Abstract
Hypoxemia during initial stabilization of patients with severe traumatic brain injury (TBI) has been associated with poorer outcomes. However, the effects of delayed hypoxemia occurring during intensive care post-TBI on outcome is unclear. Pre-clinical models of TBI have rarely shown cognitive or behavioral deficits beyond 6 weeks post-injury and commonly have not included modeling of secondary insults. We have previously developed a murine model of TBI followed by delayed hypoxemia to model the secondary insult of hypoxemia and brain hypoxia occurring in the intensive care setting. Understanding long-term effects of delayed hypoxemia post-TBI in our murine model is critical for future testing of candidate therapeutics targeting secondary brain hypoxia. For this study, forty 5-week-old male mice were randomized to controlled cortical impact (CCI; N = 24) or sham surgery (N = 16). One day later, awake animals were randomized to 60 min of hypoxemia or normoxemia. Six months after initial injury, animals underwent behavior testing (Morris water maze, social interaction, and tail suspension) before euthanasia for immunohistochemistry (IHC) assessments. At 6 months post-injury, mice experiencing CCI and hypoxemia (CCI+H) had longer swim distances to the hidden platform (51 cm) compared to CCI alone (26 cm) or sham animals (22 cm). During social interaction assessments, CCI + H mice spent less time interacting with novel stimulus mice (79 sec) than CCI alone (101 sec) or sham animals (139 sec). CCI + H had larger lesion volumes compared to CCI alone (14.0% vs. 9.9%; p < 0.003). Glial fibrillary acidic protein IHC at 6 months post-injury demonstrated increased astrogliosis in the ipsilateral white matter of CCI + H compared to CCI alone. To summarize, this clinically relevant model of delayed hypoxia post-TBI resulted in long-term behavioral deficits and evidence of exacerbated structural injury.
Keywords: : controlled cortical impact, delayed hypoxemia, long-term behavior, secondary injury, traumatic brain injury
Introduction
Traumatic brain injury (TBI) morbidity continues to be high despite advances in medical care.1 One of the primary goals of intensive care management of patients with severe TBI is the prevention or amelioration of secondary insults such as hypoxemia, hypotension, and intracranial hypertension, among others. Hypoxemia occurring in the pre-hospital or emergency room setting post-TBI has been associated with poorer outcomes.2–5 Previous investigations of pre-clinical models of hypoxemia immediately post-TBI have demonstrated exacerbations in brain edema and ischemia, hippocampal neuronal cell death, neuroinflammation, axonal injury, and short-term behavioral deficits.6–9 After initial stabilization, patients with severe TBI are at high risk of exposure to delayed hypoxemia in the intensive care setting attributed to pulmonary contusions, aspiration, pneumonia, atelectasis, and acute respiratory distress syndrome. Small retrospective studies have observed that hypoxemia within the first 24 h post-injury is common and is associated with an increase in mortality.10,11
Survivors of TBI may have lifelong impairments in cognition and behavior.12–14 Pre-clinical investigations with various animal models of TBI have improved our understanding of TBI pathophysiology and have been utilized to identify promising candidate neuroprotective therapeutics.15,16 However, the majority of animal models of TBI have not been shown to have cognitive or behavioral deficits beyond 6 weeks post-injury, whereas outcome measures in clinical trials of therapeutics for TBI are typically performed 6–12 months post-injury.17–22 We have previously developed a murine model of TBI followed by delayed hypoxemia to model the secondary insult of hypoxemia and brain hypoxia occurring in the intensive care setting.11 We observed exacerbation of white matter injury in mice experiencing TBI and delayed hypoxemia compared with TBI alone at two different time points: 48 h and 1 week post-injury. In our current investigations, we tested the hypothesis that delayed hypoxemia following controlled cortical impact (CCI) in young mice (5 weeks old) would exacerbate long-term behavioral deficits and neuropathology 6 months after initial injury.
Methods
Traumatic brain injury
All procedures were approved by the Washington University Animal Studies Committee (Protocol 2014003) and are consistent with the National Institutes of Health guidelines for the care and use of animals. Animals were housed 5/cage and had free access to water and food with 12-h light/dark cycle. Forty C57BL/6J 5-week-old male mice (Jackson Laboratory, Bar Harbor, ME) weighing 15–22.5 g were used (Fig. 1). Two mice were excluded because of excessive brain swelling after craniectomy. Fourteen mice underwent sham injury, and 24 mice underwent CCI as previously described.11,23 Briefly, mice were anesthetized with 5% isoflurane at induction, followed by maintenance at 2% isoflurane for the duration of the procedure. Buprenorphine (50 μg/kg subcutaneously s.c.) was administered before scalp incision. The head was shaved and head holders were used to stabilize the head within the stereotaxic frame (MyNeurolab, St. Louis, MO). Then, a single 5-mm craniectomy was performed by an electric drill on the left lateral side of the skull centered 2.7 mm lateral from the midline and 3 mm anterior to lambda. Animals were randomized to sham or injury after craniectomy using a computer-generated numbers randomization. For injured animals, the 3-mm electromagnetic impactor tip was then aligned with the craniectomy site at 1.2 mm left of midline, 1.5 mm anterior to the lambda suture. The impact was then delivered at 2 mm depth (velocity 5 m/s, dwell time 100 ms). The head holders were released immediately after the injury. All animals then received a loose fitting plastic cap secured over the craniectomy with Vetbond (3M, St. Paul, MN). The skin was closed with interrupted sutures and treated with antibiotic ointment before removing the mouse from anesthesia and allowing recovery on a warming pad. Two mice died immediately post-CCI.
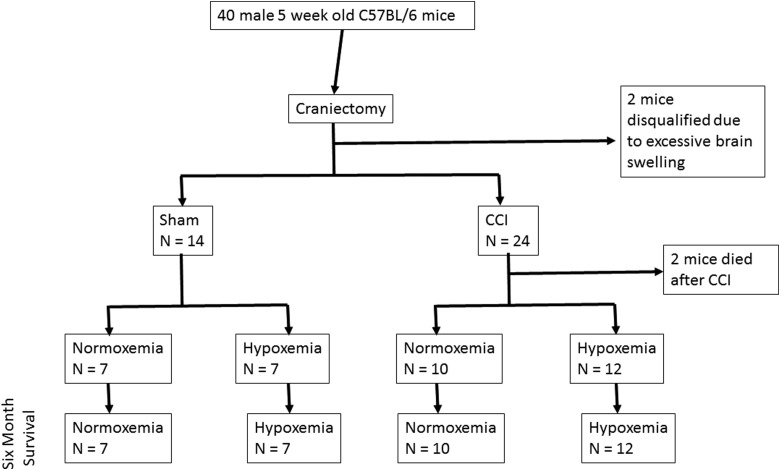
FIG. 1.
Experimental cohort design. All animals underwent craniectomy and were excluded from the study if brain swelling after the procedure was excessive. Animals were randomized to sham or CCI using a computer-generated numbers randomization. Twenty-four hours post-surgery, animals were randomized to 60 min of normoxemia or hypoxemia. Behavioral assessments followed by histological analysis were performed at 6 months. CCI, controlled cortical impact.
Delayed hypoxemia
One day after sham surgery or CCI, animals were randomized to normoxemia (room air) or hypoxemia (8% O2, 4% CO2) for 60 min. A mixture of N2, O2, and CO2 was utilized to maintain normocarbic hypoxemia.11 We have previously demonstrated that these conditions produce normocarbic hypoxemia in awake mice 24 h post-CCI or sham surgery.11 During normoxemia or hypoxemia, animals were placed in fresh cages with littermates randomized to the same treatment arm. All animals were subjected to identical transport and handling regardless of group assignment. Animals randomized to hypoxemia were placed in a Coy Labs Hypoxia Chamber (6′ × 3′ × 4′; Coy Laboratory, Grass Lake, MI), fitted with airlock, oxygen sensor, carbon dioxide sensor, and gas controllers.
Behavioral tests
Animals underwent behavioral testing 6 months after sham surgery or CCI. All tests were conducted by an experimenter blinded to group assignment. The order of tests was social interaction, Morris water maze, then tail suspension. All testing was performed during the first 4 h of the light cycle.
Social interaction
The social interaction test was adapted and modified from previous reports.21,24 Animals were individually housed for at least 1 h in the testing room for acclimation before testing. A 5-week-old male stimulus mouse was introduced for a 5-min observation period. A juvenile mouse was used to decrease the risk of aggressive behavior during the test period. Stimulus mice were used for no more than five social interaction sessions with a minimum time interval of 30 min between each session. A blinded observer scored interaction initiated by the test mouse, excluding interaction initiated by the stimulus mouse. Behaviors scored as interaction include sniffing with the nose within 1 cm of the stimulus mouse (including nose, body, and anogenital area), pawing and climbing on the stimulus mouse, and following within 2 cm of the stimulus mouse.
Morris water maze
A platform 11 cm in diameter and a pool 109 cm in diameter in a testing room with set visual cues were used as previously described.23 Animals were tested in two cohorts of 20 to maximize accuracy and ensure consistent latency times between trials. Each animal underwent four trials per day for 3 days of visible platform training, in order to control for differences in swim speed and visual acuity. No trial lasted more than 60 sec, and the animal was allowed to remain on the platform for 30 sec after completing the trial. After 3 days of rest, each animal underwent four trials per day for 5 days of hidden platform training. The visual cues were reset before hidden platform training. The platform was moved to a different quadrant and hidden slightly below the waterline after the water had been made opaque using nontoxic white tempera paint. On the final day, the platform was removed from the pool after the completion of hidden platform trials, and a 30-sec probe trial was conducted.
Tail suspension
The tail suspension test was adapted from previous investigations.21,25 Each animal was suspended with tape from a horizontal rod elevated 30 cm above a clean cage for 6 min. To prevent mice from climbing their tails, a cardstock paper cone was placed around the tail before suspension. The cones were 4.5 cm in diameter and 5.5 cm tall. A new cone was used for each mouse. A blinded observer recorded the total time each mouse was immobile during the 6-min testing period. Immobility included motionless time as well as passive swinging caused by momentum from movement.
Immunohistochemistry
Mice were killed under isoflurane anesthesia by transcardial perfusion with 0.3% heparin in phosphate-buffered saline. Whole brains were removed and fixed in 4% paraformaldehyde for 48 h, followed by equilibration in 30% sucrose for at least 48 h before sectioning. Serial 50-μm-thick coronal slices were cut on a freezing microtome starting with the appearance of a complete corpus callosum and caudally to bregma −3.08 mm. Sets of 12 sections spaced every 300 μm were mounted on glass slides and used for immunohistochemical studies.
Staining was performed on free-floating sections washed in Tris-buffered saline (TBS) between applications of primary and secondary antibodies. Endogenous peroxidase was blocked by incubating the tissue in TBS +0.3% hydrogen peroxide for 10 min. Normal goat serum (3%) in TBS with 0.25% Triton X (TBS-X) was used to block nonspecific staining for all antibodies. Slices were then incubated at 4°C overnight with one of the following primary antibodies: polyclonal rabbit anti-glial fibrillary acidic protein (GFAP; Dako North America, Carpinteria, CA) at a concentration of 1:1000, polyclonal rabbit anti-neuronal nuclei (NeuN; EMD Millipore, Billerica, MA) at a concentration of 1:4000, or polyclonal rabbit anti-ionized calcium binding adapter molecule 1 (Iba1; Wako Chemicals USA, Richmond, VA) at a concentration of 1:1000. Biotinylated goat antirabbit secondary antibodies (Vector Laboratories, Burlingame, CA) in TBS-X were used at a 1:1000 concentration to detect bound primary antibodies. Colorization was achieved using the Vectastain ABC Elite Kit (Vector Laboratories) followed by the application of 3-3′-diaminobenzidine (Sigma-Aldrich, St. Louis, MO).
Quantification of immunohistochemistry
Extent of tissue loss in the ipsilateral hemisphere for each animal was quantified using images of NeuN-stained slices acquired using Hamamatsu NanoZoomer 2.0-HT System (Hamamatsu Corporation, Middlesex, NJ). Tissue loss in the injured hemisphere was calculated as a percentage of the tissue volume in the contralateral hemisphere as described by others.26
For GFAP measurements, 5 × images of the ipsilateral corpus callosum and external capsule were obtained using the NanoZoomer. Using ImageJ (NIH, Bethesda, MD), files were converted to 32-bit images, and positive signal on each image was selected using the Max Entropy auto thresholding method. Regions of interest (ROIs) began with the most anterior slice containing hippocampal dentate gyrus and ended with the most posterior section containing corpus callosum fibers that cross the midline, yielding three to four sections for analysis per animal. The midline served as the medial boundary for the ROI, and the lateral boundary was formed by drawing a horizontal line between the ventral hippocampus and dorsal thalamus in each section. Total area of ROIs and area of positive thresholding from each slice were calculated and summed to determine total percent area of positive GFAP staining.
Stereological analysis was performed using StereoInvestigator software (version 8.2; MBF Bioscience, Williston, VT). Assessments were made by an investigator blinded to group assignment. The optical fractionator function was used to quantify target markers per cubic millimeter of tissue. For quantification of hippocampal neuronal loss in the CA3 region, a grid size of 250 × 250 μm, a counting frame of 40 × 40 μm, and a dissector height of 15 μm with a guard zone of 5 μm were used for all quantifications, resulting in 3% of the ROIs being randomly sampled. All ROIs were traced at 4 × magnification, and markers were counted at 60 × magnification.
For stereological quantification of Iba1-positive cells, the optical fractionator function was again used, with a grid size of 180 × 180 μm and a counting frame of 80 × 80 μm. The ROI began with the most anterior slice containing hippocampal dentate gyrus and ended with the most posterior section containing corpus callosum fibers that cross midline, which yielded three to four sections for analysis per animal. The midline served as the medial boundary for the ROI, whereas the lateral boundary was formed by drawing a horizontal line between the ventral hippocampus and dorsal thalamus in each coronal section. Gunderson's coefficients of error were <0.1 for all stereological quantifications. All post-processing and automated quantification algorithms are freely available upon request.
Statistical analysis
All data were analyzed using Statistica (version 12; Dell Inc., Tulsa, OK). Data results are presented as mean ± standard deviation. There was no evidence for significant deviations from normal distribution (p > 0.05 by Shapiro-Wilk tests). Data were analyzed with two-way analysis of variance with repeated measures, where necessary, followed by Tukey's tests for multiple comparisons with a significance level of p < 0.05. Effect size between CCI and CCI + H was calculated utilizing Cohen's d.27
Results
Experimental cohort
Mice were randomized to one of four groups as described above: sham (SHAM), sham+hypoxemia (SHAM+H), CCI, and CCI+hypoxemia (CCI+H; Fig. 1). At the time of surgery, there was no statistically significant difference between the weights or anesthesia times of the groups (Table 1). However, at the time of sacrifice, CCI + H weighed less than SHAM (Table 1).
Table 1.
Comparison of Weights and Anesthesia Times between Groups
| Sham | Sham+Hypoxemia | CCI | CCI+Hypoxemia | Analysis of variance | |
|---|---|---|---|---|---|
| Weight at time of CCI (g) | 18.9 (2.9) | 18.0 (2.6) | 19.4 (1.5) | 19.0 ( 1.6) | No difference between groups |
| Anesthesia time (min) | 16.5 (1.3) | 15.9 (1.7) | 17.9 (2.5) | 17.7 (2.2) | No difference between groups |
| Weight at sacrifice (g) | 34.0 (3.5) | 31.6 (2.5) | 32.3 (1.9) | 30.6 (1.8)* | Hypoxemia F(1,32) = 6.5; p < 0.02 |
| Injury F(1,32) = 2.6; p = 0.12 | |||||
| Injury*Hypoxemia F(1,32) = 1.0; p = 0.67 |
Values are mean (standard deviation).
Sham versus CCI+Hypoxemia, post-hoc Tukey, p < 0.03.
CCI, controlled cortical impact.
Effects of delayed hypoxemia after traumatic brain injury on socialization and depression-like symptoms
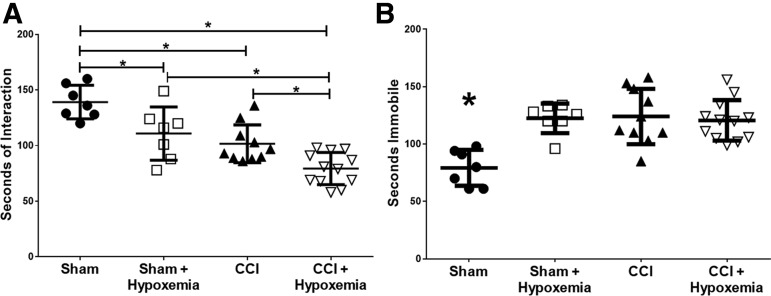
To determine the effect of delayed hypoxemia on socialization, we conducted social interaction tests 6 months post-injury. Injury and hypoxemia decreased social behaviors (Fig. 2A). Both injury status (F1, 32 = 33.4; p = 0.000002) and hypoxemia (F1, 32 = 17.9; p = 0.0002) had a significant impact on socialization. The CCI + H group interacted with the stimulus mouse significantly less than the SHAM (p = 0.0002), SHAM + H (p = 0.003), or CCI (p = 0.027) groups. The SHAM + H (p = 0.023) and CCI (p = 0.0008) groups also interacted with the novel mouse significantly less than the SHAM group. We observed a large effect size between CCI and CCI + H (Cohen's d = 1.2).
FIG. 2.
Socialization and tail suspension 6 months post-CCI and delayed hypoxemia. (A) Social interaction during a 300-sec test with a novel 5-week-old male mouse. Socialization was significantly affected by injury status (F(1,32) = 33.4; p = 0.000002) and hypoxemia (F(1,32) = 17.9; p = 0.0002). The CCI+Hypoxemia group interacted significantly less with the novel mouse than CCI alone. *p < 0.03. (B) Seconds immobile during 360 sec of tail suspension. Injury status (F(1,32) = 11.3; p = 0.002), hypoxemia (F(1,32) = 9.7; p = 0.004), and the interaction between injury status and hypoxemia (F(1,32) = 13.3; p = 0.001) had significant effects on time spent immobile. SHAM was significantly different from the SHAM + Hypoxemia (p = 0.009), CCI (p = 0.0003), and CCI+Hypoxemia (p = 0.0004) groups. *SHAM significantly different from other groups. CCI, controlled cortical impact.
In order to determine the impact of our injury model on long-term depression-like behaviors, we conducted tail suspension tests 6 months post-injury. Both injury and hypoxemia increased periods of immobility (Fig. 2B). Injury status (F1, 32 = 11.3, p = 0.002), hypoxemia (F1, 32 = 9.7, p = 0.004), and the interaction between injury and hypoxemia (F1, 32 = 13.3, p = 0.001) had significant impacts on time spent immobile during the tail suspension testing period. The SHAM group spent significantly less time immobile than the SHAM + H (p = 0.009), CCI (p = 0.0003), and CCI + H (p = 0.0004) groups. However, no difference in time spent immobile was observed between the two CCI groups, and the effect size was very small (Cohen's d = 0.l8).
Delayed hypoxemia after traumatic brain injury produces long-term deficits in learning and memory
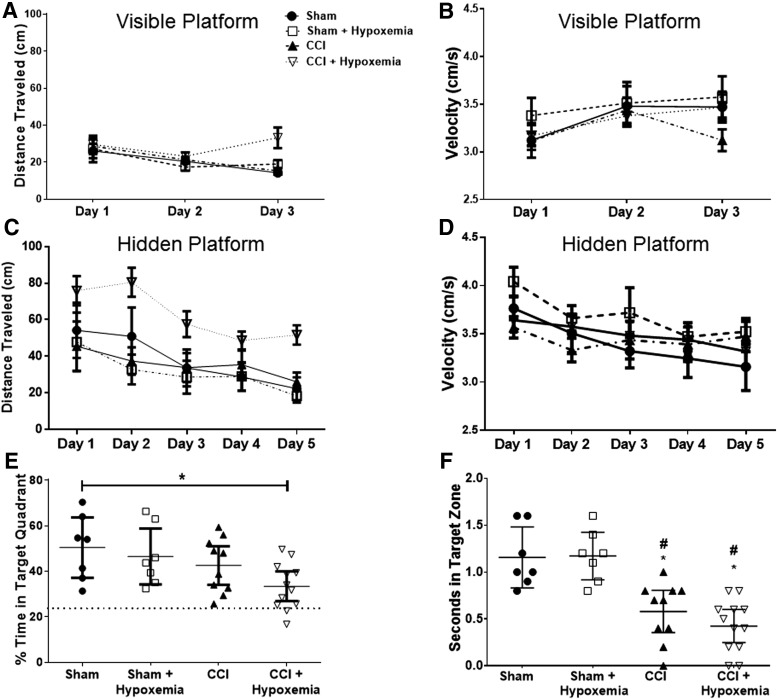
To determine the impact of delayed hypoxemia on memory and learning, we conducted Morris water maze tests 6 months post-injury. No significant effect of either injury status or hypoxemia was observed on distance to platform or velocity during the visible training period (Fig. 3A,B). Injury status (F1, 32 = 4.6; p = 0.04) and interaction of injury status and hypoxemia (F1, 32 = 6.1; p = 0.019) had significant effects on distance to platform during hidden platform training (Fig. 3C). Post-hoc Tukey's tests confirmed that the CCI + H group swam significantly longer distances on average than both the SHAM + H (p = 0.012) and CCI (p = 0.016) groups during hidden platform training. A large effect size (Cohen's d = 1.2) of hypoxemia on distance traveled by CCI mice during hidden platform training was observed. Neither injury status nor hypoxemia had a significant impact on velocity during the same period of training (Fig. 3D). The impact of delayed hypoxemia on memory and learning was further characterized by conduction of a probe test. Injury status had a significant impact on performance during the probe trial (F1, 32 = 6.4; p = 0.017; Fig. 3E). The CCI + H group spent a significantly lower percentage of time in the target quadrant compared to the SHAM group (p = 0.03). Although there was not a statistically significant difference in probe trial performance between CCI and CCI+H, a moderate effect size was demonstrated (Cohen's d = 0.77).
FIG. 3.
Memory and learning deficits six months after injury and delayed hypoxemia. (A) Distance to visible platform was not significantly different between groups. (B) Velocity to visible platform was not significantly different between groups. (C) Distance to hidden platform was significantly affected by injury (F(1,32) = 4.6; p = 0.04) and by the interaction of injury and hypoxemia (F(1,32) = 6.1; p = 0.019). Post-hoc Tukey tests confirmed that CCI+Hypoxemia traveled significantly more distance than SHAM+Hypoxemia (p = 0.012) and CCI (p = 0.016). (D) Velocity to hidden platform was not significantly different between groups. (E and F) Thirty-second probe trial after 5 days of hidden platform Morris water maze training. (E) CCI+Hypoxemia spent a significantly lower percentage of time in the target quadrant than the SHAM group (F(1,32) = 6.4; p = 0.017, followed by Tukey's test, p = 0.03). (F) Injury had a significant effect on time spent in target zone (F(1,32) = 42.23; p < 0.00001). #Compared to SHAM, p < 0.003; *compared to SHAM+Hypoxemia, p < 0.003. CCI, controlled cortical impact.
Delayed hypoxemia increases lesion size but does not reduce hippocampal neuron density
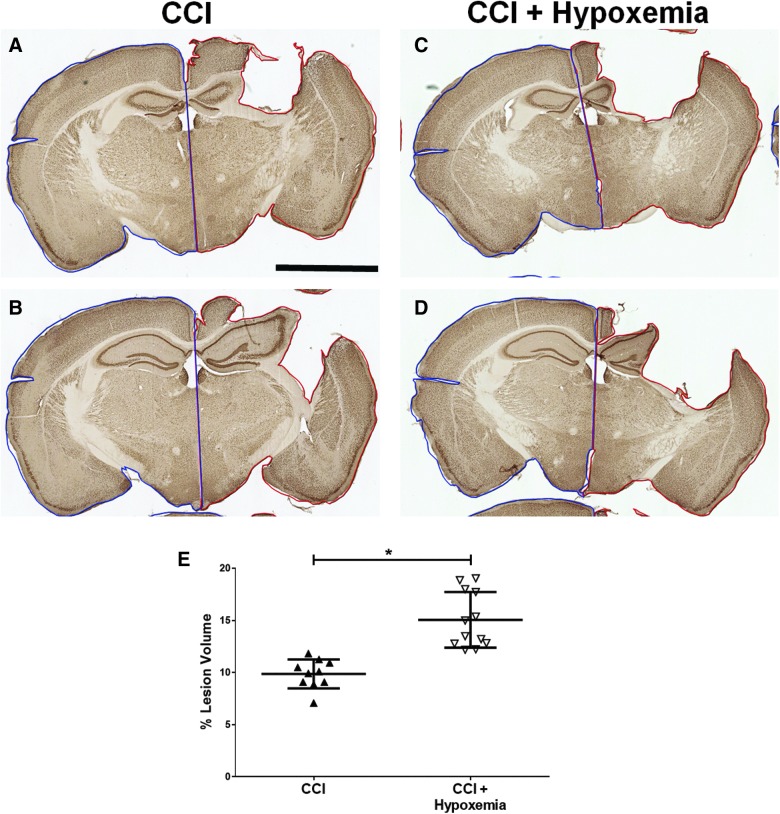
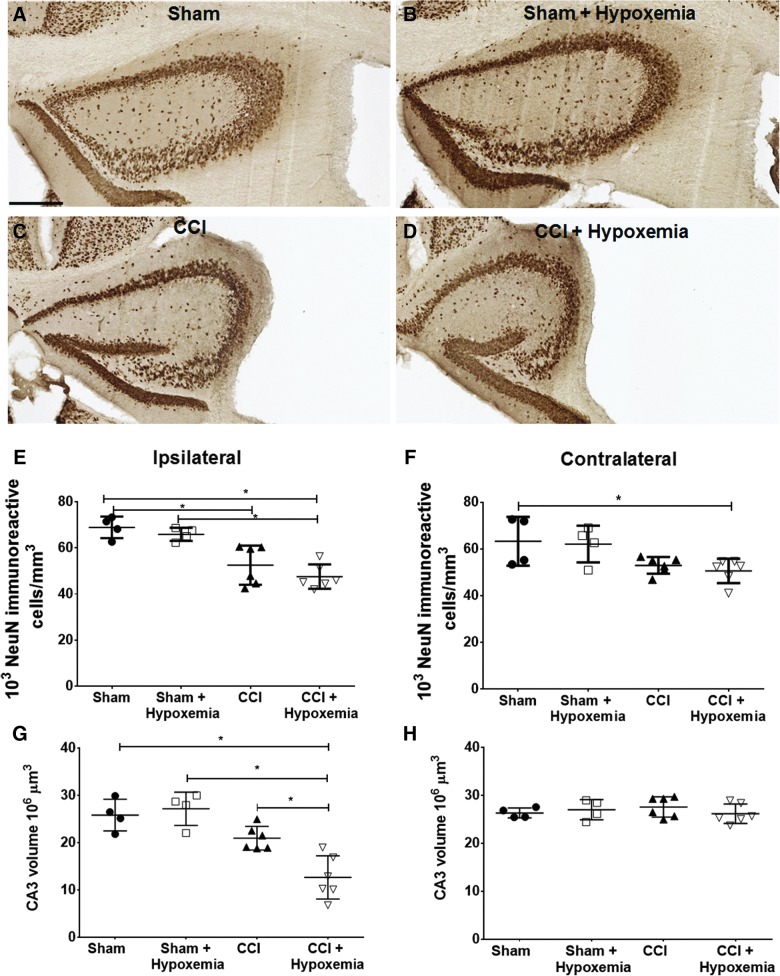
To determine the impact of delayed hypoxemia on lesion volume, we calculated ipsilateral tissue loss. The CCI + H group had significantly larger lesion volumes than CCI alone (Fig. 4) with a large effect size (Cohen's d = 1.1). Ipsilateral hippocampal volumes were reduced post-CCI, and a significant injury effect and injury*hypoxemia interaction effect were demonstrated (Fig. 5). Stereological analysis of NeuN-positive cells in the ipsilateral CA3 region of the hippocampus did not demonstrate an additive effect of hypoxemia on neuronal loss (Fig. 5E). However, stereological analysis of the contralateral CA3 region of the hippocampus revealed a reduction in NeuN-positive cells in CCI + H (Fig. 5F) and a reduction in hippocampal volumes post-injury on the ipsilateral side only (Fig. 5G,H).
FIG. 4.
Delayed hypoxemia increases lesion volume. (A–D) Exemplars of lesion volume analysis, NeuN staining. Ipsilateral slice area (red outline) and contralateral slide area (blue outline). Scale bar, 2.5 mm. (E) CCI+Hypoxemia had a significantly larger lesion volume than CCI (p = 0.002). CCI, controlled cortical impact; NeuN, neuronal nuclei. Color image is available online at www.liebertpub.com/neu
FIG. 5.
NeuN-positive cells in CA3 region of hippocampus. (A–D) Ipsilateral hippocampus. Scale bar, 250 μm. (E) Density of NeuN-positive cells in the ipsilateral CA3 region of the hippocampus was significantly impacted by injury status (F(1,16) = 39.1; p = 0.0001), but not by hypoxemia (F(1,16) = 2.1; p = 0.17) or the interaction between injury status and hypoxemia (F(1,16) = 0.128; p = 0.76). *p < 0.02 post-hoc Tukey's tests. (F) Density of NeuN-positive cells in the contralateral CA3 region of the hippocampus was significantly impacted by injury status (F(1,16) = 12.8; p = 0.003). *p < 0.05 post-hoc Tukey's test. (G) Ipsilateral CA3 hippocampal volumes were also significantly impacted by injury status (F(1,16) = 31.9; p = 0.0001) and the interaction between injury status and hypoxemia (F(1,16) = 7.6; p < 0.02). *p < 0.02 post-hoc Tukey's tests. (H) No differences in contralateral CA3 hippocampal volumes. CCI, controlled cortical impact; NeuN, neuronal nuclei. Color image is available online at www.liebertpub.com/neu
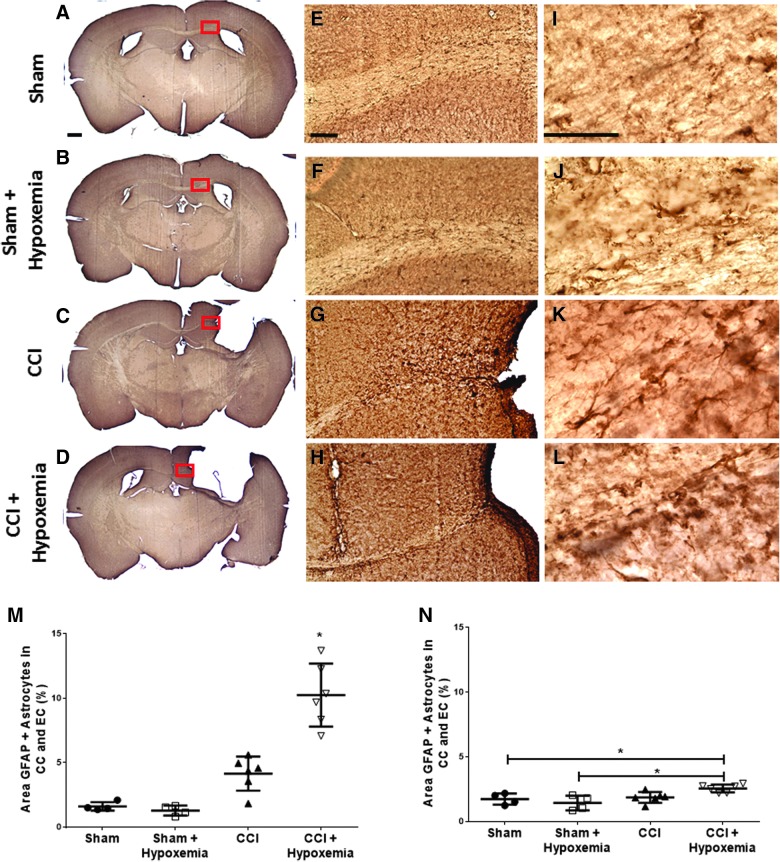
Delayed hypoxemia increases astrocytic response
To assess effects of delayed hypoxemia on astrocytic response, we performed GFAP immunohistochemical staining. GFAP-positive cell staining was increased by both injury and hypoxemia (Fig. 6). Injury status (F1, 16 = 64.5; p = 0.000001), hypoxemia (F1, 16 = 16.2; p = 0.001), and interaction between the two (F1, 16 = 20.0; p = 0.0004) all had significant impacts on level of astrocytic response. We performed a post-hoc Tukey's test and confirmed that the CCI + H group had a significantly higher level of astrocytic response than the SHAM (p = 0.0002), SHAM + H (p = 0.0002), or CCI (p = 0.0002) groups.
FIG. 6.
Increased astrocytosis 6 months post-injury and delayed hypoxemia. Immunohistochemical staining with GFAP. (A–D) Scale bar, 500 μm. Corpus callosum (CC) and external capsule (EC). (E–G) Scale bar, 100 μm. (I–L) Scale bar, 50 μm. (M) Astrocytic response in the CC and EC was significantly impacted by injury status (F(1,16) = 64.5; p = 0.000001), hypoxemia (F(1,16) = 16.2; p = 0.001), and the interaction between injury status and hypoxemia (F(1,16) = 20.0; p = 0.0004). CCI+Hypoxemia had a significantly higher percent area of GFAP staining in the ipsilateral CC and EC 6 months post-injury. *p < 0.0002, CCI+Hypoxemia compared to each of the other groups using post-hoc Tukey's test. (N) Astrocytic response in contralateral CC and EC was significantly impacted by injury status (F(1,16) = 10.6; p < 0.005) and the interaction between injury status and hypoxemia (F(1,16) = 6.7; p = 0.02). *p < 0.05 using post-hoc Tukey's test. CCI, controlled cortical impact; GFAP, glial fibrillary acidic protein. Color image is available online at www.liebertpub.com/neu
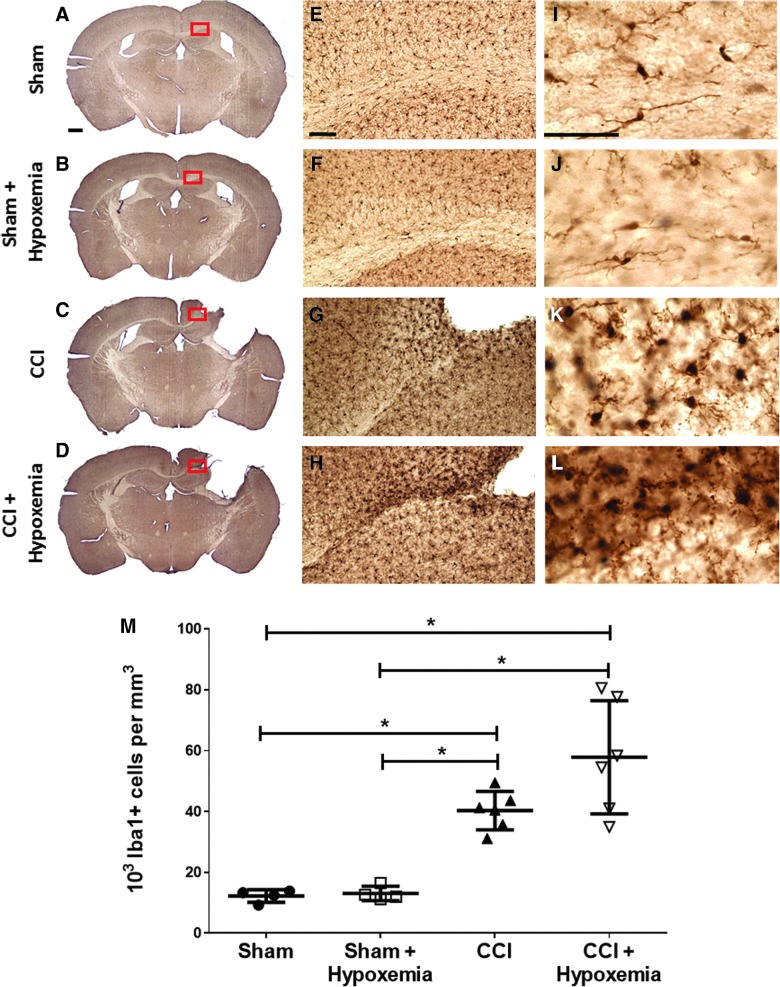
Delayed hypoxemia has no significant effect on microglial response
We performed Iba1 immunohistochemical staining to assess the effect of delayed hypoxemia on long-term microglial response (Fig. 7). However, only injury status (F1, 15 = 48.7; p = 0.000004) had a significant impact on level of microglial response. The CCI group was significantly different from both the SHAM (p = 0.008) and SHAM + H (p = 0.010) groups. The CCI + H group was also significantly different from the SHAM (p = 0.0003) and the SHAM + H (p = 0.0003) groups. The CCI and CCI + H groups were not significantly different from each other.
FIG. 7.
Microglial response not significantly impacted by delayed hypoxemia. Immunohistochemical staining with Iba1. (A–D) Scale bar, 500 μm. Corpus callosum (CC) and external capsule (EC). (E–G) Scale bar, 100 μm. (I–L) Scale bar, 50 μm. (M) Only injury status had a significant impact on microglial response (F(1,15) = 48.7; p = 0.000004). *p < 0.0003 post-hoc Tukey's tests. CCI, controlled cortical impact; Iba1, ionized calcium binding adapter molecule 1. Color image is available online at www.liebertpub.com/neu
Discussion
To summarize, at 6 months post-injury, we observed deficits in spatial learning and memory as well as socialization in mice subjected to CCI and delayed hypoxemia. Neuropathological evaluation at 6 months post-injury demonstrated increased lesion volume and white matter astrogliosis in mice subjected to CCI and delayed hypoxemia compared to CCI alone. Together, these data support the hypothesis that CCI followed by normocarbic hypoxemia 24 h later exacerbates long-term neuropathology and produces detectable behavioral deficits 6 months after initial injury.
We did not observe deficits in memory and learning with Morris water maze testing in mice 6 months after moderate CCI alone (Fig. 3). However, the addition of 1 h of hypoxemia 1 day post-CCI resulted in significant behavioral deficits in spatial learning assessed by Morris water maze during hidden platform trials. Whereas mice undergoing CCI alone were able to perform as well as sham mice with or without hypoxemia at 6 months, CCI + H demonstrated longer swim distances throughout the 5 days of hidden platform testing. Further, CCI + H were the only group to have a significantly poorer performance on probe trial compared to SHAM. The Cohen d effect size between CCI and CCI + H was large during the probe trial, suggesting that slightly larger sample sizes are needed to appropriately test the hypothesis. With no significant differences in swim velocity between the groups, these observed differences were unlikely attributable to a chronic motor deficit. To correlate the observed deficits in memory and learning with neuropathology, we performed histological analysis of the hippocampus. Hippocampal neurons in the CA3 region are vulnerable to death post-TBI, and hippocampal damage has been associated with poorer performance in Morris water maze testing.6,28–32 Specifically, hypoxemia immediately post-CCI has been observed to increase neuronal death in this ROI.6 Stereological analysis of the CA3 region of the hippocampus with NeuN immunohistochemistry did not reveal differences in neuron density between CCI and CCI+H. However, we did observe increases in lesion volume and decreases in ipsilateral CA3 volume in CCI + H compared to CCI alone, which is consistent with the behavioral deficits observed during Morris water maze testing in CCI+H. The increase in lesion volume after exposure to delayed hypoxemia supports the hypothesis that delayed hypoxemia exacerbates injury. Most likely, these observed differences are attributable to expansion of the injury penumbra in the CCI + H group, but we did not investigate whether the addition of delayed hypoxemia alters neuronal death or neurogenesis. We hope to perform future investigations at early time points to quantify neuronal death (Fluoro-Jade staining) and neurogenesis (bromodeoxyuridine labeling).
Difficulties in social and affective behavior are commonly reported as long-term sequelae in survivors of TBI.33,34 However, recent studies investigating the efficacy of neuroprotective compounds for TBI have only utilized behavior assessments in the cognitive and sensoriomotor domains.35–40 We attempted to assess the stress response utilizing the tail suspension test. Originally developed to assess the antidepressant activity of pharmacological agents, the tail suspension test has been utilized to characterize the phenotypic stress response of different genetic strains of mice.41 At 6 months post-injury, we observed increases in depression-like behavior in SHAM+H, CCI, and CCI + H compared to SHAM. Previous investigations have reported greater immobility times in mice experiencing mild TBI compared to CCI, consistent with our own findings of small differences in tail suspension between the groups.42 Interestingly, the effect of hypoxemia alone was significant with increased immobility during the tail suspension test. Our limited immunohistochemical assessments of white matter and the hippocampus did not reveal significant structural differences between SHAM and SHAM + H to correlate with these behavioral observations. It is quite possible that hypoxemia in sham animals may affect thalamic or hypothalamic pathways unrelated to CCI, which are responsible for the depression-like findings in SHAM+H.43–45
Utilizing the social interaction test with younger novel male mice, we observed decreases in interaction time in all mice who experienced TBI, similar to what others have found at earlier time points post-injury.46 CCI + H at 6 months post-injury had significantly decreased social interaction compared to the three other groups. Previous investigations have reported long-term deficits in socialization in mice experiencing repeated TBIs.42 In our studies, delayed hypoxemia may be regarded as a “second hit” similar to repetitive TBI, resulting in increased socialization deficits. Similar to tail suspension, we also observed a hypoxemia effect on sham animals. As stated above, the neuropathological correlation to our behavior findings is unclear, and changes induced by hypoxia in sham animals may not be detectable by histological assessments. These results support the use of socialization metrics in future assessments of efficacy for TBI neuroprotection of candidate therapeutics and may improve the probability of successful translation to human trials.
Immunohistochemical assessments of long-term axonal injury in a CCI model of TBI are difficult given that typical approaches such as β-amyloid precursor protein and neurofilament axonal swellings have resolved at much earlier time points.47 As surrogate markers for white matter injury, we assessed Iba1+ microglia and GFAP+ astrocytes in the ipsilateral corpus callosum and external capsule. Six months post-injury, stereological analysis of Iba1 immunohistochemistry revealed increased microglia density in animals experiencing CCI compared to SHAM. Long-term microglial activation post-TBI has been previously reported in both monkeys subsequent to experimental focal injury and humans, and our results are consistent with these previous findings.48,49 Similarly to our previous findings at 7 days post-injury, we did not observe statistically significant differences in microglia response between hypoxemia and normoxemia post-CCI as assessed by Iba1 immunohistochemistry. Assessments of GFAP immunohistochemistry in the ipsilateral white matter did demonstrate increased astrogliosis in CCI + H compared to CCI alone, though whether this increase is a direct response of astrocytes to hypoxia or just a late sequelae or marker of increased axonal injury is unclear.
Our pre-clinical platform is modeled after observations of severe TBI patients in our pediatric intensive care unit.11 In our previous study, the median age of patients experiencing delayed hypoxemia post-TBI was 12 years. We chose 5-week-old adolescent mice to be consistent with our previous clinical and pre-clinical investigations.11 However, our findings may not be generalizable to adult models. Adolescent mice have been observed to have different injury patterns and responses to repetitive TBI.50,51 Although all of our behavioral assessments were performed after the mice had reached full maturity, the long-term differences in response to CCI and delayed hypoxemia of the developing versus fully developed murine brain are unknown. Future studies on adult mice are planned to determine whether there are age-dependent responses to delayed hypoxemia.
Our work has implications for future clinically relevant therapeutic trials. Specifically, delayed hypoxemia resulting in secondary brain hypoxia post-TBI provides a novel target for neuroprotection that is less subject to the barrier of narrow temporal windows of efficacy that has plagued the translation of promising therapeutics from the bench to the bedside in the field of TBI.18,19,51–54 Patients in the intensive care setting could be rapidly administered therapeutics after hypoxemic events. It is even feasible to administer exceptionally safe neuroprotective agents preceding episodes of secondary brain hypoxia in high-risk patients. Our pre-clinical animal model has quantitative deficits in short-term neuropathology and long-term behavior to rapidly eliminate therapeutic candidates unlikely to ultimately succeed and improve the probability of effective translation to success in human studies.
There are limitations in the experimental design when translating our findings to clinical TBI. In our model, mice experience a moderate TBI attributed to limitations with increased mortality rates at higher levels of injury severity,23 whereas the majority of TBI patients in the intensive care setting at risk for delayed hypoxemia are classified as severe TBI.11 Our model of hypoxemia utilizes a 60-min period of moderate hypoxemia. Although we have previously demonstrated with immunohistochemistry that these conditions produce brain hypoxia, we have not directly measured brain tissue oxygen tension in real time as can be done in severe pediatric TBI patients.55 Another limitation to our murine model is the lack of cerebral perfusion monitoring during hypoxemia. It is quite possible that hypotension during hypoxemia could result in decreased cerebral perfusion pressure, leading to ischemia, an additional secondary insult. Future studies in a gyrencephalic model may be able to illustrate the effects of hypoxemia on systemic blood pressure, intracranial pressure, and cerebral perfusion pressure, as well as utilize clinically relevant sedation/anesthetics to more closely model hypoxemia observed in the intensive care setting.56 To control for possible confounding variables in our behavioral testing, we were extremely vigilant in randomization, blinding, and handling all groups uniformly during surgery, hypoxemia, and behavioral assessments as described in the Methods. Our investigations only included males, and future studies to determine whether there are any sex-dependent differences in behavioral response to delayed hypoxemia are planned. We performed only a limited battery of behavioral assessments, which do not cover the full spectrum of possible behavioral deficits. Future studies will include assessments of anxiety or disinhibition with open field exploration and elevated plus-maze, as well as alternative measures of emotional dysregulation such as forced swim test and sucrose preference testing.57–60
In conclusion, mice experiencing CCI followed by clinically relevant delayed hypoxemia were found to have detectable deficits in socialization, learning, and memory with increased lesion volumes and white matter astrogliosis 6 months after initial injury. Our model with long-term behavioral deficits and histological findings provides an attractive TBI platform to assess efficacy of neuroprotective compounds targeted at secondary hypoxemia and improve the successful translation of pre-clinical findings to the bedside.
Acknowledgments
This work was supported by the National Institutes of Health grants K08-NS064051 (to S.H.F.), R01 NS097721 (to S.H.F.), R01 NS065069 (to D.L.B.), and the Department of Pediatrics, Washington University School of Medicine (to S.H.F.)
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Heron M., Sutton P.D., Xu J., Ventura S.J., Strobino D.M., and Guyer B. (2010). Annual summary of vital statistics: 2007. Pediatrics 125, 4–15 [DOI] [PubMed] [Google Scholar]
- 2.Chesnut R.M., Marshall L.F., Klauber M.R., Blunt B.A., Baldwin N., Eisenberg H.M., Jane J.A., Marmarou A., and Foulkes M.A. (1993). The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 34, 216–222 [DOI] [PubMed] [Google Scholar]
- 3.Chi J.H., Knudson M.M., Vassar M.J., McCarthy M.C., Shapiro M.B., Mallet S., Holcroft J.J., Moncrief H., Noble J., Wisner D., Kaups K.L., Bennick L.D., and Manley G.T. (2006). Prehospital hypoxia affects outcome in patients with traumatic brain injury: a prospective multicenter study. J. Trauma 61, 1134–1141 [DOI] [PubMed] [Google Scholar]
- 4.Davis D.P., Dunford J.V., Poste J.C., Ochs M., Holbrook T., Fortlage D., Size M.J., Kennedy F., and Hoyt D.B. (2004). The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J. Trauma 57, 1–8; discussion, 8–10 [DOI] [PubMed] [Google Scholar]
- 5.Davis D.P., Meade W., Sise M.J., Kennedy F., Simon F., Tominaga G., Steele J., and Coimbra R. (2009). Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J. Neurotrauma 26, 2217–2223 [DOI] [PubMed] [Google Scholar]
- 6.Clark R.S., Kochanek P.M., Dixon C.E., Chen M., Marion D.W., Heineman S., DeKosky S.T., and Graham S.H. (1997). Early neuropathologic effects of mild or moderate hypoxemia after controlled cortical impact injury in rats. J. Neurotrauma 14, 179–189 [DOI] [PubMed] [Google Scholar]
- 7.Feng J.F., Zhao X., Gurkoff G.G., Van K.C., Shahlaie K., and Lyeth B.G. (2012). Post-traumatic hypoxia exacerbates neuronal cell death in the hippocampus. J. Neurotrauma 29, 1167–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellewell S.C., Yan E.B., Agyapomaa D.A., Bye N., and Morganti-Kossmann M.C. (2010). Post-traumatic hypoxia exacerbates brain tissue damage: analysis of axonal injury and glial responses. J. Neurotrauma 27, 1997–2010 [DOI] [PubMed] [Google Scholar]
- 9.Huang R.Q., Cheng H.L., Zhao X.D., Dai W., Zhuang Z., Wu Y., Liu Y., and Shi J.X. (2010). Preliminary study on the effect of trauma-induced secondary cellular hypoxia in brain injury. Neurosci. Lett. 473, 22–27 [DOI] [PubMed] [Google Scholar]
- 10.Fang R., Markandaya M., DuBose J.J., Cancio L.C., Shackelford S., and Blackbourne L.H. (2015). Early in-theater management of combat-related traumatic brain injury: a prospective, observational study to identify opportunities for performance improvement. J. Trauma Acute Care Surg. 79, 4 Suppl. 2, S181–S187 [DOI] [PubMed] [Google Scholar]
- 11.Parikh U., Williams M., Jacobs A., Pineda J.A., Brody D.L., and Friess S.H. (2016). Delayed hypoxemia following traumatic brain injury exacerbates white matter injury. J. Neuropathol. Exp. Neurol. 75, 731–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelan-Goodinson R., Ponsford J., Johnston L., and Grant F. (2009). Psychiatric disorders following traumatic brain injury: their nature and frequency. J. Head Trauma Rehab. 24, 324–332 [DOI] [PubMed] [Google Scholar]
- 13.Bryant R.A., O'Donnell M.L., Creamer M., McFarlane A.C., Clark C.R., and Silove D. (2010). The psychiatric sequelae of traumatic injury. Am. J. Psychiatry 167, 312–320 [DOI] [PubMed] [Google Scholar]
- 14.Rabinowitz A.R., and Levin H.S. (2014). Cognitive sequelae of traumatic brain injury. Psychiatr. Clin. North Am. 2014;37:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Arrastia R., Kochanek P.M., Bergold P., Kenney K., Marx C.E., Grimes C.J., Loh L.T., Adam L.T., Oskvig D., Curley K.C., and Salzer W. (2014). Pharmacotherapy of traumatic brain injury: state of the science and the road forward: report of the Department of Defense Neurotrauma Pharmacology Workgroup. J. Neurotrauma 31, 135–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith D.H., Hicks R., and Povlishock J.T. (2013). Therapy development for diffuse axonal injury. J. Neurotrauma 30, 307–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu N.K., Zhang Y.P., Zou J., Verhovshek T., Chen C., Lu Q.B., Walker C.L., Shields C.B., and Xu X.M. (2014). A semicircular controlled cortical impact produces long-term motor and cognitive dysfunction that correlates well with damage to both the sensorimotor cortex and hippocampus. Brain Res. 1576, 18–26 [DOI] [PubMed] [Google Scholar]
- 18.Skolnick B.E., Maas A.I., Narayan R.K., van der Hoop R.G., MacAllister T., Ward J.D., Nelson N.R., and Stocchetti N.; SYNAPSE Trial Investigators. (2014). A clinical trial of progesterone for severe traumatic brain injury. N. Engl. J. Med. 371, 2467–2476 [DOI] [PubMed] [Google Scholar]
- 19.Wright D.W., Yeatts S.D., Silbergleit R., Palesch Y.Y., Hertzberg V.S., Frankel M., Goldstein F.C., Caveney A.F., Howlett-Smith H., Bengelink E.M., Manley G.T., Merck L.H., Janis L.S., and Barsan W.G.; NETT Investigators. (2014). Very early administration of progesterone for acute traumatic brain injury. N. Engl. J. Med. 371, 2457–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannix R., Berglass J., Berkner J., Moleus P., Qiu J.H., Andrews N., Gunner G., Berglass L., Jantzie L.L., Robinson S., and Meehan W.P. (2014). Chronic gliosis and behavioral deficits in mice following repetitive mild traumatic brain injury. J. Neurosurg. 121, 1342–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klemenhagen K.C., O'Brien S.P., and Brody D.L. (2013). Repetitive concussive traumatic brain injury interacts with post-injury foot shock stress to worsen social and depression-like behavior in mice. PLoS One 8, e74510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox G.B., LeVasseur R.A., and Faden A.I. (1999). Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J. Neurotrauma 16, 377–389 [DOI] [PubMed] [Google Scholar]
- 23.Brody D.L., Mac Donald C., Kessens C.C., Yuede C., Parsadanian M., Spinner M., Kim E., Schwetye K.E., Holtzman D.M., and Bayly P.V. (2007). Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J. Neurotrauma 24, 657–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson J.N., Young L.J., Hearn E.F., Matzuk M.M., Insel T.R., and Winslow J.T. (2000). Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288 [DOI] [PubMed] [Google Scholar]
- 25.Steru L., Chermat R., Thierry B., and Simon P. (1985). The Tail Suspension Test—a new method for screening antidepressants in mice. Psychopharmacology 85, 367–370 [DOI] [PubMed] [Google Scholar]
- 26.Huh J.W., and Raghupathi R. (2007). Chronic cognitive deficits and long-term histopathological alterations following contusive brain injury in the immature rat. J. Neurotrauma 24, 1460–1474 [DOI] [PubMed] [Google Scholar]
- 27.Cohen J. (2013). Statistical Power Analysis for the Behavioral Sciences. Academic: New York [Google Scholar]
- 28.Baldwin S.A., Gibson T., Callihan C.T., Sullivan P.G., Palmer E., and Scheff S.W. (1997). Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical disector method for cell counting. J. Neurotrauma 14, 385–398 [DOI] [PubMed] [Google Scholar]
- 29.Hicks R.R., Smith D.H., Lowenstein D.H., Marie R.S., and Mcintosh T.K. (1993). Mild experimental brain injury in the rat induces cognitive deficits associated with regional neuronal loss in the hippocampus. J. Neurotrauma 10, 405–414 [DOI] [PubMed] [Google Scholar]
- 30.Rink A., Fung K.M., Trojanowski J.Q., Lee V.M.Y., Neugebauer E., and Mcintosh T.K. (1995). Evidence of apoptotic cell-death after experimental traumatic brain injury in the rat. Am. J. Pathol. 147, 1575–1583 [PMC free article] [PubMed] [Google Scholar]
- 31.Smith D.H., Okiyama K., Thomas M.J., Claussen B., and McIntosh T.K. (1991). Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J. Neurotrauma 8, 259–269 [DOI] [PubMed] [Google Scholar]
- 32.Morris R.G., Schenk F., Tweedie F., and Jarrard L.E. (1990). Ibotenate lesions of hippocampus and or subiculum—dissociating components of allocentric spatial-learning. Eur. J. Neurosci. 2, 1016–1028 [DOI] [PubMed] [Google Scholar]
- 33.de Sousa A., McDonald S., and Rushby J. (2012). Changes in emotional empathy, affective responsivity, and behavior following severe traumatic brain injury. J. Clin. Exp. Neuropsychol. 34, 606–623 [DOI] [PubMed] [Google Scholar]
- 34.Struchen M.A., Pappadis M.R., Sander A.M., Burrows C.S., and Myszka K.A. (2011). Examining the contribution of social communication abilities and affective/behavioral functioning to social integration outcomes for adults with traumatic brain injury. J. Head Trauma Rehabil. 26, 30–42 [DOI] [PubMed] [Google Scholar]
- 35.Shear D.A., Dixon C.E., Bramlett H.M., Mondello S., Dietrich W.D., Deng-Bryant Y., Schmid K.E., Wang K.K.W., Hayes R.L., Povlishock J.T., Kochanek P.M., and Tortella F.C. (2016). Nicotinamide treatment in traumatic brain injury: Operation Brain Trauma Therapy. J. Neurotrauma 33, 523–537 [DOI] [PubMed] [Google Scholar]
- 36.Dixon C.E., Bramlett H.M., Dietrich W.D., Shear D.A., Yan H.Q., Deng-Bryant Y., Mondello S., Wang K.K.W., Hayes R.L., Empey P.E., Povlishock J.T., Tortella F.C., and Kochanek P.M. (2016). Cyclosporine treatment in traumatic brain injury: Operation Brain Trauma Therapy. J. Neurotrauma 33, 553–566 [DOI] [PubMed] [Google Scholar]
- 37.Bramlett H.M., Dietrich W.D., Dixon C.E., Shear D.A., Schmid K.E., Mondello S., Wang K.K.W., Hayes R.L., Povlishock J.T., Tortella F.C., and Kochanek P.M. (2016). Erythropoietin treatment in traumatic brain injury: Operation Brain Trauma Therapy. J. Neurotrauma 33, 538–552 [DOI] [PubMed] [Google Scholar]
- 38.Browning M., Yan H.Q., Poloyac S., Dixon C.E., Empey P.E., Jackson T.K., Brockman E., Ma X., Janesko-Feldman K., Henchir J., Vagni V., and Kochanek P.M. (2014). Benefits of early administration of levetiracetam after controlled cortical impact in rats: Operation Brain Trauma Therapy. J. Neurotrauma 31, A108–A108 [Google Scholar]
- 39.Mondello S., Bramlett H.M., Dixon C.E., Shear D.A., Schmid K.E., Dietrich W.D., Wang K.K.W., Hayes R.L., Tortella F.C., and Kochanek P.M. (2013). Characterization of TBI models and evaluation of the therapeutic efficacy of nicotinamide, erythropoietin and cyclosporine a using biochemical markers of brain injury: results from Operation Brain Trauma Therapy. J. Neurotrauma 30, A167–A168 [Google Scholar]
- 40.Yan H.Q., Kochanek P.M., Mondello S., Ma X.C., Henchir J., Xu M., Janesko-Feldman K., Wang K.K.W., Hayes R., and Dixon C.E. (2012). Effect of nicotinamide on behavioral, neuropathological, and biomarker outcomes after controlled cortical impact in rats: an Operation Brain Trauma Therapy Consortium study. J. Neurotrauma 29, A58–A58 [Google Scholar]
- 41.Cryan J.F., Mombereau C., and Vassout A. (2005). The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 29, 571–625 [DOI] [PubMed] [Google Scholar]
- 42.Bajwa N.M., Halavi S., Hamer M., Semple B.D., Noble-Haeusslein L.J., Baghchechi M., Hiroto A., Hartman R.E., and Obenaus A. (2016). Mild concussion, but not moderate traumatic brain injury, is associated with long-term depression-like phenotype in mice. PLoS One 11, e0146886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nollet M., Gaillard P., Minier F., Tanti A., Belzung C., and Leman S. (2011). Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology 61, 336–346 [DOI] [PubMed] [Google Scholar]
- 44.Muthuswamy J., Kimura T., Ding M.C., Geocadin R., Hanley D.F., and Thakor N.V. (2002). Vulnerability of the thalamic somatosensory pathway after prolonged global hypoxic-ischemic injury. Neuroscience 115, 917–929 [DOI] [PubMed] [Google Scholar]
- 45.Shoykhet M., Simons D.J., Alexander H., Hosler C., Kochanek P.M., and Clark R.S.B. (2012). Thalamocortical dysfunction and thalamic injury after asphyxial cardiac arrest in developing rats. J. Neurosci. 32, 4972–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semple B.D., Canchola S.A., and Noble-Haeusslein L.J. (2012). Deficits in social behavior emerge during development after pediatric traumatic brain injury in mice. J. Neurotrauma 29, 2672–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friess S.H., Lapidus J.B., and Brody D.L. (2015). Decompressive craniectomy reduces white matter injury after controlled cortical impact in mice. J. Neurotrauma 32, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagamoto-Combs K., Mcneal D.W., Morecraft R.J., and Combs C.K. (2007). Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury (vol 24, pg 1719, 2007). J. Neurotrauma 24, 1889–1889 [DOI] [PubMed] [Google Scholar]
- 49.Smith C., Gentleman S.M., Leclercq P.D., Murray L.S., Griffin W.S.T., Graham D.I., and Nicoll J.A.R. (2013). The neuroinflammatory response in humans after traumatic brain injury. Neuropathol. Appl. Neurobiol. 39, 654–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semple B.D., Sadjadi R., Carlson J., Chen Y.R., Xu D., Ferriero D.M., and Noble-Haeusslein L.J. (2016). Long-term anesthetic-dependent hypoactivity after repetitive mild traumatic brain injuries in adolescent mice. Dev. Neurosci. 38, 220–238 [DOI] [PubMed] [Google Scholar]
- 51.Mannix R., Berkner J., Mei Z.R., Alcon S., Hashim J., Robinson S., Jantzie L., Meehan W.P., and Qiu J.H. (2017). Adolescent mice demonstrate a distinct pattern of injury after repetitive mild traumatic brain injury. J. Neurotrauma 34, 495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adelson P.D., Wisniewski S.R., Beca J., Brown S.D., Bell M., Muizelaar J.P., Okada P., Beers S.R., Balasubramani G.K., and Hirtz D.; Paediatric Traumatic Brain Injury Consortium. (2013). Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. Lancet Neurol. 12, 546–553 [DOI] [PubMed] [Google Scholar]
- 53.Hutchison J.S., Ward R.E., Lacroix J., Hebert P.C., Barnes M.A., Bohn D.J., Dirks P.B., Doucette S., Fergusson D., Gottesman R., Joffe A.R., Kirpalani H.M., Meyer P.G., Morris K.P., Moher D., Singh R.N., and Skippen P.W.; Hypothermia Pediatric Head Injury Trial Investigators and the Canadian Critical Care Trials Group. (2008). Hypothermia therapy after traumatic brain injury in children. N. Engl. J. Med. 358, 2447–2456 [DOI] [PubMed] [Google Scholar]
- 54.Arango M.F. and Bainbridge D. (2008). Magnesium for acute traumatic brain injury. Cochrane Database Syst. Rev. (4), CD005400. [DOI] [PubMed] [Google Scholar]
- 55.Stiefel M.F., Udoetuk J.D., Storm P.B., Sutton L.N., Kim H., Dominguez T.E., Helfaer M.A., and Huh J.W. (2006). Brain tissue oxygen monitoring in pediatric patients with severe traumatic brain injury. J. Neurosurg. 105, 281–286 [DOI] [PubMed] [Google Scholar]
- 56.Bruins B., Kilbaugh T.J., Margulies S.S., and Friess S.H. (2013). The anesthetic effects on vasopressor modulation of cerebral blood flow in an immature swine model. Anesth. Analg. 116, 838–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Handley S.L., and Mithani S. (1984). Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of fear-motivated behavior. Naunyn Schmiedebergs Arch. Pharmacol. 327, 1–5 [DOI] [PubMed] [Google Scholar]
- 58.Crawley J., and Goodwin F.K. (1980). Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol. Biochem. Behav. 13, 167–170 [DOI] [PubMed] [Google Scholar]
- 59.Porsolt R.D., Le Pichon M., and Jalfre M. (1977). Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732 [DOI] [PubMed] [Google Scholar]
- 60.Willner P., Towell A., Sampson D., Sophokleous S., and Muscat R. (1987). Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology 93, 358–364 [DOI] [PubMed] [Google Scholar]