Abstract
Sleep is a complex physiologic state, the importance of which has long been recognized. Lack of sleep is detrimental to humans and animals. Over the past decade, an important link between sleep and cognitive processing has been established. Sleep plays an important role in consolidation of different types of memory and contributes to insightful, inferential thinking. While the mechanism by which memories are processed in sleep remains unknown, several experimental models have been proposed. This article explores the link between sleep and cognition by reviewing (1) the effects of sleep deprivation on cognition, (2) the influence of sleep on consolidation of declarative and non-declarative memory, and 3) some proposed models of how sleep facilitates memory consolidation in sleep.
Keywords: Sleep, REM Sleep, Non-REM Sleep, Cognition, Memory, Learning, Consolidation, Declarative Memory, Non-declarative Memory
The question of the function of sleep has fascinated people for thousands of years, and is now a subject of both basic and clinical research. One important facet of this question is how sleep contributes to cognitive processing and learning. A clue to the role of sleep in cognition is the detrimental effect of sleep deprivation on cognitive functioning. Over the past decade, growing interest in and experimentation on the role of sleep in learning and memory has led to an explosion of research, which has established a firm connection between sleep and memory. Although there are still many unknowns, recent studies have led to a better understanding of the contribution of sleep to cognition and of possible mechanisms underlying this relationship. This article offers an overview of current literature addressing the role of sleep in memory and cognitive processing.
Neurobiology of Sleep
Sleep is composed of physiologically and neurochemically distinct stages. Sleep stages are divided, first, into rapid eye movement sleep (REM) and non-rapid eye movement sleep (non-REM), which alternate throughout the night in a roughly 90-minute cycle (Figure 1). Non-REM sleep is further divided into three stages. Stage N3, which is referred to as slow wave sleep (SWS), is prevalent during the first half of the night, while REM sleep is prevalent during the second half.†
Figure 1. The human sleep cycle.
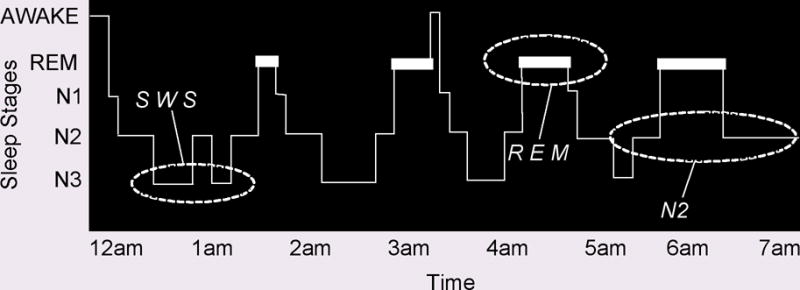
Across the night, NREM and REM sleep cycle every 90 min in an ultradian manner, while the ratio of NREM to REM sleep shifts. During the first half of the night, NREM stage N3 (SWS) dominates, while N2 and REM sleep prevail in the latter half of the night. EEG patterns also differ significantly between sleep stages, with electrical oscillations such as K complexes and sleep spindles occurring during stage 2 NREM, slow (0.5–4Hz) delta waves developing in SWS, and theta waves seen during REM.
Transitions from wake to sleep and between the stages of sleep are accompanied by complex changes in pattern of neuronal firing and neurotransmitter release [1]. Wakefulness is a time when “all systems are go” from a neurophysiological perspective; the brainstem reticular formation is active; the pons releases norepinephrine, serotonin and acetylcholine; the posterior hypothalamus releases histamine. Orexin/hypocretin neurons in the lateral and posterior hypothalamus are also most active during wakefulness and play an important role in the stabilization of both wakefulness and sleep. Production of non-REM sleep is coordinated by the ventrolateral preoptic nucleus in the anterior hypothalamus. During non-REM sleep, norepinephrine, serotonin, acetylcholine and histamine release are decreased. The initiation of REM sleep is coordinated by communication between aminergic neurons, which produce norepinephrine, serotonin and histamine, and cholinergic neurons. During REM sleep, the aminergic neurons become nearly silent, while cholinergic neurons become highly active. These profound changes in neurophysiological state seen across the sleep cycle, with changes both in the activity of neuronal networks and in the neurochemical milieu of the brain, suggest that sleep evolved as a period of altered cognitive processing.
Effects of Sleep Deprivation
One element essential to understanding the role of sleep in cognition is observing the effects of lack of sleep on cognitive processing. This is particularly relevant in our society because of the common practices of acute sleep deprivation and chronic sleep restriction. While the impact of acute, total sleep deprivation is well established [2], the effects of chronic sleep restriction have only more recently been studied under carefully controlled conditions. In one seminal study, young adults were restricted to 4, 6, or 8 hours of time in bed per night for 14 days, under controlled laboratory conditions [3] (Figure 2). Several times per day, subjects performed a psychomotor vigilance test (PVT) of attention, a digit-symbol substitution task (DSST) test of working memory, and a serial addition/subtraction test (SAST) of cognitive throughput. The performance of subjects restricted to 4 and 6 hours in bed per night worsened progressively over the 14 days on all cognitive tasks. After 14 days, their cognitive performance was similar to that of subjects who are totally sleep deprived for 24 to 48 hours. Similar findings of progressive worsening of performance on the PVT were reported in a second study in which time in bed was restricted to 3, 5, or 7 hours, over a shorter, 7 day period [4].
Figure 2. Neurobehavioral responses to varying doses of daily sleep.
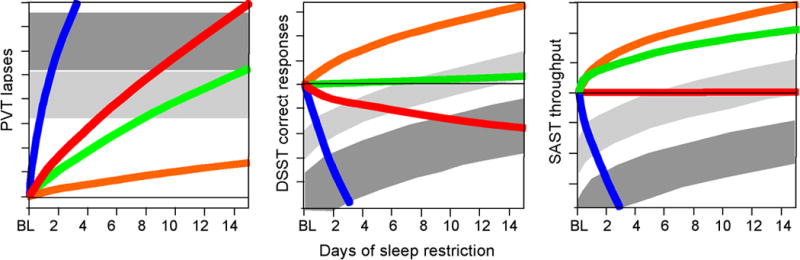
Three different neurobehavioral assays served to measure cognitive performance capability and subjective sleepiness. Each panel displays group averages for subjects in the 8 h (red lines), 6 h (green lines), and 4 h (lines) chronic sleep period conditions across 14 days, and in the 0 h (blue lines) sleep condition across 3 days. Upward corresponds to worse performance on the PVT (left), and to better performance on the DSST (center) and the SAST (right). The curves represent statistical non-linear model-based best-fitting profiles of the response to sleep deprivation. The mean ± s.e.m. ranges of performance for 1 and 2 days of total sleep deprivation are shown as light and dark gray bands, respectively, allowing comparison with the 14-day chronic sleep restriction conditions. For the DSST and SAST, these gray bands are curved parallel to the practice effect displayed by the subjects in the 8 h sleep period condition to compensate for different amounts of practice on these tasks. Adapted from Van Dongen et al. (2003).
In addition to attention and memory tasks, sleep deprived patients have also shown deficits in executive function. In particular, several studies have demonstrated impairment on neuropsychological tests that depend on the prefrontal cortex [5, 6]. Patients acutely sleep deprived for just 30 hours demonstrated deficits in verbal fluency, creative thinking and nonverbal planning.
Cognitive deficits in the setting of sleep deprivation have also been demonstrated in real-life circumstances demanding high level functioning. One such example is a series of studies performed on medical interns while “on the job”. In one of these studies, interns worked either a traditional schedule of 85 hours per week, or a modified schedule averaging 65 hours of work per week, which resulted in an additional 6 hours of sleep per week [7]. Interns working the traditional schedule had more than twice the rate of attention failures during work, as measured by the intrusion of slow rolling eye movements into wakefulness. Another study found that medical interns working in the intensive care unit on a traditional schedule consisting of extended shifts of greater than 24 hours made 36 percent more serious medical errors than interns on a modified schedule with shorter shifts and less total hours per week [8].
The question of what specific alterations in brain activation might underlie changes in performance in sleep-deprived individuals has been explored with functional magnetic resonance imaging (fMRI) [9, 10]. Brain regions activated during performance of cognitive tasks in the sleep-deprived condition differ from those activated in the rested condition, in a manner that appears to be task-dependent. During performance of an arithmetic task, several brain regions that were activated in the rested condition, including prefrontal cortex, were less activated in the sleep-deprived condition [9]. But for a verbal memory task, not only were the areas activated in the rested condition maintained during sleep-deprivation, additional novel areas became activated in the sleep-deprived condition [10]. Increased activation in one of these areas, the parietal lobes, correlated with improved performance (even though performance overall was worse in the sleep-deprived condition), suggesting that this new area of activation can play a compensatory role. In summary, sleep deprivation results in dynamic changes in patterns of brain activation. These changes may result in either increased or depressed activity in brain regions typically required for a given task. In other instances, attempts to compensate for the negative effects of lack of sleep on cognition appear to recruit entirely new regions of the brain.
Taken as a whole, these and other studies of cognitive deficits associated with both acute total sleep deprivation and chronic sleep restriction paint a picture of dramatic impairment in attention, memory and executive function caused by lack of sleep, whether tested in the laboratory or under real-life workplace conditions. This impairment is accompanied by fundamental changes in brain activation patterns, which play an important role in determining how an individual performs when deprived of sleep.
Sleep-Dependent Memory Consolidation
Over the past 10 years, a growing body of literature has supported a role for sleep in the consolidation of several types of memory. This includes both declarative and non-declarative memory. By definition, non-declarative memory includes memories that cannot be recalled to conscious awareness. One type of non-declarative memory is procedural memory (for example, how to play tennis). Declarative memory is information that can be retrieved into conscious awareness, including memory for specific life events (episodic memory; e.g., eating dinner last night) and memory for facts and general knowledge (semantic memory; e.g., the capital of France).
Non-Declarative Memory
Sleep-dependent consolidation or enhancement has been demonstrated for several non-declarative procedural tasks, including visual and auditory discrimination tasks, motor sequence tasks and a motor adaptation task. The first task to show clear sleep-dependent enhancement was a visual texture discrimination task (VDT), developed by Karni et al. In this procedural task, subjects identify the orientation of an array of three diagonal bars embedded in a background of horizontal bars [11]. Having reported that task improvement could be seen 6 to 8 hours after training [12], they went on to demonstrate that performance improved over a night of sleep, but only when subjects were allowed REM sleep [13]. These results are confirmed by an experiment that compared performance on the VDT in subjects who were retested hours after training on the same day to subjects retested after a night of sleep [12]. The study found that overnight improvement was significantly better than daytime improvement [14] (Figure 3a) and correlated with the number of hours of post-training sleep [14]. While the initial study of Karni et al [12] suggested that the simple passage of time could lead to improvement, subsequent studies found improvement only when this time included sleep. When subjects’ sleep was recorded in the laboratory, overnight improvement correlated with both the amount of SWS early in the night and of REM sleep late in the night [14]. One to six additional nights of sleep after training resulted in significantly more improvement than seen after only one night [15]. In addition, subjects who were deprived of sleep the night following training did not demonstrate significant improvement on the VDT, even after two nights of recovery sleep [15]. Thus, sleep, and not the simple passage of time, appears to be required for post-training memory consolidation and improvement in performance.
Figure 3. Sleep-dependent enhancement of performance on procedural memory task.

Three different procedural tasks show sleep dependent improvements in performance. (A) Visual discrimination task: performance improvements were seen only in groups retested after a night of sleep (green circles), and not in groups retested after equivalent amounts of daytime wake (red circles). Adapted from Stickgold et al. 2000. (B) Finger-tapping motor sequence task: performance improvements were seen only after a night of sleep (green bars). Left panel – subjects trained in the morning and retested that evening (PM) and again the next morning (AM); Center panel – the same as Left panel, except subjects wore mittens across the initial period of daytime wakefulness to eliminate interference as a possible cause of the lack of PM improvement; Right panel – subjects trained in the evening and retested the following morning and again that evening show improvement at both post-sleep tests. Adapted from Walker et al. 2002. (C) Motor adaptation to a virtual sideways force shows improved accuracy in direction of movement after sleep, but not after an equivalent period of daytime wake. Adapted from Huber et al. 2004.
As in the case of the VDT, the first hint that sleep was essential to the process of learning motor tasks came from the notion that the passage of time, in addition to practice alone, was required for improvement in motor skills [16]. In one experiment, subjects were asked to tap two five-element sequences of finger-to-thumb opposition [16]. Although improvement in speed and accuracy was seen during training, a marked further improvement was seen 24 hours later in the absence of additional training. When tested with a computerized version of this test, which involved typing the same numeric sequence (4-1-3-2-4), subjects demonstrated no further significant improvement when retested after 12 hours of daytime wake [17] (Figure 3b). However, when retested after 12 hours that contained a night of sleep, the subjects improved in speed by 20% (Figure 3b). Thus sleep, rather than the simple passage of time, appears to mediate this offline improvement in motor performance [18, 19].
Another motor task where sleep dependence has been demonstrated is a motor adaptation task. In this computerized task, subjects move a cursor on the screen between a central starting point and one of eight targets locations, while overcoming an automatic rotation in the cursor at a fixed angle from hand position [20]. When tested 8 hours after training, mean directional error was significantly reduced in a group retested after an eight hour night of sleep compared to a group retested after 8 hours of daytime wakefulness (Figure 3c).
Declarative Memory
While the role of sleep in the consolidation of declarative memory was at one time controversial, recent evidence has increasingly supported such a function. Several studies have found a significant benefit of sleep on a declarative task that involves memorization of word pairs. Two studies suggested that subjects recalled word pairs better after sleeping during just the first half of a night of sleep than just the second half [21] [22] (Figure 4a). Additionally, subjects that were permitted to sleep, either early or late in the sleep interval, performed better than subjects that stayed awake. Since the first half of the night is rich in slow wave sleep, the authors concluded that slow wave sleep is likely playing an important role in the protection or enhancement of declarative memories [21] [22]. The benefits of sleep was also seen on the word pairs task in subjects who took daytime naps that contained only non-REM sleep when compared to subjects who stayed awake between training and retest [23]. Interestingly, sleep that occurs soon after a period of learning appears to be more beneficial than sleep that is delayed for several hours after learning [24].
Figure 4. Sleep-dependent enhancement of performance on declarative memory tasks.
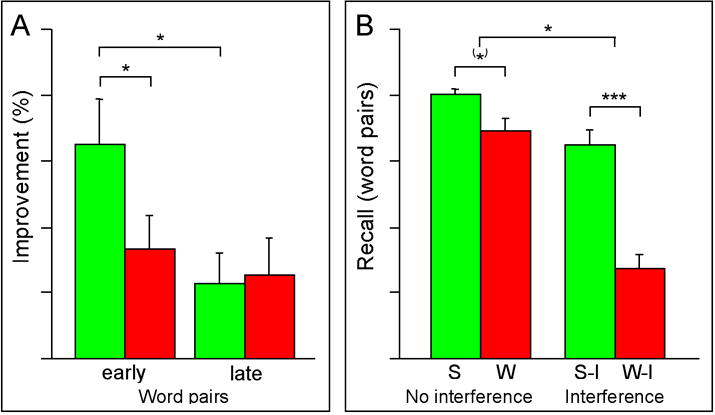
Two different memory tasks show sleep-dependent improvements in performance. (A) Benefits of early and late night sleep on the paired associates list: performance improvement was significantly greater following early sleep (green bar left) compared to the early wake control (red bar left), and compared to late sleep (green bar right). There was no significant difference between the late sleep (green bar right) and late wake (red bar right) groups. * p< 0.05; **p<0.01. Adapted from Plihal and Born (1997). (B) Paired associates task and effect of interference: there was a modest non-significant improvement in performance after sleep (green bar left compared to wake (red bar left). Performance improved significantly after sleep for the group that underwent interference training (green bar right) compared to the interference wake group (red bar right) and significantly more so than in the no interference condition (left). (*) 0.05 ≤ p ≤ 0.10, *p< 0.05, ***p<0.001. Adapted from Ellenbogen et al (2006).
Sleep also benefits declarative memory by protecting such memories from interference. Ellenbogen et al. demonstrated this concept with an experiment during which subjects learn a list of unrelated word pair associates (A-B pairs), similar to previous word pair experiments [25]. After a 12-hour interval that contained either nocturnal sleep or daytime wakefulness, subjects in the interference group learned a new list of word pairs (A-C pairs), in which words from the original list (A) were paired with new words (C). All participants were then retested on the original list. Patients that did not undergo the interference testing demonstrated a modest, non-significant benefit from sleep (Figure 4b). However, for the group that underwent interference training, there was a large and significant benefit from sleep (76% correct recall versus 32% in the “wake” group) (Figure 4b). This study supports a role of sleep not only in rote memorization of words, but also in protection of memories that are threatened by subsequent associative interference.
A growing body of literature supports a special role of sleep in the consolidation of emotional declarative memories. During one experiment, subjects viewed both neutral and emotionally arousing pictures in the evening or in the morning [26]. Subjects again viewed the pictures intermixed with new pictures twelve hours later, after either sleeping or staying awake. The subjects were asked to identify which pictures they consciously remembered from the previous session, which pictures were only familiar, and which they thought were new. Subject performance improved overall after sleep. Most striking was the influence of sleep on recognition of emotional pictures, with recognition (identification of pictures as familiar by subjects) in the subjects who slept during the 12-hour interval between the first and second viewing being 42% greater than in the wake controls, while identification of neutral pictures did not differ significantly between the wake and sleep subjects. The study thus suggests that sleep may permit preferential retention of memories based on emotional content.
In another experiment, subjects were exposed to scenes involving either negative arousing objects or neutral objects, displayed on neutral background scenes [27]. After studying the pictures during baseline training, one group of subjects was retested 30 minutes later, while two additional groups were tested after a 12-hour period either of daytime wake or containing a night of sleep. Compared to performance at 30 minutes, subjects retested after a night of sleep were significantly better at recognizing negative images they had previously seen than the subjects tested after a period of wake (Figure 5). Moreover, this improvement was not seen for the neutral images or for the neutral image backgrounds, suggesting that sleep permitted consolidation of memory for the emotional objects to the detriment of neutral memory. The emotional memory retention bias seen after periods of sleep may relate to an unconscious perception that emotional memories are most relevant to the individual; and, thus, sleep is playing a role in preserving that information which is deemed most significant.
Figure 5. The influence of sleep on emotional memory.
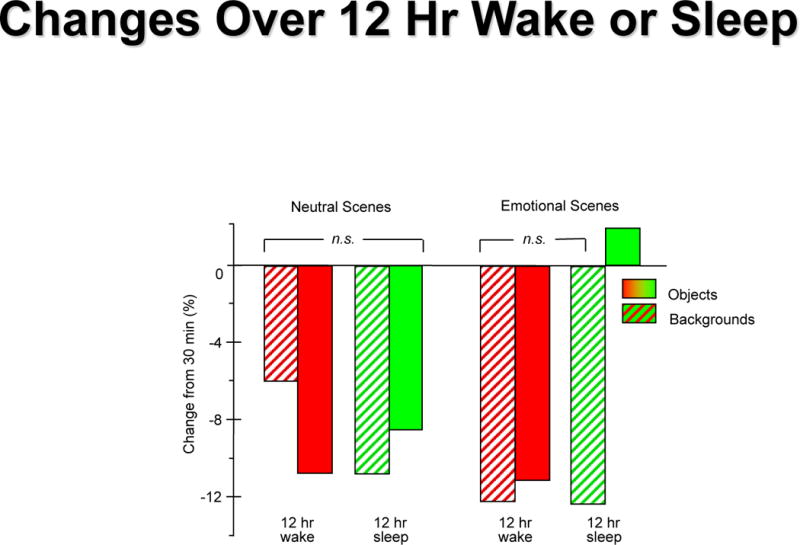
Change in recognition rate of emotional or neutral images is demonstrated 12 hours from initial encoding compared to 30 minutes after encoding. Left: Neutral objects (striped bars) and their neutral backgrounds (solid bars) both show 6-11% deterioration across the 12 hr interval, whether across a period of daytime wake or across a night with sleep; Right: The same pattern is seen for emotional objects (striped bars) and neutral backgrounds (solid bars), except that emotional objects are recognized significantly better after a night of sleep than either objects or backgrounds in any other condition.
Sleep and Insight
In addition to playing a role in the stabilization and enhancement of different types of memory, sleep has also been implicated in the process of gaining creative insight and making broad connections among learned information. In an experiment by Wagner et al, subjects were presented with a task where discovery of a hidden rule greatly improves speed of performance [28]. After initial training, subjects were retested after an 8-hour period, which contained a night of sleep, a night of wake, or a day of wake. Sleep dramatically increased the likelihood of grasping the hidden rule, with 59% of the sleep group gaining insight compared with 23% of either group that stayed awake (Figure 6a). Similarly, Ellenbogen et al. looked at the concept of “relational memory”, which involves placing learned information in a context and making inferences by relating individual pieces of information [29]. Subjects were presented with a series of “premise pairs”, such as ”choose A over B”, “B over C”, “C over D”, and “D over E”. At retest 20 minutes, 12 hours, or 24 hours later, subjects were tested on their memory for these premise pairs, but also on their ability to integrate these premise pairs to infer, for example, that they should “choose B over D”. The ability to draw inferences was greatly improved after 12 or 24 hours compared to 20 minutes. Moreover, when a delay of 12 hours over a day of wake was compared to 12 hours over a night of sleep, subjects who slept between training and retest performed better with inference pairs that had a greater degree of separation, for example inferring that they should “choose B over E” (Figure 6b).
Figure 6. Sleep-dependent development of insight.
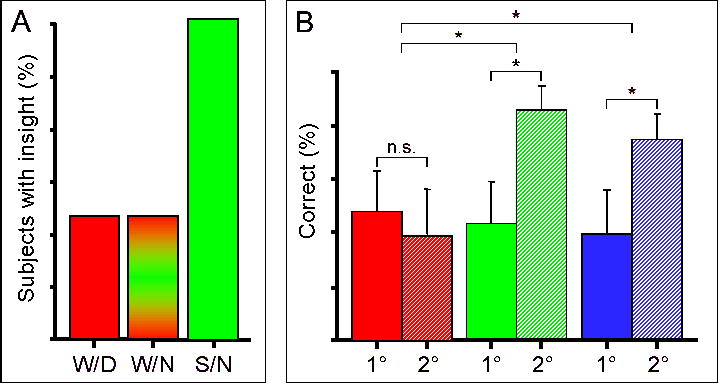
(A) Number reduction task: subjects were asked to determine the final digit in a series by processing digits from left to right according to two simple rules. However, subjects who gained insight into a hidden rule could determine the correct response after only the second digit presentation versus the sixth in those who did not gain insight. After initial training, subjects were retested after periods of wake or sleep – W/D: daytime wake; W/N: overnight sleep deprivation; S/N: nocturnal sleep. Subjects who slept had more than twice the chance of gaining insight compared to either of the wake groups. Adapted from Wagner et al (2004). (B) Inference pair performance: After initial training, subjects were tested after 12 hours of daytime wake (red), 12 hours including a night of sleep (green), or a full 24-hr day (blue). 1°: first order association (e.g., A => C); 2°: second order association (e.g., A => D). In the wake group (red), there was no difference in performance between first and second order associations. Both sleep groups (green and blue) performed significantly better on second order associations compared to first order associations. * p<0.05. Adapted from Ellenbogen et al (2007).
Sleep can also support the development and maintenance of a “big picture” view, enhancing the “gist” of a memory, at the expense of detail. This is evident in Payne et al.’s examination of the role of sleep in false memory formation [30]. Here, subjects were presented with a list of related words. However, a “critical word”, which connected all of the words in the list, was missing. For example, words like “ledge, sill, curtain and open” were present, but not “window”. Not only did subjects tested after 12 hours of sleep forget fewer of the studied words that did subjects tested after 12 hours of wake, but they also showed a numerical increase in recall of critical or gist words, which subjects falsely believe to be part of the original list, while those in the wake group showed a significant deterioration in the “recall”.
What aspect of sleep permits the incorporation of new information in these lists? Cai et al. studied the role of sleep in the development of associative networks [31]. The presence of REM sleep during a daytime nap, independent of total sleep time, enhanced performance in creative problem solving for items that subjects were exposed to prior to sleep. Another approach to understanding the processing of information in such associative networks has been to awaken subjects out of different sleep stages and quickly test their performance on various cognitive tasks [32] [33]. In one experiment, subjects were presented with a semantic priming task, which is designed to determine the strength of association between words [32]. Subjects were awakened out of REM and non-REM sleep for testing, as well as tested pre- and post-sleep. As expected, the associative strength of strongly related pairs (such as “hot – cold”) was markedly greater than that of weakly related pairs (such as “thief – wrong”) after awakenings from NREM sleep. In contrast, when subjects were awakened from REM sleep, the normally weakly related pairs showed greater associative strength than did the normally strongly related pairs. These results suggest that REM sleep may play an important role in identifying and strengthening previous weak cognitive associations. Another study compared performance on anagram word puzzles during wake and during awakenings from REM and non-REM sleep [33]. The number of anagrams solved following REM awakenings was 32% higher than those solved following non-REM awakenings, again suggesting that cognitive processing during REM may be a vital component in creative thinking and problem solving.
However, the role of REM sleep remains controversial. Individuals who have decreased amounts of REM sleep from medications or rare cases of brainstem damage appear to function normally [34]. Some studies involving suppression of REM sleep do not demonstrate impairments in learning, although creative insight has not specifically been tested [35]. The role of specific stages of sleep will be discussed in greater detail below.
Models of Memory Processing in Sleep
The mechanisms by which sleep facilitates memory consolidation are unclear. Several theoretical models have been proposed, not all of which are compatible. We review some of the major working theories here.
The first question is what processes occur in the brain during sleep that result in improved memory. One suggestion is the idea of “replay” of brain activity, i.e. reemergence of brain activity in sleep that occurred during learning in prior wakefulness. Wilson et al examined brain activity at the neuronal level in rats [36] [37]. They noted that hippocampal cells that fired together during a spatial behavioral task had an increased tendency to fire together during subsequent slow wave sleep, and tended to replicate sequences of neuronal firing representing movement along a spatial path. Peigneux et al. examined regional brain activity in humans by measuring cerebral blood flow with positron emission tomography (PET) [38]. They noted that hippocampal areas that were activated in human subjects while learning a route in a virtual town showed increased activation during subsequent slow wave sleep. Additionally, the amount of hippocampal activity seen during slow wave sleep correlated with task performance the next day. Maquet et al. also demonstrated regional activation using PET, this time with a serial reaction time task [39]. Multiple regions of the brain that were active during performance of the task were significantly more active during REM sleep in subjects who had recently executed the task than in those who had not trained on the task. These findings and those from other laboratories have led many researchers to conclude that recently encoded memories are reactivated and “replayed” during sleep, and that this replay mediates the memory processing seen to occur during sleep. In contrast to this notion that replay during sleep strengthens memories is the theory of “synaptic homeostasis”, which proposes that during sleep, particularly slow wave sleep, a process of synaptic downscaling occurs [40]. During this process total synaptic strength in the cortex is dramatically decreased, as a means of conserving energy and space within the brain. According to some formulations of this model, such downscaling could indirectly benefit learning and memory.
In addition to defining the processes, such as memory replay, that enhance specific memories during sleep, another important question is how sleep facilitates these processes. One approach to this question is to look at specific neurophysiologic features of sleep. One candidate is sleep spindles – short (~ 1 sec) bursts of rhythmic 12 to 16 Hertz (Hz) activity seen in the electroencephalogram (EEG) during non-REM sleep, which create feedback loops between the thalamus and the cortex [41]. These thalamo-cortical loops are believed to facilitate synapse formation and strengthening and thereby enhance memory [36]. Nishida and Walker observed differences in spindle activity between the “trained” and “untrained” hemisphere during a nap after subjects trained on a motor skill task [42]. The difference in spindle activity between the two hemispheres correlated with the amount of sleep-dependent improvement, with greater difference between the “learning” and “non-learning” hemispheres predicting greater improvement on the task after the nap. Other types of natural oscillation in the brain, including slow (<1 Hz) waves in the neocortex, theta (4 to 10 Hz) waves in the hippocampus, and gamma (40 to 100 Hz) waves in the cortex and thalamus, have also been implicated in sleep-dependent memory processing [43].
In addition to examining oscillatory patterns in the EEG, investigators have examined levels of hormones and neurotransmitters during sleep as possible contributors to memory consolidation. Levels of acetylcholine [44] and cortisol [45] both have been implicated in declarative memory processing during sleep.
Despite this research, the question of the mechanisms underlying sleep-dependent memory consolidation remain uncertain. It should be noted that these various models – memory replay, synaptic homeostasis, cortical oscillations, hormones and neuromodulators – are not mutually exclusive. Indeed, in the end, some or all of these processes may turn out to play a role in sleep-dependent memory processing.
Another approach to identifying mechanisms involved in sleep-dependent memory consolidation has been to investigate which stages of sleep mediate consolidation. Many studies that examine the role of sleep in memory consolidation have attempted to attribute consolidation of specific types of memory to particular stages of sleep. For example, non-declarative memory tasks have been linked to several stages of sleep, depending on the specific task in question. Consolidation of motor skills has been correlated with stage 2 sleep in the case of the motor sequence task (MST) [17] and SWS in the case of the motor adaptation task [20], while both REM sleep and SWS have been linked to performance on the visual discrimination task (VDT) [10] [11]. Declarative memory consolidation on the paired-associates word list has been correlated to non-REM sleep, specifically slow wave sleep [19] [20]. Sleep dependent improvements on other declarative tasks, where gist extraction or creative insight is required, have been correlated to REM sleep or a decrease in SWS [30, 31].
However, many of the relationships between specific sleep stages and types of memory remain unclear and controversial. Researchers have yet to clarify which types of memory are consolidated in sleep, which components of memory consolidation occur during sleep, and how these relate to particular stages of sleep. For example, while specific sleep stages have generally been thought of as mediating consolidation for different types of memories, an alternate recently proposed model suggests that individual sleep stages mediate specific components of the consolidation process, independent of memory type [46]. According to this theory, slow wave sleep plays a role in stabilizing recently encoded memories at the synaptic level, while stage 2 and REM sleep play roles in integrating the memories into larger neuronal networks at the systems level. This systems-level consolidation links memories together and captures the essence of large amounts of information. Further studies are necessary to determine whether either or conceivably both of these models are correct.
Conclusion
Sleep is composed of a series of complex neurophysiological states that play important roles in learning, memory and cognitive processing. Studies that examine the effects of sleep deprivation have noted drastic deficits in cognitive processing. With careful experimentation involving various types of memory tasks, an important role for sleep in both non-declarative and declarative memory processing has been established. In addition to simply strengthening memories and improving their resistance to interference, sleep contributes to the process of gaining insight, making connections and integrating large amounts of information. Though our understanding of these processes has dramatically increased over the past 10 years, there remain many interesting, unanswered questions. The full range of specific types of memory that are consolidated during sleep remains to be clarified, as do the particular mechanisms and aspects of sleep physiology that underlie memory consolidation and cognitive processing during sleep.
Footnotes
Prior to 2007, NREM sleep was classified into four stages, with NREM stages 3 and 4 collectively referred to as SWS.
|
| ||
| Name | Affiliation | |
|
| ||
| Matthew Walker | Univ. of California, Berkeley | mpwalker@bekeley.edu |
|
| ||
| Sara Mednick | Univ. of California, San Diego | smednick@salk.edu |
|
| ||
| Jan Born | University of Lübeck, Germany | born@kfg.uni-luebeck.de |
|
| ||
| Carlyle Smith | University of Trent, Canada | csmith@trentu.ca |
|
| ||
Contributor Information
Maryann C. Deak, Department of Medicine, Division of Sleep Medicine, Brigham and Women’s Hospital and Harvard School of Medicine, 75 Francis Street, Boston, MA 02115, USA
Robert Stickgold, Center for Sleep and Cognition, Department of Psychiatry, Harvard Medical School, Beth Israel Deaconess Medical Center E/FD 861, 330 Brookline Ave. Boston, MA 02115, USA.
References
- 1.Espana RA, Scammell TE. Sleep neurobiology for the clinician. Sleep. 2004;27(4):811–20. [PubMed] [Google Scholar]
- 2.Bonnet MH. In: Sleep Deprivation in Principles and Practice of Sleep Medicine. 4th. MHR Kryger T, Dement WC, editors. Elsevier Saunders Philadelphia; 2005. pp. 51–66. [Google Scholar]
- 3.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 4.Belenky G, Wesensten NJ, Thorne DR, Thomas ML, Sing HC, et al. Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. Journal of Sleep Research. 2003;12:1–12. doi: 10.1046/j.1365-2869.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 5.Horne JA. Sleep loss and “divergent” thinking ability. Sleep. 1988;11(6):528–36. doi: 10.1093/sleep/11.6.528. [DOI] [PubMed] [Google Scholar]
- 6.Harrison Y, Horne JA. Sleep loss impairs short and novel language tasks having a prefrontal focus. J Sleep Res. 1998;7(2):95–100. doi: 10.1046/j.1365-2869.1998.00104.x. [DOI] [PubMed] [Google Scholar]
- 7.Lockley SW, Cronin JW, Evans EE, Cade BE, Lee CJ, et al. Effect of reducing interns’ weekly work hours on sleep and attentional failures. N Engl J Med. 2004;351(18):1829–37. doi: 10.1056/NEJMoa041404. [DOI] [PubMed] [Google Scholar]
- 8.Landrigan CP, Rothschild JM, Cronin JW, Kaushal R, Burdick E, et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. N Engl J Med. 2004;351(18):1838–48. doi: 10.1056/NEJMoa041406. [DOI] [PubMed] [Google Scholar]
- 9.Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, et al. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10(18):3745–8. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- 10.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, et al. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403(6770):655–7. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 11.Karni A, Sagi D. Where practice makes perfect in texture discrimination: evidence for primary visual cortex plasticity. Proceedings of the National Academy of Science of the United States of America. 1991;88(11):4966–70. doi: 10.1073/pnas.88.11.4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 13.Karni A, Tanne D, Rubenstein BS, Askenasy JJM, Sagi D. Dependence on REM Sleep of overnight improvement of a perceptual skill. Science. 1994;265:679–682. doi: 10.1126/science.8036518. [DOI] [PubMed] [Google Scholar]
- 14.Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. Journal of Cognitive Neuroscience. 2000;12:246–254. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- 15.Stickgold R, James L, Hobson JA. Visual discrimination learning requires post-training sleep. Nature Neuroscience. 2000;2(12):1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 16.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95(3):861–8. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 18.Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proceedings of the National Academy of Science of the United States of America. 2002;99(18):11987–91. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 20.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 21.Fowler MJ, Sullivan MJ, Ekstrand BR. Sleep and memory. Science. 1973;179:302–304. doi: 10.1126/science.179.4070.302. [DOI] [PubMed] [Google Scholar]
- 22.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. Journal of Cognitive Neuroscience. 1997;9(4):534–547. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 23.Tucker MA, Hirota Y, Wamsley EJ, Lau H, Chaklader A, et al. A daytime nap containing solely non-REM sleep enhances declarative but not procedural memory. Neurobiology of Learning and Memory. 2006;86(2):241–7. doi: 10.1016/j.nlm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Gais S, Lucas B, Born J. Sleep after learning aids memory recall. Learning and Memory. 2006;13(3):259–62. doi: 10.1101/lm.132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellenbogen JM, Hulbert JC, Stickgold R, Dinges DF, Thompson-Schill SL. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Current Biology. 2006;16(13):1290–4. doi: 10.1016/j.cub.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 26.Hu P, Stylos-Allan M, Walker MP. Sleep facilitates consolidation of emotional declarative memory. Psychol Sci. 2006;17(10):891–8. doi: 10.1111/j.1467-9280.2006.01799.x. [DOI] [PubMed] [Google Scholar]
- 27.Payne JD, Stickgold R, Swanberg K, Kensinger EA. Sleep preferentially enhances memory for emotional components of scenes. Psychological Science. 2008;19:781–788. doi: 10.1111/j.1467-9280.2008.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner U, Gais S, Haider H, Verleger R, Born J. Sleep inspires insight. Nature. 2004;427(6972):352–5. doi: 10.1038/nature02223. [DOI] [PubMed] [Google Scholar]
- 29.Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proceedings of the National Academy of Science of the United States of America. 2007;104(18):7723–8. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne JD, Schacter DL, Propper RE, Huang LW, Wamsley EJ, et al. The role of sleep in false memory formation. Neurobiol Learn Mem. 2009;92(3):327–34. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai DJ, Mednick SA, Harrison EM, Kanady JC, Mednick SC. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A. 2009;11:182–93. doi: 10.1073/pnas.0900271106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stickgold R, Scott L, Rittenhouse C, Hobson JA. Sleep-induced changes in associative memory. J Cogn Neurosci. 1999;11(2):182–93. doi: 10.1162/089892999563319. [DOI] [PubMed] [Google Scholar]
- 33.Walker MP, Liston C, Hobson JA, Stickgold R. Cognitive flexibility across the sleep-wake cycle: REM-sleep enhancement of anagram problem solving. Cognitive Brain Research. 2002;14:317–324. doi: 10.1016/s0926-6410(02)00134-9. [DOI] [PubMed] [Google Scholar]
- 34.Vertes RP, Eastman KE. The case against memory consolidation in REM sleep. Behavioral and Brain Sciences. 2000;23:867–876. doi: 10.1017/s0140525x00004003. [DOI] [PubMed] [Google Scholar]
- 35.Rasch B, Pommer J, Diekelmann S, Born J. Pharmacological REM sleep suppression paradoxically improves rather than impairs skill memory. Nat Neurosci. 2009;12(4):396–7. doi: 10.1038/nn.2206. [DOI] [PubMed] [Google Scholar]
- 36.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 37.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36(6):1183–94. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 38.Peigneux P, Laureys S, Fuchs S, Collette F, Perrin F, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44(3):535–45. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 39.Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3(8):831–6. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 40.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Medicine Reviews. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:d878–99. doi: 10.2741/1043. [DOI] [PubMed] [Google Scholar]
- 42.Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2(4):e341. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sirota A, Buzsaki G. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat Syst. 2005;3(4):245–259. doi: 10.1017/S1472928807000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rasch B, Gais S, Born J. Impaired off-line consolidation of motor memories after combined blockade of cholinergic receptors during REM sleep-rich sleep. Neuropsychopharmacology. 2009;34(7):1843–53. doi: 10.1038/npp.2009.6. [DOI] [PubMed] [Google Scholar]
- 45.Plihal W, Pietrowsky R, Born J. Dexamethasone blocks sleep induced improvement of declarative memory. Psychoneuroendocrinology. 1999;24(3):313–31. doi: 10.1016/s0306-4530(98)00080-8. [DOI] [PubMed] [Google Scholar]
- 46.Stickgold R. How do I remember? Let me count the ways. Sleep Med Rev. 2009;13(5):305–308. doi: 10.1016/j.smrv.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further Reading
- Stickgold R, Ellenbogen JM. Quiet! Sleeping brain at work. Scientific American Mind. 2008 Aug-Sep;:22–29. [Google Scholar]
- Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13(5):309–321. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]


