Abstract
Background: The chronic use of antipsychotics has been associated with impaired bone mineralization, partially mediated by hyperprolactinemia. We examined if calcium and vitamin D supplementation promote bone mineral accrual in boys with risperidone-induced hyperprolactinemia.
Methods: Between February 2009 and November 2013, medically healthy, 5- to 17-year-old boys were enrolled in a 36-week double-blind, placebo-controlled study, examining the skeletal effects of supplementation with 1250 mg calcium carbonate and 400 IU of vitamin D3 in risperidone-induced hyperprolactinemia. Anthropometric, dietary, physical activity, and psychiatric assessments were conducted at baseline and week 18 and 36. Plasma prolactin and vitamin D concentrations were measured at baseline and week 36. Total body less head bone mineral content (BMC) and radius trabecular bone mineral density (BMD) were measured at baseline, week 18, and week 36, using dual-energy X-ray absorptiometry and peripheral quantitative computed tomography, respectively. Linear mixed-effects regression analysis examined the longitudinal effect of treatment on skeletal outcomes.
Results: Forty-seven boys (mean age: 11.0 ± 2.6 years) were randomized and 38 completed the study. At study entry, the average dietary calcium intake was below the recommended limit, but the average vitamin D concentration was normal. Calcium and vitamin D supplementation failed to significantly increase BMC or trabecular BMD. It also failed to affect several other skeletal and anthropometric outcomes, including plasma vitamin D concentration.
Conclusions: In this 9-month long pilot study, supplementation with a modest dose of calcium and vitamin D did not increase bone mass accrual in risperidone-treated boys with hyperprolactinemia. Alternative approaches should be investigated to optimize bone health in this population to prevent future morbidity and premature mortality. ClinicalTrials.gov Identifier: NCT00799383.
Keywords: : hyperprolactinemia, calcium, vitamin D, risperidone, children, bone mass
Introduction
Hyperprolactinemia often emerges at the onset of antipsychotic treatment, particularly for agents with potent dopamine D2 receptor antagonist activity (Peuskens et al. 2014). Following long-term use, prolactin concentration decreases, but it remains elevated in a substantial minority of patients (Findling et al. 2003; Calarge et al. 2009; Peuskens et al. 2014). This is especially true for risperidone, one of the second-generation antipsychotics most likely to induce hyperprolactinemia (Findling et al. 2003; Calarge et al. 2009).
Hyperprolactinemia may disrupt gonadotropin-releasing hormone pulsatility, impairing the release of luteinizing hormone and follicle-stimulating hormone, causing amenorrhea in females (Shibli-Rahhal and Schlechte 2009). This, in turn, can impair bone mineralization, potentially resulting in osteopenia and osteoporosis (Shibli-Rahhal and Schlechte 2009). In addition to the morbidity associated with osteoporotic fractures, mortality is increased. This is particularly relevant to patients with serious mental illness given that their life expectancy is shorter by an estimated 10–15 years (Jayatilleke et al. 2017). It suggests that efforts should be geared toward preventive measures to promote healthier aging.
In children and adolescents, bone mass rapidly accrues, especially during and after the growth spurt (Rauch and Schoenau 2001). A number of studies have shown that short- and long-term treatment with risperidone causes hyperprolactinemia in this age group (Findling et al. 2003; Calarge et al. 2009). We have also shown, in a cross-sectional study in children and adolescents, that prolactin concentration was inversely associated with trabecular bone mineral density (BMD) at the ultradistal radius (Calarge et al. 2010). However, the extent to which hyperprolactinemia might affect bone mineralization in children and adolescents remains unclear given then, in a prospective study, risperidone treatment was associated with failure to accrue bone mass, independent of prolactin concentration (Calarge et al. 2015a). This could reflect a pharmacological effect of the drug either directly on the bone or indirectly, through central pathways, perhaps involving the autonomic nervous system (Calarge et al. 2013).
To determine whether the skeletal effects of risperidone could be countered or reversed, we conducted a pilot study with calcium and vitamin D3 (Ca+VitD) supplementation. Given that antipsychotics are less often prescribed to girls and the prevailing view, at the time the study was designed, was that hyperprolactinemia mediated the skeletal effects of antipsychotics, we restricted enrollment to risperidone-treated males with hyperprolactinemia. We hypothesized that Ca+VitD supplementation would promote a larger increase in whole-body bone mineral content (BMC) and trabecular BMD at the ultradistal radius compared to placebo.
Methods
Participants: Between January 2009 and November 2013, medically healthy 5- to 17-year-old risperidone-treated boys were enrolled, through screening of the electronic medical records, referrals, and word of mouth. They were required to have been in treatment with risperidone for at least 1 year to reduce the risk of treatment discontinuation and ensure that hyperprolactinemia would be chronic. Hyperprolactinemia was defined as a prolactin concentration ≥18.4 ng/mL, confirmed on two occasions, within 1 week. Polypharmacy was allowed, but concurrent treatment with antipsychotics other than risperidone led to exclusion. Patients with chronic disorders involving a vital organ, metabolic diseases (e.g., growth hormone deficiency), skeletal diseases (e.g., Paget's disease), chronic use of drugs affecting bone metabolism (e.g., corticosteroids), a fasting random urine calcium/creatinine ratio >0.2 or a history of nephrolithiasis, malnutrition (e.g., due to chronic diarrhea and inflammatory bowel disease), congenital disorders, lead poisoning, bilateral wrist or forearm fractures, or eating disorders were excluded. In addition, participants could not have been receiving calcium or vitamin D supplementation, or multivitamins in the prior 3 months and they could not have any other medical condition that contraindicates the use of calcium or vitamin D. Participants with moderate to profound intellectual disability, with medication nonadherence (as evidenced by an undetectable combined risperidone and 9-hydroxy risperidone blood concentration), and those unable to cooperate with the bone density scans or with plans to move out of state within the following 9 months also could not enroll.
The study was approved by the local institutional review board. After study description, written consent from parents or legal guardians and assent from the participants were obtained.
Study design
The study included three phases: Phase 1 consisted of a baseline screening phase to confirm inclusion and exclusion criteria and measure prolactin concentration. If prolactin was ≥18.4 ng/mL, the participants returned for Phase 2, within a week of completing the screening phase. At that point, eligibility was reconfirmed and a second prolactin level was obtained. If, again, it was ≥18.4 ng/mL, participants were randomized to a 9-month period of supplementation with a total daily dose of 1250 mg calcium carbonate (equivalent to 500 mg of elemental calcium) along with 400 IU of vitamin D3 (cholecalciferol) or to placebo. These doses were chosen to ensure that the intake of these micronutrients met the reference daily intake (Trumbo et al. 2002). Ca+VitD and placebo were packaged in identical capsules by a compounding pharmacy, administered as one capsule twice daily. Participants were randomized 1:1, following a computer-generated sequence, and balanced by pubertal status (i.e., Tanner stage 1 vs. 2 to 5). Medication adherence was monitored by pill count. Two weeks postrandomization, participants returned for an in-person visit to measure, among other things, urinary calcium/creatinine ratio. If the ratio was >0.2, the participants were removed from the study due to concern about developing nephrolithiasis. Participants were contacted by phone at weeks 6, 12, 24, and 30, at which time their medical and psychiatric history were reviewed and adherence to study protocol was assessed. Query about adverse events were made. Participants returned for an in-person visit at weeks 18 and 36. Phase 3: at the completion of Phase 2, participants assigned to the placebo arm were offered a 9-month open treatment with Ca+VitD.
Procedures
At study entry, participants underwent a physical examination. Vital signs and anthropometric measures were obtained following standard procedures at every in-person visit (Calarge et al. 2009). The medical and pharmacy records were reviewed to document all psychotropic treatments, including the start and stop date of each drug as well as its dosage.
A best-estimate diagnosis, following the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR; American Psychiatric Association 2000), was generated based on a review of the psychiatric record, supplemented by a standardized interview of the parent using the Diagnostic Interview Schedule for Children (Shaffer et al. 2000), the Child Behavior Checklist (Achenbach and Rescorla 2001), and a clinical interview conducted by a child psychiatrist (C.A.C.).
The 2004 Block Kids Food Frequency Questionnaire was used to estimate daily calcium and vitamin D intake during the prior week (Block et al. 2000). Physical activity was assessed by asking the parent to compare the child's usual level of physical activity to their peers', using a 5-point Likert scale (Slemenda et al. 1991). Psychiatric symptom severity was assessed using the Nisonger Child Behavior Rating Form (Aman et al. 1996) and adverse events were assessed using the UKU side effects rating scale (Lingjaerde et al. 1987).
Following the protocol described previously (Calarge et al. 2015a; Calarge and Schlechte 2017), a peripheral quantitative computed tomography (pQCT) scan was obtained at the 4% and 20% sites of the nondominant radius to estimate trabecular and cortical BMD, respectively. A Stratec XCT-2000 scanner, software version 6.0 (Stratec, Inc., Pforzheim, Germany), was used. Trabecular BMD was measured as the mean density of the 85% central area of the bone's cross-section. pQCT scans with moderate to severe movement were rejected. In addition, a Hologic QDR DELPHI-4500A DXA unit (Hologic, Inc., Bedford, MA) or a Hologic Discovery A unit was used to estimate total body less head (TBLH) BMC. The Hologic software (APEX 4.0.1/13.4.1) determined total body lean and fat mass. The package also includes an automated algorithm to estimate visceral adipose tissue mass (VFat, grams) (Micklesfield et al. 2012). For some scans, manual adjustment of the regions of interest was necessary, as recommended by the manufacturer. The two DXA units were cross-calibrated and quality control and calibration of the equipment were performed daily. The bone density scans were obtained at baseline, week 18, and week 36.
At baseline and week 36, a morning, fasting blood sample was obtained to measure plasma prolactin and 25‐OH‐vitamin D by electrochemiluminescence immunoassay (Abbott, Wiesbaden, Germany).
Data analysis
Body mass index (BMI) was computed as weight/height2 (kg/m2), lean body mass index (LBMI) as lean mass/height2 (kg/m2), and fat mass index (FMI) as fat mass/height2 (kg/m2), and age- and sex-specific Z-scores were generated (Ogden et al. 2002; Weber et al. 2013). Age-sex-height-race-specific Z-scores for TBLH BMC were generated following the bone mineral density in childhood study (Zemel et al. 2011).
Differences between participants in the two treatment arms were compared using the Student's t-test or Wilcoxon rank-sum test for continuous variables and Chi-square or Fisher's exact test for categorical ones.
Linear mixed-effect regression examined the change, over time, in the outcomes of interest as a function of the treatment arms (i.e., time × treatment arm interaction effect) (Verbeke and Molenberghs 2000). All models included adjustment for age (years) at study entry and level of physical activity. Height (cm) was also included in the VFat analysis to account for differences in body size. Participant-specific random intercepts and slopes were used with an unstructured covariance matrix. Duration of study participation was the time metric in the analysis. Maximum likelihood methods were used for estimation, which yield unbiased estimates under the assumption that the missing data mechanism is ignorable (Little and Rubin 2002).
All hypothesis tests were two tailed with a significance level of p < 0.05 and analyses utilized procedures from SAS version 9.4 for Windows (SAS Institute, Inc., Cary, NC).
Results
Patient disposition
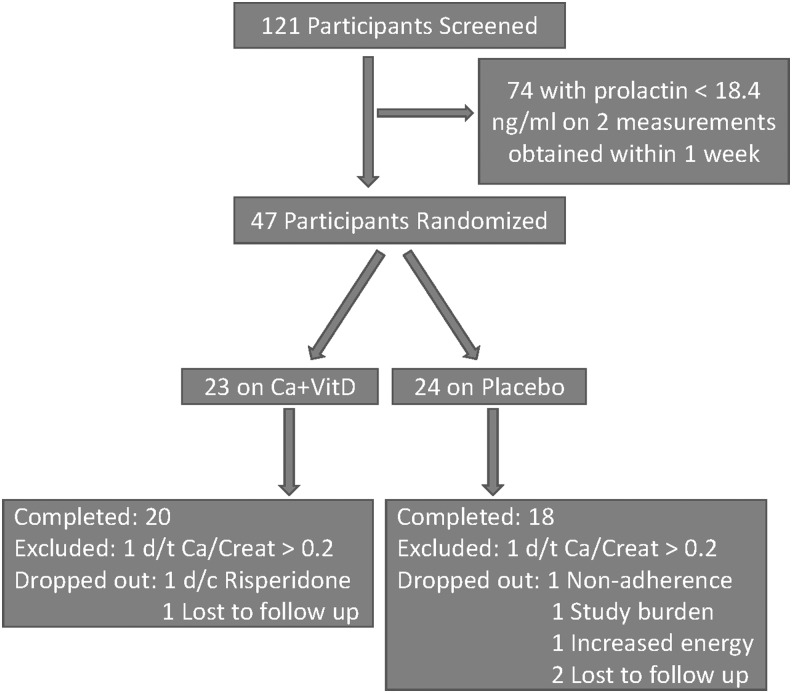
Of 121 risperidone-treated boys screened, 47 were randomized (Fig. 1). There were no significant differences in age or duration of risperidone treatment between participants who were randomized compared to those who were excluded for not having hyperprolactinemia. However, randomized participants were receiving a higher daily dose of risperidone (2.2 ± 1.8 vs. 1.4 ± 1.0, p = 0.0001) and, by design, had higher prolactin concentration (31.6 ± 12.7 vs. 15.6 ± 9.5, p < 0.0001). With regard to DSM-IV-TR disorders, the two groups were comparable except for anxiety disorders, which were more prevalent in the randomized participants (51.1% vs. 29%, p < 0.02).
FIG. 1.
Consolidated standards of reporting trials diagram showing participant allocation and attrition. Ca+VitD, calcium and vitamin D3; d/c, discontinued; d/t, due to.
Compared to those randomized to placebo, participants randomized to Ca+VitD had more adipose tissue, a higher likelihood of having a tic disorder, and higher daily intake of vitamin D at study entry (Table 1). However, plasma vitamin D concentration did not differ between the two groups.
Table 1.
Demographic and Clinical Characteristics of the Participants (Mean ± Standard Deviation, Unless Noted Otherwise)
| Placebo, n = 24 | Ca+VitD, n = 23 | p | |
|---|---|---|---|
| Age, years | 12.1 ± 3.0 | 10.8 ± 3.0 | >0.10 |
| Tanner stage (%) | |||
| I/II/III/IV/V | 48/13/0/22/17 | 43/22/9/13/13 | >0.60 |
| Race/ethnicity, n (%) | >0.10 | ||
| White | 19 (79) | 19 (86) | |
| African American | 5 (21) | 1 (5) | |
| Hispanic | 0 | 2 (9) | |
| BMI Z-score | 0.25 ± 1.05 | 0.89 ± 0.81 | <0.04 |
| Physical activity | 2.5 ± 1.1 | 2.6 ± 1.4 | >0.60 |
| Daily calcium intake, mg | |||
| At study entry | 888 ± 326 | 1071 ± 367 | <0.06 |
| At study completion | 970 ± 409 | 1000 ± 496 | >0.20 |
| Daily vitamin D intake, IU | |||
| At study entry | 196 ± 85 | 237 ± 106 | <0.03 |
| At study completion | 205 ± 107 | 204 ± 87 | >0.80 |
| Vitamin D concentration, ng/mL | |||
| At study entry | 31.2 ± 11.6 | 31.9 ± 11.9 | >0.80 |
| At study completion | 31.2 ± 35.5 | 35.5 ± 18.0 | >0.70 |
| Vitamin D status, n (%) | >0.80 | ||
| Normal, ≥30 ng/mL | 13 (54) | 13 (57) | |
| Borderline, 20–30 ng/mL | 7 (29) | 5 (22) | |
| Deficient, <20 ng/mL | 4 (17) | 5 (22) | |
| Prolactin, ng/mL | |||
| At study entry | 30.1 ± 9.0 | 33.0 ± 15.4 | >0.80 |
| At study completion | 30.3 ± 16.3 | 30.0 ± 19.1 | >0.90 |
| Psychiatric characteristics, n (%) | |||
| ADHD | 23 (96) | 22 (96) | 1.00 |
| Disruptive behavior disorder | 23 (96) | 22 (96) | 1.00 |
| Anxiety disorder | 10 (42) | 14 (61) | >0.10 |
| Autism spectrum disorder | 4 (17) | 6 (26) | >0.40 |
| Tic disorder | 0 | 4 (17) | <0.05 |
| Psychostimulant use | 19 (79) | 17 (74) | >0.60 |
| SSRIs | 9 (38) | 12 (52) | >0.30 |
| Daily risperidone dose, mg | |||
| At study entry | 2.1 ± 1.0 | 2.1 ± 1.3 | >0.70 |
| At study completion | 2.3 ± 1.4 | 2.5 ± 1.6 | >0.70 |
| Risperidone treatment duration, years | 3.8 ± 2.1 | 2.7 ± 2.0 | <0.05 |
Significant results (p < 0.05) are bolded and marginally significant results (p < 0.10) are bolded and italicized.
ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index; Ca+VitD, calcium and vitamin D3; SSRIs, selective serotonin reuptake inhibitors.
At week 2 postrandomization, two participants (one on placebo) were removed from the study due to a urinary calcium/creatinine ratio >0.2 and another seven participants dropped out of the study prematurely (Fig. 1). Adherence to the assigned treatment was comparable across the two groups (85.9 ± 15.1 vs. 85.6 ± 17.2, p > 0.70).
Psychiatric symptoms and tolerability
No significant differences in the severity of psychiatric symptoms were evident at study entry or by study end.
The UKU side effects rating scale queries about 48 symptoms. We added two, one about bloating and one about stomach aches, given that these adverse events have been reported following the use of calcium or vitamin D. Of these symptoms, only one, psychic dependence, significantly worsened during the course of the study in participants receiving Ca+VitD compared to placebo (47% vs. 13%, p < 0.04). There was a trend for the symptom related to physical dependence to also have worsened more in the Ca+VitD group (42% vs. 12%, p < 0.07). In particular, nausea/vomiting, stomach ache, bloating, diarrhea, and constipation were equally likely to occur in one group versus the other (all p-values >0.20).
Primary outcomes
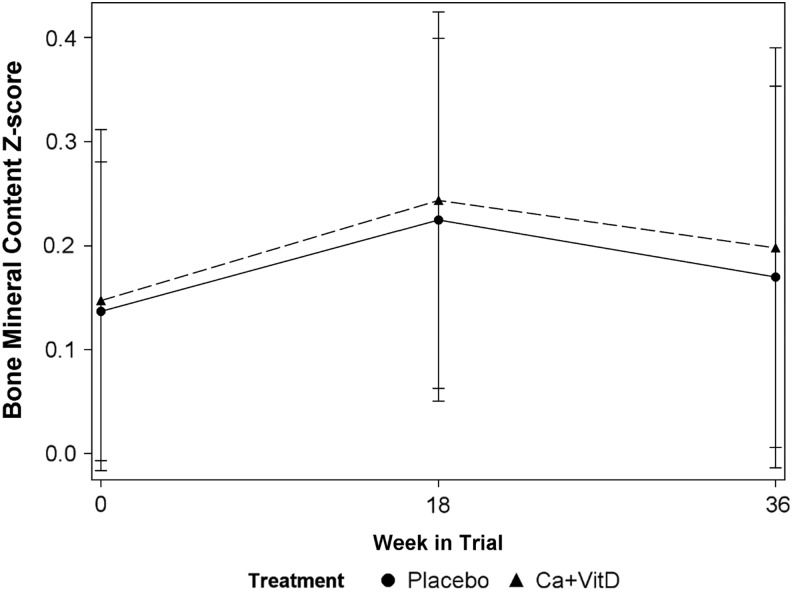
Table 2 summarizes the baseline skeletal and anthropometric characteristics of the participants. After adjusting for age at study entry (p > 0.30), physical activity (β = 0.23 ± 0.09, p < 0.02), and LBMI Z-score (β = 0.15 ± 0.07, p < 0.05), the effect of treatment assignment over time on TBLH BMC Z-score was not significant [time (in month) × treatment assignment interaction effect: β = 0.0062 ± 0.0807, p > 0.90; Fig. 2]. The effect size of the change in TBLH BCM Z-score was quite small (Cohen's d = 0.14). Similarly, after adjusting for age at study entry (p > 0.10), physical activity (p > 0.10), and forearm length (p > 0.90), the effect of treatment assignment on trabecular BMD was not significant (interaction effect: β = −4.8 ± 5.6, p > 0.30).
Table 2.
Baseline Skeletal and Anthropometric Measures (Unadjusted) Split Based on Treatment Assignment (Mean ± Standard Deviation)
| Placebo, n = 24 | Ca+VitD, n = 23 | p | |
|---|---|---|---|
| Dual-energy X-ray absorptiometry-based measures | |||
| TBLH BMC | 1259 ± 648 | 1035 ± 483 | >0.20 |
| TBLH BMC Z-score | 0.12 ± 0.68 | 0.10 ± 0.79 | >0.80 |
| FMI Z-score | 0.28 ± 0.77 | 0.79 ± 0.64 | <0.04 |
| Lean mass index Z-score | −0.79 ± 0.88 | −0.53 ± 0.79 | >0.30 |
| Visceral fat mass, g | 219 ± 103 | 242 ± 93 | >0.20 |
| pQCT-based measures | |||
| Trabecular bone mineral density, mg/cm3 | 197.2 ± 43.9 | 192.9 ± 31.1 | >0.90 |
| Strength index, mg2/mm4 | 25.3 ± 15.9 | 19.8 ± 8.1 | >0.50 |
| Cortical bone mineral density, mg/cm3 | 1063.2 ± 30.6 | 1059.6 ± 34.8 | >0.90 |
| Cortical thickness, mm | 2.3 ± 0.5 | 2.2 ± 0.3 | >0.90 |
| Periosteal circumference, mm | 32.4 ± 5.4 | 30.1 ± 4.3 | >0.20 |
| Endosteal circumference, mm | 17.7 ± 3.6 | 16.0 ± 3.4 | >0.10 |
| Polar section modulus, mm3 | 156.2 ± 76.6 | 123.8 ± 53.6 | >0.20 |
At the 4% radius site, 21 placebo and 22 Ca+VitD participants had useable pQCT data, while at the 20% radius site, 20 and 18 participants, respectively, had useable data. Age-sex-height-race-specific Z-scores for TBLH BMC were generated following the bone mineral density in childhood study. Significant results (p < 0.05) are bolded.
BMC, bone mineral content; BMI, body mass index; Ca+VitD, calcium and vitamin D3; FMI, fat mass index; pQCT, peripheral quantitative computed tomography; TBLH, total body less head.
FIG. 2.
Change in sex-age-height-race-specific total body less head bone mineral content Z-score over the study course, across calcium and vitamin D treatment arm versus placebo. Ca+VitD, calcium and vitamin D3.
Secondary outcomes
Change over time in bone strength index, cortical BMD, cortical thickness, periosteal and endosteal circumference, and polar section modulus as a result of the intervention was also not significant, after adjusting for the relevant variables (all time × treatment assignment interaction effect p-values >0.10).
Supplementation with calcium and vitamin D was not associated either with an increase in daily intake of calcium or vitamin D (all time × treatment interaction effect p-values >0.20). It also failed to increase vitamin D plasma concentration, even after accounting for treatment adherence (p > 0.40). We also could not find an effect of the intervention on BMI, FMI, or LBMI Z-scores or on visceral fat mass (p > 0.10).
Discussion
Concerns have been raised about the detrimental effects of long-term risperidone treatment on bone mass (Calarge et al. 2013, 2015a). Thus, in this pilot study, we aimed to examine whether calcium and vitamin D would promote bone mineralization in boys with risperidone-induced hyperprolactinemia. We failed to find a significant effect.
Bone mass appears to be impaired in several psychiatric conditions, including major depressive disorder, schizophrenia, and autism spectrum disorder (Wu et al. 2010; Calarge et al. 2014; Chen et al. 2016; Calarge and Schlechte 2017; Neumeyer et al. 2017). This could heighten the risk for osteoporosis with its known morbidity and mortality, and increased medical expenditure. A number of mechanisms have been proposed, such as hypercortisolemia, related to overactivity of the hypothalamic-pituitary-adrenal axis, subclinical inflammation, and activation of the sympathetic nervous system. Lifestyle factors have also been implicated, including smoking, alcohol use disorder, physical inactivity, and unhealthy dietary intake (Weaver et al. 2016). In addition, psychotropics are thought to contribute both directly as well as indirectly (Warden et al. 2010; Calarge et al. 2013). In fact, many medications can affect serotoninergic signaling with the potential to alter bone metabolism through both peripheral and central pathways (Ortuno et al. 2016). Moreover, antipsychotics, in particular, promote prolactin secretion, given their antidopaminergic activity. This, in turn, can cause hypogonadism and reduce bone mass. These medications can also induce sedation and apathy, further impeding physical activity. In fact, we have reported elsewhere that both risperidone-induced hyperprolactinemia and the use of selective serotonin reuptake inhibitors (SSRIs) are associated with lower bone mass in a group of children and adolescents very comparable to the current participants (Calarge et al. 2010). In contrast, psychostimulants were not (Calarge et al. 2015b). However, over time, SSRIs appeared not to lead to further decline in bone mass, while risperidone did, independent of prolactin concentration (Calarge et al. 2015a). Of interest, in a more recent longitudinal study in older adolescents treated only with SSRIs (without antipsychotics), SSRI treatment in males was associated with a reduction in BMC at the lumbar spine, but not in TBLH BMD (Calarge et al. 2017).
The skeletal effects of psychopathology and psychotropics are especially important to address in children and adolescents, given that this period of development is critical for bone mass accrual, that peak bone mass is a major predictor of bone mass and osteoporosis risk later in life, and that failure to accrue bone mass early in life cannot be compensated for later on (NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy 2001). As a result, we aimed to optimize bone mass with a relatively benign intervention. The failure to find an effect, despite good adherence to the assigned treatment, may reflect one of several possibilities. First, the dose of calcium and vitamin D chosen may have been too low. In our previous work in a comparable clinical sample, we had found that the dietary intake of calcium (∼1040 mg/day) was below the recommended intake of 1300 mg/day, but vitamin D intake was adequate (∼270 IU consumed daily, compared to the recommended 200 IU/day) (Trumbo et al. 2002). Thus, the doses of calcium carbonate and vitamin D we provided were meant to bring calcium intake slightly above the recommended level and ensure that every participant will be receiving a sufficient dose of vitamin D. This was indicated given that the study was conducted in northern United States, where exposure to the sun is limited during the cold months, potentially impacting vitamin D concentration (Rosecrans and Dohnal 2014). Second, it may be that the doses we used, although bringing intake up to the recommended level, may have still been insufficient to counteract the deleterious effect of psychopathology and/or psychotropics. Alternatively, it is possible that the 9-month duration of the trial was insufficient to observe an impact. However, calcium supplementation studies have shown that the largest increase in bone mass occurs within the first 6 months of the intervention (Winzenberg et al. 2006). Finally, it may be that the pathophysiological processes impairing bone mineralization in this case are unrelated to and not influenced by calcium or vitamin D, particularly when their intake is not significantly deficient to begin with. All these hypotheses should be explored in future studies. Until then, care should be taken to not overprescribe vitamin D supplements, in light of recent evidence linking excessive use (e.g., single annual dose of 500,000 IU of cholecalciferol) to increased fracture risk (Sanders et al. 2010).
This study suffers from several limitations. First, the majority of the participants had normal vitamin D concentration. This is surprising, given that the study was conducted in the upper Midwest, perhaps reflecting unmeasured characteristics of the study population (e.g., interest in this type of research or extreme hyperactivity leading to increased involvement in outdoor activities and sun exposure). At any rate, it is not clear whether the intervention would have proven more effective had it been restricted to those with vitamin D deficiency or had we used a higher dose of calcium and vitamin D. In addition, the sample size was quite limited in this pilot study, making it underpowered to meet its primary aims. However, the difference between the two treatment arms in bone mineral accrual over the course of the study was negligible (Fig. 2) and the effect size was small. We divided the group (and balanced randomization) based on whether the participant started pubertal development or not (Winzenberg et al. 2006). Whether restricting the trial to participants within a narrower range of pubertal stage would have yielded different results is unknown. Another limitation is that we only supplemented calcium and vitamin D. However, other micronutrients (e.g., vitamin E, phosphorus, and magnesium) are necessary for normal bone mineralization. Finally, we excluded females given that antipsychotics are more often prescribed to males and given the substantial sex differences in bone mineral accretion. To what extent our findings might extend to females requires independent investigation. Overall, future studies seeking to investigate whether supplementation optimizes bone mineralization in antipsychotic-treated patients should consider stratifying by the nutritional status of the micronutrient of interest (i.e., deficient vs. not), using doses larger than the reference daily intake, and including more participants with a diverse sex and racial/ethnic composition.
Conclusions
In sum, in this 9-month long pilot study, supplementation with a modest dose of calcium and vitamin D did not significantly increase bone mass accrual in risperidone-treated boys with hyperprolactinemia.
Clinical Significance
Given the potential effect of osteoporosis on morbidity and mortality, future research should continue to disentangle the effect of psychopathology from that of psychotropics on bone mineralization in children and adolescents. Moreover, alternative approaches should be investigated to optimize bone health in this population to prevent further morbidity and premature mortality.
Acknowledgments
This study was funded by the National Institutes of Health (RR024979 and K23MH085005). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agency. The authors thank the families and the staff in the University of Iowa Clinical Research Unit as well as the research team members.
Disclosures
No competing financial interests exist.
References
- Achenbach TM, Rescorla LA: Manual for the ASEBA School-Age Froms & Profiles. Burlington (Vermont), Research Center for Children, Youth & Families, 2001 [Google Scholar]
- Aman MG, Tasse MJ, Rojahn J, Hammer D: The Nisonger CBRF: A child behavior rating form for children with developmental disabilities. Res Dev Disabil 17:41–57, 1996 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision. Washington, DC: American Psychiatric Association; 2000 [Google Scholar]
- Block G, Murphy M, Roullet JB, Wakimoto P, Crawford PB, Block T: Pilot validation of a FFQ for children 8–10 years. Tucson (AZ), Fourth International Conference on Dietary Assessment Methods, 2000. (abstract) [Google Scholar]
- Calarge CA, Burns TL, Schlechte JA, Zemel BS: Longitudinal examination of the skeletal effects of selective serotonin reuptake inhibitors and risperidone in boys. J Clin Psychiatry 76:607–613, 2015a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Butcher BD, Burns TL, Coryell WH, Schlechte JA, Zemel BS: Major depressive disorder and bone mass in adolescents and young adults. J Bone Miner Res 29:2230–2237, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Ellingrod VL, Acion L, Miller DD, Moline J, Tansey MJ, Schlechte JA: Variants of the dopamine D2 receptor gene and risperidone-induced hyperprolactinemia in children and adolescents. Pharmacogenet Genomics 19:373–382, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Ivins SD, Motyl KJ, Shibli-Rahhal AA, Bliziotes MM, Schlechte JA: Possible mechanisms for the skeletal effects of antipsychotics in children and adolescents. Ther Adv Psychopharmacol 3:278–293, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Mills JA, Janz KF, Burns TL, Schlechte JA, Coryell WH, Zemel BS: The effect of depression, generalized anxiety, and selective serotonin reuptake inhibitors on change in bone metabolism in adolescents and emerging adults. J Bone Miner Res 2017. [E-pub ahead of print]; DOI: 10.1002/jbmr.3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Schlechte JA: Bone mass in boys with autism spectrum disorder. J Autism Dev Disord 47:1749–1755, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Schlechte JA, Burns TL, Zemel BS: The effect of psychostimulants on skeletal health in boys co-treated with risperidone. J Pediatr 166:1449.e1–1454.e1, 2015b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Zimmerman B, Xie D, Kuperman S, Schlechte JA: A cross-sectional evaluation of the effect of risperidone and selective serotonin reuptake inhibitors on bone mineral density in boys. J Clin Psychiatry 71:338–347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Lane HY, Lin CH: Effects of antipsychotics on bone mineral density in patients with schizophrenia: Gender differences. Clin Psychopharmacol Neurosci 14:238–249, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Kusumakar V, Daneman D, Moshang T, De Smedt G, Binder C: Prolactin levels during long-term risperidone treatment in children and adolescents. J Clin Psychiatry 64:1362–1369, 2003 [DOI] [PubMed] [Google Scholar]
- Jayatilleke N, Hayes RD, Dutta R, Shetty H, Hotopf M, Chang CK, Stewart R: Contributions of specific causes of death to lost life expectancy in severe mental illness. Eur Psychiatry 43:109–115, 2017 [DOI] [PubMed] [Google Scholar]
- Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K: The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 334:1–100, 1987 [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB: Statistical Analysis with Missing Data. New York, Wiley, 2002 [Google Scholar]
- Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL: Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 20:1109–1114, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumeyer AM, Cano Sokoloff N, McDonnell E, Macklin EA, McDougle CJ, Misra M: Bone microarchitecture in adolescent boys with autism spectrum disorder. Bone 97:139–146, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795, 2001. 11176917 [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL: Centers for Disease Control and Prevention 2000 growth charts for the United States: Improvements to the 1977 National Center for Health Statistics version. Pediatrics 109:45–60, 2002 [DOI] [PubMed] [Google Scholar]
- Ortuno MJ, Robinson ST, Subramanyam P, Paone R, Huang YY, Guo XE, Colecraft HM, Mann JJ, Ducy P: Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat Med 22:1170–1179, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuskens J, Pani L, Detraux J, De Hert M: The effects of novel and newly approved antipsychotics on serum prolactin levels: A comprehensive review. CNS Drugs 28:421–453, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch F, Schoenau E: Changes in bone density during childhood and adolescence: An approach based on bone's biological organization. J Bone Miner Res 16:597–604, 2001 [DOI] [PubMed] [Google Scholar]
- Rosecrans R, Dohnal JC: Seasonal vitamin D changes and the impact on health risk assessment. Clin Biochem 47:670–672, 2014 [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC: Annual high-dose oral vitamin D and falls and fractures in older women: A randomized controlled trial. JAMA 303:1815–1822, 2010 [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME: NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39:28–38, 2000 [DOI] [PubMed] [Google Scholar]
- Shibli-Rahhal A, Schlechte J: The effects of hyperprolactinemia on bone and fat. Pituitary 12:96–104, 2009 [DOI] [PubMed] [Google Scholar]
- Slemenda CW, Miller JZ, Hui SL, Reister TK, Johnston CC, Jr.: Role of physical activity in the development of skeletal mass in children. J Bone Miner Res 6:1227–1233, 1991 [DOI] [PubMed] [Google Scholar]
- Trumbo P, Schlicker S, Yates AA, Poos M: Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 102:1621–1630, 2002 [DOI] [PubMed] [Google Scholar]
- Verbeke G, Molenberghs G: Linear Mixed Models for Longitudinal Data. New York, Springer, 2000 [Google Scholar]
- Warden SJ, Robling AG, Haney EM, Turner CH, Bliziotes MM: The emerging role of serotonin (5-hydroxytryptamine) in the skeleton and its mediation of the skeletal effects of low-density lipoprotein receptor-related protein 5 (LRP5). Bone 46:4–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS: The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos Int 27:1281–1386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber DR, Moore RH, Leonard MB, Zemel BS: Fat and lean BMI reference curves in children and adolescents and their utility in identifying excess adiposity compared with BMI and percentage body fat. Am J Clin Nutr 98:49–56, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzenberg TM, Shaw K, Fryer J, Jones G: Calcium supplementation for improving bone mineral density in children. Cochrane Database Syst Rev 2:CD005119, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Liu J, Gallegos-Orozco JF, Hentz JG: Depression, fracture risk, and bone loss: A meta-analysis of cohort studies. Osteoporos Int 21:1627–1635, 2010 [DOI] [PubMed] [Google Scholar]
- Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, Frederick MM, Huang X, Lu M, Mahboubi S, Hangartner T, Winer KK: Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: Results of the bone mineral density in childhood study. J Clin Endocrinol Metab 96:3160–3169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]