Abstract
Alzheimer's disease (AD) is the most common cause of dementia and a great socioeconomic burden in the aging society. Compelling evidence demonstrates that molecular change characteristics for AD, such as oxidative stress and amyloid β (Aβ) oligomerization, precede by decades the onset of clinical dementia and that the disease represents a biological and clinical continuum of stages, from asymptomatic to severely impaired. Nevertheless, the sequence of the early molecular alterations and the interplay between them are incompletely understood. This review presents current knowledge about the oxidative stress-induced impairments and compromised oxidative stress defense mechanisms in AD brain and the cross-talk between various pathophysiological insults, with the focus on excessive reactive oxygen species (ROS) generation and Aβ overproduction at the early stages of the disease. Prospects for AD therapies targeting oxidant/antioxidant imbalance are being discussed, as well as for the development of novel oxidative stress-related, blood-based biomarkers for early, noninvasive AD diagnostics.
1. Complex Pathomechanisms of Alzheimer's Disease
Alzheimer's disease (AD) is a devastating, progressive neurodegenerative disorder presenting with cognitive decline and memory impairments. It can be best described as a biological and clinical continuum, covering preclinical (presymptomatic) and clinical (symptomatic) phases, with the latter traditionally subcategorized into mild cognitive impairment (MCI) due to AD (prodromal AD) and AD dementia with mild, moderate, and finally severe late stages [1, 2].
Neuropathological hallmarks of the disease are extracellular amyloid plaques and intracellular neurofibrillary tangles accumulating in the brains of AD patients [3]. Besides these AD-specific features, a large body of evidence indicates the presence of a number of other pathologies which are common for multiple neurodegenerative diseases and for normal brain aging, reviewed in [4]. These include prominent activation of inflammatory and innate immune responses as well as excessive iron deposition and mitochondrial damage [5].
The etiology of AD is incompletely understood. Most of the AD cases are late-onset, sporadic (SAD), with probably very complex origin, influenced by genetic background [6, 7], environmental exposures [8], and aging, with age being the greatest risk factor [9, 10]. Apolipoprotein E (ApoE) genotype is the second risk factor for AD after age. The ApoE exists in three isoforms: ApoE2, ApoE3, and ApoE4, with the ApoE3 being the most common variant. The risk for AD is increased 2-3-fold in the ApoE4 heterozygous carriers and 12-fold in the homozygous ones, and each ApoE4 allele lowers the AD age of onset by approximately 5 years. On the contrary, the ApoE2 isoform appears to protect against the disease [11–14].
Despite most AD cases being sporadic, 1% of cases represent an early-onset, familial subtype of the disease (FAD), which is caused by mutations in the amyloid precursor protein (APP) [15], presenilin 1 (PSEN1) [16], and presenilin 2 (PSEN2) [17] genes. Amyloidogenic processing of APP by β-secretase (BACE) and next by γ-secretase, the catalytic component of which is presenilin (PS), results in the generation of a wide spectrum of amyloid β (Aβ) peptides [18–21], with the longer forms being the most toxic, prone to aggregation and deposition in the form of amyloid plaques in the brain [2, 22, 23].
The detection of amyloid plaques in the brains of AD patients and the discovery of pathogenic mutations in APP and PSEN1 genes have led to the formulation of the amyloid cascade hypothesis, which proposes that Aβ is the main culprit in AD pathogenesis [24]. This hypothesis was later supported by the data indicating that the A673T substitution in APP, located adjacent to the BACE1 cleavage site and making the APP a less favorable substrate for BACE1, protects against cognitive decline and AD [25]. Nevertheless, further studies have demonstrated that although Aβ deposition is necessary for the development of the disease it is not sufficient, since the Aβ pathology can exist with the lack of clinical dementia [26]. In addition, several clinical trials targeting Aβ failed to provide therapeutic benefits [27]. These findings have led to the refinement of the amyloid cascade hypothesis and to the formulation of several new hypotheses, including the mitochondrial cascade and oxidative stress hypotheses, which place AD in the context of a free radical theory of aging [28–31], and the dual pathway hypothesis, which proposes the existence of common upstream drivers that cause amyloid and tau pathologies [32].
Here, we will review the current knowledge on the implication of one of those possible drivers—oxidative stress—in the development of AD pathology (Figure 1), with the major emphasis placed on the interplay between reactive oxygen species (ROS) and Aβ deposition. Furthermore, we will summarize how oxidant/antioxidant imbalance, detected in the AD brain, is reflected in peripheral cells, pointing towards the potential development of novel, noninvasive, blood-based biomarkers for early AD diagnostics and for monitoring of the response to therapeutic interventions.
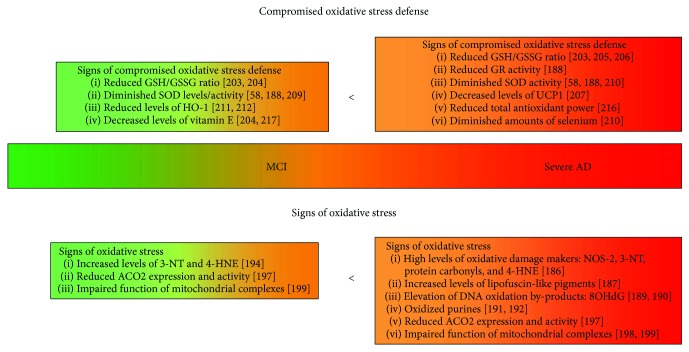
Figure 1.
Escalating signs of the antioxidant/oxidant imbalance detected in whole blood during the AD progression. The figure schematically presents the progressive impairments in the oxidative stress defense mechanisms during the progression from mild cognitive impairment (MCI) towards severe AD. These correspond to the elevation in the oxidative stress markers. The green-orange-red colour scale corresponds to healthy stage-MCI-AD progression. AD: Alzheimer's disease; MCI: mild cognitive impairment; ACO2: aconitase 2; GR: glutathione reductase; GSH/GSSG: reduced/oxidized glutathione pool; 4-HNE: 4-hydroxynonenal; NOS-2: nitric oxide synthase 2; 3-NT: 3-nitrotyrosine; 8OHdG: 8-oxo-2′-deoxyguanosine; SOD: superoxide dismutase; UCP-1: uncoupling protein 1.
2. The Definition and Causes of the Oxidative Stress
Oxidative stress is defined as an imbalance between oxidants and antioxidants, in favor of the oxidants. It leads to the disruption of redox signaling and control and/or causes damage to biomolecules [33–35]. The oxidative stress is characterized by an excessive accumulation of toxic molecules containing an oxygen atom with an unpaired electron in its outer shell, formed as end products of the normal metabolism of oxygen and known as ROS. The main source of ROS is the electron transport chain (ETC) at the mitochondrial inner membrane, where energy is generated in the form of ATP [36]. Under physiological redox conditions in the cell, ROS fulfill important roles (redox biology) in cell signaling, homeostasis, and in the immune system's response against invading pathogens. In addition, reactive oxygen and nitrogen species regulate such key biological processes at the organismal level as blood circulation, energy metabolism, reproduction, embryonic development, and remodeling of tissues through apoptotic mechanism. Finally, it has been proposed that moderate levels of ROS may induce several protective responses that prepare cells to better handle the subsequent stress. This concept is known as mitohormesis [37–40].
Given the physiological importance of the moderate ROS levels and the harmful consequences of excessive ROS generation, precise mechanisms must have been developed to regulate reducing and oxidizing conditions within the cell (cellular redox state). Aerobic organisms have integrated antioxidant systems, which include enzymatic and nonenzymatic antioxidants, usually effective in scavenging ROS and preventing their detrimental consequences. Overwhelming and/or failure of the redox control and antioxidant systems would inevitably lead to the oxidative stress.
Neurons in the brain are at extremely high risk of excessive ROS generation and oxidative damage since they show high oxygen consumption and energy production. About 20% of all organismal oxygen and 25% of all glucose are used for cerebral functions [41]. The vulnerability of neurons to the oxidative damage stems also from a relatively large amount of redox active metals, promoting ROS formation, high content of polyunsaturated fatty acids (PUFA), which are more sensitive to oxidation, and from comparatively modest levels of antioxidant enzymes [42].
Given the vulnerability of neurons to ROS, it is not surprising that oxidative stress has been widely proposed to be implicated in the initiation and the progression of a number of neurodegenerative diseases, including AD [43–47]. In line with that, markers of oxidative damage [48] to (1) lipids, indexed by thiobarbituric acid reactive substances (TBARS), alterations in PUFA, and the presence of PUFA breakdown products [45, 49, 50]; (2) proteins, marked by protein carbonyls, 3-nitrotyrosine (3-NT), and 4-hydroxy-2-nonenal (4-HNE) [51, 52]; and (3) nucleic acids [53], including nuclear [54] and mitochondrial DNA [55–57] as well as RNA [45], marked by 8-hydroxy-2′-deoxyguanosine (8OHdG) and 8-hydroxyguanosine (8-OHG), have been frequently described in the brains of MCI and AD patients. Importantly, the oxidative damage present in the brain is reflected in peripheral cells, raising a possibility for further research and the potential use of the oxidative stress biomarkers for noninvasive blood-based AD diagnostic and monitoring [58–60]. However, no validated blood-based biomarker has been established so far.
3. Oxidative Stress in AD Brain: Interplay between ROS and Aβ
Although it has been widely accepted that the presence of oxidative damage is one of the pathological hallmarks of AD, it remains uncertain whether this is a cause or a consequence of pathogenic processes occurring in the brain. The hypothesis that oxidative damage is an early event in the disease, triggering the occurrence of other pathologies, is supported by the association of the AD risk factors, including ApoE4 genotype, with higher oxidative insults. Comparison of the hippocampi from AD patients demonstrated that ApoE protein expression is reduced in ApoE4 carriers compared to ApoE3/ApoE3 controls and indicated an association between decreased ApoE protein amounts and higher oxidative stress to lipids, manifesting with increased levels of TBARS [61]. Furthermore, levels of isoprostanes, markers of lipid peroxidation [62], have been reported to be increased in cerebrospinal fluid (CSF) in ApoE4-positive healthy individuals and in AD patients [63–66]. These observations are in line with in vitro data demonstrating that different ApoE isoforms possess different antioxidant properties, with ApoE2 being the most and ApoE4 the least protective variant [67].
Other well-established risk factors for AD are insulin resistance and type 2 diabetes mellitus. Several studies have demonstrated that oxidative stress plays an important role in the pathogenesis of these conditions and that there is an overlap between pathophysiological mechanisms implicated in type 2 diabetes and AD [68–73]. Hyperinsulinemia, often seen in type 2 diabetes, may inhibit degradation of extracellular Aβ through the insulin-degrading enzyme (IDE) [74]. Moreover, the hyperglycemia may result in enhanced metabolism of glucose and in turn in increased levels of NADH and FADH2, which are used by mitochondria to generate ATP. Overproduction of NADH leads to greater proton gradient and consequently to an excessive generation of ROS [71, 75].
In addition, the increased levels of intracellular glucose lead to the nonenzymatic glycosylation of cellular proteins, lipoproteins, or nucleic acids. The resulting products are known as advanced glycation end products (AGEs). RAGE is the receptor for AGE, and it is a pattern recognition receptor that binds multiple ligands derived from damaged cell environment [76] as well as Aβ [77]. The interaction of RAGE with its ligands results in enhanced ROS generation [78], due to the activation of NADPH oxidase (NOX) [79]. Of note, RAGE has been widely implicated in the pathomechanism of AD [80]. Increased expression of RAGE has been demonstrated in the brains of AD mouse models [81]. Administration of high-AGE diet has been found to lead to spatial memory impairments, oxidative damage to vasculature, and increased levels of insoluble Aβ in mouse hippocampi [82]. Finally, polymorphisms in RAGE encoding gene, AGER, have been reported to genetically predispose to AD [83]. Another link between diabetes mellitus and AD is evidenced by the fact that oxidative stress and insulin resistance may promote transcriptional activity of Forkhead box O (FoxO) proteins [84], resulting in enhanced expression of gluconeogenic enzymes, which leads to hyperglycemia and further increase in ROS production in a vicious cycle [85].
More indications of the early involvement of the oxidative stress in AD pathogenesis stem from the data showing that markers of oxidative stress in the APP23 mice, carrying APP KM670/671NL mutation, and triple transgenic mice, harboring APP KM670/671NL, PS1 M146 V, and Tau P301L mutations, are present relatively early, before the formation of amyloid deposits [86, 87]. Furthermore, analysis of Aβ40 production in vitro and in vivo upon pharmacological or genetic inhibition of mitochondrial ETC, either by targeting complex I and/or complex III, has demonstrated that enhanced generation of ROS by mitochondria increases BACE1 activity and consequently promotes amyloidogenic APP processing and Aβ overproduction [88]. This is consistent with other studies showing that hydrogen peroxide- (H2O2-) induced oxidative stress increases the activity of BACE1 promoter in human embryonic kidney (HEK) cells [89] and results in elevated levels of secreted Aβ42 in differentiated SH-SY5Y neuroblastoma cell line [90]. Of note, intracellular Aβ42 accumulation is also observed upon H2O2 treatment, but this is apparent only during the first hour of the stimulation and decreases to the baseline later during the time course [90]. Aβ generation in the oxidative conditions might be also affected by changes in the function of the PS1/γ-secretase complex. Injection of 4-HNE or 4,4′-dithiodipyridine (DTDP) into the mouse brain has been reported to induce pathogenic shift in the arrangement of PS1 subdomains within the γ-secretase complex, resulting in enhanced generation of longer, more prone to aggregation Aβ species (Aβ42), relative to the shorter forms (Aβ40) [91, 92]. Other studies have suggested that the PS1 conformational change might be a consequence of 4-HNE modification of one of the members of the γ-secretase complex—nicastrin—in the oxidative environment [93].
Importantly, it has been reported that not only ROS can modulate Aβ production/secretion but also Aβ can reciprocally promote excessive ROS generation in a vicious cycle. Aβ overproduction, achieved by APP overexpression, has been shown to enhance ROS generation, reduce the respiratory control ratio (RCR), and diminish ATP production, suggestive of the existence of a positive ROS→Aβ feedback loop [88]. Exposure of neurons to oligomeric Aβ leads to enhanced ROS production, probably through a mechanism involving stimulation of N-methyl-D-aspartic acid (NMDA) receptors and consequent activation of NOX. ROS derived from NOX may lead to the activation of kinases, including ERK1/2, downstream activation of calcium-dependent phospholipase A2 (cPLA2), release of arachidonic acid, and perturbations in the membrane phospholipids [94–96].
The detrimental role of APP/Aβ in the induction of oxidative stress has been also reported in several in vivo studies [97]. For example, a transgenic AD mouse model, harboring APP KM670/671NL and APP V717F mutations, has been reported to present elevated levels of protein and lipid oxidation markers, such as protein carbonyls, 3-NT, and 4-HNE. Interestingly, the observed oxidative damage appears to be dependent on the methionine 35 in the Aβ peptide [98, 99]. Furthermore, intracranial imaging of APP KM670/671NL, PS1dE9 mouse brains has revealed that senile plaques are directly responsible for ROS generation [100].
4. Mitochondrial Impairments in AD Brain
There are compelling evidences that aberrant mitochondrial structure and function are important hallmarks of AD pathology. The expression and activity of mitochondrial enzymes, such as cytochrome c oxidase (COX), α-ketoglutarate dehydrogenase complex, and pyruvate dehydrogenase complex, are reduced in AD [101, 102]. Moreover, AD brain mitochondria present diminished membrane potential and increased permeability, and produce excess ROS which lead to protein, lipid, and nucleic acid damage. Furthermore, abnormal mitochondrial structure, decreased anterograde movement, and compromised mitochondrial functions have been described in neurons from an APP transgenic AD mouse model [103]. Finally, increased autophagy of mitochondria [104, 105] and altered mitochondrial structure have been observed in AD brains [106].
Although mitochondrial impairments are clearly associated with AD pathology, it is an ongoing debate whether they cause or result from an altered Aβ and tau biology. Several studies imply that mitochondrial cascade hypothesis may explain the etiology of late-onset, SAD. This hypothesis states that mitochondrial dysfunctions are the primary events leading to aberrant APP processing and Aβ deposition [28, 107]. In line with this hypothesis, chemical inhibition of oxidative energy metabolism has been reported to modulate APP processing in PC12 cells [108] and in COS cell line stably expressing APP [109, 110]. Moreover, combination of FDG- and PiB-PET imaging has demonstrated that brain hypometabolism is detected before Aβ deposition in cognitively normal individuals at risk for developing AD [111].
On the other hand, it is also possible that early alterations in Aβ biology trigger mitochondrial pathology. It has been reported that Aβ peptides interact directly with Aβ-binding alcohol dehydrogenase (ABAD) in mitochondria, and via this interaction, Aβ may cause leakage of ROS and mitochondrial dysfunctions [112]. Moreover, Aβ has been demonstrated to detrimentally impact mitochondrial enzymes, which contain iron-sulphur center, inhibit complex I and IV, reduce ATP levels, and induce mitochondrial depolarization [113–118]. Aβ may also increase the expression of heme oxygenase 1 (HO-1) enzyme which may secondarily compromise mitochondrial integrity [119], and Aβ plaques have been proposed to be a source of toxicity that induces severe mitochondrial structural and functional abnormalities in vivo in the mouse brain [120]. In addition, several studies indicate that APP and Aβ might affect fussion and fission of mitochondria. Elevated expression of mitochondrial fission genes, encoding dynamin-related protein 1 (Drp1) and fission 1 (Fis1), and reduced expression of the fussion ones, encoding mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), optic atrophy 1 (Opa1), and Tomm40, have been detected in AD brains, fibroblasts from AD patients, neurons isolated from APP transgenic mice, and cell lines overexpressing APP [121–123]. Moreover, treatments of neurons with Aβ peptides have been shown to affect mitochondrion movement [124]. All these may result in structural changes of mitochondria, decreased clearance of defective mitochondria, and enhanced neurodegeneration.
Not only Aβ but also tau might lead to mitochondrial pathology. Tau protein has been shown to play an important role in the trafficking of organelles, including mitochondria, and this tau function might be compromised in neurons in AD. In line with that, overexpression of tau in CHO and N2A cell lines as well as in neurons results in subcellular misdistribution of mitochondria, potentially via alterations in Drp1 mitochondrial location [125, 126], and genetic reduction of tau rescues aberrant mitochondrial trafficking in APP transgenic neurons [127]. Furthermore, it has been demonstrated that Aβ-triggered hyperphosphorylation of tau leads to its detachment from microtubules and abnormal mitochondria trafficking [128]. Finally, N-terminal fragment of tau has been reported to colocalize with adenine nucleotide translocator 1 (ANT1) in AD mitochondria, impair ANT function, and consequently compromise mitochondrial oxidative phosphorylation [129, 130].
Overall, these data suggest that oxidative stress is an early factor in AD pathogenesis that can lead to mitochondrial dysfunction, cell signaling impairments, and increased production of Aβ. In turn, Aβ overproduction, oligomerization, and aggregation reciprocally augment the oxidative stress. Once the positive feedback loop between ROS production and Aβ is initiated, it gradually enhances and accelerates the brain pathology.
5. Compromised Oxidative Stress Defense Mechanisms in AD Brain
On the opposite site of the ROS generation machinery stands the entire network of the oxidative stress defense mechanisms, developed to detect and control diverse forms of the oxidative stress. These include glutathione redox cycle, heat shock pathways, hemoxygenase and thioredoxin (Thx) systems, and superoxide peroxidases. Redox-regulated proteins, being part of these systems, are encoded by vitagenes, proposed to assure longevity [131, 132].
One of the most prevalent antioxidants in the cell is glutathione, which exists in a dynamic pool of thiol-reduced (GSH) and disulfide-oxidized states. GSH can react with free radicals either independently or in the reaction catalyzed by glutathione peroxidase (GPx) to form glutathione disulfide (GSSG), which can be converted back to the reduced state by glutathione reductase (GR). Alternatively, glutathione S-transferases (GST) can catalyze a reaction between GSH and nucleophilic compounds that react with thiols in proteins. Considering the antioxidant properties of GSH and its depletion under oxidative stress conditions, GSH/GSSG ratio is frequently used as an indicator of cell redox potential and oxidative stress [133, 134] and has been also analyzed to assess the oxidative stress in AD. Postmortem analysis of the human brains revealed decreased GSH levels and increased GSSG amount, resulting in diminished GSH/GSSG ratio, in postmitochondrial supernatant (PMS), mitochondrial, and synaptosomal fractions obtained from frontal cortices of MCI, as well as mild/moderate and severe AD patients compared to controls [135, 136]. Similar results have been obtained with the application of proton magnetic resonance spectroscopy for in vivo GSH analysis in human subjects. Importantly, the reduction in GSH has been detected selectively in the brain regions affected by AD pathology, such as frontal cortex and hippocampus, but not in the cerebellum, and correlated with the extent of cognitive impairments, assessed with Mini-Mental State Examination (MMSE) and clinical dementia rate (CDR) scores [137]. In addition to the altered GSH/GSSG balance, a trend towards decreased activity of GR, GPx, and GST enzymes has been detected, with statistically significant changes recorded in mitochondrial fraction from AD versus controls and in the synaptosomal fraction from both MCI and AD patients versus control individuals. Interestingly, the magnitude of the changes in the glutathione redox cycle seems to correspond with the disease severity [135, 137].
Another important antioxidant enzymes are superoxide dismutase (SOD) and catalase (Cat), which catalyze the disproportionation of superoxide to molecular oxygen and peroxide and the conversion of H2O2 to water and oxygen, respectively. The activity of these enzymes has been reported to be reduced through the entire AD continuum, from MCI to severely cognitively impaired patients, indicative of compromised oxidative stress defense mechanisms already early in the disease [135]. Of note, Karelson et al. reported an increase in SOD and Cat activities, selectively in temporal inferior, but not in the frontal inferior, sensory postcentral, and occipital cortices in AD versus control brains [136]. In addition, a trend towards reduced mRNA expression of peptide methionine sulfoxide reductase (MsrA), an enzyme converting methionine sulfoxide to methionine, and significantly diminished MsrA activity, to a much greater extent, has been detected in AD-affected brain regions, such as hippocampus, superior and middle temporal gyri, and inferior parietal lobule [138]. In addition, reduced levels of Thx, a potent ROS scavenger, have been observed in amygdala, hippocampus, and superior and middle temporal gyri in AD [139]. Reduced transcription of detoxifying genes and diminished expression of antioxidant enzymes are in line with decreased levels of nuclear factor (erythroid-derived 2)-like 2 (Nrf2), a transcription factor activated upon exposure to oxidative stress [140], in the nucleus of hippocampal neurons in human AD brains [141].
On the other hand, increased protein levels and/or activity of enzymes, which are induced as protective mechanisms against oxidative stress, have been reported. Thx reductase activity appears to be elevated in amygdala and cerebellum in AD versus controls [139]. In addition, increased HO-1 protein expression has been reported in AD, with significant changes observed only in neocortex and hippocampus but not in substantia nigra. Chronic upregulation of HO-1 may exacerbate the degenerative process by promoting pathological iron deposition and oxidative mitochondrial damage [142]. Interestingly, detailed immunofluorescence analysis of the HO-1 localization has revealed that the expression of this enzyme is elevated both in neurons and glial fibrillary acidic protein- (GFAP-) positive astrocytes and that senile plaques and neurofibrillary tangles are immunopositive for HO-1 [143]. Importantly, the percentage of double GFAP and HO-1-positive astrocytes is much higher in temporal cortex and hippocampus of MCI and AD patients compared to controls and shows a negative correlation with global cognitive performance and a positive one with AD pathology, with the latter apparent only in the temporal cortex [144]. The induction of the expression of the protective enzymes has been proposed to be a result of a chronic exposure to high levels of oxidative stress in AD [143, 144]. Overall, the described studies indicate that AD is associated with unsuccessful cellular attempts to induce oxidative stress defense mechanisms and to compensate for the oxidative damage.
6. Oxidant/Antioxidant Imbalance as a Therapeutic Target in AD
Compelling evidence presenting disrupted oxidant/antioxidant balance in AD led to the formulation of a hypothesis that compounds scavenging free radicals, and/or boosting oxidative stress defense mechanisms might provide therapeutic benefits in AD. Therefore, several antioxidants, such as N-acetylcysteine, curcumin, resveratrol, vitamin E, ferulic acid, coenzyme Q (CoQ), selenium, and melatonin, have been tested for their potential to preserve or improve cognitive performance in healthy individuals, MCI, and AD, as reviewed in [145–147]. However, despite potent effects of these compounds on cellular oxidative status and/or Aβ pathology in vitro and in vivo, the convincing evidence of their therapeutic potential in humans is lacking.
Recently, Mazzanti et al. reviewed a number of published and ongoing clinical trials with the use of curcumin and resveratrol for AD therapy [148, 149]. These compounds are known for their potent antioxidant activity in vitro and in vivo. They have a potential to scavenge nitric oxide (NO) and hydroxyl radicals, increase brain glutathione content and HO-1 expression, activate sirtuins, and prevent Aβ-mediated ROS accumulation [150]. Despite all these promising data, clinical trials demonstrated that the efficacy of these compounds in preserving or restoring cognitive functions in humans, both in healthy individuals as well as in clinical AD patients, is limited [148].
Similarly, there is no convincing evidence that supplementation with vitamin E, known to protect lipids from peroxidation, can provide therapeutic benefits in healthy individuals, MCI, and AD. Although few clinical trials have demonstrated some delay in the cognitive decline upon vitamin E administration, others have not shown any associations of the use of this compound with reduced cognitive deterioration, or even reported detrimental effects of vitamin E on neuropsychological functions of MCI and AD patients [151, 152]. Similarly, negative data have been reported in the trial of a combination of vitamin E with other antioxidants, such as vitamin C and α-lipoic acid. Despite the decrease in F2-isoprostanes in the CSF, consistent with antioxidant potential, no change in other CSF biomarkers, such as Aβ, tau, or phospho-tau, and more rapid MMSE score decline have been found in the treatment versus the placebo group [153]. In the latter study, CoQ supplementation in AD patients has been also evaluated. Unfortunately, the results have been also negative [153]. Similarly, no associations between the long-term use of antioxidants, such as vitamin E and selenium, and dementia incidence in healthy individuals were found in the large-scale primary prevention trial [154].
On the other hand, other large-scale epidemiological studies have given more promising results, suggesting that eating food reach in antioxidant supplements, beta carotene, and vitamins C and E is associated with lower risk of AD dementia [155, 156]. More hopeful data stem also from the clinical trials with glutathione precursor, N-acetylcysteine, and melatonin. It has been demonstrated that administration of N-acetylcysteine might provide modest cognitive benefits in AD patients [147]. Similarly, treatment with melatonin seems to improve sleep quality and cognitive performance in MCI patients. However, more research is needed to provide evidence of its efficacy in AD patients [157]. A recent meta-analysis of randomized double-blind clinical trials of melatonin in AD has demonstrated that the compound improves total sleep at night but has no effect on the MMSE score in AD [158].
Furthermore, as previously highlighted, alterations in mitochondrial bioenergetics, defects in mitochondrial dynamics, and ROS-mediated mitochondria damage play a key role in AD pathogenesis. Therefore, strategies that target mitochondrial dysfunctions, enhance mitochondrial bioenergetics, or reverse oxidative stress may serve as novel AD therapeutics. One of such therapeutics might be CoQ10, known also as an ubiquinone, which acts as an important cofactor of the ETC. Both in vitro and in vivo studies have demonstrated the neuroprotective potential of CoQ10 in AD [159, 160]. It has been shown that this lipophilic antioxidant compound improves cognitive functions, facilitates ATP synthesis, and upregulates mitochondrial function [161]. Furthermore, it is well documented that CoQ10 supplementation significantly increases brain CoQ10 amount, providing protection from free radical-mediated oxidative damage to biomolecules [162]. An analog of CoQ10 is idebenone. This compound consists of short chains of isoprene units, crosses blood-brain barrier easily, is well tolerated in humans, possesses good antioxidant properties, and provides neuroprotection against Aβ-induced neurotoxicity [163, 164]. Another potent antioxidant, acting as an effective inhibitor of mitochondrial permeability transition pore (mPTP), is creatine [165]. It exerts neuroprotective potential in different neurodegenerative diseases, including AD. Creatine supplementation has been shown to protect against neuronal death caused by NMDA, malonate, Aβ, and ibotenic acid [166].
In addition, due to the importance of mitochondrial dysfunctions in the pathogenesis of various disorders, scientists have focused on the engineering of therapeutic molecules that could accumulate in mitochondria. One of such compounds is MitoQ, which is the most widely used mitochondria-targeting antioxidant. MitoQ exhibits neuroprotection via scavenging peroxynitrite and superoxide and consequently protecting mitochondria against lipid peroxidation [167]. Another antioxidant acting in a similar manner is MitoVitE, also known as mitotocopherol, which is triphenylphosphonium (TPP) conjugated to α-tocopherol moiety of vitamin E via two-carbon chain. It also protects mitochondria from oxidative stress via inhibition of lipid peroxidation [168, 169]. Another used TPP derivative is MitoTEMPOL, which acts by accepting an electron from hydroxylamine. Moreover, MitoTEMPOL functions as a SOD mimetic, whose role is to convert superoxide molecules into water to detoxify ferrous iron into ferric iron. Its beneficial effects against mitochondrial dysfunction and mitochondria-mediated oxidative stress were shown in an in vitro study [170].
The question arises why, despite the clear implication of oxidative stress in AD pathology, and beneficial action of ROS targeting therapeutics in vitro and in mouse models, drugs targeting impaired oxidant/antioxidant balance quite often fail to provide benefits in clinical trials. It is possible that due to the physiological importance of moderate ROS levels and redox signaling, high doses of antioxidants, as often administered in clinical trials, are detrimental rather than beneficial for the cell physiology [39, 171]. In line with that, administration of high vitamin E doses has been reported to increase mortality [172].
Furthermore, the tested antioxidant therapeutics might present poor bioavailability and efficiency in reaching their targets due to their low solubility and absorption, together with a rapid metabolism and elimination [150, 173]. These limitations might be overcome by the development of novel delivery systems, such as adjuvants, nanoparticles, liposomes, micelles, phospholipid complexes, and nanogels. Interestingly, selenium, melanin, and cerium oxide-nanoparticles have been reported to reduce ROS levels and boost oxidative stress defense mechanisms in vitro and in vivo. Similarly, some of the preparations improving the bioavailability of curcumin and resveratrol have been reported to present therapeutic benefits, as reviewed in [148]. This raises a potential that such compounds might become novel therapeutics for oxidative stress-related disorders, including AD [174–178].
Another reason might be the inappropriate design of the clinical trials [148, 179, 180]. The number of recruited patients is usually relatively small, and the duration of the follow-up is inadequately short, preventing the possibility to detect potential improvements in cognitive functions. Moreover, the treatments are often administered to patients at the advanced AD stage with clearly developed clinical symptoms. It needs to be noted that molecular pathogenic processes in the brain precede by decades the onset of symptomatic cognitive decline. In symptomatic AD or even in MCI, the pathological changes, such as Aβ accumulation and synapse loss, are already evident and probably cannot be reversed by the use of antioxidants [1, 181]. Therefore, the therapeutics should be administered earlier, before the substantial synaptic/neuronal loss. Unfortunately, the AD field suffers from the lack of diagnostic tools which would allow the detection of the disease at its presymptomatic phase. This leads to the inadequate recruitment of patients for clinical trials and might be improved by the discovery of novel easily accessible, optimally blood-based biomarkers allowing not only the diagnosis of AD at the preclinical phase but also sensitive biomarker-based monitoring of the therapeutic efficacy of the administered drugs.
7. Diagnostic Prospects Based on Oxidative Stress Biomarkers in Blood Cells
The AD diagnostics critically needs novel biomarkers for early AD detection. These have to be cost-effective and located in easily available diagnostic tissues, which are not the case for the currently used CSF or brain imaging biomarkers, reviewed in [182]. Among the main advantages of the blood-based biomarkers are their lower risk and discomfort for patients and thus increased applicability in an ageing population. Blood-based biomarkers for early AD can be employed as a first step in large-scale screening to recruit patients who should undergo further, more sophisticated and costly testing. Moreover, they are adequate for repeated measures and hence could be useful for monitoring therapeutic outcomes in longitudinal studies. However, so far, no blood-based biomarkers have been accepted for AD diagnostics.
Novel prospects for identification of AD biomarkers in blood have emerged recently based on systemic presentation of oxidative stress. Namely, several studies have demonstrated that the imbalance in oxidative stress and antioxidant defense occurring in the brain is reflected in peripheral cells and in particular in easily accessible blood cells that could be used for early disease diagnostics and therapy monitoring, as reviewed in [59, 60, 182, 183]. Accordingly, it has been reported that ROS levels are elevated in AD lymphocytes and platelets compared to controls [184, 185]. This increase seems to correspond to higher levels of oxidative damage markers, such as nitric oxide synthase 2 (NOS-2), 3-NT, protein carbonyls, and 4-HNE [186]. Furthermore, fluorescence spectroscopy analysis of lipofuscin-like pigments (LFPs), end products of lipid peroxidation, has demonstrated increased LFP levels in AD erythrocytes compared to controls [187]. Of note, other studies have suggested that the increase in 3-NT and 4-HNE is specific only to lymphocytes from FAD patients and is not marked in the sporadic cases [188], suggesting that oxidative stress is associated with early Aβ overproduction.
Not only proteins and lipids but also nucleic acids can undergo oxidative damage. Consistently, application of high pressure liquid chromatography (HPLC) for the analysis of 8OHdG, a DNA oxidation by-product, has revealed elevation of this marker in AD versus control lymphocytes [189, 190]. Furthermore, using comet assay, modified with oxidative lesion-specific endonucleases, it has been demonstrated that AD lymphocytes contain significantly elevated levels of oxidized purines [191, 192]. All these data support the hypothesis that oxidative damage to proteins and nucleic acids in AD is a systemic event that manifests in peripheral cells in blood.
8. Mitochondrial Dysfunctions in Blood Cells as Potential AD Biomarkers
Particular emphasis in the search for novel oxidative stress-related blood-based biomarkers has been placed on mitochondria since they are the main source of oxidative stress, and their impairments may result in the leakage of superoxide radicals and consequent increased generation of reactive nitrogen and oxygen species [193]. This resulted in the report of the elevation of the protein-bound 4-HNE and 3-NT in the mitochondria isolated from lymphocytes from MCI patients, with the levels of protein-bound 4-HNE showing a significant negative correlation with the MMSE score [194]. Furthermore, several mitochondrial proteins, grouped in four categories, cell signaling, structural, cellular energetics, and cellular defense, that are altered between lymphocytes from control versus MCI versus AD patients have been identified. This points towards particular pathways that might be responsible for the initiation of cognitive decline and for the later transition from MCI to AD [195]. Another evidence of the oxidative damage to the mitochondria in blood lymphocytes in AD has been provided by the analysis of the mitochondrial aconitase 2 (ACO2), which is a Krebs cycle enzyme sensitive to free radical-mediated damage, due to the presence of an iron-sulphur group [196]. It has been demonstrated that ACO2 expression and activity are significantly lower in AD and MCI lymphocytes compared to controls and that these changes correlate with MMSE score [197].
In addition, assuming that impaired mitochondrial energetics may be causative of the excess generation of ROS, several groups have analyzed the activity of mitochondrial complexes in peripheral cells/elements, that is, lymphocytes and platelets from MCI and AD patients, but these studies resulted in contradicting data. Molina et al. reported no changes in the activity of the respiratory chain enzymes in the mitochondria isolated from lymphocytes from AD patients and control individuals [198], while later reports have shown decreased activity of complexes III and IV in the mitochondria isolated from AD platelets and decline in the activity of complex IV in these from MCI patients [199]. On the other hand, Feldhaus et al. observed increased activity of the complex II and complex IV in AD lymphocytes [200]. Despite the discrepancies, these potential impairments in the function of the respective mitochondrial complexes may manifest in the altered oxidative phosphorylation (OXPHOS), that is, mitochondrial respiration capacity, which represents the entire electron transport chain, comprised of the four complexes and F1F0ATP synthase. Indeed, basal level of respiration, total OXPHOS capacity, and RCR, indicative of mitochondria coupling state, are diminished in AD compared to control lymphocytes [201]. All these studies imply that mitochondrial energetics and oxygen consumption show impairments already early in the disease. This might lead to increased ROS generation and consequent cascade of oxidative damage at the later stages.
9. Reflection of Impaired Oxidative Stress Defense Mechanisms in Blood Cells
The alterations of the oxidative stress defense mechanisms have been also detected in peripheral tissues, including blood cells, supporting the hypothesis of the systemic nature of the disease (Table 1).
Table 1.
Impaired oxidative stress defense mechanisms in the brain and peripheral blood in AD.
|
|
|---|---|
| GSH ↓, GSSG ↑, GSH/GSSG ↓ [135, 136] | GSH ↓, GSSG ↓↑ [186, 202–204] |
| GR activity ↓, GPX activity ↓, GST activity ↓ [135, 137] | GR activity ↓ [188] GPX protein ↑, GCL protein ↑ [203, 206] |
| SOD activity ↓↑ [135, 136] | SOD mRNA ↑, SOD activity ↓ [58, 188, 209] |
| Thx protein ↓, ThxR protein ↑ [139] | Thx protein ↑, ThxR protein ↑ [186, 207] |
| HO-1 protein ↑ [143, 144] | HO-1 protein ↑, HO-1 activity ↑ [186, 207] |
| Nrf-2 in nucleus ↓ [141] | P-Nrf2 ↑ [209] |
| Cat activity ↓↑ [135, 136] | Total antioxidant status ↓ [216] |
| MsrA mRNA ↓ [138] | SIRT-1 protein ↑ [186, 207] |
| HSP60 protein ↑, HSP70 protein ↑ [186, 207] | |
| UCP1 protein ↓ [207] | |
| Vitamin E ↓ [204, 217] | |
| Selenium ↓ [218] |
The table summarizes the changes in the oxidative stress defense mechanisms reported in the brain and blood from MCI and/or AD patients relative to healthy controls; the corresponding references are given; arrows indicate direction of changes in protein levels or activities, described in detail in the text. AD: Alzheimer's disease; MCI: mild cognitive impairment; Cat: catalyse, GCL: glutamylcysteine ligase; GSH: reduced glutathione; GSSG: oxidized glutathione; GPx: glutathione peroxidase; GR: glutathione reductase; GST: glutathione S-transferase; HO-1: heme oxygenase 1; Hsp: heat shock protein; MsrA: methionine sulfoxide reductase A; Nrf-2: nuclear factor (erythroid-derived 2)-like 2; SIRT-1: sirtuin 1; SOD: superoxide dismutase; Thx: thioredoxin; ThxR: thioredoxin reductase; UCP1: uncoupling protein 1.
Reduced GSH levels with unchanged GSSG amount in lymphoblasts from FAD but not from SAD patients have been detected [202]. More recent studies have demonstrated a reduction in GSH and an increase in the GSSG in MCI and SAD lymphocytes and plasma [186, 203, 204] and a correlation between plasma GSH/GSSG ratio and MMSE score [205]. Finally, it has been reported that both FAD and SAD lymphocytes present reduced GR activity [188]. However, it might be possible that these changes represent only full-blown AD clinical pathology since the analysis of samples from young healthy individuals at risk of developing AD, due to the carriage of at least one ApoE4 allele, has demonstrated reduced GSSG levels in whole blood and increased amount of GPx and glutamylcysteine ligase (GCL) in lymphocytes when compared to the ApoE3/ApoE3 controls. These data are indicative of the existence of reductive rather than oxidative stress at the presymptomatic state of disease. The situation is reversed in MCI and clinical AD, potentially due to the exhaustion of oxidative stress defense mechanisms [203, 206].
In addition to GSH, other markers of altered oxidative stress defense mechanisms have been also studied and found to be changed in AD versus control peripheral blood samples. Levels and/or activity of sirtuin-1 (SIRT-1), Thx, HO-1, Hsp60, Hsp72, and Thx reductase (ThxR) are elevated in plasma and/or lymphocytes from AD versus control samples [186, 207]. Moreover, mRNA levels of CuZnSOD are higher in lymphocytes from AD patients when compared to healthy controls as well as Parkinson's disease patients, suggestive of disease specificity [208]. These changes probably represent cellular attempts to overcome consequences and survive constant exposure to high levels of ROS and are consistent with the activation of transcription factors, such as Nrf-2, and the phosphorylation of which at the serine 40 has been reported to be significantly increased in peripheral mononuclear blood cells (PBMC) from MCI patients and present a modest, although nonsignificant, elevation at the later stages of the disease [209]. However, the defense mechanisms seem to be not entirely functional in AD cells. Despite the increased CuZnSOD mRNA levels [208], no change in CuZnSOD and MnSOD protein levels and/or diminished Ec-CuZnSOD activity in MCI, SAD, and FAD lymphocytes [58, 188] and in SAD erythrocytes [210] has been reported. Of note, there is some inconsistency in the reports of the changes of SOD expression in different studies. Opposed to the other reports, Mota et al. demonstrated that the protein levels of SOD are significantly diminished in PBMC from MCI patients and show a trend towards an elevation in PBMC from mild AD patients [209]. Inconsistencies are also present between the reports on the HO-1 expression. While some studies demonstrated increased protein levels of HO-1 in AD lymphocytes [186], others have shown decreased HO-1 mRNA and protein levels in lymphocytes, plasma, choroid plexus, and CSF from early AD and from some amnestic MCI patients [211, 212] and augmented activity of HO-1 suppressor (HOS) in AD plasma [213]. The inconsistencies might be due to different criteria used for patient selection, population variabilities, and/or various experimental strategies applied in the studies. Nevertheless, levels of other stress defense mechanisms such as those involving mitochondrial uncoupling protein 1 (UCP1), known to reduce superoxide and H2O2 generation, are also diminished in AD plasma [207]. Consequently, the impaired oxidative stress defense mechanisms may yield cells from AD patients more vulnerable to H2O2-induced oxidative stress-triggered cell death. In line with that, AD lymphocytes cannot induce CuZnSOD mRNA expression upon H2O2 stimulation and their vulnerability to the cell death correlates with dementia severity [208, 214, 215].
Yet another evidence of the compromised defense mechanisms in AD stems from the observation of reduced total antioxidant power, a cumulative measure of the amount of all plasma antioxidants, such as vitamin E, uric acid, vitamin C, bilirubin, thiols, and glutathione, in AD plasma versus Huntington's disease control [216]. Further detailed analyses demonstrated diminished levels of vitamin E (tocopherol) in plasma from MCI and mild AD patients compared to controls [204, 217]. In addition, amounts of another micronutrient, selenium, essential component of selenocysteine and a cofactor of GPx and ThxR, important in cell defense against oxidative stress, have been shown to be reduced in plasma and erythrocytes from AD patients [210] and to correlate with cognitive decline, as evidenced in a 9-year longitudinal study in patients between 60 and 71 years of age [218].
Altogether, the above findings are supportive of the hypothesis that not only ROS generation but also the cellular oxidative stress defense mechanisms are compromised in the brain as well as in peripheral tissues in AD. The molecular alterations in blood cells that could be detected already at the preclinical AD stage might be further tested as potential easily accessible novel biomarkers for early AD.
10. Conclusions and Future Perspectives
Several evidences indicate that oxidative stress and impaired oxidative stress defense are among the main early causes of age-related neurodegeneration in AD brain. Moreover, the progression of AD seems to be associated with an interplay between oxidative stress and Aβ in a vicious cycle. Consequently, several antioxidants have been tested for their potential to preserve or improve cognitive performance in MCI and AD patients but most of them failed to provide clinical benefits. The failures of the clinical trials might be attributed to the administration of incorrect doses and/or poor bioavailability of the drugs. The latter could be improved by the development of novel nanotechnologies. Furthermore, the disappointing results of the clinical trials might be related to the inappropriate recruitment of patients, administration of drugs at the advanced AD stages, and too short follow-ups to detect clinical benefits. The selection of patients and monitoring of the clinical trials can be improved by the design of novel noninvasive biomarker-based tools allowing the detection of the disease at the presymptomatic stage and sensitive long-term monitoring of therapeutic efficacies.
Importantly, several studies have demonstrated that the oxidant/antioxidant imbalance in early AD is reflected in easily accessible blood cells. This opens new avenues for the development of noninvasive, blood-based biomarkers that could overcome the limitation of the currently employed CSF assays and brain imaging.
Acknowledgments
This work has been supported by the European Union's Horizon 2020 Research and Innovation Program under the FETOPEN Grant agreement no 737390 (ArrestAD) and carried out with the use of CePT infrastructure financed by the European Union—the European Regional Development Fund within the Operational Programme “Innovative economy” for 2007–2013.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article. No writing assistance was utilized in the production of this manuscript.
Authors' Contributions
Joanna Wojsiat and Katarzyna Marta Zoltowska contributed equally to this work.
References
- 1.Aisen P. S., Cummings J., Jack C. R., et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimer's Research & Therapy. 2017;9(1):p. 60. doi: 10.1186/s13195-017-0283-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epelbaum S., Genthon R., Cavedo E., et al. Preclinical Alzheimer’s disease: a systematic review of the cohorts underlying the concept. Alzheimers Dement. 2017;13(4):454–467. doi: 10.1016/j.jalz.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Serrano-Pozo A., Frosch M. P., Masliah E., Hyman B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine. 2011;1(1, article a006189) doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molteni M., Rossetti C. Neurodegenerative diseases: the immunological perspective. Journal of Neuroimmunology. 2017;313:109–115. doi: 10.1016/j.jneuroim.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Ayton S., Lei P., Bush A. I. Metallostasis in Alzheimer’s disease. Free Radical Biology & Medicine. 2013;62:76–89. doi: 10.1016/j.freeradbiomed.2012.10.558. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg R. N., Lambracht-Washington D., Yu G., Xia W. Genomics of Alzheimer disease: a review. JAMA Neurology. 2016;73(7):867–874. doi: 10.1001/jamaneurol.2016.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuyvers E., Sleegers K. Genetic variations underlying Alzheimer’s disease: evidence from genome-wide association studies and beyond. The Lancet Neurology. 2016;15(8):857–868. doi: 10.1016/S1474-4422(16)00127-7. [DOI] [PubMed] [Google Scholar]
- 8.Killin L. O., Starr J. M., Shiue I. J., Russ T. C. Environmental risk factors for dementia: a systematic review. BMC Geriatrics. 2016;16(1):p. 175. doi: 10.1186/s12877-016-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brookmeyer R., Gray S., Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. American Journal of Public Health. 1998;88(9):1337–1342. doi: 10.2105/AJPH.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hebert L. E., Weuve J., Scherr P. A., Evans D. A. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahoney-Sanchez L., Belaidi A. A., Bush A. I., Ayton S. The complex role of apolipoprotein E in Alzheimer’s disease: an overview and update. Journal of Molecular Neuroscience. 2016;60(3):325–335. doi: 10.1007/s12031-016-0839-z. [DOI] [PubMed] [Google Scholar]
- 12.Corder E., Saunders A., Strittmatter W., et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 13.Roses A. D. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annual Review of Medicine. 1996;47(1):387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- 14.Yu J. T., Tan L., Hardy J. Apolipoprotein E in Alzheimer’s disease: an update. Annual Review of Neuroscience. 2014;37(1):79–100. doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- 15.Goate A., Chartier-Harlin M. C., Mullan M., et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 16.Sherrington R., Rogaev E. I., Liang Y., et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 17.Rogaev E. I., Sherrington R., Rogaeva E. A., et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376(6543):775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe M. S. Processive proteolysis by γ-secretase and the mechanism of Alzheimer’s disease. Biological Chemistry. 2012;393(9):899–905. doi: 10.1515/hsz-2012-0140. [DOI] [PubMed] [Google Scholar]
- 19.Bolduc D. M., Montagna D. R., Seghers M. C., Wolfe M. S., Selkoe D. J. The amyloid-beta forming tripeptide cleavage mechanism of γ-secretase. eLife. 2016;5, article e17578 doi: 10.7554/eLife.17578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takami M., Nagashima Y., Sano Y., et al. γ-Secretase: successive tripeptide and tetrapeptide release from the transmembrane domain of β-carboxyl terminal fragment. The Journal of Neuroscience. 2009;29(41):13042–13052. doi: 10.1523/JNEUROSCI.2362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassar R., Kuhn P. H., Haass C., et al. Function, therapeutic potential and cell biology of BACE proteases: current status and future prospects. Journal of Neurochemistry. 2014;130(1):4–28. doi: 10.1111/jnc.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein A. M., Kowall N. W., Ferrante R. J. Neurotoxicity and oxidative damage of beta amyloid 1-42 versus beta amyloid 1-40 in the mouse cerebral cortex. Annals of the New York Academy of Sciences. 1999;893:314–320. doi: 10.1111/j.1749-6632.1999.tb07845.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuperstein I., Broersen K., Benilova I., et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. The EMBO Journal. 2010;29(19):3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy J. A., Higgins G. A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson T., Atwal J. K., Steinberg S., et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488(7409):96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 26.Mintun M. A., LaRossa G. N., Sheline Y. I., et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 27.Hyman B. T., Sorger P. Failure analysis of clinical trials to test the amyloid hypothesis. Annals of Neurology. 2014;76(2):159–161. doi: 10.1002/ana.24227. [DOI] [PubMed] [Google Scholar]
- 28.Swerdlow R. H., Khan S. M. A "mitochondrial cascade hypothesis" for sporadic Alzheimer’s disease. Medical Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 29.Swerdlow R. H., Burns J. M., Khan S. M. The Alzheimer’s disease mitochondrial cascade hypothesis. Journal of Alzheimer's Disease. 2010;20(Supplement 2):S265–S279. doi: 10.3233/JAD-2010-100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harman D. Aging: a theory based on free radical and radiation chemistry. Journal of Gerontology. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 31.Markesbery W. R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radical Biology & Medicine. 1997;23(1):134–147. doi: 10.1016/S0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- 32.Small S. A., Duff K. Linking Aβ and tau in late-onset Alzheimer’s disease: a dual pathway hypothesis. Neuron. 2008;60(4):534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones D. P. Redefining oxidative stress. Antioxidants & Redox Signaling. 2006;8(9-10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 34.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biology. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sies H., Berndt C., Jones D. P. Oxidative stress. Annual Review of Biochemistry. 2017;86(1):715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 36.Murphy M. P. How mitochondria produce reactive oxygen species. The Biochemical Journal. 2009;417(1):1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ristow M., Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Experimental Gerontology. 2010;45(6):410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 38.Ristow M., Schmeisser S. Extending life span by increasing oxidative stress. Free Radical Biology & Medicine. 2011;51(2):327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Ristow M. Unraveling the truth about antioxidants: mitohormesis explains ROS-induced health benefits. Nature Medicine. 2014;20(7):709–711. doi: 10.1038/nm.3624. [DOI] [PubMed] [Google Scholar]
- 40.Masoro E. J. Influence of caloric intake on aging and on the response to stressors. Journal of Toxicology and Environmental Health. Part B. 1998;1(3):243–257. doi: 10.1080/10937409809524554. [DOI] [PubMed] [Google Scholar]
- 41.Bélanger M., Allaman I., Magistretti P. J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metabolism. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Coyle J., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z., Zhou T., Ziegler A. C., Dimitrion P., Zuo L. Oxidative stress in neurodegenerative diseases: from molecular mechanisms to clinical applications. Oxidative Medicine and Cellular Longevity. 2017;2017:11. doi: 10.1155/2017/2525967.2525967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beal M. F. Aging, energy, and oxidative stress in neurodegenerative diseases. Annals of Neurology. 1995;38(3):357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 45.Nunomura A., Perry G., Aliev G., et al. Oxidative damage is the earliest event in Alzheimer disease. Journal of Neuropathology & Experimental Neurology. 2001;60(8):759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 46.Markesbery W. R., Carney J. M. Oxidative alterations in Alzheimer’s disease. Brain Pathology. 1999;9(1):133–146. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butterfield D. A. The 2013 SFRBM discovery award: selected discoveries from the butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radical Biology & Medicine. 2014;74:157–174. doi: 10.1016/j.freeradbiomed.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moreira P. I., Sayre L. M., Zhu X., Nunomura A., Smith M. A., Perry G. Detection and localization of markers of oxidative stress by in situ methods: application in the study of Alzheimer disease. Methods in Molecular Biology. 2010;610:419–434. doi: 10.1007/978-1-60327-029-8_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hajimohammadreza I., Brammer M. Brain membrane fluidity and lipid peroxidation in Alzheimer’s disease. Neuroscience Letters. 1990;112(2-3):333–337. doi: 10.1016/0304-3940(90)90226-Y. [DOI] [PubMed] [Google Scholar]
- 50.Prasad M. R., Lovell M. A., Yatin M., Dhillon H., Markesbery W. R. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochemical Research. 1998;23(1):81–88. doi: 10.1023/A:1022457605436. [DOI] [PubMed] [Google Scholar]
- 51.Smith C. D., Carney J. M., Starke-Reed P. E., et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(23):10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sultana R., Reed T., Perluigi M., Coccia R., Pierce W. M., Butterfield D. A. Proteomic identification of nitrated brain proteins in amnestic mild cognitive impairment: a regional study. Journal of Cellular and Molecular Medicine. 2007;11(4):839–851. doi: 10.1111/j.1582-4934.2007.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santos R. X., Correia S. C., Zhu X., et al. Nuclear and mitochondrial DNA oxidation in Alzheimer’s disease. Free Radical Research. 2012;46(4):565–576. doi: 10.3109/10715762.2011.648188. [DOI] [PubMed] [Google Scholar]
- 54.Gabbita S. P., Lovell M. A., Markesbery W. R. Increased nuclear DNA oxidation in the brain in Alzheimer’s disease. Journal of Neurochemistry. 1998;71(5):2034–2040. doi: 10.1046/j.1471-4159.1998.71052034.x. [DOI] [PubMed] [Google Scholar]
- 55.Mecocci P., MacGarvey U., Kaufman A. E., et al. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Annals of Neurology. 1993;34(4):609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- 56.Mecocci P., MacGarvey U., Beal M. F. Oxidative damage to mitochondrial DNA is increased in Alzheimer’s disease. Annals of Neurology. 1994;36(5):747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- 57.Mecocci P., Beal M. F., Cecchetti R., et al. Mitochondrial membrane fluidity and oxidative damage to mitochondrial DNA in aged and AD human brain. Molecular and Chemical Neuropathology. 1997;31(1):53–64. doi: 10.1007/BF02815160. [DOI] [PubMed] [Google Scholar]
- 58.Arce-Varas N., Abate G., Prandelli C., et al. Comparison of extracellular and intracellular blood compartments highlights redox alterations in Alzheimer’s and mild cognitive impairment patients. Current Alzheimer Research. 2017;14(1):112–122. doi: 10.2174/1567205013666161010125413. [DOI] [PubMed] [Google Scholar]
- 59.Wojda U. Alzheimer’s disease lymphocytes: potential for biomarkers? Biomarkers in Medicine. 2016;10(1):1–4. doi: 10.2217/bmm.15.79. [DOI] [PubMed] [Google Scholar]
- 60.Wojsiat J., Prandelli C., Laskowska-Kaszub K., Martín-Requero A., Wojda U. Oxidative stress and aberrant cell cycle in Alzheimer’s disease lymphocytes: diagnostic prospects. Journal of Alzheimer's Disease. 2015;46(2):329–350. doi: 10.3233/JAD-141977. [DOI] [PubMed] [Google Scholar]
- 61.Ramassamy C., Averill D., Beffert U., et al. Oxidative insults are associated with apolipoprotein E genotype in Alzheimer’s disease brain. Neurobiology of Disease. 2000;7(1):23–37. doi: 10.1006/nbdi.1999.0273. [DOI] [PubMed] [Google Scholar]
- 62.Cracowski J. L., Durand T., Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends in Pharmacological Sciences. 2002;23(8):360–366. doi: 10.1016/S0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 63.Glodzik-Sobanska L., Pirraglia E., Brys M., et al. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer’s disease. Neurobiology of Aging. 2009;30(5):672–681. doi: 10.1016/j.neurobiolaging.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montine T. J., Beal M. F., Cudkowicz M. E., et al. Increased CSF F2-isoprostane concentration in probable AD. Neurology. 1999;52(3):562–565. doi: 10.1212/WNL.52.3.562. [DOI] [PubMed] [Google Scholar]
- 65.Montine T. J., Peskind E. R., Quinn J. F., Wilson A. M., Montine K. S., Galasko D. Increased cerebrospinal fluid F2-isoprostanes are associated with aging and latent Alzheimer’s disease as identified by biomarkers. Neuromolecular Medicine. 2011;13(1):37–43. doi: 10.1007/s12017-010-8126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duits F. H., Kester M. I., Scheffer P. G., et al. Increase in cerebrospinal fluid F2-isoprostanes is related to cognitive decline in APOE ε4 carriers. Journal of Alzheimer's Disease. 2013;36(3):563–570. doi: 10.3233/JAD-122227. [DOI] [PubMed] [Google Scholar]
- 67.Miyata M., Smith J. D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nature Genetics. 1996;14(1):55–61. doi: 10.1038/ng0996-55. [DOI] [PubMed] [Google Scholar]
- 68.de Matos A. M., de Macedo M. P., Rauter A. P. Bridging type 2 diabetes and Alzheimer’s disease: assembling the puzzle pieces in the quest for the molecules with therapeutic and preventive potential. Medicinal Research Reviews. 2018;38(1):261–324. doi: 10.1002/med.21440. [DOI] [PubMed] [Google Scholar]
- 69.Ciudin A., Espinosa A., Simó-Servat O., et al. Type 2 diabetes is an independent risk factor for dementia conversion in patients with mild cognitive impairment. Journal of Diabetes and its Complications. 2017;31(8):1272–1274. doi: 10.1016/j.jdiacomp.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 70.Simó R., Ciudin A., Simó-Servat O., Hernández C. Cognitive impairment and dementia: a new emerging complication of type 2 diabetes-the diabetologist’s perspective. Acta Diabetologica. 2017;54(5):417–424. doi: 10.1007/s00592-017-0970-5. [DOI] [PubMed] [Google Scholar]
- 71.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World Journal of Diabetes. 2015;6(3):456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pardeshi R., Bolshette N., Gadhave K., et al. Insulin signaling: an opportunistic target to minify the risk of Alzheimer’s disease. Psychoneuroendocrinology. 2017;83:159–171. doi: 10.1016/j.psyneuen.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 73.Sandhir R., Gupta S. Molecular and biochemical trajectories from diabetes to Alzheimer’s disease: a critical appraisal. World Journal of Diabetes. 2015;6(12):1223–1242. doi: 10.4239/wjd.v6.i12.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gasparini L., Xu H. Potential roles of insulin and IGF-1 in Alzheimer’s disease. Trends in Neurosciences. 2003;26(8):404–406. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- 75.Bonnefont-Rousselot D. Glucose and reactive oxygen species. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5(5):561–568. doi: 10.1097/00075197-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 76.Piras S., Furfaro A. L., Domenicotti C., et al. RAGE expression and ROS generation in neurons: differentiation versus damage. Oxidative Medicine and Cellular Longevity. 2016;2016:9. doi: 10.1155/2016/9348651.9348651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan S. D., Chen X., Fu J., et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 78.Yan S. D., Schmidt A. M., Anderson G. M., et al. Enhanced cellular oxidant stress by the interaction of advanced glycation end products with their receptors/binding proteins. The Journal of Biological Chemistry. 1994;269(13):9889–9897. [PubMed] [Google Scholar]
- 79.Gao X., Zhang H., Schmidt A. M., Zhang C. AGE/RAGE produces endothelial dysfunction in coronary arterioles in type 2 diabetic mice. American Journal of Physiology. Heart and Circulatory Physiology. 2008;295(2):H491–H498. doi: 10.1152/ajpheart.00464.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai Z., Liu N., Wang C., et al. Role of RAGE in Alzheimer’s disease. Cellular and Molecular Neurobiology. 2016;36(4):483–495. doi: 10.1007/s10571-015-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Choi B. R., Cho W. H., Kim J., et al. Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer’s disease. Experimental & Molecular Medicine. 2014;46(2, article e75) doi: 10.1038/emm.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lubitz I., Ricny J., Atrakchi-Baranes D., et al. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Aβ deposition in an Alzheimer mouse model. Aging Cell. 2016;15(2):309–316. doi: 10.1111/acel.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeshita Y., Shibata N., Kasanuki K., et al. Genetic association between RAGE polymorphisms and Alzheimer’s disease and Lewy body dementias in a Japanese cohort: a case-control study. International Journal of Geriatric Psychiatry. 2017;32(12):1241–1246. doi: 10.1002/gps.4600. [DOI] [PubMed] [Google Scholar]
- 84.Martins R., Lithgow G. J., Link W. Long live FOXO: unraveling the role of FOXO proteins in aging and longevity. Aging Cell. 2016;15(2):196–207. doi: 10.1111/acel.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manolopoulos K. N., Klotz L. O., Korsten P., Bornstein S. R., Barthel A. Linking Alzheimer’s disease to insulin resistance: the FoxO response to oxidative stress. Molecular Psychiatry. 2010;15(11):1046–1052. doi: 10.1038/mp.2010.17. [DOI] [PubMed] [Google Scholar]
- 86.Resende R., Moreira P. I., Proença T., et al. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radical Biology & Medicine. 2008;44(12):2051–2057. doi: 10.1016/j.freeradbiomed.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 87.Hartl D., Schuldt V., Forler S., Zabel C., Klose J., Rohe M. Presymptomatic alterations in energy metabolism and oxidative stress in the APP23 mouse model of Alzheimer disease. Journal of Proteome Research. 2012;11(6):3295–3304. doi: 10.1021/pr300021e. [DOI] [PubMed] [Google Scholar]
- 88.Leuner K., Schütt T., Kurz C., et al. Mitochondrion-derived reactive oxygen species lead to enhanced amyloid beta formation. Antioxidants & Redox Signaling. 2012;16(12):1421–1433. doi: 10.1089/ars.2011.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tong Y., Zhou W., Fung V., et al. Oxidative stress potentiates BACE1 gene expression and Aβ generation. Journal of Neural Transmission. 2005;112(3):455–469. doi: 10.1007/s00702-004-0255-3. [DOI] [PubMed] [Google Scholar]
- 90.Ashabi G., Ahmadiani A., Abdi A., Abraki S. B., Khodagholi F. Time course study of Aβ formation and neurite outgrowth disruption in differentiated human neuroblastoma cells exposed to H2O2: protective role of autophagy. Toxicology In Vitro. 2013;27(6):1780–1788. doi: 10.1016/j.tiv.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Arimon M., Takeda S., Post K. L., Svirsky S., Hyman B. T., Berezovska O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiology of Disease. 2015;84:109–119. doi: 10.1016/j.nbd.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zoltowska K. M., Berezovska O. Dynamic nature of presenilin1/γ-secretase: implication for Alzheimer’s disease pathogenesis. Molecular Neurobiology. 2017 doi: 10.1007/s12035-017-0487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gwon A. R., Park J. S., Arumugam T. V., et al. Oxidative lipid modification of nicastrin enhances amyloidogenic γ-secretase activity in Alzheimer’s disease. Aging Cell. 2012;11(4):559–568. doi: 10.1111/j.1474-9726.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He Y., Cui J., Lee J. C. M., et al. Prolonged exposure of cortical neurons to oligomeric amyloid-β impairs NMDA receptor function via NADPH oxidase-mediated ROS production: protective effect of green tea (−)-epigallocatechin-3-gallate. ASN Neuro. 2011;3(1, article e00050) doi: 10.1042/AN20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shelat P. B., Chalimoniuk M., Wang J. H., et al. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. Journal of Neurochemistry. 2008;106(1):45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- 96.Serrano F., Chang A., Hernandez C., Pautler R. G., Sweatt J. D., Klann E. NADPH oxidase mediates β-amyloid peptide-induced activation of ERK in hippocampal organotypic cultures. Molecular Brain. 2009;2(1):p. 31. doi: 10.1186/1756-6606-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bilkei-Gorzo A. Genetic mouse models of brain ageing and Alzheimer’s disease. Pharmacology & Therapeutics. 2014;142(2):244–257. doi: 10.1016/j.pharmthera.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 98.Robinson R. A., Lange M. B., Sultana R., et al. Differential expression and redox proteomics analyses of an Alzheimer disease transgenic mouse model: effects of the amyloid-β peptide of amyloid precursor protein. Neuroscience. 2011;177:207–222. doi: 10.1016/j.neuroscience.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Butterfield D. A., Galvan V., Lange M. B., et al. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: requirement for methionine 35 in amyloid β-peptide of APP. Free Radical Biology & Medicine. 2010;48(1):136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garcia-Alloza M., Dodwell S. A., Meyer-Luehmann M., Hyman B. T., Bacskai B. J. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. Journal of Neuropathology & Experimental Neurology. 2006;65(11):1082–1089. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- 101.Maurer I., Zierz S., Moller H. J. A selective defect of cytochrome c oxidase is present in brain of Alzheimer disease patients. Neurobiology of Aging. 2000;21(3):455–462. doi: 10.1016/S0197-4580(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 102.Gibson G. E., Sheu K. F. R., Blass J. P. Abnormalities of mitochondrial enzymes in Alzheimer disease. Journal of Neural Transmission. 1998;105(8-9):855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 103.Calkins M. J., Manczak M., Mao P., Shirendeb U., Reddy P. H. Impaired mitochondrial biogenesis, defective axonal transport of mitochondria, abnormal mitochondrial dynamics and synaptic degeneration in a mouse model of Alzheimer’s disease. Human Molecular Genetics. 2011;20(23):4515–4529. doi: 10.1093/hmg/ddr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moreira P. I., Siedlak S. L., Wang X., et al. Increased autophagic degradation of mitochondria in Alzheimer disease. Autophagy. 2007;3(6):614–615. doi: 10.4161/auto.4872. [DOI] [PubMed] [Google Scholar]
- 105.Moreira P. I., Siedlak S. L., Wang X., et al. Autophagocytosis of mitochondria is prominent in Alzheimer disease. Journal of Neuropathology & Experimental Neurology. 2007;66(6):525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- 106.Hirai K., Aliev G., Nunomura A., et al. Mitochondrial abnormalities in Alzheimer’s disease. The Journal of Neuroscience. 2001;21(9):3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Swerdlow R. H., Burns J. M., Khan S. M. The Alzheimer’s disease mitochondrial cascade hypothesis: progress and perspectives. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2014;1842(8):1219–1231. doi: 10.1016/j.bbadis.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Webster M. T., Pearce B. R., Bowen D. M., Francis P. T. The effects of perturbed energy metabolism on the processing of amyloid precursor protein in PC12 cells. Journal of Neural Transmission. 1998;105(8-9):839–853. doi: 10.1007/s007020050098. [DOI] [PubMed] [Google Scholar]
- 109.Gabuzda D., Busciglio J., Chen L. B., Matsudaira P., Yankner B. A. Inhibition of energy metabolism alters the processing of amyloid precursor protein and induces a potentially amyloidogenic derivative. The Journal of Biological Chemistry. 1994;269(18):13623–13628. [PubMed] [Google Scholar]
- 110.Gasparini L., Racchi M., Benussi L., et al. Effect of energy shortage and oxidative stress on amyloid precursor protein metabolism in COS cells. Neuroscience Letters. 1997;231(2):113–117. doi: 10.1016/S0304-3940(97)00536-3. [DOI] [PubMed] [Google Scholar]
- 111.Murray J., H. Tsui W., Li Y., et al. FDG and amyloid PET in cognitively normal individuals at risk for late-onset Alzheimer’s disease. Advances in Molecular Imaging. 2014;04(02):15–26. doi: 10.4236/ami.2014.42003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lustbader J. W., Cirilli M., Lin C., et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer’s disease. Science. 2004;304(5669):448–452. doi: 10.1126/science.1091230. [DOI] [PubMed] [Google Scholar]
- 113.Abramov A. Y., Berezhnov A. V., Fedotova E. I., Zinchenko V. P., Dolgacheva L. P. Interaction of misfolded proteins and mitochondria in neurodegenerative disorders. Biochemical Society Transactions. 2017;45(4):1025–1033. doi: 10.1042/BST20170024. [DOI] [PubMed] [Google Scholar]
- 114.Casley C. S., Canevari L., Land J. M., Clark J. B., Sharpe M. A. β-amyloid inhibits integrated mitochondrial respiration and key enzyme activities. Journal of Neurochemistry. 2002;80(1):91–100. doi: 10.1046/j.0022-3042.2001.00681.x. [DOI] [PubMed] [Google Scholar]
- 115.Canevari L., Clark J. B., Bates T. E. β-amyloid fragment 25-35 selectively decreases complex IV activity in isolated mitochondria. FEBS Letters. 1999;457(1):131–134. doi: 10.1016/S0014-5793(99)01028-5. [DOI] [PubMed] [Google Scholar]
- 116.Casley C. S., Land J. M., Sharpe M. A., Clark J. B., Duchen M. R., Canevari L. β-amyloid fragment 25-35 causes mitochondrial dysfunction in primary cortical neurons. Neurobiology of Disease. 2002;10(3):258–267. doi: 10.1006/nbdi.2002.0516. [DOI] [PubMed] [Google Scholar]
- 117.Abramov A. Y., Canevari L., Duchen M. R. β-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. The Journal of Neuroscience. 2004;24(2):565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Picone P., Nuzzo D., Caruana L., Scafidi V., Di Carlo M. Mitochondrial dysfunction: different routes to Alzheimer’s disease therapy. Oxidative Medicine and Cellular Longevity. 2014;2014:11. doi: 10.1155/2014/780179.780179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ham D., Schipper H. M. Heme oxygenase-1 induction and mitochondrial iron sequestration in astroglia exposed to amyloid peptides. Cellular and Molecular Biology. 2000;46(3):587–596. [PubMed] [Google Scholar]
- 120.Xie H., Guan J., Borrelli L. A., Xu J., Serrano-Pozo A., Bacskai B. J. Mitochondrial alterations near amyloid plaques in an Alzheimer’s disease mouse model. The Journal of Neuroscience. 2013;33(43):17042–17051. doi: 10.1523/JNEUROSCI.1836-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang X., Su B., Siedlak S. L., et al. Amyloid-β overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(49):19318–19323. doi: 10.1073/pnas.0804871105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Manczak M., Calkins M. J., Reddy P. H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Human Molecular Genetics. 2011;20(13):2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X., Su B., Fujioka H., Zhu X. Dynamin-like protein 1 reduction underlies mitochondrial morphology and distribution abnormalities in fibroblasts from sporadic Alzheimer’s disease patients. The American Journal of Pathology. 2008;173(2):470–482. doi: 10.2353/ajpath.2008.071208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Calkins M. J., Reddy P. H. Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer’s disease neurons. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2011;1812(4):507–513. doi: 10.1016/j.bbadis.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ebneth A., Godemann R., Stamer K., et al. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. The Journal of Cell Biology. 1998;143(3):777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.DuBoff B., Gotz J., Feany M. B. Tau promotes neurodegeneration via DRP1 mislocalization in vivo. Neuron. 2012;75(4):618–632. doi: 10.1016/j.neuron.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vossel K. A., Zhang K., Brodbeck J., et al. Tau reduction prevents Aβ-induced defects in axonal transport. Science. 2010;330(6001):p. 198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Amadoro G., Corsetti V., Ciotti M. T., et al. Endogenous Aβ causes cell death via early tau hyperphosphorylation. Neurobiology of Aging. 2011;32(6):969–990. doi: 10.1016/j.neurobiolaging.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 129.Atlante A., Amadoro G., Bobba A., et al. A peptide containing residues 26-44 of tau protein impairs mitochondrial oxidative phosphorylation acting at the level of the adenine nucleotide translocator. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2008;1777(10):1289–1300. doi: 10.1016/j.bbabio.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 130.Amadoro G., Corsetti V., Atlante A., et al. Interaction between NH2-tau fragment and Aβ in Alzheimer’s disease mitochondria contributes to the synaptic deterioration. Neurobiology of Aging. 2012;33(4):833.e1–833.e25. doi: 10.1016/j.neurobiolaging.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 131.Calabrese V., Guagliano E., Sapienza M., et al. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochemical Research. 2007;32(4-5):757–773. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- 132.Dattilo S., Mancuso C., Koverech G., et al. Heat shock proteins and hormesis in the diagnosis and treatment of neurodegenerative diseases. Immunity & Ageing. 2015;12(1):p. 20. doi: 10.1186/s12979-015-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu S. C. Glutathione synthesis. Biochimica et Biophysica Acta (BBA) - General Subjects. 2013;1830(5):3143–3153. doi: 10.1016/j.bbagen.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Forman H. J., Zhang H., Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Molecular Aspects of Medicine. 2009;30(1–2):1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ansari M. A., Scheff S. W. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. Journal of Neuropathology & Experimental Neurology. 2010;69(2):155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karelson E., Bogdanovic N., Garlind A., et al. The cerebrocortical areas in normal brain aging and in Alzheimer’s disease: noticeable differences in the lipid peroxidation level and in antioxidant defense. Neurochemical Research. 2001;26(4):353–361. doi: 10.1023/A:1010942929678. [DOI] [PubMed] [Google Scholar]
- 137.Mandal P. K., Saharan S., Tripathi M., Murari G. Brain glutathione levels – a novel biomarker for mild cognitive impairment and Alzheimer’s disease. Biological Psychiatry. 2015;78(10):702–10. doi: 10.1016/j.biopsych.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 138.Gabbita S. P., Aksenov M. Y., Lovell M. A., Markesbery W. R. Decrease in peptide methionine sulfoxide reductase in Alzheimer’s disease brain. Journal of Neurochemistry. 1999;73(4):1660–1666. doi: 10.1046/j.1471-4159.1999.0731660.x. [DOI] [PubMed] [Google Scholar]
- 139.Lovell M. A., Xie C., Gabbita S. P., Markesbery W. R. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer’s disease brain. Free Radical Biology & Medicine. 2000;28(3):418–427. doi: 10.1016/S0891-5849(99)00258-0. [DOI] [PubMed] [Google Scholar]
- 140.Lee J. M., Johnson J. A. An important role of Nrf2-ARE pathway in the cellular defense mechanism. Journal of Biochemistry and Molecular Biology. 2004;37(2):139–143. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 141.Ramsey C. P., Glass C. A., Montgomery M. B., et al. Expression of Nrf2 in neurodegenerative diseases. Journal of Neuropathology and Experimental Neurology. 2007;66(1):75–85. doi: 10.1097/nen.0b013e31802d6da9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schipper H., Song W. A heme oxygenase-1 transducer model of degenerative and developmental brain disorders. International Journal of Molecular Sciences. 2015;16(3):5400–5419. doi: 10.3390/ijms16035400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schipper H. M., Cissé S., Stopa E. G. Expression of heme oxygenase-1 in the senescent and Alzheimer-diseased brain. Annals of Neurology. 1995;37(6):758–768. doi: 10.1002/ana.410370609. [DOI] [PubMed] [Google Scholar]
- 144.Schipper H., Bennett D., Liberman A., et al. Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiology of Aging. 2006;27(2):252–261. doi: 10.1016/j.neurobiolaging.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 145.Di Domenico F., Barone E., Perluigi M., Butterfield D. A. Strategy to reduce free radical species in Alzheimer’s disease: an update of selected antioxidants. Expert Review of Neurotherapeutics. 2015;15(1):19–40. doi: 10.1586/14737175.2015.955853. [DOI] [PubMed] [Google Scholar]
- 146.Jiang T., Sun Q., Chen S. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Progress in Neurobiology. 2016;147:1–19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 147.Adair J. C., Knoefel J. E., Morgan N. Controlled trial of N-acetylcysteine for patients with probable Alzheimer’s disease. Neurology. 2001;57(8):1515–1517. doi: 10.1212/WNL.57.8.1515. [DOI] [PubMed] [Google Scholar]
- 148.Mazzanti G., Di Giacomo S. Curcumin and resveratrol in the management of cognitive disorders: what is the clinical evidence? Molecules. 2016;21(9) doi: 10.3390/molecules21091243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sawda C., Moussa C., Turner R. S. Resveratrol for Alzheimer’s disease. Annals of the New York Academy of Sciences. 2017;1403(1):142–149. doi: 10.1111/nyas.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ahmed T., Javed S., Javed S., et al. Resveratrol and Alzheimer’s disease: mechanistic insights. Molecular Neurobiology. 2017;54(4):2622–2635. doi: 10.1007/s12035-016-9839-9. [DOI] [PubMed] [Google Scholar]
- 151.Fata G., Weber P., Mohajeri M. Effects of vitamin E on cognitive performance during ageing and in Alzheimer’s disease. Nutrients. 2014;6(12):5453–5472. doi: 10.3390/nu6125453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grimm M. O., Mett J., Hartmann T. The impact of vitamin E and other fat-soluble vitamins on Alzheimer’s disease. International Journal of Molecular Sciences. 2016;17(11) doi: 10.3390/ijms17111785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Galasko D. R., Peskind E., Clark C. M., et al. Antioxidants for Alzheimer disease: a randomized clinical trial with cerebrospinal fluid biomarker measures. Archives of Neurology. 2012;69(7):836–841. doi: 10.1001/archneurol.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kryscio R. J., Abner E. L., Caban-Holt A., et al. Association of antioxidant supplement use and dementia in the prevention of Alzheimer’s disease by vitamin E and selenium trial (PREADViSE) JAMA Neurology. 2017;74(5):567–573. doi: 10.1001/jamaneurol.2016.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Engelhart M. J., Geerlings M. I., Ruitenberg A., et al. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002;287(24):3223–3229. doi: 10.1001/jama.287.24.3223. [DOI] [PubMed] [Google Scholar]
- 156.Morris M. C., Evans D. A., Bienias J. L., et al. Dietary intake of antioxidant nutrients and the risk of incident Alzheimer disease in a biracial community study. JAMA. 2002;287(24):3230–3237. doi: 10.1001/jama.287.24.3230. [DOI] [PubMed] [Google Scholar]
- 157.Cardinali D. P., Furio A. M., Brusco L. I. Clinical aspects of melatonin intervention in Alzheimer’s disease progression. Current Neuropharmacology. 2010;8(3):218–227. doi: 10.2174/157015910792246209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Y. Y., Zheng W., Ng C. H., Ungvari G. S., Wei W., Xiang Y. T. Meta-analysis of randomized, double-blind, placebo-controlled trials of melatonin in Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2017;32(1):50–57. doi: 10.1002/gps.4571. [DOI] [PubMed] [Google Scholar]
- 159.Choi H., Park H. H., Koh S. H., et al. Coenzyme Q10 protects against amyloid beta-induced neuronal cell death by inhibiting oxidative stress and activating the P13K pathway. Neurotoxicology. 2012;33(1):85–90. doi: 10.1016/j.neuro.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 160.Muthukumaran K., Kanwar A., Vegh C., et al. Ubisol-Q10 (a nanomicellar water-soluble formulation of CoQ10) treatment inhibits Alzheimer-type behavioral and pathological symptoms in a double transgenic mouse (TgAPEswe, PSEN1dE9) model of Alzheimer’s disease. Journal of Alzheimer's Disease. 2017;61(1):221–236. doi: 10.3233/JAD-170275. [DOI] [PubMed] [Google Scholar]
- 161.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2004;1660(1–2):171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 162.Hargreaves I. P. Coenzyme Q10 as a therapy for mitochondrial disease. The International Journal of Biochemistry & Cell Biology. 2014;49:105–111. doi: 10.1016/j.biocel.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 163.Senin U., Parnetti L., Barbagallo-Sangiorgi G., et al. Idebenone in senile dementia of Alzheimer type: a multicentre study. Archives of Gerontology and Geriatrics. 1992;15(3):249–260. doi: 10.1016/0167-4943(92)90060-H. [DOI] [PubMed] [Google Scholar]
- 164.Weyer G., Babej-Dölle R. M., Hadler D., Hofmann S., Herrmann W. M. A controlled study of 2 doses of idebenone in the treatment of Alzheimer’s disease. Neuropsychobiology. 1997;36(2):73–82. doi: 10.1159/000119366. [DOI] [PubMed] [Google Scholar]
- 165.Dolder M., Walzel B., Speer O., Schlattner U., Wallimann T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. Journal of Biological Chemistry. 2003;278(20):17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- 166.Chaturvedi R. K., Flint Beal M. Mitochondrial diseases of the brain. Free Radical Biology & Medicine. 2013;63:1–29. doi: 10.1016/j.freeradbiomed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 167.Manczak M., Mao P., Calkins M. J., et al. Mitochondria-targeted antioxidants protect against amyloid-β toxicity in Alzheimer's disease neurons. Journal of Alzheimer's Disease. 2010;20(s2) Supplement 2:S609–S631. doi: 10.3233/JAD-2010-100564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smith R. A., Porteous C. M., Coulter C. V., Murphy M. P. Selective targeting of an antioxidant to mitochondria. European Journal of Biochemistry. 1999;263(3):709–716. doi: 10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 169.Smith R. A., Porteous C. M., Gane A. M., Murphy M. P. Delivery of bioactive molecules to mitochondria in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Trnka J., Blaikie F. H., Smith R. A. J., Murphy M. P. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radical Biology & Medicine. 2008;44(7):1406–1419. doi: 10.1016/j.freeradbiomed.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 171.Kawagishi H., Finkel T. Unraveling the truth about antioxidants: ROS and disease: finding the right balance. Nature Medicine. 2014;20(7):711–713. doi: 10.1038/nm.3625. [DOI] [PubMed] [Google Scholar]
- 172.Miller E. R., III, Pastor-Barriuso R., Dalal D., Riemersma R. A., Appel L. J., Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Annals of Internal Medicine. 2005;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 173.Belkacemi A., Doggui S., Dao L., Ramassamy C. Challenges associated with curcumin therapy in Alzheimer disease. Expert Reviews in Molecular Medicine. 2011;13, article e34 doi: 10.1017/S1462399411002055. [DOI] [PubMed] [Google Scholar]
- 174.Hajipour M. J., Santoso M. R., Rezaee F., Aghaverdi H., Mahmoudi M., Perry G. Advances in Alzheimer’s diagnosis and therapy: the implications of nanotechnology. Trends in Biotechnology. 2017;35(10):937–953. doi: 10.1016/j.tibtech.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 175.Liu Y., Ai K., Ji X., et al. Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. Journal of the American Chemical Society. 2017;139(2):856–862. doi: 10.1021/jacs.6b11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nazıroğlu M., Muhamad S., Pecze L. Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: focus on selenium nanoparticles. Expert Review of Clinical Pharmacology. 2017;10(7):773–782. doi: 10.1080/17512433.2017.1324781. [DOI] [PubMed] [Google Scholar]
- 177.Dowding J. M., Dosani T., Kumar A., Seal S., Self W. T. Cerium oxide nanoparticles scavenge nitric oxide radical (˙NO) Chemical Communications. 2012;48(40):4896–4898. doi: 10.1039/c2cc30485f. [DOI] [PubMed] [Google Scholar]
- 178.Dowding J. M., Song W., Bossy K., et al. Cerium oxide nanoparticles protect against Aβ-induced mitochondrial fragmentation and neuronal cell death. Cell Death & Differentiation. 2014;21(10):1622–1632. doi: 10.1038/cdd.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cummings J. Lessons learned from Alzheimer disease: clinical trials with negative outcomes. Clinical and Translational Science. 2017 doi: 10.1111/cts.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang J., Tan L., Yu J. T. Prevention trials in Alzheimer’s disease: current status and future perspectives. Journal of Alzheimer's Disease. 2016;50(4):927–945. doi: 10.3233/JAD-150826. [DOI] [PubMed] [Google Scholar]
- 181.Sperling R. A., Aisen P. S., Beckett L. A., et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers & Dementia. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wojsiat J., Laskowska-Kaszub K., Mietelska-Porowska A., Wojda U. Search for Alzheimer’s disease biomarkers in blood cells: hypotheses-driven approach. Biomarkers in Medicine. 2017;11(10):917–931. doi: 10.2217/bmm-2017-0041. [DOI] [PubMed] [Google Scholar]
- 183.Khan T. K., Alkon D. L. Peripheral biomarkers of Alzheimer’s disease. Journal of Alzheimer's Disease. 2015;44(3):729–744. doi: 10.3233/JAD-142262. [DOI] [PubMed] [Google Scholar]
- 184.Leutner S., Schindowski K., Frölich L., et al. Enhanced ROS-generation in lymphocytes from Alzheimer’s patients. Pharmacopsychiatry. 2005;38(6):312–315. doi: 10.1055/s-2005-916186. [DOI] [PubMed] [Google Scholar]
- 185.Vignini A., Nanetti L., Moroni C., et al. Modifications of platelet from Alzheimer disease patients: a possible relation between membrane properties and NO metabolites. Neurobiology of Aging. 2007;28(7):987–994. doi: 10.1016/j.neurobiolaging.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 186.Calabrese V., Sultana R., Scapagnini G., et al. Nitrosative stress, cellular stress response, and thiol homeostasis in patients with Alzheimer’s disease. Antioxidants & Redox Signaling. 2006;8(11–12):1975–1986. doi: 10.1089/ars.2006.8.1975. [DOI] [PubMed] [Google Scholar]
- 187.Skoumalová A., Ivica J., Šantorová P., Topinková E., Wilhelm J. The lipid peroxidation products as possible markers of Alzheimer’s disease in blood. Experimental Gerontology. 2011;46(1):38–42. doi: 10.1016/j.exger.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 188.Buizza L., Cenini G., Lanni C., et al. Conformational altered p53 as an early marker of oxidative stress in Alzheimer’s disease. PLoS One. 2012;7(1, article e29789) doi: 10.1371/journal.pone.0029789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mecocci P., Polidori M. C., Ingegni T., et al. Oxidative damage to DNA in lymphocytes from AD patients. Neurology. 1998;51(4):1014–1017. doi: 10.1212/WNL.51.4.1014. [DOI] [PubMed] [Google Scholar]
- 190.Dorszewska J., Kempisty B., Jaroszewska-Kolecka J., et al. Expression and polymorphisms of gene 8-oxoguanine glycosylase 1 and the level of oxidative DNA damage in peripheral blood lymphocytes of patients with Alzheimer’s disease. DNA and Cell Biology. 2009;28(11):579–588. doi: 10.1089/dna.2009.0926. [DOI] [PubMed] [Google Scholar]
- 191.Kadioglu E., Sardas S., Aslan S., Isik E., Karakaya A. E. Detection of oxidative DNA damage in lymphocytes of patients with Alzheimer’s disease. Biomarkers. 2004;9(2):203–209. doi: 10.1080/13547500410001728390. [DOI] [PubMed] [Google Scholar]
- 192.Mórocz M., Kálmán J., Juhász A., et al. Elevated levels of oxidative DNA damage in lymphocytes from patients with Alzheimer’s disease. Neurobiology of Aging. 2002;23(1):47–53. doi: 10.1016/S0197-4580(01)00257-3. [DOI] [PubMed] [Google Scholar]
- 193.Viña J., Lloret A., Vallés S. L., et al. Mitochondrial oxidant signalling in Alzheimer’s disease. Journal of Alzheimer's Disease. 2007;11(2):175–181. doi: 10.3233/JAD-2007-11205. [DOI] [PubMed] [Google Scholar]
- 194.Sultana R., Mecocci P., Mangialasche F., Cecchetti R., Baglioni M., Butterfield D. A. Increased protein and lipid oxidative damage in mitochondria isolated from lymphocytes from patients with Alzheimer’s disease: insights into the role of oxidative stress in Alzheimer’s disease and initial investigations into a potential biomarker for this dementing disorder. Journal of Alzheimer's Disease. 2011;24(1):77–84. doi: 10.3233/JAD-2011-101425. [DOI] [PubMed] [Google Scholar]
- 195.Sultana R., Baglioni M., Cecchetti R., et al. Lymphocyte mitochondria: toward identification of peripheral biomarkers in the progression of Alzheimer disease. Free Radical Biology & Medicine. 2013;65:595–606. doi: 10.1016/j.freeradbiomed.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bulteau A. L., Ikeda-Saito M., Szweda L. I. Redox-dependent modulation of aconitase activity in intact mitochondria. Biochemistry. 2003;42(50):14846–14855. doi: 10.1021/bi0353979. [DOI] [PubMed] [Google Scholar]
- 197.Mangialasche F., Baglioni M., Cecchetti R., et al. Lymphocytic mitochondrial aconitase activity is reduced in Alzheimer’s disease and mild cognitive impairment. Journal of Alzheimer's Disease. 2015;44(2):649–660. doi: 10.3233/JAD-142052. [DOI] [PubMed] [Google Scholar]
- 198.Molina J. A., de Bustos F., Jimenez-Jimenez F. J., et al. Respiratory chain enzyme activities in isolated mitochondria of lymphocytes from patients with Alzheimer’s disease. Neurology. 1997;48(3):636–638. doi: 10.1212/WNL.48.3.636. [DOI] [PubMed] [Google Scholar]
- 199.Valla J., Schneider L., Niedzielko T., et al. Impaired platelet mitochondrial activity in Alzheimer’s disease and mild cognitive impairment. Mitochondrion. 2006;6(6):323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Feldhaus P., Fraga D. B., Ghedim F. V., et al. Evaluation of respiratory chain activity in lymphocytes of patients with Alzheimer disease. Metabolic Brain Disease. 2011;26(3):229–236. doi: 10.1007/s11011-011-9253-y. [DOI] [PubMed] [Google Scholar]
- 201.Leuner K., Schulz K., Schütt T., et al. Peripheral mitochondrial dysfunction in Alzheimer’s disease: focus on lymphocytes. Molecular Neurobiology. 2012;46(1):194–204. doi: 10.1007/s12035-012-8300-y. [DOI] [PubMed] [Google Scholar]
- 202.Cecchi C., Latorraca S., Sorbi S., et al. Gluthatione level is altered in lymphoblasts from patients with familial Alzheimer’s disease. Neuroscience Letters. 1999;275(2):152–154. doi: 10.1016/S0304-3940(99)00751-X. [DOI] [PubMed] [Google Scholar]
- 203.Bermejo P., Martín-Aragón S., Benedí J., et al. Peripheral levels of glutathione and protein oxidation as markers in the development of Alzheimer’s disease from mild cognitive impairment. Free Radical Research. 2009;42(2):162–170. doi: 10.1080/10715760701861373. [DOI] [PubMed] [Google Scholar]
- 204.Baldeiras I., Santana I., Proença M. T., et al. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. Journal of Alzheimer's Disease. 2008;15(1):117–128. doi: 10.3233/JAD-2008-15110. [DOI] [PubMed] [Google Scholar]
- 205.Lloret A., Badía M. C., Mora N. J., Pallardó F. V., Alonso M. D., Viña J. Vitamin E paradox in Alzheimer’s disease: it does not prevent loss of cognition and may even be detrimental. Journal of Alzheimer's Disease. 2009;17(1):143–149. doi: 10.3233/JAD-2009-1033. [DOI] [PubMed] [Google Scholar]
- 206.Badía M. C., Giraldo E., Dasí F., et al. Reductive stress in young healthy individuals at risk of Alzheimer disease. Free Radical Biology & Medicine. 2013;63:274–279. doi: 10.1016/j.freeradbiomed.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 207.Cornelius C., Trovato Salinaro A., Scuto M., et al. Cellular stress response, sirtuins and UCP proteins in Alzheimer disease: role of vitagenes. Immunity & Ageing. 2013;10(1):p. 41. doi: 10.1186/1742-4933-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gatta L., Cardinale A., Wannenes F., et al. Peripheral blood mononuclear cells from mild cognitive impairment patients show deregulation of Bax and Sod1 mRNAs. Neuroscience Letters. 2009;453(1):36–40. doi: 10.1016/j.neulet.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 209.Mota S. I., Costa R. O., Ferreira I. L., et al. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer’s disease. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2015;1852(7):1428–1441. doi: 10.1016/j.bbadis.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 210.Vural H., Demirin H., Kara Y., Eren I., Delibas N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. Journal of Trace Elements in Medicine and Biology. 2010;24(3):169–173. doi: 10.1016/j.jtemb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 211.Ishizuka K., Kimura T., Yoshitake J., et al. Possible assessment for antioxidant capacity in Alzheimer’s disease by measuring lymphocyte heme oxygenase-1 expression with real-time RT-PCR. Annals of the New York Academy of Sciences. 2002;977(1):173–178. doi: 10.1111/j.1749-6632.2002.tb04814.x. [DOI] [PubMed] [Google Scholar]
- 212.Schipper H. M. Heme oxygenase-1: role in brain aging and neurodegeneration. Experimental Gerontology. 2000;35(6-7):821–830. doi: 10.1016/S0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- 213.Maes O. C., Kravitz S., Mawal Y., et al. Characterization of α1-antitrypsin as a heme oxygenase-1 suppressor in Alzheimer plasma. Neurobiology of Disease. 2006;24(1):89–100. doi: 10.1016/j.nbd.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 214.Ponce D. P., Salech F., Martin C., et al. Increased susceptibility to oxidative death of lymphocytes from Alzheimer patients correlates with dementia severity. Current Alzheimer Research. 2014;11(9):892–898. doi: 10.2174/1567205011666141001113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Behrens M. I., Silva M., Salech F., et al. Inverse susceptibility to oxidative death of lymphocytes obtained from Alzheimer’s patients and skin cancer survivors: increased apoptosis in Alzheimer’s and reduced necrosis in cancer. The Journals of Gerontology. Series A. 2012;67(10):1036–1040. doi: 10.1093/gerona/glr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Straface E., Matarrese P., Gambardella L., et al. Oxidative imbalance and cathepsin D changes as peripheral blood biomarkers of Alzheimer disease: a pilot study. FEBS Letters. 2005;579(13):2759–2766. doi: 10.1016/j.febslet.2005.03.094. [DOI] [PubMed] [Google Scholar]
- 217.Mangialasche F., Xu W., Kivipelto M., et al. Tocopherols and tocotrienols plasma levels are associated with cognitive impairment. Neurobiology of Aging. 2012;33(10):2282–2290. doi: 10.1016/j.neurobiolaging.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 218.Akbaraly T. N., Hininger-Favier I., Carrière I., et al. Plasma selenium over time and cognitive decline in the elderly. Epidemiology. 2007;18(1):52–58. doi: 10.1097/01.ede.0000248202.83695.4e. [DOI] [PubMed] [Google Scholar]