Abstract
Background: Nonsuicidal self-injury (NSSI) is common in adolescents and young adults, and few evidence-based treatments are available for this significant problem. N-acetylcysteine (NAC) is a widely available nutritional supplement that has been studied in some psychiatric disorders relevant to NSSI including mood and addictive disorders. This pilot study tested the use of NAC as a potential treatment for NSSI in youth.
Methods: Thirty-five female adolescents and young adults with NSSI aged 13–21 years were enrolled in this study that had an open-label, single-arm study design. All participants were given oral NAC as follows: 600 mg twice daily (weeks 1–2), 1200 mg twice daily (weeks 3–4), and 1800 mg twice daily (weeks 5–8). Patients were seen every 2 weeks throughout the trial, at which time youth reported the frequency of NSSI episodes. Levels of depression, impulsivity, and global psychopathology were measured at baseline and at the end of the trial using the Beck Depression Inventory-II (BDI-II), Barratt Impulsivity Scale, and Symptoms Checklist-90 (SCL-90).
Results: About two-thirds of the enrolled female youth completed the trial (24/35). NAC was generally well tolerated in this sample. NAC treatment was associated with a significant decrease in NSSI frequency at visit 6 and visit 8 compared to baseline. We also found that depression scores and global psychopathology scores (but not impulsivity scores) decreased after NAC treatment. Decrease in NSSI was not correlated with decrease in BDI-II or SCL-90 scores, suggesting these might be independent effects.
Conclusion: We provide preliminary evidence that NAC may have promise as a potential treatment option for adolescents with NSSI. The current results require follow-up with a randomized, placebo-controlled trial to confirm efficacy.
Keywords: : adolescent, nonsuicidal self-injurious behavior (NSSI), N-acetylcysteine (NAC)
Introduction
Nonsuicidal self-injury (NSSI), the deliberate act of harming oneself without suicidal intent, is a serious problem in adolescents and young adults. The onset of NSSI typically occurs during early to mid adolescence (Andover 2014). The prevalence of adolescent NSSI has been increasing in recent decades, particularly in girls (Muehlenkamp et al. 2009). A recent estimate of the international prevalence of NSSI among adolescents is 18% (Muehlenkamp et al. 2012). This behavior occurs in the context of a range of psychiatric disorders including (but not limited to) depression, anxiety, eating disorders, substance use, and borderline personality disorder. It may also occur in the absence of psychiatric disorders (Stanford and Jones 2009). NSSI is associated with long-term consequences including suicide attempts (Asarnow et al. 2011; Tang et al. 2011; Wilkinson et al. 2011; Victor and Klonsky 2014; Horwitz et al. 2015). Despite accumulating foundational knowledge to characterize NSSI, available treatment options for NSSI remain very limited. Adolescence is a notable time period for significant brain development and refinement of neural connections (Giedd et al. 1999; Giorgio et al. 2008; Fair et al. 2009) and therefore represents an important window to prevent maladaptive behaviors from becoming ingrained. With this in view, identification of successful interventions to address NSSI during adolescence could help divert adverse consequences over the lifespan.
Recent reviews have underscored the paucity of evidence for effective treatments for NSSI in adolescents (Brent et al. 2013; Hawton et al. 2015). Typically, for patients with NSSI, clinicians select treatment strategies that target the disorder that accompanies NSSI, such as depression or borderline personality disorder. Dialectical behavioral therapy (DBT), cognitive behavioral therapy (CBT), mentalization-based therapy (MBT), and some specific family-based interventions are psychotherapeutic approaches that have some preliminary support for treating self-injurious behavior (pooling both suicidal and NSSI together) in adolescents (Glenn et al. 2015; Ougrin et al. 2015). However, since these are highly specialized services that are not always available, and require a heavy time commitment on the part of the patient, parents, and providers, they may not always be the best option for all adolescents with NSSI. Alternative and supplemental options are needed.
N-acetylcysteine (NAC), a nutritional supplement that can be found in health food stores, may have some potential as an intervention for adolescent NSSI. Several literature reviews have suggested that NAC may be useful in the treatment of a range of psychiatric issues relevant to NSSI including mood disorders, addiction, and other impulsive/compulsive problems (Ng et al. 2008; Berk et al. 2013; Deepmala et al. 2015). NAC is the N-acetyl derivative of the amino acid l-cysteine, and is a precursor in the formation of the antioxidant glutathione (GSH), the primary antioxidant in the brain. The bioavailability and pharmacokinetic properties of NAC have been established (Holdiness 1991). Human research has shown that NAC crosses the blood-brain barrier (Katz et al. 2015) and acutely increases blood and brain GSH concentrations (Holmay et al. 2013). A proposed biological mechanism for NAC's positive impact on mental health is that by increasing GSH, NAC increases the capacity to reduce free radicals, thus providing a neuroprotective effect against stress-induced toxicities. Another key mechanism of NAC is its modulatory role on the glutamate transporter; this mechanism has been implicated in NAC's demonstrated alleviation of addictive behaviors (Kalivas and Volkow 2011; McClure et al. 2014). Since NSSI is a clinical problem that (a) occurs primarily in the context of negative affect (Klonsky 2007), and (b) represents a habitual behavior that can be chronic and difficult to give up (Chapman et al. 2006), NAC, which has been helpful for mood disorders and other habitual behavioral disorders (e.g., addiction), may be a promising treatment for NSSI. However, to date, there are no publications reporting results testing the efficacy of NAC for NSSI.
The purpose of this pilot study was to test the effectiveness of NAC as a treatment for NSSI in adolescents and young adults by conducting an 8-week, open-label trial. The first aim was to evaluate the association between NAC treatment and NSSI frequency and associated psychopathology. Our primary hypothesis was that adolescents would show reduced NSSI frequency during the NAC trial. Our secondary hypothesis was that NAC treatment would be associated with reductions in psychopathology including depression symptoms, impulsivity, and global psychopathology. The second study aim was to preliminarily evaluate safety and tolerability of NAC treatment in adolescents with NSSI. A final exploratory study aim was to examine baseline clinical markers of favorable NAC treatment response.
Methods
Regulatory procedures
This study was approved by the University of Minnesota Institutional Review Board. Data and safety monitoring was provided by the Clinical and Translational Science Institute at the University of Minnesota and the Federal Drug Administration (Investigational New Drug Number 10345). The study was posted on ClinicalTrials.gov NCT01111734.
Participants
Participants aged 13–21 years with NSSI were recruited for this study using advertisements in the community and clinical referrals. Participants were eligible if they had a history of engaging in at least four lifetime episodes of NSSI, with at least one episode occurring within 1 month before study entry. If they were currently taking any medications, participants were required to be on a stable dose for at least 1 month before study onset. Exclusion criteria were as follows: history of bipolar disorder, psychotic disorder, pervasive developmental disorder, or mental retardation; current, unstable medical illnesses; currently pregnant or breastfeeding; history of allergic reaction to NAC; currently taking the following medications due to interaction concerns: activated charcoal, ampicillin, carbamazepine, cephaloridine, cloxacillin, methicillin, nitroglycerin, oxacillin, penicillin G, and quinacillin.
Screening, consent/assent, and clinical evaluation
Before enrolling in the study, all participants (or their parent/legal guardian if under 18) completed a phone screening interview to determine initial eligibility. If deemed appropriate, they were invited for a baseline clinical visit. Upon arrival, after completing the informed consent and assent (when applicable) process, participants underwent a diagnostic interview, clinician-administered and self-report assessments, and a health screening and physical exam to assess for current and past medical conditions and medication use. For participants under 18 years old, the diagnostic interview consisted of the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL) (Kaufman et al. 1997), which was completed with the subject and parent/guardian separately. Both interviews were used to establish a consensus diagnosis. Participants 18 and older completed the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (First et al. 2002).
All participants completed the Inventory of Statements About Self-Injury–Lifetime version (ISAS) (Glenn and Klonsky 2011); after reviewing the results, the research clinician administered the Deliberate Self-Harm Inventory (DSHI) interview (Gratz 2001), with a focus on the type of self-harm that had been endorsed in the ISAS. In addition, participants also completed a packet of self-report measures including the following: the Deliberate Self-Harm Questionnaire: Part III Mood (DSHQ-M), Beck Depression Inventory-II (BDI-II) (Beck et al. 1996), Tanner Staging Questionnaire (Duke et al. 1980), Barratt Impulsivity Scale (BIS) (Patton et al. 1995), Symptoms Checklist-90 (SCL-90) (Derogatis and Savitz 2000; Morey 2007a, b), and a demographic questionnaire. Socioeconomic status (SES) was calculated using the Hollingshead Four-Factor Index (Hollingshead et al. 1975). Intelligence quotient (IQ) was estimated using the Wechsler Abbreviate Scale of Intelligence (WASI) (Wechsler 1999).
Study treatment and monitoring
Oral NAC dosing was as follows: weeks 1 and 2: 600 mg twice a day; weeks 3 and 4: 1200 mg twice a day; weeks 5–8: 1800 mg twice a day. Participants were given a 2-week supply of NAC at the baseline visit, and at the next three follow-up visits; participants were seen for follow-up visits at the end of weeks 2, 4, 6, and 8. Follow-up clinical assessments consisted of vital sign measurements (blood pressure, pulse, and weight), ISAS (since last visit version), DSHI (since last visit), and the Columbia Suicide Assessment Screen (Posner et al. 2008, 2011). Pill counts were used to measure medication compliance. Compliance was calculated using the following formula and applied to each visit: (Total dispensed—returned)/(days between visits × dose [# of pills]) × 100. Due to participants sometimes forgetting to return their bottles for pill counts, we calculated the average compliance for each participant by taking the average of all the visits in which we had complete information.
Outcome measures
The primary outcome measure was frequency of NSSI episodes. Notably, adolescents and young adults with NSSI will often report that they injure themselves multiple times in a row, in one “sitting” or episode. For these analyses, we focused on the number of episodes, as opposed to the number of individual injuries. We created consensus frequency scores using both the clinician-administered DSHI and the self-reported ISAS. Secondary outcome measures included depression scores as measured by the BDI-II, global psychopathology as measured by the SCL-90, and impulsivity as measured by the BIS. Safety outcome measures included vital signs and reports of side effects as reported during the follow-up clinical visits.
Statistical analysis
We first conducted summary statistics on basic demographic and clinical characteristics including age, race/ethnicity, estimated IQ, parent SES, Tanner stage, trauma history, presence of current medications, and all clinical measures listed above. Before characterizing change in NSSI, we first examined the distribution of NSSI frequency data to guide the selection of the appropriate statistical model for examining changes in NSSI frequency over visits. The baseline rate of NSSI (per 2 weeks, to match the window of time between each clinical visit) was calculated from the lifetime assessment data by dividing the number of reported lifetime episodes by the number of weeks between the first lifetime episode and study entry, and then multiplying by two. The variance in NSSI frequency at baseline was very large and the distribution was highly skewed; variance was much lower in the follow-up visits. Therefore, a repeated measures negative binomial analysis of number of episodes (with an offset for number of weeks) using Generalized Estimating Equations was conducted to examine the significance of the effect of NAC on frequency of NSSI. We initially included the following adjustors in the model: age, IQ, SES, race (minority versus nonminority), baseline BDI-II scores, baseline SCL-90 global severity index (SCL-90-GSI) scores, and the total number of types of NSSI that the participant endorsed at baseline (e.g., cutting, burning, hitting, etc.). Since age and race were related to baseline NSSI severity, they were kept in the final model. BDI-II scores, SCL-90-GSI scores, total number of types of NSSI, IQ, and SES were nonsignificant contributors and so they were not included in the final model. We examined trajectory of change in NSSI across all clinical visits: weeks 0, 2, 4, 6, and 8; and mean comparisons of each week with baseline (week 0) had p-values corrected for multiple comparisons using Stepdown Bonferroni. To examine change in secondary outcome measures, we conducted a series of linear mixed model analyses to examine treatment-related change in BDI-II, SCL-90, and BIS scores, using the same set of covariates, but here with only two time points (baseline and week 8). We also examined change in vital signs (weight, blood pressure, and pulse) from baseline to posttreatment using paired t-tests. We documented side effects and summarized them in a descriptive fashion.
Finally, we tested whether any of the clinical variables could predict response to NAC. Given the paucity of available literature on treatment of NSSI, there was no precedent for how to define responder status. In our clinical practice, we have observed that individuals report that the rate of NSSI episodes varies over time; for example, rather than a steady rate of one episode per week, there may be a cluster of episodes followed by several weeks with no episodes. Therefore, for individuals with this pattern, a focus on the 2-week window at the last visit would not be long enough to robustly estimate whether there was a true response. With this in view, we decided to consider a time window of 4 weeks to capture these patterns, and examined NSSI behaviors in weeks 5–8 versus baseline. We subtracted the baseline rate of NSSI per-4-weeks from the number of reported NSSI episodes during weeks 5–8 (so that a negative number reflects an improvement). Examination of a histogram of this change variable revealed that most (65%) of the participants showed change in the range of −3 to +3, with about 8% of participants showing a worsening in NSSI (+3 to +6), whereas about 20% of the sample improved moderately (−3 to −9) and 8% of the sample improved significantly (−9 to −21). Therefore we defined “responder” as anyone who had at least a −3 on this NSSI change variable reflecting a moderate to significant improvement in NSSI. We then conducted logistic regression analyses to determine whether any of the baseline demographic or clinical variables (baseline NSSI frequency, type of NSSI, history of suicide attempt, history of trauma, medication status, history of hospitalizations, and history of psychotherapy) would predict a positive NSSI response to NAC.
Results
Thirty-six adolescents and young adults (one male) underwent clinical assessment and enrolled into the clinical trial. Twenty-five adolescents and young adults (one male) completed the 8-week follow-up visit (Fig. 1). Because we only had one male participant, we focused the analyses on females only. Average medication compliance for the 24 female completers was 87.91%.
FIG. 1.
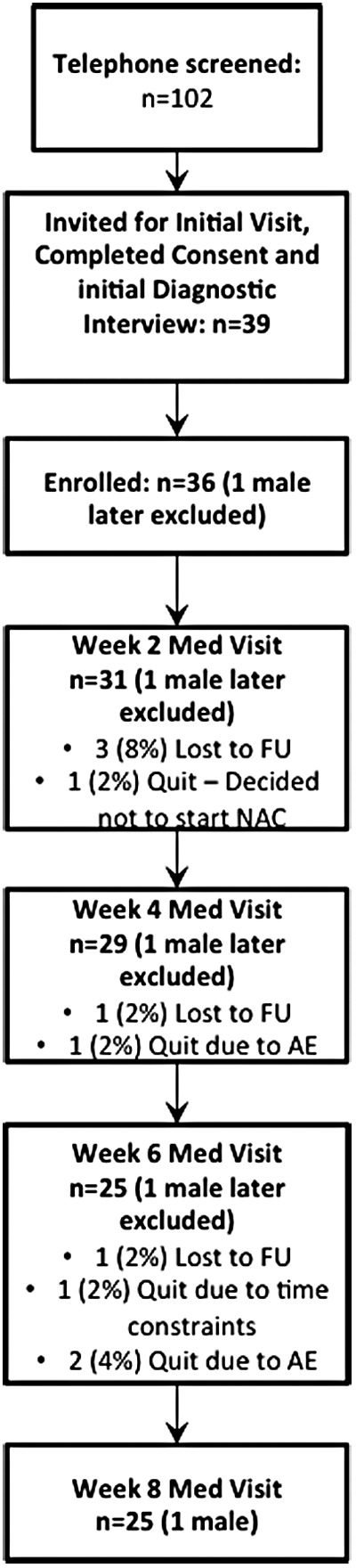
Consort diagram showing the flow of screening, recruitment, consent, enrollment, and completion of study visits for participants in this study. Thirty-six participants (one male) were enrolled and 25 (one male) participants completed the protocol. Data from the male participant were analyzed. AE, adverse events, FU, follow-up.
Clinical characteristics of this sample of adolescents and young adults with NSSI
Table 1 describes the clinical and demographic characteristics of this sample. Diagnostically, the most common type of clinical disorder in this sample was depression.
Table 1.
Summary of Demographic and Clinical Characteristics of the Study Sample
| Demographic characteristics | Baseline Statistic | Post-treatment statistic |
|---|---|---|
| Age (mean ± STD) (N = 35) | 17.43 ± 2.32 | |
| Minority (%) (N = 35) | 14.29 | |
| Hispanic (%) (N = 35) | 8.57 | |
| Parent SES (mean ± STD) (N = 31) | 46.23 ± 10.42 | |
| IQ (mean ± STD) (N = 30) | 106.20 ± 10.57 | |
| Tanner stage (mean ± STD) (N = 31) | 4.26 ± 0.89 | |
| Clinical characteristics, n (%) | ||
| Diagnosis (N = 35) | ||
| Any depressive disordera | 25 (71) | |
| Any anxiety disorderb | 33 (94) | |
| Eating disorder NOS | 1 (3) | |
| ADHD | 3 (9) | |
| Alcohol dependence | 2 (6) | |
| No current disorder | 4 (11) | |
| Number of participants currently taking medicationsc (N = 35) | 15 (43) | |
| Number of participants with a history of psychotherapy (N = 32) | 23 (72) | |
| Number of participants with past suicide attempts (N = 32) | 9 (28) | |
| Number of participants with a trauma history (N = 35) | 28 (80) | |
| NSSI type (N = 35) | ||
| Cutting | 35 (100) | |
| Scratching | 18 (51) | |
| Interfering with wound healing | 18 (51) | |
| Burning | 17 (49) | |
| Banging head or hitting self | 17 (49) | |
| Carving | 13 (37) | |
| Sticking with needles | 13 (37) | |
| Biting | 11 (31) | |
| Pinching | 8 (23) | |
| Hair pulling | 6 (17) | |
| Rubbing skin | 6 (17) | |
| Outcome measures | ||
| NSSI frequency: number of cutting episodes per week (mean ± STD) (baseline N = 35; post-treatment N = 24) | 0.74 ± 1.09 | 0.35 ± 0.83 |
| Beck Depression Inventory (mean ± STD) (baseline N = 34; post-treatment N = 23) | 31.53 ± 9.81 | 24.04 ± 15.00 |
| Barratt Impulsivity Scale (mean ± STD) (baseline N = 30; post-treatment N = 23) | 72.83 ± 10.15 | 72.61 ± 11.13 |
| Symptom Checklist-90 (SCL-90) (baseline N = 30, post-treatment N = 23) | ||
| SCL-90 Anxiety (mean ± STD) | 1.43 ± 0.93 | 1.02 ± 0.90 |
| SCL-90 Depression (mean ± STD) | 1.88 ± 0.84 | 1.56 ± 0.98 |
| SCL-90 Hostility (mean ± STD) | 1.49 ± 0.91 | 1.13 ± 0.97 |
| SCL-90 Interpersonal Sensitivity (mean ± STD) | 1.77 ± 0.88 | 1.52 ± 0.94 |
| SCL-90 Obsessive Compulsive (mean ± STD) | 1.64 ± 0.64 | 1.40 ± 0.80 |
| SCL-90 Paranoid Ideation (mean ± STD) | 1.35 ± 0.76 | 0.95 ± 0.84 |
| SCL-90 Phobic Anxiety (mean ± STD) | 0.98 ± 0.92 | 0.61 ± 0.82 |
| SCL-90 Psychoticism (mean ± STD) | 1.22 ± 0.62 | 0.83 ± 0.62 |
| SCL-90 Somatization (mean ± STD) | 1.22 ± 0.68 | 0.64 ± 0.55 |
| SCL-90 Global Severity Index (mean ± STD) | 1.49 ± 0.63 | 1.13 ± 0.72 |
Any depressive disorder included MDD (n = 20; 57%) and DD NOS (n = 5; 14%).
Any anxiety disorder included: GAD (n = 10; 29%), Anxiety Disorder NOS (n = 2; 6%), Social Phobia (n = 4; 11%), Specific Phobia (n = 5; 14%), Panic Disorder (n = 4; 11%), PTSD (n = 4; 11%), OCD (n = 4; 11%).
Current baseline medications included: fluoxetine (n = 6), buproprion (n = 5), citalopram (n = 3), sertraline (n = 4), trazodone (n = 3), escitalopram (n = 1), methylphenidate (n = 1), amphetamine (n = 2), aripiprazole (n = 1), lamotrigine (n = 1), clonazepam (n = 1), gabapentin (n = 1).
ADHD, attention-deficit/hyperactivity disorder; IQ, estimated intelligence quotient; NSSI, nonsuicidal self-injury; SES, socioeconomic status; STD, standard deviation.
Clinical change during NAC treatment: NSSI frequency
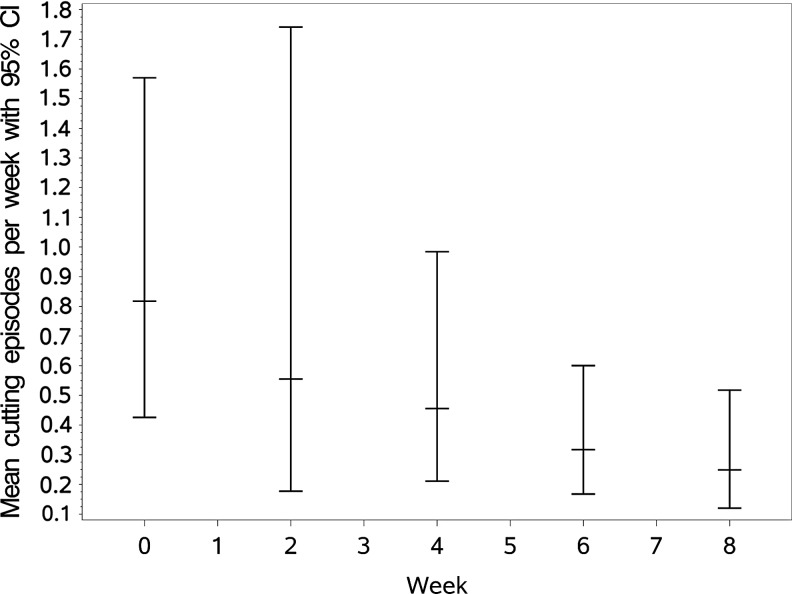
We first tested the effect of NAC on the primary outcome variable, NSSI frequency (N = 35). There was a significant decrease in NSSI frequency at visit 6 (corrected p = 0.055) and at visit 8 (corrected p = 0.022) compared to baseline (Fig. 2). Among the covariates included in the model, there was a significant effect of age, where older adolescents had less frequent cutting episodes than younger adolescents: on average 0.27 fewer cutting episodes per 2 weeks for each year older (p = 0.0009). Additionally, nonwhite adolescents had 0.87 fewer episodes per 2 weeks than white adolescents (p = 0.003).
FIG. 2.
Frequency of NSSI episodes during treatment with NAC. Model-predicted means and confidence intervals are shown for each of the clinical visits in the trial. NAC, N-acetylcysteine; NSSI, nonsuicidal self-injury.
Clinical change during NAC treatment: other clinical measures
Second, we examined the effect of NAC on the secondary outcome measures (week 8 vs. week 0 only) (N = 24). For BDI scores, there was a significant difference between baseline and week 8 in depression symptoms (p < 0.0001). In this analysis, age, race, and IQ covariates had significant effects (older age, being nonwhite, and lower IQ scores were associated with lower depression scores both at baseline and at week 8). For the SCL-90, we first analyzed the SCL90-GSI. The baseline to week 8 change in SCL-90-GSI was highly significant (p < 0.0001). Significant covariates in this analysis included age, race, IQ, and baseline BDI-II (higher SCL-90-GSI scores both at baseline and at follow-up were associated with younger age, being white, lower SES, higher IQ and higher baseline and follow-up BDI scores). To follow up on the significant overall result, we sought to better understand the key domains that were driving this finding. Therefore we conducted follow-up analyses on the individual sub-scores (depression, anxiety, hostility, paranoid ideation, interpersonal sensitivity, phobic anxiety, psychoticism, somaticism, obsessive-compulsive, and “additional items” [poor appetite]). Follow-up analyses of the sub-scores revealed significant baseline to week 8 differences in the following domains: anxiety (p = 0.0006), depression (p < 0.0001), hostility (p = 0.0166), obsessive-compulsive (p = 0.0039), phobic anxiety (p = 0.002), psychoticism (p < 0.0001), interpersonal sensitivity (p = 0.0030), somatization (p < 0.0001), and paranoid ideation (p < 0.0001). After a Bonferroni correction for multiple testing across the nine sub-scales, all sub-scales remained significant (threshold = p < 0.0056) except for hostility. Interestingly, change in NSSI frequency from week 0 to 8 was not correlated with improvement in BDI-II scores or a change in SCL-90-GSI scores (r values <0.1). There were no significant changes in impulsivity as measured by the BIS.
Safety and tolerability
There were no significant changes in blood pressure or pulse during NAC treatment for this sample. There was a small but significant increase in weight (week 0 mean = 161.6 lbs, standard deviation = 47.8 lbs; week 8 mean = 163.8 lbs; standard deviation = 48.9 lbs; n = 22). The most commonly reported side effects were headaches (n = 5) and nausea (n = 5) that ranged from mild to moderate. Other side effects included bad taste, chest pain, shortness of breath, canker sores, cold-like symptoms, cough, diarrhea, difficulty swallowing, heartburn, fever, extremity swelling and tingling, hearing changes, increased irritability and depression, mood swings, fatigue, menstrual irregularity, facial redness, urinary retention, and vomiting. See Table 2 for detailed description of all side effects and adverse events.
Table 2.
Summary of Adverse Events
| Adverse event | n (%) subjects | n (%) incidents |
|---|---|---|
| Gastrointestinal complaints | 12 (33%) | 18 (35%) |
| Nausea | 7 | 9 |
| Vomiting | 2 | 2 |
| Weight gain | 1 | 1 |
| Diarrhea | 2 | 2 |
| Bad taste | 1 | 1 |
| Acid reflux | 3 | 3 |
| Respiratory symptoms | 2 (6%) | 3 (6%) |
| Shortness of breath | 2 | 3 |
| General/ear, nose, throat | 14 (39%) | 9 (17%) |
| Cold symptoms/viral illness | 7 | 7 |
| Fever | 1 | 2 |
| Cough | 2 | 2 |
| Fatigue | 1 | 1 |
| Difficulty swallowing | 1 | 1 |
| Hearing changes | 1 | 1 |
| Headaches | 6 | 8 |
| Genitourinary complaints | 2 (6%) | 2 (4%) |
| Urinary retention | 1 | 1 |
| Menstrual irregularity | 1 | 1 |
| Psychiatric complaints | 4 (11%) | 9 (17%) |
| Irritability | 2 | 3 |
| Mood swings | 2 | 3 |
| Worsening depression | 1 | 1 |
| Panic attack | 1 | 1 |
| Suicide attempt | 1 | 1 |
| Skin and subcutaneous tissue | 6 (17%) | 8 (15%) |
| Canker sore | 2 | 2 |
| Redness | 1 | 1 |
| Burning sensation | 1 | 1 |
| Numbness | 1 | 1 |
| Tingling | 1 | 1 |
| Swelling | 1 | 2 |
| Other events | 2 (6%) | 3 (6%) |
| Ankle injury | 1 | 1 |
| Sternal pain | 1 | 1 |
| Sexual assault | 1 | 1 |
Predictors of clinical response
Twenty-four adolescents (all those who had complete data through 8 weeks) were included in these prediction analyses. Results from analyses examining potential predictors of response versus nonresponse to NAC (defined in Methods section) indicated that higher severity of NSSI at baseline (greater number of lifetime cutting episodes) predicted being a responder (p = 0.019) and was still significant after correcting for baseline age and BDI scores (15% higher odds of responding to NAC for each 10 higher number of lifetime episodes; estimate = 0.0142, p = 0.015). None of the demographic factors or baseline clinical measures including baseline BDI scores, baseline SCL-90 scores, baseline NSSI frequency, type of NSSI, history of suicide attempt, history of trauma, medication status, history of hospitalizations, and history of psychotherapy) showed a significant predictive effect for response to NAC in terms of decreased NSSI frequency.
Discussion
In this article we report preliminary evidence to suggest that NAC may be useful in reducing NSSI in adolescents and young adults. In this open-label clinical trial, adolescents and young adults showed a decrease in NSSI frequency during the 8 weeks of treatment with oral NAC. Furthermore, there were additional benefits including reduction in depression symptoms and in global psychopathology. Finally, NAC was generally well tolerated by the participants in this sample.
Although preliminary, the current findings add to the small literature on treatments for NSSI in youth. To date, one of the most commonly recommended interventions for NSSI in adolescents is DBT (e.g., Hollander 2017). DBT has been shown to reduce self-injurious behavior (both suicidal and nonsuicidal) in adults, although the strongest evidence has been for reductions in suicidal behavior rather than NSSI (Linehan et al. 2006, 2015; Stanley et al. 2007). Several early studies have now shown that DBT, MBT, CBT, and some specific family-based interventions can reduce self-injurious behavior (including NSSI) in at least some adolescents (Geddes et al. 2013; MacPherson et al. 2013; Fischer and Peterson 2014; Glenn et al. 2015; Ougrin et al. 2015). However, as noted above, these interventions have their limitations (not available in all communities, rigorous time commitment) and they may not always be the best option for all adolescents with NSSI. Alternative and supplemental options are needed.
Our findings suggest that in addition to reducing NSSI frequency, NAC treatment was associated with improvement in depressive symptoms in female adolescents and young adults with NSSI. This finding adds to a growing (and mixed) body of literature investigating NAC as a treatment for depression in the context of either major depression or bipolar disorder. Although initial studies were promising in providing early evidence suggesting NAC as potentially useful treatment for depression in unipolar (Carvalho et al. 2013) and bipolar depression (Berk et al. 2008, 2011; Magalhães et al. 2011), more recent work in adults has not supported this. In a recent double-blinded study in adults with major depression, Berk et al. (2014) reported that NAC as an adjunctive treatment over 12 weeks was no better than placebo. A recent Cochrane database review found no evidence supporting NAC as a treatment for adults with depression (Caddy et al. 2015). However, NAC's use in adolescent depression has not been investigated previously. Further, while NAC may not be sufficient to treat overall depression in adults, it may serve to reduce some aspects of depression. For example, Berk et al. (2014) noted that NAC was associated with clinical improvement in some of the secondary measures such as overall functioning and quality of life. In a study of adults with bipolar depression, NAC was associated with a reduction in suicidal ideation compared to placebo after 24 weeks of treatment (Waterdrinker et al. 2015). Taken together with the findings reported here, more work is indicated to confirm whether NAC can effectively reduce depression symptoms in adolescents with NSSI, concurrent with the reduction in NSSI itself.
The fact that both NSSI frequency and depression scores improved in our sample suggests that the two changes may be related. The relationship between depression and NSSI is evidenced in the literature by the high rates of depression symptoms among NSSI samples. Also, in a study of treatment for adolescent depression, Wilkinson et al. (2011) who reported that after 28 weeks of treatment for depression (treatments included SSRIs with or without CBT), high levels of depression symptoms were significantly associated with self-injury. In contrast, though, our analyses revealed that the change in NSSI frequency and change in depression scores were not correlated in this sample, suggesting that instead, the effects may be independent (while both dimensions of functioning improved, the changes occurred in different people). However, it should be noted that our pilot study was not designed or powered to tease apart the effects on NSSI versus the effects on depression. Future work that incorporates larger samples and a placebo arm using a causal modeling approach would be necessary to clarify the relationship between change in NSSI and change in depression in response to NAC.
The possible effectiveness of NAC in reducing NSSI and related psychopathology may be a manifestation of change through multiple neurobiological mechanisms. First, NAC is a precursor to glutathione, which is the primary antioxidant in the brain. NAC has been proposed as a promising potential treatment for mood disorders because of its antioxidant properties (Berk et al. 2013) and has been touted as a neuroprotective agent (Dodd et al. 2013). Second, NAC is a modulator of both glutamate and dopamine, both neurotransmitters of high relevance to motivation and behavior. Previous research investigating NAC as a potential treatment for addiction has shown that NAC increases the activity of cysteine-glutamate antiporters in the nucleus accumbens and abolishes reward-seeking behavior (Kalivas and Volkow 2011). While NSSI is not considered an addictive behavior, there may be some overlapping neurobiological substrates between NSSI and addiction, since NSSI is a repetitive behavior that is often maintained by the sense of relief it provides (Klonsky 2007). Attenuation of glutamatergic signaling and excitotoxicity has been proposed as a possible key mechanism for NAC's ability to ameliorate various forms of psychopathology (Berk et al. 2013). To follow-up on these ideas, research using NAC as a probe in patients with NSSI would hold promise for advancing understanding of neurobiological mechanisms of NSSI as well as providing the groundwork for continued development of biologically targeted treatments.
The current findings add to a paucity of literature regarding pharmacological options in the treatment of adolescent NSSI. Prior research investigating pharmacological interventions to target NSSI has largely taken place in the context of autism and developmental disorders, and has focused on opiate systems. The motivating principle for that approach is the idea that self-injurious acts lead to the release of endogenous endorphins, which contributes to maintenance of this behavior. For example, naltrexone, an opiate receptor blocker, has been studied as a treatment for NSSI in patients with developmental disorders. While an earlier review suggested that naltrexone showed some promise in reducing NSSI in this population (Symons et al. 2004), a subsequent review of placebo-controlled studies concluded that the evidence supporting this intervention for reducing NSSI is weak and inconclusive (Rana et al. 2013). No publications to date have examined medications targeting the opiate system in adolescents similar to the sample presented here (typically developing adolescents who primarily use NSSI as a maladaptive tool for regulating negative affect).
Historically, serotonergic and dopaminergic medications have also been explored in patients with learning disabilities and NSSI, with some positive effects (see review by Clarke 1998), but more recent research has not pursued these avenues. While selective serotonin reuptake inhibitors are commonly used for adolescents with depression, they have not been systematically examined as a treatment for NSSI. In our study, 15 of the 35 adolescents that entered our study were on a stable dose of an antidepressant medication; however, by definition these medications had not alleviated the NSSI behaviors since they met criteria for participation in our study. The presence of current psychiatric medications did not significantly predict NAC response. Due to limited power, we were not able to examine whether particular medications predicted NAC response. Plausibly, these three approaches (medications targeting the serotonin system, medications targeting the opiate system, and NAC) could be combined to more effectively address the problem by addressing multiple systems at once, pending research confirming the independent effects of each approach.
Several important limitations of this study require consideration when interpreting the findings. First and foremost, this is an open-label study with no placebo arm. Like other open-label studies, the estimated treatment effect is a mixture of a true treatment effect, the Hawthorne effect, and regression to the mean. Further, some research has suggested that children and adolescents may be even more susceptible to placebo effects than adults (Weimer et al. 2013). Also, without a placebo arm, the study can not address the problem of nonrandom drop out of study participants, which can lead to an overestimation of improvement with time. Future work using a randomized, placebo-controlled trial is needed to confirm whether NAC is effective in reducing NSSI in adolescents. A second potential limitation here is the 2-month window of time to measure reductions in NSSI. We found that some participants in our sample, similar to some others (Andover 2014), reported a baseline NSSI frequency of approximately three times per month (Table 1). While it is much easier to show an effect for individuals who engaged in NSSI several times per week, the effect is much smaller when the baseline rate is small. For a person with a baseline rate of one episode of NSSI every 2 months, the finding of abstinence from NSSI throughout the 8-week study would not necessarily represent a true NSSI reduction; rather, this would be a reflection that the window of time was too short to capture that person's naturally occurring next episode. Therefore, future studies may benefit from considering longer time windows, or focusing on youths with higher baseline frequencies, or a combination of these approaches. Third, a related point is the consideration of the optimal baseline rate of NSSI that should be used to compare against the outcome measures. In this study, we knew that all participants had at least one episode of NSSI in the month before baseline, but we did not have complete data on past-month frequency for each person. Therefore, we calculated the baseline rate as the total number of NSSI episodes divided by the number of weeks since the first episode of NSSI. While this approach has the advantage of capturing information about lifetime severity, a more optimal approach would be to gather precise data on NSSI frequency during a specific time period right before the study. Fourth, in the current study, we included both unmedicated youth (n = 20) and youth who were taking a stable dose of psychiatric medication to address co-occurring disorders (n = 15). Additional research is needed to determine whether the optimal role of NAC is as an augmentation agent or if it is sufficient to address this behavior as a monotherapy. Fifth, the optimal dose of NAC for treating NSSI and related problems in adolescents has yet to be identified. A variety of NAC doses have been tested in psychiatric research studies, ranging from 1200 to 3600 mg/day. In this study, we selected the target dose of 3600 mg/day based on (a) previous work showing that this dose was more effective than lower doses in adults with addiction (e.g., Mardikian et al. 2007), (b) a growing body of evidence that NAC is a generally safe and well-tolerated medication, and (c) personal communication from physicians prescribing this medication for skin-picking disorders suggesting that higher doses are more effective. The participants in the study found this dose to be generally well tolerated. However, it may be the case that other doses, either lower or higher, could be more effective for reducing NSSI and/or other psychopathology. Future work incorporating dose-finding studies are therefore indicated. Finally, the field of intervention research for NSSI is still in the process of identifying the optimal outcome measures. Here, we have focused on NSSI frequency, which has its limitations as discussed here related to the variability across individuals in frequency at baseline. Another potential approach is to measure urges for self-harm, using measures such as the Alexian Brothers Urge to Self-Injure Scale (ABUSI) (Washburn et al. 2010), a measure that has had some preliminary evidence showing reliability that could potentially be more sensitive to change than measuring actual NSSI episode frequency.
Conclusion
In conclusion, the current report represents the first report of the use of NAC as an intervention to address NSSI in adolescents and young adults. Given the high prevalence of this clinical problem and the paucity of available treatments, there is a need for research identifying potential new treatments. While additional research is needed to confirm the positive effects reported here, the current findings are promising given that NAC is a very well-tolerated, affordable, and widely available intervention.
Clinical Significance
NSSI is a common and serious problem in adolescents. Few evidence-based treatments are available. Since NAC is an inexpensive, widely available nutritional supplement, further research supporting its use to address this significant problem would have a potentially high impact.
Acknowledgments
The authors would like to first and foremost thank the young people and their families who volunteered their time to participate in this study. This clinical trial was primarily funded by the University of Minnesota, Academic Health Center with a Faculty Research Development grant awarded to Dr. K.R.C. and Dr. L.E.E. Additionally, many of the adolescents and young adults who participated in this study also participated in a baseline neuroimaging study that was funded by the National Institute of Mental Health (R21MH091366), which provided some additional support for the recruitment and assessment of this sample. Finally, we would like to thank Lori LaRiviere, MD for her important contributions to the study early on in the project.
Disclosures
No competing financial interests exist.
References
- Andover MS: Non-suicidal self-injury disorder in a community sample of adults. Psychiatry Res 219:305–310, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarnow JR, Porta G, Spirito A, Emslie G, Clarke G, Wagner KD, Vitiello B, et al. : Suicide attempts and nonsuicidal self-injury in the treatment of resistant depression in adolescents: Findings from the TORDIA study. J Am Acad Child Adolesc Psychiatry 50:772–781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK: Manual for the Beck Depression Inventory-II. San Antonio, TX, Psychological Corporation, 1996 [Google Scholar]
- Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI: N-acetyl cysteine for depressive symptoms in bipolar disorder—A double-blind randomized placebo-controlled trial. Biol Psychiatry 64:468–475, 2008 [DOI] [PubMed] [Google Scholar]
- Berk M, Dean O, Cotton SM, Gama CS, Kapczinski F, Fernandes BS, Kohlmann K, et al. : The efficacy of N-acetylcysteine as an adjunctive treatment in bipolar depression: An open label trial. J Affect Disord 135:389–394, 2011 [DOI] [PubMed] [Google Scholar]
- Berk M, Dean OM, Cotton SM, Jeavons S, Tanious M, Kohlmann K, Hewitt K, et al. : The efficacy of adjunctive N-acetylcysteine in major depressive disorder: A double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 75:628–636, 2014 [DOI] [PubMed] [Google Scholar]
- Berk M, Malhi GS, Gray LJ, Dean OM: The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci 34:167–177, 2013 [DOI] [PubMed] [Google Scholar]
- Brent DA, McMakin DL, Kennard BD, Goldstein TR, Mayes TL, Douaihy AB: Protecting adolescents from self-harm: A critical review of intervention studies. J Am Acad Child Adolesc Psychiatry 52:1260–1271, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R, Hawton K, Cipriani A: Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev 9:CD011612, 2015 [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Macêdo DS, Goulia P, Hyphantis TN: N-acetylcysteine augmentation to tranylcypromine in treatment-resistant major depression. J Clin Psychopharmacol 33:719–720, 2013 [DOI] [PubMed] [Google Scholar]
- Chapman AL, Gratz KL, Brown MZ: Solving the puzzle of deliberate self-harm: The experiential avoidance model. Behav Res Ther 44:371–394, 2006 [DOI] [PubMed] [Google Scholar]
- Clarke DJ: Psychopharmacology of severe self-injury associated with learning disabilities. Br J Psychiatry 172:389–394, 1998 [DOI] [PubMed] [Google Scholar]
- Deepmala JS, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R: Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci Biobehav Rev 55:294–321, 2015 [DOI] [PubMed] [Google Scholar]
- Derogatis LR, Savitz KL: The SCL-90-R and the brief symptom inventory (BSI) in primary care. In: Handbook of Psychological Assessment in Primary Care Settings, Edited by Maruish ME. Mahwah, NJ, Lawrence Erlbaum Associates, 2000, pp. 297–334 [Google Scholar]
- Dodd S, Maes M, Anderson G, Dean OM, Moylan S, Berk M: Putative neuroprotective agents in neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 42:135–145, 2013 [DOI] [PubMed] [Google Scholar]
- Duke PM, Litt IF, Gross RT: Adolescents' self-assessment of sexual maturation. Pediatrics 66:918–920, 1980 [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NUF, Church JA, Miezin FM, Schlaggar BL, Petersen SE: Functional brain networks develop from a ‘local to distributed’ organization. PLoS Comput Biol 5:e1000381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Miriam G, Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, research version, patient edition with psychotic screen (CID-I/P W/PSY SCREEN). New York, NY, Biometrics Research Institute, 2002 [Google Scholar]
- Fischer S, Peterson C: Dialectical behavior therapy for adolescent binge eating, purging, suicidal behavior, and non-suicidal self-injury: A pilot study. Psychotherapy 52:78–92, 2015 [DOI] [PubMed] [Google Scholar]
- Geddes K, Dziurawiec S, Lee CW: Dialectical behaviour therapy for the treatment of emotion dysregulation and trauma symptoms in self-injurious and suicidal adolescent females: A pilot programme within a community-based child and adolescent mental health service. Psychiatry J 2013:145219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL: Brain development during childhood and adolescence: A longitudinal MRI study. Nat Neurosci 2:861–863, 1999 [DOI] [PubMed] [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, Matthews PM, Smith SM, Johansen-Berg H: Changes in white matter microstructure during adolescence. NeuroImage 39:52–61, 2008 [DOI] [PubMed] [Google Scholar]
- Glenn CR, Franklin JC, Nock MK: Evidence-based psychosocial treatments for self-injurious thoughts and behaviors in youth. J Clin Child Adolesc Psychol 44:1–29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klonsky ED: One-year test-retest reliability of the Inventory of Statements About Self-Injury (ISAS). Assessment 18:375–378, 2011 [DOI] [PubMed] [Google Scholar]
- Gratz KL: Measurement of deliberate self-harm: preliminary data on the Deliberate Self-Harm Inventory. J Psychopathol Behav 23:253–263, 2001 [Google Scholar]
- Hawton K, Witt KG, Salisbury TLT, Arensman E, Gunnell D, Townsend E, van Heeringen K, Hazell P: Interventions for self-harm in children and adolescents. Cochrane Database Syst Rev 12:CD012013, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdiness MR: Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet 20:123–134, 1991 [DOI] [PubMed] [Google Scholar]
- Hollander M. Helping teens who cut. Using DBT to end self-injury. New York, NY: The Guilford Press, 2017. [Google Scholar]
- Hollingshead A. Four-factor index of social status. New Haven, CT: Yale University, 1975 [Google Scholar]
- Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Öz G, Cloyd JC, Tuite PJ: N-acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol 36:103–106, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz AG, Czyz EK, King CA: Predicting future suicide attempts among adolescent and emerging adult psychiatric emergency patients. J Clin Child Adolesc Psychol 44:751–761, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND: New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M, Won SJ, Park Y, Orr A, Jones DP, Swanson RA, Glass GA: Cerebrospinal fluid concentrations of N-acetylcysteine after oral administration in Parkinson's disease. Parkinsonism Relat Disord 21:500–503, 2015 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Klonsky ED: The functions of deliberate self-injury: A review of the evidence. Clin Psychol Rev 27:226–239, 2007 [DOI] [PubMed] [Google Scholar]
- Linehan MM, Comtois KA, Murray AM, Brown MZ, Gallop RJ, Heard HL, Korslund KE, Tutek DA, Reynolds SK, Lindenboim N: Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs therapy by experts for suicidal behaviors and borderline personality disorder. Arch Gen Psychiatry 63:757–766, 2006 [DOI] [PubMed] [Google Scholar]
- Linehan MM, Korslund KE, Harned MS, Gallop RJ, Lungu A, Neacsiu AD, McDavid J, Comtois KA, Murray-Gregory AM: Dialectical behavior therapy for high suicide risk in individuals with borderline personality disorder: A randomized clinical trial and component analysis. JAMA Psychiatry 72:475–482, 2015 [DOI] [PubMed] [Google Scholar]
- MacPherson HA, Cheavens JS, Fristad MA: Dialectical behavior therapy for adolescents: Theory, treatment adaptations, and empirical outcomes. Clin Child Fam Psychol Rev 16:59–80, 2013 [DOI] [PubMed] [Google Scholar]
- Magalhães PV, Dean OM, Bush AI, Copolov DL, Malhi GS, Kohlmann K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Berk M: N-acetylcysteine for major depressive episodes in bipolar disorder. Rev Bras Psiquiatr 33:374–378, 2011 [DOI] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ: An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: A pilot study. Prog Neuropsychopharmacol Biol Psychiatry 31:389–394, 2007 [DOI] [PubMed] [Google Scholar]
- McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM: Potential role of N-acetylcysteine in the management of substance use disorders. CNS Drugs 28:95–106, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey LC: Personality Assessment Inventory. 2nd ed. Lutz, FL, Psychological Assessment Resources, 2007a [Google Scholar]
- Morey LC: Personality Assessment Inventory-Adolescent. Lutz, FL, Psychological Assessment Resources, 2007b [Google Scholar]
- Muehlenkamp JJ, Claes L, Havertape L, Plener PL: International prevalence of adolescent non-suicidal self-injury and deliberate self-harm. Child Adolesc Psychiatry Mental Health 6:10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenkamp JJ, Williams KL, Gutierrez PM, Claes L: Rates of non-suicidal self-injury in high school students across five years. Arch Suicide Res 13:317–329, 2009 [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI: Oxidative stress in psychiatric disorders: Evidence base and therapeutic implications. Int J Neuropsychopharmacol 11:851–876, 2008 [DOI] [PubMed] [Google Scholar]
- Ougrin D, Tranah T, Stahl D, Moran P, Asarnow JR: Therapeutic interventions for suicide attempts and self-harm in adolescents: Systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 54:97–107.e2, 2015 [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES: Factor structure of the barratt impulsiveness scale. J Clin Psychol 51:768–774, 1995 [DOI] [PubMed] [Google Scholar]
- Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, Fisher P, et al. : Columbia-Suicide Severity Rating Scale (C-SSRS) Children's Since Last Visit. Research Foundation for Mental Hygiene, 2008
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, et al. : The Columbia-suicide severity rating scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 168:1266–1277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana F, Gormez A, Varghese S. Pharmacological interventions for self-injurious behaviour in adults with intellectual disabilities. Cochrane Database Syst Rev. 4:CD009084, 2013 [DOI] [PubMed]
- Stanford S, Jones MP: Psychological subtyping finds pathological, impulsive, and ‘normal'groups among adolescents who self‐harm. J Child Psychol Psychiatry 50:807–815, 2009 [DOI] [PubMed] [Google Scholar]
- Stanley B, Brodsky B, Nelson JD, Dulit R: Brief dialectical behavior therapy (DBT-B) for suicidal behavior and non-suicidal self injury. Arch Suicide Res 11:337–341, 2007 [DOI] [PubMed] [Google Scholar]
- Symons FJ, Thompson A, Rodriguez MC: Self-injurious behavior and the efficacy of naltrexone treatment: A quantitative synthesis. Ment Retard Dev Disabil Res Rev 10:193–200, 2004 [DOI] [PubMed] [Google Scholar]
- Tang J, Yu Y, Wu Y, Du Y, Ma Y, Zhu H, Zhang P, Liu Z: Association between non-suicidal self-injuries and suicide attempts in chinese adolescents and college students: A cross-section study. PLoS One 6:e17977, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor SE, Klonsky ED: Correlates of suicide attempts among self-injurers: A meta-analysis. Clin Psychol Rev 34:282–297, 2014 [DOI] [PubMed] [Google Scholar]
- Washburn JJ, Juzwin KR, Styer DM, Aldridge D: Measuring the urge to self-injure: Preliminary data from a clinical sample. Psychiatry Res 178:540–544, 2010 [DOI] [PubMed] [Google Scholar]
- Waterdrinker A, Berk M, Venugopal K, Rapado-Castro M, Turner A, Dean OM: Effects of N-acetyl cysteine on suicidal ideation in bipolar depression. J Clin Psychiatry 76:665, 2015 [DOI] [PubMed] [Google Scholar]
- Wechsler D: Wechsler Abbreviated Scale of Intelligence. New York, NY, The Psychological Corporation: Harcourt Brace & Company, 1999 [Google Scholar]
- Weimer K, Gulewitsch MD, Schlarb AA, Schwille-Kiuntke J, Klosterhalfen S, Enck P: Placebo effects in children: A review. Pediatr Res 74:96–102, 2013 [DOI] [PubMed] [Google Scholar]
- Wilkinson P, Kelvin R, Roberts C, Dubicka B, Goodyer I: Clinical and psychosocial predictors of suicide attempts and nonsuicidal self-injury in the adolescent depression antidepressants and psychotherapy trial (ADAPT). Am J Psychiatry 168:495–501, 2011 [DOI] [PubMed] [Google Scholar]