Haplo-cord transplantation combines the infusion of CD34 selected third party cells from a haplo-identical donor, with an umbilical cord blood graft from an unrelated donor.1–5 In the majority of cases, initial engraftment from the haplo-identical donor is superseded by dominance of the umbilical cord blood graft. But in some cases, only the haplo-graft persists. In previous studies we found that patients who received very high CD34 doses from the haplo-identical donor had impaired/delayed umbilical cord blood engraftment.6 More recently we have limited the cell dose of the haplo-graft to ~3 ×106 CD34/kgrec. But occasional cases of haplo-dominance continue to occur. Here we attempted to determine additional graft and conditioning characteristics that influence the interaction between haplo- and cord blood graft and the eventual long-term dominance of one graft over the other.
All patients were enrolled in a prospective study of reduced intensity conditioning and haplo-cord transplantation. The study was approved by the Institutional Review Board of Weill Cornell Medical College and all patients and donors provided written informed consent. The study was conducted in accordance with the Declaration of Helsinki and registered on clinical trials.gov (NCT01810588).
Cord blood units were selected based on HLA typing and cell count. As of mid-2012 we utilized high resolution HLA typing for HLA-A, B, C and DR for cord graft selection. 7 In contrast with common practice, we prioritized matching over cell dose and established a minimum cell count of 1.2 × 107 nucleated cells per kilogram of the recipient’s body.
After collection and prior to cryopreservation, haploidentical grafts were T-cell depleted using the Miltenyi CliniMACS device under an Investigational New Device (IND) permit from the United States Food and Drug Agency. The infused cell dose of the haplo graft was fixed at a minimum of 3× 106 CD34 cells/kg and a maximum of 5 × 106 CD34 cells/kg of recipient weight.
Patients received fludarabine 30mg/m2/d iv for 5 consecutive days (days -7,- 6, -5, -4, -3), rabbit antithymocyte globulin (thymoglobulin, r-ATG) at 1.5 mg/kg every other day for 3 or 4 doses, and melphalan 140 mg/m2/d for 1 dose on day-2. 88(Giralt, Thall et al. 2001) Thirteen patients were also given TBI 400 cGray, usually because of concern over disease recurrence. One patient received TBI 600 cGray. The haploidentical cells were infused on day 0 followed by cord blood later the same day or on day 1. Post-transplant GVHD prophylaxis consisted of Tacrolimus day-2 until 180 and Mycophenolate mofetil 1 gram po three times a day (TID) until day 28. CD3 and CD33 chimerism was studied as previously described.9
Univariate regression models were used to analyze the relation between continuous variables and chimerism. T-tests or ANOVA were used for categorical variables. All p-values are two-sided. Factors examined include (1) degree of CBU HLA-match-to recipient dichotomized as 4-5-6 out of 8 HLA match vs 7 or 8 out of 8 HLA match (2) UCB graft CD34/kgrec collected (2) (3) UCB TNC/kgrec infused, (4)Presence of DSA against UCB graft (5) UCB viability at the time of infusion –as defined by >90% Trypan blue exclusion vs <90% (6) Degree of Haplo-graft HLA-match to recipient HLA- dichotomized as 4–5 out of 8 HLA match vs 6–7out of 8 HLA match, (7) Presence of DSA against haplo-graft, (8) Use of TBI in conditioning, (9) CIBMTR Disease Risk index and (10) Degree of HLA match between CBU and recipient, (11) Degree of HLA match between haplo-graft and recipient, and (12) Degree of HLA match between CBU graft and haplo-graft.
Eighty-three patients with hematologic malignancies were enrolled between January 2012 and February 2015 Four died prior to engraftment including one due to infection, one from multi-organ failure and two from VOD/SOS. Of the remaining 79 patients three (4%) had complete graft failure with autologous reconstitution. Seventeen additional patients were excluded from the analysis for the following reasons: four patients had fatal infection after engraftment but before day 60; nine with evidence of early engraftment relapsed/progressed before day 60. Sixty-six patients were evaluable by day 60 of whom three (5%) had complete graft failure with autologous reconstitution (3). Three additional patients had missing data on chimerism, and one patient was lost to follow up. Fifty nine patients were engrafted, had chimerism data and were in ongoing remission by day 60. They are the subject of this analysis. Patient, disease and graft characteristics are shown in Table 1. None of the patient and none of the disease characteristics correlated with day 60 chimerism. None of the UCB characteristics correlated with day 60 UCB CD3 or CD33 chimerism. Specifically UCB graft CD34 dose at processing, UCB TNC dose at infusion, UCB viability, nor degree of UCB matching with the recipient were associated with d60 CBU chimerism.
Table 1.
Patients, disease, graft and conditioning.
| N | 59 |
| Median age (range) | 63 (18–72) |
| Male/female | 28/31 |
| Median weight (range) | 73 (41–136) |
| Disease | |
| MDS/AML vs. ALL vs. lymphoma vs. othera | 39-6-11-3 |
| Haplo-graft | |
| HLA match with host 4–5/8 vs. 6–7/8 | 53 vs. 6 |
| HLA match with UCB 1–4/8 vs. 5–7/8 | 54 vs. 5 |
| DSA present against haplo | 6 |
| UCB graft | |
| CD34 dose collected median-range (×105/kg) | 0.86 (0.13–4.3) |
| CBU viability at infusion (Trypan Blue) (range) | 97% (87–100) |
| TNC infused (×107 kg) (range) | 2.0 (0.2–8.2) |
| HLA match with host 4-5-6/8 vs. 7–8/8 | 35 vs. 24 |
| DSA present against UCB | 7 |
| Flu Mel ATG vs. Flu MEL ATG low dose TBI | 45 vs. 14 |
Other: one case of severe aplastic anemia, two cases of chronic lymphocytic leukemia.
AML: acute myeloid leukaemia; CBU: Cord blood unit; DSA: donor specific antibodies; HLA: human leukocyte antigen; MDS: myelodysplastic syndrome; TNC: total nucleated cells; UCB: umbilical cord blood.
Fifty-three of the 59 haplo-identical donors matched the recipient in 4 of 8 HLA- alleles (n=40) or 5 of 8 alleles (n=13). Twenty had 100% day 56 CBU engraftment in CD33 cells. Thirteen had between 51% and 99% CBU engraftment. Ten had between 1% and 50% CBU engraftment and seven had no evidence of CBU engraftment. Six donors had either one HLA-mismatch (n=3) or 2 mismatches (n=3) with the recipient. Two of the six had no evidence of d56 CBU engraftment in CD33 cells and the other 4 had less than 50% CD33 engraftment. The average day 56 chimerism in CD33 cells for patients with ≤5/8 haplo HLA match was 68% versus an average of 14% UCB chimerism for the six patients with 6/8 or 7/8 HLA match (p=0.001). (Figure 1) A similar but less pronounced pattern was seen for chimerism in CD3 lineage. The average day 56 chimerism in CD3 cells for patients with ≤5/8 haplo HLA match was 79% versus an average of 50% UCB chimerism for the six patients with 6/8 or 7/8 HLA match (p=0.05). Interestingly the degree of matching between the two grafts - UCB graft and haplo graft – did not predict for degree of chimerism.
Figure 1.
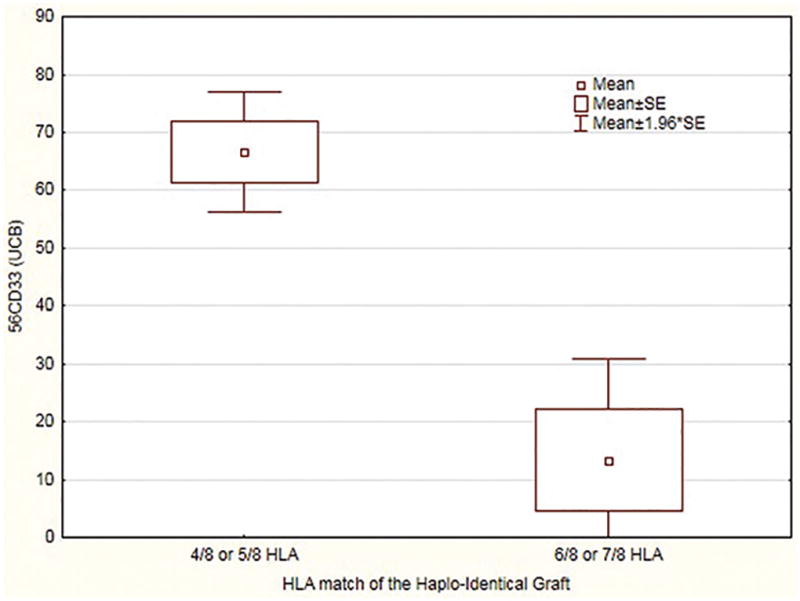
Relation between HLA-match of Haplo-graft and percentage day 56 UCB Chimerism in CD33 lineage
The infusion of several grafts leads to complex mechanisms of acceptance and rejection between host and graft, and mutually between grafts. In the case of double umbilical cord blood transplantation usually only one graft survives and murine studies strongly suggest that graft vs graft mechanisms are primarily responsible for rejection of the losing graft.10 In the case of haplo-cord transplant we have previously shown that excessive CD34 doses of the haplo-graft prevent engraftment of the cord blood graft. Here we show that in addition to haplo-graft dose, the degree of matching between haplo graft and host is important. Presumably host vs graft reactions directed against HLA-antigens play a role in rejection of the haplo-graft. When these reactions are attenuated because of close HLA-matching, the haplo-graft tends to dominate. A more dominant haplo-graft then prevents the establishment of the cord blood graft, possibly through veto-effects. We did not find an effect of inter-graft matching on the chimerism outcome, probably because mismatching between umbilical cord blood and haplo-graft affects rejection reactions in a bidirectional fashion. We also did not find a major effect of UCB graft-host HLA matching and cord blood engraftment. In addition to the haplo-graft cell dose and the match between haplo-graft and host, several additional mechanisms appear to contribute to UCB graft rejection/loss. Poor quality of the umbilical cord graft may contribute, but is less frequent when optimal search algorithms are utilized.11 Rejection is increased in the presence of DSA, and grafts targeted by DSA are best avoided.12 The group from NIH showed that KIR ligand mismatch between haplo-graft and cord blood graft may play a role in rejection of the cord blood graft.13 Lastly, use of ATG may also affect graft outcomes, particularly if post-transplant levels are high.14,15 Further studies on the relation between ATG pharmacokinetics and outcome are ongoing.
In summary, here we demonstrated that the establishment of an effective UCB graft is very dependent on the characteristics of the haplo-graft. In addition to haplo graft cell dose, a high degree of HLA-match between the haplo-graft and the host results in increased persistence. The outcome of the competition between haplo-graft and cord blood graft, provides some insight into the complex biological interactions between grafts but is also of great clinical importance. Indeed, in related studies we showed that –at least in AML and MDS –persistence of the UCB graft is essential for disease control.9 We continue to limit haplo-graft cell dose to 5×10^6 CD34 cells/kg but now also avoid haplo-grafts that are more than 5/8 matched in order to assure eventual dominance of the UCB graft. Importantly – and in contrast to the experience with single or double cord blood transplant- rate of engraftment or durability of cord blood engraftment appears to depend minimally on the cell dose of the cord blood graft. This has allowed us to use smaller, well matched cord blood grafts that are associated with low incidence of graft vs host disease and excellent long-term outcomes.5 Our study is limited because of relatively small sample size, but since we now avoid the use of one and two HLA mismatched haplo-identical relatives as donors, we are unlikely to be able to provide additional evidence or larger patient numbers.
Acknowledgments
KVB received study support from Miltenyi Biotech.
Footnotes
Conflict of Interest:
Coauthors report no conflict.
References
- 1.Lindemans CA, van Besien K. Topping it up: methods to improve cord blood transplantation outcomes by increasing the number of CD34+ cells. Cytotherapy. 2015;17(6):723–729. doi: 10.1016/j.jcyt.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 2.Liu H, van Besien K. Alternative donor transplantation--"mixing and matching": the role of combined cord blood and haplo-identical donor transplantation (haplo-cord SCT) as a treatment strategy for patients lacking standard donors? Curr Hematol Malig Rep. 2015;10(1):1–7. doi: 10.1007/s11899-014-0245-y. [DOI] [PubMed] [Google Scholar]
- 3.Kwon M, Bautista G, Balsalobre P, et al. Haplo-cord transplantation using CD34+ cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2014;20(12):2015–2022. doi: 10.1016/j.bbmt.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118(24):6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Besien K, Hari P, Zhang MJ, et al. Reduced intensity haplo plus single cord transplant compared to double cord transplant: improved engraftment and survival free of progression and GVHD (GRFS) Haematologica. 2016 doi: 10.3324/haematol.2015.138594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SB, Liu H, Shore T, et al. Frequency and Risk Factors Associated with Cord Graft Failure (CGF) after Transplant with Single Unit Umbilical Cord Cells Supplemented by Haploidentical Cells (Haplo-Cord) with Reduced-Intensity Conditioning. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eapen M, Klein JP, Ruggeri A, et al. Impact of allele-level HLA matching on outcomes after myeloablative single unit umbilical cord blood transplantation for hematologic malignancy. Blood. 2014;123(1):133–140. doi: 10.1182/blood-2013-05-506253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giralt S, Thall PF, Khouri I, et al. Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood. 2001;97(3):631–637. doi: 10.1182/blood.v97.3.631. [DOI] [PubMed] [Google Scholar]
- 9.van Besien K, Koshy N, Gergis U, et al. Cord Blood Chimerism And Relapse After Haplo-Cord Transplantation. Leuk Lymphoma. 2016 doi: 10.1080/10428194.2016.1190970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eldjerou LK, Chaudhury S, Baisre-de Leon A, et al. An in vivo model of double-unit cord blood transplantation that correlates with clinical engraftment. Blood. 2010;116(19):3999–4006. doi: 10.1182/blood-2010-03-276212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117(8):2332–2339. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshihara S, Taniguchi K, Ogawa H, Saji H. The role of HLA antibodies in allogeneic SCT: is the type-and-screen strategy necessary not only for blood type but also for HLA? Bone Marrow Transplantation. 2012 doi: 10.1038/bmt.2011.249. [DOI] [PubMed] [Google Scholar]
- 13.Tian X, Wilder J, Gormley N, et al. NK Cell KIR Ligand Mismatches Influence Engraftment Following Combined Haploidentical and Umbilical Cord Blood (UCB) Transplantation In Patients With Severe Aplastic Anemia (SAA) Blood. 2013;122(21):2038–2038. [Google Scholar]
- 14.Lindemans CA, Chiesa R, Amrolia PJ, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123(1):126–132. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 15.Lindemans CA, Te Boome LC, Admiraal R, et al. Sufficient Immunosuppression with Thymoglobulin Is Essential for a Successful Haplo-Myeloid Bridge in Haploidentical-Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(10):1839–1845. doi: 10.1016/j.bbmt.2015.06.001. [DOI] [PubMed] [Google Scholar]


