Abstract
Significance: Particulate matter (PM) air pollution is a leading cause of global cardiovascular morbidity and mortality. Understanding the biological action of PM is of particular importance in improvement of public health.
Recent Advances: Both fine (PM <2.5 μM) and ultrafine particles (<0.1 μM) are widely believed to mediate their effects through redox regulated pathways. A rather simplistic graded ramp model of redox stress has been replaced by a more sophisticated understanding of the role of oxidative stress in signaling, and the realization that many of the observed effects may involve disruption and/or enhancement of normal endogenous redox signaling and induction of a potent immune-mediated response, through entrainment of multiple reactive oxygen species (ROS).
Critical Issues: The molecular events by which pulmonary oxidative stress in response to inhalational exposure to air pollution triggers inflammation, major ROS (e.g., superoxide, hydroxyl radical, nitric oxide, and peroxynitrite) generated in air pollution exposure, types of oxidative tissue damage in target organs, contributions of nonimmune and immune cells in inflammation, and the role of protective proteins (e.g., surfactant, proteins, and antioxidants) are highly complex and may differ depending on models and concomitant disease states.
Future Directions: While the role of oxidative stress in the lung has been well demonstrated, the role of oxidative stress in mediating systemic effects especially in inflammation and injury processes needs further work. The role of antioxidant defenses with chronic exposure will also need further exploration. Antioxid. Redox Signal. 28, 797–818.
Keywords: : air pollution, particulate matter, oxidative stress, redox reaction, reactive oxygen species
Background
The paradigm of oxidative stress-mediated cellular toxicity has dominated our understanding of particulate matter (PM) air pollution-mediated inflammation and health effects for several decades (82, 118). The traditional model of understanding has been that of a graded response paradigm, where low levels of oxidant stress mediate physiological effects, while high levels mediate toxicity (179). Over the last two decades, this rather simplistic model has been gradually replaced by a more nuanced understanding of the role of redox mechanisms where redox-active mediators, including reactive oxygen species (ROS) and reactive nitrogen species, act in a complex manner as both site-specific mediators of cell signaling and central regulators of inflammatory response (27, 71, 113). This emerging perspective suggests that many of the observed effects of particulates could involve disruption of endogenous redox signaling and/or potentiation of endogenous sources of ROS resulting in exaggerated responses. Endogenous ROS come from different sources that include the mitochondrial respiratory chain, NADPH oxidases (NOXs), nitric oxide synthases (NOS), cyclooxygenases, lipoxygenases, xanthine oxidase, and cytochrome P450s (71, 113, 172). The focus in this review is to examine recent studies in animals and humans that have contributed to a refined understanding of the role of ROS in air pollution mediated cardiovascular (CV) and metabolic disease. The American Heart Association (AHA), European scientific statements, and several expert documents on air pollution provide the foundation for the extensive literature on air pollution-mediated CV events, and thus, this review will not attempt to recapitulate any of this evidence (18, 109, 110, 118). While Wilson et al. discuss the role of oxidative stress in diesel exhaust-associated CV disease in the same forum issue (174), we focus our review on the pathways through which systemic effects occur with ambient air pollution exposure. We address mechanisms surrounding pulmonary transduction of air pollution systemic effects, including the role of reactive biologic intermediates generated in response to air pollution exposure. The evidence for oxidant stress pathways in mediating systemic effects in humans, based on evidence from epidemiologic studies, human panel studies, and controlled exposure studies, will be presented.
Air Pollutant Considerations
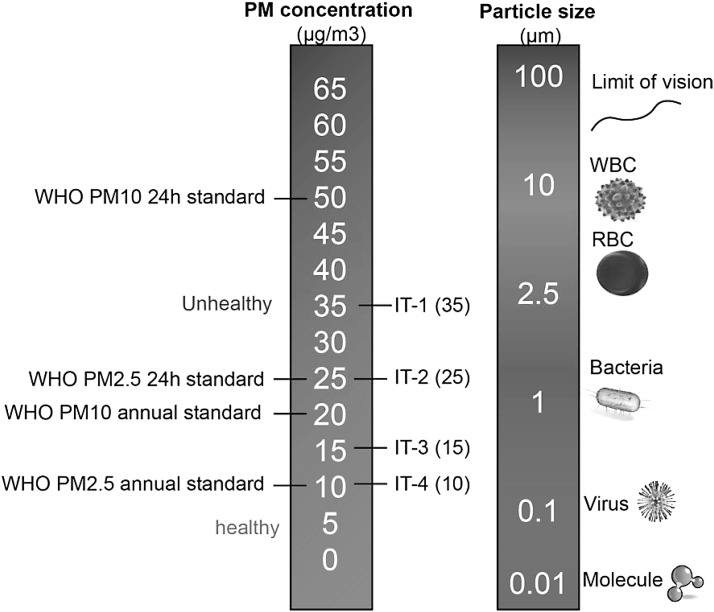
Airborne particulates constitute a heterogeneous complex mixture, differing based on source and varying with time, and atmospheric conditions (18). A number of factors affect the toxicity of particles, including size, shape, structure, surface reactivity, solubility, biopersistence, and “leachable” components (114, 122, 123, 139). Considering these factors, classification of particles based on physicochemical attributes is not easy and for lack of better ways, the most common method to characterize PM is based on size (Fig. 1). The most important size-fractions are particulate matter <10 μM (PM10), particulate matter <2.5 μM (PM2.5), and particulate matter <0.1 μM (PM0.1; also called ultrafine particles, UFPs). Coarse particles typically originate from natural sources such as dust from earth cover, road and tire abrasion, construction work, and agricultural sources. This component may also contain substantial amounts of lipopolysaccharides. The fine and ultrafine fractions tend to be dominated by anthropogenic sources such as power, combustion, mining, and other industrial sources. Size is an important determinant of locus of deposition (upper vs. lower airways) and toxicity, as the smaller particles have a larger surface-to-mass ratio and also compositionally are replete with reactive components (121, 122, 139). UFPs carry an abundance of soluble components on the particle surface, such as transition metal ions and organic compounds, including polycyclic aromatic hydrocarbons (PAHs) that may mediate systemic effects (122–124). PAHs are a group of hydrocarbons—organic compounds that are produced from the burning of organic substances such as coal, oil, gasoline, trash, tobacco, wood, and charcoal-broiled meat. They are catalyzed by cytochrome P450 and dihydrodiol dehydrogenase and generate ROS (92, 126). Exposure to PAHs has been implicated in CV disease related to smoking and environmental exposures (134). Source apportionment studies demonstrate that the organic carbon fraction and sulfate had the strongest evidence for associations with the CV disease endpoints, with much weaker evidence for elemental carbon and silicon (12, 17, 99, 131, 169).
FIG. 1.
Particulate matter sizes and air pollution thresholds and guidelines. Air pollution standards were obtained from WHO 2005 air quality guidelines. IT, interim targets; PM2.5, particulate matter <2.5 μM; PM10, particulate matter <10 μM.
Current challenges in interpretation of studies of air pollution-mediated toxicity
Clarifying the mechanisms by which particles trigger inflammatory and redox pathways are at the core of particle toxicology and biological effects. There are several difficult questions pertaining to better understanding of the role of ROS in the pathogenesis of air pollution-mediated effects. These are enumerated below and are considered throughout the article.
(i) Contribution of endogenous cellular sources of ROS: ROS may originate not only directly from the particles, but also from various intracellular sources. The best evidence for the contribution of endogenous cellular sources at least in animal models is proof that manipulation of ROS pathways through knockout or other models modulates effects of air pollution exposure (31, 63, 89, 136, 178, 184).
(ii) Delineating contributions of ROS from immune cells versus nonimmune cells: The precise delineation of the role of ROS from immune-mediated cells to nonimmune cells such as endothelial and epithelial cells may be important to understand locus of effects and potential targets for intervention (106).
(iii) Role of pulmonary oxidative stress in mediation of systemic responses: Many elements within the PM could directly elicit oxidative damage to the airway and lung tissues. The inflammation and ROS in the lung likely help to remove the injurious stimuli and initiate tissue repair. However, the persistence of pulmonary inflammation and imbalance in ROS and antioxidant response may cause systemic effects. The role of pulmonary oxidative stress and various damage-associated molecular patterns (DAMPs), including oxidatively modified lipoproteins, oxDNA, ssRNA, dsRNA, HMGB1, and mitochondrial protein, and their impact by binding to various receptors (Toll-like receptors [TLRs] and Receptor for Advanced Glycation End Products, [RAGE]) in triggering systemic cytokine and chemokines are important areas of research (35, 36, 59, 106).
PM exposure methodological considerations
Mechanisms obtained from simple cell culture studies and in vitro models although useful to define specific pathways, are of limited utility in predicting systemic responses outside of the lung, especially given the fact that the cells in consideration likely never see these particles (51, 139, 166). Intratracheally administered doses rely on numerous assumptions and often result in uneven intrapulmonary distribution of the particles bypassing the upper airway tracts. Small rodent models, almost exclusively used in research, differ in their breathing patterns, nasal anatomy, and filtering mechanisms from humans, making extrapolation of results difficult. Concentrator systems provide the best compromise as they allow physiologic exposure to higher concentrations over prolonged periods and mimic the physiologic route of entry. Both a strength and limitation, however, are that both concentrations and composition can vary considerably from day-to-day. In addition, only certain particle size ranges are typically concentrated, whereas ambient air contains a mixture of particle sizes and gases. Potential interactions between PM and gaseous copollutants are therefore excluded, unless the latter is reintroduced (22, 93). Other methods of controlled inhalation exposures include diesel engine exhaust (diluted and aged mixtures of high numbers of fresh combustion UFP with vapor-phase components), roadside aerosols, and wood burning sources and are useful in examining effects of source-specific emissions (94, 98, 100). Human studies involving direct exposure although valuable, occur over a few hours and these responses may not be representative of prolonged exposure. Moreover, exposure health risk of experimental subjects, specifically those with underlying heart and lung diseases, and high expense are difficult issues. Panel studies in humans and analysis of surrogate endpoints also provide windows into potential pathways, but are associative. In this review, any reference to PM2.5 exposure is equivalent to exposures to concentrated ambient PM2.5 (CAP) in whole body chambers, unless otherwise specified.
Evidence for Systemic Oxidative Stress with Air Pollution Exposure: Insights from Animal Studies into Sources
Evidence of oxidative stress involvement in the lungs has been noted extensively previously (81). In addition, evidence of oxidative stress has been noted systemically in many organs (30, 54, 180, 184, 186, 192) (Table 1 and Fig. 2). Acute exposure studies have shown a relationship between the vascular dysfunction in systemic microvessels and the release of myeloperoxidase from leukocytes into the vasculature within only hours after the pulmonary instillation of PM (120). In a seminal investigation involving ApoE−/− fed high-fat diet, chronic exposure to CAP exacerbated vascular oxidant stress and promoted atherosclerosis progression (155). The proatherogenic effects of ambient UFP versus PM2.5 in genetically susceptible ApoE−/− mice in a mobile whole-body exposure facility close to a Los Angeles freeway have also been compared (4). Exposure to UFP resulted in a more pronounced atherosclerotic effect (compared to PM2.5), inhibition of anti-inflammatory capacity of high-density lipoprotein (HDL), and greater systemic oxidative stress as evidenced by increased hepatic malondialdehyde (MDA) and upregulation of Nrf2-regulated antioxidant genes (4).
Table 1.
Animal Studies Investigating the Mechanisms by Which Oxidative Stress Mediates Adverse Health Effect of Particulate Matter <2.5 μM
| Authors/year | Animal | Exposure method | Exposure length | ROS targets | Main findings |
|---|---|---|---|---|---|
| Sun et al./2008 (158) | Male Sprague-Dawley rat | Whole-body exposure | 10 Weeks | O2•− generation by DHE staining and lucigenin-enhanced chemiluminescence | O2•− production in aortic rings was markedly enhanced in PM2.5-exposed rat compared with the FA group. This effect was abolished by PEG-SOD or NADPH oxidase inhibitor treatment. mRNA level of NAPDH oxidase subunit p22phox and p47phox significantly increased in the aortic tissues of PM-exposed rats. |
| Xu et al./2010 (184) | 4-Week-old C57BL/6 and p47phox−/− | Whole-body exposure | 10 Weeks | O2•− anion by lucigenin-enhanced chemiluminescence | O2•− production was significantly increased in the epididymal fat (visceral fat), but not in the subcutaneous fat location of the mice exposed to PM2.5 compared with the FA group. This effect was abolished in p47phox−/− mice. |
| Xu et al./2011 (181) | 4-Week-old C57BL/6 mice | Whole-body exposure | 9 Months | DHE fluorescence, 3-nitrotyrosine, Nrf2, Nqo1, and Gclm | Long-term PM2.5 exposure significantly induced superoxide production as determined by DHE staining, increased 3-nitrotyrosine expression in BAT depots, increased Nrf2, Nqo1, and Gclm gene expression in both WAT and BAT. |
| Xu et al./2011 (186) | Male ApoE−/− mice | Whole-body exposure | 2 Months | DHE fluorescence | DHE fluorescence density increased, while UCP-1 level decreased in BAT of mice exposed to PM2.5 |
| Kampfrath et al./2011 (63) | C57BL/6; Nox2−/−; Tlr4Lps-d; BALB/c; c-fmsYFP mice | Whole-body exposure | O2•− anion by lucigenin-enhanced chemiluminescence; oxPAPC (POVPC, PGPC) | O2•− production increased in aorta and perivascular fat of mice exposed to PM2.5. TLR4 deficiency attenuated, while Nox2 deficiency abrogated the effects of PM2.5 on O2•− production. oxPAPC level increased in the BAL fluid of PM2.5-exposed mice. The increase of oxPAPC was attenuated in PM2.5-exposed Tlr4-deficient mice. | |
| Wold et al./2012 (175) | C57BL/6 mice | Whole-body exposure | 9 Months | Total antioxidant capacity in inhibiting ABTS oxidation | Total antioxidant capacity in the plasma was significantly decreased in the plasma of PM2.5 mice. |
| Davel et al./2012 (30) | Male Wistar rat | Harvard-ambient particle concentrator | 2 Weeks | DHE fluorescence, eNOS, SOD | DHE fluorescence density and protein expression of Cu/Zn- and Mn-SOD increased in the pulmonary artery, while eNOS decreased in the artery after PM2.5 exposure. |
| Sun et al./2013 (153) | Sprague-Dawley rat | Whole-body exposure (CAPs, O3, or CAPs+O3) | 2 Weeks | iNOS | CAPs and/or O3 exposure resulted in increased iNOS immunofluorescence signal in white adipose tissue of high fructose-treated mice exposed to CAPs, O3, and CAPs+O3 compared to the normal diet air groups. |
| Liu et al./2014 (88) | KKay mice | Whole-body exposure | 8–13 Weeks | oxPAPC | The ratio of ox-PAPC/PAPC was increased twofold in the brain of PM2.5-exposed mice compared to FA-exposed mice. |
| Rao et al./2014 (136) | ApoE−/− or LDLR−/− mice | Whole-body exposure | 6 or 3 Months | 7-Ketocholesterol | PM2.5 increased 7-ketocholesterol in plasma IDL/LDL fraction and in aortic plaque concomitant with progression of atherosclerosis. |
| Li et al./2015 (78) | Male Wistar rat | Intratracheal instillation (0.375, 1.5, 6.0, and 24.0 mg/kg body weight) | First, third, fifth, seventh, and ninth day | SOD, MAD, iNOS, NO, GST, c-fos, c-jun | SOD activity decreased, MAD, iNOS, NO, GST, c-fos, and c-jun increased in lung tissues after PM2.5 exposure. |
| Zheng et al./2015 (192) | Male C57 BL/6 and p47phox−/− mice | Whole-body exposure | 10 Weeks | DHE fluorescence | PM2.5 exposure significantly induced ROS (indicated by fluorescence density) in the liver tissues of the WT mice, PM2.5-triggered ROS production was significantly reduced in the p47phox−/− mice. |
| Haberzettl et al./2016 (50) | C57BL/6 (WT) and ecSOD Tg mice (lung specific) | Whole-body exposure | 9 or 30 Days | eNOS, Akt phosphorylation, IkBa | Treatment with the antioxidant TEMPOL or lung-specific overexpression of ecSOD prevented PM2.5-induced suppression of insulin-stimulated Akt/eNOS phosphorylation and IκBα levels in the aorta. |
| Xu et al./2016 (180) | C57BL/6J and Nrf2−/− male mice | Nose- and mouth-type inhalation exposure system | 24 Weeks | SOD activity and O2•− level in hypothalamus | SOD activity decreased, O2•− level increased in hypothalamus of PM2.5-exposed WT mice, and even further in Nrf2−/− mice. |
ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); Akt, protein kinase B; BAL, bronchoalveolar lavage; BAT, brown adipose tissue; BC, black carbon; CAPs, concentrated ambient PM2.5; CAT, catalase; DHE, dihydroethidium; eNOS, endothelial nitric oxide synthase; FA, filtered air; Gclm, glutamate-cysteine ligase modifier subunit; GST, glutathione S-transferase; IDL, intermediate-density lipoprotein; IkBa, inhibitor of kappa B; iNOS, inducible nitric oxide synthase; LDL, low-density lipoprotein; MDA, malondialdehyde; mRNA, messenger RNA; NO, nitric oxide; Nqo1, NAD(P)H dehydrogenase (quinone 1); Nrf2, nuclear factor, erythroid derived 2, like 2; oxPAPC, oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine; PAPC, 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine; PEG-SOD, polyethylene glycol-SOD; PGPC, 1-palmitoyl-2-glutaroyl-sn-glycero-3-phosphorylcholine; PM, particulate matter; PM2.5, particulate matter <2.5 μM; POVPC, 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphorylcholine; ROS, reactive oxygen species; SOD, superoxide dismutase; TBA-RS, thiobarbituric acid reactive substances; TLR4, Toll-like receptor 4; UCP-1, uncoupling protein-1; WAT, white adipose tissue; WT, wild type.
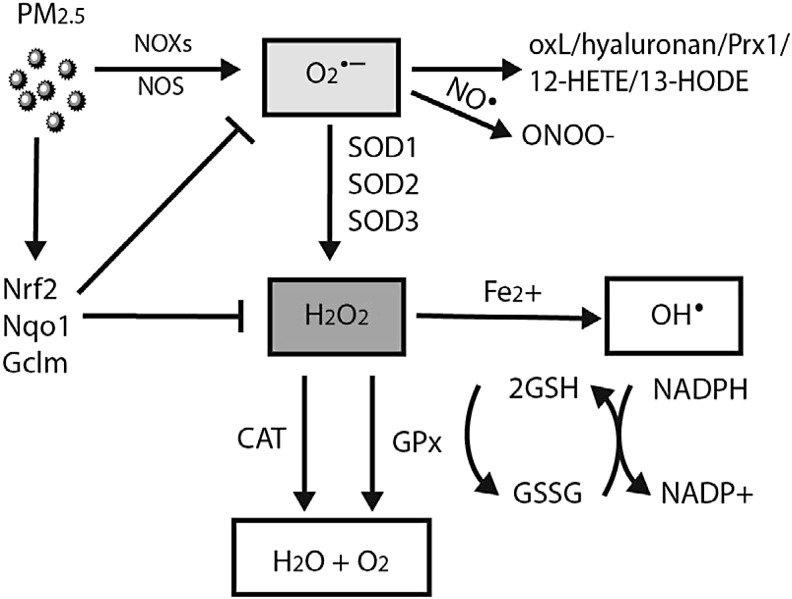
FIG. 2.
Generation and clearance of reactive oxygen species in air pollution. CAT, catalase; Gclm, glutamate-cysteine ligase modifier subunit; GPx, glutathione peroxidase; GSH, glutathione; GSSG, glutathione disulfide; NO, nitric oxide; NOS, nitric oxide synthases; NOXs, NADPH oxidase; Nqo1, NAD(P)H dehydrogenase, quinone 1; Nrf2, nuclear factor erythroid 2-related factor 2; oxL, oxidized lipids; Prx1, peroxiredoxin-1; SOD, superoxide dismutase.
In Sprague-Dawley rats exposed to PM2.5 for 10 weeks, before angiotensin II infusion, exposure increased superoxide (O2•−) production in the aorta as measured by in situ dihydroethidium (DHE) staining, as well as enhancing the vasoconstriction to phenylephrine compared to the filtered air (FA) group (158). The O2•− production was abolished by NAD(P)H oxidase inhibitor apocyanin and NOS inhibitor N-omega-nitro-l-arginine methyl ester (L-NAME), suggesting that O2•−-mediated reduction in bioavailability might be an important mechanism inducing the adverse vascular effect. Further analysis demonstrated that NOX subunits rac1, p22phox, and p47phox were increased in the aortic tissues of PM2.5-exposed animals (158). In these studies, a substantial drop in the expression of tetrahydrobiopterin (BH4), a cofactor of NOS, was seen in the heart and mesenteric vasculature, consistent with uncoupled endothelial NOS as a source of redox stress (158). Later studies also found an increase of inducible NOS in animals exposed to PM2.5 or PM2.5/ozone combine exposure (153, 190). Therefore, NOS may be an important source of aortic ROS production in response to air pollution. We have also detected increased O2•− production in the visceral adipose tissue but not subcutaneous fat in mice exposed to PM2.5. Deficiency of p47phox abolished the enhancement of O2•− production in the visceral adipose tissue from PM2.5-exposed animals (184). Both fine PM and ultrafine ambient PM have been observed to result in dysfunctional HDL that loses its anti-inflammatory properties, antioxidant capacity, reduced ability to protect against low-density lipoprotein (LDL)-induced monocyte migration, and decreased paraoxonase activity (189). Exposure to UFPs such as diesel may result in 5-lipoxygenase-mediated formation of 12-HETE and 13-HODE, peroxidation products in the plasma and liver, but interestingly not in the lung (79). Increases in other oxidases such as cytochrome P450s and glutathione S-transferase, along with the increases in inflammatory markers and oxidative stress markers (nitric oxide [NO] and MAD), have also been observed in the lung of rats intratracheally exposed to PM2.5 (78). To date, although there are several studies demonstrating abnormalities in mitochondrial numbers and morphology, including alterations in mitochondrial gene expression, there are no definitive studies demonstrating a clear contribution of mitochondrial sources, at least with long-term concentrated ambient exposures (181, 183, 186).
Unfolded Protein Response and Endoplasmic Reticulum Stress
Oxidative stress, endoplasmic reticulum (ER) stress, and inflammation usually coexist in the pathogenesis of multiple cardiometabolic diseases such as diabetes and atherosclerosis (57, 74). Unfolded protein response (UPR)/ER stress is an evolutionarily conserved and sophisticated cellular response to alleviate protein misfolding (171). Various environmental stressors can disrupt ER protein-folding resulting in the accumulation of “unfolded” proteins and ER stress (53). The activation of UPR involves three ER transmembrane stress sensors: inositol-requiring enzyme 1α (IRE1α), PKR-like ER kinase (PERK), and activating transcription factor 6α (ATF6α) (194). These ER stress sensors are normally bound to immunoglobulin protein (BiP) and are inactive, but in response to unfolded/misfolded proteins, BiP dissociates to bind unfolded/misfolded proteins initiating downstream signaling (49).
Recent studies have suggested that UPR/ER stress is involved in PM2.5 exposure-induced adverse effects (69, 101, 133). After 10 weeks of whole-body exposure (mean PM2.5 concentration 74.6 μg/m3), both oxidative stress and ER stress were detected in the lung and liver tissues of C57BL/6 mice (69). To test the hypothesis that ROS are required for PM2.5-induced UPR signaling in macrophages, cells overexpressing manganese superoxide dismutase (SOD) or dominant negative Rac 1 (N17Rac1) were exposed to PM2.5 particles in the media. PM2.5 exposure increased levels of phosphorylated eIF2α, CHOP, and GADD34 in the control RAW264.7 cells but not in cells expressing Mn-SOD or dominant negative N17Rac1, suggesting that PM2.5-induced ER stress depends on the production of ROS (69). A subsequent study confirmed the upregulation of a number of genes associated with ER stress along with enhanced infiltration of macrophage in the white adipose tissue from PM2.5-exposed animals, suggesting that activation of ER stress via ROS pathways may occur in systemic tissues (101). Phosphorylation of eIF2α was increased in the liver along with induction of CHOP/GADD153, a C/EBP homologous transcription factor, associated with apoptosis in the lung and liver (69). This study also demonstrated a critical role for NOX-dependent oxidant stress in the activation of PM2.5-induced ER stress, as p47−/− mice were protected against ER stress. Mendez et al. also reported that inhalational exposure to PM2.5 chronically (10 months) induces UPR/ER stress, lipid deposition, and adipocyte differentiation changes in adipose tissue (101). There was an increase of expression of ER stress-associated genes (such as BiP/GRP78, Xbp-1, and Edem1) in white adipose tissue of PM2.5-exposed mice, along with an increased size of adipocytes. ER stress may also regulate adipocyte lipid metabolism and inflammation (33, 65). Indeed, expression of the genes involved in lipogenesis, lipid transport (cluster of differentiation 36 [CD36]), TG synthesis, and adipocyte differentiation/lipid droplet formation in white adipose were affected by exposure to PM2.5 (101). In a subsequent study, exposure to PM2.5 led to activation of c-Jun N-terminal kinase (JNK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and TLR4, and suppression of the insulin receptor substrate 1 (IRS1)-mediated signaling in the liver, with fat deposition and glycogen depletion consistent with a nonalcoholic steatohepatitis picture (69). PM2.5 exposure repressed expression of the peroxisome proliferator-activated receptor PPARγ and PPARα in the liver, suggesting that inflammatory activation, perhaps through ER stress, may modulate an inflammatory response that then leads to downregulation of PPARα and PPARγ leading to inflammation and reduction of fatty acid oxidation and hepatic glycogen production (69).
Xu et al. observed that in response to PM2.5 exposure, ApoE−/− mice (96.89 μg/m3, 2 months) reduced the expression of brown adipocyte-specific genes, while white adipocyte-specific genes were differentially upregulated in brown adipose tissue (BAT) (186). Thus, exposure to air pollutants and likely PM2.5-mediated ROS and ER stress may adversely affect adipose tissue lipid metabolism and brown to white adipose transition. Indeed, in additional careful experiments performed in metabolic cages, PM2.5 exposure resulted in reduced VO2 and VCO2 levels, consistent with a reduction in metabolism. These effects were associated with reduction in uncoupled protein-1 (UCP-1) expression in BAT consistent with an impact of CAP on thermogenesis (87). Whether mitochondrial ROS sources are additionally involved remains to be determined, but parenthetically, chronic inhalational PM2.5 exposure results in reduction in BAT mitochondria and downregulation of BAT genes, including UCP-1 (181, 186).
Antioxidant Mechanisms and Redox Balance with Air Pollution Exposure
In contrast to the rather robust in vivo evidence base supporting the upregulation of ROS pathways in response to air pollution exposure, the regulation and expression of antioxidant defenses with in vivo are relatively sparse. There are a multitude of endogenous pathways that scavenge a range of ROS, including enzymatic systems (such as SOD, catalase [CAT], glutathione peroxide, thioredoxin reductases, NADPH quinone oxidoreductase 1 [NQO1], and methionine sulfoxide reductases) and nonenzymatic entities (such as glutathione [GSH], vitamins A, C, and E, and flavonoids) (97). Many of these defense mechanisms are regulated at the transcriptional and post-transcriptional levels. For example, SOD, glutathione S-transferase A2 (GSTA2), and NQO1, the major detoxication enzymes, contain antioxidant response elements in their promoter region. In response to oxidative stress, Keap1 dissociates from Nrf2, releasing it to bind to the antioxidant response element of target antioxidant genes (61, 143). The magnitude of antioxidative response may depend on the duration, concentration, and toxicity of exposure, and susceptibility of the subjects to air pollutants. For instance, antioxidants may increase in the early phase to limit oxidative damage, while long-term exposure or high-level exposure or highly toxic air pollutants may cause exhaustion of endogenous antioxidant responses. In this regard, there is a paucity of understanding of temporal regulation of antioxidant responses at the transcriptional and post-transcriptional level.
Two weeks of exposure to PM2.5 resulted in vascular oxidative stress and upregulated the expression of Cu/Zn-SOD and Mn-SOD together with a reduction in endothelial nitric oxide synthase (eNOS) and vasorelaxation in the pulmonary artery (30). Findings by Xu et al. indicate an increase in messenger RNA (mRNA) expression of Nrf2 and downstream genes Nqo1 and glutamate-cysteine ligase modifier subunit (Gclm) in C57BL/6 mice exposed to CAP for 10 months (181). The contribution of locus and tissue-specific Nrf2 in attenuating PM2.5 effects are important questions and would need to be examined. At least in in vitro studies, stimulation of Nrf2−/− dendritic cells with PM, augmented oxidative stress, and cytokine production compared with resting or Nrf2+/+ cells. In contrast to Nrf2+/+ cells, coincubation of Nrf2−/− dendritic cells with PM and the antioxidant N-acetyl cysteine attenuated PM-induced upregulation of CD80 and CD86 (173). These findings suggest that broad transcriptional regulators of antioxidant responses may regulate initiation and maintenance of T cell proliferation in response to antigenic stimulation to components of PM.
Mechanisms by Which Oxidative Stress Mediates Effects of PM in the Lung
The molecular events by which pulmonary oxidative stress triggers inflammation, along with the contributions and interactions between nonimmune and immune cells, the role of protective proteins (e.g., surfactants, proteins, and antioxidants) is highly complex and may differ depending on models (39, 111, 149). In the human condition, the situation is decidedly more complex, with the ultimate effect depending on particle fate (e.g., lung clearance vs. retention), sequestration, intracellular distribution, pathways of potential systemic transmission, and ultimately on multiple host factors, including susceptibility (39, 149). The importance of oxidant stress mechanisms and the contribution of alveolar macrophages to generation of cytokines and chemokines have been previously extensively reviewed and we touch on broad themes (11, 55, 106, 113, 168).
Role of direct ROS generating capacity of particle/particle constituents and the oxidant stress paradigm
It is often difficult to determine if particle-derived ROS formation or secondary endogenous ROS by cells contribute to toxicity. This is important in the correct interpretation of the direct toxic effects of particles, where prior studies have attempted to correlate the oxidative capacity of particles in cell-free systems and their ability to induce inflammatory responses or other effects in cells, animals, or humans (51, 139, 166). The effects of PM in animal models with inhalational exposure may vary depending on the composition and sources of particulates and since UFPs have more reactive components and greater ROS potential, they may be expected to have greater systemic effects (3). For example, Manhattan PM2.5 composed of traffic-related UFP has larger effects on atherosclerosis (89% increase) (190) compared with those in Sterling Forest, New York (58–68% increase) (155, 157). Araujo et al. also reported that UFP-exposed mice developed 25% greater atherosclerotic plaques compared with those exposed to PM2.5 (4). In humans, the evidence suggests an inconsistent correlation between oxidative capacity of particles and their effects in humans (166). In the graded ramp model of oxidative stress, low levels of ROS result in activation of antioxidant and phase II defenses (114). When the antioxidant response is inadequate, pathological oxidative stress can initiate a variety of pulmonary inflammatory responses. For example, ROS in the lungs have been shown to augment the signal transduction of membrane ligands (e.g., epidermal growth factor by disrupting phosphatases) and pattern recognition (e.g., TLRs) (9, 24, 56, 58, 83) that lead to the increased expression of a variety of cytokines and chemokines. With higher levels of ROS, there could be activation of kinase pathways (MAPK) and transcription factors such as NF-κB and AP1 leading to increased synthesis of inflammatory proteins. At extreme levels of ROS, there may be direct alterations in membrane permeability and mitochondrial damage. This rather simplistic model does not account for host defense mechanisms, including antioxidant response that may curb the effects of ROS.
PM interaction with cellular membranes
Carbon nanoparticles have been reported to alter the composition of lipid rafts in airway epithelial cells, by increasing the content of ceramides (sphingolipids) (129). Components such as PAHs may alter the fluidity of cellular membranes and affect lipid raft formation (164). Since lipid rafts play a central role in aggregation of receptor complexes, the alterations in this key membrane component could affect a variety of signaling pathways and cellular functions. Notably, this response seems to be mediated through activation of the cytosolic aryl hydrocarbon receptor (AhR) and inhibition of cholesterol synthesis (163, 164). PAHs have also been found to increase the fluidity of cellular model membranes directly, and benzo[a]pyrene (B[a]P) may interact with carbonyl groups of phospholipids (67, 86). Negatively charged surface groups on the surface of particles may interact with positively charged moieties on the head group of membrane phospholipids resulting in alteration of membrane bilayer and induction of membrane permeability. Crystalline particles such as asbestos and quartz while efficiently taken up by pulmonary macrophages without toxicity (due to coating by protective proteins, including surfactants), lysed in the acidic environment of the lysozyme, where their surface is stripped away resulting in lysosomal membrane damage and rupture. Membrane binding may induce both interleukin-1β (IL-1β) transcription and cleavage of pro-IL-1β to mature IL-1β through combined activation of NF-κB and ROS-mediated stimulation of the nucleotide-binding oligomerization domain (NOD)-like receptor containing pyrin domain 3 (NLRP3) resulting in so called frustrated phagocytosis (66, 72, 119, 159).
Activation of membrane-associated surface receptors
Membrane-associated receptors have been noted to be involved in a variety of in vitro studies in recognizing particles and particle components (148). Transient receptor potential channels (TRP) have been implicated in particle sensing and may be activated by combustion particles or soluble organics (transient receptor potential cation channel, subfamily A, member 1 [TRPA1], and transient receptor potential cation channel, subfamily V, member 1 [TRPV1]) (68, 140). Some of these receptors appear to be activated directly by combustion particles or soluble organics, such as TRPA1 and TRPV1, while others such as TRPV4 have been suggested to be activated more indirectly through transactivation (40, 52, 150). TRP-mediated calcium signaling, at least in the case of TRPV4, seems to regulate inflammatory gene transcription through ERK1/2 cascade (77). The precise role of ROS in regulation of TRP channels is not clear.
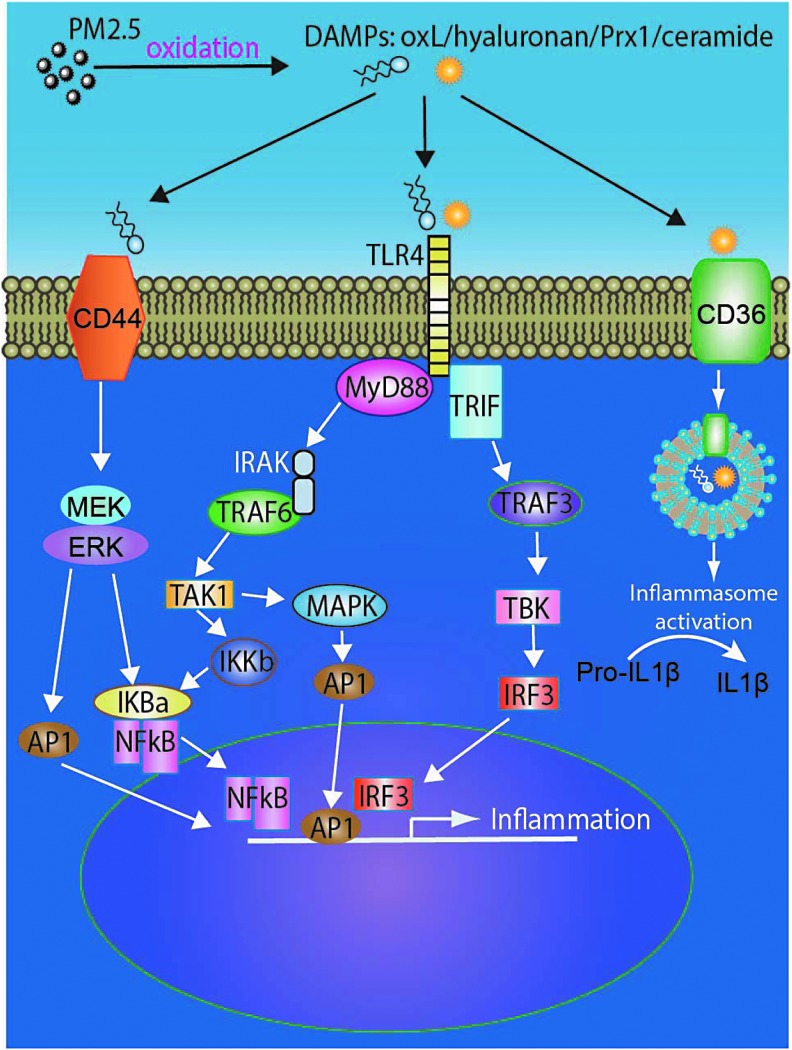
Multiple families of pattern recognition receptors (PRRs) exist and include C-type lectin receptors, TLRs, and pentraxins that survey the extracellular milieu, as well as the nucleotide-binding domain leucine-rich repeats (NLRs) and RIG-I-like receptors (RLRs), which detect intracellular signals. Biological components of PM2.5 such as endotoxin, DNA, may activate TLRs directly or indirectly through secondary mediators such as ROS (Fig. 3) (10, 60, 148). Scavenger receptors such as CD36 are also involved in sensing PM-generated oxidation products and may cooperate with TLRs for mediation of effects (146). For instance, oxidized lipid derivatives such as 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine (PAPC) and 7-ketocholesterol may be recognized by CD36 and phagocytosed by macrophage and ultimately synergize with TLR4-dependent pathways to amplify inflammation (136). ROS as an activator of TLR and NLRP3 has been suggested by many studies, in which inhibition and/or deficiency of NOX-derived ROS prevented TLR activation or ATP-induced caspase-1 activation and IL-1β production in alveolar macrophages (26, 36). In an important study, innate immune signaling via TLR4-TIR-domain-containing adapter-inducing interferon-β (TRIF)-TNF receptor-associated factor (TRAF)-6 was shown to be a key genetic pathway that determines the susceptibility to acute lung failure in vivo (59). In the same study, oxidized phospholipids generated in the lung in response to acute lung injury were shown to activate the TLR4 signaling cascade. Local lung injury not only triggered activation of the oxidative stress but also induced upregulation of TLR4 and amplified inflammatory response. Deficiency of p47phox significantly ameliorated the generation of ROS and production of oxidized phospholipids.
FIG. 3.
TLR signaling mediated by PM2.5. AP1, activator protein 1; DAMP, damage-associated molecular pattern; IκB, nuclear factor of kappa light polypeptide gene enhancer in B cell inhibitor; IKKβ, IκB kinase β; IRF3, interferon regulatory transcription factor 3; MAPK, mitogen-activated protein kinase; MyD88, myeloid differentiation primary response gene 88; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; TAK1, TGF-β-activated kinase 1; TBK, TANK-binding kinase; TLR, Toll-like receptor; TRAF, TNF receptor-associated factor; TRIF, TIR-domain-containing adapter-inducing interferon-β.
Although multiple PRR families in conjunction with uptake receptors such as CD36 converge in the regulation of cytokine and chemokine transcription, the NLR family is more specifically responsible for maturation of proinflammatory cytokines IL-1β or IL-18 especially in response to crystalline or foreign biomaterials (38, 96, 146). The activation of NlrP3 appears to be a two-step mechanism, with the primary signal from the activation of TLRs and transcriptional upregulation of NLRP3 and pro-IL-1β via NF-κB. Secondary signals come from multiple pathways: K+ efflux via P2X7 receptor activation, ER stress, mitochondrial dysfunction, NOX, frustrated phagocytosis, and lysosomal rupture pathways, all of which appear to converge in the production of ROS. The hypothesis that ROS could serve as an NLRP3-activating trigger was initially proposed when NOX-derived ROS prevented ATP-induced caspase-1 activation and IL-1β production in alveolar macrophages (26). Knockdown of the p22phox subunit of NOX significantly suppressed IL-1β release in THP1 cells in response to asbestos and monosodium urate challenge, both of which are classic triggers for NLRP3 activation (36). The crystal structure of NLRP3 contains a highly conserved disulfide bond connecting the PYD domain and the nucleotide-binding site domain, which is sensitive to redox alterations (5).
Mechanisms by Which Pulmonary Oxidative Stress Is Systemically Transduced
The mechanisms underlying initiation of systemic inflammation in response to air pollution and the involvement of oxidative stress in this transduction are still evolving but almost certainly have to involve the lung in some capacity. Hypothesized mechanisms include the following. (i) Release of inflammatory cytokines and chemokines systemically (46, 63). (ii) Recruitment of a systemic innate immune response (42, 46, 63, 89, 188). (iii) Secondary antigens generated in response to oxidation, by antigen-presenting cells that may then present antigens to the T cells in the draining lymph node and activate adaptive immunity (32). (iv) Leachable components such as transition metals and organic secondary intermediates such as oxidized phospholipids, quinines, semiquinones, and aldehydes generated in the lung that may overflow into the circulation (34, 63, 85). (v) Activation of central nerve system pathways via TRP receptors, C-fibers, taste receptors, or other afferent mechanisms that may facilitate systemic inflammation (91, 133, 150). (vi) Small particles (especially UFPs) entering blood stream and impair endothelial function by direct interaction with the endothelium.
Reactive intermediates generated in response to air pollution exposure and role in systemic responses
PM-induced oxidation may also result in a number of oxidatively modified molecules, which serve as a secondary mediator to induce systemic effects. These mediators include oxidatively modified proteins, lipids, surfactants, and matrix proteins.
To illustrate the role of oxidized lipids as a secondary mediator, we examined the oxidative stress response in the lungs and oxidized lipid generation in mice exposed chronically (>20 weeks) to concentrated PM2.5. Airborne PM2.5 markedly increased oxidized derivatives of PAPC in the bronchoalveolar lavage (BAL) fluid. Both 1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine (POVPC) and 1-palmitoyl-2-glutaryl phosphatidylcholine (PGPC) increased with PM2.5 exposure. Incubation of PM2.5 by itself with BAL had no effect on oxidized phospholipids, arguing against direct particle-mediated ROS generation and suggesting an endogenous mechanism of oxidation of PAPC (63). The production of NOX-derived O2•− was increased in monocytes, aortic tissue, and perivascular fat from wild-type (WT) mice exposed to concentrated PM2.5, accompanied by impaired vascular function suggesting a systemic oxidative stress response. In another study, an increase of oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (oxPAPC) was noted in the brain of PM2.5-exposed mice, as measured by mass spectrometry (88).
To delineate the involvement of TLR4 and NOX pathways in the generation of oxidized lipids, and recruitment of a systemic inflammatory response, Nox2−/−, WT mice, and mice deficient in TLR4 (Tlr4Lps-d [TLR4d]) were exposed to air pollution. PM2.5 increased inflammatory monocytes (CD11b+Ly6Chi) in the peripheral circulation in TLR4wt mice, an effect diminished in TLR4d mice (16). PM2.5 exposure-associated vascular O2•− production, vascular dysfunction, and macrophage infiltration were reversed in TLR4d mice and in Nox2−/− mice (63). TLR4wt mice demonstrated an increase in TNFα, MCP-1, and IL12p70 and a decrease of IL-10 levels in the lung, while TLR4 deficiency attenuated these responses. Increase in plasma levels of TNFα and MCP-1 with PM2.5 exposure was normalized in TLR4d mice. Incubation of bone marrow-derived macrophages to oxidized PAPC recapitulated a stereotypical inflammatory response reminiscent of the in vivo effects in the lung with PM2.5 exposure, including phosphorylation of a cytosolic subunit of NOX p47 and interleukin-1 receptor-associated kinase (IRAK) phosphorylation, abolished by TLR deficiency. Using atherosclerosis-prone ApoE−/− mice, we demonstrated that PM2.5 exposure also increased 7-ketocholesterol, an oxidatively modified form of cholesterol, in plasma LDL and intermediate-density lipoprotein fractions concomitant with progression of atherosclerosis. In addition, CD36, a scavenger receptor recognizing specific oxidized phospholipids and lipoproteins, was upregulated in PM2.5-exposed mice in peripheral monocytes and within plaque macrophages. Increased CD36 expression resulted in foam cell formation and accumulation of 7-ketocholesterol in atherosclerotic plaque, while CD36 deficiency in myeloid cells abolished PM2.5-induced 7-ketocholesterol accumulation, foam cell formation, and plaque progression in LDLR−/− mice (136).
Gaseous air pollutants such as ozone have been demonstrated to have important effects on immune activation (153, 193). Garantziotis reported that O3 exposure increased hyaluronan levels in the lavage fluid and enhanced airway hyperactivity in C57BL/6 mice. Short-fragment hyaluronan (100–400 kDa) but not high-molecular-weight hyaluronan activated macrophages and induced airway hyperactivity in CD44 (a hyaluronan surface receptor)- and TLR4/myeloid differentiation primary response gene 88 (MyD88)-dependent mechanisms (43–45, 84). Mice deficient in CD44 (43, 44), TLR4, MyD88, or TIRAP (84) attenuated O3-induced airway hyperactivity and production of proinflammatory cytokines, including TNFα, IL-1β, MCP-1, IL-6, and keratinocyte chemoattractant (CXCL1).
Recruitment of a systemic innate immune response in response to PM
A role for ROS
Multiple early studies have demonstrated that acute exposure results in recruitment of an innate bone marrow response, but the identity of these cells and specific populations was unclear (46, 162). Using a model of fluorescently labeled monocytes under control of a c-fms (receptor for macrophage-colony stimulating factor, M-CSF), Sun et al. confirmed that intratracheal PM2.5 enhanced YFP+ migration, adhesion to the mesenteric microcirculation, and accumulation in visceral fat (156). These results were redemonstrated with chronic exposure to ambient PM2.5 where significantly more adherent YFP+ cells were found in the cremasteric endothelial wall in response to chronic PM2.5 (63). Xu et al., while investigating the effects of PM2.5 exposure over 10 weeks in C57Bl/6 mice, detected increased macrophage infiltration in visceral adipose tissue and vascular dysfunction (184). Deficiency of p47phox improved abnormalities in insulin resistance, vascular function, and reduced visceral inflammation (F4/80+ macrophages) in response to PM2.5, suggesting a proximal role for NOX in this process (184). TLR4 and NOX appear to mediate the effect of PM2.5 as deficiency of TLR4 diminished the effect of PM2.5 on Ly6Chigh cells (F4/80+, CD11b+, CD115+). The preponderant majority of PM2.5-mediated circulating Ly6Chigh cells appeared to originate from the bone marrow. Deficiency of TLR4 and Nox2 abolished the effects of PM2.5 on systemic tissues, suggesting a cooperation of these pathways in mediating exposure effects (63).
We examined the role of CCR2 and chemokine (C-X-C motif) receptor 3 (CXCR3) in two separate experiments involving separate groups of WT C57BL/6, CCR2−/−, and CXCR3−/− knockout mice, exposed to variable but chronic periods to PM2.5 or FA. In the first article, PM2.5 exposure resulted in increased CD11b+CD11c+ macrophages and CXCR3+ T cells in the lung, mediastinal lymph nodes, and spleen (32). While circulating CD4+ cell numbers remained unchanged, CD8+ cell numbers increased in WT-PM2.5 mice. The number of activated CD44+CD62L−CD4+ T cells in the lung was also increased in PM2.5-exposed WT mice. CXCR3 deficiency completely prevented the movement of CXCR3+CD4+ and CXCR3+CD8+ T cells into the lung, reduced the numbers of activated T cells (CD44+CD62L− CD4+), in response to PM2.5, and also reduced increases in CD11c+CD11b+ dendritic cells in the lung seen in response to PM2.5. CXCR3 deficiency attenuated the total number of CD4+ and CD8+ T cells exposed to PM2.5 seen in WT and was associated with increased retention of these populations in the spleen, suggesting impairment of homing to the lung. Central memory cell populations (CCR7+ CD44+CD62+) were increased with PM2.5 in both WT and CXCR3−/− mice compared with FA groups, suggesting that CXCR3 is not involved in the trafficking of this subset of cells (32). Consistent with the prior study by Kampfrath et al., PM2.5-exposed mice had significantly higher ratios of oxidized derivatives of PAPC (POVPC and PGPC) in the BAL fluid, with the ratio of PGPC/POVPC to PAPC nearly doubling with exposure to PM2.5 (63). These findings suggest robust innate and adaptive immune activation in response to the air pollution, likely via oxidative stress-mediated transformation of intermediate proteins with recruitment via CXCR3 mechanisms. In a subsequent article, we tested the involvement of CCR2 in mobilization of innate immune cell populations. CCR2−/− or WT C57Bl/6 mice were exposed by inhalation to either FA or PM2.5 (PM) 17 weeks. We noted an increase in circulating CD11b+Gr-1low7/4hi cells, the inflammatory subtype in response to PM2.5 exposure, seen previously (63). The levels of CD11b+Gr-1low7/4hi in circulation following PM2.5 inhalation were significantly reduced in CCR2−/− mice with a corresponding decrease in the spleen. F4/80+ adipose tissue macrophages were increased in visceral adipose of WT mice but not in CCR2−/− exposed to PM2.5. PPARγ, a transcription factor required for alternate macrophage differentiation, was downregulated in visceral adipose tissue (VAT) of WT-PM2.5 mice, but was only partially downregulated in CCR2-PM2.5 mice. Nrf1 levels were significantly lower in the WT-PM group than that in the WT-FA group, and this was partially restored in CCR2−/− PM2.5 mice. CCR2−/− mice demonstrated reduction in whole-body insulin resistance and improvements in hepatic lipid accumulation in the liver. This occurred via SREBP1c-mediated transcriptional reprogramming, decreased fatty acid uptake, and suppression of hepatic p38 MAPK activity likely related to decreased hepatic infiltration of CCR2+ inflammatory cells in the CCR2−/− mice exposed to PM2.5. Abnormal phosphorylation levels of protein kinase B (AKT), AMPK in visceral adipose tissue in response to PM2.5 exposure, and adipose tissue macrophage content in WT mice exposed to PM were not present in CCR2−/− mice. In contrast to the studies by Kampfrath et al., vascular function impaired by PM2.5 was not significantly different between CCR2−/− and WT mice (63, 89).
A recent study by Haberzettl et al. provides definitive evidence that oxidative stress emanating from the lung may modulate systemic responses. In this work, exposure to concentrated PM2.5 using an exposure chamber for 9 or 30 days reduced insulin-stimulated Akt/eNOS activation in the lung and circulation system. Thirty-day PM2.5 exposure also increased adipose tissue inflammation and systemic glucose intolerance in WT mice. Treatment with antioxidant TEMPOL or overexpression of lung-specific extracellular SOD prevented PM2.5-induced suppression of Akt/eNOS activation and inflammation (50).
Activation of central nerve system pathways
The activation of autonomic nervous system has also been suggested to be involved in the systemic transmission of the adverse effect of air pollution and oxidative stress (21, 135). Air pollutants have been shown to permeate into the olfactory bulb through the olfactory nerve and induce inflammation in the central nervous system. In addition, the TRPA1 receptors in airway sensory neurons can also sense the environmental toxicants and aerogenic oxidants, resulting in neurogenic inflammation (150). We observed increased hippocampal inflammation and impaired spatial learning memory in animals exposed to chronic PM2.5 (41). In follow-up studies, we have provided evidence that activation of sympathetic nervous system and hypothalamic inflammation occurs in response to PM2.5 exposure, with inhibition of central IκB kinase β (IKKβ) preventing the adverse effects of air pollution on peripheral inflammation and abnormalities in insulin resistance and changes in whole-body metabolism (88, 191). In addition, PM particles may directly permeate via the olfactory nerve into the central nervous system (13, 112). Indeed, disruption of antioxidative response by deleting Nrf2 enhanced nerve injury and hypothalamus oxidative stress, while nanoceria, an anti-inflammatory and antioxidant stress biomaterial, restrained PM2.5-induced metabolic syndrome and inflammation (180). We also demonstrated that PM2.5 exposure increased the level of oxPAPC, an oxidatively modified lipid that has been shown to activate TLR/NF-κB pathway (36, 63), in the brain (88).
Endothelial dysfunction induced by direct interaction between PM and endothelial cells
Air pollution exposure-associated endothelial dysfunction has been evidenced in both humans and animals and considered to be an instigating factor for CV disease (28). Ultrafine components UFPs are able to penetrate lung epithelium and enter the bloodstream where they could mediate systemic effects (115, 117, 147, 161). Findings in both humans and mice suggest that nanoparticles inhaled into the lung could rapidly cross the alveolar membrane and appear in the circulation (115, 117). Individual toxic components such as heavy metals, hydrocarbons, and other organic chemicals on the surface of circulating UFPs may directly interact with vascular endothelium, causing oxidative stress and endothelium damage (116). Both endothelial vasodilation and endogenous fibrinolysis were found to be impaired in healthy adults exposed to dilute diesel exhaust (300 μg/m3) (104). We have previously demonstrated redox alterations in the vessel wall through NOX pathways, which in turn may increase aortic vascular tone through O2•−-mediated upregulation of the Rho/ROCK pathway (158). Increased peripheral vasoconstriction due to short-term exposure to PM2.5 may result in elevation in systemic blood pressure and increased cardiac afterload, and over a longer term result in left ventricular hypertrophy, myocardial fibrosis, and alteration in coronary flow reserve (175).
Evidence of the Association Between Particulate Matter and Oxidative Stress in Humans
Evidence from panel studies
The demonstration of systemic oxidative stress is difficult in humans as the techniques are relatively simple and can be applied only on plasma or airway fluid. The assays used to assess the footprint of systemic “oxidative stress” or damage may significantly influence the results. As such, the clinical evidence for increased oxidative stress following exposure to air pollutants is limited, and existing studies somewhat inconsistent (Table 2). Nevertheless, a recently published meta-analysis suggests that there is a robust relationship between exposure to particulate air pollution and increases in oxidative DNA adducts and oxidized lipids in man (107). The studies that have demonstrated a positive relationship include an increase in urinary excretion of free 8-iso-prostaglandin2α among healthy adults following a 4-h exposure to concentrated wood smoke (8), and an increase in plasma antioxidant capacity 24 h after exposure to diesel exhaust in a group of healthy volunteers after a 1-h exposure (167). Other investigators (127) have observed significant differences in expression of genes involved in oxidative stress pathways due to diesel exhaust exposure. In a study that examined the effect of ultrafine traffic particles on oxidative stress-induced damage to DNA in healthy young adults exposed to low concentrations of ambient urban particles (PM2.5 and PM2.5–10 mass of 9.7 and 12.6 μg/m3, respectively) in an exposure chamber above a busy road with high traffic density, increased levels of DNA strand breaks and formamidopyrimidine-DNA glycosylase sites in monocytes were seen without any change in the DNA repair enzyme 7,8-dihydro-8-oxoguanine-DNA glycosylase (14). Similar to their previous findings with ambient exposure (170), the results seemed to suggest that short-term exposure to UFP may result in damage to DNA. Results from the same investigators failed to demonstrate significant biomarker signals for lipid or protein oxidative damage after similar near-roadway exposures (15).
Table 2.
Association of Air Pollution and Oxidative Stress in Human Studies
| Authors/year | Location | Subjects | Exposure | ROS targets | Main findings |
|---|---|---|---|---|---|
| Sørensen et al./2003 (152) | Central Copenhagen | 50 Students | BC, PM2.5 | HBGGS, HBAAS, PLAAS, MDA | Significant effect of BC exposure (p < 0.01) and moderate effect of PM2.5 (p = 0.061) were positively associated with PLAAS concentration. A 3.7% increase in MDA concentrations in personal PM2.5 exposure was found for women but not for men. |
| Schwartz et al./2005 (144) | Boston, MA | 497 Men, mean age: 73 | PM2.5 48 h | GSTM1 | Change per 10 μg/m3 GSTM1-present −3.6% (95% CI: −40.5, 56.2), GSTM1-null −34% (95% CI: −53, −7.2). |
| Park et al./2006 (125) | Boston, MA | 518 Men, mean age: 73 | PM2.5 48 h | HFE | The difference in effect of PM2.5 on the high-frequency component of HRV between persons with and without HFE variants was significant (p for interaction = 0.02). |
| Chahine et al./2007 (25) | Boston, MA | 539 Men, mean age: 73 | PM2.5 48 h | GSTM1 HO1 promoter | PM2.5 was significantly associated with SDNN, HF, and LF in subjects with GSTM1 deletion but not in subjects with GSTM1. Similarly, significant association with HRV measure in subjects with any long repeat variant of HO1, but not in any short repeat variant. |
| Mordukhovich et al./2009 (108) | 457 Men | 7-Day moving averages of BC and PM2.5 | GSTM1, GSTP1, GSTT1, NQO1, catalase, and HO1 | Positive association between BP and PM2.5, but not between BP and PM2.5; no association between /PM2.5 and BP by oxidative gene variants. | |
| Baja et al./2010 (6) | Boston, MA | 580 Men | BC, PM2.5, SO2, and O3 | GSTM1, GSTT1, GSTP1, NAD(P)H dehydrogenase, NQO1, CAT, HO1 | BC but not PM2.5 was associated with longer QTc. Association between BC and QTc was stronger among participants who had higher GSSs. |
| Ren et al./2010 (138) | Boston, MA | 1000 Men, mean age: 72 | 7-Day moving averages of PM2.5 | HFE, NQO1, CAT, GSTM1, GSTP1, GSTT1, HO1 | Association between PM2.5 and increases in total plasma homocysteine (2.2%, 95% CI: 0.6–3.9%) was mediated by HFE, C282Y, and CAT. |
| Madrigano et al./2010 (95) | Boston, MA | 809 Men, mean age: 74 | PM2.5 1–3 days | GSTM1, HO1, HFE | Polymorphisms in GSTM1, HO1, or HFE genes did not modify the relationship between PM2.5 exposure and serum concentrations of sICAM-1 or sVCAM-1 (effect estimates not reported). |
| Levinsson et al./2014 (76) | West Sweden | 119 AMI and 1310 controls | NO2 | GSTP1, GSTT1, GSTCD | NO2 was significantly associated with increased risk of AMI (OR: 1.78; 95% CI: 1.04–3.03). No obvious interaction between genetic variants in the GST genes and air pollution exposure for hypertension and AMI. |
| Lee et al./2014 (75) | Boston, MA | 21 Participants, mean age: 44 years | PM2.5 48 h | 8-OHdG (DNA oxidation) | Significantly greater PM2.5-associated nighttime SDNN reductions were observed among subjects in the upper 20th percentile of 8-OHdG by −25.3% compared with the lower 80th percentile (p < 0.0001). |
| Roy et al./2014 (142) | Beijing, China | 125 Young adults, mean age: 24 years | CO, PM2.5, NO2, SO2, EC, OC, and sulfate | FeNO, nitrite, and MDA (exhaled breath), 8-OHdG and MDA (urine) | The pulmonary inflammation and oxidative stress pathway is the first to respond to ambient air pollution exposure (within 24 h) and the hemostasis pathway responds gradually over a 2–3-day period. |
| Wu et al./2015 (177) | Beijing, China | 40 Male university students | PM2.5 and compositions | oxLDL | There were a 1.9% increase (95% CI: 0.2–3.7%) and a 1.8% (95% CI: 0.2–3.4%) increase in oxLDL, for each interquartile range increase in iron (1 day, 0.51 μg/m3) and nickel (2 day, 2.5 ng/m3) in PM2.5, respectively. |
| Ambroz et al./2016 (2) | Prague | 342 Mothers, 344 newborns | PM2.5 | 8-oxodG (DNA oxidation), 15-F2t-IsoP (lipid peroxidation) | Authors showed that PM2.5 concentrations could be a significant predictor for 8-oxodG and 15-F2t-IsoP levels. |
| Li et al./2016 (80) | Boston, MA | 2035 Participants | PM2.5, BC, SO42−, NOx, and O3 | Myeloperoxidase (blood), 8-epi-PGF2α (urine) | Positive associations of short-term PM2.5 and BC exposure with myeloperoxidase; 2- to 7-day moving averages of PM2.5 and sulfate were positively associated with 8-epi-PGF2α. |
| Byun et al./2016 (20) | Quincy, MA | 48 Men | PM2.5 | mtDNA methylation (blood) | Interaction of PM2.5 exposure and D-loop mtDNA promoter was significantly associated with adverse effect of HRV. |
8-epi-PGF2α, 8-epi-prostaglandin F2α; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; 8-oxodG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; 15-F2t-IsoP, 15-f2t-isoprostane; AMI, acute myocardial infarction; BP, blood pressure; CI, confidence interval; FeNO, fractional exhaled nitric oxide; GSS, glutathione synthetase; GSTCD, glutathione S-transferase C-terminal domain containing; GSTM1, glutathione S-transferase M1; GSTP1, glutathione S-transferase P1; GSTT1, glutathione S-transferase theta-1; HBAAS, 2-aminoadipic semialdehyde in hemoglobin; HBGGS, gamma-glutamyl semialdehyde in hemoglobin; HF HRV, high-frequency heart rate variability; HFE, high iron Fe; HO1, heme oxygenase-1; HRV, heart rate variability; LF HRV, low-frequency heart rate variability; NO2•, nitrogen dioxide; oxLDL, oxidized low-density lipoprotein; PLAAS, 2-aminoadipic semialdehyde in plasma protein; QTc, corrected QT interval; SDNN, standard deviation of all normal RR intervals; sICAM-1, intercellular adhesion molecule 1; sVCAM-1, vascular cell adhesion protein 1.
Although not entirely consistent, the available studies demonstrate that acute exposure to PM, perhaps even at ambient levels, may induce acute systemic oxidative stress in human subjects under certain circumstances that may depend on host susceptibility factors. To investigate the effect of air pollution on oxidative stress markers, the association between plasma oxidized low-density lipoprotein (oxLDL) level and traffic-related air pollution, determined by the distance from the patient's residence to a major road and by airway macrophage carbon load, was studied in a cross-sectional study of nonsmoking adult outpatients with diabetes. Each interquartile range increase (0.25 μm2) in airway macrophage carbon load was associated with a 7.3 U/L (95% confidence interval [CI]: 1.3–13.3 U/L) increase in plasma oxLDL, and each doubling in distance from patient residence to major roads was associated with decrease in plasma oxLDL (62). In a study of 40 healthy university students before and after relocating from a suburban campus to an urban campus with high air pollution levels in Beijing, China, oxLDL was associated with iron and nickel concentration in PM2.5 particles (177). In another study carried out in Copenhagen, the association between personal PM2.5 exposure and MDA, a marker for lipid peroxidation, was examined in 50 students. A 3.7% increase in the concentration of MDA per 10 μg/m3 increase in personal PM2.5 exposure was found in women, although no significance was observed in men (152). The levels of urine 8-isoprostane, MDA in breath condensate, and alveolar NO were increased in humans exposed to wood smoke-derived particulate air pollution, along with the increase of serum amyloid A, a CV risk factor (7, 8). In addition, increased levels of oxidized nucleic acids such as 8-hydroxy-2′-deoxyguanosine (8-OHdG, also known as 8-oxo-7,8-dihydro-2′-deoxyguanosine [8-oxodG]) and 8-oxoguanine (8-oxoguanine) have also been observed in human subjects exposed to particulate air pollution (1, 2, 29, 75, 142, 160). Mitochondrial DNA is also a primary target of oxidative stress in response to environmental stimulation. Byun et al. measured personal PM2.5 exposure and blood mitochondrial DNA methylation in 48 healthy men in Massachusetts. They reported that the level of blood mitochondrial DNA methylation in the D-loop promoter was associated with PM2.5 level and heart rate variability (HRV) (20). Collectively, these data provide evidence that oxidative stress markers are associated with a variety of exposures in humans.
Epidemiological evidence of oxidative stress in particulate air pollution exposure
Larger epidemiologic studies investigating the susceptibility to air pollution have provided some evidence that oxidative stress plays an essential role in air pollution exposure. There are several studies conducted in the same population of elderly Caucasian men (The Normative Aging Study) that have studied the effects of polymorphisms in different antioxidant genes. By examining the association between PM2.5 and the high-frequency component of HRV in 497 participants in the Normative Aging Study, Schwartz et al. noticed an association between decreased high-frequency component and increased PM2.5 exposure during prior 48 h only in subjects without glutathione-S-transferase M1 (GSTM1) allele (144). Similarly, only subject with GT long tandem repeat polymorphism in the heme oxygenase-1 (HO1) promoter, but not those with short repeat variant, showed associations between PM2.5 and HRV measures (normal-to-normal intervals, high frequency, and low frequency) (25). Polymorphisms in other antioxidative genes, including high iron Fe (HFE) C282Y, CAT (rs2300181), glutathione Stransferase P1 (GSTP1), and glutathione S-transferase theta-1 (GSTT1) also modified the effects of PM2.5 (125, 138). However, there were also some studies that showed a negative impact of certain variants in antioxidative genes on certain effects of PM2.5 such as blood pressure (76, 108).
Controlled exposure studies
Several controlled exposure studies demonstrate that acute exposure to PM2.5 and dilute diesel exhaust results in rapid vascular dysfunction that manifests as endothelial dysfunction or transient constriction of a peripheral conduit vessel that is reversible at least in some studies (16, 19, 103, 104, 128, 167). In some of these studies, fine CAP exposure diminished conduit artery endothelial-dependent vasodilatation 24 h (but not immediately) postexposure (19). PM2.5 mass and TNF-α level postexposure were associated with the degree of endothelial dysfunction, suggesting that systemic inflammation induced by higher levels of particles was likely responsible (19). However, depending on the location and composition of exposure, concentrated PM2.5 may not always induce endothelial dysfunction, underscoring the importance of composition in determining vascular responses (102).
In studies of UFP, composed of elemental carbon, a 2-h exposure impaired peak forearm blood flow response to ischemia 3.5 h later. There were no other vascular changes or alterations at other time points (145). Several studies have also shown that dilute diesel exhaust can impair peripheral resistance vessel responses to agonists and reactive hyperemia responses (104, 128). The blunted responses to acetylcholine persisted for 24 h in healthy adults (167). In contrast, bradykinin- and sodium nitroprusside (SNP)-mediated vasodilatation and bradykinin-induced acute plasma tissue plasminogen activator (tPA) release were not altered 24 h later. In subsequent studies, patients with stable coronary artery disease exposed to dilute diesel exhaust for 1 h during intermittent exercise demonstrated reduced bradykinin-mediated tPA release; however, microvascular endothelial function was not impaired (103). This may perhaps be related to some degree of pre-existing endothelial dysfunction in these patients that prevented additional impairment. However, exercise-induced ST-segment depression and ischemic burden were significantly greater during diesel compared to FA exposure (103). In some studies, even a 24-h-long exposure to ambient pollution shunted into a chamber next to a busy street did not impair microvascular endothelial function assessed by digital tonometry (15). This exposure to near-roadway air, consisting of ambient UFP and PM2.5, also did not alter biomarkers of inflammation or markers of protein and lipid oxidation.
In a randomized controlled clinical trial, individuals with metabolic syndrome were exposed to diesel exhaust, 200 mg/m3 of fine PM, and FA for 120 min on days separated by ≥2 weeks, systolic blood pressure increased at all of the points measured during and after diesel exhaust exposure; the mean effect peaked between 30 and 60 min after exposure initiation (3.8 mm Hg [95% CI: −0.4 to 8.0 mm Hg] and 5.1 mm Hg [95% CI: 0.7–9.5 mm Hg], respectively). The alterations of endothelial function have been speculated to represent enhanced degradation of NO. This latter concept was tested in a randomized double-blind crossover study; healthy nonsmokers were exposed to diesel exhaust or FA. Bilateral forearm blood flow was measured during intrabrachial infusions of acetylcholine and SNP in the presence of an NO synthase inhibitor NG-monomethyl-l-arginine (L-NMMA) coinfused with the NO donor SNP to maintain basal blood flow. Following diesel exhaust inhalation, plasma nitrite concentrations were increased (68 ± 48 vs. 41 ± 32 nmol/L; p = 0.006) despite similar l-NMMA-induced reductions in basal blood flow compared to air (73). In the presence of the NO clamp, ACh and SNP caused local dose-dependent vasodilatation of the forearm, which was not affected by diesel exhaust inhalation (p > 0.05 for both). However, following exposure to diesel exhaust, systemically administered l-NMMA caused a greater increase in blood pressure (p = 0.048) and central arterial stiffness suggesting that in conduit vessels, reduced NO bioavailability has more marked effects that cannot be adequately compensated for by increased basal NO release (73).
Effects of air pollution prevention on disease
Multiple studies have demonstrated that an improvement in air quality results in a favorable reduction in cardiopulmonary disease. Pope et al. showed in 2009 that reduction in air pollution was associated with as much as 15% of the overall increase in life expectancy (130). Data from personalized intervention studies are also available, demonstrating that use of domestic air-filtration devices, particle-filtration masks, and car air filtration/air conditioning leads to meaningful reduction in CV surrogates such as systolic blood pressure, improvements in microvascular function, autonomic tone, and lower levels of inflammatory biomarkers in adults exposed to PM2.5 (110).
Polymorphisms in several oxidative stress-related genes such as GSTM1, GSTP1, GSTT1, HFE C282Y, and CAT are found to be associated with the vulnerability to PM2.5 (125, 138, 144). There have been a few intervention studies that have demonstrated that the harmful effects of air pollution could be modulated by intake of antioxidant and anti-inflammatory nutrients. Canova et al. showed that serum levels of antioxidants (vitamin C, uric acid, and vitamin E) modified the effect of PM10 on asthma/chronic obstructive pulmonary disease exacerbations (23). Vitamin C and E supplementation for 6 months normalized biomarkers of oxidative stress in individuals exposed to coal electric power plant-derived PM to control levels, suggesting a protective effect of vitamins C and E against PM-associated oxidative insult (132). Omega-3 polyunsaturated fatty acid intake from fish oil has been associated with increased levels of antioxidant proteins such as SOD and GSH. In a randomized controlled trial in Mexico City, the elderly residents of a nursing home supplemented with 2 g/day of fish oil showed a 7% decrease in high-frequency component of HRV/standard deviation increase in indoor PM2.5, compared with a 54% decrease in those individuals supplemented with 2 g/day of soy oil (141). In another randomized, controlled exposure study, 4 weeks of fish oil supplement attenuated concentrated ambient fine/ultrafine PM-induced HRV changes and increases in very low-density lipoprotein/triglyceride (165). These studies appear to suggest that common interventions thought to be protective from a CV standpoint can be beneficial in protecting against air pollution.
Conclusions and Future Direction
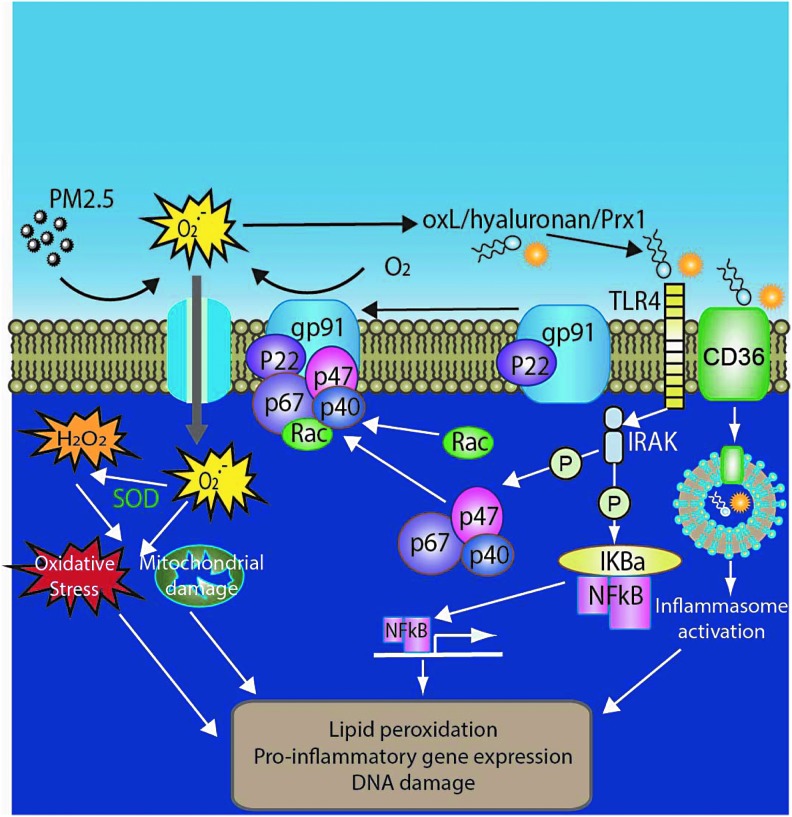
Taken together, oxidative stress appears to be a common thread in the effects of air pollution (Fig. 4). While the role of oxidative stress is compelling in the lung, the role of oxidative stress in mediating systemic effects and its role in processes such as inflammation and injury need further work. The role of endogenous antioxidant defenses particularly with chronic exposure will need further exploration. The importance of personal interventions to reduce air pollution and their effects on key oxidative stress pathways, including endogenous antioxidant defense mechanisms, are important emerging areas in future research. Finally, the effects of targeted interventions that disrupt oxidant stress pathways and/or enhance antioxidant defenses in reducing effects of air pollution are also important areas of interest.
FIG. 4.
PM2.5-mediated oxidative stress. CD36, cluster of differentiation 36; Rac, Ras-related C3 botulinum toxin substrate.
Abbreviations Used
- Akt
protein kinase B
- BAL
bronchoalveolar lavage
- BAT
brown adipose tissue
- BiP
bound to immunoglobulin protein
- CAP
concentrated ambient PM2.5
- CAT
catalase
- CD36
cluster of differentiation 36
- CI
confidence interval
- CV
cardiovascular
- CXCR3
chemokine (C-X-C motif) receptor 3
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- FA
filtered air
- GSH
glutathione
- GSTM1
glutathione-S-transferase M1
- GSTP1
glutathione S-transferase P1
- GSTT1
glutathione S-transferase theta-1
- HDL
high-density lipoprotein
- HFE
high iron Fe
- HRV
heart rate variability
- IL-1β
interleukin-1β
- LDL
low-density lipoprotein
- L-NMMA
NG-monomethyl-l-arginine
- MDA
malondialdehyde
- MyD88
myeloid differentiation primary response gene 88
- N17Rac1
dominant negative Rac 1
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLR
nucleotide-binding domain leucine-rich repeat
- NLRP3
nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3
- NO
nitric oxide
- NOS
nitric oxide synthases
- NOX
NADPH oxidase
- NQO1
NADPH quinone oxidoreductase 1
- O2•−
superoxide
- oxLDL
oxidized low-density lipoprotein
- oxPAPC
oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- PAH
polycyclic aromatic hydrocarbon
- PAPC
1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphorylcholine
- PGPC
1-palmitoyl-2-glutaryl phosphatidylcholine
- PM
particulate matter
- PM2.5
particulate matter <2.5 μM
- PM10
particulate matter <10 μM
- POVPC
1-palmitoyl-2-(5-oxovaleroyl)-sn-glycero-3-phosphocholine
- PRR
pattern recognition receptors
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- TLR
Toll-like receptor
- tPA
tissue plasminogen activator
- TRP
transient receptor potential channel
- TRPA1
transient receptor potential cation channel, subfamily A, member 1
- TRPV1
transient receptor potential cation channel, subfamily V, member 1
- UCP1
uncoupled protein-1
- UFPs
ultrafine particles
- UPR
unfolded protein response
Acknowledgments
This work was supported by grants from the NIH (R01ES015146, R01ES019616, K99ES026241, and T32DK098107). J.Z. was supported by K01DK105108 and 17GRNT33670485.
References
- 1.Allen J, Trenga CA, Peretz A, Sullivan JH, Carlsten CC, and Kaufman JD. Effect of diesel exhaust inhalation on antioxidant and oxidative stress responses in adults with metabolic syndrome. Inhal Toxicol 21: 1061–1067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambroz A, Vlkova V, Rossner P, Jr., Rossnerova A, Svecova V, Milcova A, Pulkrabova J, Hajslova J, Veleminsky M, Jr., Solansky I, and Sram RJ. Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. Int J Hyg Environ Health 219: 545–556, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Araujo JA. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual Atmos Health 4: 79–93, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo JA, Barajas B, Kleinman M, Wang X, Bennett BJ, Gong KW, Navab M, Harkema J, Sioutas C, Lusis AJ, and Nel AE. Ambient particulate pollutants in the ultrafine range promote early atherosclerosis and systemic oxidative stress. Circ Res 102: 589–596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae JY. and Park HH. Crystal structure of NALP3 protein pyrin domain (PYD) and its implications in inflammasome assembly. J Biol Chem 286: 39528–39536, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baja ES, Schwartz JD, Wellenius GA, Coull BA, Zanobetti A, Vokonas PS, and Suh HH. Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the normative aging study. Environ Health Perspect 118: 840–846, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barregard L, Sallsten G, Andersson L, Almstrand AC, Gustafson P, Andersson M, and Olin AC. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occup Environ Med 65: 319–324, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Barregard L, Sallsten G, Gustafson P, Andersson L, Johansson L, Basu S, and Stigendal L. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhal Toxicol 18: 845–853, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Becker S, Dailey L, Soukup JM, Silbajoris R, and Devlin RB. TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicol Appl Pharmacol 203: 45–52, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Becker S, Fenton MJ, and Soukup JM. Involvement of microbial components and toll-like receptors 2 and 4 in cytokine responses to air pollution particles. Am J Respir Cell Mol Biol 27: 611–618, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Becker S, Mundandhara S, Devlin RB, and Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: further mechanistic studies. Toxicol Appl Pharmacol 207: 269–275, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Bell ML, Ebisu K, Peng RD, Samet JM, and Dominici F. Hospital admissions and chemical composition of fine particle air pollution. Am J Respir Crit Care Med 179: 1115–1120, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block ML, Elder A, Auten RL, Bilbo SD, Chen H, Chen JC, Cory-Slechta DA, Costa D, Diaz-Sanchez D, Dorman DC, Gold DR, Gray K, Jeng HA, Kaufman JD, Kleinman MT, Kirshner A, Lawler C, Miller DS, Nadadur SS, Ritz B, Semmens EO, Tonelli LH, Veronesi B, Wright RO, and Wright RJ. The outdoor air pollution and brain health workshop. Neurotoxicology 33: 972–984, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brauner EV, Forchhammer L, Moller P, Simonsen J, Glasius M, Wahlin P, Raaschou-Nielsen O, and Loft S. Exposure to ultrafine particles from ambient air and oxidative stress-induced DNA damage. Environ Health Perspect 115: 1177–1182, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brauner EV, Moller P, Barregard L, Dragsted LO, Glasius M, Wahlin P, Vinzents P, Raaschou-Nielsen O, and Loft S. Exposure to ambient concentrations of particulate air pollution does not influence vascular function or inflammatory pathways in young healthy individuals. Part Fibre Toxicol 5: 13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brook RD, Brook JR, Urch B, Vincent R, Rajagopalan S, and Silverman F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105: 1534–1536, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Brook RD. and Rajagopalan S. Air pollution and cardiovascular events. N Engl J Med 356: 2104–2106, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Brook RD, Rajagopalan S, Pope CA. 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC. Whitsel L, and Kaufman JD; American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation 121: 2331–2378, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold D, Wellenius G, Mittleman MA, Rajagopalan S, and Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension 54: 659–667, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byun HM, Colicino E, Trevisi L, Fan T, Christiani DC, and Baccarelli AA. Effects of air pollution and blood mitochondrial dna methylation on markers of heart rate variability. J Am Heart Assoc 5: e003218, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calderon-Garciduenas L, Solt AC, Henriquez-Roldan C, Torres-Jardon R, Nuse B, Herritt L, Villarreal-Calderon R, Osnaya N, Stone I, Garcia R, Brooks DM, Gonzalez-Maciel A, Reynoso-Robles R, Delgado-Chavez R, and Reed W. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicol Pathol 36: 289–310, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Campen MJ, Lund AK, Doyle-Eisele ML, McDonald JD, Knuckles TL, Rohr AC, Knipping EM, and Mauderly JL. A comparison of vascular effects from complex and individual air pollutants indicates a role for monoxide gases and volatile hydrocarbons. Environ Health Perspect 118: 921–927, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canova C, Dunster C, Kelly FJ, Minelli C, Shah PL, Caneja C, Tumilty MK, and Burney P. PM10-induced hospital admissions for asthma and chronic obstructive pulmonary disease: the modifying effect of individual characteristics. Epidemiology 23: 607–615, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Cao D, Tal TL, Graves LM, Gilmour I, Linak W, Reed W, Bromberg PA, and Samet JM. Diesel exhaust particulate-induced activation of Stat3 requires activities of EGFR and Src in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L422–L429, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Chahine T, Baccarelli A, Litonjua A, Wright RO, Suh H, Gold DR, Sparrow D, Vokonas P, and Schwartz J. Particulate air pollution, oxidative stress genes, and heart rate variability in an elderly cohort. Environ Health Perspect 115: 1617–1622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, and Ojcius DM. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem 282: 2871–2879, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daiber A, Oelze M, Steven S, Kröller-Schön S, and Münzel T. Taking up the cudgels for the traditional reactive oxygen and nitrogen species detection assays and their use in the cardiovascular system. Redox Biol 12: 35–49, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, and Münzel T. Targeting vascular (endothelial) dysfunction. Br J Pharmacol 174: 1591–1619, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danielsen PH, Brauner EV, Barregard L, Sallsten G, Wallin M, Olinski R, Rozalski R, Moller P, and Loft S. Oxidatively damaged DNA and its repair after experimental exposure to wood smoke in healthy humans. Mutat Res 642: 37–42, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Davel AP, Lemos M, Pastro LM, Pedro SC, de Andre PA, Hebeda C, Farsky SH, Saldiva PH, and Rossoni LV. Endothelial dysfunction in the pulmonary artery induced by concentrated fine particulate matter exposure is associated with local but not systemic inflammation. Toxicology 295: 39–46, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Deiuliis JA, Kampfrath T, Ying Z, Maiseyeu A, and Rajagopalan S. Lipoic acid attenuates innate immune infiltration and activation in the visceral adipose tissue of obese insulin resistant mice. Lipids 46: 1021–1032, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deiuliis JA, Kampfrath T, Zhong J, Oghumu S, Maiseyeu A, Chen LC, Sun Q, Satoskar AR, and Rajagopalan S. Pulmonary T cell activation in response to chronic particulate air pollution. Am J Physiol Lung Cell Mol Physiol 302: L399–409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng J, Liu S, Zou L, Xu C, Geng B, and Xu G. Lipolysis response to endoplasmic reticulum stress in adipose cells. J Biol Chem 287: 6240–6249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dominici F, Peng RD, Ebisu K, Zeger SL, Samet JM, and Bell ML. Does the effect of PM10 on mortality depend on PM nickel and vanadium content? A reanalysis of the NMMAPS data. Environ Health Perspect 115: 1701–1703, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dostert C, Meylan E, and Tschopp J. Intracellular pattern-recognition receptors. Adv Drug Deliv Rev 60: 830–840, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, and Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320: 674–677, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. This reference has been deleted.
- 38.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, and Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464: 1357–1361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EPA US. Air Quality Criteria for Particulate Matter. October 2004. 600/P-99/002aF-bF, 2004. www.epa.gov/ttn/naaqs/standards/pm/s_pm_2006_cd.html November 7, 2007
- 40.Fariss MW, Gilmour MI, Reilly CA, Liedtke W, and Ghio AJ. Emerging mechanistic targets in lung injury induced by combustion-generated particles. Toxicol Sci 132: 253–267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, and Nelson RJ. Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry 16: 987–995, 973, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujii T, Hayashi S, Hogg JC, Mukae H, Suwa T, Goto Y, Vincent R, and van Eeden SF. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am J Respir Cell Mol Biol 27: 34–41, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, and Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 284: 11309–11317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 44.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, and Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem 291: 19257–19258, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, and Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med 181: 666–675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 46.Goto Y, Ishii H, Hogg JC, Shih CH, Yatera K, Vincent R, and van Eeden SF. Particulate matter air pollution stimulates monocyte release from the bone marrow. Am J Respir Crit Care Med 170: 891–897, 2004 [DOI] [PubMed] [Google Scholar]
- 47. This reference has been deleted.
- 48. This reference has been deleted.
- 49.Grootjans J, Kaser A, Kaufman RJ, and Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol 16: 469–484, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haberzettl P, O'Toole TE, Bhatnagar A, and Conklin DJ. Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ Health Perspect 124: 1830–1839, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han X, Corson N, Wade-Mercer P, Gelein R, Jiang J, Sahu M, Biswas P, Finkelstein JN, Elder A, and Oberdörster G. Assessing the relevance of in vitro studies in nanotoxicology by examining correlations between in vitro and in vivo data. Toxicology 297: 1–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hazari MS, Haykal-Coates N, Winsett DW, Krantz QT, King C, Costa DL, and Farraj AK. TRPA1 and sympathetic activation contribute to increased risk of triggered cardiac arrhythmias in hypertensive rats exposed to diesel exhaust. Environ Health Perspect 119: 951–957, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hettiarachchi KD, Zimmet PZ, and Myers MA. Dietary toxins, endoplasmic reticulum (ER) stress and diabetes. Curr Diabetes Rev 4: 146–156, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Hiraiwa K. and van Eeden SF. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators Inflamm 2013: 619523, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hogg JC. and van Eeden S. Pulmonary and systemic response to atmospheric pollution. Respirology 14: 336–346, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Hollingsworth JW. 2nd, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, and Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med 170: 126–132, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang YC, Wu W, Ghio AJ, Carter JD, Silbajoris R, Devlin RB, and Samet JM. Activation of EGF receptors mediates pulmonary vasoconstriction induced by residual oil fly ash. Exp Lung Res 28: 19–38, 2002 [DOI] [PubMed] [Google Scholar]
- 59.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, and Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inoue K, Takano H, Yanagisawa R, Hirano S, Ichinose T, Shimada A, and Yoshikawa T. The role of toll-like receptor 4 in airway inflammation induced by diesel exhaust particles. Arch Toxicol 80: 275–279, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, and Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobs L, Emmerechts J, Hoylaerts MF, Mathieu C, Hoet PH, Nemery B, and Nawrot TS. Traffic air pollution and oxidized LDL. PLoS One 6: e16200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kampfrath T, Maiseyeu A, Ying Z, Shah Z, Deiuliis JA, Xu X, Kherada N, Brook RD, Reddy KM, Padture NP, Parthasarathy S, Chen LC, Moffatt-Bruce S, Sun Q, Morawietz H, and Rajagopalan S. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res 108: 716–726, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. This reference has been deleted.
- 65.Kawasaki N, Asada R, Saito A, Kanemoto S, and Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci Rep 2: 799, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kingsbury SR, Conaghan PG, and McDermott MF. The role of the NLRP3 inflammasome in gout. J Inflamm Res 4: 39–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Korchowiec B, Corvis Y, Viitala T, Feidt C, Guiavarch Y, Corbier C, and Rogalska E. Interfacial approach to polyaromatic hydrocarbon toxicity: phosphoglyceride and cholesterol monolayer response to phenantrene, anthracene, pyrene, chrysene, and benzo[a]pyrene. J Phys Chem B 112: 13518–13531, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Kurhanewicz N, McIntosh-Kastrinsky R, Tong H, Ledbetter A, Walsh L, Farraj A, and Hazari M. TRPA1 mediates changes in heart rate variability and cardiac mechanical function in mice exposed to acrolein. Toxicol Appl Pharmacol 324: 51–60, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laing S, Wang G, Briazova T, Zhang C, Wang A, Zheng Z, Gow A, Chen AF, Rajagopalan S, Chen LC, Sun Q, and Zhang K. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol 299: C736–C749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. This reference has been deleted.
- 71.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 4: 181–189, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Lamkanfi M. and Kanneganti TD. Nlrp3: an immune sensor of cellular stress and infection. Int J Biochem Cell Biol 42: 792–795, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Langrish JP, Unosson J, Bosson J, Barath S, Muala A, Blackwell S, Soderberg S, Pourazar J, Megson IL, Treweeke A, Sandstrom T, Newby DE, Blomberg A, and Mills NL. Altered nitric oxide bioavailability contributes to diesel exhaust inhalation-induced cardiovascular dysfunction in man. J Am Heart Assoc 2: e004309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee J. and Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol Chem 289: 1203–1211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee MS, Eum KD, Fang SC, Rodrigues EG, Modest GA, and Christiani DC. Oxidative stress and systemic inflammation as modifiers of cardiac autonomic responses to particulate air pollution. Int J Cardiol 176: 166–170, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levinsson A, Olin AC, Modig L, Dahgam S, Bjorck L, Rosengren A, and Nyberg F. Interaction effects of long-term air pollution exposure and variants in the GSTP1, GSTT1 and GSTCD genes on risk of acute myocardial infarction and hypertension: a case-control study. PLoS One 9: e99043, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J, Kanju P, Patterson M, Chew WL, Cho SH, Gilmour I, Oliver T, Yasuda R, Ghio A, Simon SA, and Liedtke W. TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ Health Perspect 119: 784–793, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li R, Kou X, Xie L, Cheng F, and Geng H. Effects of ambient PM2.5 on pathological injury, inflammation, oxidative stress, metabolic enzyme activity, and expression of c-fos and c-jun in lungs of rats. Environ Sci Pollut Res Int 22: 20167–20176, 2015 [DOI] [PubMed] [Google Scholar]
- 79.Li R, Navab K, Hough G, Daher N, Zhang M, Mittelstein D, Lee K, Pakbin P, Saffari A, Bhetraratana M, Sulaiman D, Beebe T, Wu L, Jen N, Wine E, Tseng CH, Araujo JA, Fogelman A, Sioutas C, Navab M, and Hsiai TK. Effect of exposure to atmospheric ultrafine particles on production of free fatty acids and lipid metabolites in the mouse small intestine. Environ Health Perspect 123: 34–41, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li W, Wilker EH, Dorans KS, Rice MB, Schwartz J, Coull BA, Koutrakis P, Gold DR, Keaney JF, Jr., Lin H, Vasan RS, Benjamin EJ, and Mittleman MA. Short-term exposure to air pollution and biomarkers of oxidative stress: the Framingham Heart Study. J Am Heart Assoc 5: e002742, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li XY, Gilmour PS, Donaldson K, and MacNee W. Free radical activity and pro-inflammatory effects of particulate air pollution (PM10) in vivo and in vitro. Thorax 51: 1216–1222, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li XY, Gilmour PS, Donaldson K, and MacNee W. In vivo and in vitro proinflammatory effects of particulate air pollution (PM10). Environ Health Perspect 105 Suppl 5: 1279–1283, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Z, Carter JD, Dailey LA, and Huang YC. Pollutant particles produce vasoconstriction and enhance MAPK signaling via angiotensin type I receptor. Environ Health Perspect 113: 1009–1014, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li Z, Potts-Kant EN, Garantziotis S, Foster WM, and Hollingsworth JW. Hyaluronan signaling during ozone-induced lung injury requires TLR4, MyD88, and TIRAP. PLoS One 6: e27137, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 85.Liberda EN, Cuevas AK, Gillespie PA, Grunig G, Qu Q, and Chen LC. Exposure to inhaled nickel nanoparticles causes a reduction in number and function of bone marrow endothelial progenitor cells. Inhal Toxicol 22 Suppl 2: 95–99, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liland NS, Simonsen AC, Duelund L, Torstensen BE, Berntssen MH, and Mouritsen OG. Polyaromatic hydrocarbons do not disturb liquid-liquid phase coexistence, but increase the fluidity of model membranes. Chem Phys Lipids 184: 18–24, 2014 [DOI] [PubMed] [Google Scholar]
- 87.Liu C, Bai Y, Xu X, Sun L, Wang A, Wang TY, Maurya SK, Periasamy M, Morishita M, Harkema J, Ying Z, Sun Q, and Rajagopalan S. Exaggerated effects of particulate matter air pollution in genetic type II diabetes mellitus. Part Fibre Toxicol 11: 27, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu C, Fonken LK, Wang A, Maiseyeu A, Bai Y, Wang TY, Maurya S, Ko YA, Periasamy M, Dvonch T, Morishita M, Brook RD, Harkema J, Ying Z, Mukherjee B, Sun Q, Nelson RJ, and Rajagopalan S. Central IKKbeta inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol 11: 53, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu C, Xu X, Bai Y, Wang T-Y, Rao X, Wang A, Sun L, Ying Z, Gushchina L, Maiseyeu A, Morishita M, Sun Q, Harkema JR, and Rajagopalan S. Air pollution-mediated susceptibility to inflammation and insulin resistance: influence of CCR2 pathways in mice. Environ Health Perspect 122: 17–26, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. This reference has been deleted.
- 91.Liu C, Ying Z, Harkema J, Sun Q, and Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol 41: 361–373, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lodovici M. and Bigagli E. Oxidative stress and air pollution exposure. J Toxicol 2011: 487074, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lucking AJ, Lundback M, Barath SL, Mills NL, Sidhu MK, Langrish JP, Boon NA, Pourazar J, Badimon JJ, Gerlofs-Nijland ME, Cassee FR, Boman C, Donaldson K, Sandstrom T, Newby DE, and Blomberg A. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation 123: 1721–1728, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Lund AK, Lucero J, Lucas S, Madden MC, McDonald JD, Seagrave JC, Knuckles TL, and Campen MJ. Vehicular emissions induce vascular MMP-9 expression and activity associated with endothelin-1-mediated pathways. Arterioscler Thromb Vasc Biol 29: 511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Madrigano J, Baccarelli A, Wright RO, Suh H, Sparrow D, Vokonas PS, and Schwartz J. Air pollution, obesity, genes and cellular adhesion molecules. Occup Environ Med 67: 312–317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Malik AF, Hoque R, Ouyang X, Ghani A, Hong E, Khan K, Moore LB, Ng G, Munro F, Flavell RA, Shi Y, Kyriakides TR, and Mehal WZ. Inflammasome components Asc and caspase-1 mediate biomaterial-induced inflammation and foreign body response. Proc Natl Acad Sci U S A 108: 20095–20100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martindale JL. and Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol 192: 1–15, 2002 [DOI] [PubMed] [Google Scholar]
- 98.Mauderly JL, Barrett EG, Day KC, Gigliotti AP, McDonald JD, Harrod KS, Lund AK, Reed MD, Seagrave JC, Campen MJ, and Seilkop SK. The National Environmental Respiratory Center (NERC) experiment in multi-pollutant air quality health research: II. Comparison of responses to diesel and gasoline engine exhausts, hardwood smoke and simulated downwind coal emissions. Inhal Toxicol 26: 651–667, 2014 [DOI] [PubMed] [Google Scholar]
- 99.Maynard D, Coull BA, Gryparis A, and Schwartz J. Mortality risk associated with short-term exposure to traffic particles and sulfates. Environ Health Perspect 115: 751–755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McDonald JD, Doyle-Eisele M, Campen MJ, Seagrave J, Holmes T, Lund A, Surratt JD, Seinfeld JH, Rohr AC, and Knipping EM. Cardiopulmonary response to inhalation of biogenic secondary organic aerosol. Inhal Toxicol 22: 253–265, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Mendez R, Zheng Z, Fan Z, Rajagopalan S, Sun Q, and Zhang K. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Transl Res 5: 224–234, 2013 [PMC free article] [PubMed] [Google Scholar]
- 102.Mills NL, Robinson SD, Fokkens PH, Leseman DL, Miller MR, Anderson D, Freney EJ, Heal MR, Donovan RJ, Blomberg A, Sandstrom T, MacNee W, Boon NA, Donaldson K, Newby DE, and Cassee FR. Exposure to concentrated ambient particles does not affect vascular function in patients with coronary heart disease. Environ Health Perspect 116: 709–715, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mills NL, Tornqvist H, Gonzalez MC, Vink E, Robinson SD, Soderberg S, Boon NA, Donaldson K, Sandstrom T, Blomberg A, and Newby DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med 357: 1075–1082, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, Boon NA, Donaldson K, Blomberg A, Sandstrom T, and Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112: 3930–3936, 2005 [DOI] [PubMed] [Google Scholar]
- 105. This reference has been deleted.
- 106.Miyata R. and van Eeden SF. The innate and adaptive immune response induced by alveolar macrophages exposed to ambient particulate matter. Toxicol Appl Pharmacol 257: 209–226, 2011 [DOI] [PubMed] [Google Scholar]
- 107.Moller P. and Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect 118: 1126–1136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mordukhovich I, Wilker E, Suh H, Wright R, Sparrow D, Vokonas PS, and Schwartz J. Black carbon exposure, oxidative stress genes, and blood pressure in a repeated-measures study. Environ Health Perspect 117: 1767–1772, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Münzel T, Sörensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, and Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J 38: 557–564, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Münzel T, Sörensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD, and Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J 38: 550–556, 2017 [DOI] [PubMed] [Google Scholar]
- 111.NAAQS. National Ambient Air Quality Criteria Standards. 2008. www.epa.gov/air/criteria.html September 1, 2008
- 112.Nakane H. Translocation of particles deposited in the respiratory system: a systematic review and statistical analysis. Environ Health Prev Med 17: 263–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nathan C. and Cunningham-Bussel A. Beyond oxidative stress: an immunologist's guide to reactive oxygen species. Nat Rev Immunol 13: 349–361, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nel A, Xia T, Madler L, and Li N. Toxic potential of materials at the nanolevel. Science 311: 622–627, 2006 [DOI] [PubMed] [Google Scholar]
- 115.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, Vanbilloen H, Mortelmans L, and Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation 105: 411–414, 2002 [DOI] [PubMed] [Google Scholar]
- 116.Nemmar A, Hoylaerts MF, Hoet PH, and Nemery B. Possible mechanisms of the cardiovascular effects of inhaled particles: systemic translocation and prothrombotic effects. Toxicol Lett 149: 243–253, 2004 [DOI] [PubMed] [Google Scholar]
- 117.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, and Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med 164: 1665–1668, 2001 [DOI] [PubMed] [Google Scholar]
- 118.Newby DE, Mannucci PM, Tell GS, Baccarelli AA, Brook RD, Donaldson K, Forastiere F, Franchini M, Franco OH, Graham I, Hoek G, Hoffmann B, Hoylaerts MF, Künzli N, Mills N, Pekkanen J, Peters A, Piepoli MF, Rajagopalan S, and Storey RF. Expert position paper on air pollution and cardiovascular disease. Eur Heart J 36: 83–93, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, Lowell CA, Ling CC, Amrein MW, and Shi Y. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity 29: 807–818, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nurkiewicz TR, Porter DW, Barger M, Millecchia L, Rao KM, Marvar PJ, Hubbs AF, Castranova V, and Boegehold MA. Systemic microvascular dysfunction and inflammation after pulmonary particulate matter exposure. Environ Health Perspect 114: 412–419, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oberdörster G. Nanotoxicology: in vitro-in vivo dosimetry. Environ Health Perspect 120: 45, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Oberdorster G, Ferin J, and Lehnert BE. Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect 102: 173–179, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Oberdorster G, Oberdorster E, and Oberdorster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect 113: 823–839, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Oberdorster G. and Utell MJ. Ultrafine particles in the urban air: to the respiratory tract—and beyond? Environ Health Perspect 110: A440–A441, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park SK, O'Neill MS, Wright RO, Hu H, Vokonas PS, Sparrow D, Suh H, and Schwartz J. HFE genotype, particulate air pollution, and heart rate variability: a gene-environment interaction. Circulation 114: 2798–2805, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Penning TM, Ohnishi ST, Ohnishi T, and Harvey RG. Generation of reactive oxygen species during the enzymatic oxidation of polycyclic aromatic hydrocarbon trans-dihydrodiols catalyzed by dihydrodiol dehydrogenase. Chem Res Toxicol 9: 84–92, 1996 [DOI] [PubMed] [Google Scholar]
- 127.Peretz A, Peck EC, Bammler TK, Beyer RP, Sullivan JH, Trenga CA, Srinouanprachnah S, Farin FM, and Kaufman JD. Diesel exhaust inhalation and assessment of peripheral blood mononuclear cell gene transcription effects: an exploratory study of healthy human volunteers. Inhal Toxicol 19: 1107–1119, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, Carlsten C, Wilkinson CW, Gill EA, and Kaufman JD. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect 116: 937–942, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peuschel H, Sydlik U, Grether-Beck S, Felsner I, Stockmann D, Jakob S, Kroker M, Haendeler J, Gotic M, Bieschke C, Krutmann J, and Unfried K. Carbon nanoparticles induce ceramide- and lipid raft-dependent signalling in lung epithelial cells: a target for a preventive strategy against environmentally-induced lung inflammation. Part Fibre Toxicol 9: 48, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pope CA. 3rd, Ezzati M, and Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 360: 376–386, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pope CA. 3rd, Rodermund DL, and Gee MM. Mortality effects of a copper smelter strike and reduced ambient sulfate particulate matter air pollution. Environ Health Perspect 115: 679–683, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Possamai FP, Junior SA, Parisotto EB, Moratelli AM, Inacio DB, Garlet TR, Dal-Pizzol F, and Filho DW. Antioxidant intervention compensates oxidative stress in blood of subjects exposed to emissions from a coal electric-power plant in South Brazil. Environ Toxicol Pharmacol 30: 175–180, 2010 [DOI] [PubMed] [Google Scholar]
- 133.Rajagopalan S. and Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes 61: 3037–3045, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramos KS. and Moorthy B. Bioactivation of polycyclic aromatic hydrocarbon carcinogens within the vascular wall: implications for human atherogenesis. Drug Metab Rev 37: 595–610, 2005 [DOI] [PubMed] [Google Scholar]
- 135.Rao X, Patel P, Puett R, and Rajagopalan S. Air pollution as a risk factor for type 2 diabetes. Toxicol Sci 143: 231–241, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rao X, Zhong J, Maiseyeu A, Gopalakrishnan B, Villamena FA, Chen LC, Harkema JR, Sun Q, and Rajagopalan S. CD36-dependent 7-ketocholesterol accumulation in macrophages mediates progression of atherosclerosis in response to chronic air pollution exposure. Circ Res 115: 770–780, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. This reference has been deleted.
- 138.Ren C, Park SK, Vokonas PS, Sparrow D, Wilker E, Baccarelli A, Suh HH, Tucker KL, Wright RO, and Schwartz J. Air pollution and homocysteine: more evidence that oxidative stress-related genes modify effects of particulate air pollution. Epidemiology 21: 198–206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rivera Gil P, Oberdörster G, Elder A, Puntes V, and Parak WJ. Correlating physico-chemical with toxicological properties of nanoparticles: the present and the future. ACS Nano 4: 5227–5231, 2010 [DOI] [PubMed] [Google Scholar]
- 140.Robertson S, Thomson AL, Carter R, Stott HR, Shaw CA, Hadoke PW, Newby DE, Miller MR, and Gray GA. Pulmonary diesel particulate increases susceptibility to myocardial ischemia/reperfusion injury via activation of sensory TRPV1 and beta1 adrenoreceptors. Part Fibre Toxicol 11: 12, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Romieu I, Tellez-Rojo MM, Lazo M, Manzano-Patino A, Cortez-Lugo M, Julien P, Belanger MC, Hernandez-Avila M, and Holguin F. Omega-3 fatty acid prevents heart rate variability reductions associated with particulate matter. Am J Respir Crit Care Med 172: 1534–1540, 2005 [DOI] [PubMed] [Google Scholar]
- 142.Roy A, Gong J, Thomas DC, Zhang J, Kipen HM, Rich DQ, Zhu T, Huang W, Hu M, Wang G, Wang Y, Zhu P, Lu SE, Ohman-Strickland P, Diehl SR, and Eckel SP. The cardiopulmonary effects of ambient air pollution and mechanistic pathways: a comparative hierarchical pathway analysis. PLoS One 9: e114913, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rushmore TH, King RG, Paulson KE, and Pickett CB. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A 87: 3826–3830, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schwartz J, Park SK, O'Neill MS, Vokonas PS, Sparrow D, Weiss S, and Kelsey K. Glutathione-S-transferase M1, obesity, statins, and autonomic effects of particles: gene-by-drug-by-environment interaction. Am J Respir Crit Care Med 172: 1529–1533, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shah AP, Pietropaoli AP, Frasier LM, Speers DM, Chalupa DC, Delehanty JM, Huang LS, Utell MJ, and Frampton MW. Effect of inhaled carbon ultrafine particles on reactive hyperemia in healthy human subjects. Environ Health Perspect 116: 375–380, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, and Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol 14: 812–820, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, and Morita T. Translocation pathway of the intratracheally instilled ultrafine particles from the lung into the blood circulation in the mouse. Toxicol Pathol 34: 949–957, 2006 [DOI] [PubMed] [Google Scholar]
- 148.Shoenfelt J, Mitkus RJ, Zeisler R, Spatz RO, Powell J, Fenton MJ, Squibb KA, and Medvedev AE. Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. J Leukoc Biol 86: 303–312, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Simkhovich BZ, Kleinman MT, and Kloner RA. Air pollution and cardiovascular injury epidemiology, toxicology, and mechanisms. J Am Coll Cardiol 52: 719–726, 2008 [DOI] [PubMed] [Google Scholar]
- 150.Simon SA. and Liedtke W. How irritating: the role of TRPA1 in sensing cigarette smoke and aerogenic oxidants in the airways. J Clin Invest 118: 2383–2386, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. This reference has been deleted.
- 152.Sørensen M, Daneshvar B, Hansen M, Dragsted LO, Hertel O, Knudsen L, and Loft S. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect 111: 161–166, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sun L, Liu C, Xu X, Ying Z, Maiseyeu A, Wang A, Allen K, Lewandowski RP, Bramble LA, Morishita M, Wagner JG, Dvonch J, Sun Z, Yan X, Brook RD, Rajagopalan S, Harkema JR, Sun Q, and Fan Z. Ambient fine particulate matter and ozone exposures induce inflammation in epicardial and perirenal adipose tissues in rats fed a high fructose diet. Part Fibre Toxicol 10: 43, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. This reference has been deleted.
- 155.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, Aguinaldo JG, Fayad ZA, Fuster V, Lippmann M, Chen LC, and Rajagopalan S. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA 294: 3003–3010, 2005 [DOI] [PubMed] [Google Scholar]
- 156.Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, and Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119: 538–546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun Q, Yue P, Kirk RI, Wang A, Moatti D, Jin X, Lu B, Schecter AD, Lippmann M, Gordon T, Chen LC, and Rajagopalan S. Ambient air particulate matter exposure and tissue factor expression in atherosclerosis. Inhal Toxicol 20: 127–137, 2008 [DOI] [PubMed] [Google Scholar]
- 158.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, and Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol 28: 1760–1766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sun Y, Rogers JA, Shaw JM, Seidler PF, Stauth SA, Parviz BA, Jang J, Grozea D, Herner SB, Kumar T, Gerstner EG, Balon F, Hatton R, Shannon JM, and Conference D. How frustration leads to inflammation? Science 320: 2–3, 2008 [Google Scholar]
- 160.Suzuki J, Inoue Y, and Suzuki S. Changes in the urinary excretion level of 8-hydroxyguanine by exposure to reactive oxygen-generating substances. Free Radic Biol Med 18: 431–436, 1995 [DOI] [PubMed] [Google Scholar]
- 161.Takenaka S, Karg E, Roth C, Schulz H, Ziesenis A, Heinzmann U, Schramel P, and Heyder J. Pulmonary and systemic distribution of inhaled ultrafine silver particles in rats. Environ Health Perspect 109 Suppl 4: 547–551, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tan WC, Qiu D, Liam BL, Ng TP, Lee SH, van Eeden SF, D'Yachkova Y, and Hogg JC. The human bone marrow response to acute air pollution caused by forest fires. Am J Respir Crit Care Med 161(Pt 1): 1213–1217, 2000 [DOI] [PubMed] [Google Scholar]
- 163.Tekpli X, Holme JA, Sergent O, and Lagadic-Gossmann D. Importance of plasma membrane dynamics in chemical-induced carcinogenesis. Recent Pat Anticancer Drug Discov 6: 347–353, 2011 [DOI] [PubMed] [Google Scholar]
- 164.Tekpli X, Holme JA, Sergent O, and Lagadic-Gossmann D. Role for membrane remodeling in cell death: implication for health and disease. Toxicology 304: 141–157, 2013 [DOI] [PubMed] [Google Scholar]
- 165.Tong H, Rappold AG, Diaz-Sanchez D, Steck SE, Berntsen J, Cascio WE, Devlin RB, and Samet JM. Omega-3 fatty acid supplementation appears to attenuate particulate air pollution-induced cardiac effects and lipid changes in healthy middle-aged adults. Environ Health Perspect 120: 952–957, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tonne C, Yanosky JD, Beevers S, Wilkinson P, and Kelly FJ. PM mass concentration and PM oxidative potential in relation to carotid intima-media thickness. Epidemiology 23: 486–494, 2012 [DOI] [PubMed] [Google Scholar]
- 167.Tornqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, Macnee W, Donaldson K, Soderberg S, Newby DE, Sandstrom T, and Blomberg A. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med 176: 395–400, 2007 [DOI] [PubMed] [Google Scholar]
- 168.Valavanidis A, Vlachogianni T, Fiotakis K, and Loridas S. Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health 10: 3886–3907, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vedal S, Campen MJ, McDonald JD, Larson TV, Sampson PD, Sheppard L, Simpson CD, and Szpiro AA. National Particle Component Toxicity (NPACT) initiative report on cardiovascular effects. Res Rep Health Eff Inst 5–8, 2013 [PubMed] [Google Scholar]
- 170.Vinzents PS, Moller P, Sörensen M, Knudsen LE, Hertel O, Jensen FP, Schibye B, and Loft S. Personal exposure to ultrafine particles and oxidative DNA damage. Environ Health Perspect 113: 1485–1490, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Walter P. and Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086, 2011 [DOI] [PubMed] [Google Scholar]
- 172.Wende AR, Young ME, Chatham J, Zhang J, Rajasekaran NS, and Darley-Usmar VM. Redox biology and the interface between bioenergetics, autophagy and circadian control of metabolism. Free Radic Biol Med 100: 94–107, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Williams MA, Rangasamy T, Bauer SM, Killedar S, Karp M, Kensler TW, Yamamoto M, Breysse P, Biswal S, and Georas SN. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J Immunol 181: 4545–4559, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wilson SJ, Miller MR, and Newby DE. Effects of diesel exhaust on cardiovascular function and oxidative stress. Antioxid Redox Signal 28: 819–836, 2018 [DOI] [PubMed] [Google Scholar]
- 175.Wold LE, Ying Z, Hutchinson KR, Velten M, Gorr MW, Velten C, Youtz DJ, Wang A, Lucchesi PA, Sun Q, and Rajagopalan S. Cardiovascular remodeling in response to long-term exposure to fine particulate matter air pollution. Circ Heart Fail 5: 452–461, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. This reference has been deleted.
- 177.Wu S, Yang D, Wei H, Wang B, Huang J, Li H, Shima M, Deng F, and Guo X. Association of chemical constituents and pollution sources of ambient fine particulate air pollution and biomarkers of oxidative stress associated with atherosclerosis: a panel study among young adults in Beijing, China. Chemosphere 135: 347–353, 2015 [DOI] [PubMed] [Google Scholar]
- 178.Wu Z, Liu MC, Liang M, and Fu J. Sirt1 protects against thrombomodulin down-regulation and lung coagulation after particulate matter exposure. Blood 119: 2422–2429, 2012 [DOI] [PubMed] [Google Scholar]
- 179.Xia T, Li N, and Nel AE. Potential health impact of nanoparticles. Annu Rev Public Health 30: 137–150, 2009 [DOI] [PubMed] [Google Scholar]
- 180.Xu MX, Zhu YF, Chang HF, and Liang Y. Nanoceria restrains PM2.5-induced metabolic disorder and hypothalamus inflammation by inhibition of astrocytes activation related NF-kappaB pathway in Nrf2 deficient mice. Free Radic Biol Med 99: 259–272, 2016 [DOI] [PubMed] [Google Scholar]
- 181.Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, and Sun Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci 124: 88–98, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. This reference has been deleted.
- 183.Xu X, Rao X, Wang T-Y, Jiang SY, Ying Z, Liu C, Wang A, Zhong M, Deiuliis JA, Maiseyeu A, Rajagopalan S, Lippmann M, Chen LC, and Sun Q. Effect of co-exposure to nickel and particulate matter on insulin resistance and mitochondrial dysfunction in a mouse model. Part Fibre Toxicol 9: 40, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M, Chen LC, Rajagopalan S, and Sun Q. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol 30: 2518–2527, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. This reference has been deleted.
- 186.Xu Z, Xu X, Zhong M, Hotchkiss IP, Lewandowski RP, Wagner JG, Bramble LA, Yang Y, Wang A, Harkema JR, Lippmann M, Rajagopalan S, Chen LC, and Sun Q. Ambient particulate air pollution induces oxidative stress and alterations of mitochondria and gene expression in brown and white adipose tissues. Part Fibre Toxicol 8: 20, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. This reference has been deleted.
- 188.Yatera K, Hsieh J, Hogg JC, Tranfield E, Suzuki H, Shih CH, Behzad AR, Vincent R, and van Eeden SF. Particulate matter air pollution exposure promotes recruitment of monocytes into atherosclerotic plaques. Am J Physiol Heart Circ Physiol 294: H944–H953, 2008 [DOI] [PubMed] [Google Scholar]
- 189.Yin F, Lawal A, Ricks J, Fox JR, Larson T, Navab M, Fogelman AM, Rosenfeld ME, and Araujo JA. Diesel exhaust induces systemic lipid peroxidation and development of dysfunctional pro-oxidant and pro-inflammatory high-density lipoprotein. Arterioscler Thromb Vasc Biol 33: 1153–1161, 2013 [DOI] [PubMed] [Google Scholar]
- 190.Ying Z, Kampfrath T, Thurston G, Farrar B, Lippmann M, Wang A, Sun Q, Chen LC, and Rajagopalan S. Ambient particulates alter vascular function through induction of reactive oxygen and nitrogen species. Toxicol Sci 111: 80–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ying Z, Xu X, Bai Y, Zhong J, Chen M, Liang Y, Zhao J, Liu D, Morishita M, Sun Q, Spino C, Brook RD, Harkema JR, and Rajagopalan S. Long-term exposure to concentrated ambient PM2.5 increases mouse blood pressure through abnormal activation of the sympathetic nervous system: a role for hypothalamic inflammation. Environ Health Perspect 122: 79–86, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zheng Z, Zhang X, Wang J, Dandekar A, Kim H, Qiu Y, Xu X, Cui Y, Wang A, Chen LC, Rajagopalan S, Sun Q, and Zhang K. Exposure to fine airborne particulate matters induces hepatic fibrosis in murine models. J Hepatol 63: 1397–1404, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zhong J, Allen K, Rao X, Ying Z, Braunstein Z, Kankanala SR, Xia C, Wang X, Bramble LA, Wagner JG, Lewandowski R, Sun Q, Harkema JR, and Rajagopalan S. Repeated ozone exposure exacerbates insulin resistance and activates innate immune response in genetically susceptible mice. Inhal Toxicol 28: 383–392, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhong J, Rao X, Xu JF, Yang P, and Wang CY. The role of endoplasmic reticulum stress in autoimmune-mediated beta-cell destruction in type 1 diabetes. Exp Diabetes Res 2012: 238980, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]