Abstract
Background
Dynactin p150Glued, the largest subunit of the dynactin macromolecular complex, binds to both microtubules and tubulin dimers through the N-terminal cytoskeleton-associated protein and glycine-rich (CAP-Gly) and basic domains, and serves as an anti-catastrophe factor in stabilizing microtubules in neurons. P150Glued also initiates dynein-mediated axonal retrograde transport. Multiple missense mutations at the CAP-Gly domain of p150Glued are associated with motor neuron diseases and other neurodegenerative disorders, further supporting the importance of microtubule domains (MTBDs) in p150Glued functions. However, most functional studies were performed in vitro. Whether p150Glued is required for neuronal function and survival in vivo is unknown.
Methods
Using Cre-loxP genetic manipulation, we first generated a line of p150Glued knock-in mice by inserting two LoxP sites flanking the MTBD-coding exons 2 to 4 of p150Glued–encoding Dctn1 gene (Dctn1LoxP/), and then crossbred the resulting Dctn1LoxP/ mice with Thy1-Cre mice to generate the bigenic p150Glued (Dctn1LoxP/LoxP; Thy1-Cre) conditional knockout (cKO) mice for the downstream motor behavioral and neuropathological studies.
Results
P150Glued expression was completely abolished in Cre-expressing postnatal neurons, including corticospinal motor neurons (CSMNs) and spinal motor neurons (SMNs), while the MTBD–truncated forms remained. P150Glued ablation did not affect the formation of dynein/dynactin complex in neurons. The p150Glued cKO mice did not show any obvious developmental phenotypes, but exhibited impairments in motor coordination and rearing after 12 months of age. Around 20% loss of SMNs was found in the lumbar spinal cord of 18-month-old cKO mice, in company with increased gliosis, neuromuscular junction (NMJ) disintegration and muscle atrophy. By contrast, no obvious degeneration of CSMNs, striatal neurons, midbrain dopaminergic neurons, cerebellar granule cells or Purkinje cells was observed. Abnormal accumulation of acetylated α-tubulin, and autophagosome/lysosome proteins was found in the SMNs of aged cKO mice. Additionally, the total and cell surface levels of glutamate receptors were also substantially elevated in the p150Glued-depleted spinal neurons, in correlation with increased vulnerability to excitotoxicity.
Conclusion
Overall, our findings demonstrate that p150Glued is particularly required to maintain the function and survival of SMNs during aging. P150Glued may exert its protective function through regulating the transportation of autophagosomes, lysosomes, and postsynaptic glutamate receptors in neurons.
Electronic supplementary material
The online version of this article (10.1186/s13024-018-0242-z) contains supplementary material, which is available to authorized users.
Keywords: Dynactin p150Glued, Dynein, Microtubule binding domain, Motor neuron, Neurodegeneration, Autophagy, Lysosome, Glutamate receptor, Excitotoxicity
Background
Impairments in intracellular transport are often associated with neurological disorders, including Alzheimer’s disease, Huntington’s disease, Parkinson’s disease and amyotrophic lateral sclerosis (ALS) [1]. Dynein mediates the retrograde transport of cargos from microtubule plus ends to the minus ends, while the dynactin protein complex is proposed to promote the processivity of dynein motor proteins moving along the microtubules, as well as expand the variety of dynein cargoes [2, 3]. Dynactin consists of more than 20 subunits, with its largest subunit p150Glued to interact with both microtubules and dynein motor protein complex [2]. P150Glued, encoded by the full-length DCTN1 gene, weights around 150 kD and contains the N-terminal CAP-Gly and basic domains, followed by the coiled-coil 1 (CC1) and CC2 domains [2]. Both the CAP-Gly and basic domains exhibit microtubule binding affinity, and together form the tandem MTBDs [4]. On the other hand, the CC1 and CC2 domains mediate the interactions with dynein intermediate chain (DIC) and the other dynactin subunits [2]. The MTBDs of p150Glued are required for cell division by providing essential attachment to the microtubules in spindle formation and chromosome movement [5], p150Glued inhibition causes cell proliferation arrest [6] and germline deletion of p150Glued leads to early embryonic lethality [7]. Multiple missense mutations in the CAP-Gly domain of p150Glued have been linked to a slowly progressive, autosomal dominant form of lower motor neuron disease without sensory symptoms [8]; Perry syndrome, which consists of parkinsonism with severe mental depression and central hypoventilation [9]; and progressive supranuclear palsy [10]. P150Glued is proposed as an anti-catastrophe factor in maintaining the stability of microtubules in neurons, and facilitate the dynein-mediated axonal retrograde transport in neuronal cultures [11]. These disease-causal mutations seem to weaken the microtubule binding affinity of p150Glued [3]. However, the functional significance of p150Glued has not been critically evaluated in neurons in living animals.
In addition to p150Glued, DCTN1 also encodes p135 and other short splicing variants [12]. P135 lacks the coding exons 2 to 5, resulting in a complete loss of the CAP-Gly domain and a large portion of basic domain. P150Glued is expressed in all types of mammalian cells, while p135 is more abundant in neurons [13]. Dynein/dynactin is required for the mitosis as germline deletion of p150Glued caused early embryonic lethality and apoptosis in p150Glued knockout mice [7]. However, p135 can compensate for the most dynactin activity in p150glued-deficient post-mitotic cells [12]. These observations raise questions about the overall importance of p150Glued in neurons, despite mutations in its CAP-Gly domain are associated with multiple neurological disorders. One possible scenario is that p150Glued proteins are particularly needed to maintain the integrity of microtubule network and the efficiency of axonal retrograde transport in the large projection neurons with long axons, such as the CSMNS and SMNs.
In this study, we utilized Cre-loxP system [14] to selectively deplete p150Glued but keep p135 expression in CSMNs and SMNs. To our surprise, genetic ablation of p150Glued in postnatal neurons did not cause overt behavioral and neuropathological phenotypes in mice. Only moderate motor deficits and SMN loss were observed in aged p150Glued cKO mice. The p150Glued-lacking neurons appeared to be more susceptible to excitotoxicity in correlation with abnormal augmentations of total and surface expression of glutamate receptors, a potential pathogenic mechanism of SMN degeneration in p150Glued cKO mice.
Methods
Generation of Dctn1LoxP/ knock-in mice and Dctn1LoxP/LoxP; Cre conditional knockout mice
Genomic DNA fragments containing Dctn1 gene locus were isolated from a mouse genomic DNA library (Stratagene). A 9.3 kb KpnI/AflII fragment carrying exons 2–4 of Dctn1 was subcloned into the pBluescript vector for later modifications. To construct the targeting vector, the Dctn1 clone was modified by inserting the first loxP site and a neomycin (Neo) selection cassette flanked with two Frt sites in intron 1, and the second loxP site in intron 4 (Fig. 1a). The targeting vector was linearized at a unique NotI site and transfected into 129/SvJ ES cells. After neomycin resistance (Neo) positive selection with G418 for 7 days, ES clones that had undergone homologous recombination were picked and screened by PCR, Southern blot analysis and partial genome sequencing (Fig. 1b). Two positive ES clones were expanded and injected into blastocysts. The resulting male chimera mice were bred with wild-type C57BL/6 J female mice to obtain Dctn1LoxP-Frt-Neo-Frt mice. The Dctn1LoxP-Frt-Neo-Frt mice were then crossbred with FLPe knock-in mice [15] (JAX, stock number: 003946) to remove Neo selection marker and get Dctn1LoxP/ mice. Finally, the Dctn1LoxP/ mice were crossed with Thy1-Cre [16] (JAX, Stock Number: 006143) or Cre/Esr1 [17] (JAX, Stock Number: 004682) mice to delete exons 2 to 4 and thereby the MTBD-containing p150Glued from the Cre-expressing cells. The mice were housed in a 12 h light/dark cycle and fed regular diet ad libitum. All mouse work followed the guidelines approved by the Institutional Animal Care and Use Committees of the National Institute on Aging, NIH.
Fig. 1.

Genetic deletion of p150Glued in Dctn1LoxP/; Cre mice. a The schematic diagram depicts the generation of Dctn1LoxP/; Cre mice in which the exon 2–4 of Dctn1 gene is deleted. b Amino acid sequence highlights residues encoded by exon 2 (light blue), exon 3 (brown), and exon 4 (navy blue). The CAP-Gly domain was marked by solid line. The basic domain was indicated by dash line. c Southern blot of genomic DNAs isolated from mouse embryonic stem (ES) cell clones shows a correct gene targeting of the Dctn1 gene locus in Dctn1+/LoxP-Frt-Neo-Frt ES cell clones. As predicted, the insertion of the LoxP-Frt-Neo-Frt gene-targeting cassette into the Dctn1 gene locus generated a new 3.8 kb BamHI fragment in Dctn1+/LoxP-Frt-Neo-Frt ES cell clones. d-e Western blot analysis shows the protein levels of p150Glued, p135+, p62, p50 and ARP1 in cultured Dctn1+/+, Dctn1+/+; Cre/Esr1, Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Cre/Esr1 cortical neurons at 7 days in vitro (DIV). Actin was used as loading control. Bar graphs show the quantification of p150Glued and p135+ levels normalized with actin (n = 3 per genotype). Data were presented as mean ± SEM. One-way ANOVA plus Tukey’s post hoc test was used for statistical analysis. ***p < 0.001 for difference against Dctn1LoxP/LoxP; Cre/Esr1, while no difference was found among Dctn1+/+, Dctn1+/+; Cre/Esr1 and Dctn1LoxP/LoxP
Genotyping
Genomic DNA was prepared from tail biopsy using DirectPCR Lysis Reagent (Viagen Biotech) and subjected to PCR amplification using specific sets of PCR primers for each genotype, including Dctn1LoxP/ knock-in mice (mDCTN1-Ex4-F, CAG CTG CAA AGA CCA GCA AA; mDCTN1-Ex5-R: CAC ACC ACC TTC TTA GGC TTC A), Cre transgenic mice (CRE-F, CAT TTG GGC CAG CTA AAC AT; CRE-R, TGC ATG ATC TCC GGT ATT GA).
Sucrose density gradient fractionation and sedimentation analysis
Neuronal cells or neural tissues were homogenized in 50 mM Tris-HCl, pH 7.5, containing 150 mM NaC1, 1 mM EDTA and Protease Inhibitor Cocktail (Thermo Scientific). The cytosolic extracts were prepared by centrifugation at 16,000 × g for 15 min at 4 °C, and further clarified at 100,000 × g for 15 min. The resulting supernatant was layered over a 12 ml, 5–20% sucrose density gradient and centrifuged at 120,000 × g for 18 h at 4 °C. 1-ml gradient fractions were collected and equal volumes of all fractions analyzed by SDS-PAGE followed by Western blotting.
Behavior tests
Open-field test. As described previously [18], the ambulatory and rearing activities of male mice were measured by the Flex-Field Activity System (San Diego Instruments). Flex-Field software was used to trace and quantify mouse movement in the unit as the number of beam breaks per 30 min.
Rotarod test. As described previously [19], male mice were placed onto a rotating rod with auto-acceleration from 0 rpm to 40 rpm for 1 min (San Diego Instruments). The length of time the mouse stayed on the rotating rod was recorded. Three measurements were taken for each animal during each test.
Hindlimb clasping test. In the hindlimb clasping test, male mice were suspended by the tail and observed for 10 s. The severity of hindlimb clasping phenotype was scored as follows: 0 = both hindlimbs consistently spread outward and away from the abdomen, 1 = one hindlimb retracted toward the abdomen, 2 = both hindlimbs partially retracted toward the abdomen, 3 = both hindlimbs completely retracted toward the abdomen. For each mouse, three separate tests were taken over 3 h, and the averaged hindlimb clasping severity score of the three tests was calculated.
Histology, immunohistochemistry and light microscopy
For neuropathology study, mice were perfused with 4% paraformaldehyde (PFA) in cold phosphate buffered saline (PBS), brains and spinal cords were collected, post-fixed overnight, submerged in 30% sucrose in PBS for at least 72 h, and sectioned at 40–50 μm thickness using CM1950 cryostat (Leica). For muscle pathology analysis, mice were euthanized, gastrocnemius muscles were dissected, snap frozen in 2-methylbutane (Sigma-Aldrich) cooled in dry ice, sectioned at 20 or 40 μm thickness, and fixed in 4% PFA in PBS for 15 min. For Nissl staining, sections were stained with 0.1% Cresyl Violet (Sigma-Aldrich). For HE staining, hematoxylin and eosin stain kit (Vector Laboratories) were used as suggested by manufacturers. For immunostaining, antibodies specific to p150Glued N-terminus (amino acid 3–202, 1:200, BD Biosciences), p150Glued C-terminus (amino acid 1266–1278, 1:500, Abcam), neuron-specific nuclear protein (NeuN, 1:1000, Millipore), CTIP2 (1:200, Abcam), choline acetyltransferase (CHAT, 1:500, Millipore), Glial Fibrillary Acidic Protein (GFAP, 1:1000, Abcam), Ionized calcium-Binding Adaptor Molecule-1 (IBA1, 1:1000, Wako Chemicals), acetylated α-tubulin (ac-TUBA, 1:500, Abcam), Lysosomal Associated Membrane Protein 2 (LAMP2, 1:200, Abcam), Tyrosine Hydroxylase (TH, 1:500, Pel-Freeze) and Calbindin D28K (1:200, Abcam) were used as suggested by manufacturers. For immunofluorescence study, Alexa Fluor 488-, 546- or 647-conjugated secondary antibody (1:500, Invitrogen) was used to visualize the staining. Alexa Fluor 488-conjugated α-bungarotoxin (1:500, Invitrogen) was applied to label postsynaptic acetylcholine receptors of NMJs. Fluorescence images were captured using LSM 780 laser-scanning confocal microscope (Zeiss). The paired images in all the figures were collected at the same gain and offset settings. Post collection processing was applied uniformly to all paired images. The images were presented as either a single optic layer after acquisition in z-series stack scans at 1.0 μm intervals from individual fields or displayed as maximum-intensity projections to represent confocal stacks. For immunohistochemistry study, Vectastain Elite ABC Kit and DAB Kit (Vector Laboratories) were used to visualize the staining. Bright field images were captured by Axio microscope Imager A1 (Zeiss).
Image analysis
For the quantitative assessment of various marker protein distributions, images were taken using identical settings and exported to ImageJ (NIH) for imaging analysis. Images were converted to an 8-bit color scale (fluorescence intensity from 0 to 255) using ImageJ. The areas of interest were first selected with Polygon or Freehand selection tools and then subjected to measurement by mean optical intensities or area fractions. The mean intensity for the background area was subtracted from the selected area to determine the net mean intensity.
Stereology
Stereology for CSMNs, striatal neurons, SMNs and midbrain dopaminergic neurons was performed as described previously [19, 20]. To examine the number of CSMNs and striatal neurons, series of sagittal brain sections (40 μm per section thickness, every ninth section, nine sections per case) were processed for Nissl staining or CTIP2 immunohistochemistry. The motor cortex was designated as layer V neurons in the anteromedial cortex, and outlined according to the mouse brain in stereotaxic coordinates. To examine the number of SMNs, series of coronal spinal cord sections (50 μm per section, every tenth section from L3 to L5, ten sections per case) were processed for Nissl staining or CHAT immunohistochemistry. To examine the number of midbrain dopaminergic neurons, series of coronal sections across the midbrain (40 μm per section, every fourth section from Bregma − 2.54 to − 4.24 mm, ten sections per case) were processed for TH immunohistochemistry. For the unbiased stereological estimation of CSMNs, striatal neurons, SMNs and midbrain dopaminergic neurons, the number of CTIP2 positive or Nissl-stained large (>15 μm in diameter) cortical neurons in layer V, Nissl-stained neurons in stratum, CHAT positive or Nissl-stained large (>35 μm in diameter) spinal neurons in ventral horn, and TH-positive neurons in substantia nigra pars compacta (SNpc) were assessed using the Optical Fractionator function of Stereo Investigator 10 (MicroBrightField). Six mice were used per genotype at each time point. Counters were blinded to the genotypes of the samples. The sampling scheme was designed to have a coefficient of error less than 10% in order to obtain reliable results.
Preparation of postsynaptic density (PSD) fraction
PSD fractions were prepared from mouse brains as described previously [18]. All procedures were performed at 4 °C. Mouse brains were isolated and homogenized in 10 volumes of ice-cold Buffer A (0.32 M sucrose, 5 mM HEPES, pH 7.4, 1 mM MgCl2, 0.5 mM CaCl2, Protease and Phosphatase Inhibitor Cocktail) with Teflon homogenizer (12 strokes). The homogenate was spun at 1400 × g for 10 min. Supernatant (S1’) was saved and pellet (P1’) was homogenized again with Teflon homogenizer in another 10 volumes of Buffer A (5 strokes). After centrifugation at 700 × g for 10 min, the supernatant (S1’) was collected and pooled with S1, and the pellet (P1’) were saved as crude nuclear fraction. Pooled S1 and S1’ were centrifuged at 13,800 × g for 10 min to collect the crude synaptosomal pellet (P2) and the supernatant (S2), which contains the cytosol and light membranes. S2 was centrifuged at 100,000 × g for 15 min to separate the cytosolic fraction (Cyt) and the light membrane fraction (LM). P2 was resuspended in 10 volumes of Buffer B (0.32 M sucrose, 6 mM Tris, pH 8.0, supplemented with Protease and Phosphatase Inhibitor Cocktail) with Teflon homogenizer (5 strokes). The P2 suspension was loaded onto a discontinuous sucrose gradient (0.85 M/1 M/1.15 M sucrose solution in 6 mM Tris, pH 8.0), and centrifuged at 82,500 × g for 2 h. Synaptosome fraction (Syn) was collected from the layer between 1 M and 1.15 M sucrose, supplemented with 0.5% Triton X-100 and mixed for 15 min. The suspension was spun at 32,800 × g for 20 min, and the resulting pellet was saved as PSD fraction (PSD). P1’, LM and PSD fractions were lysed in 1% SDS buffer. SDS were added into Cyt and Syn fraction to 1% final concentration. Equal amount of total protein from each fraction were resolved in SDS-PAGE and applied to western blot analysis.
Primary neuron culture
Mouse primary neuron cultures were prepared from the cortex or spinal cord of newborn (postnatal day 0, P0) pups, as described previously [21, 22]. Briefly, individual cortex or spinal cord was dissected and subjected to papain digestion (5 U/ml, Worthington Biochemicals) for 40 min at 37 °C. The digested tissue was carefully triturated into single cells using increasingly smaller pipette tips. The cells were then centrifuged at 250 × g for 5 min and resuspended in warm Basal Medium Eagle (BME, Sigma-Aldrich) supplemented with 5% heat-inactivated fetal bovine serum (FBS; Invitrogen), 1× N2/B27 supplement (100× stock, Invitrogen), 1× GlutaMax (100× stock, Invitrogen), 0.45% D-glucose (Sigma-Aldrich), 10 U/ml penicillin (Invitrogen), and 10 μg/ml streptomycin (Invitrogen). The dissociated neurons were seeded in plated in Biocoat Poly-D-Lysine Cellware plate (BD Biosciences), and maintained at 37 °C in a 95% O2 and 5% CO2 humidified incubator. Twenty-four hours after seeding, the cultures were switched to serum-free medium supplemented with 1 μM cytosine β-D-arabinofuranoside (Sigma-Aldrich) to suppress the proliferation of glia and 1 μM 4-hydroxytamoxifen (4-OHT, Sigma-Aldrich) to induce CRE recombinase activity. Starting from 5 days in vitro (DIV), culture medium was changed twice every week.
Biotinylation of neuronal surface proteins
Spinal neurons were cultured at the density of 1.2 × 106 per well in Poly-D-Lysine coated 6-Well plate (BD Biosciences) and treated with 0 or 10 μM glutamate (Sigma-Aldrich) on 20 DIV. After 24-h treatment, neurons were washed with PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBS/CM), and then incubated with 1 ml biotin solution (0.5 mg/ml Sulfo-NHS-SS-Biotin in cold PBS/CM, Thermo Fisher Scientific) for 20 min at 4 °C and then washed subsequently with PBS/CM, 0.1 M glycine solution, and TBS (25 mM Tris-HCl, pH 7.4, containing 137 mM NaCl) buffer. Cells were harvested in 250 μl RIPA buffer (Sigma) supplemented with protease inhibitor and phosphatase inhibitor cocktail, lysed on ice for 30 min, and centrifuged at 16,000 × g for 15 min at 4 °C. Resulting supernatant containing equal amount of total protein was incubated with 50 μl neutral-avidin agarose (Pierce) at 4 °C for 2 h with gently rotating. After washing in RIPA buffer for 5 times, the biotin-labeled surface protein was eluted with SDS sample buffer by heating at 70 °C for 10 min. Total proteins and isolated biotinylated surface proteins were analyzed by western blotting.
Assessment of cell survival by 3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) assay
Spinal neurons were cultured at the density of 1.0 × 105 per well in Poly-D-Lysine coated 96-Well plate (BD Biosciences) and treated with 0 or 10 μM glutamate (Sigma-Aldrich) on 20 DIV. After 24-h treatment, the cells were incubated with 0.5 mg/ml MTT (Sigma-Aldrich) at 37 °C for 4 h. After the media were removed, dimethyl sulfoxide (DMSO, 100 μl) was added to each well to solubilize the formazan crystals generated by viable mitochondrial succinate dehydrogenase from MTT. The absorbance at 570 nm was measured using a SpectraMax M5 Multi-Mode Microplate Reader (Molecular Devices) as the MTT reducing activity of the cells.
Western blot analysis
Neurons or tissues were homogenized and sonicated in RIPA buffer (Sigma-Aldrich) or 1% SDS lysis buffer (50 mM Tris–HCl, 150 mM NaCl, 2 mM EDTA, pH 7.5, and 1% SDS) supplemented with Protease Inhibitor Cocktails (Thermo Scientific). Lysates were clarified by centrifugation at 15000 × g for 15 min at 4 °C. The supernatants were quantified for protein content using the bicinchoninic acid (BCA) assay kit (Thermo Fisher Scientific) and separated by 4–12% NuPage BisTris-PAGE (Invitrogen) using MES or MOPS running buffer (Invitrogen). The separated proteins were then transferred to nitrocellulose membranes using the iBlot Dry Blotting system (Invitrogen) and incubated with specific primary antibodies. The antibodies used for western blot analysis included p150Glued N-terminus (amino acid 3–202, 1:1000, BD Biosciences), p150Glued C-terminus (amino acid 1266–1278, 1:1000, Abcam), dynactin subunit p62 (1:1000, Abcam), dynactin subunit p50 (1:5000, BD Biosciences), dynactin subunit Actin Related Protein 1 (ARP1, 1:1000, Sigma-Aldrich), dynein heavy chain (DHC, 1:500, Santa Cruz Biotechnology), dynein intermediate chain (DIC, 1:1000, Sigma-Aldrich), dynein light chain (DLC, 1:500, Santa Cruz Biotechnology), α-tubulin (TUBA, 1:10000, Abcam), acetylated α-tubulin (ac-TUBA, 1:10000, Abcam), tyrosinated α-tubulin (tyro-TUBA, 1:10000, Abcam), detyrosinated α-tubulin (detyro-TUBA, 1:10000, Abcam), Autophagy Related Protein ATG3 (ATG3, 1:1000, Cell Signaling), ATG5 (1:1000, Cell Signaling), ATG7 (1:1000, Cell Signaling), autophagy related protein LC3 (1:1000, Abcam), Lysosomal Associated Membrane Protein 1 (LAMP1, 1:1000, BD Biosciences), LAMP2 (1:1000, Abcam), synaptophysin (SYP, 1:10000, Millipore), Postsynaptic Density Protein 95 (PSD95, 1:1000, Sigma-Aldrich), AMPA receptor subunit GLUR1 (1:1000, Abcam), GLUR2 (1:1000, BD Biosciences), NMDA receptor subunit NR2A (1:250, BD Biosciences), NR2B ((1:500, BD Biosciences), and β-actin (1:5000, Sigma-Aldrich). Protein signals were visualized by IRDye secondary antibodies and Odyssey system (LI-COR Biosciences), and quantified with NIH ImageJ software.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software). Data were presented as mean ± SEM. Statistical significance was determined by comparing means of different groups using unpaired t-test, one-way or two-way ANOVA followed by the post hoc test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Selective depletion of p150Glued expression in neurons
To selectively disrupt the expression of p150Glued, we initially generated Dctn1LoxP knock-in mice by inserting two LoxP sites in the first and fourth introns of mouse Dctn1 gene locus, respectively, resulting in a complete loss CAP-Gly domain and partial loss of basic domain (Fig. 1a-c). The Dctn1LoxP knock-in mice were then crossbred with Cre/Esr1 mice to generate Dctn1LoxP/LoxP; Cre/Esr1 pups and littermate controls for neuronal cultures. The administration of tamoxifen activated the estrogen receptor (ESR) tagged-Cre and removed the floxed exons, resulting in a selective ablation of p150Glued expression in cortical neurons cultured from postnatal day 0 (P0) Dctn1LoxP/LoxP; Cre/Esr1 pups (Fig. 1d, e). P150Glued proteins were identified by an antibody against the N-terminal 200 amino acids of p150Glued. By contrast, the levels of p135 and other N-terminal truncated p150Glued, called collectively as p135+, were substantially increased (Fig. 1d, e). The p135+ proteins were recognized by an antibody against the C-terminus of p150Glued. On the other hand, the other dynactin subunits–p62, p50, and ARP1 showed comparable expression levels in Dctn1LoxP/LoxP; Cre/Esr1 and control neuronal cultures (Fig. 1d, e). Therefore, genetic depletion of p150Glued increases p135+ expression, but does not affect the other dynactin subunit levels in neurons.
A lack of p150Glued does not affect the formation of dynein and dynactin complexes
To evaluate the impact of p150Glued on the formation of dynein/dynactin complexes, we fractionated the cell lysates from Dctn1LoxP/LoxP; Cre/Esr1 and control Dctn1LoxP/LoxP cortical neuron cultures in a sucrose gradient column and compared the distribution patterns of dynein and dynactin subunits in different fractions. In control cell lysates, all the dynactin subunits and dynein DIC subunit concentrated in fractions 3 to 6 (Fig. 2a). The same distribution pattern of dynein and dynactin subunits was also observed in the p150Glued-lacking cell lysates (Fig. 2b). Thus, a lack of p150Glued does not affect the formation of dynein/dynactin complexes.
Fig. 2.

Dynein/dynactin complex remains intact in Dctn1LoxP/LoxP; Cre/Esr1 neurons. a-b Sucrose density gradient centrifugation shows p150Glued and p135+ predominantly migrate at fraction 4 and 5 in a 5–20% sucrose gradient similar to the other dynactin subunits p62, p50, and ARP1, as well as the dynactin-interacting dynein subunit DIC in the control Dctn1LoxP/LoxP (a) and p150Glued cKO Dctn1LoxP/LoxP; Cre/Esr1 cortical neuron cultures (b) at 7 DIV
Generation of p150Glued conditional knockout mice that lack p150Glued expression in the postnatal forebrain and spinal neurons
Our previous study suggests the G59S missense mutation in the MTBDs of p150Glued might cause the autosomal dominant motor neuron disease through a dominant-negative mechanism [7]. On the one hand, the G59S substitution disrupts the normal folding and destabilizes the mutant p150Glued proteins. On the other hand, the residual mutant proteins might dimerize with the wild-type proteins and thereby compromises the overall function of p150Glued. However, the exact pathogenic mechanisms of G59S mutation remain controversial [1]. To test the hypothesis that p150Glued is required for the survival of motor neurons, we decided to remove p150Glued from both CSMNs in the frontal cortex and SMNs in the ventral spinal cords by generating Dctn1LoxP/LoxP; Thy1-Cre cKO mice. In line with the early study [16], Cre was widely expressed by neurons in the forebrain regions (Additional file 1: Figure S1A), including olfactory bulb, frontal cortex, striatum, and hippocampus, as well as in the spinal cord (Additional file 1: Figure S1B). Although the expression of Cre was detectable in the brain extracts of P0 pups, a substantial reduction of p150Glued expression became apparent in 2-month-old Dctn1LoxP/LoxP; Thy1-Cre mice (Additional file 1: Figure S1C). The depletion of p150Glued restrictive to adult neurons may avoid any potential developmental defects in the p150Glued cKO mice.
Immuno-staining showed endogenous p150Glued proteins were highly expressed by neurons in the olfactory bulb, cerebral cortex, hippocampus, midbrain, and hindbrain regions of control Dctn1LoxP/LoxP mice (Fig. 3a). Following the expression pattern of Cre (Additional file 1: Figure S1A), the levels of p150Glued were substantially depleted in the olfactory bulb, frontal cortex, and hippocampus of Dctn1LoxP/LoxP; Thy1-Cre cKO mice (Fig. 3a). More importantly, p150Glued was absent in the CSMNs of cKO mouse brains, while the p135+ remained (Fig. 3b). Here the CSMNs were indicated by staining with CSMN-specific marker protein CTIP2 [23]. P150Glued was also abundantly expressed by spinal neurons, including the SMNs in the ventral horns, but disappeared in the cKO spinal cords (Fig. 3c, d). In contrast, the p135+ proteins were still present in the SMNs of cKO mice (Fig. 3d). Therefore, we generated a line of p150Glued cKO mice that depleted the expression of p150Glued in the adult CSMNs and SMNs, while kept p135 and other MTBD-lacking variants.
Fig. 3.

Selective deletion of neuronal p150Glued in brain and spinal cord of Dctn1LoxP/LoxP; Thy1-Cre mice. a Representative images show p150Glued (green) and NeuN (blue) staining in the sagittal brain sections of 3-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Arrows point to olfactory bulb (OB), cortex (CX) and hippocampus (HP) with intense p150Glued staining in Dctn1LoxP/LoxP mice and weak p150Glued staining in Dctn1LoxP/LoxP; Thy1-Cre mice. Scale bar: 500 μm. b Representative images show p150Glued (green) and p150Glued/p135+ (purple) expression in the CTIP2-positive (red) CSMNs of 3-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Scale bar: 500 μm. c Representative images show p150Glued (green) and CHAT (blue) staining in the lumbar spinal cord sections of 3-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Arrows point to the CHAT-positive SMNs. Scale bar: 500 μm. d Representative images show p150Glued (green) and p150Glued/p135+ (red) expression in the NeuN-positive SMNs of 3-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Scale bar: 20 μm
P150Glued conditional knockout mice develop late-onset motor impairments
The Dctn1LoxP/LoxP; Thy1-Cre p150Glued cKO mice were born at a normal Mendelian ratio, developed normally, and had similar body weights as littermate controls (Fig. 4a). The cKO mice also displayed normal ambulatory movement in the open-field tests (Fig. 4b), but showed markedly reduced rearing at 18 months of age (Fig. 4c), and stayed less time on the rotating rods compared to the controls starting at 12 months of age (Fig. 4d). These data suggest that a lack of p150Glued affects the motor control of aged animals.
Fig. 4.

Dctn1LoxP/LoxP; Thy1-Cre mice develop abnormal motor phenotypes. a-d Male Dctn1LoxP/LoxP, Dctn1+/+; Thy1-Cre and Dctn1LoxP/LoxP; Thy1-Cre mice (n ≥ 10 per genotype per time point) were measured for body weight (a); tested for the ambulatory movement (b) and rearing movement (c) in Open-field; and examined for the latency to fall in Rotarod test (d) at 1, 3, 6, 12 and 18 months of age. Data were presented as mean ± SEM. One-way ANOVA plus Tukey’s post hoc test was used for statistical analysis. c At 18 months of age, *p < 0.05 for difference against Dctn1LoxP/LoxP; Thy1-Cre, while no difference was found between Dctn1LoxP/LoxP and Dctn1+/+; Thy1-Cre. d At 12 or 18 months of age, *p < 0.05 or ***p < 0.001 for difference against Dctn1LoxP/LoxP; Thy1-Cre, respectively, while no difference was found between Dctn1LoxP/LoxP and Dctn1+/+; Thy1-Cre
P150Glued conditional knockout mice develop late-onset, selective degeneration of SMNs
To investigate whether p150Glued depletion causes motor neuron loss, we used the unbiased stereology approach to count the numbers of lumbar SMNs and CSMNs in 9 and 18-month-old control Dctn1LoxP/LoxP mice and Dctn1LoxP/LoxP; Thy1-Cre cKO mice. Series of evenly sampled lumbar spinal sections were stained with SMN marker CHAT or histological dye Nissl for neuron counting (Fig. 5a). There was modest but statistically significant reduction of CHAT-positive SMN numbers in the lumbar spinal cord of 18-month-old cKO mice (Fig. 5b). The decrease of lumbar SMN numbers in 18-month-old cKO mice was also observed in the Nissl-stained sections (Fig. 5b). On the other hand, no significant alterations of lumbar SMN numbers were found in 9-month-old cKO mice (Fig. 5b). Additionally, no apparent changes of CTIP2-positive or Nissl-stained CSMN numbers were detected in the motor cortex of 18-month-old cKO mice (Fig. 5c, d). Given that endogenous p150Glued are ubiquitously expressed throughout central neuronal system, we investigated whether p150Glued depletion led to neurodegeneration in other motor function-related brain regions, including midbrain, striatum and cerebellum. Unbiased stereology revealed that no obvious loss of TH-positive dopaminergic (DA) neurons in substantia nigra pars compacta (SNpc) (Additional file 1: Figure S2A, B) and striatal neurons in 18-month-old cKO mice (Additional file 1: Figure S2C-E). Furthermore, neither the thickness of molecular layer (ML) and granule cell layer (GCL), or the density of Calbindin D28K-positive Purkinje cells in the cerebellum of 18-month-old cKO mice was obviously altered (Additional file 1: Figure S2C, F-I). These results demonstrate that p150Glued depletion in adult neurons causes preferentially SMN loss in aged animals.
Fig. 5.
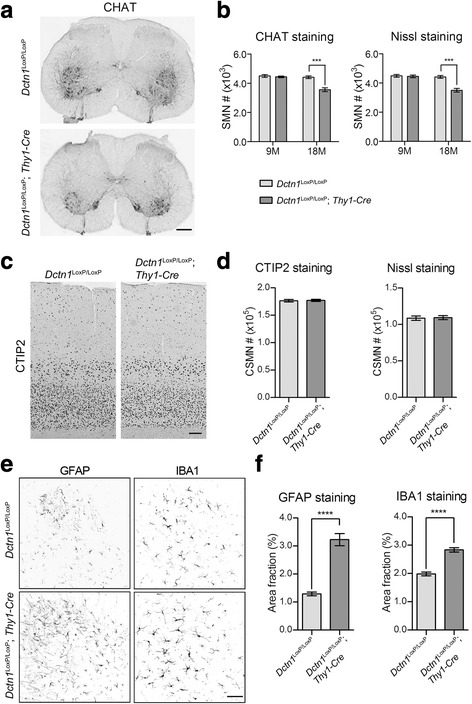
Dctn1LoxP/LoxP; Thy1-Cre mice display loss of spinal motor neurons and gliosis. a Representative images show CHAT staining in the lumbar spinal cord of Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice at 18 months of age. Scale bar: 500 μm. b Unbiased stereological estimation of the number of CHAT-positive SMNs and Nissl-stained SMNs in the lumbar spinal cord of 9 and 18-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice (n = 6 per genotype per time point). Data were presented as mean ± SEM. Two-way ANOVA plus Bonferroni’s post hoc test was used for statistical analysis. At 18 months of age, ***p < 0.001 for difference between Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre. There were significant main effects for age (CHAT staining: F(1, 20) = 25.51, p < 0.0001; Nissl staining: F(1, 20) = 25.18, p < 0.0001), genotype (CHAT staining: F(1, 20) = 22.46, p = 0.0001; Nissl staining: F(1, 20) = 21.85, p = 0.0001) and age-genotype interaction (CHAT staining: F(1, 20) = 17.08, p = 0.0005; Nissl staining: F(1, 20) = 18.47, p = 0.0004). c Representative images show CTIP2 staining in the motor cortex of Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice at 18 months of age. Scale bar: 500 μm. d Unbiased stereological estimation of the number of CTIP2-positive and Nissl-stained large pyramidal neurons in the layer V motor cortex of 18-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice (n = 6 per genotype per time point). Data were presented as mean ± SEM. Unpaired t-test showed no statistical significance (p > 0.05). e Representative images show GFAP and IBA1 staining in the ventral horn of lumbar spinal cord of Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice at 18 months of age. Scale bar: 500 μm. f The areas occupied by GFAP-positive astrocytes and IBA1-positive microglia in the ventral horn of lumbar spinal cord of Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice at 18 months of age (n = 6 animals and 10 sections per animal). Data were presented as mean ± SEM; ****p < 0.0001. Unpaired t-test was used for statistical analysis
P150Glued depletion induces astrogliosis and microgliosis in the spinal cord of aged p150Glued cKO mice
Given that astrogliosis and microgliosis are often in accompany with motor neurodegeneration in the spinal cord [24], we stained the lumbar spinal sections with antibodies against astrogliosis marker GFAP and microgliosis marker IBA1. In line with previous findings [24], we observed a substantial induction of astrogliosis and microgliosis in the spinal cord of 18-month-old cKO mice (Fig. 5e, f).
P150Glued ablation causes severe neuromuscular defects in aged p150Glued cKO mice
To investigate whether p150glued depletion impairs the connections between SMN axon terminals and the innervated muscles, we examined the neuromuscular junctions (NMJs) of gastrocnemius muscles of 18-month-old control and p150Glued cKO mice. α-Bungarotoxin (BTX), which specifically labels acetylcholine receptors at the postsynaptic sites, was applied to label the NMJs (Fig. 6a). Meanwhile, acetylated α-tubulin (ac-TUBA), which is enriched in axons, was used to mark the axon terminals (Fig. 6a). While the NMJs in control mice remained mostly intact, the majority of NMJs in p150Glued cKO mice were either partially or fully fragmented, in company with severe denervation (Fig. 6a, b). The hindlimb clasping test has been used to rate the extent of neuromuscular function impairment in rodent models of motor neuron diseases [25]. 18-month-old male control and p150Glued cKO mice were suspended by the tail for 10 s and the severity of hindlimb clasping was scored. Control mice splayed their hindlimbs outward and away from the abdomen with no clasping reflex, while p150Glued cKO mice retracted their hindlimbs toward the abdomen with substantially higher hindlimb clasping severity score than control mice (Fig. 6c, d). Given that denervation often induce skeletal muscle atrophy in motor neuron diseases, we measured muscle weight and examined gross muscle pathology of control and p150Glued cKO mice. We observed a substantial decrease of gastrocnemius muscle weight in 24-month-old male p150Glued cKO mice (Fig. 6e). Hematoxylin and eosin (HE) staining of gastrocnemius cross sections revealed that 24-month-old male control mice had a polygonal shaped muscle fibers with homogenous fiber size, whereas p150Glued cKO mice exhibited significantly smaller size of muscle fiber in accompany with pathological hallmarks of myofiber de−/regenerating and inflammatory cells infiltration (Fig. 6f, g). These results demonstrate that a loss of p150glued caused substantial NMJ disruption and muscle atrophy in aged mice.
Fig. 6.

Dctn1LoxP/LoxP; Thy1-Cre mice show NMJ fragmentation. a Representative images show α-bungarotoxin (BTX, green), p150Glued (red) and ac-TUBA (purple) staining in the gastrocnemius muscles of Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice at 18 months of age. Scale bar: 20 μm. b Bar graph shows the percentage of intact, partially fragmented, and fully fragmented NMJs in the gastrocnemius muscles of Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice at 18 months of age (n = 6 animals and 10 sections per animal). Data were presented as mean ± SEM; ****p < 0.0001. Unpaired t-test was used for statistical analysis. c Photographs display posture of 18-month-old male Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice in the hindlimb clasping test. d Bar graph shows the hindlimb clasping severity score of 18-month-old male Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice (n = 10–11 per genotype). Data were presented as mean ± SEM; ***p < 0.001. Unpaired t-test was used for statistical analysis. e Quantification of gastrocnemius muscle weight from 24-month-old male Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice (n = 10–11 per genotype). Data were presented as mean ± SEM; **p < 0.01. Unpaired t-test was used for statistical analysis. f Representative images show hematoxylin and eosin (HE) staining of gastrocnemius cross sections from 24-month-old male Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Note the very high amount of small muscle fibers as well as myofiber de−/regeneration (arrow head) and inflammatory cells infiltration (arrow) in Dctn1LoxP/LoxP; Thy1-Cre mice. Scale bar: 50 μm. g Quantification of gastrocnemius muscle fiber areas from 24-month-old male Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice (n = 6 mice per genotype and 6 sections per mouse). Data were presented as mean ± SEM; ****p < 0.0001. Unpaired t-test was used for statistical analysis
A loss of p150Glued increases α-tubulin acetylation
Microtubules are formed by the polymerization of α- and β-tubulin dimers [26]. Various post-translational modifications (PTMs) of α-tubulin affect the microtubule dynamics [27]. To assess the impact of p150Glued depletion on α-tubulin PTMs, we examined the levels of α-tubulin acetylation, tyrosination, and detyrosination by western blots in the spinal cord homogenates of 9-month-old Dctn1LoxP/LoxP; Thy1-Cre cKO mice and littermate Dctn1LoxP/LoxP mice (Fig. 7a). We observed a substantial increase of acetylated α-tubulin levels in the p150Glued cKO samples (Fig. 7a, b). We further confirm this finding by immuno-staining the lumbar spinal sections of 9-month-old mice. There were a few spots in the ventral root SMN axon bundles positive to acetylated α-tubulin staining in the control spinal cords (Fig. 7c). By contrast, a drastic upregulation of acetylated α-tubulin was found in the SMN axons of cKO mice (Fig. 7d). These data demonstrate that p150Glued depletion leads to substantial increase of acetylated α-tubulin levels in the SMN axons, suggesting potential alterations of microtubule stability in p150Glued-lacking SMNs.
Fig. 7.

P150Glued deficiency enhances tubulin acetylation in spinal motor neurons. a-b Western blot analysis shows the expression of acetylated (ac-), tyrosinated (tyro-), detyrosinated (detyro-) and total alpha tubulin (TUBA) in the spinal cord homogenate of 9-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Actin was used as loading control. Bar graphs show the quantification of ac-TUBA, tyro-TUBA and detyro-TUBA level normalized with total TUBA (n = 8 per genotype). Data were presented as mean ± SEM, ***p < 0.001. Unpaired t-test was used for statistical analysis. c, d Representative images show p150Glued (green), ac-TUBA (red) and CHAT (blue) staining in the lumbar spinal cord of 9-month-old Dctn1LoxP/LoxP (c) and Dctn1LoxP/LoxP; Thy1-Cre (d) mice. Four mice per genotype and 10 sections per mouse were examined. Arrows point to CHAT-positive ventral roots with weak ac-TUBA staining in Dctn1LoxP/LoxP mice and substantial ac-TUBA staining in Dctn1LoxP/LoxP; Thy1-Cre mice. Scale bars: 500 μm (low-magnification images), 20 μm (high-magnification images)
A loss of p150Glued promotes autophagosome and lysosome protein accumulation
Dynein/dynactin complexes mediate the transport of autophagosomes and lysosomes in cells [28]. Early studies show impairments of autophagosome and lysosome trafficking in the SMNs of transgenic mice overexpressing disease-related p150Glued G59S mutation, resulting in cytoplasmic accumulation of autophagosome and lysosome-enriched proteins [29, 30]. Accordingly, we examined the levels of autophagosome and lysosome marker proteins in the spinal cord lysates of 9-month-old p150Glued control and cKO mice by western blots. Substantial increases of autophagosome protein LC3II [31] and lysosome proteins LAMP1 and LAMP2 [32] were found in the cKO spinal cords (Fig. 8a, b). However, no apparent alterations of other autophagosome proteins ATG3, ATG5, ATG7, and LC3I were detected (Fig. 8a, b). Since the LAMP2 antibody also works well in immunostaining of brain tissues, we further checked the distribution pattern of LAMP2-postive signals in the SMNs of 9-month-old control and cKO mice. We observed markedly increased LAMP2 signals in the perinuclear regions of cKO SMNs (Fig. 8c). Together, our results reveal the impairments of autophagosome and lysosome transportation in the p150Glued-depleted SMNs.
Fig. 8.

P150Glued deficiency alters autophagosome and lysosome marker protein expression in spinal motor neurons. a Western blot analysis shows the expression of ATG3, ATG5, ATG7, LC3, LAMP1 and LAMP2 in the spinal cord homogenate of 9-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Actin was used as loading control. b Bar graphs show the quantification of ATG3, ATG5, ATG7, LC3, LAMP1 and LAMP2 level normalized with actin (n = 10 per genotype). Data were presented as mean ± SEM; **p < 0.01, ***p < 0.001. Unpaired t-test was used for statistical analysis. c Representative images show LAMP2 (green), CHAT (red) and p150Glued (purple) staining in the lumbar spinal cord of 9-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Four mice per genotype and 10 sections per mouse were examined. Scale bar: 20 μm
P150Glued and other dynactin subunits are more enriched in the postsynaptic sites compared to dynein motor proteins
Neurons communicate with each other through synapses, where the transmitters released from the presynaptic axon terminals bind to the receptors located in the opposite postsynaptic sites in dendritic spines, small protrusions from dendritic shaft [33]. P150Glued is enriched in the axon terminals and facilitate the loading of cargos to dynein-mediated axonal retrograde transport in developing neurons [34]. To investigate whether p150Glued plays any role in the postsynaptic sites, we purified postsynaptic density (PSD)-enriched fractions from control mouse brains. The purity of extracted PSD fractions was verified by the presence of high levels of PSD95 proteins, but not the presynaptic protein synaptophysin (Fig. 9). Interestingly, p150Glued, p135, and other dynactin subunits were found in the PSD fractions, whereas dynein heavy chain (DHC), DIC, and tubulins were undetectable (Fig. 9). On the other hand, low levels of dynein light chain (DLC) were present in the PSD fraction (Fig. 9). These data suggest that p150Glued and dynactin may mediate the transport of postsynaptic cargos from the postsynaptic sites to dynein motor complex associated with microtubules in the dendrites.
Fig. 9.
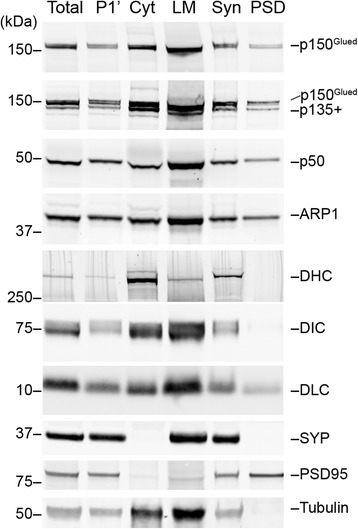
P150Glued and other dynactin subunits are present in the postsynaptic sites. Subcellular fractionation of 3-month-old wild-type mouse brains shows the distribution of dyanctin subunits p150Glued, p135+, p50 and ARP1, dynein subunits DHC, DIC and DLC, synaptophysin (SYP), postsynaptic density protein 95 (PSD95), and α-tubulin in total brain lysates (Total), crude nuclear fraction (P1’), cytosol (Cyt), light membrane fraction (LM), synaptosomes (Syn), and postsynaptic density (PSD)
P150Glued depletion leads to increased cell surface targeting of glutamate receptors and increased vulnerability to glutamate-induced excitotoxicity
Excitotoxicity mediated by glutamate and its receptors has been implicated in the pathogenesis of motor neuron diseases [35]. Using western blots, we found the levels of glutamate receptors GLUR1, GLUR2, NR2A, and NR2B, as well as postsynaptic density protein 95 (PSD95) were all substantially elevated in the spinal cord lysates of 9-month-old Dctn1LoxP/LoxP; Thy1-Cre cKO mice (Fig. 10a, b). By contrast, we did not observe any obvious changes of presynaptic protein synaptophysin expression (Fig. 10a, b). To investigate whether more glutamate receptors are transported to the synapses, we isolated the biotin-labeled cell surface proteins from cultured spinal neurons, and found substantial elevation of glutamate receptor GLUR1, GLUR2, NR2A and NR2B present at the membrane fractions (Fig. 10c, d). In correlation with the increased cell surface targeting of glutamate receptors, p150Glued cKO neurons showed more severe cell death after treated with 10 μm glutamate (Fig. 10e). Therefore, p150Glued depletion causes more postsynaptic accumulation of glutamate receptors, making p150Glued-lacking neurons more susceptible to excitotoxicity.
Fig. 10.

P150Glued deficiency increases protein expression of glutamate receptors and vulnerability to glutamate-mediated excitotoxicity in spinal neurons. a Western blot analysis shows the expression of AMPAR subunits GLUR1 and GLUR2, NMDAR subunits NR2A and NR2B, postsynaptic density protein 95 (PSD95) and synaptophysin (SYP) in the spinal cord homogenate of 9-month-old Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Thy1-Cre mice. Actin was used as loading control. b Bar graphs show the quantification of GLUR1, GLUR2, NR2A, NR2B, PSD95 and SYP level normalized with actin (n = 10 per genotype). Data were presented as mean ± SEM; **p < 0.01, ***p < 0.001. Unpaired t-test was used for statistical analysis. c Western blot analysis shows the levels of biotinylated cell surface and total AMPAR subunits GLUR1 and GLUR2, NMDAR subunits NR2A and NR2B in cultured Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Cre/Esr1 spinal neurons at 21 DIV. Actin was used as loading control. d Bar graphs show the quantification of total and surface GLUR1, GLUR2, NR2A and NR2B level normalized with actin, and the ratio of GLUR1, GLUR2, NR2A and NR2B at cell surface versus total proteins (n = 8 per genotype). Data were presented as mean ± SEM; **p < 0.01, ***p < 0.001. Unpaired t-test was used for statistical analysis. (E) Primary spinal neurons were treated with glutamate, and the viability of these neurons was measured by the MTT assay. Box & whiskers graphs show survival rate (percentage) of cultured Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Cre/Esr1 spinal neurons (21 DIV) treated with 0 or 10 μM glutamate for 24 h (N = 40 per genotype per condition). Data were presented as mean ± SEM. One-way ANOVA plus Tukey’s post hoc test was used for statistical analysis. ***p < 0.001 for difference between Dctn1LoxP/LoxP neurons treated with 0 and 10 μM glutamate, ***p < 0.001 for difference between Dctn1LoxP/LoxP; Cre/Esr1 neurons treated with 0 and 10 μM glutamate, **p < 0.01 for difference between Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Cre/Esr1 neurons treated with 10 μM glutamate, while no difference was found between Dctn1LoxP/LoxP and Dctn1LoxP/LoxP; Cre/Esr1 neurons treated with 0 μM glutamate
Discussion
In the current study, we generated and characterized a line of p150Glued cKO mice to critically evaluate the roles of CAP-Gly domain-containing dynactin p150Glued in postnatal neurons. A loss of p150Glued in postnatal neurons did not affect the assembly of dynein/dynactin motor protein complex in neurons, but caused rather selective SMN degeneration and movement impairments in aged mice. The depletion of p150Glued also led to substantial elevation of acetylated α-tubulin, glutamate receptors, and proteins involved in autophagosome and lysosome pathways in the cKO neurons. Finally, p150Glued ablation rendered spinal neurons more susceptible to excitotoxicity. Considering all the disease-causative mutations compromise the MTBD functions of p150Glued, increased excitotoxicity may underlie a common pathogenic mechanism of p150Glued mutations in diverse neurodegenerative diseases.
The absence of more drastic morphological and functional phenotypes in p150Glued cKO neurons suggests that p135 and other MTBD-lacking p150Glued splicing variants may largely substitute the functions of p150Glued in neurons. The CC1 domain of p150Glued mediates the interaction with DIC and other dynein motor protein complex [2]. Since p135 and other p150Glued short forms contain the CC1 domain, it is not surprising that dynein and dynactin complex remains intact and appears mostly functional in neurons. P150Glued thereby is likely devoted to some special tasks in neurons. Neurons, especially the large projection neurons like CSMNs and SMNs, contain long axons. Microtubules form the backbone of these axons. P150Glued is proposed to play a critical role in maintaining the stability of microtubules by serving as an anti-catastrophe factor in neuronal cultures [11]. However, we did not observe any substantial loss of axons in brain and spinal cord of presyptomatic p150Glued cKO mice. Instead, we found a substantial augmentation of acetylated α-tubulin in neural tissues, including the axons of SMNs. It has been well documented that the acetylation of α-tubulin improves the stability of microtubules [36]. Therefore, the increased acetylation of α-tubulin may serve as a compensatory mechanism to maintain the integrity of microtubules in the absence of p150Glued. It will be interesting to delineate the signaling pathways leading to the increased α-tubulin acetylation in p150Glued cKO neurons.
In addition to maintaining microtubule stability, p150Glued is also proposed as a key player in uploading various cargos to the dynein motor complex in the axon terminals before being transported to the soma [34]. For example, live imaging studies show p150Glued captures the cargos and loads to the microtubule plus ends for the minus-end-directed transport [37]. Accordingly, we found a lack of p150Glued caused substantial disruption of NMJ structures in p150Glued cKO mice. Moreover, our studies suggest that p150Glued also plays a role in uploading postsynaptic glutamate receptors to dynein-mediated transport in dendrites. As results, p150Glued depletion led to increased accumulation of glutamate receptors in the postsynaptic sites, which makes the p150Glued cKO neurons more vulnerable to glutamate stimulation, suggesting increased excitotoxicity may contribute to the eventual loss of SMNs in p150Glued cKO mice. Future studies will be needed to investigate whether the disease-causative mutations also make neurons subject to excitotoxicity.
The dynein/dynactin complex is critical for organelle transport from cell periphery to the perinuclear sites along the microtubule networks, such as the centripetal movement of autophagosomes, lysosomes and endosomes [2]. It has been shown in the previous studies that overexpressing the motor neuron disease–causative G59S mutant p150Glued impairs the autophagosome and lysosome pathways [1, 30]. In line with these earlier findings, we observed similar defects in abnormal accumulation of autophagosome and lysosome proteins in p150Glued-lacking SMNs. The disruption of autophagosome and lysosome-mediated protein turnover thereby could also lead to the SMN loss in aging.
While in this study we focused on the roles of p150Glued in CSMNs and SMNs, the floxed MTBD of p150Glued can also be removed from the other neuron and cell types at different time points based on the spatial and temporal expression pattern of Cre DNA recombinase. The availability of p150Glued cKO mice therefore provides a useful tool to study the functional significance of the CAP-Gly domain of p150Glued in vivo.
Conclusions
Multiple missense mutations in the CAP-Gly domain of dynactin p150Glued have been linked to lower motor neuron disease, and other degenerative neurological disorders. These disease-causative mutations seem to weaken the microtubule binding affinity of p150Glued. However, the functional significance of p150Glued has not been critically evaluated in vivo. In the present study, we generated and characterized a line of p150Glued cKO mice to critically evaluate the roles of CAP-Gly domain-containing dynactin p150Glued in postnatal neurons. The depletion of p150Glued caused selective spinal motor neuron degeneration and movement impairments in aged mice. Substantial elevation of acetylated α-tubulin, proteins involved in autophagosome and lysosome pathways, and glutamate receptors were observed in the cKO neurons, while higher glutamate receptor levels were correlated with increase susceptibility of spinal neurons to excitotoxicity. Increased excitotoxicity may underlie a common pathogenic mechanism of p150Glued mutations in diverse neurodegenerative diseases.
Additional files
Figure S1. Distribution of CRE in the brain and spinal cord of Thy1-Cre mice and depletion of p150Glued in adult cKO mice. Figure S2. No apparent loss of midbrain dopaminergic neurons, striatal neurons, cerebellar granule cells and Purkinje cells in aged Dctn1LoxP/LoxP; Thy1-Cre mice. (DOCX 4180 kb)
Acknowledgements
The authors thank Cai lab members for their various supports, China Scholarship Council (CSC) for its International Exchange Program, and Beijing Municipal Administration of Hospitals for its High-level Talents Program.
Funding
This work was supported in part by the intramural research programs of National Institute on Aging (AG000946), National Natural Science Foundation of China (No. 81601117), Beijing Natural Science Foundation (No. 7152077 and 7184221), Beijing Hundreds and Thousands of Talents Project (No. 2017A14), and Beijing Nova Program (No. xx2018099).
Authors’ contributions
HC, JY, CL. HS conceived the research and designed the experiments. JY, CL, HS, CX, LS, C-XL, JD, and YL performed experiments. HC and JY analyzed data and wrote manuscript. CL critically reviewed manuscript. All authors read and approved the final manuscript.
Ethics approval
All animal procedures conformed to the NIH guide for the ethical care and use of laboratory animals. Animal protocols were approved by the Institutional Animal Care and Use Committee of National Institute on Aging.
Consent for publication
All authors have read the manuscript and indicated consent for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13024-018-0242-z) contains supplementary material, which is available to authorized users.
References
- 1.Levy JR, Holzbaur EL. Cytoplasmic dynein/dynactin function and dysfunction in motor neurons. Int J Dev Neurosci. 2006;24(2–3):103–111. doi: 10.1016/j.ijdevneu.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Schroer TA. Dynactin. Annu Rev Cell Dev Biol. 2004;20:759–779. doi: 10.1146/annurev.cellbio.20.012103.094623. [DOI] [PubMed] [Google Scholar]
- 3.Ayloo S, Lazarus JE, Dodda A, Tokito M, Ostap EM, Holzbaur EL. Dynactin functions as both a dynamic tether and brake during dynein-driven motility. Nat Commun. 2014;5:4807. doi: 10.1038/ncomms5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Culver-Hanlon TL, Lex SA, Stephens AD, Quintyne NJ, King SJ. A microtubule-binding domain in dynactin increases dynein processivity by skating along microtubules. Nat Cell Biol. 2006;8(3):264–270. doi: 10.1038/ncb1370. [DOI] [PubMed] [Google Scholar]
- 5.Robinson RW, Snyder JA. Colocalization studies of Arp1 and p150Glued to spindle microtubules during mitosis: the effect of cytochalasin on the organization of microtubules and motor proteins in PtK1 cells. Cell Biol Int. 2006;30(7):631–639. doi: 10.1016/j.cellbi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhapparova ON, Bryantseva SA, Dergunova LV, Raevskaya NM, Burakov AV, Bantysh OB, Shanina NA, Nadezhdina ES. Dynactin subunit p150Glued isoforms notable for differential interaction with microtubules. Traffic. 2009;10(11):1635–1646. doi: 10.1111/j.1600-0854.2009.00976.x. [DOI] [PubMed] [Google Scholar]
- 7.Lai C, Lin X, Chandran J, Shim H, Yang WJ, Cai H. The G59S mutation in p150(glued) causes dysfunction of dynactin in mice. J Neurosci. 2007;27(51):13982–13990. doi: 10.1523/JNEUROSCI.4226-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puls I, Jonnakuty C, LaMonte BH, Holzbaur EL, Tokito M, Mann E, Floeter MK, Bidus K, Drayna D, Oh SJ, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33(4):455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- 9.Farrer MJ, Hulihan MM, Kachergus JM, Dachsel JC, Stoessl AJ, Grantier LL, Calne S, Calne DB, Lechevalier B, Chapon F, et al. DCTN1 mutations in Perry syndrome. Nat Genet. 2009;41(2):163–165. doi: 10.1038/ng.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustavsson EK, Trinh J, Guella I, Szu-Tu C, Khinda J, Lin CH, Wu RM, Stoessl J, Appel-Cresswell S, McKeown M et al: DCTN1 p.K56R in progressive supranuclear palsy. Parkinsonism Relat Disord 2016, 28:56–61. [DOI] [PubMed]
- 11.Lazarus JE, Moughamian AJ, Tokito MK, Holzbaur EL. Dynactin subunit p150(glued) is a neuron-specific anti-catastrophe factor. PLoS Biol. 2013;11(7):e1001611. doi: 10.1371/journal.pbio.1001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixit R, Levy JR, Tokito M, Ligon LA, Holzbaur EL. Regulation of dynactin through the differential expression of p150Glued isoforms. J Biol Chem. 2008;283(48):33611–33619. doi: 10.1074/jbc.M804840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tokito MK, Howland DS, Lee VM, Holzbaur EL. Functionally distinct isoforms of dynactin are expressed in human neurons. Mol Biol Cell. 1996;7(8):1167–1180. doi: 10.1091/mbc.7.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin S, Ziese M, Sternberg N. A novel role for site-specific recombination in maintenance of bacterial replicons. Cell. 1981;25(3):729–736. doi: 10.1016/0092-8674(81)90180-X. [DOI] [PubMed] [Google Scholar]
- 15.Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28(3–4):106–110. doi: 10.1002/1526-968X(200011/12)28:3/4<106::AID-GENE30>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Dewachter I, Reverse D, Caluwaerts N, Ris L, Kuiperi C, Van den Haute C, Spittaels K, Umans L, Serneels L, Thiry E, et al. Neuronal deficiency of presenilin 1 inhibits amyloid plaque formation and corrects hippocampal long-term potentiation but not a cognitive defect of amyloid precursor protein [V717I] transgenic mice. J Neurosci. 2002;22(9):3445–3453. doi: 10.1523/JNEUROSCI.22-09-03445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 18.Parisiadou L, Yu J, Sgobio C, Xie C, Liu G, Sun L, Gu XL, Lin X, Crowley NA, Lovinger DM, et al. LRRK2 regulates synaptogenesis and dopamine receptor activation through modulation of PKA activity. Nat Neurosci. 2014; [DOI] [PMC free article] [PubMed]
- 19.Lin X, Parisiadou L, Sgobio C, Liu G, Yu J, Sun L, Shim H, Gu XL, Luo J, Long CX, et al. Conditional expression of Parkinson's disease-related mutant alpha-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci. 2012;32(27):9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aliaga L, Lai C, Yu J, Chub N, Shim H, Sun L, Xie C, Yang WJ, Lin X, O'Donovan MJ, et al. Amyotrophic lateral sclerosis-related VAPB P56S mutation differentially affects the function and survival of corticospinal and spinal motor neurons. Hum Mol Genet. 2013;22(21):4293–4305. doi: 10.1093/hmg/ddt279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai H, Lin X, Xie C, Laird FM, Lai C, Wen H, Chiang HC, Shim H, Farah MH, Hoke A, et al. Loss of ALS2 function is insufficient to trigger motor neuron degeneration in knock-out mice but predisposes neurons to oxidative stress. J Neurosci. 2005;25(33):7567–7574. doi: 10.1523/JNEUROSCI.1645-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai C, Xie C, McCormack SG, Chiang HC, Michalak MK, Lin X, Chandran J, Shim H, Shimoji M, Cookson MR, et al. Amyotrophic lateral sclerosis 2-deficiency leads to neuronal degeneration in amyotrophic lateral sclerosis through altered AMPA receptor trafficking. J Neurosci. 2006;26(45):11798–11806. doi: 10.1523/JNEUROSCI.2084-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45(2):207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 24.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 25.McGoldrick P, Joyce PI, Fisher EM, Greensmith L. Rodent models of amyotrophic lateral sclerosis. Biochim Biophys Acta. 2013;1832(9):1421–1436. doi: 10.1016/j.bbadis.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Weisenberg RC. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- 27.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 2011;12(12):773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 28.Fu MM, Nirschl JJ, Holzbaur EL. LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Dev Cell. 2014;29(5):577–590. doi: 10.1016/j.devcel.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levy JR, Sumner CJ, Caviston JP, Tokito MK, Ranganathan S, Ligon LA, Wallace KE, LaMonte BH, Harmison GG, Puls I, et al. A motor neuron disease-associated mutation in p150Glued perturbs dynactin function and induces protein aggregation. J Cell Biol. 2006;172(5):733–745. doi: 10.1083/jcb.200511068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laird FM, Farah MH, Ackerley S, Hoke A, Maragakis N, Rothstein JD, Griffin J, Price DL, Martin LJ, Wong PC. Motor neuron disease occurring in a mutant dynactin mouse model is characterized by defects in vesicular trafficking. J Neurosci. 2008;28(9):1997–2005. doi: 10.1523/JNEUROSCI.4231-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26(31):8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273(5274):501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 33.Blanpied TA, Ehlers MD. Microanatomy of dendritic spines: emerging principles of synaptic pathology in psychiatric and neurological disease. Biol Psychiatry. 2004;55(12):1121–1127. doi: 10.1016/j.biopsych.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Moughamian AJ, Holzbaur EL. Dynactin is required for transport initiation from the distal axon. Neuron. 2012;74(2):331–343. doi: 10.1016/j.neuron.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(Suppl 1):S3–S9. doi: 10.1002/ana.21543. [DOI] [PubMed] [Google Scholar]
- 36.Portran D, Schaedel L, Xu Z, Thery M, Nachury MV. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat Cell Biol. 2017;19(4):391–398. doi: 10.1038/ncb3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaughan PS, Miura P, Henderson M, Byrne B, Vaughan KT. A role for regulated binding of p150(glued) to microtubule plus ends in organelle transport. J Cell Biol. 2002;158(2):305–319. doi: 10.1083/jcb.200201029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Distribution of CRE in the brain and spinal cord of Thy1-Cre mice and depletion of p150Glued in adult cKO mice. Figure S2. No apparent loss of midbrain dopaminergic neurons, striatal neurons, cerebellar granule cells and Purkinje cells in aged Dctn1LoxP/LoxP; Thy1-Cre mice. (DOCX 4180 kb)


