Abstract
Objective
To demonstrate that ephrin-B2 (the ligand of EphB2 receptor) antagonizes the pathogenic effects of patients’ N-methyl-D-aspartate receptor (NMDAR) antibodies on memory and synaptic plasticity.
Methods
One hundred twenty-two C57BL/6J mice infused with cerebrospinal fluid (CSF) from patients with anti-NMDAR encephalitis or controls, with or without ephrin-B2, were investigated. CSF was infused through ventricular catheters connected to subcutaneous osmotic pumps over 14 days. Memory, behavioral tasks, locomotor activity, presence of human antibodies specifically bound to hippocampal NMDAR, and antibody effects on the density of cell-surface and synaptic NMDAR and EphB2 were examined at different time points using reported techniques. Short- and long-term synaptic plasticity were determined in acute brain sections; the Schaffer collateral pathway was stimulated and the field excitatory postsynaptic potentials were recorded in the CA1 region of the hippocampus.
Results
Mice infused with patients’ CSF, but not control CSF, developed progressive memory deficit and depressive-like behavior along with deposits of NMDAR antibodies in the hippocampus. These findings were associated with a decrease of the density of cell-surface and synaptic NMDAR and EphB2, and marked impairment of long-term synaptic plasticity without altering short-term plasticity. Administration of ephrin-B2 prevented the pathogenic effects of the antibodies in all the investigated paradigms assessing memory, depressive-like behavior, density of cell-surface and synaptic NMDAR and EphB2, and long-term synaptic plasticity.
Interpretation
Administration of ephrin-B2 prevents the pathogenic effects of anti-NMDAR encephalitis antibodies on memory and behavior, levels of cell-surface NMDAR, and synaptic plasticity. These findings reveal a strategy beyond immunotherapy to antagonize patients’ antibody effects.
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is an inflammatory disorder of the brain that results in prominent neurological and psychiatric symptoms in association with immunoglonulin G (IgG) antibodies against the GluN1 subunit of the receptor.1 In recent years, several studies have provided evidence that the antibodies alter synaptic function and likely result in the clinical syndrome.2–4 Approximately 80% of the patients improve with immunotherapy or sometimes spontaneously indicating that, despite the severity and protracted course of the disease, the altered NMDAR signaling is largely reversible.1,5–7 To date, the antibody effects have been mainly studied in cultured neurons exposed to patients’ cerebrospinal fluid (CSF), demonstrating internalization of NMDAR and a decrease in their surface density and NMDAR-mediated currents.2,4 At the synapse, the antibodies disrupt the interaction between NMDAR and ephrin type B2 receptor (EphB2), displacing the NMDAR to extrasynaptic sites before they are internalized.3 This process of NMDAR internalization is antibody-titer dependent, reversible upon removing the antibodies, and highly specific for NMDAR, without per se affecting the density of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR).2 Furthermore, antibody-treated neurons failed to increase the levels of AMPAR after chemical induction of long-term potentiation (LTP), suggesting that the mechanisms of plasticity were altered.3 Disruption of long-term plasticity was also suggested by a study in which the antibodies were applied for 5 minutes onto slices of normal mouse hippocampus.8 However, none of these studies determined whether long-term synaptic plasticity was altered in the brain of mice with symptoms related to NMDAR antibodies or whether the mechanisms of short-term plasticity were affected at the presynaptic level.
A definite link between development of memory and behavioral alterations and antibody-mediated decrease of NMDAR was recently established in a mouse model of cerebroventricular infusion of patients’ CSF antibodies.9 The study showed a correlation between the intensity of the symptoms and the time course of antibody administration as well as between the reversibility of the symptoms and the restoration of levels of NMDAR after discontinuing the antibody infusion. In the current study, we first reproduced these findings using CSF from another group of patients and then applied the model to investigate whether the antibodies alter synaptic plasticity using electrophysiological paradigms of short- and long-term plasticity at the Schaffer collateral pathway. Additionally, given that previous work showed that ephrin-B2 antagonized the antibody-mediated changes in cell-surface dynamics of NMDAR,3 we have investigated in vivo whether this ligand prevents development of symptoms and antagonizes the mechanisms of disease in sets of experiments examining memory and behavioral responses, levels of cell-surface and synaptic NMDAR and EphB2, and synaptic plasticity in hippocampal networks. The results show a marked antibody-mediated impairment of the mechanisms of long-term synaptic plasticity, revealing a strategy to prevent the pathogenic effect of the antibodies that may lead to novel therapies.
Materials and Methods
Animals, Preparation of CSF, and Cerebroventricular Infusion of Antibodies
One hundred twenty-two male C57BL/6J mice (Charles River Laboratories, Wilmington, MA), 8 to 10 weeks old (25–30 g), were used for the studies. Some animals were used for more than one study (51 for memory and behavior, 60 for immunohistochemistry, and 29 for electrophysiological studies). Animal care, anesthesia, insertion of bilateral ventricular catheters (model 3280PD-2.0/SP; coordinates: 0.2mm anterior and 1.00mm lateral from bregma, depth 2.2mm; PlasticsOne Inc., Roanoke, VA), and connection of each catheter to a subcutaneous osmotic pump for continuous infusion of CSF (volume 100 μl, flow rate 0.25 μl/h for 14 days; Alzet, Cupertino, CA) have been reported.9 The CSF infused was pooled from samples of 34 patients with high-titer IgG GluN1 antibodies (all >1:320) and 12 patients with normal pressure hydrocephalus without NMDAR antibodies (control samples). The patients used as controls had a history of rapidly progressive (median, 4 months; range, 2–9) memory deficits, cognitive decline, gait unsteadiness, or sphincter dysfunction; none of them had CSF inflammatory changes or autoantibodies.
To avoid the presence of other antibodies or small molecules that may have biological activity, patients’ CSF samples were selected and prepared using the following studies: (1) confirmation that patients’ CSF only had NMDAR antibodies by immunoabsorption of a representative sample of pooled CSF with HEK293 cells expressing the GluN1 subunit of the NMDARs showing abrogation of reactivity with mouse brain and NMDAR (tested in a cell-based assay [CBA]); (2) demonstration of the absence of antibodies against EphB2 receptor (confirmed by CBA, data not shown); and (3) CSF filtration (Amicon Ultracel 30K; Sigma-Aldrich, St. Louis, MO), dialysis against phosphate-buffered saline (PBS), and normalization to a physiological concentration of 2mg of IgG/dl, as reported.9
Four experimental groups were established: mice infused with patients’ CSF without or with ephrin-B2 (50ng of ephrin-B2 added to the 100 μl of CSF in each osmotic pump; two pumps per mouse; Sino Biological Inc, North Wales, PA), and mice infused with control CSF without or with ephrin-B2. The total dose of ephrin-B2 (100ng infused over 14 days) was based in a previous study using a single hippocampal injection (eg, 10ng/1 day).3 Animal procedures were conducted in accord with standard ethical guidelines (European Communities Directive 86/609/EU) and approved by the local ethical committees.
Cognitive Tasks and Locomotor Activity
These tasks were aimed to assess memory (novel object recognition [NOR]) discrimination index) and depressive-like behavior (tail suspension, and forced swimming tests) along with locomotor activity (local motor, horizontal and vertical activity). The selection and timing of these tasks were based on the findings of our previous study showing that patients’ CSF antibodies, but not control CSF, caused memory deficits and depressive-like behaviors without significant change of locomotor activity.9 All behavioral tasks were performed by researchers blinded to experimental conditions.
Immunohistochemistry, Confocal Microscopy, and Immunoprecipitation
All techniques related to immunolabeling of live cultures of dissociated rodent hippocampal neurons,2 immunoabsorption of patients’ samples with antigen-expressing HEK cells,10 brain tissue processing, quantitative brain tissue immunoperoxidase staining, extraction of human IgG bound to mice brain, and determination of NMDAR specificity of brain-extracted IgG have been previously reported on.9 To quantify the effects of patients’ antibodies on total cell-surface and synaptic NMDAR clusters and PSD95, nonpermeabilized 5-μm brain sections were blocked with 5% goat serum, incubated with human CSF antibodies (1:20; used here as primary NMDAR antibody) for 2 hours at room temperature (RT), washed with PBS, permeabilized with Triton X-100 0.3% for 10 minutes at RT, and serially incubated with rabbit polyclonal anti-PSD95 (1:250, ab18258; Abcam, Cambridge, MA) overnight at 4 °C and the corresponding secondary antibodies, Alexa Fluor 594 or 488 goat antihuman Fab-specific IgG (109-585-097 or 109-545-097; Jackson Immunoresearch Laboratories, Inc., West Grove, PA) and Alexa Fluor 488 goat antirabbit IgG (A-11008; Thermo Fisher Scientific, Waltham, MA), all diluted 1:1,000, for 1 hour at RT. Clusters of EphB2 were identified using non-permeabilized tissue and a goat anti-EphB2 as primary antibody (1:100, AF467; R&D Systems, Minneapolis, MN) overnight at 4 °C, followed by a secondary antibody, Alexa Fluor 594 donkey antigoat IgG (A-11058, 1:1000; Thermo Fisher Scientific), for 1 hour at RT. Slides were mounted and results scanned with a confocal microscope (Zeiss LSM710; Carl Zeiss GmbH, Jena, Germany) as reported.9 Standardized z-stacks including 50 optical images were acquired from 18 hippocampal regions per animal, including CA1, CA2, CA3, and dentate gyrus, as reported.9 For cluster density analysis, a spot detection algorithm from Imaris suite 7.6.4 (Bitplane, Zurich, Switzerland) was used. Density of clusters in each hippocampal region was expressed as spots/μm3. Synaptic localization is defined as colocalization of NMDAR with postsynaptic PSD95, and synaptic cluster density is expressed as colocalized spots/μm3. For animals treated with patients’ CSF with or without ephrin-B2 or control CSF with ephrin-B2, the mean densities of all 18 hippocampal regions were calculated for total and synaptic NMDAR and EphB2, and normalized to the mean density of the 18 hippocampal regions in animals treated with control CSF (= 100%).
To demonstrate the binding of ephrin-B2 to EphB2, nonpermeabilized brain sections were obtained from animals infused for 5 days with patients’ CSF with or without ephrin-B2, processed as above and sequentially incubated with rabbit anti-ephrin-B2 antibodies (1:500, ab131536; Abcam) for 2 hours at 4 °C, washed, goat anti-EphB2 antibody (1:200, AF467; R&D Systems) overnight at 4 °C, washed, and the secondary antibodies, Alexa Fluor 594 donkey antigoat followed by goat-anti-rabbit 488 (A-11058 or A-11008; Thermo Fisher Scientific), all diluted 1:1,000, for 1 hour at RT. For each animal, three identical image stacks representing CA1 were examined. Intensity of ephrin-B2 immunostaining was calculated with ImageJ software (National Institutes of Health [NIH], Bethesda, MD), and colocalization of clusters of ephrin-B2 and EphB2 was determined with Imaris suite 7.6.4 (Bitplane). Results were normalized to the mean values obtained in animals treated with patients’ CSF + ephrin-B2 (100%).
To determine the phosphorylation of EphB2 by ephrin-B2, permeabilized brain tissue sections were blocked as above and sequentially incubated with rabbit anti-phospho-EphB2 (dilution 1:50, E22-65BR; SignalChem, Richmond, British Columbia, Canada) overnight at 4 °C and a secondary antibody, Alexa 488 goat anti-rabbit (1:1,000, A-11008; Thermo Fisher Scientific), for 1 hour at RT. Slides were then mounted and scanned with confocal microscope as above.
To demonstrate the specificity of neuronal-bound IgG, we used an immunoprecipitation method similar to that previously reported.11 In brief, cultures of live hippocampal neurons were incubated for 1 hour with aliquots of the samples (patients’ or control CSF with or without ephrin-B2) used for mice cerebroventricular infusion, washed, lysed, and neuronal-bound IgG precipitated with protein A and G sepharose beads. Immunoprecipitates were then run in a gel and incubated with a rabbit antibody specific for the NR1 subunit of the NMDAR (#G8913; Sigma-Aldrich), diluted 1:1,000, for 1 hour at room temperature, followed by a biotinylated anti-rabbit antibody (diluted 1:1,000, BA-1000; Vector Laboratories, Burlingame, CA), and the reactivity developed with the avidin-biotin peroxidase technique.
Electrophysiological Studies
Seventeen to 23 days after activation of osmotic pumps, mice were deeply anesthetized with isofluorane and decapitated. Brains were removed, placed in ice-cold, high-sucrose extracellular artificial fluid 1 (artificial CSF [aCSF] 1; 40mM of NaCl, 25mM of NaHCO3, 10mM of glucose, 150mM of sucrose, 4mM of KCl, 1.25mM of NaH2PO4, 0.5mM of CaCl2, and 7mM of Mg2Cl; purged with 95% O2/5% CO2 [pH 7.35]) and subdivided into the hemispheres. Thick (400-μm) coronal slices of hippocampus were obtained with a vibratome (VT1200 S; Leica Microsystems, Wetzlar, Germany) and transferred into an incubation beaker with extracellular aCSF 2 appropriate for electrophysiological recordings (aCSF 2; 124mM of NaCl, 26mM of NaHCO3, 10mM of glucose, 3.4mM of KCl, 1.2mM of NaH2PO4, 2mM of CaCl2, and 2mM of MgSO4; purged with 95% O2/5% CO2 [pH 7.35]). Slices were kept at 34 °C for 60 minutes and subsequently at RT for at least 60 additional minutes. For field potential measurements, single slices were then transferred into a measurement chamber perfused with aCSF 2 at 1.3 to 2.5ml/min at 32 °C to 33 °C (control CSF: number of acute slices: n = 7, prepared from brain hemisections of 4 mice; control CSF + ephrin-B2: n = 7, from hemisections of 6 mice; patients’ CSF: n = 7, from hemisections of 4 mice; patients’ CSF + ephrin-B2: n = 5, from hemisections of 5 mice). A custom made bipolar stimulation electrode was placed in the Schaffer collateral pathway. Recording electrodes were made with a puller (P-1000; Sutter Instrument Company, Novato, CA) from thick-walled borosilicate glass with a diameter of 2mm (Science Products, Hofheim, Germany). The recording electrode filled with aCSF 2 was placed in the dendritic branching of the CA1 region for local field potential measurement (field excitatory postsynaptic potential; fEPSP). A stimulus isolation unit (Isoflex; A.M.P.I, Jerusalem, Israel) was used to elicit stimulation currents between 70 and 260 μA). Before baseline recordings for long-term potentiation (LTP) measurements, input-output curves were recorded for each slice at 0.03Hz (control CSF: n = 8 slices from N = 5 mice; control CSF + ephrin-B2: n = 8, N = 6; patients’ CSF: n = 9, N = 5; patients’ CSF + ephrin-B2: n = 7, N = 5). The stimulation current was then adjusted in each recording to evoke fEPSP at which the population spike could first be distinguished from the field potential and was then reduced by 10%.12 The final intensities of stimulation ranged from 60% to 70% of maximum fEPSP slopes and were unchanged in between the experimental groups. After baseline recordings for 25 minutes with 0.03Hz, LTP was induced by theta-burst stimulation (TBS; 10 theta bursts of four pulses of 100Hz with an interstimulus interval of 200ms repeated 10 times with 0.03Hz).13 After LTP induction, fEPSPs were recorded for additional 65 minutes with 0.03Hz. Paired-pulse fEPSPs in the test pathway were measured directly before and 30 minutes after LTP induction with 0.03Hz and an interstimulus interval of 100ms. The fEPSP of the first paired-pulse stimulus was included in fEPSP analysis. All recordings were filtered at 2.9 and 10kHz using Bessel filters of the amplifier. Traces were analyzed by IGOR Pro software (WaveMetrics, Lake Oswego, OR).
Statistical Analysis
Behavioral tests with multiple time points (NOR, locomotor activity) were analyzed using repeated-measures two-way analysis of variance (ANOVA). The tail suspension test (behavioral paradigm with single time point) was analyzed with one-way ANOVA. All experiments were assessed for outliers, but none were identified, so measurements were pooled per time point and treatment (patient or control CSF). Human IgG intensity from different time points and electrophysiological data (LTP-induced changes in fEPSP slope and absolute fEPSP slope values) were analyzed with two-way ANOVA. Intensity of reactivity of patients’ antibodies with cultures of neurons, confocal cluster densities (GluN1, PSD95, and EphB2) at one time point, and assessment of fEPSP stimulation strength were analyzed using one-way ANOVA. Electrophysiological data from the paired-pulse experiment were analyzed with repeated-measures two-way ANOVA. Post-hoc analyses for all experiments used Bonferroni correction for multiple testing. The EphB2 activation experiment was assessed by two-tailed t test. A p value of <0.05 was considered significant. The α-error was set at 0.05. All tests were done using GraphPad Prism software (version 6; GraphPad Software Inc., La Jolla, CA).
Results
Patients’ Antibodies Cause Memory and Behavioral Deficits That Are Prevented by Ephrin-B2
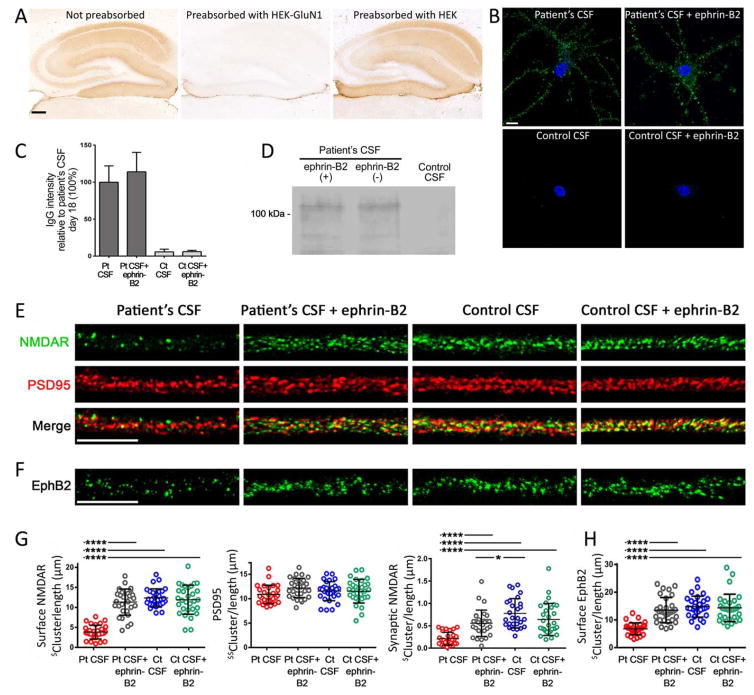
We first confirmed that (1) pooled patients’ CSF only had GluN1 NMDAR antibodies (Fig 1A), (2) ephrin-B2 did not block binding of patients’ antibodies (Fig 1B,C), and (3) binding of patients’ antibodies to the neuronal surface was specific for NMDARs (eg, patients’ antibodies precipitated this receptor; Fig 1D). We also confirmed in cultured neurons that patients’ antibodies removed NMDARs from synapses causing their internalization2 and that these effects were antagonized by ephrin-B23 (Fig 1E–G). Next, we used patients’ CSF samples for cerebroventricular infusions to mice. Animals infused for 14 days with patients’ CSF antibodies, but not control CSF, showed a progressive impairment of memory (NOR discrimination index) that was maximal on day 18 and recovered on day 25 (Fig 2A,B). Compared with animals infused with control CSF, those infused with patients’ CSF developed depressive-like behavior (longer time of immobility during the tail suspension test [TST] on day 10; Fig 2C) that recovered after the CSF infusion stopped (assessed by forced swimming test [FST] on day 20; Fig 2D). The reason for using two different tests to assess a similar task is that mice cannot be re-exposed to TST or FST test because of behavioral learning acquired during the first exposure. Although these tests are considered equivalent,14,15 future studies should confirm this in our model.
FIGURE 1.
Soluble ephrin-B2 does not interfere with antibody binding and prevents antibody-induced reduction of surface NMDAR and EphB2 clusters in cultures of neurons. (A) Sections of mouse hippocampus incubated with pooled CSF from patients with anti-NMDAR encephalitis not preabsorbed (left), and pre-absorbed with HEK293 cells expressing (middle) and not expressing (right) the GluN1 subunit of the NMDARs. Preabsorption with GluN1 abrogates patients’ CSF reactivity with brain; scale bar = 200 μm. (B) Cultured rat hippocampal neurons incubated for 1 hour with patients’ or control CSF with or without ephrin-B2 show similar immunolabeling by patients’ antibodies regardless of the presence of ephrin-B2; scale bar = 10 μm. (C) Quantification of intensity of CSF IgG reactivity (10 dendrites per condition); bars show the mean intensity + standard error of the mean in percentage relative to patients’ CSF IgG reactivity. Significance assessed by one-way analysis of variance (ANOVA; p <0.0001) with Bonferroni post-hoc correction; ****p <0.0001. (D) Immunoprecipitation of the neuronal antigen bound to patients’ IgG showed that the target was the NMDAR (band at ~110kDa); the same result was obtained in neurons incubated with patients’ CSF with or without ephrin-B2. (E) Representative dendrite of hippocampal neurons immunostained for surface NMDAR (green) and PSD95 (red) after 24-hour treatment with patients’ CSF antibodies (Pt CSF) or control CSF (Ct CSF) without or with ephrin-B2. Patients’ antibodies reacted with surface NMDAR in nonpermeabilized neurons; synaptic NMDARs were defined by the colocalization of reactivity with PSD95 (yellow). (F) Immunostaining of surface EphB2 after 24-hour treatment with Pt CSF or Ct CSF without or with ephrin-B2. Scale bars = 10 μm. (G) Quantification of the density of surface and synaptic NMDAR: The presence of ephrin-B2 prevented the antibody-mediated reduction of surface and synaptic NMDAR clusters. PSD95 was not affected by ephrin-B2. (H) Quantification of the density of EphB2: The presence of ephrin-B2 prevented the antibody-mediated reduction of EphB2 (n = 10 cells per condition; three independent experiments). Cluster density analysis was performed with a spot detection algorithm from Imaris suite 7.6.4 (Bitplane). ($) in Y axis represents the number of surface clusters (NMDAR or EphB2) or synaptic clusters (NMDAR) per dendrite (measured three-dimensionally [3D])/length of the dendrite; ($$) represents the number of intracellular clusters of PSD95 (measured in 3D)/length of dendrite. Significance of treatment effect was assessed by one-way ANOVA (p <0.0001 for NMDAR, synaptic NMDAR, and EphB2) with Bonferroni post-hoc correction; ****p <0.0001. CSF = cerebrospinal fluid; IgG = immunoglobulin G; NMDAR = N-methyl-D-aspartate receptor.
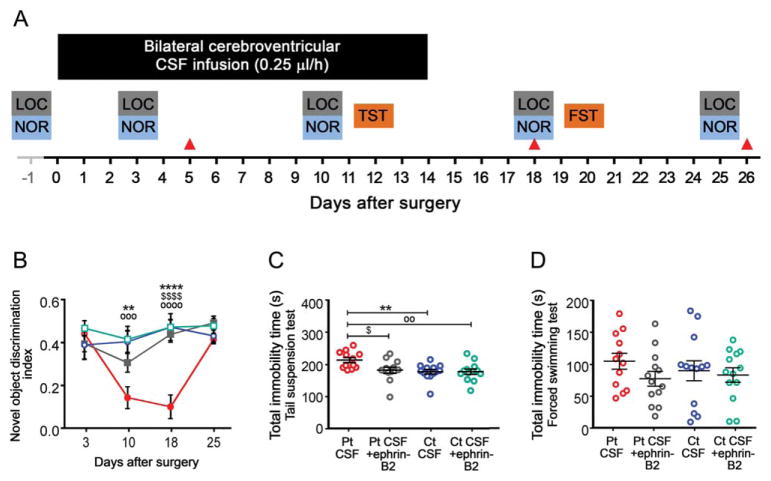
FIGURE 2.
Intraventricular infusion of CSF from patients with NMDAR antibodies causes deficits in memory and depressive-like behavior that are prevented by ephrin-B2. (A) Schedule of cognitive testing and animal sacrifice. Memory (novel object recognition [NOR] discrimination index), depressive-like behavior (tail suspension test [TST] and forced swimming test [FST]), and locomotor activity (LOC) were assessed blinded to treatment at the indicated days. The NOR discrimination index was assessed in open field in four different cohorts of mice. Animals were habituated the day before surgery (baseline) to NOR and LOC. Red arrow heads indicate the days of sacrifice for studies of effects of antibodies in brain. (B) NOR index in open-field paradigm in animals treated with patients’ CSF antibodies (Pt CSF; solid circles), Pt CSF + ephrin-B2 (open circles), control CSF (Ct CSF; solid squares), or Ct CSF + ephrin-B2 (open squares). A high index indicates better object recognition memory. (C) Total time of immobility in TST during the infusion period (day 12). (D) Total time of immobility in FST (day 20). Data are presented as mean ± standard error of the mean (median ± interquartile range IQR in C and D). Number of animals: patients’ CSF, n = 12; patients’ CSF + ephrin-B2, n = 13; control CSF, n = 13; and control CSF + ephrin-B2, n = 13. Significance of treatment effect was assessed by repeated-measures two-way analysis of variance (ANOVA; p = 0.0001; B) with post-hoc testing with Bonferroni adjustment (asterisks), or one-way ANOVA (p = 0.0032) and Bonferroni post-hoc correction (C). Patients’ CSF versus control CSF: **p <0.01; ****p <0.0001; patients’ CSF versus patients’ CSF + ephrin-B2: $p <0.05; $$$$p <0.0001; patients’ CSF versus control CSF + ephrin-B2: ∘∘p <0.01; ∘∘∘p <0.001; ∘∘∘∘p <0.0001. CSF = cerebrospinal fluid; NMDAR = N-methyl-D-aspartate receptor.
In contrast to the above-mentioned findings, mice that received patients’ CSF along with ephrin-B2 showed a mild, not significant, decrease of memory on day 10 and normal memory on day 18. In addition, mice that received ephrin-B2 did not develop depressive-like behavior (Fig 2B,C; Supplementary Table 1A,B). No significant difference in motor activity was noted among the different groups (Supplementary Table 2). Overall, these findings confirm those of our previous study and demonstrate, for the first time, that the alteration of memory and behavior caused by patients’ NMDAR antibodies can be largely prevented by administration of ephrin-B2.
Ephrin-B2 Does Not Alter the Specific Binding of Patients’ Antibodies to Mouse NMDAR
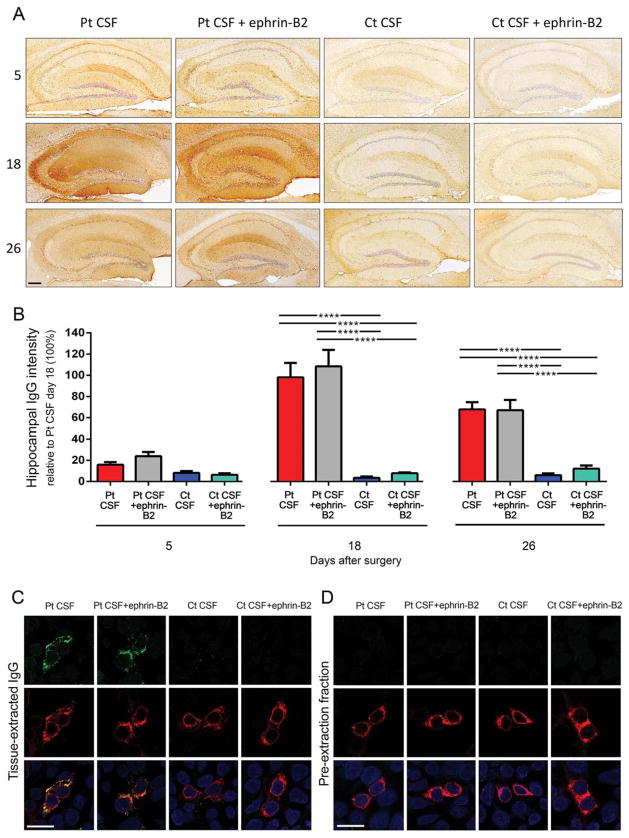
Mice infused with patients’ CSF, but not control CSF, showed progressive accumulation of brain-bound human IgG, predominantly in the hippocampus (Fig 3A,B). Compared with mice not receiving ephrin-B2, those infused with this ligand showed increased density of ephrin-B2 bound to EphB2 and increased levels of EphB2 phosphory-lated (data not shown). The dynamics and the degree of IgG binding to the brain were not altered by adding ephrin-B2 to patients’ CSF (Fig 3A,B; Supplementary Table 3). NMDAR specificity of brain-bound human IgG (confirmed in immunoabsorption experiments before infusion in mice; Fig 1A) was further determined by acid extraction of the IgG bound to hippocampus and testing it with a CBA for NMDAR. IgG extracted from hippocampus of mice infused with patients’ CSF with or without ephrin-B2, but not control CSF, was specific for NMDAR (Fig 3C). Confirmation that extracted IgG represented antibodies bound to the hippocampus and not unbound antibodies (eg, present in blood vessels) was provided by the absence of reactivity in the pre-extraction fraction (tissue wash fraction before applying acid; Fig 3D).
FIGURE 3.
Animals infused with patients’ CSF have a progressive increase of human anti-NMDAR IgG bound to hippocampus that is not altered by ephrin-B2. (A) Immunostaining of human IgG in sagittal hippocampal sections of mice infused with patients’ CSF antibodies (Pt CSF), Pt CSF + ephrin-B2, control CSF (Ct CSF), and Ct CSF + ephrin-B2, sacrificed at the indicated experimental days. In animals infused with patients’ CSF and patients’ CSF + ephrin-B2, there is a gradual increase of IgG immunostaining until day 18, followed by a decrease of immunostaining. Scale bar: A = 200 μm. (B) Quantification of intensity of human IgG immunostaining in hippocampus of mice infused with patients’ CSF (red bars), patients’ CSF + ephrin-B2 (gray bars), control CSF (blue bars), and control CSF + ephrin-B2 (cyan bars) sacrificed at the indicated time points. For all quantifications, mean intensity of IgG immunostaining in the group with the highest value (animals treated with patients’ CSF and sacrificed at day 18) was defined as 100%. All data are presented as mean ± standard error of the mean. For each time point, 5 animals of each experimental group were examined. Significance of treatment effect was assessed by two-way analysis of variance (ANOVA; time points, treatment, and interaction, all p <0.0001), and post-hoc analyses were performed with Bonferroni correction; ****p <0.0001. (C and D) Demonstration that the human IgG in mouse brain has NMDAR specificity: HEK293 cells expressing the GluN1 subunit of the NMDAR immunolabeled with acid-extracted IgG fractions (top row in C) or pre-extraction fractions (top row in D) from hippocampus of mice infused with patients’ CSF antibodies (Pt CSF), Pt CSF + ephrin-B2, control CSF (Ct CSF), or Ct CSF + ephrin-B2 at day 18. The intense reactivity with GluN1-expressing cells was noted in acid-extracted IgG fractions from Pt CSF and Pt CSF + ephrin-B2 groups (C); none of the pre-extraction fractions from any animal group showed GluN1 reactivity (D). The second row in (C) and (D) shows the reactivity with a monoclonal GluN1 antibody, and the third row the colocalization of immunolabeling. Scale bars = 10 μm. Pt CSF (n = 5), Pt CSF + ephrin-B2 (n = 5), Ct CSF (n = 5), and Ct CSF + ephrin-B2 (n = 5). CSF = cerebrospinal fluid; IgG = immunoglobulin G; NMDAR = N-methyl-D-aspartate receptor.
These experiments show that ephrin-B2 did not alter the specific binding of patients’ antibodies to mouse hippocampal NMDAR, although it prevented the memory and behavioral deficits caused by the antibodies.
The Antibody-Mediated Reduction of Synaptic NMDAR and EphB2 Is Antagonized by Ephrin-B2
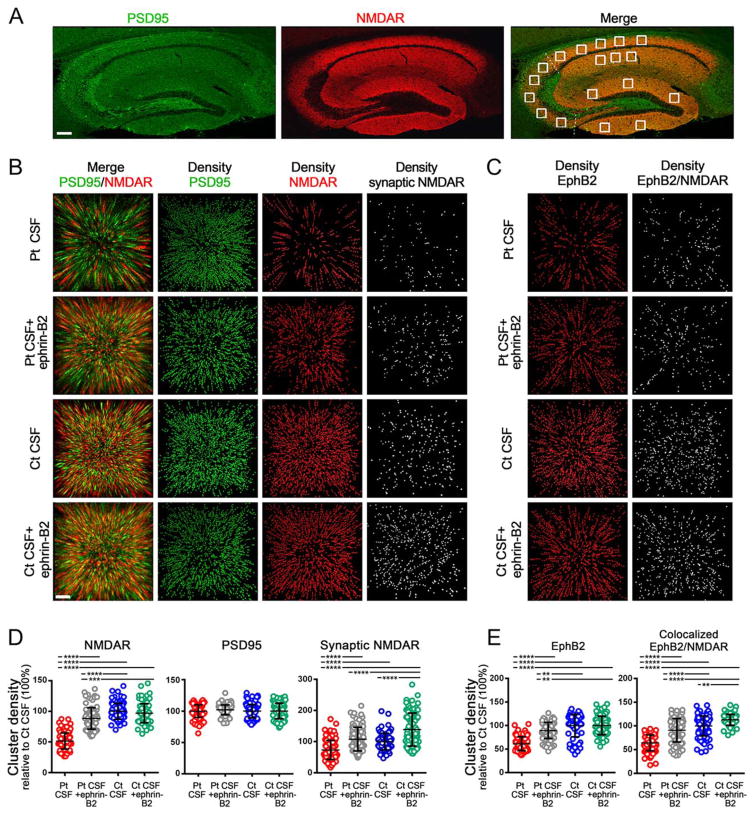
To determine whether the protective effects of ephrin-B2 occurred in vivo, we infused this ligand along with patients’ antibodies into the cerebroventricular system of our mouse model. We then compared the effects of patients’ antibodies on the density of total and synaptic NMDAR in the hippocampus of mice infused with patients’ or control CSF, with or without ephrin-B2. Eighteen hippocampal areas with 50 z-sections per area, representing a total of 900 sections per animal (5 animals per group), were investigated (Fig 4A). Animals infused with patients’ CSF antibodies, but not control CSF, showed a significant decrease of the density of total and synaptic NMDAR clusters. These effects were largely prevented when ephrin-B2 was coinfused with patients’ antibodies (Fig 4B,D).
FIGURE 4.
Soluble ephrin-B2 antagonizes the antibody-mediated reduction of NMDAR and EphB2 in mice hippocampus. (A) Hippocampus of mouse immunolabeled for PSD95 and NMDAR. Images were merged (merge) and postprocessed to demonstrate colocalizing clusters. Squares in “merge” indicate the analyzed areas in CA1, CA2, CA3, and dentate gyrus. Each square is a three-dimensional (3D) stack of 50 sections. Scale bar = 200 μm. (B) 3D projection and analysis of the density of total clusters of PSD95 and NMDAR, and synaptic clusters of NMDAR (defined as NMDAR clusters colocalizing with PSD95) in a CA3 region (square in A “merge”) from a representative animal of each experimental group. Merged images (merge: PSD95 [green]/NMDAR [red]) were postprocessed and used to calculate the density of clusters (density = spots/μm3). Scale bar = 2 μm. (C) Density of total clusters of EphB2 and EphB2 colocalizing with NMDAR. Scale bar = 2 μm. (D) Quantification of the density of total (left) and synaptic (right) NMDAR clusters, and (E) total EphB2 and EphB2 colocalizing with NMDAR at day 18 in a pooled analysis of hippocampal areas (CA1, CA2, CA3, and dentate gyrus) in animals treated with patients’ CSF antibodies (Pt CSF; red), Pt CSF + ephrin-B2 (gray), control CSF (Ct CSF; blue), and control Ct CSF + ephrin-B2 (cyan). Mean density of clusters in control CSF treated animals was defined as 100%. Data are presented as scatterplot + mean ± standard error of the mean. For each condition, 5 animals were examined (18 hippocampal areas per animal = 90 hippocampal areas per condition). Significance of treatment effect was assessed by one-way analysis of variance (p <0.0001) and by post-hoc analysis with Bonferroni correction; **p <0.01; ***p <0.001; ****p <0.0001. CSF = cerebrospinal fluid; NMDAR = N-methyl-D-aspartate receptor.
Patients’ antibodies also caused a decrease of the density of EphB2 that was prevented by coinfusion of ephrin-B2 (Fig 4C,E) similar to the results observed with cultured neurons (Fig 1F,H). Furthermore, we observed an increase in the density of colocalized EphB2/NMDAR in animals receiving ephrin-B2 either with patients’ or control CSF (Fig 4E). This increase of colocalized EphB2/NMDAR, but not the total number of cell-surface EphB2 in the control group, is in line with studies showing that activation of EphB2 facilitates interaction with NMDAR and increases formation of EphB2/NMDAR clusters.16 Our findings confirm that CSF antibodies from patients with anti-NMDAR encephalitis cause a decrease of cell-surface and synaptic NMDAR in vitro and in mice, and show that these molecular effects as well as the resulting memory and behavioral deficits are prevented by the activation of EphB2 with ephrin-B2 in vivo.
Ephrin-B2 Antagonizes Antibody-Mediated Impairment of Hippocampal Long-Term Synaptic Plasticity
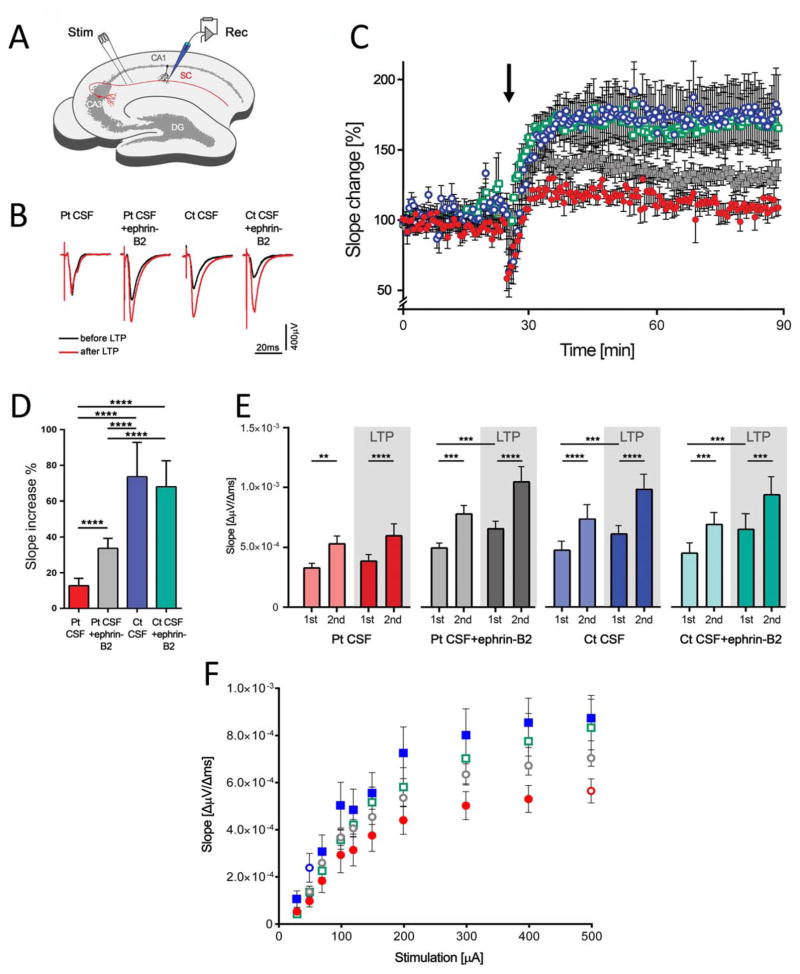
Acute brain slices from mice infused with patients’ or control CSF, without or with ephrin-B2, were used to record fEPSPs in the CA1 region of the hippocampus (Fig 5A). Animals infused with patients’ CSF showed a substantial reduction of LTP compared with animals infused with control CSF, as shown by analysis of fEPSP slope change (Fig 5B,C). Quantitative analysis comparing median changes in slope values of minutes 40 to 90 of the recordings (15 minutes after TBS) demonstrated a reduced potentiation of fEPSP in mice receiving patients’ CSF in comparison to those receiving control CSF (Fig 5D). Coadministration of patients’ CSF antibodies with ephrin-B2 partially abrogated the NMDAR antibody-mediated impairment of potentiation (Fig 5C,D; p <0.0001). Administration of ephrin-B2 together with control CSF had no effects on the potentiation of fEPSP.
FIGURE 5.
Patients’ antibodies cause severe impairment of long-term synaptic plasticity in the hippocampus that is partially prevented by ephrin-B2. (A) The Schaffer collateral pathway (SC, red) was stimulated (Stim) and field potentials were recorded in the CA1 region of the hippocampus (Rec). Long-term potentiation (LTP) was induced by theta-burst stimulation (TBS); DG = dentate gyrus; CA = Cornu Ammonis. (B) Example traces of individual recordings showing average traces of baseline recording before LTP induction (black traces) and after LTP (red traces). Slope and peak amplitude of fEPSPs are increased after TBS in mice infused with control CSF (Ct CSF) and Ct CSF + ephrin-B2, whereas manifestation of LTP is strongly impaired in animals infused with patients’ CSF antibodies (Pt CSF). In mice infused with Pt CSF + ephrin-B2, the increase of slope is improved. Note that initial peak amplitude of fEPSP may vary within individual recordings. (C) Time course of fEPSP recordings demonstrating robust changes in fEPSP slope in the Ct CSF (n = 7 recordings, blue open circles) and Ct CSF + ephrin-B2 group (n = 7, cyan open squares), which is stable throughout the recording period after TBS (arrow). In animals chronically infused with Pt CSF (n = 7, red solid circles), the induction of synaptic LTP is markedly impaired. Recordings from the Pt CSF + ephrin-B2 group (n = 5, gray solid squares) show partially resolved effects on synaptic plasticity after LTP induction. (D) Quantitative analysis of LTP-induced changes in fEPSPs in the plateau interval after TBS depicted as comparison to each individual baseline value (slope increase as median values ± standard error of the mean in the consolidation phase during the last 50 minutes of each recording, starting 15 minutes after TBS). Chronic application of Pt CSF results in marked reduction of LTP (13.3 ± 4.1% slope increase vs 73.6 ± 19.3% and 68.3 ± 14.7% in Ct CSF and Ct CSF + ephrin-B2, respectively). Coadministration of soluble ephrin-B2 improved fEPSP potentiation to levels of 33.7 ± 5.5%. Significance of treatment effect was assessed by two-way analysis of variance (ANOVA; p <0.0001 for treatment group) and by post-hoc analysis with Bonferroni correction; ***p <0.001. (E) Patients’ antibodies do not alter short-term plasticity, as revealed by paired-pulse facilitation. Short-term plasticity in the Schaffer collateral-CA1 synaptic region shows paired pulse facilitation as measured by mean slope values of the first (1st) and second (2nd) stimulus in the group of mice infused with control CSF (Ct CSF, blue), Ct CSF + ephrin-B2 (cyan), patients’ CSF antibodies (Pt CSF, red), or Pt CSF + ephrin-B2 (grey) before (pale color) and after (dark color) LTP induction. Analysis of 2nd versus 1st stimulation reveals a significant increase of fEPSPs in all groups and at both time points before LTP induction (Ct CSF: p <0.0001; Ct CSF + ephrin-B2: p = 0.0002; Pt CSF: p = 0.0012; Pt CSF + ephrin-B2: p = 0.0004) and after LTP (Ct CSF: p <0.0001; Ct CSF + ephrin-B2: p = 0.0009; Pt CSF: p <0.0001; Pt CSF + ephrin-B2: p <0.0001). Comparison of fEPSPs after the first stimulus before and after LTP induction shows a significant increase in the Pt CSF + ephrin-B2 (p = 0.0007) and both control CSF groups (Ct CSF: p = 0.0003; Ct CSF + ephrin-B2: p = 0.0004), but not in the Pt CSF group (p = 0.21). Analysis was performed using repeated-measures two-way ANOVA (p <0.0001 for treatment groups) and post-hoc analysis with Bonferroni correction; **p <0.01; ***p <0.001; ****p <0.0001. (F) Analysis of fEPSP absolute slope values depending on stimulus amplitude. Increasing stimulation leads to higher slope values reaching a plateau phase at stimulation strength >400 μA (maximum slope and peak amplitude of fEPSP). The fEPSP slope is significantly reduced in mice infused with patients’ CSF antibodies (Pt CSF; red circles). In the Pt CSF + ephrin-B2 group (gray circles), the fEPSP slope is partially restored in comparison to mice infused with Pt CSF. Blue and cyan squares indicate animals infused with control CSF (Ct CSF) and Ct CSF + ephrin-B2, respectively. Two-way ANOVA showed p <0.0001 for increasing stimulation and treatment; post-hoc analysis with Bonferroni correction showed difference between the groups of mice infused with Pt CSF compared with Ct CSF with or without ephrin-B2 (p <0.0001); Pt CSF compared with Pt CSF + ephrin-B2 (p = 0.001); and Pt CSF + ephrin-B2 compared with Ct CSF (p = 0.0007). CSF = cerebrospinal fluid.
In contrast to severe reduction of hippocampal LTP, short-term plasticity was not affected in animals infused with patients’ CSF antibodies. We performed a paired-pulse protocol before and after TBS in the CA3-CA1 synapse. As expected, fEPSP recordings showed significant paired-pulse facilitation (Fig 5E) according to increased presynaptic release probability. This effect was preserved in mice receiving patients’ CSF regardless of the presence of ephrin-B2. Moreover, the fEPSP slope values of the first of the paired stimuli before and after TBS did not change in mice infused with patients’ CSF, but they significantly increased in mice infused with patients CSF and ephrin-B2, consistent with the antibody-induced impairment of LTP shown in Figure 5C. Input-output curves of fEPSPs reflecting glutamatergic transmission in the CA1/Schaffer collateral pathway at increasing stimulus intensities revealed an exponential rise of fEPSP slope values to a maximum plateau phase that is finally reached by complete stimulation of the fiber tract. In animals that received patients’ CSF, absolute fEPSP slope values were reduced at nearly all stimulation intensities and also in the plateau phase compared to those of control animals. Coinjection of ephrin-B2 led to improvement of antibody-induced reduction of fEPSP slope (Fig 5F; p = 0.001 compared to patients’ CSF). fEPSPs in slices of mice infused with control CSF and ephrin-B2 showed no differences compared to animals infused with control CSF alone. Thus, we found severe impairment of postsynaptic, but not presynaptic, plasticity after TBS in animals that received patients’ CSF.
Discussion
We have used a mouse model of chronic cerebroventricular infusion of patients’ NMDAR antibodies to demonstrate the antibody pathogenicity at multiple levels from behavior to synaptic plasticity, and that all antibody-mediated effects can be at least partially prevented by administration of ephrin-B2, suggesting a novel molecular intervention with potential therapeutic implications.
Our results with CSF from a new group of patients with anti-NMDAR encephalitis accurately reproduced those of our previous study,9 as expected, knowing the limited GluN1 epitope repertoire in patients with this disorder.17 A novel finding was that patients’ antibodies caused a reduction of EphB2, a receptor that stabilizes the NMDAR at the synapsis.18
Previous work with cultured neurons showed that patients GluN1 antibodies interfered with NMDAR signaling by reducing NMDAR-mediated miniature excitatory postsynaptic currents (mEPSCs).2,17 GluN1 is the obligatory subunit for formation of functional NMDAR, and GluN1 knockout mice die within hours after birth.19 Hippocampal CA1 region-specific GluN1 knockouts show impaired spatial and temporal learning with severe impairment of formation of LTP in the Schaffer collateral/CA1 synapse, demonstrating the role of NMDAR in establishing synaptic plasticity and memory formation.20,21 Our findings show that similar hippocampal network alterations occur when the NMDAR is targeted by GluN1 antibodies from patients with anti-NMDAR encephalitis. Indeed, LTP recordings in the CA3-CA1 synapses of acute brain slices of our mouse model showed severe impairment of hippocampal synaptic plasticity. This finding possibly accounts for the memory and behavioral deficits observed in our animal model. Long-term impairment of synaptic plasticity by altered NMDAR function may result in reduction of activity-induced incorporation of high-conductance AMPARs in synaptic receptor fields, which is one of the main postsynaptic mechanisms of LTP.22 In contrast to these postsynaptic alterations, paired-pulse facilitation was unaffected accounting for largely intact presynaptic release mechanisms.23
A study using bilateral single injection of patients’ CSF into the dentate gyrus of rats showed impairment of spatial memory along with a reduction of NMDAR-evoked excitatory postsynaptic potentials and long-term potentiation.24 However, these alterations appeared to be irreversible and similar to those of a commercial GluN1 antibody, the binding of which requires cell permeabilization, suggesting that antibody-independent factors may have been involved.4 Another study that aimed to model seizures in mice by single cerebroventricular injection of patients’ IgG showed no spontaneous epileptic activity, but a decrease of seizure threshold.25 The NMDAR specificity of these effects (eg, change of synaptic NMDAR cluster density or NMDAR synaptic currents) was not investigated.
Eph receptors are a family of receptor tyrosine kinases that modulate LTP probably through their interaction with NMDAR and stabilization and clustering of this receptor in the postsynaptic membrane.16,26–28 Ephrin signaling, such as ephrin-B2 binding to the EphB2, is important to establish LTP in CA3-CA1 synapses for which downstream kinase signaling upon EphB activation is not critical as intracellular truncated forms of EphB do not interfere with LTP.26,29 In our mouse model, we hypothesize that autoantibody-induced disruption of the direct extracellular interaction of NMDAR with EphB216 results in reduction of synaptically located NMDAR, leading to memory and behavioral deficits and reduced LTP. Moreover, ligand-dependent direct interaction of EphB2 and NMDAR may also influence phosphorylation status or subunit composition of NMDAR and, in this way, modulate synaptic plasticity.18 In cultured neurons, the antibody-mediated disruption of the cross-talk of NMDAR and EphB2 leading to NMDAR synaptic displacement and internalization is efficiently antagonized by stimulation of EphB2.3 Here, we provide evidence that in mice receiving patients’ antibodies, treatment with ephrin-B2 is able to partially prevent the antibody-induced impairment of LTP and glutamatergic transmission in the hippocampus.
Direct quantification of ephrin-B2-induced restoration of NMDAR-mediated individual currents awaits further experimental evidence by evaluating synaptic currents using patch-clamp experiments at the single-cell level. These effects ex vivo are supported by the morphological findings demonstrating abrogation of the antibody-induced reduction of NMDAR and EphB2 in the ephrin-B2-treated mice. Thus, stabilization of NMDAR expression in the postsynaptic receptor fields mediated by stimulation of Eph2B receptors may account for the rescue of memory and behavioral deficits in this animal model.
Different from the current model in which the antibodies are infused by a pump that stops after 14 days, in the human disease the exposure of brain to central nervous system (CNS) antibodies decreases slowly over months.30,31 A relevant source of CNS antibodies are the plasma cells demonstrated in pathological studies32,33 and associated with intrathecal synthesis of antibodies.1,6 Current treatments (plasma exchange, intravenous immune globulin, or rituximab) have limited efficacy on removing antibodies and plasma cells from the CNS. Thus, prolonged exposure of the human brain to NMDAR antibodies may disturb synaptic networks, as suggested by our observed decrease of synaptic NMDAR and alteration of synaptic plasticity in our model. It is important to keep in mind that a period of 14 days in 2-month-old mice is equivalent to 60 weeks in humans.34 Additionally, in the human disease, the presence of inflammatory cells, mediators of inflammation, and frequent clinical complications (eg, intensive care unit complications, autonomic instability) may modify the outcome of the disorder.35
An important finding of this study is that all antibody-mediated deficits, from memory to synaptic plasticity, were substantially prevented by ephrin-B2, providing a potential treatment strategy with peptides or small-molecule agonists of EphB2. There is precedence for the clinical use of agonists of other types of Ephrin receptors, such as the EphA2 agonist, doxazosin, which is used for treatment of benign prostate hypertrophy, but in experimental models also inhibits tumor cell migration and metastases.36 In antibody-mediated disorders of the neuro-muscular junction, such as myasthenia gravis or Lambert-Eaton syndrome, the discovery of the physiopathological underpinnings led to the development of drugs that antagonize the effects of the corresponding antibodies (eg, anti-cholinesterases and 3,4-diaminopyridine).37 One envisions a similar result in anti-NMDAR and perhaps other auto-immune encephalitis, in which the combined use of immunotherapy and small molecules crossing the blood–brain barrier and antagonizing antibody effects could represent a future treatment approach. For anti-NMDAR encephalitis, such drugs may lead to a faster control of the severe symptoms of the disease, stabilizing the function of the NMDAR in the synapses.
Supplementary Material
Acknowledgments
This study was supported, in part, by Instituto Carlos III/FEDER (FIS 15/00377 [to F.G.], FIS 14/00203 and CIBERER [to J.D.], and RETICS-RTA and RD12/0028/0023 [to R.M.]), NIH RO1NS077851 (to J.D.), MINECO (SAF2014-59648-P; to R.M.), European Commission (HEALTH-F2-2013-602891; to R.M.), Fundació Cellex (to J.D.), the Netherlands Organisation for Scientific Research (NWO, Veni incentive; to M.T.), an Erasmus MC fellowship (to M.T.), and the German Research Council (DFG; GE 2519/3-1 and CRC-TR 166/1 B2 [to C.G.]).
We thank Dr Melike Lakadamyali (ICFO-Institut de Ciències Fotòniques, Barcelona) and Dr Myrna Rosenfeld (IDIBAPS, University of Barcelona) for their critical review of the manuscript and useful suggestions and Dr Thaís Armangué (IDIBAPS, University of Barcelona) for her comments on the statistical analysis.
Footnotes
Additional supporting information can be found in the online version of this article
Author Contributions
J.D. and C.G. were responsible for conception and design of the study. F.M., M.P.-P., E.M.-G., R.F., and J.D. were responsible for acquisition and analysis of animal behavior. J.P., M.J.T., P.J., F.G., and J.D. were responsible for acquisition and analysis of immunohistochemistry and confocal microscopy. H.H., B.G., L.R., and C.G. were responsible for acquisition and analysis of electrophysiological studies. J.P., H.H., C.G., and J.D. were responsible for drafting of the manuscript and figures. J.P., H.H., and F.M. contributed equally. C.G. and J.D. share seniority.
Potential Conflicts of interest
Dr Dalmau holds a patent for the use of NMDA receptor as an autoantibody test. Dr Dalmau has received a research grant from Euroimmun Inc.
References
- 1.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mikasova L, De Rossi P, Bouchet D, et al. Disrupted surface crosstalk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain. 2012;135:1606–1621. doi: 10.1093/brain/aws092. [DOI] [PubMed] [Google Scholar]
- 4.Moscato EH, Peng X, Jain A, et al. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol. 2014;76:108–119. doi: 10.1002/ana.24195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viaccoz A, Desestret V, Ducray F, et al. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. 2014;82:556–563. doi: 10.1212/WNL.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Tanaka K, Sun P, et al. Suppression of synaptic plasticity by cerebrospinal fluid from anti-NMDA receptor encephalitis patients. Neurobiol Dis. 2012;45:610–615. doi: 10.1016/j.nbd.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Planaguma J, Leypoldt F, Mannara F, et al. Human N-methyl D-aspartate receptor antibodies alter memory and behaviour in mice. Brain. 2015;138:94–109. doi: 10.1093/brain/awu310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai M, Hughes EG, Peng X, Dalmau J. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grover LM, Kim E, Cooke JD, Holmes WR. LTP in hippocampal area CA1 is induced by burst stimulation over a broad frequency range centered around delta. Learn Mem. 2009;16:69–81. doi: 10.1101/lm.1179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Schmuckermair C, Gaburro S, Sah A, et al. Behavioral and neuro-biological effects of deep brain stimulation in a mouse model of high anxiety- and depression-like behavior. Neuropsychopharmacology. 2013;38:1234–1244. doi: 10.1038/npp.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kordjazy N, Haj-Mirzaian A, Amiri S, et al. Involvement of N-methyl-d-aspartate receptors in the antidepressant-like effect of 5-hydroxytryptamine 3 antagonists in mouse forced swimming test and tail suspension test. Pharmacol Biochem Behav. 2015;141:1–9. doi: 10.1016/j.pbb.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Dalva MB, Takasu MA, Lin MZ, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 17.Gleichman AJ, Spruce LA, Dalmau J, et al. Anti-NMDA receptor encephalitis antibody binding is dependent on amino acid identity of a small region within the GluN1 amino terminal domain. J Neurosci. 2012;32:11082–11094. doi: 10.1523/JNEUROSCI.0064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nolt MJ, Lin Y, Hruska M, et al. EphB controls NMDA receptor function and synaptic targeting in a subunit-specific manner. J Neurosci. 2011;31:5353–5364. doi: 10.1523/JNEUROSCI.0282-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forrest D, Yuzaki M, Soares HD, et al. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron. 1994;13:325–338. doi: 10.1016/0896-6273(94)90350-6. [DOI] [PubMed] [Google Scholar]
- 20.Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- 21.Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- 22.Zamanillo D, Sprengel R, Hvalby O, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 23.Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K. Facilitation at single synapses probed with optical quantal analysis. Nat Neurosci. 2002;5:657–664. doi: 10.1038/nn867. [DOI] [PubMed] [Google Scholar]
- 24.Wurdemann T, Kersten M, Tokay T, et al. Stereotactic injection of cerebrospinal fluid from anti-NMDA receptor encephalitis into rat dentate gyrus impairs NMDA receptor function. Brain Res. 2015;1633:10–18. doi: 10.1016/j.brainres.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Wright S, Hashemi K, Stasiak L, et al. Epileptogenic effects of NMDAR antibodies in a passive transfer mouse model. Brain. 2015;138:3159–3167. doi: 10.1093/brain/awv257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henderson JT, Georgiou J, Jia Z, et al. The receptor tyrosine kinase EphB2 regulates NMDA-dependent synaptic function. Neuron. 2001;32:1041–1056. doi: 10.1016/s0896-6273(01)00553-0. [DOI] [PubMed] [Google Scholar]
- 27.Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. [DOI] [PubMed] [Google Scholar]
- 28.Lisabeth EM, Falivelli G, Pasquale EB. Eph receptor signaling and ephrins. Cold Spring Harb Perspect Biol. 2013:5. doi: 10.1101/cshperspect.a009159. pii: a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grunwald IC, Korte M, Wolfer D, et al. Kinase-independent requirement of EphB2 receptors in hippocampal synaptic plasticity. Neuron. 2001;32:1027–1040. doi: 10.1016/s0896-6273(01)00550-5. [DOI] [PubMed] [Google Scholar]
- 30.Gresa-Arribas N, Titulaer MJ, Torrents A, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13:167–177. doi: 10.1016/S1474-4422(13)70282-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leypoldt F, Hoftberger R, Titulaer MJ, et al. Investigations on CXCL13 in anti-N-methyl-D-aspartate receptor encephalitis: a potential biomarker of treatment response. JAMA Neurol. 2015;72:180–186. doi: 10.1001/jamaneurol.2014.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez-Hernandez E, Horvath J, Shiloh-Malawsky Y, et al. Analysis of complement and plasma cells in the brain of patients with anti-NMDAR encephalitis. Neurology. 2011;77:589–593. doi: 10.1212/WNL.0b013e318228c136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bien CG, Vincent A, Barnett MH, et al. Immunopathology of autoantibody-associated encephalitides: clues for pathogenesis. Brain. 2012;135:1622–1638. doi: 10.1093/brain/aws082. [DOI] [PubMed] [Google Scholar]
- 34.Flurkey K, Currer JM, Harrison DE. The mouse in aging research. In: Fox JG, et al., editors. The Mouse in Biomedical Research. Burlington, MA: American College Laboratory Animal Medicine (Elsevier); 2015. pp. 637–672. [Google Scholar]
- 35.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petty A, Myshkin E, Qin H, et al. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS One. 2012;7:e42120. doi: 10.1371/journal.pone.0042120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newsom-Davis J. Therapy in myasthenia gravis and Lambert-Eaton myasthenic syndrome. Semin Neurol. 2003;23:191–198. doi: 10.1055/s-2003-41135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.