Abstract
Skeletal muscle dysfunction contributes to exercise limitation in COPD. In this study cigarette smoke exposure was hypothesized to increase expression of the inflammatory cytokine, TNF-α, and down stream molecules that regulate oxygen transport and muscle function. Furthermore, we hypothesized that highly-vascularized oxidative skeletal muscles would be more susceptible to the damaging effects of cigarette smoke compared to a less well-vascularized glycolytic muscle. To test these hypotheses mice were exposed to daily periods of cigarette smoke over 8 and 16-weeks resulting in 157% (8 wks) and 174% (16 wks) increases in serum TNF-α. Separately TNF-α administered to C2C12 myoblasts was found to dose dependently reduce PGC-1α mRNA. The vascular PGC-1α target molecule, VEGF, was also down-regulated but only in the soleus, which exhibited capillary regression and an oxidative to glycolytic fiber-type transition. The apoptosis PGC-1α target genes, atrogin-1 and MuRF1, were upregulated and to a greater extent in the soleus compared to the EDL. Citrate synthase (soleus −19%, EDL −17%) and β-hydroxyacyl CoA dehydrogenase (β-HAD) (soleus −22%, EDL −19%) decreased similarly in both muscle types. There was loss of body and gastrocnemius complex mass, with rapid soleus but not EDL fatigue and diminished exercise endurance. These data suggest that in response to smoke exposure, TNF-α mediated down-regulation of PGC-1α may be a key step leading to vascular and myocyte dysfunction, effects that are more evident in oxidative than glycolytic skeletal muscles.
Introduction
Cigarette smoking is a well-known risk factor for the development of COPD (Barnes, 2003). In addition to the damaging effects cigarette smoke exerts on the lung, several associated extrapulmonary pathologies have been found to occur in peripheral organs (Jobin et al., 1998; Maltais et al., 1998; Rabinovich et al., 2001; Schols et al., 1996). For instance some COPD patients and animal models exposed to cigarette smoke present a decrease in skeletal muscle fiber size, reduced oxidative enzyme activity, capillary regression and a shift in muscle fiber composition from oxidative to glycolytic fiber types (Gosker et al., 2008; Jobin et al., 1998; Whittom et al., 1998). However, the underlying mechanism responsible for these changes in the cigarette smoke associated muscle pathology has not been fully elucidated.
One consequence of cigarette smoke exposure is the presence of chronic systemic inflammation (Barreiro et al., 2008; Gosker et al., 2008; Schols et al., 1996). In particular, the inflammatory cytokine, TNF-α, has been associated with cachexia, or muscle wasting, that occurs in subpopulations of COPD patients (Di Francia et al., 1994; Schols et al., 1996; Takabatake et al., 2000). Circulating TNF-α levels have also been shown to increase in mice exposed to periods of cigarette smoke over the course of 6 months (Gosker et al., 2008). Following this long-term period of smoke exposure, mice revealed a loss of body mass, a fiber type transition in the soleus characterized by less MHC IIA and decreased oxidative enzyme activities (Gosker et al., 2008). More direct evidence, indicating that TNF-α expressed in the lung may contribute to the pathological mechanism leading skeletal muscle mass loss, comes from a lung-specific TNF-α transgenic mouse model. TNF-α overexpressing mice exhibit emphysema and chronic inflammation. In addition these TNF-α overexpressing mice have an impaired ability to regenerate skeletal muscle following hind limb suspension-induced atrophy (Langen et al., 2006).
A transcriptional co-factor that could potentially mediate changes in both vascular and myocyte-specific cellular function in COPD is PGC-1α (Handschin et al., 2007; Leick et al., 2009). PGC-1α functions as a co-factor with ERR to regulate the exercise-induced angiogenic factor, VEGF (Arany et al., 2008; Breen et al., 1996), and PGC-1α knockout mice have recently been reported to exhibit decreases in both skeletal muscle VEGF levels and capillary to fiber ratio (Leick et al., 2009). Furthermore, PGC-1α gene deleted mice are unable to increase VEGF expression in response to acute exercise or elicit a skeletal muscle exercise-induced angiogenic response (Leick et al., 2009). PGC-1α also has the potential to regulate several target genes that regulated myocyte function including those involved in mitochondrial biogenesis, skeletal muscle fiber type switching and atrophy (Adhihetty et al., 2009; Arany et al., 2005; Handschin et al., 2007; Lin et al., 2002). Thus, PGC-1α is a potential upstream regulator of both vascular and myocyte cellular functions and insufficient PGC-1α levels could contribute to impaired muscle performance in smoke-induced COPD.
To further understand the possible roles of, and links between, TNF-α and PGC-1α in cigarette smoke-induced skeletal muscle impairment, we assessed circulating TNF-α levels, hind-limb muscle PGC-1α expression, and target effects of PGC-1α (capillarity, oxidative metabolism, atrophy, fiber type transition and contractile capacity) in mice exposed to daily periods of cigarette smoke for either 8 or 16 weeks. This covers a period prior to destruction of the lung (emphysema) (Gosker et al., 2008; Hautamaki et al., 1997; Wright and Churg, 1990), and was chosen to minimize the possible influences of pulmonary dysfunction itself on exercise performance. In addition, we examined the direct effect of TNF-α on PGC-1α expression isolated myocytes. Furthermore, because PGC-1α plays a role in mitochondrial biogenesis and capillary regulation (Arany et al., 2008; Handschin et al., 2007; Lin et al., 2002), the soleus, composed mostly of oxidative fibers with a high mitochondrial content and vessel density, was compared to the extensor digitorum longus (EDL), which is essentially glycolytic (lower mitochondrial and capillary number) (Spangenburg and Booth, 2003), with the expectation that the soleus would be more affected.
Materials and Methods
Animals
Eight weeks old male C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, MA). The Institutional Animal Care and Use Committee at the University of California, San Diego approved the use of animals in this study.
C2C12 cell culture
The murine skeletal muscle cell line C2C12 was obtained from the ATCC (# CRL1772) and cultured in DMEM with 10% fetal calf serum. To induce differentiation, myoblasts were platted at an initial density of 3 × 105 cells/well in 6-well culture dishes for 24 h, allowing them to reach 80% confluency. Then, medium was replaced with DMEM containing 2% horse serum for 5 days. When applicable, 1, 10, or 100 ng/ml mouse TNF-α (Invitrogen, Carlsbad, CA) was added once to the culture dishes. After 24 h incubation, myoblasts were harvested for mRNA or Western blot analysis.
Cigarette Smoke Exposure
Non-filtered 3R4F research cigarettes were purchased from Kentucky Tobacco Research and Development Center, University of Kentucky. A modified cigarette smoke chamber was designed based on previously published protocols (Chen et al., 2006). In brief, mice were kept inside an 18-liter plastic chamber. A rodent ventilator was used to draw on the lighted cigarette and deliver the smoke into the chamber such that each cigarette was consumed over 5 minutes. Mice were exposed to two smoking periods (5 cigarettes consecutively for each period with a 30-min fresh air exposure in between) each day, 6 days a week. Mice of the same strain and age were continuously exposed to room air as the control group. Tissue samples were collected 24 hours after the last smoke exposure.
VEGF and TNFα ELISA
VEGF and TNF-α were measured by ELISA specific for mouse VEGF (R&D, Minneapolis MN) and TNF-α (Pierce, Rockford IL), respectively, following the manufacture’s protocols.
Real-time RT-PCR assay for measurement of target mRNAs
Total cellular RNA from soleus and EDL was isolated with TriPure Isolation Reagent (Invitrogen) and purified and DNase I treated with RNAeasy Mini Kit (QIAGEN, Germany). A Thermoscript kit (Invitrogen) was used for reverse transcription from total RNA. The RT products were amplified with an RT2 Real-time SYBR Green Kit (SuperArray, Fredrick MD) and MxP3000 Real Time PCR System (Stratagene, La Jolla CA). Primers used for mPGC-1α (NCBI accession number: NM_008904) amplification were: Forward: 5′-CGGAAATCATATCCAACCAG-3′, reverse: 5′-TGAGGACCGCTAGCAAGTTTG-3′; mTNFα (NM_013693) Forward: 5′-CCGATGGGTTGTACCTTGTC-3′, Reverse: 5′-TGGAAGACTCCTCCCAGGTA-3′; and for mVEGF (M95200) were: Forward: 5′-CGAAACCATGAACTTTCTGC-3′, Reverse: 5′-CCTCAGTGGGCACACACTCC-3′; mAtrogin1 (AF441120) Forward: 5′-CTGGCAGGGAGGAGCCTAATGAATC-3′, Reverse: 5′-GGGAGTGGCAAAGCCGTCTC-3; mMuRF1 (DQ229108) Forward 5′-TGGAAACGCTATGGAGAACC-3′, Reverse 5′-ATTCGCAGCCTGGAAGATG-3′.
Capillarity Measurements
Soleus and EDL were surgically removed from mice under anesthesia, positioned vertically, mounted with OCT and quickly frozen in liquid N2-cooled isopentane and then stored at −80 °C. Ten micron frozen cross-sections were stained with a combined alkaline phosphatase (AP) and dipeptidylpeptidase (DPP) staining technique to reveal capillaries (Mrazkova et al., 1986). We have previously confirmed the accuracy for capillary identification using this method by comparison to von Willebrand factor immunohistochemistry and CD31 (Tang et al., 2004). Two parameters of capillarity were determined: (1) the capillary number per fiber (capillary/fiber ratio); and (2) the number of capillaries per mm2 of fiber area (capillary density). A minimum of 100 randomly chosen muscle fibers over the entire cross-section of soleus and EDL were counted per sample.
Muscle Preparation for Enzyme Assays and Myosin Heavy Chain (MHC) Analysis
According to a modified method of Gosker et al. (Gosker et al., 2008), the soleus (7.6±0.37 mg) and EDL (8.2±0.33 mg) were frozen in liquid N2, and stored at −80°C. Muscles were homogenized in a buffer (50μl/mg) containing 250 mM sucrose, 2 mM EDTA, 10 mM TRIS, pH 7.4. After a 1-minute sonication period, the homogenized samples were centrifuged at 10,000g, 4°C, for 10 min. The supernatant from this spin was used for citrate synthase (CS) and β-hydroxyacyl CoA dehydrogenase (β-HAD) assays. The remaining pellet was re-suspended in 50μl of ice-cold extraction buffer (100 mM Na4O7P2 10H2O, 5 mM EDTA, 1 mM DTT, pH8.5), incubated on ice for 30 min, and centrifuged at 10,000 g, 4°C for 10 min. Supernatant from the second spin was used for the MHC analysis.
Citrate Synthase (CS) and β-hydroxyacyl CoA dehydrogenase (β-HAD) activity assays
CS activity in the homogenates was measured (indirectly with the oxaloacetate method) with a Citrate Synthase Assay Kit (Sigma, St. Louis, MO), following the manufacture’s protocol (Srere, 1969). The activity of β-HAD was measured by the NADH method (Bergmeyer, 1974). Briefly, every reaction mixture (total volume 500μl) for CS measurement included 320μl 100mM-Tris buffer (pH8.3), 50μl 1mM-DTNB, 80μl 3mM-Acetayl CoA, 20μl homogenate, and 30μl 10mM-Oxaloacetate. The β-HAD reaction included 430μl assay buffer (0.25 mM NADH, 1mM EDTA, 50 mM Tris pH 7.0), 50μl homogenate, 10μl Triton −100, and 10μl 5mM-Acetoacetyl-CoA. Reaction products were assayed by spectrophotometer (Beckman DU-640) at 412 nm for CS, and 340 nm for β-HAD, respectively, at 1 min intervals at 25°C over 5 minutes. The enzyme activities are expressed as the units (U) catalyzing μmole of substrate /mg wet tissue /min.
Western blot for PGC-1α
Homogenized muscle protein samples (30 μg) were separated on Tris-glycine gels and transferred to Proban BA 85 nitrocellulose membrane (Schleicher and Schuell, Dassel, Germany). Western blots were visualized with enhanced chemiluminescence fluid (ECF; Amersham). The primary antibody used in this project was an anti-PGC-1α with 1:500 dilution (Cat #ab72230, Abcam, Cambridge, MA). The density of each band was divided by the average density of the control group to quantify fold changes induced by smoke exposure.
Myosin Heavy Chain (MHC) Isoform Analyses
MHC isoforms were analyzed by SDS-PAGE (Mizunoya et al., 2008). Briefly, 1 μg of supernatant total protein was electrophoresed at 70 V, 4°C for 24 h on 8% SDS-PAGE (acrylamide:Bis 99:1, 35% v:v glycerol, 0.4% w:v SDS, 0.1 M glycine, and 0.2 M Tris-HCl, pH 8.8) with a 6% stacking gel (Acrylamide:Bis 50:1, 30% v:v glycerol, 0.4% w:v SDS, 4 mM EDTA, and 70 mM Tris–HCl pH 6.7). The lower running buffer consisted of 0.05 M Tris, 75 mM glycine, and 0.05% w:v SDS. The upper running buffer was at 6× the concentration of the lower running buffer with 0.12% v:v β-mercaptoethanol (Kohn and Myburgh, 2006). After electrophoresis, gels were stained with a silver staining kit (Bio-Rad, Hercules CA.) and analyzed with Image J software (http://www.nih.gov). The densities of individual MHC bands are expressed as a percentage of the total density of all detectable MHC bands in each sample.
Measurement of fatigue in isolated muscles
The method used to evaluate fatigue in isolated muscles was that of Del Prete et al. (Del Prete et al., 2008). Isolated soleus and EDL were individually suspended in Krebs solution aerated with 95% O2 and 5% CO2 at room temperature (23° C). One end of each muscle was anchored to an FT-10 force transducer (Grass, Quincy MA). The muscle was then stimulated at the optimal original length using single twitch pulses via electrodes positioned on the two sides of the muscle with 1mm distance. Stimulation was generated with an S48 stimulator (Grass, Quincy, MA) as follows: train duration: 500msec for soleus, 300msec for EDL; rate: 50Hz for soleus, 70Hz for EDL; and for both muscles, voltage: 10V; stim duration: 0.2msec; stim rate: 15/min for 2min, 20/min for 2min, 30/min for 2min, and 60/min for 2 min. The fatigue resistance was calculated as the time to reach 50% of the initial force generated by electrically stimulated contractions.
Treadmill tests for maximal speed and endurance
Mice were exercised on an Omnipacer Treadmill LC4 (Omnitech Electronics Inc., Columbus, OHIO) on a 10° incline while breathing room air. Maximal Speed: To determine the maximal running capacity mice were subjected to an incremental speed running test on a treadmill. Mice were initially exercised at a speed of 24 m/min and this was increased by 2 m/min every 30 seconds until exhaustion. Endurance: Both control and smoke-exposed mice were run on a treadmill at 20 m/min (60% of the average maximal speed of smoke-exposed mice) at a 10° inclination and time to exhaustion was recorded. Exhaustion was defined in both tests as consistent refusal to run in spite of contact with the electrical grid at the rear of the treadmill.
Statistics
All experimental data are expressed as the mean ± SD, and were analyzed by an ANOVA. When this produced a significant result, Tukey’s post-hoc test was used for pair-wise comparison. Values of p<0.05 were considered to demonstrate significant differences.
Results
TNF-α-induced inhibition of PGC-1α expression in C2C12 cells
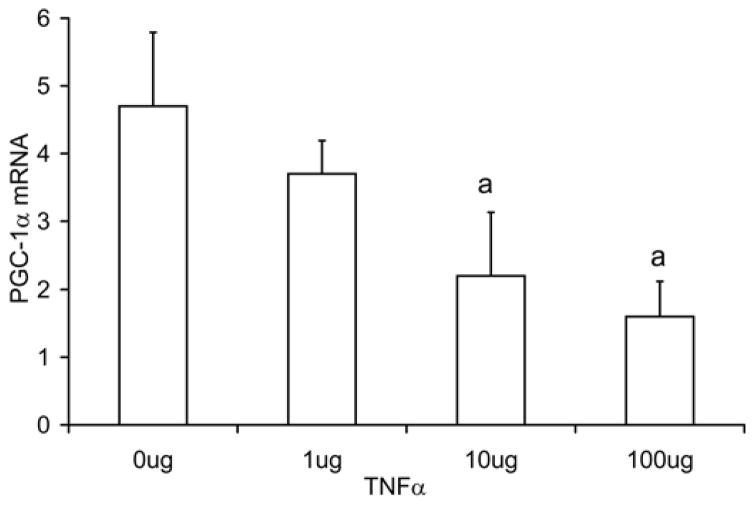
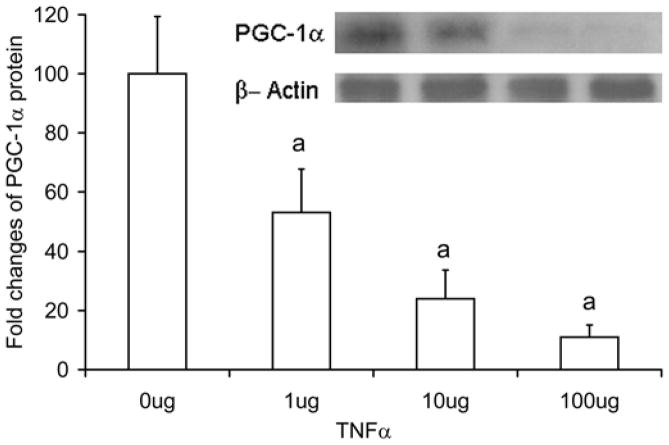
In Fig. 1a, the PGC-1α mRNA levels are reported for cultured C2C12 cells incubated with 1, 10, and 100 ng/ml of TNF-α for 24 hours. In TNF-α treated C2C12 cells, PGC-1α mRNA levels were decreased at doses of 10 ng/ml (Control 4.7±1.1 relative units, TNF-α 2.2±0.9 relative units, p=0.013) and 100ng/ml (1.6±0.5 relative units, p=0.002), representing reduction of 53% and 66%, respectively. In the same TNF-α treated cells, PGC-1α protein showed a greater inhibition (Fig. 1b) with a 47% decrease (p=0.009) in the 1 ug TNF-α group, 76% decrease in the 10 ug group (p<0.001), and 89% decrease in the 100 ug group (p<0.001), respectively.
Fig. 1. TNF-α decreases PGC-1α mRNA level in C2C12 cells.
C2C12 cells were treated with TNFα (1, 10, and 100ng/ml) for 24 hours. PGC-1α mRNA levels measured with real time RT-PCR (a), and in protein levels measured by Western blot (b). Values represent the mean ± SD, a=p<0.05 from control group, n=4.
Serum TNF-α and skeletal muscle PGC-1α levels
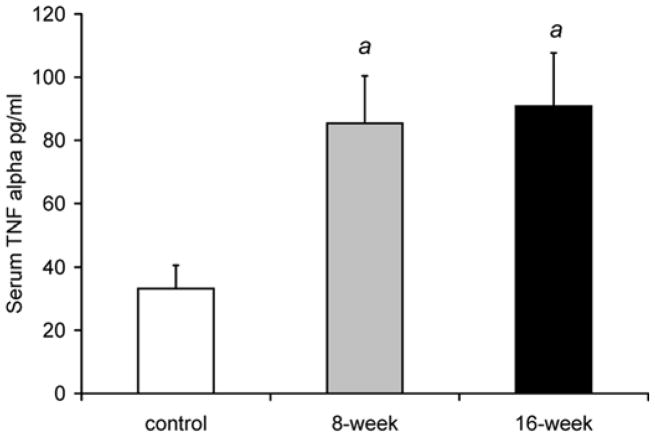
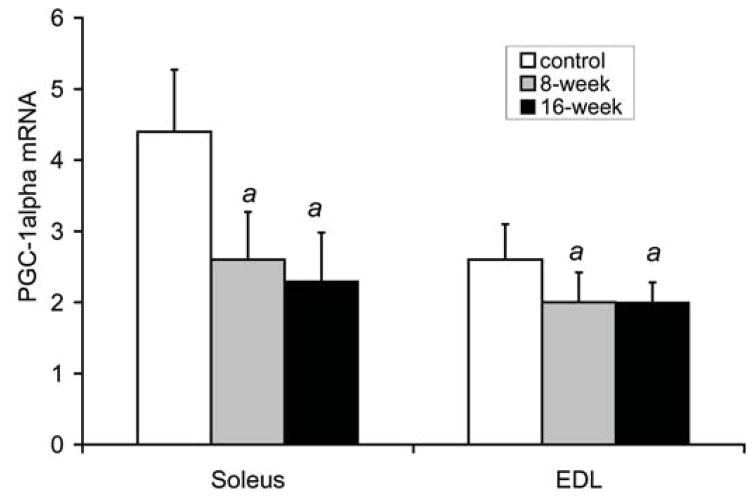
Fig. 2a shows that serum TNFα levels were higher by 157% after 8 weeks, and by 174% after 16 weeks of smoke exposure compared to control, non-exposed mice [control: 33.2±7.4 pg/ml, 8 weeks: 85.4±15.0 (p<0.001), and 16 weeks: 90.8±16.9 (p<0.001)]. Fig. 2b shows that PGC-1α mRNA levels in the soleus were lower by 42% after 8 weeks of smoke exposure (2.6±0.7, relative units, p=0.002) and by 48% after 16 weeks (2.3±0.7, p=0.001) compared to the control group (4.4±0.9). In the EDL, PGC-1α mRNA was lower by 24% after 8 weeks (2.0±0.4 relative units, p=0.044), and by 23% after 16 weeks in the smoke exposed groups (2.0±0.3 p=0.028) compared to control (2.6±0.5).
Fig. 2. Increased serum TNF-α and decreased skeletal muscle PGC-1α in mice exposed to cigarette smoke.
C57Bl/6J mice exposed to daily periods of whole body cigarette smoke for 8 and 16 weeks, (a) plasma TNFα levels (b) soleus and EDL PGC-1α mRNA levels. Values are the mean ± S.D. a= significantly (p<0.05) from control, b= significantly (p<0.05) from 8-week result, n=6.
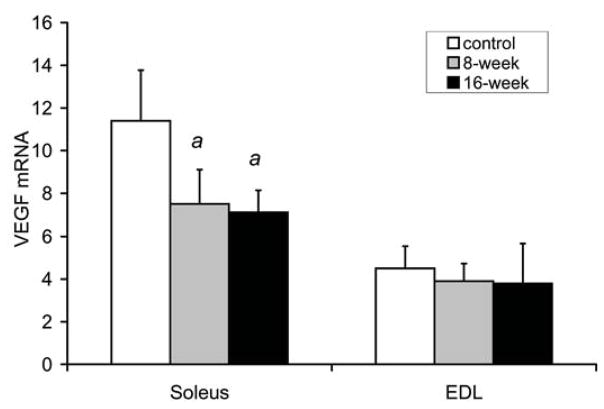
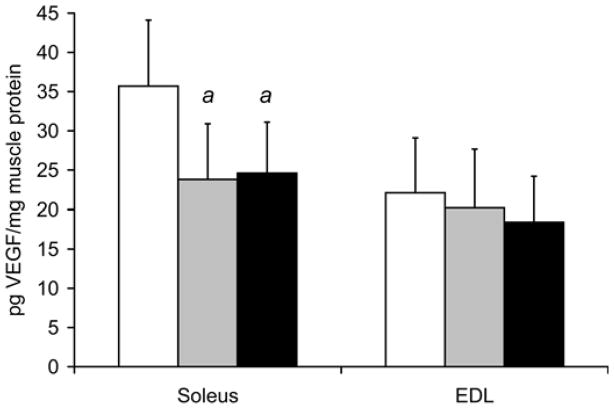
VEGF levels in the soleus and EDL
Fig. 3a shows VEGF mRNA levels in the soleus were decreased by 35% at 8 weeks (7.5±1.6 relative units, p=0.007), and by 38% (7.1±1.3, p=0.003) at 16 weeks in the smoke exposed mice compared to soleus isolated from control mice (11.4±2.4). However, smoke exposure did not alter VEGF mRNA levels in the EDL (3.9±0.8 relative units after 8 weeks, and 3.8±1.9 after 16 weeks vs. 4.5±1.0 in controls). In the same samples used to measure VEGF mRNA levels (Fig. 3b), VEGF protein in the soleus was found to be 33% lower at 8 weeks (23.8±7.1 pg/mg muscle, p=0.024), and decreased by 31% (24.6±6.5, p=0.027) at 16 weeks in the smoke exposed mouse group compared to control, non-exposed mice (35.7±8.4). As seen for mRNA levels, VEGF protein concentrations in the EDL were not affected by smoke exposure (20.2±7.5 pg/mg muscle after 8 weeks, and 18.4±5.8 after 16 weeks vs. 22.1±7.0 in controls).
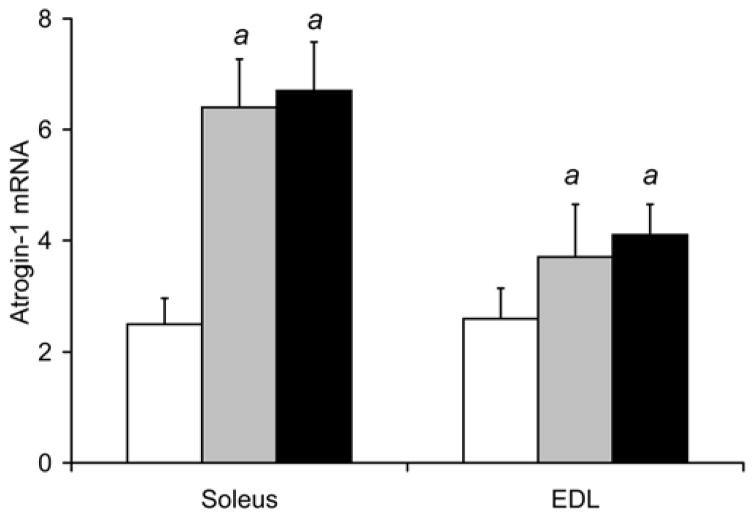
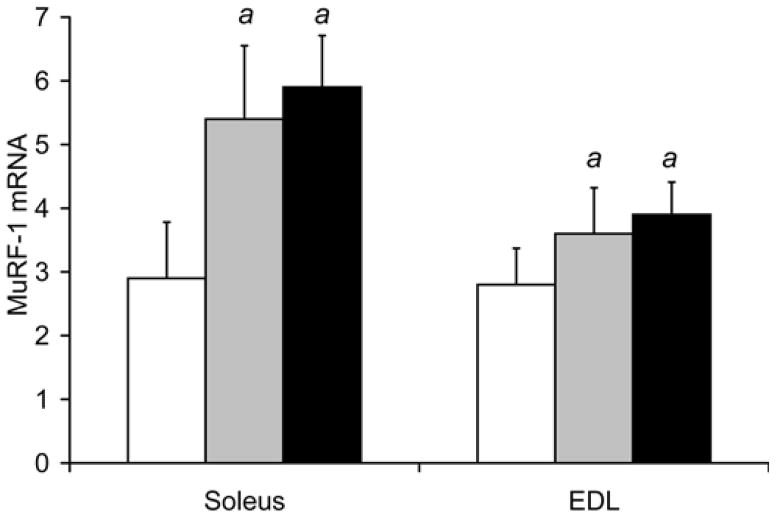
Fig. 3. Smoke exposure leads to a decrease in VEGF and increase in atrogin-1 and MuRF-1 gene expression in locomotor skeletal muscles.
Following 8 and 16 weeks of repeated exposures to cigarette smoke, VEGF mRNA (a) and protein (b) levels were measured in soleus and EDL by real time RT-PCR and ELISA, respectively. In the same soleus and EDL muscle samples atrogin-1 mRNA (c) and MuRF1 mRNA (d) were also measured by real time RT-PCR. Values are the mean ± SD. a= significantly (p<0.05) from control, b= significantly (p<0.05) from 8-week result, n=6.
Gene expression of muscle ubiquitin ligases, atrogin-1 and MuRF
Fig. 3c shows that soleus atrogin-1 mRNA levels were higher after 8 weeks of smoke exposure by 151% (6.4±0.9 relative units, p<0.001), and by 163% after 16 weeks (6.7±0.9, p<0.001) compared to control soleus levels (2.5±0.5 relative units). In the EDL, atrogin-1 mRNA was 42% higher after 8 weeks (3.7±1.0 relative units, p=0.037) and 58% higher after 16 weeks (4.1±0.6, p=0.001) in smoke-exposed mice compared to control, non-exposed mice (2.6±0.6).
In the same soleus muscle samples (Fig 3d), soleus MuRF-1 mRNA levels were found to be 82% higher after 8 weeks (5.4±1.1 relative units, p=0.002), and 101% higher after 16 weeks of daily smoke exposure (5.9±0.8, p<0.001) compared to soleus isolated from control mice (2.9±0.9). In the EDL, MuRF-1 mRNA was increased by 31% after 8 weeks (3.6±0.72 relative units, p=0.05), and 39% after 16 weeks in the smoke-exposed group (3.9±0.5, p=0.006) compared to control (2.8±0.6).
Muscle Capillarity
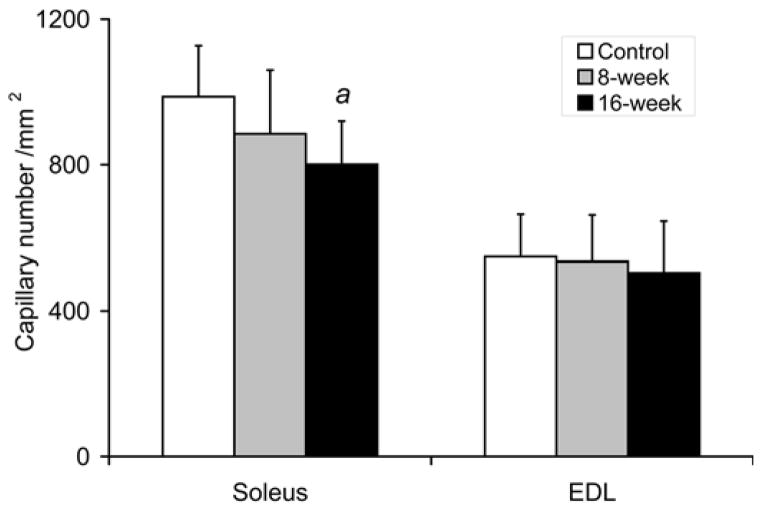
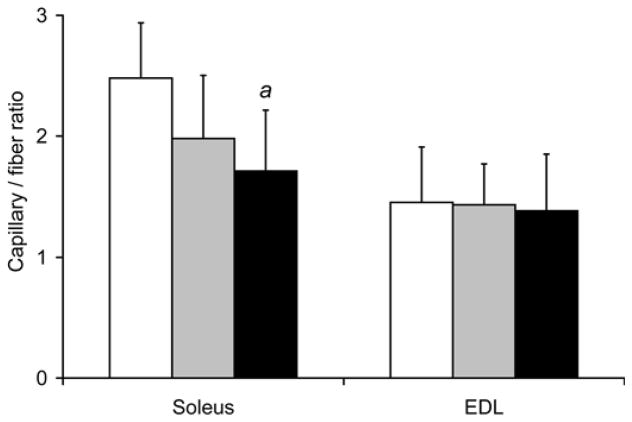
As shown in Fig. 4, the capillary density in soleus isolated from 16-week smoke exposed mice was decreased by 19% (801±119/mm2 vs. 987±140/mm2 in controls, p=0.033). Soleus capillary/fiber ratio was also decreased by 31% (1.71±0.50 vs. 2.48±0.46 in controls, p=0.020). Capillary density and capillary/fiber ratio after 8 weeks of smoke exposure were not significantly different from control muscles. Capillary density and capillary/fiber ratio in EDL were also not different between the smoke-exposed and non-exposed mice at either time point.
Fig. 4. Reduced soleus capillarity in mice exposed to cigarette smoke.
(a) Soleus capillary number /mm2 and (b) capillary/fiber ratio were evaluated in the soleus and EDL after repeated bouts of cigarette smoke exposure for 8 and 16 weeks. Values presented are the mean ± S.D. a= significantly (p<0.05) from control, b= significantly (p<0.05) from 8-week result, n=6.
Oxidative enzyme activities
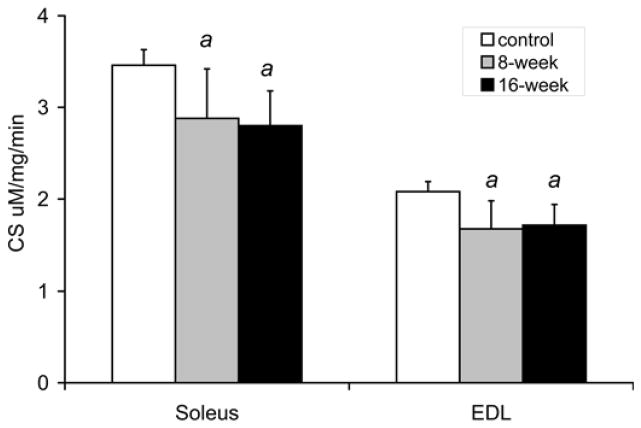
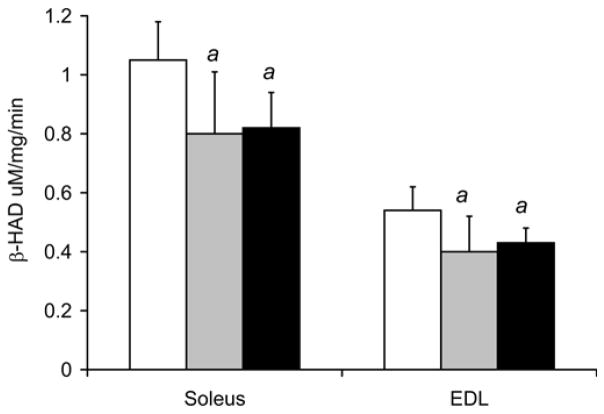
Figure 5 shows that smoke exposure altered oxidative enzyme activities in both the soleus and EDL. In control mice, the EDL, which contains predominantly glycolytic fibers, had lower CS and β-HAD activities compared to the more oxidative soleus (EDL-2.08±0.11 citrate synthase U vs. Sol - 3.46±0.17 U, p<0.001, and: EDL - 0.54±0.08 β-HAD U vs. 1.05±0.13 U, p<0.001, respectively). In response to repeated bouts of smoke exposure, soleus CS activity levels decreased by 17% to 2.88±0.54 U (p=0.032) after 8 weeks, and by 19% to 2.80±0.38 U (p=0.003) after 16 weeks. Similarly, soleus β-HAD activity decreased by 23% to 0.80±0.21 U (p=0.036) after 8 weeks, and by 22% to 0.82±0.12 U (p=0.011) after 16 weeks. In EDL, CS activity also decreased by 19% to 1.68±0.30 U (p=0.011) after 8 weeks, and by 17% to 1.72±0.22 U (p=0.005) after 16 weeks. EDL β-HAD activity decreased by 26% to 0.40±0.12 U (p=0.041) after 8 weeks and by 19% to 0.43±0.08 U (p=0.048) after 16 weeks.
Fig. 5. Decreased CS and β-HAD activities in both the soleus and EDL of mice exposed to cigarette smoke.
(a) CS and (b) β-HAD activity levels were measured in soleus and EDL muscles from cigarette smoke exposed and non-exposed, control mice at 8 and 16 weeks. The values presented are the mean ± S.D. a= significantly (p<0.05) from control, b= significantly (p<0.05) from 8-week result, n=6.
Muscle fiber type distribution
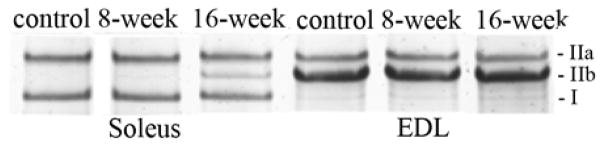
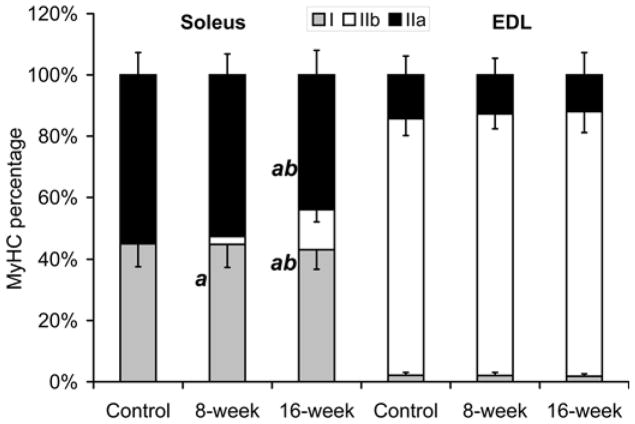
In soleus, a fiber type shift from type IIa to type IIb occurred in response to smoke-exposure (Fig. 6). The percentage of type IIa MHC decreased from 54.4±7.3% to 44.0±8.0% (p=0.04) while type IIb MHC increased from 0.53±0.37% to 2.56±1.34 (p=0.006) at 8 weeks and to 12.9±3.8% (p<0.001) at 16 weeks. There was no change in the percentage of type I MHC in the soleus in response to cigarette smoke exposure. The MHC distribution in the EDL of control mice was 14.3±6.1% MHC IIa and 83.6±5.5% MHC IIb and was not altered by cigarette smoke exposure.
Fig. 6. Cigarette smoke exposure resulted in a MHC transition from type IIa to IIb in the soleus.
(a) Representative gel electrophoresis separation and silver staining of MHC isoforms in the soleus and EDL. (b) The levels of each MHC were quantified by densitometry. The bar graphs represent the mean ±S.D. of the relative densitometry units. a= significantly (p<0.05) from control, b= significantly (p<0.05) from 8-week result, n=6.
Whole body and locomotor skeletal muscle mass
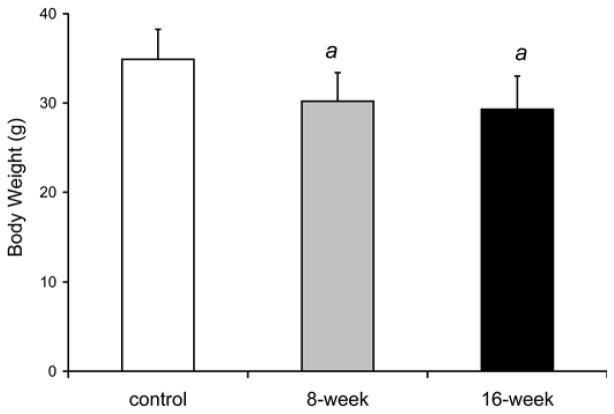
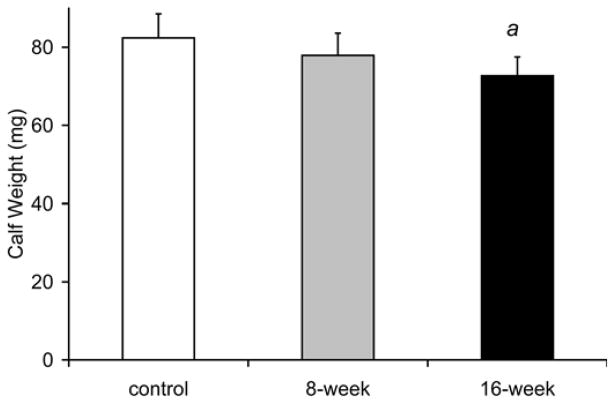
Fig. 7 showed that whole body weight was 13% lower at 8 weeks [30.2±3.2g (p=0.033)] and 16% lower at 16 weeks in smoke-exposed mice [29.3±3.7g (p=0.021)] compared to control mice of the same age [34.9±3.4 g]. Similarly, the wet weights of the gastrocnemius complex (soleus, plantaris and gastrocnemius) were reduced by 12 % after 16 weeks of smoke exposure (72.8±4.8 mg vs. 82.4±6.2 mg in controls, p=0.013; Fig. 7). At 8 weeks (76.3±4.3 mg), muscle weights in the smoke exposed mice were not significantly different from the control group (p=0.072). The ratio of gastrocnemius complex weight to body weight was not different at anytime point between the smoke-exposed and control mouse groups.
Fig. 7. Decreased body weight and gastrocnemius complex muscle mass observed in cigarette smoke-exposed mice.
(a) Cigarette smoke-exposed and non-exposed mouse body weight (g). (b) Gastrocnemius complex containing the soleus, plantaris and gastrocnemius weight (mg). Values presented are the mean weights ± SD. a= significantly (p<0.05) from control, b= significantly (p<0.05) from 8-week result, n=6.
Analysis of isolated muscle fatigue in vitro and exercise endurance in vivo
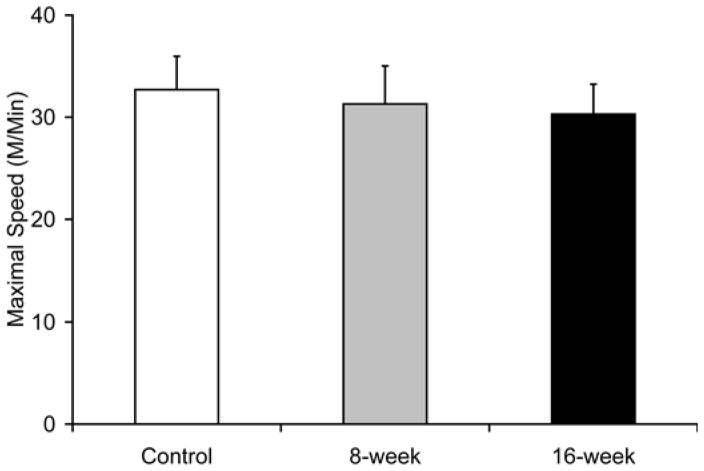
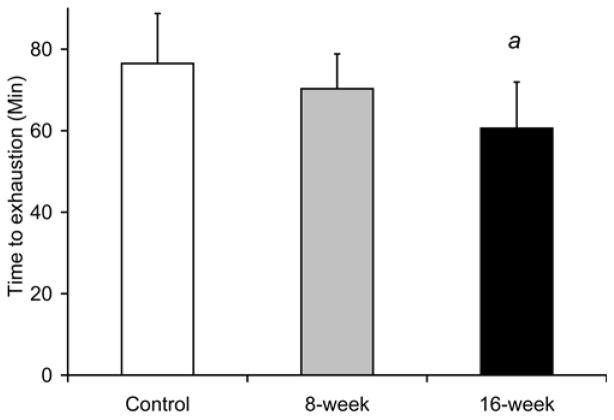
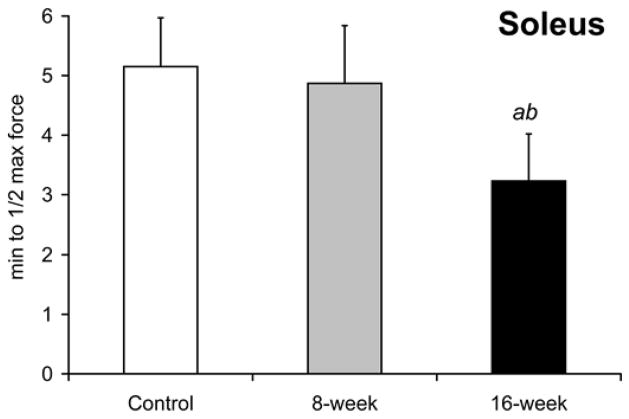
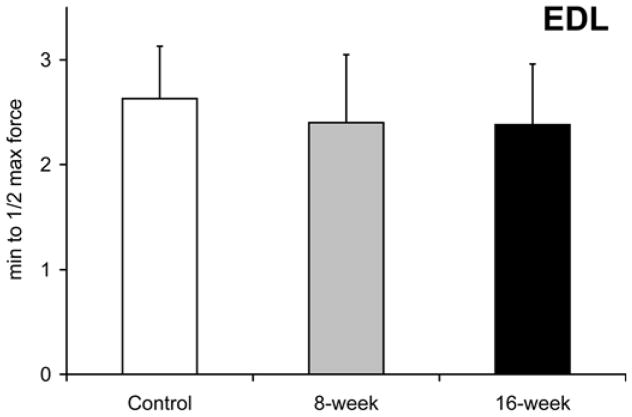
Smoke exposure did not significantly influence maximal treadmill running speed at either the 8- or 16-week time points [31.3±3.7 m/min at 8-week and 30.3±2.8 m/min in 16-week vs. 30.3±2.9 m/min in controls, Fig. 8a]. However, the time to reach exhaustion when subjected to a treadmill running bout at 60% of maximal speed was lower by 21% after 16 weeks (60.3±11.3 min vs. 76.5±12.3 min in controls, p=0.039 Fig. 8b), with no detectable decrease in the 8-week exposed mice (70.3±8.6 min, p=0.34). In vitro, soleus fatigue resistance was lower by 37% after 16 weeks (3.2±0.8 min vs. 5.2±0.8 min, p=0.002, Fig 8c), while no effect was seen in 8-week smoke-exposed mice (4.9±1.0 min, p=0.60). EDL fatigue resistance was unaffected at either time point [2.4±0.7 min (p=0.50) and 2.4±0.6 min (p=0.44) vs. 2.6±0.7 min in controls Fig. 8d).
Fig. 8. Endurance capacity and muscle contraction is impaired in soleus of mice exposed to cigarette smoke.
(a) The VO2 maximal speed in an incremental treadmill running test was recorded for mice in cigarette smoke-exposed and control groups. (b) Endurance exercise capacity was evaluated by the time to reach exhaustion when mice were run at 60% of the average VO2 max speed. The contractile performance of individual muscle types, independent of capillary O2 supply, was evaluated in isolated soleus (c) and EDL (d) muscles that were electrically stimulated to contract in vitro. The time to reach 50% of the initial force was used as an index of fatigue. (d). Mean ± SD, a= significantly (p<0.05) from control, b= significantly (p<0.05) from 8-week result, n=6.
Discussion
Early COPD-like locomotor skeletal muscle phenotype in smoke-exposed mice
Skeletal muscle wasting and functional impairments have been recognized as a powerful predictor of mortality associated with COPD (Rabe et al., 2007). Furthermore these changes seem to be independent of worsening lung function (Hansen et al., 2006; Schols et al., 1998). In humans suffering from COPD, skeletal muscle changes include 1) the loss of muscle strength and mass; 2) capillary rarefaction and lower locomotor skeletal muscle VEGF levels; 3) muscle oxidative to glycolytic fiber type transition; and 4) an impaired muscle energy metabolism and decreased PGC-1α levels; and 5) muscle atrophy associated with increased expression of the atrophy genes, atrogin-1 and MuRF1. In the experiments presented in this current study, mice exposed to just 16-week cigarette smoke reproduced many of the muscle abnormalities seen in humans (Gosker et al., 2009; Jobin et al., 1998; Maltais et al., 1998; Remels et al., 2007; Whittom et al., 1998). Mice exposed to cigarette smoke exposure have higher circulating levels of TNF-α, and TNF-α treated myocytes as well locomotor muscles from smoke exposed animals revealed lower PGC-1α levels. The PGC-1α target-gene, that regulates skeletal muscle capillarity, namely VEGF, is reduced and thus smoke-induced changes in gene expression are accompanied by evidence of decreased oxygen availability (capillarity), energy utilization, atrophy-related gene expression and muscle performance. These smoke-induced alterations were prominent in a skeletal muscle with greater reliance on oxidative metabolism. The overall outcome of this smoke-induced skeletal muscle phenotype is exercise limitation.
The potential role of TNF-α in PGC-1α-dependent cigarette smoke-induced muscle dysfunction
A key finding of the present study is that TNF-α has the potential to down regulate the transcriptional co-factor, PGC-1α, in skeletal myocytes. TNF-α has been proposed to decrease skeletal muscle mass by activating the ubiquitin-proteasome pathway and thus accelerating muscle protein degradation (Attaix et al., 2008; Tisdale, 2009). However, activation of the ubiquitin-proteasome does not fully explain the muscle impairments observed in COPD patients. The ubiquitin-proteasome pathway non-selectively degrades myosin in all types of skeletal muscle (Attaix et al., 2008; Tisdale, 2009). However, muscle mass loss and dysfunction in COPD patients and smoke exposed mice predominantly occurs skeletal muscles composed of oxidative fiber types (Gosker et al., 2008; Jobin et al., 1998; Whittom et al., 1998; Wuyam et al., 1992). Thus, a specific mechanism is invoked to replace oxidative fibers types with less energy efficient glycolytic fibers.
TNF-α regulation of PGC-1α could potential be a key step in development of the smoke-induced muscle pathology that selectively affects oxidative muscles. First, VEGF is a target gene of the PGC-1α, and studies in PGC-1α knockout mice have suggested that PGC-1α is necessary for exercise induced VEGF expression as well as capillary adaptation to exercise training (Arany et al., 2008; Leick et al., 2009). PGC-1α has also been shown to play a vital role in the biogenesis of muscle mitochondria and maintenance of oxidative type fibers (Adhihetty et al., 2009; Handschin et al., 2007). Importantly, the smoke-induced deleterious effects on muscle function occur after just a few months of smoke exposure and before emphysema-like morphological changes are thought to take place in the lung. Thus, it is likely that the consequences of TNF-α induced down regulation of PGC-1α may be mediated by a combination of both a loss of VEGF-dependent capillaries supplying oxygen to the muscle and altered regulation of genes important for mitochondrial biogenesis, type IIa fiber preservation and prevention of muscle atrophy. (Handschin et al., 2007; Sandri et al., 2006). The predominant effects of smoke exposure in the soleus vs. the EDL suggest that maintaining skeletal muscle capillarity and mitochondrial capacity are necessary for efficient aerobic exercise that relies on oxidative metabolism to generate high energy phosphates.
Differential response of oxidative vs. glycolytic locomotor skeletal muscles to the effects of cigarette smoke exposure
In this study, all of the changes in gene expression, vascular and myocyte morphology and function were either more pronounced or occurred selectively in the soleus and not in the EDL. The exception to this distinction between soleus and EDL muscles was the decreased in oxidative enzyme activities. However, when both the soleus and EDL muscle from smoke exposed mice were tested in vitro for a decrement in contractile function, the soleus displayed a rapid fatigue response to smoke exposures while the EDL was unaffected. This in vitro assay of isolated muscles does not depend on blood flow or the number of capillaries supplying oxygen to the muscle and thus reflects the intrinsic mitochondrial capacity and contractile properties of the muscle fibers. Taken together, our data suggest that impairment of both oxygen availability and muscle contractile function, but not metabolic oxidative enzyme capacity, contribute to impaired muscle performance and reduced exercise endurance after smoke exposure.
Summary
This study demonstrates that mice exposed to cigarette smoke for just a few months exhibit skeletal muscle capillary regression, oxidative enzyme capacity attenuation, oxidative to glycolytic fiber type transition, rapid fatigue and muscle atrophy. Oxidative muscle types, as opposed to glycolytic, are preferentially affected Together these changes limit oxygen availability and energy utilization by locomotor skeletal myocytes and reduce exercise tolerance. Our in vitro experiments show that TNF-α decreases PGC-1α expression in skeletal myocytes which supports the possibility that smoking-induced increase in circulating TNF-α directly down-regulate skeletal muscle PGC-1α which then impairs vascular endothelial cell and myocyte cell function through PGC-1α target-gene expression. Thus, PGC-1α may be a promising candidate to target for the development of novel therapies aimed at reversing muscle wasting and restoring activity levels in COPD patients.
Acknowledgments
This work was funded by a grant from the NIH 5RO1 HL 84281, NIH P01 HL091830-01A1 and TRDRP 12RT-0062.
References
- Adhihetty PJ, Uguccioni G, Leick L, Hidalgo J, Pilegaard H, Hood DA. The role of PGC-1{alpha} on mitochondrial function and apoptotic susceptibility in muscle. Am J Physiol Cell Physiol. 2009 doi: 10.1152/ajpcell.00070.2009. [DOI] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451(7181):1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1(4):259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Attaix D, Combaret L, Bechet D, Taillandier D. Role of the ubiquitin-proteasome pathway in muscle atrophy in cachexia. Curr Opin Support Palliat Care. 2008;2(4):262–266. doi: 10.1097/spc.0b013e3283196ac2. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. New concepts in chronic obstructive pulmonary disease. Annu Rev Med. 2003;54:113–129. doi: 10.1146/annurev.med.54.101601.152209. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Schols AM, Polkey MI, Galdiz JB, Gosker HR, Swallow EB, Coronell C, Gea J. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax. 2008;63(2):100–107. doi: 10.1136/thx.2007.078030. [DOI] [PubMed] [Google Scholar]
- Bergmeyer H. Methods in Enzymatic Analysis. New York: Academic; 1974. [Google Scholar]
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81(1):355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- Chen H, Hansen MJ, Jones JE, Vlahos R, Bozinovski S, Anderson GP, Morris MJ. Cigarette smoke exposure reprograms the hypothalamic neuropeptide Y axis to promote weight loss. Am J Respir Crit Care Med. 2006;173(11):1248–1254. doi: 10.1164/rccm.200506-977OC. [DOI] [PubMed] [Google Scholar]
- Del Prete Z, Musaro A, Rizzuto E. Measuring mechanical properties, including isotonic fatigue, of fast and slow MLC/mIgf-1 transgenic skeletal muscle. Ann Biomed Eng. 2008;36(7):1281–1290. doi: 10.1007/s10439-008-9496-x. [DOI] [PubMed] [Google Scholar]
- Di Francia M, Barbier D, Mege JL, Orehek J. Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1453–1455. doi: 10.1164/ajrccm.150.5.7952575. [DOI] [PubMed] [Google Scholar]
- Gosker H, Remels A, Sliwinski P, Polkey M, Galdiz J, Wouters E, Langen R, Schols A. Altered Body Composition and Muscle Anabolic, Catabolic and Metabolic Gene Expression in COPD Patients with Elevated Skeletal Muscle Inflammation. Am J Respir Crit Care Med. 2009;179:A4200. [Google Scholar]
- Gosker HR, Langen RC, Bracke KR, Joos GF, Brusselle GG, Steele C, Ward KA, Wouters EF, Schols AM. Extrapulmonary manifestations of COPD in a mouse model of chronic cigarette smoke exposure. Am J Respir Cell Mol Biol. 2008 doi: 10.1165/rcmb.2008-0312OC. [DOI] [PubMed] [Google Scholar]
- Handschin C, Chin S, Li P, Liu F, Maratos-Flier E, Lebrasseur NK, Yan Z, Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282(41):30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Gualano RC, Bozinovski S, Vlahos R, Anderson GP. Therapeutic prospects to treat skeletal muscle wasting in COPD (chronic obstructive lung disease) Pharmacol Ther. 2006;109(1–2):162–172. doi: 10.1016/j.pharmthera.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277(5334):2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- Jobin J, Maltais F, Doyon JF, LeBlanc P, Simard PM, Simard AA, Simard C. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil. 1998;18(6):432–437. doi: 10.1097/00008483-199811000-00005. [DOI] [PubMed] [Google Scholar]
- Kohn TA, Myburgh KH. Electrophoretic separation of human skeletal muscle myosin heavy chain isoforms: the importance of reducing agents. J Physiol Sci. 2006;56(5):355–360. doi: 10.2170/physiolsci.RP007706. [DOI] [PubMed] [Google Scholar]
- Langen RC, Schols AM, Kelders MC, van der Velden JL, Wouters EF, Janssen-Heininger YM. Muscle wasting and impaired muscle regeneration in a murine model of chronic pulmonary inflammation. Am J Respir Cell Mol Biol. 2006;35(6):689–696. doi: 10.1165/rcmb.2006-0103OC. [DOI] [PubMed] [Google Scholar]
- Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JF, Hidalgo J, Pilegaard H. PGC-1{alpha} mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00076.2009. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Maltais F, Jobin J, Sullivan MJ, Bernard S, Whittom F, Killian KJ, Desmeules M, Belanger M, LeBlanc P. Metabolic and hemodynamic responses of lower limb during exercise in patients with COPD. J Appl Physiol. 1998;84(5):1573–1580. doi: 10.1152/jappl.1998.84.5.1573. [DOI] [PubMed] [Google Scholar]
- Mizunoya W, Wakamatsu J, Tatsumi R, Ikeuchi Y. Protocol for high-resolution separation of rodent myosin heavy chain isoforms in a mini-gel electrophoresis system. Anal Biochem. 2008;377(1):111–113. doi: 10.1016/j.ab.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Mrazkova O, Grim M, Carlson BM. Enzymatic heterogeneity of the capillary bed of rat skeletal muscles. Am J Anat. 1986;177(2):141–148. doi: 10.1002/aja.1001770203. [DOI] [PubMed] [Google Scholar]
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- Rabinovich RA, Ardite E, Troosters T, Carbo N, Alonso J, Gonzalez de Suso JM, Vilaro J, Barbera JA, Polo MF, Argiles JM, Fernandez-Checa JC, Roca J. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(7):1114–1118. doi: 10.1164/ajrccm.164.7.2103065. [DOI] [PubMed] [Google Scholar]
- Remels AH, Schrauwen P, Broekhuizen R, Willems J, Kersten S, Gosker HR, Schols AM. Expression and content of PPARs is reduced in skeletal muscle of COPD patients. Eur Respir J. 2007 doi: 10.1183/09031936.00144106. [DOI] [PubMed] [Google Scholar]
- Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103(44):16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51(8):819–824. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- Spangenburg EE, Booth FW. Molecular regulation of individual skeletal muscle fibre types. Acta Physiol Scand. 2003;178(4):413–424. doi: 10.1046/j.1365-201X.2003.01158.x. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate Synthase. Methods Enzymol. 1969;13:3–5. [Google Scholar]
- Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 2004;18(1):63–69. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev. 2009;89(2):381–410. doi: 10.1152/physrev.00016.2008. [DOI] [PubMed] [Google Scholar]
- Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, Belleau R, Maltais F. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc. 1998;30(10):1467–1474. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- Wright JL, Churg A. Cigarette smoke causes physiologic and morphologic changes of emphysema in the guinea pig. Am Rev Respir Dis. 1990;142(6 Pt 1):1422–1428. doi: 10.1164/ajrccm/142.6_Pt_1.1422. [DOI] [PubMed] [Google Scholar]
- Wuyam B, Payen JF, Levy P, Bensaidane H, Reutenauer H, Le Bas JF, Benabid AL. Metabolism and aerobic capacity of skeletal muscle in chronic respiratory failure related to chronic obstructive pulmonary disease. Eur Respir J. 1992;5(2):157–162. [PubMed] [Google Scholar]