventions. No other clinically relevant changes in biochemical indices or vital signs.
Table 4.
Adverse events
| Exenatide | Placebo | |
|---|---|---|
| Serious adverse events | ||
|
| ||
| Fall* | 2 | 0 |
| Atrial flutter† | 1 | 0 |
| Acute urinary retention | 1 | 0 |
| Collapse | 1 | 0 |
| Significant weight loss‡ | 0 | 1 |
| Faecal impaction | 0 | 1 |
| Postural hypotension | 1 | 0 |
|
| ||
| Adverse events | ||
|
| ||
| Injection site reaction | 27 | 26 |
| Weight loss from baseline§ | 24 | 18 |
| 0–2 kg | 11 | 10 |
| 2–4 kg | 2 | 3 |
| >4 kg | 11 | 5 |
| Nausea | 16 | 10 |
| Other pain | 13 | 11 |
| Constipation | 12 | 11 |
| Increased time off medication | 8 | 12 |
| Diarrhoea | 8 | 6 |
| Weight gain from baseline§ | 7 | 11 |
| Lower-urinary-tract symptoms | 6 | 7 |
| Sleep disorder | 3 | 6 |
| Abdominal pain | 5 | 3 |
| Increased dystonia | 3 | 5 |
| Back pain | 2 | 5 |
| Upper-respiratory-tract infection | 5 | 3 |
| Dyskinesia | 2 | 3 |
| Loss of appetite | 3 | 1 |
| Anxiety | 2 | 1 |
| Freezing | 1 | 2 |
| Urinary-tract infection | 0 | 3 |
| Hyperamylasaemia | 1 | 1 |
| Rash | 1 | 1 |
| Vomiting | 2 | 0 |
| Fever | 1 | 0 |
| Worsening tremor | 0 | 1 |
| Miscellaneous | 64 | 46 |
One fall occurred in an individual before randomisation.
Occurred before the first dose of exenatide.
Defined as decrease in body-mass index of >10% in 12 weeks.
Figures for weight loss or gain are presented according to numbers of individuals with weight change at 48 weeks rather than number of individual events across all timepoints.
Three patients discontinued the study drug before 48 weeks but continued follow-up assessments as per protocol. One patient in the exenatide group had asymptomatic hyperamylasaemia at 12 weeks (predefined as a rise greater than 50% above baseline concentrations and the laboratory reference range), and thus study drug was withdrawn. Two patients in the placebo group discontinued injections, one after 9 weeks because of worsening anxiety, the other after 36 weeks because of dyskinesia. An emergency unblinding procedure was necessary for one patient in the placebo group, who developed pancreatic cancer shortly after the end of the trial monitoring period.
Discussion
In this randomised, double-blind, placebo-controlled, parallel-group, single-centre trial, patients with Parkinson’s disease of moderate severity given exenatide for 48 weeks had a significant advantage of 3·5 points on part 3 of the MDS-UPDRS in an off-medication state compared with those given placebo 12 weeks after stopping exenatide. However, no significant differences were noted between the exenatide and placebo groups in scores on parts 1–4 of the MDS-UPDRS (in the on-medication state), or in assessments of cognition, mood, dyskinesia, non-motor symptoms, or quality of life. Frequency and severity of adverse events did not differ significantly between the two groups and were not significantly related to changes in motor scores.
Because of the fluctuating nature of symptom severity according to dopaminergic treatment, we did all study assessments in the early morning in the practically defined off-medication state. Patients with moderate disease experiencing wearing-off effects were preferentially recruited over those with de-novo or early-stage disease to increase the speed of recruitment and to minimise inclusion of patients with atypical forms of parkinsonism, the number of recruiting centres (thereby reducing costs), the risk of differential dropout among treatment-naive patients receiving placebo, and floor effects on rating assessment scales.
The simple washout trial design enabled faster and more cost-efficient data collection than would have been possible with more complex and expensive pivotal trial designs, such as delayed start, randomised withdrawal, or long-term simple approaches. The single-centre nature eliminated inter-site variability in data collection, thereby potentially facilitating the detection of significant effects despite the small sample size, and resulted in an extremely low dropout rate (data for only one participant were missing for the primary outcome). That patients could seek medication adjustments via their treating clinicians throughout the trial, similar to routine clinical practice in Parkinson’s disease, could also have contributed to patient retention.
Exenatide was well tolerated. Previously recognised adverse effects, including gastrointestinal symptoms and injection-site reactions, occurred in similar frequencies in this patient group as have been previously reported in trials of people with diabetes,14 and did not affect compliance. Early observational studies15,16 suggested that exenatide could be associated with pancreatic cancer; however, a recent study showed no significant associations.17 Asymptomatic hyperamylasaemia was reported in one patient in the exenatide group, necessitating drug withdrawal. Exenatide can induce amylase secretion in vitro, and increased amylase concentrations have been reported in patients with type 2 diabetes treated with similar drugs.18 This effect is a possible explanation for the hyperamylasaemia, although the contribution of other comorbid disorders cannot be excluded. Patients in the exenatide group lost a mean of 2·6 kg, which reversed on drug cessation. Excessive weight loss (>10% of BMI during 12 weeks) necessitated temporary withdrawal of the study drug in only one patient, who was in the placebo group.
Our study had several limitations. To ensure preservation of blinding of the rating of Parkinson’s disease severity, recording of adverse events and measurement of vital signs and weight was done by independent clinicians. However, patients might have been partly unblinded to their treatment allocation as a result of adverse effects (although injection site reactions were similar across both groups). Furthermore, the small size of our study meant that, despite randomisation with a block design according to Hoehn and Yahr status, the exenatide group had higher MDS-UPDRS part 3 scores and lower LED at baseline than the placebo group, necessitating adjustment in the primary analysis (although this adjustment was prespecified). Our statistical analyses suggest that none of the differences in outcome measures can be explained by differences in adverse events, baseline disease severity, or adjustment to conventional Parkinson’s disease medications.
To allow us to recruit patients already treated with dopaminergic replacement, we were compelled to use the practically defined off-medication MDS UPDRS part 3 scores as our primary outcome. Although this measure provides a better insight into disease severity than do on-medication scores, additional variability in scores could be due to differences in timing since last dose of Parkinson’s disease medication, despite the consistent instructions given to patients and all assessments being done at consistent times in the morning. This possibility deserves consideration, especially because the differences we noted in off-medication scores were not supported by significant differences in clinical secondary outcomes. This absence of significant differences between groups is likely to be partly because of the major effects of dopaminergic replacement on any scores assessed in the on-medication state (which reflects the usual situation of patients). Whether the absences of significant differences between groups in off-medication timed tests or Hauser diaries could be related to differences in the sensitivity or precision within these measures, the small sample size, or disease stage of the population needs to be further explored.
We noted little evidence of any placebo effect in the control group. By contrast, in the exenatide group, improvements in MDS-UPDRS part 3 scores were already detectable at 12 weeks, suggesting possible symptomatic effects on Parkinson’s disease. Furthermore, the benefits noted in the exenatide group were greater at 48 weeks than at 60 weeks, which is again potentially indicative of a symptomatic effect. Nonetheless, the persisting improvements in MDS-UPDRS part 3 scores at 60 weeks suggests that exenatide could have a longer-lasting effect on disease severity beyond conventional drug effects on dopaminergic receptors.
The demonstration that exenatide might have novel symptomatic effects is an important discovery in treatment of Parkinson’s disease. Preclinical studies5,19 suggest that exenatide can normalise dopaminergic function in lesioned rodents, but whether symptomatic effects relate to improvement in functioning in surviving dopaminergic neurons or to changes to the pharmacokinetics of levodopa or other dopaminergic therapies requires further study. Beyond the identification of a drug that could have novel symptomatic effects in Parkinson’s disease, our original aim and study design was to assess whether the long-lasting advantages we previously noted in an open-label trial might be reproducible in a placebo-controlled study. We have reported significant between-group differences in our primary outcome, and thus further investigation into exenatide as a disease-modifying treatment for Parkinson’s disease is warranted.
Distinguishing between long-lasting symptomatic effects and effects on underlying disease pathophysiology has been previously discussed, but no simple solutions have emerged.20,21 Most notably, rasagiline, which is approved for symptomatic treatment in Parkinson’s disease, showed inconclusive results in a delayed-start study22 designed to assess effects on disease progression. In our trial, it might be tempting to view persistent benefits detectable after the washout period as evidence of disease modification. Although exenatide was undetectable in the serum at 60 weeks, the 12-week washout period might have been insufficient to eliminate unexpected long-lasting symptomatic effects, which could have contributed to the benefits in motor function and other modalities. Indeed, the severity of Parkinson’s disease can be altered by symptomatic therapies that induce preservation of healthy behaviours, such as exercise, which can have long-term effects without changing the underlying neuropathological process.23
The possibility that exenatide has neuroprotective effects is supported by robust preclinical studies, which suggest that exenatide affects pathological mechanisms relevant to Parkinson’s disease.24 These effects include inhibitory effects on inflammation,5,8 promotion of mitochondrial biogenesis,25,26 neurotrophic effects,27,28 stimulation of neurogenesis,7 and restoration of neuronal insulin signalling.29 Whether some or all of these mechanisms contributed to the clinical effects in our study cannot be definitively established, but one or several of these mechanisms could have acted in synergy to promote cell survival, preserve compensatory responses, and prevent maladaptive responses.
In our DaTscan analysis, we used statistical parametric mapping, which is a modern approach for the statistical analysis of imaging changes that allows for adjustment of baseline differences30 and has been used previously in clinical trials in Parkinson’s disease.31 Although overall uptake of DaTscan fell in both groups, a quantitative analysis suggested a possible reduced rate of decline in the exenatide group. However, because this signal was detectable only at uncorrected height thresholds of p=0·0034 or less, these data would benefit from larger confirmatory studies or studies of patients at an earlier disease stage when the rate of change of DaTscan uptake is greater,32 making group differences more readily detectable.
Exenatide did not seem to significantly improve disease severity or participants’ quality of life beyond effects resulting from dopaminergic replacement. A long-term simple multi-site trial design will be necessary to establish the long-term effects of exenatide treatment on daytime function in Parkinson’s disease and specifically whether exenatide can delay the development of levodopa-refractory symptoms. Furthermore, since the development of exenatide, additional GLP-1 receptor agonists have been developed that are based on the structure of either exendin-4 or human GLP-1. Comparative clinical efficacy data to support the use of one drug over another are scarce, but some studies33,34 suggest significant differences in glycaemic control and frequency of adverse events with different GLP-1 receptor agonists in diabetes trials, and preliminary data35,36 suggest that some might exert greater neuroprotective effects than others. Our study has shown for the first time (to our knowledge) that exenatide given at a dose licensed for type 2 diabetes can cross the blood–brain barrier and access the CSF in concentrations equivalent to those in preclinical models of Parkinson’s disease associated with advantageous outcomes.6,27 However, further studies of the safety, efficacy, and CNS penetration of other members of this drug class, in parallel with mechanism-of-action studies, will help to clarify the eventual role that GLP-1 receptor agonists might have in Parkinson’s disease. Furthermore, the potential relevance of these drugs to other neurodegenerative disorders (eg, Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, multiple sclerosis) and other neurological diseases (eg, cerebrovascular disorders, traumatic brain injury) is being assessed in preclinical studies and clinical trials.34
We have replicated the findings from our previous open-label study and shown that, compared with placebo, exenatide treatment is associated with positive and persistent effects on off-medication motor scores as measured by MDS-UPDRS part 3. Whether this drug acts as a novel symptomatic agent, influences compensatory responses or behaviours, or has neuroprotective effects on underlying pathology is unclear, but there is a strong indication that GLP-1 receptor agonists may have a useful role in future treatment of Parkinson’s disease.
Research in context.
Evidence before this study
We searched PubMed with the terms “Parkinson’s disease”, “glucagon-like peptide-1”, “exenatide”, “trial”, “neuroprotection”, and “disease modification” for articles published in English on or before Dec 4, 2016 (the date of our final search), in any field. We identified several preclinical studies of exenatide, a glucagon-like peptide-1 agonist, which showed neuroprotective and neurorestorative effects in experimental animal-toxin models of Parkinson’s disease. We also identified a proof-of-concept study of exenatide as a possible disease-modifying treatment in patients with Parkinson’s disease. In this open-label trial, 21 patients who received 12 months of exenatide injections in addition to their regular drugs had a mean improvement of 2·7 points on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale part 3, compared with a deterioration of 2·2 points in 24 patients in the control group who received their regular drugs only (mean difference 4·9, 95% CI 0·3–9·4; p=0·037). Furthermore, patients treated with exenatide had a significant improvement on a cognitive assessment scale compared with those in the control group (mean difference 5·0, 95% CI 9·2–0·8; p=0·006). Persistent significant benefits were noted in the exenatide group compared with the control group in motor disability and cognitive function 12 months after the withdrawal of exenatide. However, because a placebo control was not used, these data could not be interpreted as proof of efficacy.
Added value of this study
To our knowledge, ours is the first randomised, placebo-controlled trial of exenatide as a potential disease-modifying drug in Parkinson’s disease. After 48 weeks, patients given 2 mg exenatide weekly had a significant advantage in terms of the primary outcome, the Movement Disorders Society Unified Parkinson’s Disease Rating Scale part 3, compared with those given placebo. This difference between groups was still significant after a 12-week drug washout period. Our study is also the first to show that exenatide, when given at licensed diabetes doses, crosses the blood–brain barrier and is detectable in cerebrospinal fluid in concentrations similar to those in preclinical models of Parkinson’s disease, which are associated with advantageous outcomes. Exenatide was well tolerated, although injection site reactions and gastrointestinal symptoms were noted.
Implications of all the available evidence
We have replicated the results of our previous clinical study and shown that patients with Parkinson’s disease who were given exenatide had improvements in the practically defined off-medication motor scores of Parkinson’s disease compared with those given placebo. Whether exenatide affects the underlying pathophysiology of Parkinson’s disease or simply induces long-lasting symptomatic effects remains uncertain. However, these results represent a major new avenue for investigation in the treatment of Parkinson’s disease.
Acknowledgments
This study was funded by the Michael J Fox Foundation for Parkinson’s Research and coordinated by University College London’s Comprehensive Clinical Trials Unit. This work was done partly at UCL and UCL Hospital and was funded in part by the Department of Health National Institute for Health Research Biomedical Research Centres funding scheme. The analysis of exenatide concentrations was partly supported by the Intramural Research Program of the US National Institutes of Health’s National institute on Aging. We thank the patients and their families who participated in the trial, and Vincenzo Libri and Rajeshree Khengar from the Leonard Wolfson Experimental Neuroscience Centre.
Footnotes
Contributors
DA, SSS, KC, and TF were involved in statistical analysis and data interpretation. DA, NB, and LZ recruited and followed up patients. DA and JD were involved in DaTscan acquisition and data analysis. DA, KM, MB-J, DL, SH, IA-O, TTW, PL, AJL, ST, and TF were responsible for study oversight. YL and NHG acquired exenatide pharmacokinetic data. TF was the principal investigator and oversaw study design. DA wrote the first draft of the Article, which all authors critically revised and commented on. All authors approved the final Article.
Declaration of interests
AJL reports grants from the Frances and Renee Hock Fund, consulting fees from Britannia Pharmaceuticals (Genus) and BIAL Portela, and honoraria from Profile Pharma, Teva, Lundbeck, BIAL, Roche, Britannia, UCB, Nordiclnfu Care, NeuroDerm, and Decision Resources. TTW has received honoraria from Britannia Pharmaceuticals. PL has received honoraria from Medtronic and St Jude Medical. NHG is a named inventor on a National Institutes of Health patent describing the use of GLP-1 receptor agonists in neurodegenerative disorders. All rights to this patent belong solely to the US Government. TF has received honoraria from Profile Pharma, BIAL, AbbVie, Genus, Medtronic, and St Jude Medical. All other authors declare no competing interests.
Contributor Information
Dilan Athauda, Sobell Department of Motor Neuroscience, University College London Institute of Neurology and the National Hospital for Neurology and Neurosurgery, London, UK.
Kate Maclagan, Comprehensive Clinical Trials Unit, University College London, London, UK.
Simon S Skene, Comprehensive Clinical Trials Unit, University College London, London, UK.
Martha Bajwa-Joseph, Comprehensive Clinical Trials Unit, University College London, London, UK.
Dawn Letchford, Comprehensive Clinical Trials Unit, University College London, London, UK.
Kashfia Chowdhury, Comprehensive Clinical Trials Unit, University College London, London, UK.
Steve Hibbert, Comprehensive Clinical Trials Unit, University College London, London, UK.
Natalia Budnik, Leonard Wolfson Experimental Neuroscience Centre, London, UK.
Luca Zampedri, Leonard Wolfson Experimental Neuroscience Centre, London, UK.
John Dickson, Institute of Nuclear Medicine, University College London Hospitals NHS Trust, London, UK.
Yazhou Li, Translational Gerontology Branch, Intramural Research Program, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA.
Iciar Aviles-Olmos, Sobell Department of Motor Neuroscience, University College London Institute of Neurology and the National Hospital for Neurology and Neurosurgery, London, UK.
Prof Thomas T Warner, Queen Square Brain Bank, London, UK.
Prof Patricia Limousin, Sobell Department of Motor Neuroscience, University College London Institute of Neurology and the National Hospital for Neurology and Neurosurgery, London, UK.
Prof Andrew J Lees, Sobell Department of Motor Neuroscience, University College London Institute of Neurology and the National Hospital for Neurology and Neurosurgery, London, UK.
Nigel H Greig, Translational Gerontology Branch, Intramural Research Program, National Institute on Aging, National Institutes of Health, Baltimore, MD, USA.
Susan Tebbs, Comprehensive Clinical Trials Unit, University College London, London, UK.
Prof Thomas Foltynie, Sobell Department of Motor Neuroscience, University College London Institute of Neurology and the National Hospital for Neurology and Neurosurgery, London, UK.
References
- 1.Athauda D, Foltynie T. The ongoing pursuit of neuroprotective therapies in Parkinson disease. Nat Rev Neurol. 2014;11:25–40. doi: 10.1038/nrneurol.2014.226. [DOI] [PubMed] [Google Scholar]
- 2.Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–69. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- 3.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267:7402–05. [PubMed] [Google Scholar]
- 4.Rampersaud N, Harkavyi A, Giordano G, Lever R, Whitton J, Whitton PS. Exendin-4 reverses biochemical and behavioral deficits in a pre-motor rodent model of Parkinson’s disease with combined noradrenergic and serotonergic lesions. Neuropeptides. 2012;46:183–93. doi: 10.1016/j.npep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation. 2008;5:19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Perry T, Kindy MS, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. PNAS. 2009;106:1285–90. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertilsson G, Patrone C, Zachrisson O, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res. 2008;86:326–38. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- 8.Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol. 2009;202:431–39. doi: 10.1677/JOE-09-0132. [DOI] [PubMed] [Google Scholar]
- 9.Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123:2730–36. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aviles-Olmos I, Dickson J, Kefalopoulou Z, et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J Parkinsons Dis. 2014;4:337–44. doi: 10.3233/JPD-140364. [DOI] [PubMed] [Google Scholar]
- 11.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–84. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 13.Ouchi Y, Yoshikawa E, Sekine Y, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–75. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 14.MacConell L, Gurney K, Malloy J, Zhou M, Kolterman O. Safety and tolerability of exenatide once weekly in patients with type 2 diabetes: an integrated analysis of 4328 patients. Diabetes Metab Syndr Obes. 2015;8:241–53. doi: 10.2147/DMSO.S77290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150–56. doi: 10.1053/j.gastro.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh S1, Chang HY, Richards TM, Weiner JP, Clark JM, Segal JB. Glucagon like peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus. A population-based matched case-control study. JAMA Intern Med. 2013;173:534–539. doi: 10.1001/jamainternmed.2013.2720. [DOI] [PubMed] [Google Scholar]
- 17.Azoulay L, Filion KB, Platt RW, et al. Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ. 2016;352:581. doi: 10.1136/bmj.i581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs—FDA and EMA assessment. N Engl J Med. 2014;370:794–97. doi: 10.1056/NEJMp1314078. [DOI] [PubMed] [Google Scholar]
- 19.Abuirmeileh A, Harkavyi A, Rampersaud N, et al. Exendin-4 treatment enhances L-DOPA evoked release of striatal dopamine and decreases dyskinetic movements in the 6-hydoxydopamine lesioned rat. J Pharm Pharmacol. 2012;64:637–43. doi: 10.1111/j.2042-7158.2011.01394.x. [DOI] [PubMed] [Google Scholar]
- 20.Kieburtz K, Olanow CW. Advances in clinical trials for movement disorders. Mov Disord. 2015;30:1580–87. doi: 10.1002/mds.26371. [DOI] [PubMed] [Google Scholar]
- 21.Athauda D, Foltynie T. Challenges in detecting disease modification in Parkinson’s disease clinical trials. Parkinsonism Relat Disord. 2016;32:1–11. doi: 10.1016/j.parkreldis.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 22.Olanow CW, Rascol O, Hauser R, et al. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361:1268–78. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- 23.Ward CD. Does selegiline delay progression of Parkinson’s disease? A critical re-evaluation of the DATATOP study. J Neurol Neurosurg Psychiatry. 1994;57:217–20. doi: 10.1136/jnnp.57.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21:802–18. doi: 10.1016/j.drudis.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Kang MY, Oh TJ, Cho YM. Glucagon-like peptide 1 increases mitochondrial biogenesis and function in INS-1 rat insulinoma cells. Endocrinol Metab. 2014;30:216–20. doi: 10.3803/EnM.2015.30.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Zhang Y, Li L, Hölscher C. Neuroprotective effects of geniposide in the MPTP mouse model of Parkinson’s disease. Eur J Pharmacol. 2015;768:21–27. doi: 10.1016/j.ejphar.2015.09.029. [DOI] [PubMed] [Google Scholar]
- 27.Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002;302:881–88. doi: 10.1124/jpet.102.037481. [DOI] [PubMed] [Google Scholar]
- 28.Perry T, Lahiri DK, Chen D, et al. A novel neurotrophic property of glucagon-like peptide 1: a promoter of nerve growth factor-mediated differentiation in PC12 cells. J Pharmacol Exp Ther. 2002;300:958–66. doi: 10.1124/jpet.300.3.958. [DOI] [PubMed] [Google Scholar]
- 29.Athauda D, Foltynie T. Insulin resistance and Parkinson’s disease: a new target for disease modification? Prog Neurobiol. 2016;145:98–120. doi: 10.1016/j.pneurobio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Kas A, Payoux P, Habert M-O, et al. Validation of a standardized normalization template for statistical parametric mapping analysis of 123 I-FP-CIT images. J Nucl Med. 2007;48:1459–67. doi: 10.2967/jnumed.106.038646. [DOI] [PubMed] [Google Scholar]
- 31.Whone AL, Watts RL, Stoessl AJ, et al. Slower progression of Parkinson’s disease with ropinirole versus levodopa: the REAL-PET study. Ann Neurol. 2003;54:93–101. doi: 10.1002/ana.10609. [DOI] [PubMed] [Google Scholar]
- 32.Pirker W, Djamshidian S, Asenbaum S, et al. Progression of dopaminergic degeneration in Parkinson’s disease and atypical parkinsonism: a longitudinal beta-CIT SPECT study. Mov Disord. 2002;17:45–53. doi: 10.1002/mds.1265. [DOI] [PubMed] [Google Scholar]
- 33.Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013;381:117–24. doi: 10.1016/S0140-6736(12)61267-7. [DOI] [PubMed] [Google Scholar]
- 34.Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 35.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. doi: 10.1186/1471-2202-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu W, Jalewa J, Sharma M, Li G, Li L, Hölscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2015;303:42–50. doi: 10.1016/j.neuroscience.2015.06.054. [DOI] [PubMed] [Google Scholar]