Abstract
Intervertebral disc (IVD) degeneration (IDD) and herniation (IDH) can result in low back pain and impart significant socioeconomic burden. These pathologies involve detrimental alteration to the nucleus pulposus (NP) either via biochemical degradation or extrusion from the IVD, respectively. Thus, engineering living NP tissue utilizing biomaterial scaffolds that recapitulate native NP microarchitecture, biochemistry, mechanical properties and which support cell viability represents an approach to aiding patients with IDD and IDH. To date, an ideal biomaterial to support NP regeneration has yet to be developed; however, one promising approach to generating biomimetic materials is to employ the decellularization (decell) of xenogeneic NP tissue to remove host DNA while maintaining critical native extracellular matrix (ECM) components. Herein, 13 different procedures were evaluated in an attempt to decell bovine caudal IVD NP tissue. An optimal method was identified which was confirmed to effectively remove bovine DNA, while maintaining physiologically relevant amounts of glycosaminoglycan (GAG) and type-II collagen. Unconfined static and dynamic compressive mechanical properties of scaffolds approached values reported for human NP and viability of human amniotic stem cells (hAMSCs) was maintained on non-crosslinked and EDC/NHS treated scaffolds for up to 14 days in culture. Taken together, NP tissue obtained from bovine caudal IVDs can be successfully decelled in order to generate a biomimetic scaffold for NP tissue regeneration.
Keywords: Nucleus Pulposus, Decellularization, Biomimetic Scaffold, Tissue Engineering, Intervertebral Disc Degeneration
1. Introduction
Low back pain (LBP) poses a significant socioeconomic burden with a lifetime prevalence of 84% and estimated expenditures of $85.9 billion in the U.S.1,2 Although the cause of LBP is difficult to pinpoint; it has been demonstrated to originate from degenerating or herniated intervertebral discs (IVDs).3 IVD degeneration (IDD) is a multifactorial process which manifests initially as biochemical degradation of the nucleus pulposus (NP); a highly hydrated tissue primarily composed of type II collagen and the proteoglycan aggrecan, which together allow the IVD to support compressive loads via the generation of intradiscal pressures (IDPs) during activities of daily living.4–6 IVD herniation (IDH) is commonly the result of an increase in IDP due to abrupt loading, which exceeds the strength of the restraining annulus fibrosus (AF) region of the IVD leading to extrusion of NP tissue from the IVD. Both pathologies can result in decreased IVD heights and irritation of adjacent nerve roots leading to the generation of back and leg pain as well as limb weakness.3,7,8
Current therapies for IDD and IDH are palliative and merely delay invasive surgical management in the form of discectomy, spinal fusion and total disc replacement. While these procedures may temporarily relieve pain, they do not attempt to replace, restore or regenerate healthy NP tissue. Additionally, there are concerns with the use of current surgical methodologies which may promote re-herniation, altered spinal biomechanics and accelerated degeneration in adjacent IVDs.4,7,9
Regenerative medicine approaches to IVD repair have been investigated both in vitro and in vivo. Injection of growth factors and stem cells alone into the NP have demonstrated limited success likely due their short half-life, leakage and inability to maintain an appropriate phenotype due to the altered tissue ECM microarchitecture, respectively.10–14 Tissue engineering (TE) strategies combining scaffolds and an alternative healthy cell source (i.e. stem cells) together provide a promising avenue for developing viable NP tissue constructs. Success of such approaches rely largely upon the development of a biomaterial scaffold that mimics the native NP biochemistry and mechanical properties which can function to deliver, protect and provide instructive cues to stem cells such that they attain the appropriate phenotype and produce NP-specific ECM. Examples of biomaterial scaffolds investigated for NP TE to date include pre-formed and injectable hydrogels composed of type II collagen-hyaluronic acid and cross-linked alginate.15,16
An alternative scaffold formation method gaining widespread use in many TE applications includes the decellularization (decell) of a target tissue; a process which aims to remove host cells from the tissue while maintaining intact, tissue-specific ECM. One primary advantage of this approach is that the remaining ECM provides cues that can advantageously affect migration, proliferation, differentiation and subsequent tissue-specific ECM production by seeded cells.17 When developing new decell procedures to create scaffolds one must objectively establish acceptance criteria a priori to verify that successful decell has occurred and that ECM components critical to the function of the scaffold are maintained. Accordingly, defined metrics and threshold values to assess residual xenogenic DNA have been established and should be used as guidance.17,18 Moreover, proteoglycan/glycosaminoglycan content of decell scaffolds should be on the order of magnitude to that found in human lumbar IVDs.19
Given the similarity in size, geometry, resting stress and greater average NP glycosaminoglycan content found in the bovine caudal (tail) IVD as compared to human lumbar IVDs it was hypothesized that successful decell of this tissue would yield a biomaterial scaffold that could mimic key physico-chemical properties found in native human NP. Thus, the objectives of this study were to; 1) develop an optimized decell procedure by which to create a biomaterial from bovine caudal IVD NP, 2) histologically, biochemically and mechanically characterize the resultant biomaterial scaffold and 3) evaluate its cytotoxicity prior to and following chemical crosslinking and seeding with human amniotic mesenchymal stem cells (hAMSCs).
2. Materials and Methods
2.1 Tissue Harvest
Bovine tails were harvested from 2-3 years old cows at a local abattoir and were transported on wet ice to the lab. Betadine® was applied to the exterior of the tail to remove bacteria. Excess fascia and muscle were removed from the tails with a scalpel; the location of IVDs was visually confirmed prior to excision immediately adjacent to the endplates via bone cutters. An 8mm biopsy punch was used to remove NP tissue from the center of the IVD. Fresh NP samples were stored at −20°C and served as controls for experiments. Samples for decell were immediately placed either in ethanol or various decellularization solutions.
2.2 Decellularization Methods
Thirteen different decell treatment methods consisting of varying chemical decell solutions, inclusion of ultrasonication (40kHz), and treatment durations were evaluated (Table1). All decell solutions (except decell 12) were made in 50mM Tris buffer (pH 7.5) containing 2% antibiotic/antimycotic. The decell methods were adapted from Mercuri et al. as described previously.20,21 Briefly, all samples (10 samples/100mL of solution) were placed in their respective decell solutions and ultrasonicated for 10 minutes prior to undergoing constant agitation (150RPM) on an orbital shaker at room temperature. Every twenty-four hours thereafter decell solutions were changed and samples were ultrasonicated for an additional 10 minutes. This process was repeated for a total of either three or six days. At the completion of the decell process, all samples were rinsed in sequential changes of distilled water, 70% ethanol and distilled water again; each for 30 minutes under orbital agitation (150RPM) at room temperature prior to undergoing nuclease treatment (720mU/ml DNase and RNase) at 37°C for 48 hours. All nuclease solutions were made in 1× PBS (pH 7.5) containing 5mM magnesium chloride. Following nuclease treatment all samples were thoroughly rinsed in distilled water for one hour under agitation.
Table 1.
Comparison of decellularization methods investigated.
| Decellularization Solution | Nuclease Solutions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group |
Number of Sonications |
Time in Decell Solution |
70 % EtOH Treatment Time |
EDTA | Triton X-100 | Deoxycholic Acid | Sodium Azide | SDS | DNase | RNase | Summary of Findings |
| Decell 1 | 6 | 3 | - | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 7.19 ng/mg Average [GAG): 52.63 μg/mg Histology: Visable Nuclei and Faded Blue ECM |
| Decell 2 | 12 | 3 | - | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 15.26 ng/mg Average [GAG]: 59.81 μg/mg |
| Decell 3 | 6 | 6 | - | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 10.21 ng/mg Average [GAG]: 70.67 μg/mg |
| Decell 4 | 12 | 6 | - | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 7.53ng/mg Average [GAG]: 63.04 μg/mg Histology:Visable Nuclei and Deep Blue ECM |
| Decell 5 | 6 | 3 | - | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 1440 mU/mL | 1440 mU/mL | Average [DNA]: 11.59 ng/mg Average [GAG]: 83.09 μg/mg |
| Decell 6 | 3 | 3 | - | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 10.45 ng/mg Average [GAG]: 57.99 μg/mg |
| Decell 7* | 3 | 3 | - | 0.4% (w/v) | 1.2% (v/v) | 2% (w/v) | 0.04% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 33.61 ng/mg Average [GAG]: 216.98 μg/mg Histology: Empty Lacunae and Faded Blue ECM |
| Decell 8 | 3 | 3 | 30 min | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 15.59 ng/mg Average [GAG]: 108.42 μg/mg |
| Decell 9 | 3 | 3 | 60 min | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 9.07 ng/mg Average [GAG]: 184.53 μg/mg |
| Decell 10* | 3 | 3 | 45 min | 0.2% (w/v) | 0.6% (v/v) | 1% (w/v) | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 33.08 ng/mg Average [GAG]: 208.93 μg/mg Histology: Nuclei Present and Faded Blue ECM |
| Decell 11 | 3 | 3 | - | 0.2% (w/v) | 0.6% (v/v) | - | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 15.22 ng/mg Average [GAG]: 133.59 μg/mg Histology: Nuclei Present and Intense Blue ECM |
| Decell 12 | 3 | 3 | - | 2.21 mM | - | - | - | 0.4% (w/v) | 720 mU/mL | 720 mU/mL | Average [DNA]: 23.31 ng/mg Average [GAG]: 71.92 μg/mg Histology: Nuclei Present and Faded Blue ECM |
| Decell 13* | 3 | 3 | - | 0,4% (w/v) | 1.2% (v/v) | - | 0.02% (w/v) | - | 720 mU/mL | 720 mU/mL | Average [DNA]: 9.07 ng/mg Average [GAG]: 205.5 μg/mg Histology: Empty Lacunae and Deep Blue ECM |
2.3 Decellularization Efficacy Screening
The efficacy of each decell method was initially screened via quantitative biochemical assays used to measure residual bovine DNA and glycosaminoglycan (GAG) content via PicoGreen® (Thermo Fisher Scientific) and DMMB assays, respectively. Additionally, histological analyses were performed on OCT embedded cryosections to detect the presence of residual bovine cell nuclei via Alcian blue counterstained with nuclear fast red. Acceptance criteria were established a priori to indicate successful decell which included: 1) an average residual DNA content of less than or equal to 50ng.
DNA/mg dry tissue, 2) an average residual GAG content of greater than or equal to 200μg GAG/mg dry tissue and 3) no histological evidence of intact cell nuclei within the decelled NP tissue. All samples that met the acceptance criteria were further evaluated for residual DNA via agarose gel electrophoresis.
2.3.1 DMMB Analysis for GAG
Fresh (n=6) and decell treated bovine NP tissue samples (n=6/decell treatment) were frozen for 24 hours at −80°C prior to lyophilization. Sample dry weights were recorded for data normalization prior to complete digestion in PBE buffer containing papain (5mM L-cysteine, 100mM dibasic phosphate buffer, 5mM EDTA, 12.50μg/mL papain, pH 7.5) for 24 hours at 65°C. Fresh and decelled samples were diluted by 1000x and 100x, respectively in PBE buffer prior to combining 50μL of each sample with 200μL of DMMB reagent (40mM NaCl, 40mM Glycine, 46μM DMMB, pH 3.0) in a 96-well plate. Absorbance was read at 525nm and GAG content was determined from a standard curve developed from known concentrations of chondroitin-6-sulfate.
2.3.2 PicoGreen® Analysis for dsDNA
Total double stranded DNA content of fresh (n=6) and decell treated bovine NP tissue samples (n=6/decell treatment) were quantified using a PicoGreen® assay according to manufacturer’s instructions. Briefly, papain digested samples were thawed and diluted 2x in a 1X TE buffer prior to combining 100μL of sample with 100μL of PicoGreen® reagent in a black-walled 96-well plate. Fluorescence was detected using excitation and emission wavelengths of 480nm and 520nm, respectively. DNA content was determined from a standard curve developed from known concentrations of lambda DNA supplied by the assay manufacturer.
2.3.3 Agarose Gel Electrophoresis
DNA was purified from aliquots of papain digested samples of both fresh (n=3) and decell treated bovine NP tissue samples (n=3/decell treatment) using a Qiagen DNeasy® Blood & Tissue extraction kit according to manufacturer’s instructions. A total of 30μl of purified DNA sample was loaded into each well of an agarose gel containing ethidium bromide. Two concentrations of agarose gels were used in order to detect high and low molecular weight ranges of residual DNA. A wide range DNA ladder (300-24,000bp, exACTGene 24kb) was ran on a 1% agarose gel at 100V for 60 minutes. A low range DNA ladder (10-300bp, O’GeneRuler Low Range DNA) was run on a 5% agarose gel at 75V for 60 minutes. Gels were imaged in a bio-imager (Bio-Rad) using an ethidium bromide filter to detect the presence of DNA bands. All samples were run against a DNA standard.
2.4 Optimal Bovine NP Decellularization Procedure
Outcomes from the initial screening studies described above indicated that treatment of bovine NP tissue with a combination of a chemical decellularization solution coupled with ultrasonication and constant agitation (150RPM) for a duration 72 hours at room temperature resulted in successful decell according to the acceptance criteria. The optimal decell solution tested (decell treatment 13) contained 1.2% Triton X-100, 0.2% EDTA, 0.02% sodium azide, and 50mM Tris all in 400mL DI water with a pH of 7.5. The addition of 10 minute ultrasonication (40kHz) periods immediately following sample submersion into the decell solution and every 24 hours thereafter for a total of 3 days prior to 48 hours of nuclease treatment at 37°C resulted in an NP matrix that was devoid of intact cell nuclei and residual DNA while maintaining an average GAG content of ≥200μg/mg dry sample. The resulting biomaterial scaffold material is hereafter referred to as the acellular bovine nucleus pulposus (ABNP) scaffold, which was further characterized as follows:
2.5 Water Content Analysis on ABNP Scaffold
Percent water content was determined by the following equation: [(Wwet−Wdry)/Wwet] × 100, where Wwet was sample wet weights and Wdry was the sample dry weight determined following lyophilization.
2.6 Hydroxyproline Analysis on ABNP Scaffold
Collagen content of fresh bovine NP (n=3) and ABNP scaffolds (n=6) were quantified using a hydroxyproline (HYP) assay according to the manufacturer’s instructions (Sigma – MAK008). Hydrolyzed samples from both fresh and ABNP scaffolds were diluted 100x in 12M HCl and 10μL of each sample were analyzed at 550nm. HYP content was calculated using a standard curve developed from serial dilutions of a known concentration of HYP. HYP Values were normalized to sample dry weight.
2.7 Histology and Immunohistochemistry (IHC) on ABNP Scaffold
Samples of fresh bovine NP and ABNP scaffolds were fixed in 10% neutral buffered formalin for 24 hours. Fixed samples were then washed in 1X PBS with 0.01% sodium azide for 30 minutes and then soaked in a 15% sucrose solution at 4°C overnight. Subsequently, samples were placed in a 30% sucrose solution for 2 hours at 4°C, and then placed in a 50:50 30% sucrose and OCT solution for 2 hours at room temperature. Finally, samples were placed in OCT for 2 hours then transferred to OCT molds for cryosectioning at −20°C until solid. Cryosections of 8μm thickness were collected on positively charged slides and stored at −20°C until staining. All imaging was performed using a Zeiss AxioVert.A1 inverted microscope with Axiovision software.
2.7.1 Immunohistochemistry for Type II Collagen
Frozen sections of fresh bovine NP (n=3) and ABNP scaffolds (n=3) were fixed for 20 minutes in cold acetone. Slides were rinsed twice in TBS for 5 minutes, permeabilized in 0.025% Triton X-100, non-specific binding and endogenous peroxidases were blocked with normal serum and a solution of 0.3% hydrogen peroxide in 0.3% normal serum, respectively. A rabbit polyclonal antibody towards bovine type II collagen (Abcam – ab78482; 1:50 dilution) was incubated overnight at 4°C prior to thorough rinsing and incubation at room temperature for 30 minutes with a secondary biotinylated antibody and avidin biotin complex according to manufacturer’s instructions (Vectastain® ABC Elite Kit Rabbit IgG - Vector Labs). A DAB substrate kit (Vector Labs: SK4100) was used to visualize positive staining according to manufacturer’s instructions prior to counterstaining with a dilute hematoxylin solution for 30 seconds. To account for non-specific binding of the secondary antibody; select samples did not receive primary antibody. Additional positive and negative controls for type II collagen staining included chondrogenically induced hAMSC cell pellets and decellularized porcine pericardium, respectively (data not shown).
2.7.2 Alcian Blue and Nuclear Fast Red Histology Staining
Frozen sections of fresh bovine NP (n=6) and ABNP scaffolds (n=6) were rinsed in tap prior to being placed in 3% acetic acid for 1 minute followed by a 1% alcian blue in 3% acetic acid (pH 2.5) solution for 3 minutes. Slides were rinsed in tap water for 1 minute and counterstained in 0.1% nuclear fast red for 2 minutes.
2.7.3 DAPI Nuclear Staining
Frozen slides of fresh bovine NP (n=3) and ABNP scaffolds (n=3) were rinsed in 1X PBS for 1 minute and placed in a 300mM DAPI solution for 5 minutes in the dark. Slides were washed in PBS and then immediately transported for imaging under a fluorescent microscope fitted with a 460nm filter.
2.7.4 Ethidium Bromide DNA Staining
Samples of fresh bovine NP (n=3) and ABNP scaffolds (n=3) were placed in a solution of 2mM ethidium bromide in PBS for 30 minutes. Tissues were then thinly sectioned using a scalpel blade and imaged under a fluorescent microscope.
2.8 Mechanical Testing
2.8.1 Sample Preparation and Testing Conditions
Fresh bovine NP and ABNP samples were frozen at −20°C, embedded in OCT and sectioned to a thickness of approximately 6 mm for all mechanical testing. Prior to removing samples from OCT, a 6 mm diameter biopsy punch was used to ensure consistent sample diameters which resulted in a 1:1 diameter to height ratio. Subsequently, all samples (both fresh bovine NP and ABNP) were allowed to thaw and free swell in the testing tank (which contained a solution of 1X PBS and 1% protease inhibitor) for at least one hour at room temperature (25°C). All samples were tested while submerged in a solution of 1X PBS and protease inhibitor using a Bose Electrofoce® 3200 series mechanical test frame equipped with a 1000g load cell fitted with a testing tank according to methods adapted from previously described reports.22,23
2.8.2 Unconfined Compression
Unconfined compressive stress relaxation experiments were performed on fresh bovine NP (n=5) and ABNP scaffolds (n=5) to determine the compressive properties of the specimens once equilibrium relaxation was attained. Briefly, a compression platen was brought in contact with a stationary testing platform and the displacement measurement was zeroed prior to pre-loading. Pre-loading consisted of compressing the specimens in displacement control until a 0.05N compressive load was achieved (which resulted in approximately 12% total strain). Platen displacement was held constant for 20 minutes allowing for the sample to equilibrate. Sample heights were determined at the end of pre-loading based on the initial zeroed displacement by the test software. Subsequently, stress relaxation testing was conducted which consisted of subjecting the preconditioned samples to a final strain of 16% (based on sample heights determined after preconditioning) using 4% strain increments (starting at 8%) held for 20 minutes each until the change in load was less than 0.025N/minute. Data was collected in WinTest 7 using level crossing with each change of 0.025N being recorded. NIH Image J software was used to measure and confirm sample height and diameter at the applied peak strain and at equilibrium. Intrinsic equilibrium Young’s modulus of the solid matrix was determined through the equilibrium response during stress relaxation at 8%, 12%, and 16% applied strains by dividing the equilibrium stress by the respective applied strain. Percent relaxation was determined at each applied strain using the following equation: [(σp – σe)/σp] × 100, where σp = peak stress and σe = equilibrium stress. Poisson’s ratio (υ), shear modulus (μ), and aggregate modulus (HA) were determined assuming isotropic, homogeneous biphasic theory for cartilaginous materials as previously reported.24 Briefly, Poisson’s ratio was measured directly from unconfined compression testing using an optical method. Briefly, direct lateral images of specimens were obtained at equilibrium following compression to 8 and 12% strain, respectively. Images of each specimen were obtained using a digital camera and a stationary metric calibrator located inside the testing tank. NIH Image J software was used in conjunction with the metric calibrator in order to determine equilibrium lateral expansion of the specimen immediately adjacent to the lower testing platform. Shear and aggregate moduli were determined through their relationships with both intrinsic equilibrium Young’s modulus and Poisson’s ratio via the following equations, respectively: and .24
2.8.3 Unconfined Dynamic Mechanical Analysis (DMA)
DMA testing was used to determine the complex, storage, and loss modulus as well as phase angle of a second set of fresh bovine NP (n=6) and ABNP scaffolds (n=6). Similar to unconfined compression testing, samples were pre-loaded to 0.05N under displacement control and were then allowed to equilibrate for 20 minutes with the platen displacement being held constant. The height and width of each sample was confirmed from a direct lateral digital image taken at the end of the preconditioning period. Height data was input into the DMA testing software within WinTest 7. Samples were then preconditioned to 5% strain for 10 cycles prior to initiation of the test program, which was set to apply a cyclic strain of 8% (based on initial sample height) at a frequency of 0.01, 0.1, 1, and 10Hz with a 20-minute relaxation between each test frequency.
2.9 Cell Studies
2.9.1 ABNP Scaffold Cytotoxicity
Scaffolds were sterilized in 0.01% peracetic acid followed by washing three times for 15 minutes each in 1X sterile PBS. Scaffolds were then placed in a solution containing 50% FBS, 48% DMEM, and 2% AB/AM for 24 hours. Human amniotic mesenchymal stem cells (hAMSCs; passage 2) were obtained from consenting patients immediately following the delivery via elective cesarean section of full-term babies (Pro00031185-Greenville Health System). Stem cells were isolated within 4 hours of delivery according to procedures established in our lab. Stem cells were seeded via drop-wise addition to the surface of the scaffolds at a density of 2,000 hAMSCs/mm2. Seeded scaffolds were incubated at 37°C for 3 hours to allow time for cells to attached prior to the addition of media (DMEM containing 10% FBS and 1% Ab/Am). Media was refreshed every 3 days. On days 3, 7, and 14 a live/dead assay was performed (n=3 per time point) was performed according to manufacturer’s instructions (Biotium). ABNP scaffolds were sectioned using a scalpel blade and imaged under a fluorescent microscope. Three representative images of both live and dead staining were taken for each scaffold. Quantification of cells was performed by manually counting live and dead cells in each image. Percent viability was determined by dividing the number of live cells by the total number of cells.
2.9.2 ABNP Scaffold Crosslinking for Stem Cell Injection Studies
ABNP scaffolds were crosslinked in a solution of 30mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide HCL (EDC)/6mM n-hydroxysuccinimide (NHS) in 50mM beta-morpholinoethansulfonurea hydrate (MES) buffer (pH 5.5) for 30 minutes. Scaffolds were subsequently washed three times for 15 minutes each, sterilized in 0.1% peracetic acid, and washed again three times at 15 minutes each in 1X sterile PBS then were placed in a solution of 50% FBS, 48% DMEM, and 2% AB/AM for 24 hours prior to cell seeding/injection.
2.9.3 ABNP Scaffold Cell Injection
Injection of hAMSCs into ABNP scaffolds was investigated due to the observation that hAMSCs were not able to infiltrate the scaffold after 14 days of culture following seeding on the scaffold surface (described previously in section 2.9.1). Thus, a pilot study was initiated to determine if hAMSCs could be injected into the ABNP scaffolds. Preliminary studies were performed to confirm appropriate needle bore size for injection (i.e. what was the smallest bore needle diameter that could be used to inject stem cells without killing them due to shearing). A 28G needle was selected based on the results of the preliminary hAMSC viability studies after injection through various syringe needle diameters (data not shown). Scaffolds were injected with 150μL of hAMSC suspension (4×106 cells/mL). Cell injected scaffolds were cultured for 3 and 14 days and cell viability was assessed using live/dead staining and fluorescent microscopy.
2.10 Statistical Analysis
Results are represented as a mean ± standard error of the mean (SEM). For quantitative biochemical data obtained during decellularization screening studies, viability comparisons and mechanical data comparing across strain levels within each study group (ABNP vs Fresh); a one-way analysis of variance (ANOVA) using a Tukey’s honest significant difference post hoc analysis for all pairwise comparisons was used. Mechanical data was also analyzed via a Students two-tailed t-test assuming equal variance in order to determine if differences between study groups (i.e. ABNP vs Fresh) existed within each applied strain (for stress relaxation testing) or test frequency (for DMA testing), respectively. Significance was defined in all cases as p<0.05.
3. Results
3.1 Decellularization Optimization Screening Analysis
The effectiveness of decellularization procedures was initially screened by evaluating residual bovine DNA concomitant with the retention of GAG, an NP ECM component critical to proper IVD function. An overview of the decellularization procedures investigated and screening results are summarized in Table 1. Optimal decell procedures were defined as those that resulted in a tissue that contained less than 50ng/mg DNA and maintained at least 200μg/mg of GAG. Accordingly, three decellularization procedures were identified based on these criteria: treatment groups 7, 10, and 13, respectively.
3.2 Biochemical Analysis of Optimum Decellularization Treatments
3.2.1 DNA Quantification
Evaluation of residual double stranded bovine DNA demonstrated significant reductions (p<0.01) in treatment groups 7, 10, & 13 as compared to fresh NP tissue. These treatment groups contained 33.61±9.49ng, 33.08±11.57ng, and 9.07±1.95ng DNA per mg sample dry weight, respectively (Figure 1A). Interestingly, samples treated with SDS, a method commonly employed for tissue decellularization did not show statistical reductions in DNA content in treated NP tissue (71.31±30.59ng DNA per mg dry weight). Compared to fresh bovine NP tissue (125.45±11.81ng DNA per mg sample dry weight) there was a 73.2±7.56%, 73.63±9.22%, & 92.77±1.54% reduction in DNA content for groups 7, 10, and 13, respectively.
Figure 1.
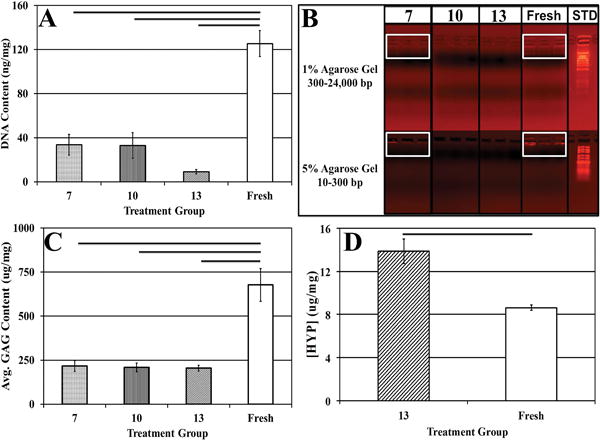
(A) DNA quantification demonstrating significant reductions in DNA content after decellularization as compared to fresh bovine NP tissue. (B) Agarose gel electrophoresis depicting the presence of intact DNA in fresh bovine NP and in treatment group 7 indicated in white boxes, and the lack of DNA fragments in treatment groups 10 and 13. (C) GAG and (D) [HYP] content of fresh and decellularized bovine NP subjected to treatments 7, 10, and 13. Horizontal bars connecting groups indicate significant differences (p<0.05).
3.2.2 Qualitative Measurement of DNA
No residual DNA fragments between 10-24,000 base pairs (bp) were detected in procedures 10 and 13, while residual fragments (indicated by light bands) appeared in the range of 300bp for samples in treatment group 7 (Figure 1B).
3.2.3 GAG Quantification and Histological Evaluation
GAG quantification of decellularized bovine NP tissue demonstrated the retention of 216.98±31.06μg, 208.93±24.96μg, and 205.5±16.00μg GAG per mg sample dry weight using methods 7, 10, and 13 respectively (Figure 1C). Expectedly, these values were statistically lower than values obtained for fresh bovine NP tissue (677.66±93.22μg GAG per mg sample dry weight) (Figure 1C). Decellularization resulted in a reduction in average GAG content of 67.98±4.58%, 69.17±3.68%, & 69.68±2.36% in treatment groups 7, 10, and 13 respectively. These biochemical findings were corroborated by Alcian blue staining (Figure 2) which illustrated that the ECM in all decellularization samples was stained less intensely blue which indicated a reduction in GAG and a disrupted microarchitecture compared to fresh NP tissue samples. These histological observations were also likely the result of ABNP swelling which occurs during the decell process. Taken together with the aforementioned data, treatment group 13 was selected as the optimal decellularization method investigated herein, thus further biochemical, histological and mechanical analysis was performed on samples subjected to this decellularization method and hereafter the biomaterial is termed “acellular bovine nucleus pulposus” (ABNP) scaffold.
Figure 2.
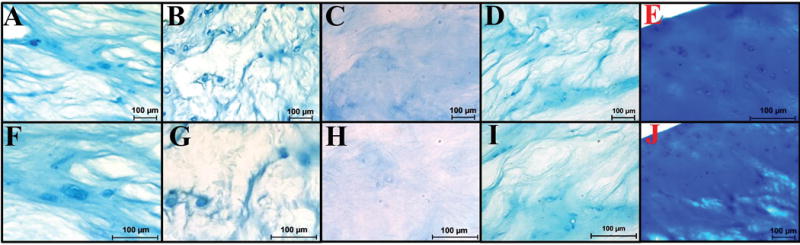
Alcian blue counterstained with nuclear fast depicting GAG (blue) and cell nuclei (red) (A-E 200×, F-J 400×) (A&F) Treatment group 7. (B&G) Treatment group 10. (C&H) Treatment group 12. (D&I) Treatment group 13. (E&J) Fresh bovine NP.
3.2.4 ABNP Scaffold Hydroxyproline Content
ABNP scaffolds had significantly higher HYP content per dry mass when compared to fresh bovine NP tissue (p<0.05) (Figure 1D). More specifically, ABNP scaffolds contained nearly double the average amount of HYP as compared to fresh bovine NP tissue (13.87±1.14 μg HYP/mg sample dry weight and 8.63±0.24 μg HYP/mg sample dry weight, respectively). Additionally, the GAG to HYP ratio was calculated to be approximately 15:1 for decellularized samples whereas fresh bovine NP tissue had an apparent ratio of 79:1.
3.2.5 Assessment of Type II Collagen of ABNP Scaffolds
IHC for collagen type 2 (Col-II) confirmed the presence of this ECM protein within both ABNP scaffolds and fresh bovine NP. Histological images illustrated intense brown (positive) staining in both groups throughout the ECM and pericellular regions as compared to negative controls (Figure 3A&E).
Figure 3.
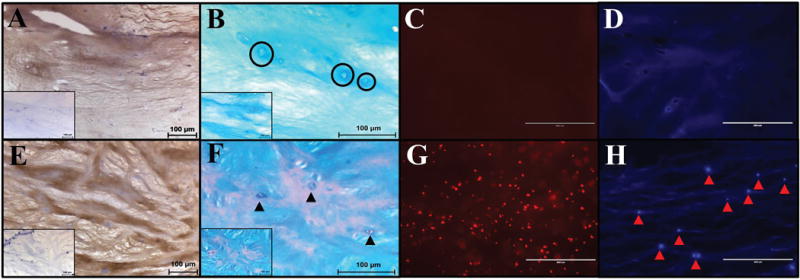
Histological analysis of ABNP scaffolds (A–D) compared to fresh bovine NP tissue (E-H). (A&E) Immunohistochemistry for Col-II (brown = positive, light blue = nuclei) (Insert = negative control) (200×). (B&F) Alcian blue counterstained with nuclear fast red depicting GAG (blue) and cell nuclei (red) (400×, insert 200×). Black circles in ABNP indicate empty lacunae; black arrowheads in fresh bovine NP indicate cell nuclei. (C&G) Ethidium bromide staining (fluorescent red = DNA) indicated a lack of DNA fragments in the ABNP as compared to fresh tissue. (D&H) DAPI staining (fluorescent blue = nuclei) indicated a lack of nuclei in ABNP as compared to fresh tissue (arrow heads = intact cell nuclei) (200×).
3.2.5 Macroscopic & Histological Evaluation of ABNP Scaffolds
Macroscopically both fresh bovine NP and the ABNP scaffolds (Figure 5) maintained their firm shape and exhibited an average water content of 76.58±1.47% and 94.56±0.37%, respectively. Qualitative histology of the fresh bovine NP tissue depicted the presence of cells in clusters/islands. The fresh bovine NPs also stained more intensely with alcian blue (indicating the presence of GAG within the ECM) as illustrated by a deeper blue staining as compared to ABNP scaffolds (Figure 3B&F), which corroborated DMMB results. Additionally, empty lacunae were visible throughout the ECM of the ABNP scaffolds and ethidium bromide staining confirmed the absence of nucleic acids in ABNP samples (Figure 3D). However, nuclear material was present in the fresh bovine NP as indicated by positive DAPI staining (Figure 3H).
Figure 5.
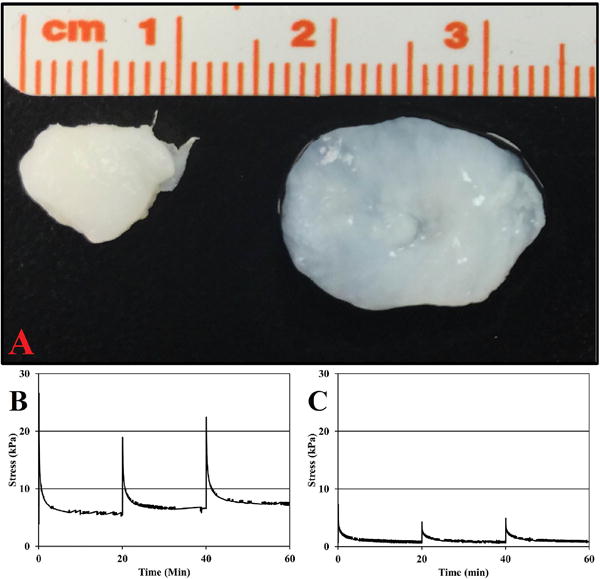
(A) Macroscopic image of (left) fresh bovine NP and (right) ABNP Scaffold. (B & C) Representative unconfined compression stress relaxation curves for (B) fresh bovine NP and (C) ABNP scaffold illustrating relaxation profiles following the sequential application of 8, 12, and 16% strain, respectively.
3.3 Mechanical Analysis
3.3.1 Unconfined Compression
All results, with the exception of Poisson’s ratio, are listed with respect to 8%, 12%, and 16% applied strain. Mean unconfined intrinsic equilibrium Young’s modulus of ABNP scaffolds (10.13±1.58 kPa, 7.59±1.00 kPa, and 5.85±0.90 kPa) were significantly different at only the 8% strain increment (p<0.05) compared to fresh bovine NP (42.18±13.36 kPa, 29.87±9.79 kPa, and 24.76±8.65 kPa). Percent relaxation of ABNP scaffolds (91.86±1.49%, 83.56±2.78%, and 84.67±2.74%) was not significantly different compared to fresh bovine NP (86.82±3.72%, 77.20±4.02%, and 77.75±4.10%) (Figure 4). Apparent Poisson’s ratio from 8-12% strain of ABNP scaffolds (0.142±0.026) was not significantly different compared to values obtained for fresh bovine NP (0.126±0.059). Shear modulus (4.44±0.69 kPa, 3.33±0.44 kPa, and 2.56±0.40 kPa) and aggregate modulus (10.62±1.66 kPa, 7.97±1.05 kPa, and 6.14±0.95 kPa) values for ABNP scaffolds were significantly different at only the 8% strain increment (p<0.05) compared to fresh bovine NP tissue (18.73±5.93 kPa, 13.26±4.35 kPa, and 11.00±3.84 kPa) and (43.77±13.86 kPa, 30.99±10.15 kPa, and 25.69±8.98 kPa), respectively.
Figure 4.
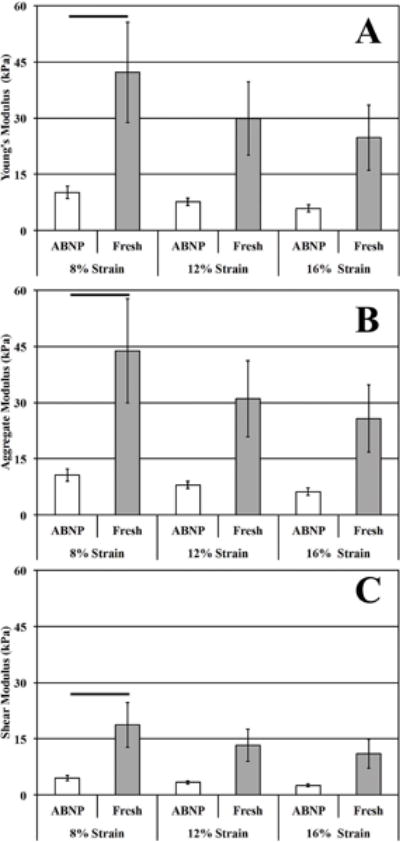
Equilibrium mechanical properties determined following stress relaxation testing to defined applied strain end-points. (A) Intrinsic Young’s modulus, (B) aggregate modulus, and (C) shear modulus. Horizontal bars connecting groups indicate significant differences (p<0.05).
3.3.3 Dynamic Mechanical Analysis (DMA)
Complex, storage, and loss moduli as well as the phase angle were evaluated across a range of frequencies using DMA (Table 2). No statistical differences were observed between the two groups at any of the frequencies tested. Moduli values for each study group demonstrated a test frequency dependent relationship. The range of values observed for ABNP scaffold for the complex, storage, and loss moduli and phase angle were 6.59-13.52kPa, 6.35-12.11kPa, 1.68-4.80kPa, and 14.99-26.44°, respectively over the frequencies tested (Table 2 & Figure 6). Fresh bovine NP values were found within the ranges of 6.36-13.99kPa, 5.99-13.13kPa, 2.12-4.55kPa, and 15.36-20.16°, respectively (Table 2 & Figure 6).
Table 2.
Dynamic viscoelastic properties of ABNP scaffold and fresh bovine NP tissue.
| Complex Modulus (kPA) | ||||
|---|---|---|---|---|
| Frequency (Hz) | 0.01 | 0.1 | 1 | 10 |
| ABNP | 6.59 ± 1.64 | 8.02 ± 1.96 | 9.96 ± 2.22 | 13.52 ± 2.82 |
| Fresh | 6.36 ± 1.13 | 8.56 ± 1.40 | 10.92 ± 1.59 | 13.99 ± 2.00 |
| Storage Modulus (kPA) | ||||
| Frequency (Hz) | 0.01 | 0.1 | 1 | 10 |
| ABNP | 6.35 ± 1.62 | 7.85 ± 1.92 | 10.25 ± 2.65 | 12.11 ± 2.85 |
| Fresh | 5.99 ± 1.11 | 8.23 ± 1.38 | 10.51 ± 1.56 | 13.13 ± 1.90 |
| Loss Modulus (kPA) | ||||
| Frequency (Hz) | 0.01 | 0.1 | 1 | 10 |
| ABNP | 1.68 ± 0.29 | 1.83 ± 0.32 | 2.45 ± 0.41 | 4.80 ± 0.89 |
| Fresh | 2.12 ± 0.30 | 2.29 ± 0.36 | 2.86 ± 0.48 | 4.55 ± 0.83 |
| Phase Angle (Degrees) | ||||
| Frequency (Hz) | 0.01 | 0.1 | 1 | 10 |
| ABNP | 17.3 ± 1.34 | 14.99 ± 1.17 | 16.66 ± 1.59 | 26.44 ± 3.53 |
| Fresh | 20.16 ± 1.61 | 15.93 ± 1.77 | 15.36 ± 1.96 | 18.90 ± 2.37 |
Figure 6.
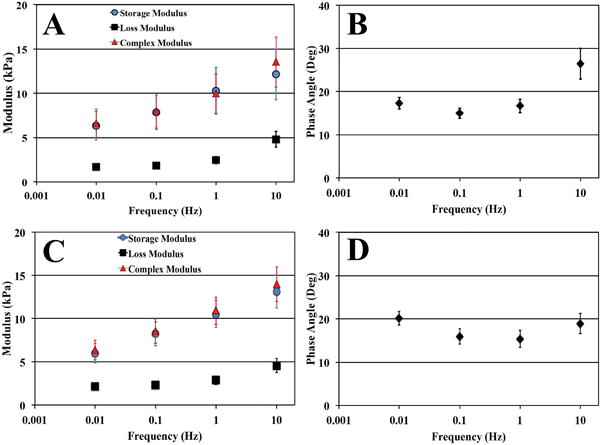
Graphical representation of dynamic mechanical analysis of ABNP (A, B) compared to fresh bovine (C, D) NP tissue test to 8% compressive strain. (A, C) Plots of the average complex (E*, triangles), storage (E’, circles) and loss (E”, Squares) moduli as well as (B, D) phase angle (δ) of test samples over the frequencies tested. No significant differences were found comparing values obtained from ABNP scaffolds and fresh bovine NP tissue.
3.4 Cell Studies
3.4.1 ABNP Cytotoxicity Study
ABNP scaffold cytotoxicity was evaluated by seeding human amniotic mesenchymal stem cells (hAMSCs) onto the surface of the ABNP scaffold and cultured to 14 days. Live/dead fluorescence indicated cell viability to be 93.97±1.90%, 87.14±4.30%, and 95±1.46% at days 3, 7, and 14, respectively (Figure 7) indicating that the scaffold itself was not cytotoxic. There was no noticeable cell migration/penetration into the scaffolds throughout the duration of the study, as hAMSCs appeared to attach and spread on the surface of the scaffolds with increasing time in culture (Figure 7D).
Figure 7.
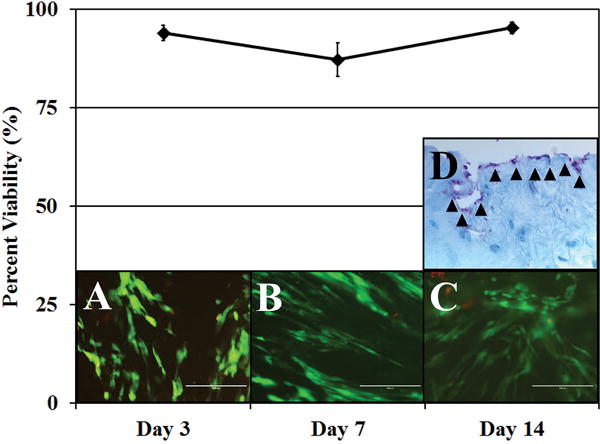
Live/Dead staining on ABNP scaffolds seeded on the surface with hAMSCs for 3, 7, and 14 days illustrating percent cell viability. (Top) Graph of the percent cell viability. (A–C) Representative live/dead images of hAMSC seeded scaffolds (live cells = green, dead cells = red) at following 3, 7 and 14 days of culture, respectively. (D) Representative alcian blue stained ABNP scaffold seeded with hAMSCs (arrow heads) following 14 days of culture illustrating that hAMSCs remained on the seeded surface of the ABNP scaffold and did not migrate in (glycosaminoglycan = blue, cell nuclei = purple). All images were obtained at 200× total magnification.
3.4.2 Cell Injection and Scaffold Cytotoxicity
Cell injection into non-crosslinked and EDC/NHS treated ABNP scaffolds was investigated due to the fact that hAMSCs could not infiltrate the scaffolds when seeded onto the surface. Thus, the stem cells were injected via a 28G syringe in order to minimize scaffold disruption due to needle stick. Viable cells were clearly visible within and around the injection path (Figure 8A-D). Viability of hAMSCs injected within non-crosslinked ABNP scaffolds were 91±4.99% and 97±1.44%, at day 3 and 14, respectively. Comparatively, viability on EDC/NHS crosslinked samples was 73±7.92% and 65±8.12%, respectively at day 3 and 14 (Figure 8E). In general, viability was maintained in both treatment groups over time in culture (i.e. there were no statistical significant differences in viability with respect to time within each study group); however, viability was significantly decreased (p<0.05) in the crosslinked scaffolds as compared to non-crosslinked ABNP at day 14 which would suggest that the crosslinking process needs to be optimized in order to reduce cytotoxicity. Of note, all injected hAMSCs maintained a round morphology reminiscent of NP-like cells within the scaffolds.
Figure 8.
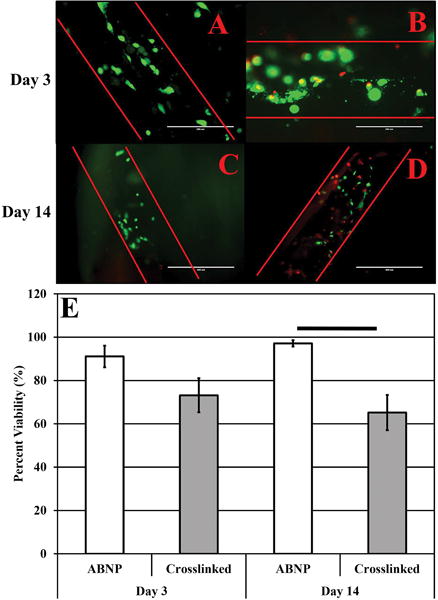
(upper panel) Representative live/dead images of non-crosslinked (A, C) and EDC/NHS treated (B, D) ABNP scaffolds after 3 (200× total magnification) and 14 (100× total magnification) days in culture, respectively. (lower panel) (E) Graphical representation of hAMSC viability within non-crosslinked (“ABNP”) and EDC/NHS treated (“crosslinked”) ABNP after 3 and 14 days in culture. Horizontal bars connecting groups indicate statistical differences (p<0.05).
4. Discussion
Herein it has been demonstrated that an optimal decell procedure can be developed to effectively remove cells and their remnants from bovine caudal NP tissue which resulted in the development of a biomaterial scaffold that contained glycosaminoglycan and collagen type II and had similar mechanical properties as compared values reported in literature for human NP tissue. Furthermore, the scaffold was shown to be cytocompatible with human amniotic mesenchymal stem cells (hAMSCs). Taken together, these results suggest that this biomaterial could be used in conjunction with stem cells as a surrogate to regenerate NP tissue that has succumbed to degeneration or extrusion in patients with IDD and IDH, respectively.
The process of decellularization is a ‘top-down’ approach to biomaterial scaffold fabrication which begins with selecting an appropriate starting material which, when decellularized, would ideally result in a scaffold with similar physico-chemical properties as the target tissue to be replaced/regenerated. This is of critical importance for tissue regeneration as preservation of native tissue ECM within a scaffold has been shown to provide instructive cues that influence cell migration, proliferation, and differentiation.25,26
Considering these points, bovine caudal IVD NP tissue was targeted for decell as it has been shown to have a similar average GAG content comparable to healthy human NP, which can vary from 300-600μg GAG/mg dry tissue weight.19 Bovine NP has also been shown to have similar biochemical composition, resting stress, and height to diameter ratio when compared to human lumbar IVDs and thus was deemed to be an optimal starting material for NP scaffold formation.27
4.1 Decellularization Optimization
Striking a balance between maintenance of native ECM and removing host cells is a primary challenge faced when decelling a tissue; this is particularly evident when attempting to decell NP tissue in which the host cells are encased within a highly hydrated proteoglycan rich matrix. In fact, several groups have attempted to decell NP tissue from various species utilizing a multitude of methods including chemical and physical methods.20,25,28,29 While some have demonstrated the ability to retain key NP ECM components in physiologically relevant quantities and ratios, in many such investigations published results suggested that the resident NP cells were merely devitalized yet remained sequestered within the tissue ECM.29 Conversely, others have demonstrated successful removal of NP cell DNA via disruption of the native NP ECM via freeze-thaw cycles and subsequent ‘tissue grinding’ to create an injectable scaffold for NP regeneration.28 The resulting scaffold material did have a significant reduction in GAG, which is highly susceptible to leaching, and mechanical characteristics of the resultant material were not characterized.
Herein a multitude of decell solutions containing various concentrations of detergents coupled with mechanical agitation (in the form of orbital shaking and ultrasonication) were evaluated over different durations of time; a process which was initially modeled after the work described by Mercuri et al.20 A departure from the original decell protocol used for porcine NP tissue was investigated in an attempt to further reduce the amount of residual host DNA while maintaining GAG content and NP ECM. Additionally, the departure from using porcine tissue has its advantages including a lack of the porcine antigenic epitope α-gal and material properties of bovine tissue, which more closely resemble that of human NP tissue. Key differences in decell solutions investigated herein included; 1) the inclusion of an ethanol pre-wash prior to decell which was used to fully dehydrate the NP tissue prior to immersion in decell solution such that full uptake of the detergents into the interstice of the dehydrated tissue occurred, 2) increased concentrations of chelating and non-ionic detergents (EDTA and Triton X-100, respectively) were evaluated in order to improve decell efficacy, decrease processing time and minimize ECM disruption, and 3) elimination of the ionic detergent deoxycholic acid which appeared to improve resultant biomaterial scaffold micro-architecture.
4.2 Confirmation of Decellularization
The removal of cellular components is imperative for biomaterials developed from the decellularization of xenogeneic tissues. Despite the healthy IVD being considered an immune privilege tissue, the results of degeneration and surgery can create a site of inflammatory reaction.30 The results described herein are in alignment with Gilbert and Crapo et al who provided benchmarks defining minimal criteria for effective tissue decellularization (<50ng DNA/mg dry tissue weight, <200bp DNA fragment length, lack of visible nuclear material via DAPI or H&E staining).25,31 Judicious histological and biochemical analysis of the ABNP scaffold illustrated a complete absence of intact residual cell nuclei and of high and low molecular weight host DNA fragments and quantitative analysis suggest that the average DNA content of the biomaterial scaffold was less than 10ng/mg dry tissue weight indicating successful decell.
4.3 Biochemistry of the ABNP Scaffold
NP tissue biochemistry is severely altered during IDD; average GAG content has been reported to be approximately 87μg/mg dry weight and as low as 18μg/mg dry weight.19 The retention of this key NP ECM component, along with collagen type II, is critical for the mechanical function of the NP tissue,32–34 which creates the osmotic properties and swelling pressures required to support compressive loads and prevents nerve and blood vessel in-growth into the IVD.35–38 Characterization of ABNP scaffolds provided evidence that significant amounts of GAG and the presence of collagen type II were found and resulted in a GAG:HYP ratio of approximately 15:1, which although lower than that reported for healthy NP tissue (27:1), is higher than that found in other NP scaffolds reported in literature.28,39–41 This could prove significant given that starting with these relatively high quantities of ECM may support earlier NP tissue regeneration as less ECM would need to be produced by seeded cells in order to achieve proper mechanical function of the tissue.
4.4 Viscoelastic Properties of the ABNP Scaffold
The viscoelastic properties of the native human NP have been shown to influence both the mechanical behavior of the IVD and the mechanics of the entire functional spinal unit.42,43 With this in mind, an NP scaffold should attempt to mimic or re-establish the native properties of the human NP. Static and dynamic unconfined compressive properties of human NP tissue have been reported in the literature (equilibrium modulus: 5.39±2.56kPa, percent relaxation: 65.77±11.30%, storage modulus: 25-125kPa, loss modulus: 10-47kPa and phase angle: 15-27°).22,23,44 The ABNP scaffold mechanical properties approach these target values, even in the absence of cell seeding suggesting that the biomaterial may be able to mechanically function immediately upon implantation. Expectedly however, the percent relaxation of ABNP scaffolds was significantly higher than that reported for human NP likely owing to increased porosity and GAG loss from the ABNP ECM. Furthermore, values of Poisson’s ratio obtained for both ABNP scaffolds and fresh bovine NP are in agreement with values reported by others (0.125-0.185) which adds further validation to the optical testing method used herein.45,46 Of note, both ABNP and fresh bovine NP displayed a decrease in equilibrium properties with increasing applied strain. Similar phenomenon has been reported by others when testing human NP tissue at similar applied strains and resultant aggregate modulus values of ABNP scaffolds mirror values reported by others when testing NP tissue obtained from human IVDs.22 One could speculate that such phenomenon could be due to the fact that the samples were allowed to freely swell prior to testing which would result in a maximum expansion of the ECM and intermolecular crosslinks. Thus, the application of increasing axial strains and resultant radial deformations could cause increasing damage to the ECM micro-architecture and an increasing inability to resist applied loads/deformations. It should be noted, however that the applied axial strains used during the stress relaxation testing are on the higher end of strains expected in vivo and the unconfined nature of the testing herein does not adequately reflect the constrained conditions as would be experienced when implanted within the IVD. The former point is a limitation of the current study as confined compression testing may yield results more reflective of in vivo conditions.
In general, it is envisioned that the mechanical characteristics of the ABNP scaffolds will increase upon incorporation a healthy alternative cell source, which can produce ECM, in combination with chemical crosslinking (studies ongoing in our lab).
4.5 In vitro Cell Studies
In order to be used to regenerate healthy NP tissue, the ABNP scaffold must be cell friendly. To confirm that the ABNP scaffolds were not cytotoxic and could support a healthy alternative cell source, human amniotic stem cells (hAMSCs) were seeded onto or were injected into non-crosslinked and EDC/NHS crosslinked scaffolds. It has become evident that healthy cells are needed to aid in NP tissue regeneration as they have been shown to aid in tissue remodeling and improve both the biochemical and viscoelastic characteristics of NP tissue.40,47–52 Furthermore, the use of autologous NP cells is not a feasible alternative as these cells are found in limited quantities and their harvest would likely damage the IVD potentially leading to further degeneration. Additionally, NP cells harvested from degenerated IVDs exhibit altered phenotypes with a reduced capacity for ECM synthesis.53 Thus, stem cells are commonly used as an alternative source for tissue engineering/regenerative medicine applications. Herein, human amniotic mesenchymal stem cells (hAMSCs), a novel and largely under investigate cell source for NP tissue regeneration were investigated due to their large cell yields at harvest (especially in comparison to bone marrow and adipose derived counterparts), their multipotent and immunomodulatory characteristics as well as their potential to be used in allogeneic applications.47,50,54,55,56 Herein, it has been demonstrated that stem cell viability was maintained on ABNP scaffolds for up to 14 days in culture on scaffolds with and without crosslinking. Of note however, increased cytotoxicity was observed on scaffolds treated with EDC/NHS. EDC/NHS crosslinking was employed as this chemistry is water soluble, has the ability to crosslink/stabilize GAGs and reduce the propensity of their degradation due to proteases commonly found within the degenerate IVD.53,57–61 The results obtained suggest that EDC/NHS results in a significant reduction in hAMSC viability on ABNP scaffolds and thus studies are currently on-going in our lab to identify crosslinker concentrations which; 1) optimize ABNP scaffold resistance to degradation by enzymes commonly observed during IDD, 2) enhance ABNP scaffold mechanical properties, while 3) minimizing cytotoxicity effects.
5. Conclusion
In conclusion, an effective and efficient decellularization procedure has been developed which when applied to bovine caudal IVD NP tissue resulted in the formation of a mechanically competent biomimetic scaffold supportive of human stem cell viability. Taken together, this material may be used in conjunction with human stem cells for regenerative medicine approaches in patients with IDD or IDH in order to mitigate progressive dysfunction of the IVD. On-going studies using the ABNP scaffolds include optimizing EDC/NHS crosslinking, in vitro studies characterizing hAMSC gene expression and ECM deposition within the scaffolds and in vivo implantation studies investigating the efficacy of the biomaterial with hAMSCs to mitigate IDD progression in an established large animal model.
Acknowledgments
The authors would like to acknowledge Ms. Cynthia Shepard for her assistance in preparing ABNP scaffolds and the Department of Maternal Fetal Medicine at Greenville Health System for their help in obtaining amniotic membranes for hAMSC isolation. Research support has been provided to JM by the National Institute of General Medical Sciences of the National Institutes of Health (award numbers: 5P20GM103444-07 and 2P20GM103499-15) and departmental funds allocated to Dr. Mercuri.
Footnotes
Author Contributions
Christopher Fernandez: Conception and design, collection/assembly of data, data interpretation, manuscript preparation, final approval of manuscript.
Alan Marionneaux: Data interpretation, manuscript preparation, final approval of manuscript
Sonny Gill: Data interpretation, final approval of manuscript
Jeremy Mercuri: Conception and design, data interpretation, manuscript preparation, final approval of manuscript
References
- 1.Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non-specific low back pain. Lancet. 2012;379:482–491. doi: 10.1016/S0140-6736(11)60610-7. [DOI] [PubMed] [Google Scholar]
- 2.Martin BI, et al. Expenditures and health status among adults with back and neck problems. JAMA. 2008;299:656–664. doi: 10.1001/jama.299.6.656. [DOI] [PubMed] [Google Scholar]
- 3.Luoma K, et al. Low Back Pain in Relation to Lumbar Disc Degeneration. Spine (Phila Pa 1976) 2000;25 doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 4.Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–62. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivan SS, Wachtel E, Roughley P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim Biophys Acta. 2014;1840:3181–9. doi: 10.1016/j.bbagen.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Campana S, et al. Relationships between viscoelastic properties of lumbar intervertebral disc and degeneration grade assessed by MRI. J Mech Behav Biomed Mater. 2011;4:593–9. doi: 10.1016/j.jmbbm.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Hanley EN, Shapiro DE. The development of low-back pain after excision of a lumbar disc. J Bone Joint Surg Am. 1989;71:719–21. [PubMed] [Google Scholar]
- 8.Lorme KJ. THE BIOMECHANICS OF BACK PAIN. Australas Chiropr Osteopat. 2003;11:755–762. [Google Scholar]
- 9.Whatley BR, Wen X. Intervertebral disc (IVD): Structure, degeneration, repair and regeneration. Mater Sci Eng C. 2012;32:61–77. [Google Scholar]
- 10.Wang F, Shi R, Cai F, Wang YT, Wu XT. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015;24:2479–2495. doi: 10.1089/scd.2015.0158. [DOI] [PubMed] [Google Scholar]
- 11.Le Maitre CL, Baird P, Freemont AJ, Hoyland JA. An in vitro study investigating the survival and phenotype of mesenchymal stem cells following injection into nucleus pulposus tissue. Arthritis Res Ther. 2009;11:R20. doi: 10.1186/ar2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leckie SK, et al. Injection of human umbilical tissue–derived cells into the nucleus pulposus alters the course of intervertebral disc degeneration in vivo. Spine J. 2013;13:263–272. doi: 10.1016/j.spinee.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2010;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woiciechowsky C, et al. Regeneration of nucleus pulposus tissue in an ovine intervertebral disc degeneration model by cell-free resorbable polymer scaffolds. J Tissue Eng Regen Med. 2014;8:811–820. doi: 10.1002/term.1582. [DOI] [PubMed] [Google Scholar]
- 15.Priyadarshani P, Li Y, Yang S, Yao L. Injectable hydrogel provides growth-permissive environment for human nucleus pulposus cells. J Biomed Mater Res Part A. 2015 doi: 10.1002/jbm.a.35580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou AI, Akintoye SO, Nicoll SB. Photo-crosslinked alginate hydrogels support enhanced matrix accumulation by nucleus pulposus cells in vivo. Osteoarthritis Cartilage. 2009;17:1377–84. doi: 10.1016/j.joca.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in Biologic Scaffold Materials. J Surg Res. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iatridis JC, MacLean JJ, O’Brien M, Stokes IAF. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila Pa 1976) 2007;32:1493–1497. doi: 10.1097/BRS.0b013e318067dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercuri JJ, Gill SS, Simionescu DT. Novel tissue-derived biomimetic scaffold for regenerating the human nucleus pulposus. J Biomed Mater Res A. 2011;96:422–35. doi: 10.1002/jbm.a.33001. [DOI] [PubMed] [Google Scholar]
- 21.Mercuri JJ, et al. Regenerative potential of decellularized porcine nucleus pulposus hydrogel scaffolds: stem cell differentiation, matrix remodeling, and biocompatibility studies. Tissue Eng Part A. 2013;19:952–66. doi: 10.1089/ten.TEA.2012.0088. [DOI] [PubMed] [Google Scholar]
- 22.Freeman AL, Buttermann GR, Beaubien BP, Rochefort WE. Compressive properties of fibrous repair tissue compared to nucleus and annulus. J Biomech. 2013;46:1714–21. doi: 10.1016/j.jbiomech.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 23.Cloyd JM, et al. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur Spine J. 2007;16:1892–8. doi: 10.1007/s00586-007-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Annu Rev Biomed Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- 25.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Londono R, Badylak S. Biologic Scaffolds for Regenerative Medicine: Mechanisms of In vivo Remodeling. Ann Biomed Eng. 2014:1–16. doi: 10.1007/s10439-014-1103-8. [DOI] [PubMed] [Google Scholar]
- 27.Perie DS, Maclean JJ, Owen JP, Iatridis JC. Correlating material properties with tissue composition in enzymatically digested bovine annulus fibrosus and nucleus pulposus tissue. Ann Biomed Eng. 2006;34:769–77. doi: 10.1007/s10439-006-9091-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Illien-Jünger S, et al. Development of a bovine decellularized extracellular matrix-biomaterial for nucleus pulposus regeneration. J Orthop Res. 2015;34:876–88. doi: 10.1002/jor.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan LKY, et al. Decellularized bovine intervertebral disc as a natural scaffold for xenogenic cell studies. Acta Biomater. 2013;9:5262–72. doi: 10.1016/j.actbio.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert TW, Freund JM, Badylak SF. Quantification of DNA in Biologic Scaffold Materials. J Surg Res. 2009;152:135–139. doi: 10.1016/j.jss.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwale F, Roughley P, Antoniou J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater. 2004;8:58–64. doi: 10.22203/ecm.v008a06. [DOI] [PubMed] [Google Scholar]
- 33.Bibby S, Jones D, Lee R, Yu J, Urban J. The pathophysiology of the intervertebral disc. Jt Bone Spine. 2001;68:537–542. doi: 10.1016/s1297-319x(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 34.Sowa GA, et al. Alterations in gene expression in response to compression of nucleus pulposus cells. Spine J. 2011;11:36–43. doi: 10.1016/j.spinee.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middleditch A, O J. Functional Anatomy of the Spine. 2005 [Google Scholar]
- 36.Sztrolovics R, Alini M, Roughley P, Mort J. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;241:235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivan SS, Wachtel E, Roughley P. Structure, function, aging and turnover of aggrecan in the intervertebral disc. Biochim Biophys Acta. 2014;1840:3181–9. doi: 10.1016/j.bbagen.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Johnson WEB, et al. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- 39.Van Dijk BGM, Potier E, Ito K. Long-term culture of bovine nucleus pulposus explants in a native environment. Spine J. 2013;13:454–463. doi: 10.1016/j.spinee.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Yuan M, et al. Effects of nucleus pulposus cell-derived acellular matrix on the differentiation of mesenchymal stem cells. Biomaterials. 2013;34:3948–61. doi: 10.1016/j.biomaterials.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Rahaman MN, Bal BS. Modulating Notochordal Differentiation of Human Induced Pluripotent Stem Cells Using Natural Nucleus Pulposus Tissue Matrix. PLoS One. 2014;9:e100885. doi: 10.1371/journal.pone.0100885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J. 2013;13:243–62. doi: 10.1016/j.spinee.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nerurkar NL, Elliott DM, Mauck RL. Mechanical design criteria for intervertebral disc tissue engineering. J Biomech. 2010;43:1017–30. doi: 10.1016/j.jbiomech.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umehara S, et al. Effects of Degeneration on the Elastic Modulus Distribution in the Lumbar Intervertebral Disc. Spine (Phila Pa 1976) 1996;21 doi: 10.1097/00007632-199604010-00007. [DOI] [PubMed] [Google Scholar]
- 45.Farrell MD, Riches PE. On the Poisson’s Ratio of the Nucleus Pulposus. J Biomech Eng. 2013;135:104501. doi: 10.1115/1.4025180. [DOI] [PubMed] [Google Scholar]
- 46.Jurvelin JS, Buschmann MD, Hunziker EB. Optical and mechanical determination of poisson’s ratio of adult bovine humeral articular cartilage. J Biomech. 1997;30:235–241. doi: 10.1016/s0021-9290(96)00133-9. [DOI] [PubMed] [Google Scholar]
- 47.Dai J, et al. Dynamic compression and co-culture with nucleus pulposus cells promotes proliferation and differentiation of adipose-derived mesenchymal stem cells. J Biomech. 2014;47:966–972. doi: 10.1016/j.jbiomech.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 48.Wang F, Shi R, Cai F, Wang YT, Wu XT. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015;24:2479–2495. doi: 10.1089/scd.2015.0158. [DOI] [PubMed] [Google Scholar]
- 49.Liu Y, Fu S, Rahaman MN, Mao JJ, Bal BS. Native nucleus pulposus tissue matrix promotes notochordal differentiation of human induced pluripotent stem cells with potential for treating intervertebral disc degeneration. J Biomed Mater Res A. 2014;8-11 doi: 10.1002/jbm.a.35243. [DOI] [PubMed] [Google Scholar]
- 50.Ni L, et al. Effects of hypoxia on differentiation from human placenta-derived mesenchymal stem cells to nucleus pulposus-like cells. Spine J. 2014;14:2451–8. doi: 10.1016/j.spinee.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 51.Huang YC, Leung VYL, Lu WW, Luk KDK. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J. 2013;13:352–62. doi: 10.1016/j.spinee.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Sakai D, Andersson GBJ. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243–256. doi: 10.1038/nrrheum.2015.13. [DOI] [PubMed] [Google Scholar]
- 53.Yang SH, Hu MH, Sun YH, Lin FH. Differential phenotypic behaviors of human degenerative nucleus pulposus cells under normoxic and hypoxic conditions: influence of oxygen concentration during isolation, expansion, and cultivation. Spine J. 2013;13:1590–6. doi: 10.1016/j.spinee.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Keeley R, Topoluk N, Mercuri J. Tissues Reborn: Fetal Membrane-Derived Matrices and Stem Cells in Orthopedic Regenerative Medicine. Crit Rev Biomed Eng. 2014;42:249–270. doi: 10.1615/critrevbiomedeng.2014011591. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Jiang F, Liang Y, Shen M, Chen N. Human Amnion-Derived Mesenchymal Stem Cells Promote Osteogenic Differentiation in Human Bone Marrow Mesenchymal Stem Cells by Influencing the ERK1/2 Signaling Pathway. Stem Cells Int. 2016;2016:4851081. doi: 10.1155/2016/4851081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim EY, Lee KB, Kim MK. The potential of mesenchymal stem cells derived from amniotic membrane and amniotic fluid for neuronal regenerative therapy. BMB Rep. 2014;47:135–140. doi: 10.5483/BMBRep.2014.47.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4) Matrix Biol. 2002;21:499–511. doi: 10.1016/s0945-053x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 58.Tian Y, et al. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-κB. Am J Pathol. 2013;182:2310–2321. doi: 10.1016/j.ajpath.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang M, et al. Alterations of ADAMTSs and TIMP-3 in human nucleus pulposus cells subjected to compressive load: Implications in the pathogenesis of human intervertebral disc degeneration. J Orthop Res. 2012;30:267–273. doi: 10.1002/jor.21507. [DOI] [PubMed] [Google Scholar]
- 60.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 61.Vo NV, et al. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13:331–41. doi: 10.1016/j.spinee.2012.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]


